Summary
Cardiovascular disease remains a leading cause of death, but stakeholders have recently raised concerns about the pace of innovation and investment in developing new therapeutics. Here, the authors characterized temporal trends in cardiovascular research and development over the past 2 decades and the likelihood of successful completion of pre-approval clinical trials. The authors also evaluated the reasons for discontinuation, novelty, and rates of trial results publication for cardiovascular therapies in late-stage development. Between 1990 and 2012, the number of new cardiovascular drugs entering clinical trials declined across all stages of development (p < 0.001 for linear trends). There was no evidence for a difference in probability of successful progression to the next stage of development between cardiovascular and noncardiovascular drugs. Small and medium-sized companies sponsored 43%, 38%, and 31% of new Phase 1, Phase 2, and Phase 3 trials, respectively. Roughly one-half of the drugs in Phase 3 trials were categorized as targeting a novel biological pathway. The number of cardiovascular trials sponsored by small and medium-sized companies and the number of novel drugs entering Phase 3 trials increased over time. Most drugs were discontinued in Phase 3 due to inadequate efficacy (44%) or safety issues (24%), but the Phase 3 trial results for only one-half of the discontinued drugs were published in peer-reviewed journals. These results shed light on important shifts in research and development activity and confirm the perceived challenges in cardiovascular translational research.
Key Words: cardiovascular drug development, regulatory science, translational research
Abbreviations and Acronyms: ATC, Anatomical Therapeutic Chemical; CI, confidence interval; CNS, central nervous system; FDA, Food and Drug Administration; HR, hazard ratio; LDL, low-density lipoprotein; PCSK9, proprotein convertase subtilisin/kexin type 9
The development of new prescription drugs and their adoption into clinical practice have been associated with significant reductions in cardiovascular mortality over the past 2 decades (1). Despite this progress, cardiovascular disease is a leading cause of death in the developing world and still accounts for 1 in 3 deaths in the United States 1, 2, 3, 4, 5. The productivity of translational research in this field has recently come under scrutiny amidst concerns over the declining pipeline of novel therapies (6). Proposed explanations for the discrepancy between the slowdown in innovation and burden of disease include the rising cost of conducting large cardiovascular outcome trials, stagnating financial investment, and diminished commercial attractiveness of the cardiovascular field owing to availability of low-cost generic medications 6, 7. Several high-profile failures of clinical development have contributed to this perception. For example, in 2012, a large Phase 3 trial of varespladib, a secretory phospholipase A2 inhibitor hypothesized to improve cardiovascular outcomes, was halted when an interim analysis found that the drug was in fact associated with an increased risk of myocardial infarction (8).
There are limited data on trends in cardiovascular research and development and the factors associated with the success of new therapies in clinical trials. It has been previously reported that the number of new cardiovascular drugs approved by the U.S. Food and Drug Administration (FDA) has declined in recent years 6, 9. A contraction in the pool of cardiovascular drugs under development has also been reported (10), but trends in new drugs that have entered clinical testing or those that have been discontinued remain undefined.
In this study, we describe temporal trends in cardiovascular drug development over the past 2 decades, analyze the likelihood that investigational cardiovascular drugs successfully complete pre-approval clinical trials, and characterize the novelty of drug pathways, reasons for discontinuation, and rates of publishing trial results for new drugs in late-stage development.
Methods
Data sources and extraction
We analyzed data from a large commercial database of drug development activity (Citeline Pharmaprojects, Informa plc, London, United Kingdom), which tracks in real time the pipeline of pharmaceutical research and development projects. This database covers more than 50,000 products for all diseases from pre-clinical to commercialization stage and is widely used by industry and researchers to analyze trends in drug development 11, 12, 13, 14, 15. Using methods described previously (16), we selected for analysis all products that had entered Phase 1 clinical trials between January 1, 1990, and December 31, 2012 (N = 4,715). For each product, we extracted key information, including generic and proprietary names, sponsor, primary indication, mechanism of action (if known), start and end dates of each phase of clinical testing, date of regulatory approval (if applicable), and date and reason for discontinuation (e.g., failure to demonstrate efficacy, safety concerns, commercial/financial). On the basis of the primary indication, each product was mapped to an Anatomical Therapeutic Chemical (ATC) code, which categorizes drugs according to the organ or system on which they act and their therapeutic and chemical characteristics. We focused on drugs intended to treat disorders of the cardiovascular system (ATC code C), such as antihypertensive, antiarrhythmic, antianginal, and lipid-lowering agents, and disorders of blood and blood-forming organs (ATC code B), such as blood fraction and plasma substitutes, and anticoagulant, antithrombotic, antifibrinolytic and antianemic agents. We also compared rates of cardiovascular drugs entering clinical trials with those of cancer drugs (ATC code L01) and central nervous system (CNS) drugs (ATC code N, except N01 and N02) (11). We categorized all sponsors in our study cohort into large pharmaceutical companies, defined as companies with gross revenues >$1 billion, and small and medium-sized companies. Next, we searched Medline, EMBASE, and Web of Science for peer-reviewed publications of trial results, and search engines, press releases, and other publicly available sources for the stated reasons (if any) for discontinuation of drug development.
Finally, 2 investigators (T.J.H. and J.C.L.) categorized cardiovascular drugs that entered Phase 3 trials during our study period as targeting a “novel pathway” or “other target.” Consistent with prior studies by the FDA and others 17, 18, 19, we defined a novel pathway as a target or biological pathway for which the FDA had not yet approved a therapeutic agent by the pivotal trial start year. Changes in formulation (e.g., the first oral alternative to existing intravenously administered products) and new combinations of existing drugs (with or without a new agent) were considered novel pathways. Changes in chirality (e.g., a purified single enantiomer form of an already-approved racemic drug) were not considered to be a novel pathway. Any disagreements (representing ∼5% of cases) were resolved by consensus.
All data were initially downloaded on June 28, 2013, and information on publication status and novelty was updated through March 1, 2016. This study was not submitted for institutional review board review, because it is based on publicly available data and involved no patient health records.
Outcome measures
We first studied temporal trends in the number of new Phase 1, 2, and 3 clinical trials started for investigational cardiovascular drugs over time and compared these trends to those for drugs intended to treat cancer and CNS disorders. We also evaluated the proportion of such trials started by small and medium-sized companies. Because the absolute number of new trials would not capture differential rates of development activity among therapeutic areas, the primary outcome was the proportion of new cardiovascular drug trials relative to all new clinical trials in a given year.
Our second outcome of interest was the likelihood of survival of cardiovascular drugs, defined as the probability of successfully proceeding from one clinical trial phase to the subsequent phase of development (e.g., Phase 1 to Phase 2). Because companies often discontinue development projects without public disclosure, we used a conservative approach to identify “implicitly” discontinued products by assuming that projects with no development reported for 3 calendar years or more from the start date for Phase 1 and 5 calendar years or more for Phase 2 and Phase 3 were discontinued. Our third outcome of interest was the proportion of new Phase 3 trials started for drugs targeting novel pathways over time. Finally, we analyzed the reasons for discontinuation of cardiovascular drugs in late-stage development (i.e., during or after Phase 3 trials) and the rate of publication of the results from these trials.
Statistical analysis
We calculated the proportion of new Phase 1, 2, and 3 clinical trials for cardiovascular drugs relative to all new clinical trials and compared to those for cancer and CNS drugs, the proportion of new trials started by small and medium-sized companies, and the proportion of Phase 3 trials started for drugs targeting novel pathways. We used linear regression for trend analysis of continuous variables, and we used the Fisher exact test to compare the difference in proportion of new trials started by small and medium-sized companies for cardiovascular versus noncardiovascular drugs.
To determine the probability of progression of drugs in development, we constructed Cox proportional hazards regression models for each phase change (i.e., Phase 1 to 2, Phase 2 to 3, and Phase 3 to regulatory filing). A key assumption of the Cox proportional hazards model is the proportionality of hazards. Although no violations of proportionality were observed in the Phase 3 model, the assumption of proportional hazards was not met for the Phase 1 and Phase 2 models, indicating that the estimated hazard ratios (HRs) from these models should be interpreted as the average HR over time. For the models corresponding to progression from Phase 2 to Phase 3 and from Phase 3 to regulatory filing, we restricted our analysis to products that entered testing by January 1, 2008, because inclusion of more recent trials may bias our results due to our 5-year discontinuation threshold. As a sensitivity analysis, we used logistic regression and also repeated our analysis excluding hematologic drugs (i.e., only ATC code C).
Next, we constructed multivariable linear regression to examine factors associated with drugs categorized as targeting novel pathways. Models included all variables of interest regardless of statistical significance: firm type (an indicator variable for large pharmaceutical company vs. small and medium-sized companies), indicators for therapeutic area, and a continuous time variable. Finally, we used descriptive statistics to summarize the reasons for cardiovascular drug discontinuations as due to efficacy, safety, or commercial or other reasons.
Statistical analyses were performed using Stata version 12 (StataCorp, College Station, Texas). Two-tailed p values <0.05 were considered statistically significant.
Results
Our study cohort comprised 347 cardiovascular drugs that entered Phase 1 testing between 1990 and 2012, of which 239 (69%) were categorized under ATC code C, corresponding to drugs for the cardiovascular system (e.g., antihypertensive agents) and 108 (31%) were categorized under ATC code B, corresponding to hematologic drugs (e.g., antithrombotic agents and blood substitutes). The most common types of products under development were antihypertensive agents (18%, 19%, and 25% of new cardiovascular Phase 1, 2, and 3 trials, respectively), lipid-lowering agents (22%, 20%, and 12%), and anticoagulants (9%, 6%, and 9%).
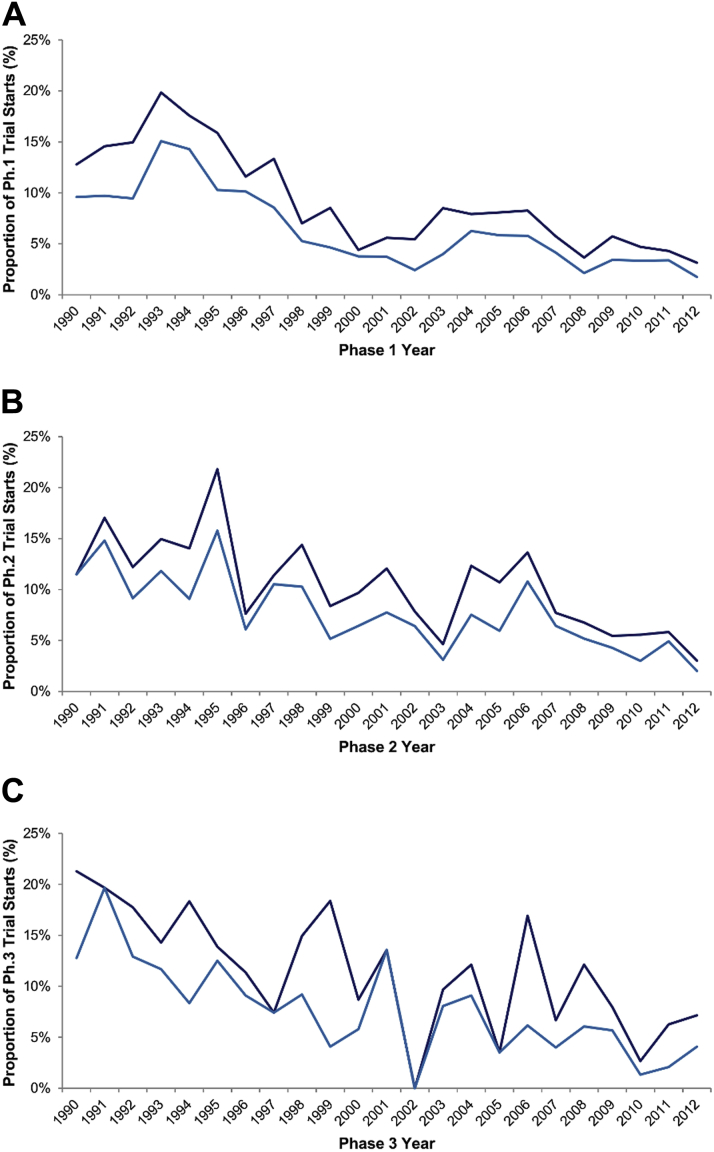
The number of new cardiovascular drugs entering clinical trials in all stages of development declined over time (p < 0.001 for linear trend in Phases 1 to 3) (Figure 1). Between 1990 and 1995, 108 of 679 (16%) Phase 1 trials were initiated for cardiovascular drugs, compared with 125 of 2,366 (5%) Phase 1 trials between 2005 and 2012. Similarly, cardiovascular drugs accounted for 21% of all Phase 3 trials in 1990 but only 7% in 2012. This decline was similar to that for CNS drugs, whereas the number of new cancer drugs increased over the same period (Figure 2).
Figure 1.
Temporal Trends in Cardiovascular Drugs Entering Clinical Trials, 1990 to 2012
Temporal trends in all new cardiovascular (Anatomical Therapeutic Chemical [ATC] codes B and C; shown in dark blue) and cardiovascular (ATC code C only; shown in light blue) trials as a proportion of trials for all products in Phase 1 (A), Phase 2 (B), and Phase 3 (C), 1990 to 2012. Ph. = phase.
Figure 2.
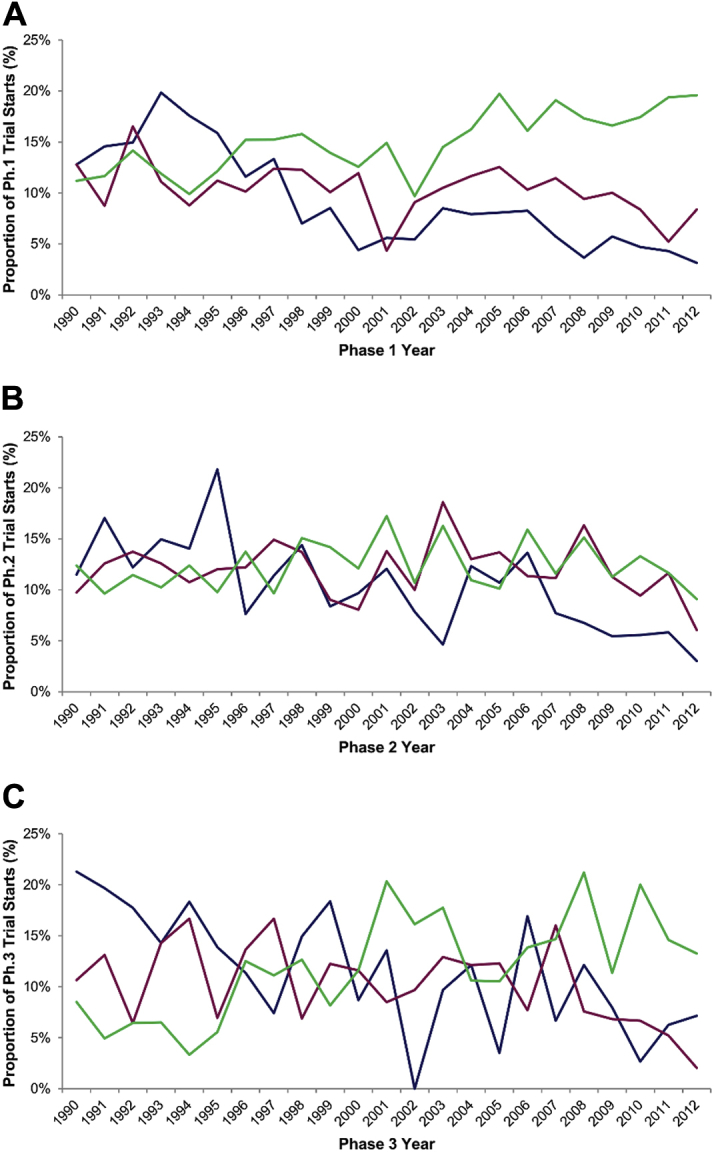
Temporal Trends in Cardiovascular, Cancer, and Central Nervous System Drugs Entering Clinical Trials, 1990 to 2012
Temporal trends in cardiovascular (Anatomical Therapeutic Chemical [ATC] codes B and C; shown in blue), cancer (shown in green), and central nervous system (shown in red) trials as a proportion of trials for all products in Phase 1 (A), Phase 2 (B), and Phase 3 (C), 1990 to 2012. Ph. = phase.
Large pharmaceutical companies sponsored most clinical trials for investigational cardiovascular drugs. Overall, small and medium-sized companies accounted for 43% of new Phase 1 trials for cardiovascular drugs, 38% of new Phase 2 trials, and 31% of new Phase 3 trials. In all 3 phases, the proportion of cardiovascular trials started by small and medium-sized companies was significantly smaller than that for noncardiovascular trials (p < 0.001 for all phases), but the number of cardiovascular trials sponsored by small and medium-sized companies grew over time (Supplemental Figure 1).
Progress of cardiovascular drugs through development
We found no evidence for a difference in probability of survival (defined as successful progression to the next stage of development) between cardiovascular and noncardiovascular drugs in Phase 1 (HR: 1.13; 95% confidence interval [CI]: 0.78 to 1.38; p = 0.41), Phase 2 (HR: 0.74; 95% CI: 0.05 to 1.18; p = 0.29), and Phase 3 (HR: 1.09; 95% CI: 0.54 to 1.43; p = 0.70) (Figure 3). In a sensitivity analysis excluding hematologic drugs (ATC code B), similar results were obtained, with no significant differences in probability observed between cardiovascular and noncardiovascular drugs in all phases of development (p > 0.06). Repeating our analysis with logistic regression yielded substantively similar results.
Figure 3.
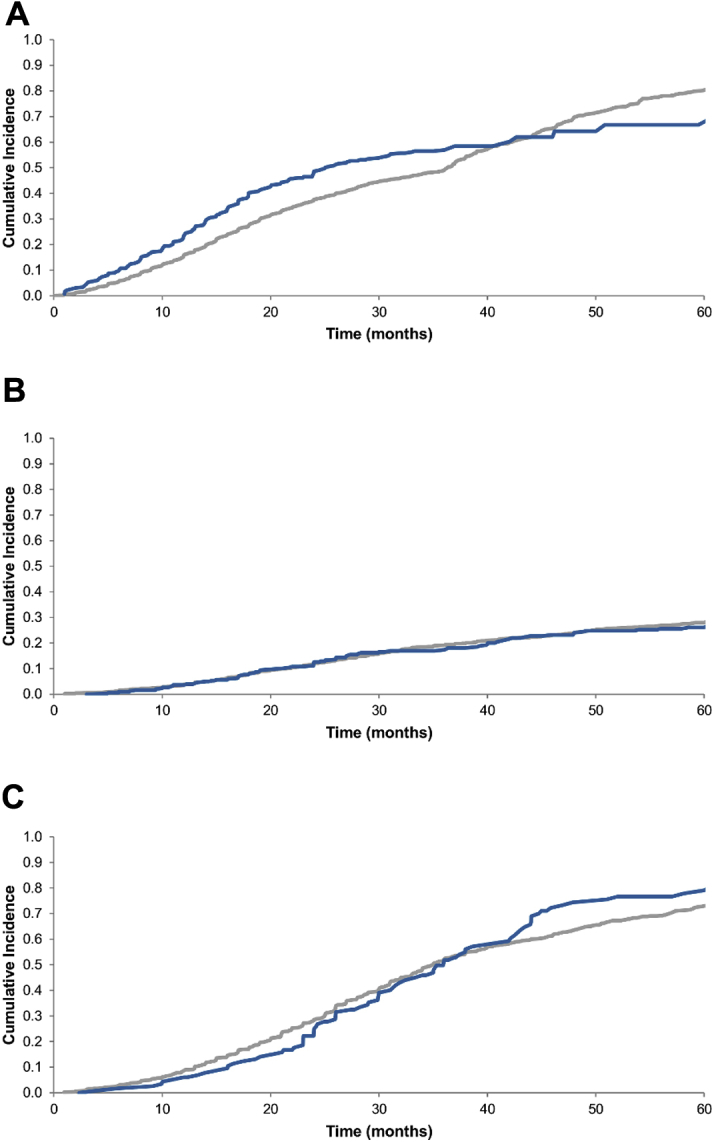
Cumulative Incidence Curves of Time to Progression to the Next Development Phase by Time Elapsed After Trial Start for Cardiovascular Drugs Compared With Noncardiovascular Drugs in Phase 1, 2, and 3 Trials
Cumulative incidence curves of progression to the next development phase for cardiovascular (Anatomical Therapeutic Chemical [ATC] codes B and C; shown in blue) and noncardiovascular (shown in grey) drugs in Phase 1 (A), Phase 2 (B), and Phase 3 (C) trials.
Drugs targeting novel biological pathways
Overall, 50% (89 of 177) of cardiovascular drugs entering Phase 3 trials were categorized as targeting a novel biological pathway. Over time, the rate of novel drugs entering Phase 3 trials increased, with novel drugs accounting for 27% of Phase 3 cardiovascular trials in 1990 to 1991 and 57% in 2012 (p = 0.004 for linear trend) (Supplemental Figure 2). In multivariable analyses, none of the studied variables were significant predictors of novel drug status.
Discontinuation of development for efficacy and safety reasons
Among 63 cardiovascular drug discontinuations in Phase 3, the reasons for discontinuing development were identifiable for 54 (86%) (Figure 4). Clinical development for 28 (44%) cardiovascular drugs was discontinued due to poor efficacy, and 15 (24%) were discontinued due to safety issues, including 7 drugs that were associated with an increased risk of death. A further 11 (17%) drugs were discontinued for commercial or other strategic reasons. The results from the Phase 3 trials were published in peer-reviewed journals for roughly one-half (32 of 63, 51%) of these drugs with discontinued development.
Figure 4.
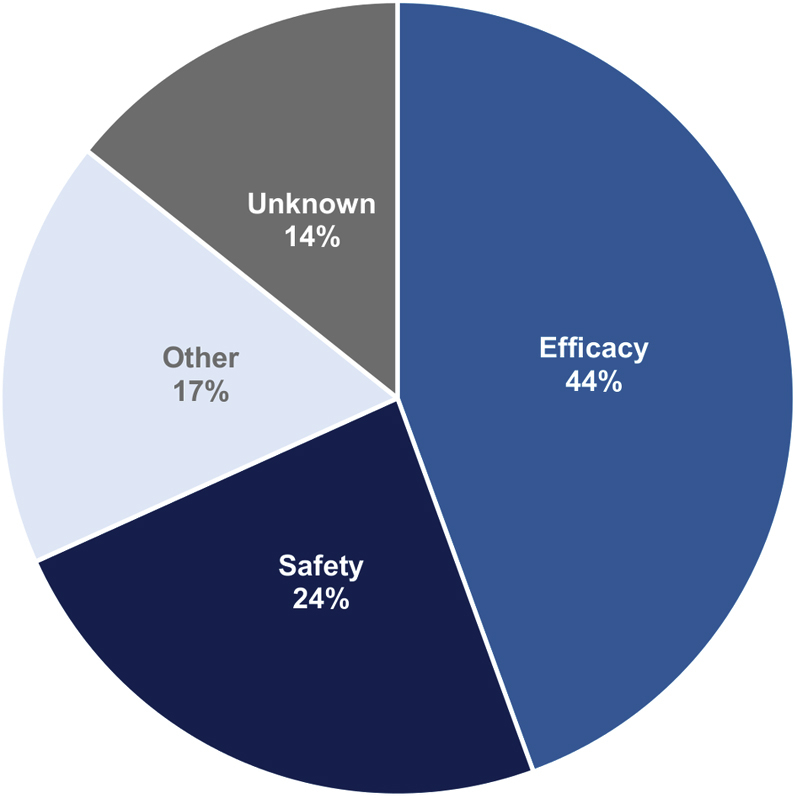
Discontinuation of Development of Cardiovascular Drugs in Phase 3, by Cause
Selected examples of discontinuations of cardiovascular drugs due to efficacy or safety reasons are shown in Table 1 20, 21, 22, 23. For example, the development of several lipid-modulating agents was discontinued after disappointing results from large controlled trials. A Phase 3 trial of torcetrapib, a cholesteryl ester transfer protein (CETP) inhibitor, was prematurely terminated in 2006 after a significantly increased risk of death was observed in treated patients (23). Nolomirole, a neurohormonal agent and selective dopaminergic D2 and adrenergic α2 agonist, failed to show benefit in reducing or slowing time to death or hospitalization among patients with heart failure (21).
Table 1.
Selected Investigational Cardiovascular Drugs Discontinued for Efficacy or Safety Reasons
| Drug Name (Ref. #) | Indication (Sponsor) | Key Trial Characteristics |
Reason for Discontinuation | ||
|---|---|---|---|---|---|
| Design | Comparator | Primary Endpoints | |||
| Cariporide (20) | Unstable angina and non–ST-segment elevation MI∗ (Sanofi-Aventis) | Randomized, double-blind | Placebo | Death or MI at 36 days | Failure to demonstrate efficacy (no significant improvement vs. placebo) |
| Darusentan | Treatment-resistant hypertension (Gilead/Abbott) | Randomized, double-blind | Placebo | Changes in sitting systolic and diastolic blood pressures | Failure to demonstrate efficacy in second pivotal trial (no significant change in blood pressure) |
| Lotrafiban (21) | Acute coronary syndrome (GlaxoSmithKline) | Randomized, double-blind | Placebo | Death, MI, stroke, severe recurrent ischemia, and urgent revascularization | Halted due to safety concerns (increased risk of death and serious bleeding) |
| Nolomirole (22) | Heart failure (Chiesi) | Randomized, double-blind | Placebo | Time to death or hospitalization for heart failure | Failure to demonstrate efficacy (no significant improvement vs. placebo) |
| Torcetrapib (23) | Prevention of cardiovascular disease (Pfizer) | Randomized, double-blind | Atorvastatin | Time to first major cardiovascular event† | Halted due to safety concerns (increased risk of death and cardiovascular events) |
MI = myocardial infarction.
The inclusion criteria for the cariporide trial also included patients undergoing a high-risk percutaneous coronary intervention or coronary artery bypass surgery.
The primary outcome was the time to the first occurrence of a major cardiovascular event, a composite that included 4 components: death from coronary heart disease (defined as fatal MI excluding procedure-related events, fatal heart failure, sudden cardiac death, or other cardiac death), nonfatal MI (excluding procedure-related events), stroke, and hospitalization for unstable angina.
Discussion
This study sheds light on several important shifts in cardiovascular research and development activity that have occurred over the past 2 decades. We found that the share of new cardiovascular drugs entering clinical trials has fallen since 1990, both in absolute terms and in comparison to drugs in other therapeutic areas, such as the development of new cancer therapeutics. In parallel, over time, small and medium-sized companies have sponsored a greater proportion of trials for new therapies.
Our findings confirm the perceived challenges in cardiovascular translational research, extending previously reported declines in successful cardiovascular drug approvals (6) to show that there have been fewer new investigational cardiovascular drugs across all stages of clinical development, particularly in late-stage development. Although the numbers may be declining overall, we also found a relative growth in the number of drugs entering late-stage testing that targeted novel biological pathways, suggesting that the observed contraction in cardiovascular research output may be driven by fewer follow-on drugs. This trend is consistent with a prior study that found a decrease over the past decade in the number of FDA approvals for drugs that were not first-in-class (17).
Cardiovascular drugs do not appear to be any less likely to successfully complete clinical trials than other drugs, even in Phase 3. Although they can be time consuming and costly to conduct, Phase 3 comparative clinical trials also provide the highest level of evidence on safety and efficacy to justify utilization in the intended patient population. As such, the consensus of stakeholders from industry, academia, and regulators was that “despite a temptation to use surrogate endpoints to decrease sample sizes and shorten the duration of clinical trials (ostensibly to reduce the likelihood of drug-development failures)[…]we must continue to promote large, pragmatic trials to measure clinical outcomes when evaluating new cardiovascular therapies” (6). For example, a recent FDA advisory committee stressed that low-density lipoprotein (LDL) cholesterol levels were not a reliable surrogate for cardiovascular benefit, and that timely completion of outcome trials was imperative (24). Among cardiovascular drugs for which the development was halted in Phase 3, our study found that most were discontinued due to inadequate efficacy, underscoring the importance of rigorous trials in elucidating the risk–benefit balance of new treatments.
In some cases, early and compelling evidence of superior efficacy, compared with existing treatment options, tilts the risk–benefit balance towards faster patient access, and various regulatory programs can be leveraged to appropriately expedite the development and approval of these treatments. For example, in March 2014, the PARADIGM-HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial of sacubitril/valsartan (Entresto, Novartis, Basel, Switzerland) was stopped early when investigators reported that the combination significantly reduced the risks of death and of hospitalization for heart failure (25). In April, the sponsor notified the FDA, which subsequently granted fast-track designation (which provides rolling review of the new drug application, more frequent communication with FDA reviewers, and other actions to expedite development) and priority review (which requires the FDA to make a decision within 6 months, rather than the standard 10-month timeline). The first full approval was granted by the FDA in July 2015, with approvals by authorities in Canada and Europe in October and November 2015, respectively.
Advances in translational science may yield further breakthroughs, and policymakers seeking to promote innovation in cardiovascular drug development should prioritize new incentives for such research, particularly given the demonstrated potential of recent cardiovascular translational efforts. For example, the discovery of proprotein convertase subtilisin/kexin type 9 (PCSK9) (26), identification of mutations in PCSK9 that reduce LDL cholesterol 27, 28, 29, and the ensuing validation of PCSK9 as a therapeutic target for the treatment of hypercholesterolemia 30, 31 represent a recent success in the efficient translation of academic research into novel therapeutics. After the initial discovery in 2003, the first human clinical trial of a monoclonal antibody to PCSK9 was started in 2009 (32). In 2015, recognizing the potential benefit of these drugs for high-risk patients, the FDA approved alirocumab and evolocumab before the large cardiovascular outcome trials were completed. Other inhibitors of PCSK9 are now under development, including a RNA interference drug 33, 34 and a therapeutic vaccine (35).
Our results also indicate that the pivotal trial results are frequently not published for drugs in which development is discontinued. This paucity of information, particularly on development failures, is problematic for investigators, as understanding why a drug fails may yield insights for other drugs in the same class and potentially guide efforts to repurpose failed drugs for new indications. Such information could also be useful to patients, who otherwise may be exposed to futile treatments or to unnecessary harms 36, 37. In addition, even “failed” trials can yield valuable insights on the pathophysiology of disease and the validity of experimental systems and surrogate endpoints (such as in the case of torcetrapib and the use of LDL cholesterol as a surrogate for cardiovascular outcomes). To improve reporting rates, recently, the National Institutes of Health issued a proposed regulation to require that trials for unapproved drugs are registered and that results are deposited in a public repository. If enacted, this rule may further incentivize investigators to share, at a minimum, summary study results for all clinical trials (38).
Study limitations
We used publicly available sources and commercial databases to identify the reasons for discontinuation, but additional factors may have played a role in these failures. However, we were able to identify the stated reasons for discontinuation of development for most (86%) of the drugs in our study cohort. In addition, although our study period was chosen to allow sufficient time for follow-up, it is possible that some of the discontinued drugs may be approved, and more trial results may be published in the future.
Conclusions
Over the past 2 decades, fewer investigational cardiovascular drugs have entered clinical trials across all stages of development, though recently, more therapies have targeted novel biological pathways and have been sponsored by small and medium-sized companies. Most cardiovascular drugs fail in Phase 3 clinical trials due to inadequate efficacy or safety concerns, but cardiovascular drugs do not appear to be more likely to fail than drugs for other diseases. Given the increasing burden of cardiovascular disease globally, the declining pipeline of new therapies is concerning. Policymakers should focus their efforts on supporting research aimed at improving gaps in the understanding of the pathophysiological bases for cardiovascular disorders, as well as facilitating translational efforts to develop new cardiovascular therapeutics.
Footnotes
Mr. Hwang has been an employee of Blackstone and Bain Capital, which has invested in health care companies. Dr. Lauffenburger has received unrestricted research funding payable to her institution from AstraZeneca. Dr. Franklin has received research funding from the Patient-Centered Outcomes Research Institute (PCORI) and Merck & Co.; and has consulted for Aetion, Inc., a software company. Dr. Kesselheim has received research funding from the Greenwall Foundation, Harvard Program in Therapeutic Science, Laura and John Arnold Foundation, and the FDA Office of Generic Drugs and Division of Health Communication.
Appendix
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S. Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V. Global burden of cardiovascular disease: time to implement feasible strategies and to monitor results. J Am Coll Cardiol. 2014;64:520–522. doi: 10.1016/j.jacc.2014.06.1151. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy K.S. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350:2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 5.Gaziano T.A. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112:3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 6.Fordyce C.B., Roe M.T., Ahmad T. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol. 2015;65:1567–1582. doi: 10.1016/j.jacc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Garber A.M. An uncertain future for cardiovascular drug development? N Engl J Med. 2009;360:1169–1171. doi: 10.1056/NEJMp0808414. [DOI] [PubMed] [Google Scholar]
- 8.Nicholls S.J., Kastelein J.J., Schwartz G.G. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 9.Darrow J.J., Kesselheim A.S. Drug development and FDA approval, 1938-2013. N Engl J Med. 2014;370:e39. doi: 10.1056/NEJMp1402114. [DOI] [PubMed] [Google Scholar]
- 10.Pammolli F., Magazzini L., Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 2011;10:428–438. doi: 10.1038/nrd3405. [DOI] [PubMed] [Google Scholar]
- 11.Kesselheim A.S., Hwang T.J., Franklin J.M. Two decades of new drug development for central nervous system disorders. Nat Rev Drug Discov. 2015;14:815–816. doi: 10.1038/nrd4793. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter D., Zucker E.J., Avorn J. Drug-review deadlines and safety problems. N Engl J Med. 2008;358:1354–1361. doi: 10.1056/NEJMsa0706341. [DOI] [PubMed] [Google Scholar]
- 13.Adams C.P., Brantner V.V. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 2006;25:420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 14.Lincker H., Ziogas C., Carr M., Porta N., Eichler H.G. Regulatory watch: where do new medicines originate from in the EU? Nat Rev Drug Discov. 2014;13:92–93. doi: 10.1038/nrd4232. [DOI] [PubMed] [Google Scholar]
- 15.Hwang T.J., Kesselheim A.S. Vaccine pipeline has grown during the past two decades with more early-stage trials from small and medium-size companies. Health Aff (Millwood) 2016;35:219–226. doi: 10.1377/hlthaff.2015.1073. [DOI] [PubMed] [Google Scholar]
- 16.Hwang T.J., Carpenter D., Kesselheim A.S. Target small firms for antibiotic innovation. Science. 2014;344:967–969. doi: 10.1126/science.1251419. [DOI] [PubMed] [Google Scholar]
- 17.Lanthier M., Miller K.L., Nardinelli C., Woodcock J. An improved approach to measuring drug innovation finds steady rates of first-in-class pharmaceuticals, 1987-2011. Health Aff (Millwood) 2013;32:1433–1439. doi: 10.1377/hlthaff.2012.0541. [DOI] [PubMed] [Google Scholar]
- 18.Kesselheim A.S., Wang B., Franklin J.M., Darrow J.J. Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: cohort study. BMJ. 2015;351:h4633. doi: 10.1136/bmj.h4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eder J., Sedrani R., Wiesmann C. The discovery of first-in-class drugs: origins and evolution. Nat Rev Drug Discov. 2014;13:577–587. doi: 10.1038/nrd4336. [DOI] [PubMed] [Google Scholar]
- 20.Théroux P., Chaitman B.R., Danchin N. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations: main results of the GUARDIAN trial. Circulation. 2000;102:3032–3038. doi: 10.1161/01.cir.102.25.3032. [DOI] [PubMed] [Google Scholar]
- 21.Topol E.J., Easton D., Harrington R.A. Randomized, double-blind, placebo-controlled, international trial of the oral IIb/IIIa antagonist lotrafiban in coronary and cerebrovascular disease. Circulation. 2003;108:399–406. doi: 10.1161/01.CIR.0000084501.48570.F6. [DOI] [PubMed] [Google Scholar]
- 22.Torp-Pedersen C., Køber L., Carlsen J.E. A randomised trial of a pre-synaptic stimulator of DA2-dopaminergic and alpha2-adrenergic receptors on morbidity and mortality in patients with heart failure. Eur J Heart Fail. 2008;10:89–95. doi: 10.1016/j.ejheart.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Barter P.J., Caulfield M., Eriksson M. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 24.Everett B.M., Smith R.J., Hiatt W.R. Reducing LDL with PCSK9 inhibitors—the clinical benefit of lipid drugs. N Engl J Med. 2015;373:1588–1591. doi: 10.1056/NEJMp1508120. [DOI] [PubMed] [Google Scholar]
- 25.McMurray J.J., Packer M., Desai A.S. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 26.Seidah N.G., Benjannet S., Wickham L. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abifadel M., Varret M., Rabès J.P. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J., Pertsemlidis A., Kotowski I.K., Graham R., Garcia C.K., Hobbs H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J., Boerwinkle E., Mosley T.H., Jr., Hobbs H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 30.Rashid S., Curtis D.E., Garuti R. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci U S A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall S.S. Genetics: a gene of rare effect. Nature. 2013;496:152–155. doi: 10.1038/496152a. [DOI] [PubMed] [Google Scholar]
- 32.Stein E.A., Mellis S., Yancopoulos G.D. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 33.Frank-Kamenetsky M., Grefhorst A., Anderson N.N. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald K., Frank-Kamenetsky M., Shulga-Morskaya S. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crossey E., Amar M.J., Sampson M. A cholesterol-lowering VLP vaccine that targets PCSK9. Vaccine. 2015;33:5747–5755. doi: 10.1016/j.vaccine.2015.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters D.D., Chau K.H. Tribulations of recent cardiology trials, the audacity of hope, and HOPE-3. Can J Cardiol. 2016;32:275–277. doi: 10.1016/j.cjca.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Hakala A., Kimmelman J., Carlisle B., Freeman G., Fergusson D. Accessibility of trial reports for drugs stalling in development: a systematic assessment of registered trials. BMJ. 2015;350:h1116. doi: 10.1136/bmj.h1116. [DOI] [PubMed] [Google Scholar]
- 38.Hudson K.L., Collins F.S. Sharing and reporting the results of clinical trials. JAMA. 2015;313:355–356. doi: 10.1001/jama.2014.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.