Abstract
Background and aims
The association between minimally elevated coronary artery calcification (CAC) and cerebrovascular disease is not well known. We assessed whether individuals with minimal CAC (Agatston scores of 1–10) have higher ischemic stroke or transient ischemic attack (TIA) frequencies compared with those with no CAC. We also investigated the relative prevalence of carotid atherosclerosis in these two groups.
Methods
A total of 3924 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) without previous cardiovascular events, including stroke, and with baseline CAC scores of 0–10 were followed for the occurrence of incident ischemic stroke/TIA. We used carotid ultrasound to detect carotid artery plaques and to measure the intima-media thickness (IMT).
Results
During a median follow-up of 13.2 years, 130 participants developed incident ischemic stroke/TIA. There was no significant difference in the ischemic stroke/TIA incidence between those with minimal CAC and no CAC (3.7 versus 2.7 per 1000 person-years). In participants with minimal CAC, we observed a significant association of the condition with an internal carotid artery (ICA) that had a greater-than-average IMT (ICA-IMT; β = 0.071, p = 0.001) and a higher odds ratio (OR) for carotid artery plaques (OR 1.46; with a 95% confidence interval [CI] of 1.18–1.80; p < 0.001).
Conclusions
A CAC score of 0–10 is associated with a low rate of ischemic stroke/TIA, and thus a minimal CAC score is not a valuable predictive marker for ischemic stroke/TIA. A minimal CAC score may, however, provide an early and asymptomatic sign of carotid artery disease.
Keywords: Ischemic stroke/Transient ischemic attack, Coronary artery calcification, Carotid artery lesion, Carotid artery intima-media thickness
1. Introduction
Coronary artery calcification (CAC) is an established marker of subclinical atherosclerosis and an independent predictor of future coronary heart disease (CHD)1–3, cardiovascular disease (CVD)4 and all-cause mortality5. Individuals with even minimal CAC scores may have a higher risk of CHD and atherosclerotic cardiovascular disease including stroke compared with those without CAC, which suggests that patients with minimal CAC represent a distinct risk group 6–9. In contrast, the negative predictive value of the absence of CAC is also well established for major adverse cardiovascular events 10, and individuals with an absence of CAC have a low mortality rate regardless of risk factors.3, 11 Similar associations with ischemic stroke/transient ischemic attack (TIA) have been reported for coronary atherosclerosis as assessed by CAC.12–17 A recent publication from the Multi-Ethnic Study of Atherosclerosis (MESA) showed an increase in ischemic stroke/TIA risk in individuals with a low CAC score of 1–99 compared with those without CAC.15, 18 In contrast, the prognostic value of a minimal CAC score of 1–10, compared with no CAC, relative to the incidence of ischemic stroke/TIA has not been fully investigated. We hypothesize that individuals with minimal CAC could have a higher risk of ischemic stroke/TIA compared with those without CAC. Moreover, carotid artery atherosclerosis, which potentially causes ischemic stroke/TIA, could be greater in individuals with minimal CAC relative to those without CAC because of a positive association of CAC and carotid artery atherosclerosis.19, 20
In this study, we assessed whether individuals with minimal CAC (corresponding to an Agatston score of 1–10) have similar or higher ischemic stroke/TIA event rates when compared with those without CAC. We also investigated the prevalence of carotid artery atherosclerosis in individuals with minimal or no CAC.
2. Materials and methods
2.1. Study population
The MESA design has been described in detail.21 The MESA is a prospective observational cohort of 6814 men and women who, at baseline, were free of clinical cardiovascular disease and were 45–84 years old. Patient history of clinical cardiovascular disease was assessed by self-reported information of a physician-diagnosed heart attack or angina or the prescribed use of nitroglycerin; physician-diagnosed stroke or TIA; physician-diagnosed heart failure; current atrial fibrillation or having undergone procedures related to cardiovascular disease (coronary artery bypass graft surgery, angioplasty, valve replacement, pacemaker or defibrillator implantation, any surgery on the heart or arteries). The participants were recruited from six field centers in Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York and St. Paul, Minnesota. The specific racial/ethnic groups recruited were Caucasian, African-American, Hispanic and Chinese. Participants were enrolled from July 2000 through September 2002. The study was approved by the Institutional Review Boards at each site, and all participants gave written informed consent. In this study, we included 3924 MESA participants with baseline CAC scores of 0–10.
2.2. Risk factors
As part of the baseline examination, staff at each of the six centers collected information about cardiovascular risk factors. Medical history, anthropometric measurements and laboratory data were collected during the first examination of individuals from the MESA cohort (July 2000 to August 2002). Information about age, gender, ethnicity and medical history was obtained through questionnaires. “Current smoking” was defined as cigarette smoking in the last 30 days, whereas “former smoker” was defined as an individual who had not smoked in the last 30 days but had smoked ≥100 cigarettes in his/her lifetime. Diabetes was defined as fasting glucose of ≥7.0 mmol/l (126 mg/dl) or use of hypoglycemic drugs. Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg or use of antihypertensive medication. Total cholesterol, high-density lipoprotein (HDL) cholesterol and triglyceride levels from blood samples obtained after a 12-h fast were measured at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, Minnesota). Low-density lipoprotein (LDL) cholesterol was calculated with the Friedewald equation.22
2.3. CAC measurements
The MESA methods for computed tomography (CT) scanning and interpretation have been published.23 CAC was assessed at all six MESA sites during the first examination of individuals from the MESA cohort by using either a cardiac-gated electron-beam CT scanner (Chicago, Los Angeles and New York field centers) or a multi-detector CT system (Baltimore, Forsyth County and St. Paul field centers). We used the average Agatston score from the two scans in all analyses, and the results were calibrated against a phantom containing known densities of calcium hydroxyapatite. All CT scans were read by a trained radiologist or cardiologist at a central reading center (Los Angeles Biomedical Research Institute at Harbor UCLA), who were blinded to all clinical and demographic patient information.
2.4. Carotid artery assessments
At the first examination, B-mode ultrasonography was used to image the near and far walls of the right and left distal CCA, carotid bulb and proximal ICA using a Logiq 700 ultrasound system (General Electric Medical Systems, 13 MHz transducer). The carotid bifurcations and ICAs were examined thoroughly at 9 MHz from both longitudinal and transverse approaches to identify the thickest regions. Images were stored on super-VHS videotape and digitized on a workstation at the Tufts Medical Center Ultrasound Reading Center. Methods for measuring and interpreting carotid intima-media thickness (IMT) have been reported.24 Carotid artery assessments were made by blinded researchers. The mean maximal IMTs of the CCA (CCA-IMT) and carotid bulb + internal carotid artery (ICA) (ICA-IMT) were obtained by averaging the bilateral maximal measurements from the near and far walls at each projection. Carotid lesions were screened in either the CCA or ICA/carotid bulb. Carotid lesions was classified into six categories, numbered 0–5, with a 0 indicating no lesion, and 1–5 indicating various levels of stenosis (1, 1–24%; 2, 25–49%; 3, 50–74%; 4, 75–99%; 5, 100%) and, moreover, we further subdivided these categories into those with and without stenotic lesion, which were defined as the presence or absence of carotid artery plaques, respectively.25
The inter-scan reproducibility was obtained by having the same reader blindly read a baseline scan and a separate repeat ultrasound examination. The correlation coefficients were 0.92 (95% CI: 0.89 to 0.94) for the CCA-IMT (n = 143) and 0.90 (95% CI: 0.86–0.95) for the ICA-IMT (n = 140). Moreover, for carotid plaque presence/absence, intra-reader reproducibility was κ=0.89 (95% Cl, 0.85–0.92).
2.5. Stroke/TIA
Participants were followed from baseline examination (2000–2002) through 2016. They were contacted by telephone every 9–12 months to inquire about interim hospital admissions, cardiovascular outpatient diagnoses and deaths. To verify self-reported diagnoses, information was collected from death certificates and medical records for all hospitalizations and outpatient cardiovascular diagnoses.26 Stroke was defined as the rapid onset of documented focal neurological deficit lasting 24 h or until death, or, if the deficit lasted <24 h, a diagnosis of stroke was confirmed if there was a clinically relevant lesion on brain imaging (typically CT or magnetic resonance imaging) and no nonvascular cause. TIA was defined as a focal neurological deficit lasting <24 h without detection of stroke by brain imaging. Patients with focal neurological deficits secondary to brain trauma, tumor, infections or other non-vascular cause were excluded. Stroke/TIA events were adjudicated by a MESA committee that included cardiologists, physician-epidemiologists and neurologists. The reviewers were blinded to the study data. If a disagreement between reviewers persisted after review and adjudication, the case was discussed and a consensus reached. A detailed description of the adjudication process has been published.21
2.6. Statistical analysis
The association of CAC score subsets with the presence of carotid artery plaque was assessed using multivariable-adjusted logistic regression, and with CCA-IMT and ICA-IMT data using multivariable linear regression analysis. The values were adjusted for age, gender, race, body mass index (BMI), diabetes mellitus, systolic/diastolic blood pressure, total cholesterol, HDL cholesterol, cigarette smoking status, use of blood pressure–lowering agents, family history of heart attack, family history of stroke and statin use. Event rates were estimated by dividing the number of events by the number of person-years at risk. Only the first stroke/TIA event for each participant was included. The multivariable-adjusted Cox proportional hazards model was used to assess the hazard ratio (HR) of minimal CAC with the incidence of stroke/TIA. The model was adjusted for age, gender and race/ethnicity.
3. Results
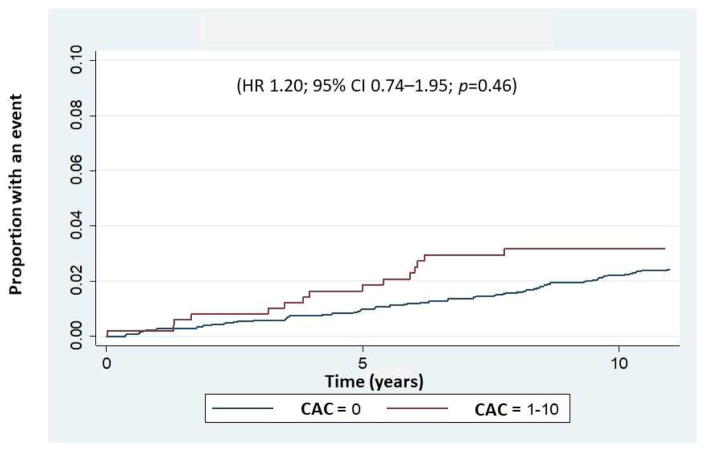
This study population consisted of 3924 asymptomatic individuals without known ischemic stroke/TIA at baseline (mean age: 58.4±9.3 years; 38.2% males). Of the 3924 individuals, 3416 (87%) had a CAC score of zero and 508 (13%) individuals had minimal CAC (CAC score of 1–10). As shown in Table 1, individuals with a minimal CAC score were more likely to be male, older and Caucasian and to have hypertension, dyslipidemia and a family history of heart attack. Overall, 130 ischemic stroke/TIA events (3.3%) including 99 ischemic strokes and 31 TIAs were observed in the total study population over a median 13.2-year follow-up. In 111 (3.3%) individuals with a CAC score of zero and 19 (3.7%) with a minimal CAC score, ischemic stroke/TIA occurred with annual incident rates per 1000 persons of 2.70 and 3.22, respectively (Fig. 1). Although individuals with a minimal CAC score had a slightly higher incidence of ischemic stroke/TIA compared with those with a CAC score of zero, the association was not significant in either an unadjusted model (HR 1.20, 95% CI 0.74–1.95, p=0.46), or in a model adjusted for age, gender and race/ethnicity (HR 1.05, 95% CI 0.64–1.73, p=0.84). In addition, using ischemic stroke alone as the outcome, the unadjusted HR for minimal CAC was 1.25 (95% CI 0.72–2.17, p=0.422) and the HR adjusted for age, gender and race was 1.06 (95% CI 0.61–1.86, p=0.83).
Table 1.
Baseline demographics and traditional risk factors between individuals with a CAC score of zero and those with a minimal CAC score
| Variable | All individuals (n=3924) mean±SDa or N (%) | CAC score=0 (n=3416) mean±SDa or N (%) | CAC score=1–10 (n=508) mean±SDa or N (%) | p-value |
|---|---|---|---|---|
| Age (years) | 58.4±9.3 | 58.0±9.1 | 61.6±9.9 | <0.001 |
| Male | 1498 (38.2) | 1249 (36.6) | 249 (49.0) | <0.001 |
| Race/ethnicity | 0.001 | |||
| Caucasian | 1336 (34.1) | 1126 (33.0) | 210 (41.3) | |
| Chinese | 463 (11.8) | 400 (11.7) | 63 (12.4) | |
| African American | 1203 (30.7) | 1072 (31.4) | 131 (25.8) | |
| Hispanic | 922 (23.5) | 818 (24.0) | 104 (20.5) | |
| Hypertension | 1425 (36.3) | 1200 (35.1) | 225 (44.3) | <0.001 |
| LDL cholesterol (mg/dl) | 116.7±31.1 | 116.0±30.7 | 120.9±33.0 | 0.001 |
| HDL cholesterol (mg/dl) | 52.2±15.0 | 52.5±15.0 | 50.4±14.5 | 0.003 |
| Lipid-lowering medication | 463 (11.8) | 362 (10.6) | 101 (19.9) | <0.001 |
| Smoking status | 0.073 | |||
| Never | 2161 (55.3) | 1905 (56.0) | 256 (50.6) | |
| Former | 1220 (31.2) | 1045 (30.7) | 175 (34.6) | |
| Current | 526 (13.5) | 451 (13.3) | 75 (14.8) | |
| Diabetes | 375 (9.6) | 318 (9.4) | 57 (11.2) | 0.181 |
| Family history of heart attack | 1414 (38.0) | 1203 (37.2) | 211 (43.5) | 0.008 |
| Carotid lesions | <0.001 | |||
| 0, no lesion | 2747 (71.0) | 2449 (72.8) | 298 (59.0) | |
| 1, 1–24% | 885 (22.9) | 728 (21.6) | 157 (31.1) | |
| 2, 25–49% | 224 (5.8) | 181 (5.4) | 43 (8.5) | |
| 3, 50–74% | 5 (0.13) | 3 (0.09) | 2 (0.4) | |
| 4, 75–99% | 7 (0.18) | 4 (0.12) | 3 (0.6) | |
| 5, 100% | 3 (0.08) | 1 (0.03) | 2 (0.4) |
SD: standard deviation.
Fig. 1.
Kaplan-Meier failure curve showing time to first occurrence of an ischemic stroke/TIA event during the follow-up period of 13.2 years.
Data were analyzed for individuals with a CAC score of zero and for those with a CAC score of 1–10.
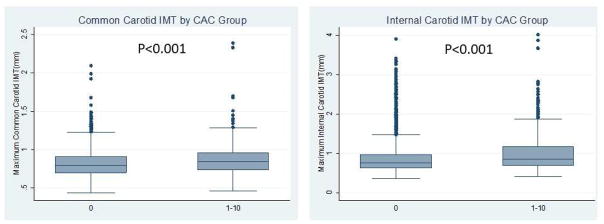
A comparison of maximum CCA-IMT and ICA-IMT values between the subjects with a CAC score of zero and those with a minimal CAC score is shown in Fig. 2. Compared with individuals with a CAC score of zero, those with a minimal CAC score had significantly higher values for the ICA-IMT (0.89±0.42 mm vs. 1.03±0.54 mm, p<0.001) and CCA-IMT (0.82±0.17 mm vs.0.86±0.20 mm, p<0.001), and a higher prevalence of carotid artery plaques (27.2% vs. 41.0%, p<0.001) (Table 1).
Fig. 2.
Box plots representing the CCA-IMT and ICA-IMT measurements for groups with different CAC scores.
The upper edge of the box indicates the 75th percentile, the lower edge indicates the 25th percentile and the middle line indicates the 50th percentile; whiskers (lines extending from the box top and bottom) indicate the highest and the lowest values that are not outliers or extreme values, and circles represent outliers.
Multivariable-adjustment analysis showed no correlation between a minimal CAC score and a greater-than-average CCA-IMT (coefficient 0.009, 95% CI −0.005 to 0.023, p=0.223). In contrast, compared with those with a CAC score of zero, individuals with a minimal CAC score showed a greater-than-average ICA-IMT (coefficient 0.071, 95% CI 0.031–0.111, p=0.001) and higher odds of having a carotid artery plaque (OR 1.457, 95% CI 1.182–1.795, p<0.001) (Tables 2 and 3).
Table 2.
Unadjusted and multivariable linear regression analysis of CAC score and maximum CCA-IMT and ICA-IMT values.
| Model | Univariate β (SE)a, p-value | Age, gender and race/ethnicity adjusted β (SE)a, p-value | Multivariable-adjustedc β (SE)a, p-value |
|---|---|---|---|
| Maximum CCA-IMT | |||
| CAC score=0 | Reference | Reference | Reference |
| CAC score=1–10 | 0.044 (0.008), <0.001 | 0.017 (0.007), 0.023 | 0.009 (0.007), 0.223 |
| Maximum ICA-IMT | |||
| CAC score=0 | Reference | Reference | Reference |
| CAC score=1–10 | 0.144 (0.021), <0.001 | 0.102 (0.021), <0.001 | 0.071 (0.020), 0.001 |
β (SE): beta coefficient (standard error);
NA: not applicable;
Multivariable-adjusted: adjusted for age, gender, race, BMI, diabetes mellitus, systolic blood pressure, total cholesterol, HDL cholesterol, cigarette smoking status, blood pressure–lowering agent use, family history of stroke/myocardial infarction (MI) and statin use.
Table 3.
Unadjusted and multivariable logistic regression analysis of the presence of carotid lesions and CAC scores.
| Model | Univariate ORa (95% CIb), p-value | Age, gender and race/ethnicity adjusted ORa (95% CIb), p-value | Multivariable-adjustedc ORa (95% CIb), p-value |
|---|---|---|---|
| Carotid lesion | |||
| CAC score=0 | Reference | Reference | Reference |
| CAC score=1–10 | 1.87 (1.55–2.27), <0.001 | 1.62 (1.32–1.98), <0.001 | 1.46 (1.18–1.80), <0.001 |
OR, odds ratio;
CI, confidence interval.
Adjusted for age, gender, race, BMI, diabetes mellitus, systolic blood pressure, total cholesterol, HDL cholesterol, cigarette smoking status, use of blood pressure–lowering agents, family history of stroke/MI and statin use.
4. Discussion
This large, population-based, multi-ethnic study with over a decade of follow-up demonstrated a low rate of ischemic stroke/TIA among individuals with either the absence of CAC or minimal CAC scores. Meanwhile, there was no significant difference in the ischemic stroke/TIA incidence between those two groups, suggesting that a minimal CAC score is not suitable for identifying individuals at high risk of ischemic stroke and TIA, even though those individuals are potentially at higher risk for CHD/CVD when compared with individuals with CAC scores of zero. Meanwhile, individuals with minimal CAC scores had significantly higher ICA-IMT and CCA-IMT values, which was consistent with our previous findings.6 Moreover, the presence of a minimal CAC score showed a significant correlation with an increased probability of having carotid artery plaques and larger ICA-IMT values after adjusting for conventional cardiovascular risk factors, suggesting that a minimal CAC score may provide an early and asymptomatic sign of carotid artery disease.
In the current study, we focused on ischemic stroke/TIA and showed a non-significant association between the CAC score and ischemic stroke/TIA incidence. The association was not significant even in an unadjusted analysis, and there was only a 5% increase in the HR in a model adjusted for age, gender and race/ethnicity. Potentially, CAC may not be the best marker to assess ischemic stroke/TIA. An increased CAC score may, therefore, be a stronger predictor of CHD than of ischemic stroke/TIA, which may vary simply due to the extent of the vascular territory that is affected. Whereas several studies have shown a positive association of the CAC score with the occurrence of ischemic stroke/TIA 14, 15, 18, 27, there are no available data concerning the prognostic value of CAC for ischemic stroke/TIA in those with a minimal CAC score. We observed annualized ischemic stroke/TIA rates of 2.70 per 1000 persons for individuals with a zero CAC score and 3.22 per 1000 persons for those with a minimal CAC score. Both rates are comparable to those observed in the general population.28 These results support a classification scheme in which individuals with minimal CAC scores are placed into the same low-risk category for ischemic stroke/TIA as those having a CAC score of zero.
Our study showed a significant association between a minimal CAC score and a prevalence of carotid artery plaque and a higher ICA-IMT. These results support a previous investigation that found an increase in incident CHD among subjects with a minimal CAC.6 Several studies have shown the robustness of the relationship between carotid artery plaque/ICA-IMT and CHD/CVD.17, 29–31 A recent MESA study confirmed the predictive value of the presence of carotid artery plaque for CHD/CVD as compared with conventional risk factor models only.17 Moreover, recent studies have shown a positive association between ICA-IMT and CHD/CVD and confirmed the predictive, reclassifying or discriminative value of this measurement for the risk of CHD/CVD. 25, 32, 33 Our data suggest that individuals with minimally increased CAC scores might have a higher risk for CHD/CVD but apparently do not have an increased risk of ischemic stroke/TIA. A carotid artery evaluation even in low-risk individuals with CAC scores of 0–10 may provide predictive value for the early detection of subclinical CHD/CVD.
The strengths of our study include its large sample size, multi-ethnic nature of the cohort, adjudicated ischemic stroke/TIA, and the long duration of follow-up. Some limitations of the present report should, however, be addressed. First, the number of ischemic stroke/TIA was relatively small— there were only 19 ischemic stroke/TIA events in the minimal CAC score group, so these results must be interpreted cautiously. The numbers were also inadequate to evaluate the ethnic-specific risk of minimal CAC. In addition, 63% of the individuals with CAC score of zero and 51% of those with minimal CAC were female. The observation that females are overrepresented in the overall sample (62%) may have affected the lower event rate in the study. Second, as in other similar studies, the analysis did not consider the effect of any change of preventive medical therapy during the follow-up, and this may have affected our event rate and results. Third, a carotid artery assessment was not performed on all individuals with a CAC score of 0–10. The completion rate for carotid assessment was 97% (3807/3924). Another limitation is possible residual confounding as this was an observational study.
Combining CAC with other measures of subclinical atherosclerosis such as carotid IMT or plaque presence may be more promising for discriminating ischemic stroke/TIA.34,35, 36 Potentially, increased carotid artery IMT and the presence of carotid plaque are associated with intracranial atherosclerosis37, which is strongly associated with ischemic stroke/TIA.38 However, carotid artery IMT has not been routinely recommended for atherosclerotic cardiovascular disease risk assessment due to a lack of sufficient evidence in the current guidelines.39 The available data were based on a single meta-analysis that concentrated on IMT measurements made using different techniques and locations in the CCA.40 Even among individuals with a CAC score of 0–10, the number of adverse events showed a slight tendency to increase given a higher carotid artery burden.41 Given the positive association of the atherosclerotic burden between IMT and CAC as described in the current study, CAC may be an effective tool to identify individuals with minimal CAC scores who would benefit from carotid IMT examination. Further research for evaluating the clinical usefulness of combining minimal CAC and carotid artery measurements to predict ischemic stroke/TIA is clearly warranted, and our group is currently working on such investigations.
Risk of cerebrovascular events in individuals with a CAC score of 0–10 is unknown.
CAC score of 0–10 indicated a low rate of ischemic stroke/transient ischemic attack (TIA) events.
Ischemic stroke/TIA risk did not differ between those with minimal CAC and no CAC.
Carotid artery disease was significantly associated with a minimal CAC score.
CAC score may provide an early and asymptomatic sign of carotid artery disease.
Acknowledgments
Financial support
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079 and UL1-TR-001420 from NCATS.
The authors would like to thank the investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Conflict of interest
Matthew J. Budoff: NIH, General Electric Healthcare. The other authors have nothing to disclose.
Author contributions
KO, RN, RLM, WB, IC, NN, SR, HQ, MK and MJB designed the study, and KO and RN and RLM wrote the initial draft of the manuscript. RLM, JFP and RLS contributed to the analysis and interpretation of data and assisted in the preparation of the manuscript. All authors have contributed to data collection and interpretation and critically reviewed the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Nasir K, McClelland RL, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2009;53:345–352. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman MG, Blaha MJ, Krumholz HM, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanishi R, Li D, Blaha MJ, et al. The relationship between coronary artery calcium score and the long-term mortality among patients with minimal or absent coronary artery risk factors. Int J Cardiol. 2015;185:275–281. doi: 10.1016/j.ijcard.2015.03.146. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158:554–561. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Valenti V, BOH, Heo R, et al. A 15-Year Warranty Period for Asymptomatic Individuals Without Coronary Artery Calcium: A Prospective Follow-Up of 9,715 Individuals. JACC Cardiovasc Imaging. 2015;8:900–909. doi: 10.1016/j.jcmg.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi PH, Blaha MJ, Budoff MJ, et al. The 10-Year Prognostic Value of Zero and Minimal CAC. JACC Cardiovasc Imaging. 2017;10:957–958. doi: 10.1016/j.jcmg.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Nasir K, Rubin J, Blaha MJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–473. doi: 10.1161/CIRCIMAGING.111.964528. [DOI] [PubMed] [Google Scholar]
- 12.Vliegenthart R, Hollander M, Breteler MM, et al. Stroke is associated with coronary calcification as detected by electron-beam CT: the Rotterdam Coronary Calcification Study. Stroke. 2002;33:462–465. doi: 10.1161/hs0202.103071. [DOI] [PubMed] [Google Scholar]
- 13.Elias-Smale SE, Odink AE, Wieberdink RG, et al. Carotid, aortic arch and coronary calcification are related to history of stroke: the Rotterdam Study. Atherosclerosis. 2010;212:656–660. doi: 10.1016/j.atherosclerosis.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Hermann DM, Gronewold J, Lehmann N, et al. Coronary artery calcification is an independent stroke predictor in the general population. Stroke. 2013;44:1008–1013. doi: 10.1161/STROKEAHA.111.678078. [DOI] [PubMed] [Google Scholar]
- 15.Gibson AO, Blaha MJ, Arnan MK, et al. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc Imaging. 2014;7:1108–1115. doi: 10.1016/j.jcmg.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Yoon YE, Kim YJ, et al. Presence and extent of coronary calcified plaque evaluated by coronary computed tomographic angiography are independent predictors of ischemic stroke in patients with suspected coronary artery disease. Int J Cardiovasc Imaging. 2015;31:1469–1478. doi: 10.1007/s10554-015-0709-8. [DOI] [PubMed] [Google Scholar]
- 17.Gepner AD, Young R, Delaney JA, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015:8. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gepner AD, Young R, Delaney JA, et al. Comparison of Carotid Plaque Score and Coronary Artery Calcium Score for Predicting Cardiovascular Disease Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017:6. doi: 10.1161/JAHA.116.005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KB, Budoff MJ, Zavodni A, et al. Coronary artery calcium is associated with degree of stenosis and surface irregularity of carotid artery. Atherosclerosis. 2012;223:160–165. doi: 10.1016/j.atherosclerosis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Sillesen H, Muntendam P, Adourian A, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5:681–689. doi: 10.1016/j.jcmg.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 24.Polak JF, O’Leary DH. Carotid Intima-Media Thickness as Surrogate for and Predictor of CVD. Glob Heart. 2016;11:295–312. e293. doi: 10.1016/j.gheart.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki R, Xie J, Cheung N, et al. Retinal microvascular signs and risk of stroke: the Multi-Ethnic Study of Atherosclerosis (MESA) Stroke. 2012;43:3245–3251. doi: 10.1161/STROKEAHA.112.673335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BJ, Choi SY, Lee SH, et al. Advanced coronary artery calcification is associated with ischemic stroke. Cerebrovasc Dis. 2010;30:93–100. doi: 10.1159/000314711. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plichart M, Celermajer DS, Zureik M, et al. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. The Three-City Study. Atherosclerosis. 2011;219:917–924. doi: 10.1016/j.atherosclerosis.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Polak JF, Pencina MJ, Pencina KM, et al. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polak JF, Pencina MJ, Meisner A, et al. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalent cardiovascular disease: comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. J Ultrasound Med. 2010;29:1759–1768. doi: 10.7863/jum.2010.29.12.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gronewold J, Bauer M, Lehmann N, et al. Coronary artery calcification, intima-media thickness, and ankle-brachial index are complementary stroke predictors. Stroke. 2014;45:2702–2709. doi: 10.1161/STROKEAHA.114.005626. [DOI] [PubMed] [Google Scholar]
- 35.Naghavi M, Falk E, Hecht HS, et al. From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Osawa K, Nakanishi R, Budoff MJ. Is there a role for coronary artery calcification scoring in primary prevention of cerebrovascular disease? Atherosclerosis. 2017;257:279–287. doi: 10.1016/j.atherosclerosis.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40:1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bos D, Portegies ML, van der Lugt A, et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam Study. JAMA Neurol. 2014;71:405–411. doi: 10.1001/jamaneurol.2013.6223. [DOI] [PubMed] [Google Scholar]
- 39.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 41.Baber U, Mehran R, Sartori S, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65:1065–1074. doi: 10.1016/j.jacc.2015.01.017. [DOI] [PubMed] [Google Scholar]