Abstract
Immunotherapy has achieved breakthrough status in many advanced stage malignancies and is rapidly becoming the fourth arm of cancer treatment. Although cancer immunotherapy has generated significant excitement because of the potential for complete and sometimes durable responses, there is also the potential for severe and occasionally life-threatening toxicities, including cytokine release syndrome and severe auto-immunity. A large body of work also points to a “meta-inflammatory” state in obesity associated with impairment of immune responses. Since immune checkpoint blockade (and other cancer immunotherapies) have altered the landscape of immunotherapy in cancer, it is important to understand how immune responses are shaped by obesity and how obesity may modify both immunotherapy responses and potential toxicities.
Keywords: Obesity, cancer, immunotherapy, T cells, macrophages, T-regs, MDSCs, cytokines, toxicity
Introduction
Recent breakthrough developments in cancer immunotherapy such as checkpoint inhibitors and chimeric antigen receptor (CAR) T cells underscore the clinical promise of immunotherapy. Yet, despite the exciting success of these therapies, only a fraction of patients respond to treatment and there is a risk for significant toxicities. This is particularly pertinent with strong stimulatory immunotherapies such as CAR T cells and high-dose interleukin (IL)-2 but is also observed with oncolytic viruses and check-point inhibitors targeting CTLA4 and PD-1/PD-L1. In fact, recent clinical studies have indicated that identical regimens can have markedly different outcomes depending on the age of the patient[1]. Therefore, a critical issue for the expansion of cancer immunotherapy is determining factors predictive of response and toxicity, and unlike traditional chemotherapy or targeted therapy, key aspects of the patient’s immune system (including immune-modifying factors such as obesity) are likely to be as important as tumor-related factors in determining response and toxicity. It is also critical that preclinical modeling used to mechanistically assess these various regimens takes these factors into account.
The obesity epidemic, particularly in the developed world, has reached dramatic proportions. According to a 2014 World Health Organization report, more than 600 million people worldwide are obese (as defined by a body mass index of ≥ 30 kg/m2). Moreover, the prevalence of obesity has more than doubled since 1980, and somewhat shockingly, obesity and obesity-related diseases account for more deaths worldwide than starvation and under-nutrition[2]. Notably, in the US, approximately 69% of individuals are overweight (BMI ≥ 25 kg/m2), and roughly 36% are obese (BMI ≥ 30 kg/m2)[3]. Perhaps more significantly, approximately 30% of US children are overweight or obese, and these alarming trends are now affecting European as well as middle and low-income countries[4]. Furthermore, the link between obesity and cancer is well-established, and obesity is expected to surpass tobacco as the greatest risk factor for carcinogenesis in the developed world by 2020[5]. Importantly, obesity has also been linked with worse survival in multiple cancer types, including kidney, colorectal, esophago-gastric, breast, liver, and pancreas[6].
Immunologically, obesity is known to be a major driver of a number of inflammatory processes including macrophage dysregulation, chronic cytokine production, suppression of both adaptive and innate immune arms and accelerating immune aging in a process termed “inflammaging”[7–10]. An extensive and growing body of basic and translational research has also demonstrated that obesity has significant metabolic effects on the host, and importantly for this review, obesity not only directly impacts cancer initiation and promotion but also impacts immune homeostasis and the elimination, equilibrium, and escape phases of immune-editing[7, 8, 11]. Yet, much less is known about the impact of obesity on the induction, regulation and maintenance of adaptive immune responses, particularly in the setting of cancer immunotherapy. Given the increasing prevalence of obesity among cancer patients and the growing importance of immunotherapy in cancer therapy, a more comprehensive understanding of the impact of obesity on immune homeostasis in relation to the responses and toxicity observed with immunotherapy is critically needed. In this review, we will provide an overview of the metabolic and immune derangements observed in obesity with a focus on how these derangements can impact the efficacy as well as the adverse events observed with cancer immunotherapy.
Pathophysiology of Obesity and Cancer
Although obesity has been defined as body mass index (BMI) ≥ 30 kg/m2 for purposes of disease-tracking and monitoring, it is important to acknowledge that excess adiposity represents a continuum in which somewhat arbitrary categories have been established by medical/ scientific convention, and the metabolic and immunological manifestations of obesity may vary from individual to individual based on other factors. For this reason, the true impact of obesity on clinical health outcomes is somewhat controversial, and some experts have declared an “obesity paradox,” namely that low levels of obesity are beneficial and either directly (via causal mechanisms) or indirectly (via correlation with other factors such as better socio-economic status or freedom from other health conditions) associated with superior health outcomes[12, 13]. In addition, the relative contribution of diet, physical activity, body fat distribution, age and duration of obesity likely contribute to the variable penetrance and phenotype of obesity-related disease and immune effects across the spectrum of patients and studies. These factors may account for some of the variability observed in pre-clinical and clinical studies of the impact of obesity on immune function.
Although adipose tissue is primarily a storage organ for excess energy/fat intake, evolving data have pointed to important roles for adipose tissue in endocrine function and immune homeostasis due to cytokine production, especially in obese individuals where there appears to be metabolic/fat dysfunction. Importantly, there appear to be significant differences in fat metabolism and secondary effects based on male and female sex. For example, although women tend to have more body fat than men, the pattern of adipose tissue deposition is different. Key studies performed in mice have highlighted the physiological/ pathophysiological significance of fat in different anatomical locations[14–16]. These studies have been corroborated in humans and led investigators to draw important distinctions between subcutaneous fat and visceral adipose tissue (VAT) and have further suggested that VAT is directly correlated with the adverse effects of obesity (including metabolic syndrome, fatty liver, and insulin resistance), while subcutaneous fat is not (and may even be protective against obesity-related syndromes and diseases)[17].
Similarly, the cancer-initiating and promoting effects of obesity also appear to be mediated by sex hormones. However unlike metabolic diseases, data suggest a greater risk of carcinogenesis from obesity in women than men[5]. A likely mechanism, at least in part, is aromatase activity in adipose tissue which increases systemic estrogen levels, and increased aromatase activity is observed with obesity[18]. Additionally, endometrial and breast cancer, two obesity-related malignancies, have been reproducibly associated with estrogen excess, and the link between greater estrogen production, endometrial cancer, and obesity has been well documented in the European Prospective Investigation into Cancer and Nutrition study[19, 20].
Although excess food intake is clearly one of the most important factors influencing adiposity, there are also complex neuro-hormonal pathways involved (Figure 1). To date, much research has focused on characterizing the interplay and cross-talk between brain and gut hormones (such as leptin, cholecystokinin, insulin, and glucagon) as a result of food intake and over-feeding. Leptin and insulin (among others) are critical hormones secreted in the gut and adipose tissue which interact with receptors in the hypothalamus and have potent effects on food intake, satiety signals, energy expenditure and levels of stored fat[21, 22]. Recent reviews have provided comprehensive discussions of the roles of adiponectin, leptin, and PPARγ in adipogenesis, cancer progression, and immune cross-talk[7, 21, 23, 24].
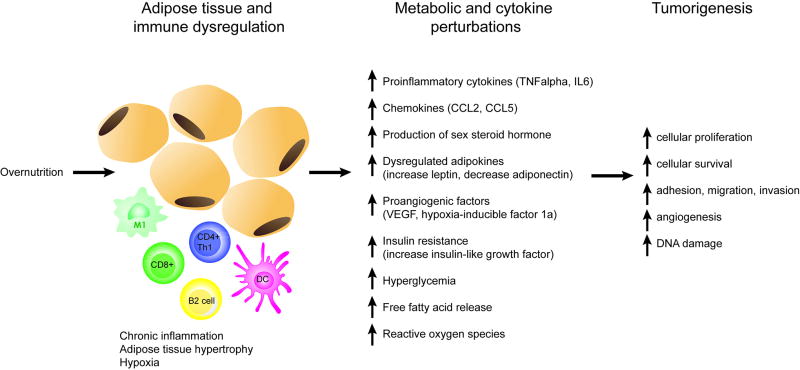
Figure 1. Pathophysiology of obesity and cancer.
Diet-induced obesity drives tumorigenesis through several mechanisms. Overnutrition drives adipocyte hypertrophy, stress, and apoptosis, resulting in immune dysregulation, pro-inflammatory cell infiltration of adipose tissue, hypoxia, and chronic low-grade systemic inflammation. Several factors of obesity-induced metabolic and cytokine perturbations including release of proinflammatory cytokines, chemokines, dysregulated adipokines, increased sex steroid hormone, proangiogenic factors, and reaction oxygen species promote tumorigenesis and growth.
Seminal studies have also highlighted the key differences between white adipose tissue (specialized for energy storage), brown adipose tissue (metabolically active in thermogenesis and protective against diabetes), and beige adipose tissue which demonstrates a molecular and genetic profile distinct from both white and brown fat[25–29]. Wu et al. and others have highlighted the plasticity and responsiveness of beige fat in response to cold stress, suggesting that beige fat may be an attractive target for metabolic/anti-obesity therapy given this plasticity[30]. Although Fabbiano et al. showed that calorie restriction stimulated an increase in beige fat, a decrease in white fat, and increases in anti-inflammatory immune parameters (M2 macrophages, Th2 cytokines, and eosinophil numbers) in both lean C57BL/6 and BALB/c mice, there are limited data characterizing the impact of obesity on immune function in different types of adipose tissue[31].
Mouse models of obesity
For over one hundred years, mouse models have been the foundation for basic discoveries in immunology and immunotherapy, and there is no question that mouse models remain fundamentally important today[32].. A fundamental advance in the study of obesity was the discovery/development of mice on the C57BL/6 background lacking the leptin gene (ob/ob mice)[33, 34]. Without leptin, ob/ob mice eat excessively and become profoundly obese routinely achieving weights of 50 – 60 grams due to excessive eating and lack of appetite regulation [35]. Leptin also has direct effects on immune homeostasis and regulation, and these immune effects confound studies of the immune-modifying effects of obesity since there are both direct effects from absence of leptin and indirect effects from obesity-mediated inflammation [23, 36, 37].
Another important consideration in the use of ob/ob mice is the potential limited generalizability of the model since human obesity is primarily the result of excessive calorie consumption as well as type of foods consumed rather than leptin deficiency. Therefore, many researchers prefer models of diet-induced obesity (DIO) which are accomplished by providing chow that is very high in fat (60% kcal), or a Western diet which includes high sugar content, compared with normal chow that is typically less than 10% fat[38]. A drawback of these DIO models of obesity is that they require time (typically several months) for optimal weight gain, and these DIO mice (and their controls) will therefore be older when they are studied, bringing age into play as a potential variable in immune phenotype and function (see Table 1). In fact, age is key confounding variable in studies of obesity since there is an established correlation between age and increased adiposity[39]. Our own work has shown that aged mice (15 – 18 months) are approximately 1.5 – 2 fold heavier than young (< 6 months old) mice, and this weight gain is coincident with increased visceral fat stores[40]. Moreover, we have shown that aged mice are equally susceptible as ob/ob leptin knockout mice to the lethal toxicity of anti-CD40/IL-2 immunotherapy, but these effects could be reversed by the effects of calorie restriction starting at 14 weeks of age and reaching 40% restriction by 16 weeks[40, 41].
Table 1.
Similarities and Differences in Etiology and Assessment of Obesity Across Four Species
| Species | Definition of Obesity |
Induction of Obesity | Other variables that impact immune readouts |
|
|---|---|---|---|---|
|
| ||||
| Mouse | Varies | Genetic | Monogenic: ob/ob; db/db Polygenic | Age |
| Average ± 3 SD of control | Diet composition | |||
| Duration of diet | ||||
|
|
||||
| Imaging modalities | Diet-induced | High-fat diet (30 – 60% fat) Western diet (cafeteria) | Housing conditions | |
| Female vs Male | ||||
| Source of vendor | ||||
|
|
||||
| Spontaneous | Mouse strain (Resistant vs Susceptible) | |||
|
| ||||
| Canine | Body condition score ≥ 8 | Spontaneous | Age | |
| Environmental factors | ||||
| Behavioral factors | ||||
| Diet composition | ||||
| Female vs Male | ||||
|
| ||||
| Non-human primate | BMI > 30 kg/m2 | Diet-induced | Western diet | Age |
| Waist-to-hip ratio | Diet composition | |||
|
|
||||
| Spontaneous | Duration of diet | |||
| Housing conditions | ||||
| Female vs Male | ||||
|
| ||||
| Human | BMI > 30 kg/m2 | Genetic | Monogenic: lep deficient | Age |
| Waist circumference | Environmental factors | |||
|
|
||||
| Waist-to-hip ratio | Spontaneous | Behavioral factors | ||
| Waist-to height ratio | Diet composition | |||
| Body fat percentage | Female vs Male | |||
Mouse strain is also an important factor in studies using the DIO model. For example, BALB/c mice are frequently resistant to diet-induced obesity, and after 20 weeks on a high-fat diet (HFD), some studies have demonstrated that only 45–55% of BALB/c weigh significantly more than normal diet controls[42]. C57BL/6 mice experience more uniform weight gain and become significantly heavier than control mice, sometimes after as little as 8–10 weeks in DIO models. Interestingly, male DIO mice appear to gain more weight than females. In DIO models, fat calories clearly appear to drive obesity, although the causative role of dietary fat as the principal cause of human obesity is controversial, and some authors have attributed obesity to other causes besides excess fat consumption[43, 44]. Therefore, some have argued that it is important to incorporate other dietary components when modeling human eating habits of obesity and immune dysfunction. Finally, the occurrence of a diabetic state (as shown by glucose intolerance) in models over time further complicates interpretation of results.
For that reason, some investigators favor the so-called nonalcoholic steatohepatitis (NASH) diet as a more representative diet for pre-clinical modelling of human obesity. The NASH uses a HFD (45% kcal, 40% kcal from trans fats) which is also high in fructose and sucrose[45]. The NASH diet induces weight gain and adipose tissue development and is accompanied by systemic inflammation with elevated TNF and IL-6 levels[46]. Consequently, this diet may more accurately model the spectrum of metabolic and immunological complications observed in human obese patients, although there are no studies which perform a side-by-side comparison of the impact of a HFD vs. NASH diet on mouse physiology, biochemistry, and immune function.
A modification of the NASH diet is the cafeteria diet. In the cafeteria diet model, rodents are provided a mixture of standard processed foods available as part of the Western diet such as cookies, candy, cheese, and processed meats. These food items approximate the composition of salt, sugar, and fat which typically comprise the contemporary diets of obese human subjects[47]. However, a drawback of the cafeteria diet is the lack of standardization since animals may choose a different selection of foods each day. Therefore, diets cannot be accurately replicated for future studies making this type of diet a problematic choice for carefully controlled experiments[48]. Finally, although numerous other mouse models of obesity are available (including monogenic and polygenic), these models are less widely utilized for a variety of reasons including cost, availability, and generalizability [48, 49].
From an experimental design perspective, HFD in mice should be carefully matched to a control diet to adjust for potential confounding factors. In many cases, a chow diet (e.g., cereal-based) is used as a low-fat control diet in DIO studies. However, chow diets contain plant-derived ingredients which are subject to changes in the growing season and will vary in composition at the time of harvest. Purified ingredients, on the other hand, are highly refined and contain just a single nutrient. These ingredients have little variability and therefore provide consistency between batches[50, 51]. An important caveat of DIO studies using HFD is the variation in the extent of fat utilized and the duration of DIO diet between investigators. A variety of different HFD have been used with fat fractions varying between 20% and 60% of calories delivered as fat, and importantly, a human diet of 60 kcal% fat is extreme and almost never observed clinically in patients with obesity. Yet, diets with 60 kcal% fat are commonly used to induce obesity in mouse models since mice tend to gain weight quickly and reproducibly with this approach. Another disadvantage of using a 60% HFD is that it is difficult to reverse the side effects of obesity with this approach (compared to diets with a lower percentage of fat)[50]. This may be important when studying the plasticity and reversibility of the immune-modifying effects of obesity.
The type of fat is another important factor that should be considered when choosing HFD diets for an animal study. Many HFD used in a laboratory setting contain a high amount of saturated fat, such as lard, beef tallow, or coconut oil. Each type of fat has a different effect on animals. For example, when fish oil is added to diets of animals that are fed similar amounts of fat, they do not gain as much weight as those fed diets with more saturated fat[52, 53]. In addition, these animals are more insulin sensitive[54, 55]. Furthermore, diverse fatty acids can affect phenotype through a variety of mechanisms, including gene transcription, eicosanoid production, and expression of membrane receptors.
Finally, as in people, there are diverse manifestations of obesity in mice which may directly or indirectly influence experimental results, especially when evaluating immune responses and immune-mediated toxicities. For example, in our unpublished studies, we have observed that C57BL/6 mice are particularly susceptible to dermatitis and an oily coat the longer they are receiving a HFD. Not only may these be manifestations of a pro-inflammatory state secondary to obesity, but they may predispose the mice to further insults such as skin and soft tissue infections which can amplify inflammatory signals. In controlled experiments, these factors may introduce additional variables that may confound results.
Multi-species Evaluation of Immune-Modifying Effects of Obesity
Although data from mouse models have been the cornerstone of mechanistic studies in scientific research for many years, intrinsic characteristics of mouse models create challenges for clinical translation, especially given the influence of age, obesity, and pathogen exposure on immune phenotype. For that reason, the “typical” cancer patient phenotype is not completely reflected in many mouse models which frequently rely on young, lean, and specific-pathogen free (SPF) mice. Therefore, evaluating the impact of obesity on immune phenotypes in large, outbred models has key relevance for understanding the nature of immunotherapy responses and toxicity.
Dog
As with humans, obesity in dogs is increasingly prevalent. In fact, the parallel evolutionary history of humans and dogs and the shared exposure of environmental risk factors have led to many similarities in disease prevalence and incidence between humans and dogs[32, 56]. In the United States, it is estimated that approximately 25% of dogs are obese[57]. In dogs, obesity is categorized using a body condition score (BCS). The BCS system is a scale of 1–9, with 1 being emaciated, 4 – 5 being ideal, 6 – 7 being overweight, and 8 – 9 being obese[58, 59]. As in humans, canine obesity is associated with the development of insulin resistance, altered lipid profiles, and mild hypertension, which are ameliorated by weight loss. Furthermore, overweight dogs are more likely to suffer from diabetes mellitus and, similar to humans, this can impact lifespan [57, 60].
Recent studies in dogs are shedding some light on the presence of low-grade inflammation in overweight or obese dogs. As with humans, investigators have observed that IL-6 and TNF-α are elevated in obesity-induced low-grade inflammation[61, 62]. In addition, there is evidence that T cell proliferative capacity is reduced in dogs in the setting of obesity, although interestingly, no differences in B-cell responses were observed[63]. In our laboratory, we have also observed impaired T cell responses to in vitro stimulation in obese versus lean dogs (BCS >6 versus <4, respectively, manuscript submitted). Although the data from dogs to date are not as robust regarding the consequences of obesity on canine immunity, these studies provide evidence of the value of the canine model for a multi-species evaluation of obesity-related immune characterization. However, since the etiology of obesity in dogs is multifactorial and heterogeneous (HFD, sedentary lifestyle, high fructose/sucrose intake, among other factors), studies of immune phenotype and immune responses in obese dogs are also potentially subject to variable results (see Table 1).
Non-human Primate
There are currently seven national primate research centers funded by the National Institutes of Health (NIH) whose purpose is to model human diseases, behavior, and infection. Non-human primate (NHP) research encompasses more than 20 species of NHPs with rhesus macaque being the most commonly studied[64]. The close phylogenetic relationship and similarity in reproduction, development, anatomy, physiology, and social structure among NHP and humans make NHP an invaluable biomedical model, though the outbred nature of colonies, cost, small litter size, and average lifespan of two decades limit the use of NHP as a model. In immunology, the NHP primate model has several significant advantages: 1) greater proximity to human immunity by genetics and anatomy, 2) development of mature immune system that is not found in specific-pathogen-free housed mice, and 3) greater similarity of host pathogens.
As with humans, NHP have been shown to spontaneously develop obesity, metabolic syndrome, and type 2 diabetes, and their metabolic features mirror humans. In NHP, obesity can be defined by BMI and abdominal circumference or by ratios of the waist-to-hip and waist-to-thigh, correlating well with results of imaging studies which measures body fat[65]. While spontaneous models of obesity can arise though ad libitum feeding on standard colony diet in age-dependent manner, obesity-induced models in NHP can also be generated by providing a proto-typical Western diet (high fat/high cholesterol)[66, 67]. Although NHP consuming high fat and high carbohydrate diets can take several years to become obese, this method offers a more controlled method of inducing obesity, although other factors that predispose to spontaneous obesity such as genetics and age may also influence the results.
Overall, the impact of obesity on the NHP immune system is not well characterized. Notable studies using caloric restriction have shown improved survival in NHP suggesting a role of obesity in accelerating cellular senescence and physiological decline[68]. However, caloric restriction in rodents has more definitively shown benefits in health span and metabolism[69, 70], and there are conflicting studies on the impact of caloric restriction on life span in NHP, likely due to differences in study design[71, 72]. Messaoudi et al. have shown that restrictive diets starting in early adulthood reverse T cell senescence and promote a higher frequency of naïve T cells, a greater T cell receptor diversity, and greater T cell proliferation in rhesus macaques[73]. Conversely, Li et al. assessed the impact of DIO on immune function in NHP[74]. Adult monkeys (9 – 12 years old) were fed a HFD (32% fat plus high sucrose) for 5 years and ranged in weight from 14.4 to 21 kg (compared to normal weight of 6 – 11 kg). Obese subjects had higher levels of serum cytokines (e.g. CD40, CD40 ligand, and IL-6) and effector (CD28-CD95+) and central memory (CD28+CD95+) responses after anti-CD3 stimulation[74]. In our own studies, we compared T cell responses from peripheral blood of spontaneously obese NHP (> 12 kg) compared to lean NHP (5 – 8 kg), observing reduced proliferation with mitogen stimulation of PBMCs from obese subjects and decreased naïve-to-memory T cell ratios compared to lean (in review). As in DIO rodent studies, data from different studies should be interpreted in context since age of onset of obesity, length and type of obesogenic diet, and timing of readouts are all variables which may influence results.
Immunological Effects of Obesity
A growing body of literature has demonstrated the link between obesity, increased adipose tissue, macrophage dysfunction, and other disruptions in immune homeostasis[75–78]. These inflammatory and immunological changes not only impact the end-organ pathology and metabolic dysfunction observed in obesity but also have relevance for the host response to cancer immunotherapy which is dependent on a delicate balance between response and toxicity in patients. Although there are conflicting data on the impact of obesity on tumor growth kinetics in mice, our own unpublished data (similar to others) demonstrate that tumor growth is accelerated in DIO mice[79–81]. Several hypotheses exist regarding the mechanism of accelerated tumor growth in obese mice, including direct metabolic effects via fatty acid oxidation and increased growth factors as well as indirect effects via impaired immunosurveillance (Figure 2). Since accelerated tumor growth has been observed in both syngeneic and xenogeneic models, it suggests a direct effect on tumor growth via IGF1, growth hormone, or insulin rather than an immune-mediated mechanism.
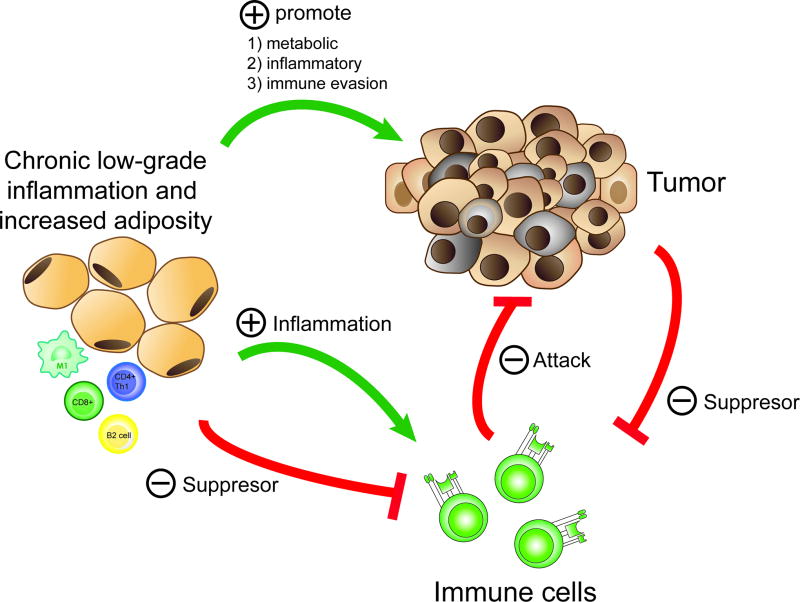
Figure 2. Obesity-cancer-immune system triad.
Obesity directly promotes tumor progression through metabolic and inflammatory disturbances and indirectly through suppression of immune response. The impact of obesity on immune system can be both promotion of inflammation and suppression of response.
Growing data also indicate that obesity results in a loss of diversity and skewed predominance of certain microbial species in the GI tract, assessed by 16s rRNA sequencing of fecal samples =[82–84]. Since differences of environmental/microbiome factors have recently been implicated in response to cancer therapy in mice, including immunotherapy, further work is needed to assess how obesity and aging impact the spectrum of responses and toxicity which are observed[85–87]. The importance of the microbiome to the field of cancer immunotherapy has been underscored by increasing studies demonstrating that microbiota can differentially promote or resist tumor progression with and without immunotherapy. Furthermore, recent high impact studies of human patients receiving checkpoint blockade have observed that specific bacterial species correlate with immunotherapy response via greater frequencies of both antigen-presenting cells and CD8 T cells, suggesting a possible mechanism for how the microbiome can shape immunotherapy responses. These studies have further reinforced the significance of their findings in mouse experiments where fecal transfer of specific bacterial species have reversed resistance to immunotherapy[85, 88, 89]. Microbiome studies are, therefore, a key element of ongoing studies of how obesity impacts the spectrum of responses and toxicity in cancer immunotherapy.
It has also long been known that immune cells, in particular macrophages and Tregs, can be identified in adipose tissue. As adipocytes increase in size in the setting of obesity, they become apoptotic and surrounded by macrophages to form the crown-like structures that have become a hallmark of adipose inflammation[21]. Subsequently, key observations by Hotamisligil et al. among others have shown that adipose tissue is an important source of tumor necrosis factor (TNF)-α and other cytokines[90], and the elaboration of TNF by adipose tissue is magnified in obesity[91, 92]. Importantly, not only was secretion of these pro-inflammatory cytokines found to have effects at both local and distant sites, later work showed that the dominant source of these adipose-derived cytokines was the resident macrophages within the adipose tissue rather than adipocytes or other immune cells[75].
The increased adipose tissue deposition which occurs in obesity is therefore widely believed to stimulate both an influx of macrophages into adipose tissue as well as a transition from an M2/anti-inflammatory phenotype to an M1/pro-inflammatory one. Parallel with the influx of macrophages and the simultaneous transition to an increased M1 phenotype is a decrease in the prevalence of tissue resident Tregs in adipose tissue in obesity. This efflux and/or culling of Tregs in obesity is paralleled by a transition from a Th2 to a Th1 phenotype which further promotes a pro-inflammatory phenotype[93].
In adipose tissue of lean subjects, Tregs are one of the most prevalent cell types and a critical regulator of immune homeostasis. Han et al. and others have shown that up to 40% of CD4+ T cells in the adipose tissue of 6 – 9 month old C57BL/6 mice are FoxP3+[94], and it these tissue-resident immunosuppressive Treg cells which promote an anti-inflammatory M2-macrophage phenotype under lean conditions. Similarly, pre-clinical experiments have shown that Treg depletion produces insulin resistance and predisposes to obesity, and depletion of resident Treg populations has been observed as an early event in obesity and DIO models. In contrast, it is widely recognized that immunosuppressive Tregs are increased in a substantial proportion of cancers, underscoring the importance of studies to assess this cell population in obesity and in the response to cancer immunotherapy.
Lysaght et al. have observed activated CD4+ and CD8+ T cells as key players in inflammatory macrophage stimulation and inflammatory cytokine production[95] in obesity-associated inflammation. Overall, the preponderance of the data supports a cascade whereby obesity initiates necrosis of adipocytes with release of DAMPs and other macrophage attractants followed by M1 macrophage activation and subsequent recruitment of CD4+ and CD8+ T cells with efflux or repression of Tregs.
Effects of obesity on immune subsets
T cells
Obesity has been previously demonstrated to induce a meta-inflammatory state with increased pro-inflammatory cytokines such as TNFα and IL6, elevated levels of glucose, insulin, leptin, fatty acids and fatty oxidation, which have all been demonstrated to directly impact T cell responses. PD-1 expression has recently been reported to be upregulated on T cells in visceral adipose tissue in obese mice although similar findings were not observed at other anatomical sites[96]. Naive T cells are usually metabolically quiescent, while effector T cells are rapidly proliferating and therefore are heavily dependent on glycolysis and anaerobic metabolism to meet their significant metabolic requirements. Subsequently, during the contraction phase, memory T cells (and Tregs) return to an aerobic, Krebs cycle-based metabolism for their energy needs. Disruption of these metabolic pathways in obesity with excess fatty acids and greater areas of malperfusion/hypoxia may therefore skew normal T cell homeostasis and transit through the naïve-effector-memory pathways. Similarly, whether activated T cells are insulin resistant in obesity is unknown. Interestingly, data suggest that obesity may promote increased effector T cell function as T cells upregulate the insulin receptor in obesity states leading to increased uptake and metabolism of essential nutrients[97].
Impairment in T cell and DC interaction in priming has also been reported in obesity, and suboptimal adaptive immunity may explain obesity-associated increased susceptibility to infection and decreased response to vaccination. In rodent models, DIO mice have impaired production of type 1 interferons, delayed pro-inflammatory cytokine upregulation, increased lung pathology scores, and higher mortality rates after infection with influenza virus compared to lean controls[98]. DIO mice also have impaired adaptive immune response to pandemic H1N1 vaccination, exhibiting lower antibody responses, increased viral lung titers, and greater lung pathology after influenza A (H1N1) infection[99]. However, DIO mice do not have impaired memory CD8 generation or function.
NK and B cells
Overall, the majority of studies performed to date underscore the dominant role of pro-inflammatory macrophages and T cells in the pathophysiology and immune dysfunction present in obesity. However, important observations have also been made regarding the key immuno-regulatory roles played by other immune effector cells, such as natural killer (NK) and B cells. For example, using wild-type C57BL/6 mice fed a 50% HFD, Wensveen et al. demonstrated that VAT-resident NK cells activate macrophages leading to insulin resistance after only 4 weeks via expression of NCR1 ligands on adipocytes and IFN-γ secretion by subsequently activated NK cells[78]. This study highlights the cross-talk between local stress signals in adipose tissue, polarization of M1 macrophages, and amplification of inflammatory signals via cytotoxic T and NK cells.
DeFuria et al. showed that B cells from DIO mice (60% fat for 16 weeks) produce elevated levels of pro-inflammatory cytokines, including IL-6, TNF-α, and IFN-γ, and that lean mice and B cell-null mice demonstrated significantly lower adipose tissue Tregs compared to obese mice, suggesting that B cells are also important regulators of inflammatory pathways in obesity[100]. Similarly, Frasca et al. showed that the B cell pools of obese subjects are enriched with pro-inflammatory memory B cells while at the same time showing reductions in anti-inflammatory B cells. They also showed that these enriched pro-inflammatory B cells enhance production of IL-17 and IFN-γ producing T cells[101]. Taken together, these studies emphasize the pleiotropic effects of obesity on the immune system to initiate and promote an inflammatory phenotype.
Myeloid-Derived Suppressor Cells
MDSCs are a heterogeneous group of immune cells from the myeloid lineage which expand in cancer and have been associated with key immunosuppressive and immune evasion mechanisms. Clinical and experimental evidence has shown that high infiltration of MDSCs in the tumor microenvironment is associated with adverse patient prognosis and resistance to therapies[102, 103]. Overall, the phenotype of MDSCs is heterogenous and plastic, but two predominant MDSC populations have been described: monocytic MDSCs(CD11b+GR1midLY6ChiLY6G−CD49d+ in mice and CD33+CD14+HLA-DRlow/− in humans) and granulocytic/ polymorphonuclear MDSCs (CD11b+GR1hiLY6ClowLY6G+CD49d− in mice and LIN−CD11b+HLA-DR−CD33+CD15+CD66b+in humans)[103–105]. MDSCs exert their immunosuppressive functions in multiple ways, including nutrient depletion and generation of reactive-oxygen species, both of which appear to preferentially induce senescence, anergy, and hyporesponsiveness of antigen-activated T cells. In addition, MDSCs can disrupt effector cell homing. For example, Hanson et al. demonstrated that MDSCs can cleave CD62L on the surface of naive CD4+ and CD8+ T cells via ADAM17 (disintegrin and metalloproteinase domain-containing protein 17) inhibiting T cell trafficking to lymph nodes[106]. Finally, Huang et al. and Serafini et al. have shown that MDSCs can induce Tregs and thereby promote immune evasion and tumor immune escape[107, 108].
The chronic inflammatory state in obesity has been proposed to promote MDSC accumulation. Mouse studies, in particular, have observed increased MDSCs in the setting of obesity, including both ob/ob as well as diet-induced obese mice[102]. Clements et al., for example, demonstrated an increased percentage of MDSCs in the peripheral blood of both BALB/c and C57BL/6 female mice after 12 weeks of high-fat diet[109]. This effect was partially mediated through a leptin-dependent manner. Furthermore, Clements et al. demonstrated that MDSC-depletion reduced tumor growth and increased survival in obese BALB/c mice bearing 4T1 tumors. Hale et al. observed elevated levels of intra-tumoral MDSCs in Renca-bearing BALB/c mice who were fed a HFD and developed diet-induced obesity[110]. In these mice, the frequency of PD-L1-positive MDSCs was also increased in obesity. In vitro blockade of PD-L1 on MDSCs obtained from 4T1-bearing mice reduced the ability of MDSCs to suppress T cell proliferative responses to antigen, suggesting that HFD/obesity supports tumor progression through PD-L1 upregulation on MDSCs. Interestingly, MDSC accumulation may also promote insulin sensitivity in obesity, indicating a possible positive role in the case of glucose intolerance/ diabetes but a negative role in cancer progression[109, 111]. In humans, there are few studies examining the impact of obesity on MDSC accumulation. However, a small cohort study from Bao and colleagues observed an increase in MDSCs in the peripheral blood of overweight/obese male Chinese subjects (BMI > 25 kg/m^2) compared to lean male healthy controls (BMI < 25 kg/m^2)[112].
Impact of obesity on cancer immunotherapy toxicity
The anti-CD28 trial (TGN1412) in the United Kingdom in 2006 in which all 6 patients participating in the phase I clinical trial experienced severe CRS and organ failure dramatically underscored the potential toxicity of stimulatory immunotherapies[113, 114]. Similarly, although less severe, the cumulative clinical experience with immune checkpoint inhibitors and CAR T-cells has also demonstrated the risks of severe toxicity that may occur with these treatments[1]. The diverse impact of standard cancer therapies such as surgery, radiotherapy, and chemotherapy on immune phenotype and function, particularly when combined with immunotherapy, is also poorly characterized. Therefore, given the potential for unexpected toxicities to emerge in unique patient populations, it is important to understand the impact of obesity on end-organ function since the immune-pathology related to obesity may interact with or be exacerbated by immunotherapy.
To date, clinical studies have largely overlooked the impact of obesity on cancer immunotherapy outcomes, and this raises questions whether the clinical experience will mirror the pre-clinical observations of obesity increasing immune exhaustion and potentially exacerbating immune-mediated toxicity[40]. However, a key recent publication evaluating over 2000 patients with metastatic melanoma demonstrated statistically significant improvements in progression-free and overall survival among obese patients (BMI ≥30) treated with immunotherapy, providing important clinical data to support the hypothesis that obesity may impact responses to immunotherapy, potentially in unexpected and favorable ways[115]. Similarly, research into the safety and utility of immunotherapy for obese cancer patients has been sparse. Clinical trials have identified age as a risk factor for toxicity, especially cytokine-release syndrome after high-dose IL-2 infusion[116]. In addition, two recent phase III clinical trials evaluating PD-1 blockade for patients with multiple myeloma (KEYNOTE-183 and KEYNOTE-185 testing pembrolizumab in combination with lenalidomide, pomalidomide, or dexamethasone – https://www.fda.gov/Drugs/DrugSafety/ucm574305.htm, accessed January 15, 2018) were halted prematurely because of an increased number of deaths in the pembrolizumab arms. Although age is associated with increased adiposity and loss of muscle/ lean body mass, total body weight does not necessarily reflect obesity, which is more accurately measured by the percentage of visceral adipose tissue and body mass index. Ultimately, because obesity has such significant effects on the immune milieu, the impact of obesity on immunotherapy responses remains a key area for future clinical studies, especially given the large and growing body of basic and translational studies demonstrating the impact of obesity on key parameters of immune responses, including exhaustion, memory responses, and immune dysfunction.
For example, ongoing unpublished work in our lab suggests that obesity increases immune exhaustion (PD-1 expression) in T cell repertoires, especially memory T cell subsets. In addition, diet-induced obesity accelerates age-associated thymic involution, resulting in decreased naïve T cell frequency in circulation and increased effector-memory T cells and decrease TCR diversity in the spleen of 9month-old mice[117]. Human obese patients showed a negative correlation between circulating TRECs and BMI. Obesity has also been associated with impaired lymphatic cell transport and DC migration to peripheral lymph nodes and abnormal lymph node architecture[118].
These data imply that a phenotype with pre-existing inflammation may impact immunotherapy toxicities such as CAR-T cell therapy which can be complicated by cytokine release syndrome and macrophage activation syndrome[119]. It would be of benefit to retrospectively stratify treated patients by BMI to determine if toxicity severity correlates with pretreatment BMI. Determining whether immune-mediated adverse events are magnified in obese patients receiving immunotherapy is therefore a key area for both basic and translational research.
Impact of obesity on cancer immunotherapy responses
Alterations of T cell populations in obese adipose tissue are indicative of an active adaptive immune response. Given that new T cell specificities can be generated only in a functional thymus and that the peripheral proliferation of preexisting T cell clones allows only limited immunological vigilance, it is possible that the restriction of TCR diversity in obesity may account for the impaired adaptive immunity in obesity[117, 120].
For example, in a mouse model of renal cell carcinoma, combination therapy with an adenoviral vector expressing TRAIL and inflammatory CpG reduced tumor burden in normal weight mice but not obese recipients[121]. Because this treatment relies on antigen-presenting DCs to present tumor antigen to CD8+ T cells, the lack of efficacy observed in obese mice was attributed to excessive tumor infiltration by inhibitory DCs which produced less TNF and IL-12 and thereby failed to stimulate protective immunity in cytotoxic CD8+ T cells. These data highlight the potential for obesity to impact the efficacy of immunotherapy.
Concluding Remarks
Improved understanding of the immunological landscape in obesity and cancer will undoubtedly allow development of further breakthroughs but more importantly will allow a more accurate reflection on potential clinical outcomes, both positive and negative, when immunotherapy is applied. There is a critical need to better understand “inflammaging” that occurs in obesity and how it will impact responses. The use of multiple species (mouse, dog, NHP, humans) is expected to improve translational studies on the dominant and shared effects of obesity on immunotherapy to allow for improved overall assessment and drive clinical directions. A better understanding of the intersection between obesity and immunotherapy outcomes is critical to the field which is rapidly becoming the standard of care.
Summary Sentence.
This review focuses on how inflammation and immune homeostasis are impacted by obesity and what the implications of these perturbations are for the burgeoning field of cancer immunotherapy.
Acknowledgments
This work was supported in part by National Institute for Health/National Cancer Institute grants 1R01CA195904-01.
Abbreviations Page
- BCS
body condition score
- BMI
body mass index
- CAR
chimeric antigen receptor
- CNS
central nervous system
- COX
cyclo-oxygenase
- CRS
cytokine release syndrome
- CTLA-4
cytotoxic T lymphocyte-associated antigen-4
- DAMP
damage-associated molecular proteins
- DC
dendritic cell
- DIO
diet-induced obesity
- EAE
experimental autoimmune encephalomyelitis
- GVHD
graft-versus-host disease
- HFD
high fat diet
- HPA
hypothalamic-pituitary-adrenal
- IFN
interferon
- IL
interleukin
- ILC
innate lymphoid cell
- MHC
major histocompatibility complex
- ML
milliliter
- MOG
myelin oligodendrocyte glycoprotein
- NASH
non-alcoholic steatohepatitis
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NG
nanogram
- NIH
National Institutes of Health
- NK
natural killer
- NHP
non-human primate
- PPARγ
peroxisome proliferator-activated receptor-γ
- ROS
reactive oxygen species
- STAT3
signal transducer and activator of transcription 3
- TNF
tumor necrosis factor
- Treg/Tregs
regulatory T cell/cells
- US
United States
- VAT
visceral adipose tissue
Footnotes
Authorship:
RJC – conception and design, analysis and interpretation of data, writing the manuscript
CTL - conception and design, analysis and interpretation of data, writing the manuscript
JMTB – conception and design, analysis and interpretation of data, writing the manuscript
WJM – conception and design, analysis and interpretation of data, critical revision of the manuscript
Conflict of Interest Disclosure:
The authors declare no conflicts of interest.
References
- 1.Neelapu SS, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2017 doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312(2):189–90. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligibel JA, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–74. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calle EE, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015;23(3):512–8. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–12. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 9.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wensveen FM, et al. The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45(9):2446–56. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 11.Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol. 2014;5:379. doi: 10.3389/fimmu.2014.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens. 2013;7(1):85–94. doi: 10.1016/j.jash.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. 2014;25(3):454–61. doi: 10.1097/EDE.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, et al. Dynamic differences in oxidative stress and the regulation of metabolism with age in visceral versus subcutaneous adipose. Redox Biol. 2015;6:401–8. doi: 10.1016/j.redox.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Tran TT, et al. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–20. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbaramaiah K, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Dossus L, et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort--a factor analysis. Am J Epidemiol. 2013;177(8):787–99. doi: 10.1093/aje/kws309. [DOI] [PubMed] [Google Scholar]
- 20.Zeleniuch-Jacquotte A, et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. Br J Cancer. 2001;84(7):975–81. doi: 10.1054/bjoc.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng T, et al. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421–49. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10(7):739–43. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 23.Abella V, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 24.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 25.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowell BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366(6457):740–2. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 28.Almind K, et al. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104(7):2366–71. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmons JA, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104(11):4401–6. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabbiano S, et al. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab. 2016;24(3):434–46. doi: 10.1016/j.cmet.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Park JS, et al. Canine cancer immunotherapy studies: linking mouse and human. J Immunother Cancer. 2016;4:97. doi: 10.1186/s40425-016-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy AJ, et al. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3(3–4):156–66. doi: 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Procaccini C, et al. Leptin signaling: A key pathway in immune responses. Curr Signal Transduct Ther. 2009;4(1):22–30. doi: 10.2174/157436209787048711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lord GM, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 38.Lin S, et al. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24(5):639–46. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, et al. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring) 2014;22(10):2147–55. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirsoian A, et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med. 2014;211(12):2373–83. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turturro A, et al. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 42.Prpic V, et al. Adaptive changes in adipocyte gene expression differ in AKR/J and SWR/J mice during diet-induced obesity. J Nutr. 2002;132(11):3325–32. doi: 10.1093/jn/132.11.3325. [DOI] [PubMed] [Google Scholar]
- 43.Cohen DA, et al. Not enough fruit and vegetables or too many cookies, candies, salty snacks, and soft drinks? Public Health Rep. 2010;125(1):88–95. doi: 10.1177/003335491012500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozaffarian D. Foods, obesity, and diabetes-are all calories created equal? Nutr Rev. 2017;75(suppl 1):19–31. doi: 10.1093/nutrit/nuw024. [DOI] [PubMed] [Google Scholar]
- 45.Tetri LH, et al. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G987–95. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanuri G, et al. Role of tumor necrosis factor alpha (TNFalpha) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J Nutr Biochem. 2011;22(6):527–34. doi: 10.1016/j.jnutbio.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Sampey BP, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011;19(6):1109–17. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett P, Mercer JG, Morgan PJ. Preclinical models for obesity research. Dis Model Mech. 2016;9(11):1245–1255. doi: 10.1242/dmm.026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;(Unit 5):61. doi: 10.1002/0471141755.ph0561s58. Chapter 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warden CH, Fisler JS. Obesity: from animal models to human genetics to practical applications. Prog Mol Biol Transl Sci. 2010;94:373–89. doi: 10.1016/s1877-1173(10)94013-1. [DOI] [PubMed] [Google Scholar]
- 51.Warden CH, Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008;7(4):277. doi: 10.1016/j.cmet.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Storlien LH, Huang XF. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Physiol Endocrinol Metab. 2002;282(6):E1352–9. doi: 10.1152/ajpendo.00230.2001. [DOI] [PubMed] [Google Scholar]
- 53.Storlien LH, et al. Diet composition and insulin action in animal models. Br J Nutr. 2000;83(Suppl 1):S85–90. doi: 10.1017/s0007114500001008. [DOI] [PubMed] [Google Scholar]
- 54.Ide T. Interaction of fish oil and conjugated linoleic acid in affecting hepatic activity of lipogenic enzymes and gene expression in liver and adipose tissue. Diabetes. 2005;54(2):412–23. doi: 10.2337/diabetes.54.2.412. [DOI] [PubMed] [Google Scholar]
- 55.Bashir S, et al. Amelioration of obesity-associated inflammation and insulin resistance in c57bl/6 mice via macrophage polarization by fish oil supplementation. J Nutr Biochem. 2016;33:82–90. doi: 10.1016/j.jnutbio.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Galibert F, et al. Toward understanding dog evolutionary and domestication history. C R Biol. 2011;334(3):190–6. doi: 10.1016/j.crvi.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Chandler M, et al. Obesity and Associated Comorbidities in People and Companion Animals: A One Health Perspective. J Comp Pathol. 2017;156(4):296–309. doi: 10.1016/j.jcpa.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Laflamme DP, Kuhlman G, Lawler DF. Evaluation of weight loss protocols for dogs. J Am Anim Hosp Assoc. 1997;33(3):253–9. doi: 10.5326/15473317-33-3-253. [DOI] [PubMed] [Google Scholar]
- 59.Mawby DI, et al. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc. 2004;40(2):109–14. doi: 10.5326/0400109. [DOI] [PubMed] [Google Scholar]
- 60.Tvarijonaviciute A, et al. Obesity-related metabolic dysfunction in dogs: a comparison with human metabolic syndrome. BMC Vet Res. 2012;8:147. doi: 10.1186/1746-6148-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baric Rafaj R, et al. Plasma markers of inflammation and hemostatic and endothelial activity in naturally overweight and obese dogs. BMC Vet Res. 2017;13(1):13. doi: 10.1186/s12917-016-0929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frank L, et al. Increasing body condition score is positively associated interleukin-6 and monocyte chemoattractant protein-1 in Labrador retrievers. Vet Immunol Immunopathol. 2015;167(3–4):104–9. doi: 10.1016/j.vetimm.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Van de Velde H, et al. Proliferation capacity of T-lymphocytes is affected transiently after a long-term weight gain in Beagle dogs. Vet Immunol Immunopathol. 2013;152(3–4):237–44. doi: 10.1016/j.vetimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 64.Phillips KA, et al. Why primate models matter. Am J Primatol. 2014;76(9):801–27. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz SM, Kemnitz JW. Age- and gender-related changes in body size, adiposity, and endocrine and metabolic parameters in free-ranging rhesus macaques. Am J Phys Anthropol. 1992;89(1):109–21. doi: 10.1002/ajpa.1330890110. [DOI] [PubMed] [Google Scholar]
- 66.Mubiru JN, et al. A preliminary report on the feeding of cynomolgus monkeys (Macaca fascicularis) with a high-sugar high-fat diet for 33 weeks. J Med Primatol. 2011;40(5):335–41. doi: 10.1111/j.1600-0684.2011.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kavanagh K, et al. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 2007;15(7):1675–84. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 68.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9(4):683–8. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- 70.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215(4538):1415–8. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 71.Mattison JA, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–21. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Messaoudi I, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103(51):19448–53. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, et al. Targeting oxidized LDL improves insulin sensitivity and immune cell function in obese Rhesus macaques. Mol Metab. 2013;2(3):256–69. doi: 10.1016/j.molmet.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 76.Han MS, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–22. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wensveen FM, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16(4):376–85. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 79.Brandon EL, et al. Obesity promotes melanoma tumor growth: role of leptin. Cancer Biol Ther. 2009;8(19):1871–9. doi: 10.4161/cbt.8.19.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yakar S, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147(12):5826–34. doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- 81.Nunez NP, et al. Obesity provides a permissive milieu in inflammation-associated carcinogenesis: analysis of insulin and IGF pathways. Methods Mol Biol. 2009;512:29–37. doi: 10.1007/978-1-60327-530-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winer DA, et al. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016;23(3):413–26. doi: 10.1016/j.cmet.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torres-Fuentes C, et al. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747–756. doi: 10.1016/S2468-1253(17)30147-4. [DOI] [PubMed] [Google Scholar]
- 85.Vetizou M, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sivan A, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iida N, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Routy B, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 89.Gopalakrishnan V, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 91.Kirchgessner TG, et al. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;100(11):2777–82. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uysal KT, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 93.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 94.Han JM, et al. Insulin inhibits IL-10-mediated regulatory T cell function: implications for obesity. J Immunol. 2014;192(2):623–9. doi: 10.4049/jimmunol.1302181. [DOI] [PubMed] [Google Scholar]
- 95.Lysaght J, et al. Pro-inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer Lett. 2011;312(1):62–72. doi: 10.1016/j.canlet.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 96.Shirakawa K, et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126(12):4626–4639. doi: 10.1172/JCI88606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maciver NJ, et al. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84(4):949–57. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park HL, et al. Obesity-induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Hum Vaccin Immunother. 2014;10(5):1181–6. doi: 10.4161/hv.28332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim YH, et al. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. J Infect Dis. 2012;205(2):244–51. doi: 10.1093/infdis/jir731. [DOI] [PubMed] [Google Scholar]
- 100.DeFuria J, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110(13):5133–8. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frasca D, et al. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) 2016;24(3):615–25. doi: 10.1002/oby.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ostrand-Rosenberg S. Myeloid derived-suppressor cells: their role in cancer and obesity. Curr Opin Immunol. 2018;51:68–75. doi: 10.1016/j.coi.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol. 2018;200(2):422–431. doi: 10.4049/jimmunol.1701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bronte V, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanson EM, et al. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183(2):937–44. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang B, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 108.Serafini P, et al. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clements VK, et al. Frontline Science: High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol. 2018;103(3):395–407. doi: 10.1002/JLB.4HI0517-210R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hale M, et al. Obesity triggers enhanced MDSC accumulation in murine renal tumors via elevated local production of CCL2. PLoS One. 2015;10(3):e0118784. doi: 10.1371/journal.pone.0118784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xia S, et al. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286(26):23591–9. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bao Y, et al. Increased monocytic CD14(+)HLADRlow/− myeloid-derived suppressor cells in obesity. Mol Med Rep. 2015;11(3):2322–8. doi: 10.3892/mmr.2014.2927. [DOI] [PubMed] [Google Scholar]
- 113.Bakacs T, Mehrishi JN, Moss RW. Ipilimumab (Yervoy) and the TGN1412 catastrophe. Immunobiology. 2012;217(6):583–9. doi: 10.1016/j.imbio.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 114.Eastwood D, et al. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. Br J Clin Pharmacol. 2013;76(2):299–315. doi: 10.1111/bcp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McQuade JL, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schwartz RN, Stover L, Dutcher JP. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16(11 Suppl 13):11–20. [PubMed] [Google Scholar]
- 117.Yang H, et al. Obesity accelerates thymic aging. Blood. 2009;114(18):3803–12. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weitman ES, et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8(8):e70703. doi: 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maude SL, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119–22. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang H, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.James BR, et al. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol. 2012;189(3):1311–21. doi: 10.4049/jimmunol.1100587. [DOI] [PMC free article] [PubMed] [Google Scholar]