Theoretical models of autism posit that impaired social skills may be related to reduced motivation arising from aberrancies in reward processing systems in the brain. Supekar et al. report that structural and functional deficits in the mesolimbic reward pathway contribute to impaired social skills in childhood autism.
Keywords: autism, nucleus accumbens, ventral tegmental area, HARDI, social motivation
Abstract
Lack of interest in social interaction is a hallmark of autism spectrum disorder. Animal studies have implicated the mesolimbic reward pathway in driving and reinforcing social behaviour, but little is known about the integrity of this pathway and its behavioural consequences in children with autism spectrum disorder. Here we test the hypothesis that the structural and functional integrity of the mesolimbic reward pathway is aberrant in children with autism spectrum disorder, and these aberrancies contribute to the social interaction impairments. We examine structural and functional connectivity of the mesolimbic reward pathway in two independent cohorts totalling 82 children aged 7–13 years with autism spectrum disorder and age-, gender-, and intelligence quotient-matched typically developing children (primary cohort: children with autism spectrum disorder n = 24, typically developing children n = 24; replication cohort: children with autism spectrum disorder n = 17, typically developing children n = 17), using high angular resolution diffusion-weighted imaging and functional MRI data. We reliably identify white matter tracts linking—the nucleus accumbens and the ventral tegmental area—key subcortical nodes of the mesolimbic reward pathway, and provide reproducible evidence for structural aberrations in these tracts in children with autism spectrum disorder. Further, we show that structural aberrations are accompanied by aberrant functional interactions between nucleus accumbens and ventral tegmental area in response to social stimuli. Crucially, we demonstrate that both structural and functional circuit aberrations in the mesolimbic reward pathway are related to parent-report measures of social interaction impairments in affected children. Our findings, replicated across two independent cohorts, reveal that deficits in the mesolimbic reward pathway contribute to impaired social skills in childhood autism, and provide fundamental insights into neurobiological mechanisms underlying reduced social interest in humans.
Introduction
Impairments in social communication are a defining phenotypic feature of autism spectrum disorder (ASD) (Kanner, 1943). Children with the disorder demonstrate reduced preference and orientation to social stimuli (Dawson et al., 1998; Swettenham et al., 1998; Baranek, 1999; Riby and Hancock, 2008; Nakano et al., 2010; Pierce et al., 2011; Jones and Klin, 2013; Pierce et al., 2016; Franchini et al., 2017), diminished interest in collaborative social activities (Liebal et al., 2008), deficits in reciprocal social interaction (Kanner, 1943), and find social situations less pleasant than their typically developing peers (Chevallier et al., 2012a; Ruta et al., 2017). A prominent theory of ASD posits that that affected individuals find social stimuli less rewarding than their neurotypical peers, leading to impaired social skills (Chevallier et al., 2012b). The mesolimbic reward pathway, which evaluates, regulates, and reinforces appetitive behaviours through dopaminergic signalling, is a core brain system for processing reward value (O’Connell and Hofmann, 2011). Although multiple studies using preclinical animal models of autism point to a link between aberrant mesolimbic reward pathway and dysfunction in reciprocal social interactions (Katayama et al., 2013; Gunaydin et al., 2014; Squillace et al., 2014; Bariselli et al., 2016; Krishnan et al., 2017), little is known about the integrity of this core reward circuit in ASD. Moreover, the relevance of findings in animal models to human clinical studies of childhood ASD remains largely unexplored.
The mesolimbic reward pathway reciprocally connects the ventral tegmental area (VTA) of the midbrain and the nucleus accumbens (NAc) of the striatum via white matter tracts along the medial forebrain bundle (Haber and Knutson, 2010). The VTA is a dense site of dopaminergic neurons that primarily project to the NAc. Multiple lines of animal research have shown that neural connections between VTA and NAc are essential for detecting and modulating responses to rewarding stimuli (Lenz and Lobo, 2013; Abraham et al., 2014; Lammel et al., 2014; Hikida et al., 2016), and that lesioning and stimulation of the medial forebrain bundle influences reward-seeking behaviours (Krotewicz and Romaniuk, 1998; Boylan et al., 2007; Berridge and Kringelbach, 2015). Critically, the NAc and VTA regions also respond to social stimuli and have been shown to regulate social motivation in animals (Vanderschuren et al., 1995; Gordon et al., 2002; Trezza et al., 2011; van Kerkhof et al., 2013, 2014). In humans, functional MRI studies have reported NAc engagement during processing of social stimuli in neurotypical adults (Knutson et al., 2001; Spreckelmeyer et al., 2009), and reduced NAc activation in both children (Scott-Van Zeeland et al., 2010) and adults with ASD (Critchley et al., 2000; Scott-Van Zeeland et al., 2010; Delmonte et al., 2012; Dichter, 2012; Assaf et al., 2013; Kohls et al., 2013). However, it is not known whether the structural and functional integrity of connections between the NAc and VTA is aberrant in children with ASD, and whether this aberrant integrity contributes to social deficits in affected children, thus limiting our understanding of brain circuit mechanisms underlying childhood ASD. This is perplexing since autism is widely characterized as a neurodevelopmental disorder of altered brain connectivity (Supekar et al., 2013; Uddin et al., 2013).
Our study had four major objectives: (i) to develop a robust protocol for delineating white matter tracts connecting the NAc and VTA in children; (ii) to investigate whether the structural integrity of these tracts is aberrant in children with ASD; (iii) to investigate whether functional interactions between the NAc and VTA during the processing of social stimuli are aberrant in children with ASD; and (iv) to investigate whether structural and functional aberrations of the mesolimbic reward pathway are linked to behavioural deficits in social interaction in children with ASD. Here, we use structural and functional imaging techniques with well-defined clinical-behavioural assays to address these objectives. The knowledge gained here has implications for (i) identifying circumscribed neurobiological mechanisms of social dysfunction in children with ASD; and (ii) cross-species translational research by clarifying the relevance of preclinical animal models to human clinical studies of childhood ASD.
A major challenge in investigating the mesolimbic reward pathway in the human brain is that standard diffusion tensor imaging (DTI) protocols are ill-suited for quantitatively assessing white matter tracts linking the NAc and VTA (Mori and Zhang, 2006; Alexander and Seunarine, 2012; Emsell, 2016). Furthermore, current analytical procedures are limited in their ability to accurately localize NAc and VTA. To address this, we used a high angular resolution diffusion-weighted imaging (HARDI) protocol (Vos et al., 2016) and an analysis pipeline capable of resolving crossing fibres and detecting white matter tracts that link deep brain structures, including the NAc and VTA. We analysed HARDI data from 41 children with ASD and 41 age-, sex-, and IQ-matched typically developing children, from two independent cohorts—the primary cohort and the replication cohort. In addition, we investigated functional connectivity between NAc and VTA using functional MRI data acquired in children from the primary cohort while they performed tasks involving perception of social and non-social stimuli.
Materials and methods
Participants
Primary cohort
Twenty-four children with ASD and 24 age-, gender-, and IQ-matched typically developing children were included in the HARDI part of this study (Supplementary Tables 1 and 3). Sixteen children with ASD and 20 age-, gender-, and IQ-matched typically developing children were included in the functional MRI part of this study (Supplementary Tables 2 and 4).
Replication cohort
Seventeen children with ASD and 17 age-, gender-, and IQ-matched typically developing children were included in the HARDI part of this study (Supplementary Tables 1 and 3).
Cohort data collection
The primary and the replication cohorts were part of separate data collection efforts. Specifically, the primary cohort data were obtained as part of a study investigating brain systems underlying social information processing in autism while the secondary cohort data were obtained as part of a study examining structural connectivity of large-scale brain networks in autism. Additionally, primary and replication cohort data were obtained from two different 3 T GE Signa MRI scanners located in adjacent suites in the Stanford Richard M. Lucas Center for Imaging. T1-weighted MRI data and HARDI (Frank, 2002; Descoteaux et al., 2009; Fillard et al., 2011; Vos et al., 2016) data from both cohorts, and functional MRI data from the primary cohort were acquired from each child.
In both cohorts, children with ASD received an autism diagnosis based on scores from the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 2000) and/or the Autism Diagnostic Observation Schedule (ADOS) (Luyster et al., 2009) following criteria established by the National Institutes of Health (Lainhart, 2006).
Imaging
The T1 and HARDI acquisition protocols were identical across the primary and replication cohorts.
Structural MRI acquisition and preprocessing
For each subject, a high resolution T1-weighted spoiled gradient recalled inversion recovery 3D MRI sequence was acquired for selecting the regions of interest. Structural MRI data acquisition and preprocessing procedures are described in detail in the Supplementary material.
HARDI acquisition, preprocessing and quality control
For each subject, a HARDI pulse sequence was acquired. HARDI data acquisition, preprocessing and quality control procedures are described in detail in the Supplementary material.
HARDI region of interest selection
Regions of interest for the NAc and amygdala were extracted from the FreeSurfer-based (Fischl et al., 2002) segmentation of each individual’s T1-weighted image, and regions of interest for the VTA were generated from midbrain peaks in the ‘REWARD’ (Delgado et al., 2000) contrast z-map distributed as part of the Human Connectome Project (Van Essen et al., 2012). The region of interest selection procedure is described in detail in the Supplementary material.
HARDI tract analysis
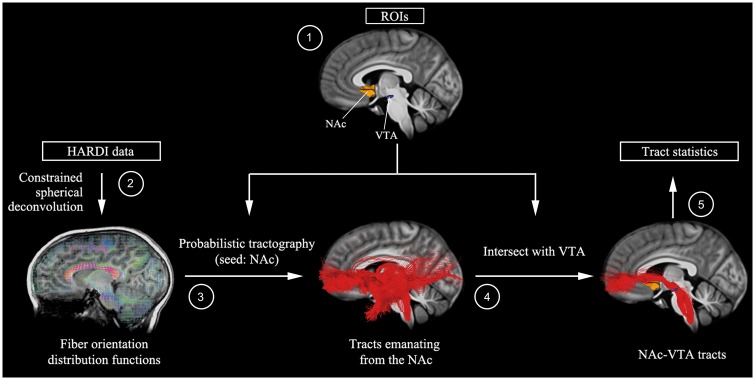
Our analytical pipeline for the preprocessed HARDI data is summarized in Fig. 1. Probabilistic tractography was performed by seeding 10 000 points in each voxel of a region of interest and generating streamlines using the iFOD2 (second order integration over fibre orientation distributions) algorithm (Tournier et al., 2010) in MRtrix3. Tractography parameters are described in detail in the Supplementary material. Fibre density—the source- and target-volume-normalized fraction of streamlines emanating from the seed region of interest and intersecting the ipsilateral target region of interest—was then computed for each child and used as a measure of structural integrity. In separate analyses, the NAc was seeded and targets in the VTA and amygdala were used to measure NAc-VTA and NAc-amygdala fibre densities, respectively. Fibre density was compared between the ASD and typically developing groups. Correlation and multivariate sparse regression analyses were used to determine the relationships between the structural connectivity and the ASD symptom severity (see Supplementary material for details). These analyses were conducted in each of the two cohorts separately.
Figure 1.
Overall HARDI mesolimbic reward pathway analysis pipeline. (1) NAc region of interest (ROI) was demarcated using FreeSurfer-based segmentations of each individual’s T1-weighted anatomical image, and then warped to diffusion space using parameters from a nonlinear co-registration between T1 and fractional anisotropy images implemented in the advanced normalization tools software package (Avants et al., 2011). A VTA region of interest was determined using midbrain activation peaks from a reward (gambling) task performed by 448 participants as part of the Human Connectome Project (Van Essen et al., 2012)—this approach allowed us to reliably identify VTA voxels that are most sensitive to reward. VTA regions of interest were transformed to individual subject diffusion space using a 2D template-based approach to maximize anatomical precision (see Supplementary material for details). (2) Fibre orientation distribution functions were computed by constrained spherical deconvolution with a maximum harmonic degree of 10, using MRtrix3 (Tournier et al., 2012). (3) Probabilistic tractography was then performed by seeding 10 000 points in each voxel of the NAc and generating streamlines using the iFOD2 algorithm implemented in MRtrix3 (Tournier et al., 2010). (4) Tracts emanating from the NAc were intersected with ipsilateral VTA region of interest to identify NAc-VTA tracts. (5) Fibre density—the target-volume (VTA) normalized fraction of streamlines emanating from the NAc and intersecting ipsilateral VTA—was then computed for each child.
Functional MRI data acquisition, preprocessing and analysis
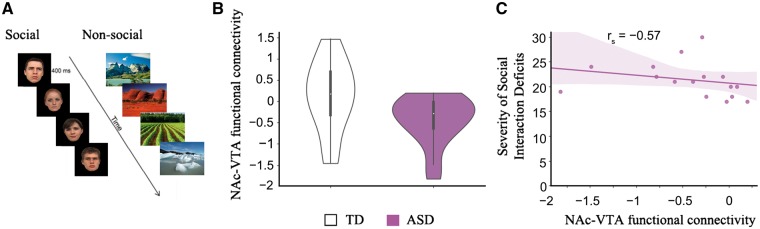
In the primary cohort, during the functional MRI scan, each participant performed an event-related task involving perception of social and non-social stimuli. Each participant viewed either social stimuli (faces) or non-social stimuli (scenes) or control stimuli (scrambled face or scene) (Fig. 4A). Given our goal of contrasting differential functional connectivity responses to social versus non-social stimuli, we performed task-based functional connectivity analysis using the generalized psychophysiological interaction (gPPI) model (McLaren et al., 2012) and preprocessed functional MRI data from NAc, VTA, and amygdala regions of interest. Functional MRI data acquisition, experimental design, preprocessing, region of interest selection, and functional connectivity analysis procedure is described in detail in the Supplementary material. We performed between-group t-tests on differences between functional connectivity values for face and scene stimuli. Correlation and multivariate sparse regression analyses were used to determine the relationships between functional connectivity and the ASD symptom severity (Supplementary material)
Figure 4.
Aberrant functional connectivity in mesolimbic reward pathway for social stimuli and its association with social interaction impairments in children with ASD. (A) Each participant viewed social stimuli (faces) and non-social stimuli (scenes). (B) Functional connectivity between the NAc and VTA during face relative to scene processing was disrupted in ASD. In contrast to their typically developing (TD) peers, children with ASD showed lower functional connectivity between the NAc and the VTA when viewing faces compared to scenes. (C) Children in the ASD group who had lower NAc-VTA functional connectivity during face relative to scene processing exhibited more severe social interaction impairments, as measured by ADI-R social interaction subscale.
Statistical analysis
Statistical significance (P < 0.05, two-tailed) for between-group analyses was determined using two-sample t-test. The strength of brain–behaviour relations was examined using the ADI-R, which is based on a child’s full developmental history. Additional analyses using ADOS, which assesses current symptom status, are reported in the Supplementary material. The relation between white matter integrity or functional connectivity, and social deficits was assessed using the social interaction subscale of the ADI-R. Additional analyses examined links with two other ADI-R subscales: restricted and repetitive behaviours and language–communication deficits. Brain–behaviour relations were first examined using Spearman correlations, and the specificity of findings with respect to the three ADI-R subscales was then investigated using a rigorous machine learning approach involving multivariate sparse regression with cross-validation (Tibshirani, 1996; see Supplementary material for details). To objectively quantify evidence for or against replication, we performed Bayesian analyses for t-tests and correlations using the R code (http://www.josineverhagen.com/?page_id=76) provided by Verhagen and colleagues (Verhagen and Wagenmakers, 2014; Wagenmakers et al., 2016).
Data availability
Data will be made available through the National Database for Autism Research (https://ndar.nih.gov).
Results
Identification of white matter tracts connecting the key nodes of the mesolimbic reward pathway
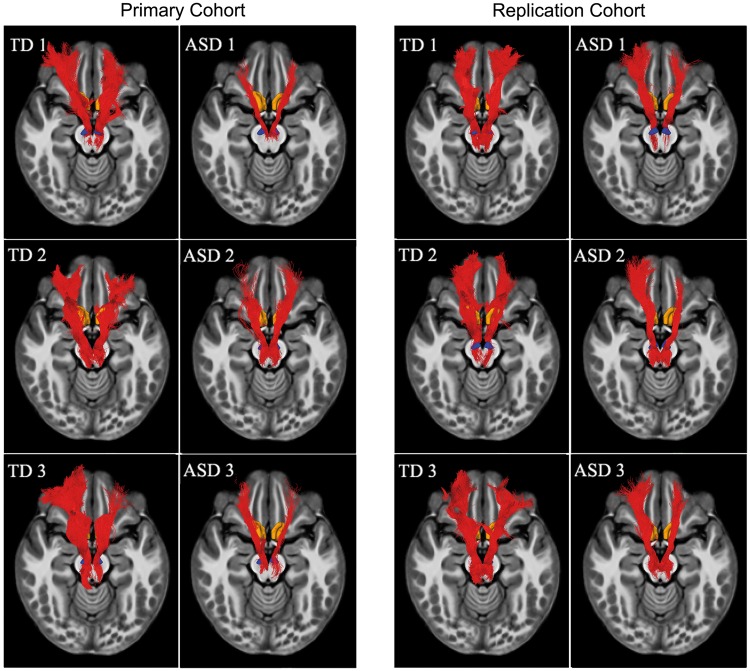
Using the HARDI data analyses procedures illustrated in Fig. 1, white matter tracts connecting the key nodes of mesolimbic reward pathway could be reliably detected in each child, in both the primary and replication cohorts. Single subject tracts connecting the NAc and VTA are shown in Fig. 2.
Figure 2.
Mesolimbic reward pathway in children with ASD and typically developing children. Using analytical procedures illustrated in Fig. 1, white matter tracts connecting the NAc and the VTA, the major subcortical nodes of the mesolimbic reward pathway, could be reliably detected in each child in both cohorts. Six exemplar participants (three ASD, three typically developing) from the primary cohort, and six exemplar participants (three ASD, three typically developing) from the replication cohort. In all cases, the NAc-VTA tracts were detected and showed similar trajectory. The tracts are shown in subject space. TD = typically developing children.
Structural deficits in mesolimbic reward pathway in children with ASD
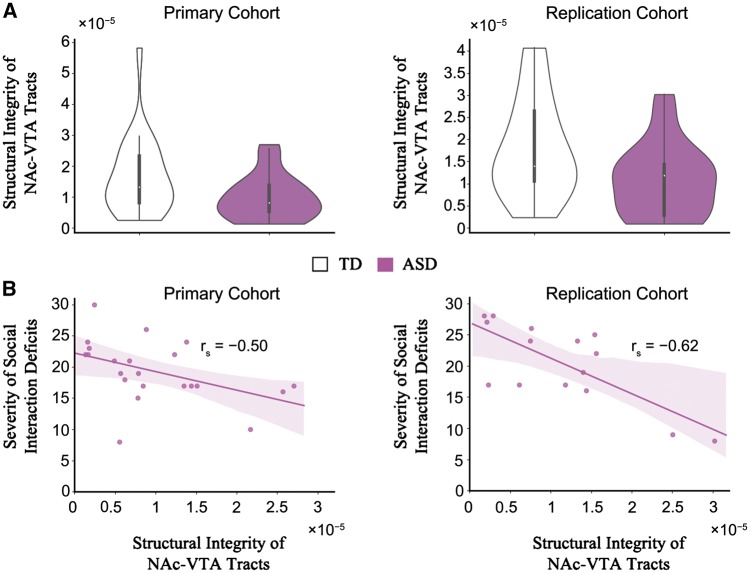
Within each cohort, we first compared the density of the identified NAc-VTA tracts between ASD and typically developing groups. The density of the NAc-VTA tracts was significantly lower in children with ASD compared to typically developing (TD) children, in the primary cohort [meanASD = 9.90 × 10−6, standard deviation (SD)ASD = 7.24 × 10−6, meanTD = 1.64 × 10−5, SDTD = 1.22 × 10−5, t(46) = − 2.24, P = 0.03, Bayes factor = 2.10, Cohen’s d = − 0.65; Fig. 3A]. Notably, we also observed this result in the replication cohort [meanASD = 1.09 × 10−5, SDASD = 8.27 × 10−6, meanTD = 1.80 × 10−5, SDTD = 1.14 × 10−5, t(32) = − 2.07, P = 0.04, Bayes factor = 1.64, Cohen’s d = − 0.71; Fig. 3A]. To objectively quantify evidence for or against replication, we performed additional Bayesian analyses for t-tests (Verhagen and Wagenmakers, 2014) and found that the Bayes factor for replication was high (BF10 = 5.83), indicating evidence in favour of the presence of effect (observed in the primary cohort) in the replication cohort. These results reliably demonstrate that the mesolimbic reward pathway is structurally aberrant in children with ASD. To investigate the anatomical specificity of this finding to the mesolimbic reward pathway, we examined white matter tracts connecting the NAc and the amygdala, a subcortical region that plays a crucial role in socio-affective processing (Kling and Brothers, 1992; Pessoa and Adolphs, 2010), with strong links to the NAc. Consistent with animal tract tracing studies that have reported monosynaptic links between the NAc and the amygdala (McDonald, 1991; Wright et al., 1996), white matter tracts connecting the NAc and amygdala could be reliably detected in all individual participants in both cohorts, using our HARDI analysis pipeline (Supplementary Fig. 1A–C). However, in both cohorts, we found no significant differences between ASD and typically developing groups in the density of NAc-amygdala tracts [primary cohort: meanASD = 4.36 × 10−6, SDASD = 3.92 × 10−6, meanTD = 4.46 × 10−6, SDTD = 2.24 × 10−6, t(46) = − 0.11, P = 0.91, Bayes factor = 0.29, Cohen’s d = − 0.03; replication cohort: meanASD = 4.04 × 10−6, SDASD = 1.70 × 10−6, meanTD = 4.10 × 10−6, SDTD = 2.02 × 10−6, t(32) = − 0.10, P = 0.92, Bayes factor = 0.33, Cohen’s d = − 0.03; Supplementary Fig. 1D] demonstrating the specificity of our findings to the mesolimbic reward pathway.
Figure 3.
Structural deficits in mesolimbic reward pathway and its association with social interaction impairments in children with ASD. (A) Density of the NAc-VTA tracts was significantly lower in children with ASD compared to typically developing children, in both cohorts (see main text for details). (B) Children in the ASD group who had lower density of the NAc-VTA tracts exhibited more severe social interaction impairments, as measured by ADI-R social interaction subscale, in both cohorts (see main text for details). TD = typically developing children.
Structural deficits in mesolimbic reward pathway are associated with social interaction impairments in children with ASD
Next, we examined the relation between NAc-VTA tract integrity and social interaction impairments in children with ASD and found that children in the ASD group who had lower density of the NAc-VTA tracts exhibited more severe social interaction impairments as measured by ADI-R social interaction subscale. Notably, this association was found in both cohorts separately [primary cohort: rs(22) = − 0.50, P = 0.02, Bayes factor = 3.57; replication cohort: rs(15) = − 0.62, P = 0.01, Bayes factor = 4.24; Fig. 3B], and in the combined cohort [primary cohort + replication cohort: rs(37) = − 0.49, P = 0.002, Bayes factor = 17.67]. To objectively quantify evidence for or against replication, we performed additional Bayesian analyses for correlations (Wagenmakers et al., 2016) and found that the Bayes factor for replication was high (BF10 = 37.95), indicating evidence in favour of the presence of effect (observed in the primary cohort) in the replication cohort. Crucially, we did not find a relationship between density of the NAc-VTA tracts and other ASD symptoms measured by other ADI-R subscales including restricted and repetitive behaviours [primary cohort: rs(22) = − 0.06, P = 0.80, Bayes factor = 0.39; replication cohort: rs(15) = − 0.10, P = 0.73, Bayes factor = 0.46] and language deficits [primary cohort: rs(22) = − 0.10, P = 0.66, Bayes factor = 0.41; replication cohort: rs(15) = − 0.48, P = 0.08, Bayes factor = 1.42]. To further examine the specificity of our brain–behaviour relationships we used a machine-learning approach: multivariate sparse regression combined with cross validation. Results from this analysis were consistent with the results from the correlation analysis, namely: in children with ASD, the ADI-R Social Interaction subscale scores and not the ADI-R Communication and Language or the ADI-R Restricted and Repetitive Behaviors subscale scores, were uniquely associated with fibre density of NAc-VTA tracts, in both the primary and replication cohort (Supplementary Tables 5 and 6). We also did not find a significant relationship between the density of the NAc-amygdala tracts and ADI-R social interaction subscale [primary cohort: rs(22) = − 0.19, P = 0.39, Bayes factor = 0.50; replication cohort: rs(15) = − 0.12, P = 0.67, Bayes factor = 0.46; Supplementary Fig. 1E]. These results highlight the specificity of our findings with respect to social interaction impairments and the mesolimbic reward pathway in children with ASD.
Aberrant functional connectivity in mesolimbic reward pathway for social stimuli in children with ASD
While there have been reports of atypical functional activation in the NAc in response to social stimuli in ASD (Scott-Van Zeeland et al., 2010; Delmonte et al., 2012; Kohls et al., 2013), nothing is known regarding functional interactions between the NAc and VTA in this context. To address this, we examined task-related functional connectivity from data acquired from 36 children from the primary cohort who underwent functional MRI scanning while performing an event-related task involving perception of social (face) and non-social (scene) stimuli (Fig. 4A). Functional connectivity between the NAc and VTA for the contrast faces versus scenes was estimated using a gPPI model (McLaren et al., 2012). gPPI analysis revealed that children with ASD, in contrast to their typically developing peers, showed decreased functional connectivity between the NAc and VTA during face relative to scene processing [meanASD = − 0.44, SDASD = 0.57, meanTD = 0.13, SDTD = 0.82, t(34) = − 2.34, P = 0.03, Bayes factor = 2.53, Cohen’s d = − 0.79; Fig. 4B]. We also computed functional connectivity between the NAc and the amygdala and found no significant group differences during face relative to scene processing [meanASD = − 0.11, SDASD = 0.78, meanTD = 0.05, SDTD = 0.74, t(34) = − 0.63, P = 0.53, Bayes factor = 0.38, Cohen’s d = − 0.21]. Additional analyses confirmed the robustness of our findings against potential motion-related confounds (Supplementary Fig. 2 and Supplementary material). These results demonstrate that atypical functional interactions between key nodes of the mesolimbic reward pathway in response to social stimuli in ASD.
Functional deficits in mesolimbic reward pathway are associated with social interaction impairments in children with ASD
Lastly, we sought to determine whether functional connectivity measures between the NAc and VTA are related to social interaction deficits. To this end, we examined the relation between functional interactions between the NAc and VTA in response to social stimuli and social interaction impairments again using the ADI-R social interaction subscale. This analysis revealed that children in the ASD group who had lower functional connectivity between the NAc and VTA in response to social stimuli exhibited more severe social interaction impairments [rs(16) = − 0.57, P = 0.02, Bayes factor = 3.02; Fig. 4C]. We did not find a relationship between NAc-VTA functional connectivity during face processing and other ASD symptoms measured by the ADI-R subscales including restricted and repetitive behaviours [rs(16) = − 0.18, P = 0.51, Bayes factor = 0.50] and deficits in language [rs(16) = − 0.07, P = 0.79, Bayes factor = 0.44].
To examine the specificity of our brain–behaviour relationships further, we used a machine-learning approach: multivariate sparse regression combined with cross-validation. Results from this analysis were consistent with the results from the correlation analysis, namely: in children with ASD, the ADI-R Social Interaction subscale scores and not the ADI-R Communication and Language or the ADI-R Restricted and Repetitive Behaviors subscale scores, were uniquely associated with NAc-VTA functional connectivity (Supplementary Table 7). We also did not find a significant relationship between NAc-amygdala functional connectivity and ADI-R social interaction subscale [rs(16) = − 0.44, P = 0.10, Bayes factor = 1.21]. These results highlight the specificity of our findings with respect to social interaction impairments and social information processing in the mesolimbic reward pathway in children with ASD.
Discussion
Our study addresses important gaps in knowledge of dysfunctional brain circuits associated with a core characteristic of ASD. Using advanced HARDI techniques, we provide novel evidence that children with ASD have reduced structural connectivity in the mesolimbic pathway linking the NAc and VTA, two brain areas critical for processing social reward. We also found that the weak connectivity contributes to impaired social interaction abilities such that children with ASD with weaker connectivity exhibited more severe social deficits. Notably, we replicated these findings in an independent (‘replication’) cohort of children with ASD and typically developing children. Although previous structural connectivity studies in adults with ASD, using standard DTI, have reported abnormalities in other white matter tracts within regions important for social information processing including the uncinate and arcuate fasciculi, cingulum bundle, inferior longitudinal fasciculus, and the inferior fronto-occipital fasciculus (Ameis and Catani, 2015; Catani et al., 2016), direct links between these structural impairments and social deficits have been missing (Walker et al., 2012). In contrast to studies in humans, research using animal models has consistently linked dysfunction in the mesolimbic dopaminergic reward pathway with atypical social function. For instance, both juvenile and adult animal models have demonstrated that (i) the NAc and VTA are responsive to social stimuli (Vanderschuren et al., 1995; Gordon et al., 2002; van Kerkhof et al., 2013); (ii) deficits in reciprocal social interactions arise from aberrant dopaminergic and glutamatergic neurotransmission (Katayama et al., 2013; Squillace et al., 2014; Bariselli et al., 2016; Krishnan et al., 2017); and (iii) that social play behaviour is modulated by dopaminergic signalling, in the mesolimbic reward pathway (Panksepp et al., 1984; Niesink and Van Ree, 1989; Manduca et al., 2016). A recent study used optogenetic manipulation of the mesolimbic reward pathway to show a causal role for this pathway in initiating and maintaining social interactions (Gunaydin et al., 2014). Our study extends these preclinical findings by showing that structural connectivity between the core nodes of the mesolimbic reward pathway is not only weaker in children with ASD but is also associated with individual differences in social interaction impairments in affected children. Notably, we did not find structural deficits in children with ASD in white matter tracts connecting the NAc and the amygdala, a region widely reported to be dysfunctional in ASD (Amaral et al., 2008). This finding is consistent with evidence from a majority of neuroimaging studies that have reported no aberrant neural responses in the amygdala during reward processing in individuals with ASD (Bottini, 2018). One possibility that is supported by our findings is that the predominant projections from the amygdala—specifically its basolateral nucleus—to the NAc are glutamatergic (Russo and Nestler, 2013) not dopaminergic, unlike the NAc-VTA pathway, which has a more specific link to social reward processing (Janak and Tye, 2015). The specificity of our findings with respect to the mesolimbic reward system provides support for a primary social reward deficit in ASD (Chevallier et al., 2012b), and highlights a circumscribed neurobiological signature of ASD.
To our knowledge, no study to date has systematically investigated the structural connectivity of the mesolimbic reward pathway linking the NAc and VTA in humans and its clinical relevance to ASD and other neurodevelopmental disorders has remained unexplored. Here, we leveraged advanced HARDI techniques that overcome shortcomings of previous DTI studies and resolves complex subcortical fibre tracts in ways that were not previously possible. Furthermore, our protocol strikes a balance between the spatial and angular resolutions necessary to achieve high-quality reconstructions of white matter pathways and the acquisition time feasible for clinical and paediatric populations (Vos et al., 2016). Additionally, we used rigorous quality control procedures and a replication framework to address recent concerns about false positives and HARDI data quality (Koldewyn et al., 2014; Yendiki et al., 2014; Maier-Hein et al., 2017). Crucially, using HARDI, we were able to reliably identify white matter tracts connecting the NAc and VTA in each individual child.
Another key finding of our study highlights aberrant functional connectivity of the mesolimbic reward pathway during social information processing in children with ASD. Previous studies have demonstrated reward-related deficits in individuals with ASD, including decreased activation of the NAc during both social and non-social reward tasks (Kohls et al., 2013). Here we used a passive viewing task with no explicit reward and found that functional connectivity between the NAc and VTA was diminished in children with ASD relative to their typically developing peers during passive viewing of social, but not non-social stimuli. Notably, this analysis revealed that, similar to our HARDI structural findings, reduced functional connectivity in response to social stimuli was associated with social interaction impairments in children with ASD. These results demonstrate that mesolimbic reward circuit functioning is impaired in the context of processing socially-relevant information in ASD even in the absence of explicit rewards. Again, these results were specific to NAc-VTA connectivity during face processing and to social interaction abilities, but not with other core ASD symptoms.
In sum, disrupted brain connectivity is currently one of the most prominent neurobiological models of autism (Supekar et al., 2013; Uddin et al., 2013; Hahamy et al., 2015) and our study has addressed previously identified unresolved issues and critical gaps in our knowledge (Vasa et al., 2016), by (i) linking the disrupted connectivity model to a leading hypothesized mechanism of social dysfunction in ASD—the social motivation theory; (ii) investigating/testing a hypothesis across multiple levels—brain structure and function; (iii) clarifying the clinical relevance of animal work in ASD; and (iv) providing findings that are reproducible across independent datasets.
Limitations and future work
First, as in extant empirical brain imaging studies, our sample was limited to children with high-functioning ASD. Further research is needed to investigate whether our study findings are also present in more severely affected individuals. Second, our study primarily included male participants, consistent with a higher male prevalence of the disorder. Considering that previous research has demonstrated sex differences in clinical symptoms of ASD, further studies are needed to determine whether females show similar profiles of deficits in the mesolimbic reward processing system as males. Third, our study reports significant structural and functional deficits in the mesolimbic reward pathway in childhood ASD, using frequentist statistics. Future work using larger sample sizes is required to further examine the replicability of these findings. Finally, we found that social impairments as measured by the ADI-R, but not the ADOS, were related to structural and functional connectivity deficits of the reward pathway in children with ASD. Unlike the ADOS, which is an observational measure of the child’s current functioning, the ADI-R covers a child’s full developmental history. Analysis of differential changes in brain–behaviour relations over time using both current and past symptoms remains an important question for future research, and will require longitudinal data.
Conclusion
Our study provides strong evidence that circumscribed disruptions of the mesolimbic reward pathway, linking the NAc and VTA, underlie social interaction impairments in childhood ASD (Piven et al., 1996; Seltzer et al., 2004). These findings observed in multiple modalities, converge on and extend an emerging body of work in preclinical mouse models of autism showing that disruptions to the mesolimbic reward system diminish initiation and maintenance of social interactions (Boylan et al., 2007; Bariselli et al., 2016). Based on the results presented here, we propose that abnormal structural connectivity contributes significantly to social deficits in ASD via aberrant signalling and context-dependent functional interactions between the NAc and the VTA. Our findings, replicated across two independent cohorts, provide support for impaired reward processing circuitry that is consistent with the social motivation theory of autism. It is noteworthy that despite the complex phenotypic features of the disorder, a core pathway in the human brain not only contributes significantly to social deficits but also is a biological index of heterogeneity of a core clinical symptom of childhood ASD. Our findings suggest that targeted treatments focusing on reward and motivation may alleviate social impairments in affected children.
More broadly, our study has established an important new link between animal models and clinical applications by implementing quantitatively rigorous procedures for investigating white matter pathways in childhood ASD along with careful quality control and behavioural characterization, constrained hypothesis testing, and, importantly, replication in an independent sample. In doing so, we hope to have provided a template for future studies that facilitate a tighter link between basic and translational neuroscience (Ecker and Murphy, 2014).
Supplementary Material
Acknowledgements
We thank Roland Bammer for assistance with the HARDI protocol, and Julia Kang for proofreading the manuscript. The authors also thank the anonymous reviewers for their helpful suggestions.
Glossary
Abbreviations
- ADI-R
Autism Diagnostic Interview-Revised
- ASD
autism spectrum disorder
- HARDI
high angular resolution diffusion weighted imaging
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Funding
This research was supported by a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation to K.S. (22579), a National Institutes of Health Career Development Award to SQ (K99MH105601), postdoctoral fellowships from the Autism Science Foundation and the Child Health Research Institute (Lucile Packard Foundation for Children’s Health; Stanford CTSA UL1 TR001085) to A.P., and grants from the Swiss National Foundation to MS (163859), the Simons Foundation for Autism Research to V.M. (308939), and the National Institutes of Health to V.M. (MH084164).
References
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol Learn Mem 2014; 108: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D, Seunarine K. Mathematics of crossing fibers. In: Jones D, editor. Diffusion MRI. Oxford, England: Oxford University Press; 2012. [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci 2008; 31: 137–45. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Catani M. Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder Cortex 2015; 62: 158–81. [DOI] [PubMed] [Google Scholar]
- Assaf M, Hyatt CJ, Wong CG, Johnson MR, Schultz RT, Hendler T et al. . Mentalizing and motivation neural function during social interactions in Autism spectrum disorders. Neuroimage Clin 2013; 3: 321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011; 54: 2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Dev Disord 1999; 29: 213–24. [DOI] [PubMed] [Google Scholar]
- Bariselli S, Tzanoulinou S, Glangetas C, Prevost-Solie C, Pucci L, Viguie J et al. . SHANK3 controls maturation of social reward circuits in the VTA. Nat Neurosci 2016; 19: 926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015; 86: 646–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini S. Social reward processing in individuals with autism spectrum disorder: a systematic review of the social motivation hypothesis. Res Autism Spectr Disord 2018; 45: 9–26. [Google Scholar]
- Boylan CB, Blue ME, Hohmann CF. Modeling early cortical serotonergic deficits in autism. Behav Brain Res 2007; 176: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Budisavljevic S, Howells H, Thiebaut de Schotten M, Froudist-Walsh S et al. . Frontal networks in adults with autism spectrum disorder. Brain 2016; 139 (Pt 2): 616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Grezes J, Molesworth C, Berthoz S, Happe F. Brief report: selective social anhedonia in high functioning autism. J Autism Dev Disord 2012a; 42: 1504–9. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci 2012b; 16: 231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM et al. . The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain 2000; 123 (Pt 11): 2203–12. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord 1998; 28: 479–85. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 2000; 84: 3072–7. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, Fagan AJ et al. . Social and monetary reward processing in Autism spectrum disorders. Mol Autism 2012; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux M, Deriche R, Knosche TR, Anwander A. Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans Med Imaging 2009; 28: 269–86. [DOI] [PubMed] [Google Scholar]
- Dichter GS. Functional magnetic resonance imaging of Autism spectrum disorders. Dialogues Clin Neurosci 2012; 14: 319–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Murphy D. Neuroimaging in autism–from basic science to translational research. Nat Rev Neurol 2014; 10: 82–91. [DOI] [PubMed] [Google Scholar]
- Emsell L. Diffusion tensor imaging. New York, NY: Springer Science; 2016. [Google Scholar]
- Fillard P, Descoteaux M, Goh A, Gouttard S, Jeurissen B, Malcolm J et al. . Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. Neuroimage 2011; 56: 220–34. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al. . Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–55. [DOI] [PubMed] [Google Scholar]
- Franchini M, Glaser B, Wood de Wilde H, Gentaz E, Eliez S, Schaer M. Social orienting and joint attention in preschoolers with Autism spectrum disorders. PLoS One 2017; 12: e0178859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LR. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med 2002; 47: 1083–99. [DOI] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res Bull 2002; 57: 651–9. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A et al. . Natural neural projection dynamics underlying social behavior. Cell 2014; 157: 1535–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A, Behrmann M, Malach R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat Neurosci 2015; 18: 302–9. [DOI] [PubMed] [Google Scholar]
- Hikida T, Morita M, Macpherson T. Neural mechanisms of the nucleus accumbens circuit in reward and aversive learning. Neurosci Res 2016; 108: 1–5. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 2015; 517: 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 2013; 504: 427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child 1943; 2: 217–50. [PubMed] [Google Scholar]
- Katayama T, Okamoto M, Suzuki Y, Hoshino KY, Jodo E. Phencyclidine affects firing activity of ventral tegmental area neurons that are related to reward and social behaviors in rats. Neuroscience 2013; 240: 336–48. [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers LA. The amygdala and social behavior. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss; 1992. p. 353–77. [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neuroscience 2001; 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I et al. . Reward system dysfunction in Autism spectrum disorders. Soc Cogn Affect Neurosci 2013; 8: 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Yendiki A, Weigelt S, Gweon H, Julian J, Richardson H et al. . Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc Natl Acad Sci USA 2014; 111: 1981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Stoppel DC, Nong Y, Johnson MA, Nadler MJ, Ozkaynak E et al. . Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature 2017; 543: 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotewicz M, Romaniuk A. Social interactions, brain monoamines, and GABA alterations in MFB-lesioned cats. Pharmacol Biochem Behav 1998; 60: 533–8. [DOI] [PubMed] [Google Scholar]
- Lainhart JE. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet C Semin Med Genet 2006; 142C: 33–9. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014; 76 (Pt B): 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res 2013; 255: 44–54. [DOI] [PubMed] [Google Scholar]
- Liebal K, Colombi C, Rogers SJ, Warneken F, Tomasello M. Helping and cooperation in children with autism. J Autism Dev Disord 2008; 38: 224–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC et al. . The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30: 205–23. [PubMed] [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K et al. . The autism diagnostic observation schedule-toddler module: a new module of a standardized diagnostic measure for Autism spectrum disorders. J Autism Dev Disord 2009; 39: 1305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J et al. . The challenge of mapping the human connectome based on diffusion tractography. Nat Commun 2017; 8: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJ, Trezza V. Dopaminergic neurotransmission in the nucleus accumbens modulates social play behavior in rats. Neuropsychopharmacology 2016; 41: 2215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 1991; 44: 15–33. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 2012; 61: 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006; 51: 527–39. [DOI] [PubMed] [Google Scholar]
- Nakano T, Tanaka K, Endo Y, Yamane Y, Yamamoto T, Nakano Y et al. . Atypical gaze patterns in children and adults with Autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proc Biol Sci 2010; 277: 2935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesink RJ, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology 1989; 28: 411–18. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 2011; 519: 3599–639. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev 1984; 8: 465–92. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci 2010; 11: 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Conant D, Hazin R, Stoner R, Desmond J. Preference for geometric patterns early in life as a risk factor for autism. Arch Gen Psychiatry 2011; 68: 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, Malige A. Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biol Psychiatry 2016; 79: 657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. J Am Acad Child Adolesc Psychiatry 1996; 35: 523–9. [DOI] [PubMed] [Google Scholar]
- Riby DM, Hancock PJ. Viewing it differently: social scene perception in Williams syndrome and autism. Neuropsychologia 2008; 46: 2855–60. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013; 14: 609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta L, Fama FI, Bernava GM, Leonardi E, Tartarisco G, Falzone A et al. . Reduced preference for social rewards in a novel tablet based task in young children with Autism spectrum disorders. Sci Rep 2017; 7: 3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res 2010; 3: 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10: 234–47. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K et al. . Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci 2009; 4: 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squillace M, Dodero L, Federici M, Migliarini S, Errico F, Napolitano F et al. . Dysfunctional dopaminergic neurotransmission in asocial BTBR mice. Transl Psychiatry 2014; 4: e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE et al. . Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep 2013; 5: 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A et al. . The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. J Child Psychol Psychiatry 1998; 39: 747–53. [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Methodol 1996; 58: 267–88. [Google Scholar]
- Tournier JD, Calamante F, Connely A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Stockholm, Sweden: International Society for Magnetic Resonance Medicine; 2010. p. 1670. [Google Scholar]
- Tournier JD, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 2012; 22: 53–66. [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci 2011; 31: 6362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci 2013; 7: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Mostofsky SH, Ewen JB. The disrupted connectivity hypothesis of autism spectrum disorders: time for the next phase in research. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R et al. . The Human Connectome Project: a data acquisition perspective. Neuroimage 2012; 62: 2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof LW, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJ. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology 2013; 38: 1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof LW, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LJ. Cellular activation in limbic brain systems during social play behaviour in rats. Brain Struct Funct 2014; 219: 1181–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res 1995; 680: 148–56. [DOI] [PubMed] [Google Scholar]
- Verhagen J, Wagenmakers EJ. Bayesian tests to quantify the result of a replication attempt. J Exp Psychol Gen 2014; 143: 1457–75. [DOI] [PubMed] [Google Scholar]
- Vos SB, Aksoy M, Han Z, Holdsworth SJ, Maclaren J, Viergever MA et al. . Trade-off between angular and spatial resolutions in in vivo fiber tractography. Neuroimage 2016; 129: 117–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, Verhagen J, Ly A. How to quantify the evidence for the absence of a correlation. Behav Res Methods 2016; 48: 413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Gozzi M, Lenroot R, Thurm A, Behseta B, Swedo S et al. . Diffusion tensor imaging in young children with autism: biological effects and potential confounds. Biol Psychiatry 2012; 72: 1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci 1996; 16: 1877–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 2014; 88: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available through the National Database for Autism Research (https://ndar.nih.gov).