SUMMARY
The treatment of colorectal cancer metastatic to the liver is increasingly complex as a result of changes in the patient population, advances in preoperative staging, changing definitions of resectability, advances in surgical technique and the expanding chemotherapeutic armamentarium. Management of these patients within a multidisciplinary team is increasingly important and associated with better outcomes. In patients with irresectable hepatic metastases, high secondary resection rates can be achieved with multiagent chemotherapy when managed in conjunction with a liver specialist. Perioperative mortality rates are reducing but morbidity remains high, and enhanced recovery could help reduce morbidity. Despite the advancing age and comorbidity of the patient population, multimodal management is likely to lead to further improvements in perioperative and long-term outcomes.
Practice Points.
At diagnosis, 25% of patients have hepatic metastases, and up to 50% of patents will develop them during the course of the disease.
Colorectal cancer screening could lead to an increase in patients with resectable metastases.
A globally aging population means that we can expect our patient population to be older with more comorbidities.
Hepatic metastases are resectable if all macroscopic disease can be resected while preserving an adequate future liver remnant.
Extrahepatic disease is not a contraindication as long as it is potentially resectable.
Secondary resection should be a treatment goal in patients with irresectable hepatic metastases.
The role of chemotherapy in patients with resectable disease is unclear.
Advanced techniques are allowing more patients to undergo hepatic resection.
Mortality following hepatectomy is now approaching 1%.
Morbidity is common and enhanced recovery and laparoscopic surgery may offer strategies to address this.
The 5-year survival for patients undergoing surgery for colorectal liver metastases is now approaching 50%.
The 10-year survival for patients undergoing surgery for colorectal liver metastases is now 25%, and for these patients it can be seen as a cure.
The number of treatments for patients with metastatic colorectal cancer has expanded rapidly over the past two decades. In particular, surgery for colorectal cancer metastatic to the liver has evolved from a treatment only considered in a few highly selected patients to a standard treatment considered in all eligible patients. While this progress in treating metastatic colorectal cancer has led to improvements in survival, it has meant that treatment pathways are increasingly complex. This review attempts to summarize the current and future perspectives in the surgical management of colorectal liver metastases within Europe.
Incidence & prevalence
Colorectal cancer is common with 1.2 million new diagnoses annually worldwide and 608,000 attributable deaths [1]. The UK incidence of colorectal cancer diagnoses in 2012 was expected to be 55.6 and 36.7 per 100,000 population in men and women, respectively [2]. The liver is the dominant site of metastatic spread, with 25% of patients having liver metastases at diagnosis and up to 50% developing these during the course of the disease [3]. As the liver is often the first site of blood-borne metastatic spread, resection of these metastases before distant systemic spread occurs can be curative [4].
▪ Changing patterns of disease
When considering a treatment, and how delivery is likely to change in the future, it is important to consider how the target population may change as a result of treatment advances, global population changes and changes in health policy. The expanding chemotherapeutic armamentarium, advancing surgical technique and expanding criteria of what is seen as resectable are likely to lead to an expanding patient population. The other major factor to be considered in predicting our future patient population is the effect of global aging on patient case mix [5].
▪ Aging populations
Our population is becoming increasingly elderly, with the over 70 years age group expected to make up approximately 20% of the UK population by 2020 [6]. Cancer in general, and colorectal cancer in particular, are diseases directly linked to age, with 70% of colorectal cancer diagnoses occurring after the age of 65 years and 50% after the age of 70 years [6]. Thus, we can expect more cases in this increasingly aged population [6]. This has significant implications for practice since age is associated with poorer long-term oncologic and short-term peritherapeutic outcomes [7]. Managing these patients is challenging, and current evidence suggests that we are poor at tailoring treatment to these patients, a fact that may be underpinning the poorer outcomes [8]. A UK Department of Health review has identified the accurate assessment and management of older patients as a key target for health improvement [9].
Criteria for resection
Early hepatectomies for colorectal liver metastases were only carried out in selected physiologically fit patients believed to have the best prognostic features: one to three unilobar metastases; no extrahepatic disease; presenting at least 12 months after resection of the primary tumor and where resection was possible with at least a 1-cm margin. Using these criteria, only 10% of patients with liver-only metastases were considered resectable [10].
Gradually these early resection criteria were challenged with evidence demonstrating that patients beyond these limited criteria could achieve long-term survival [11]. This led to consensus statements from the American Hepato–Pancreato–Biliary Association and a pan-European group [12,13]. These statements recommended resection if all disease could be resected while preserving an adequate future liver remnant. Suggested contraindications to resection were quickly challenged with long-term survival being achieved in patients following nodal resection and resection of metastasis involving the inferior vena cava [14].
The latest UK national guidance from 2011 recommends that treatment of colorectal liver metastases should be offered by way of resection with or without ablation if a patient is fit enough, and that complete resection or ablation can be achieved leaving adequate future liver remnant. Extrahepatic disease is not a contraindication to resection as long as the disease is potentially resectable [15]. There are no absolute contraindications to resection issued in this guidance, but the relative contraindications to liver resection or ablation in normal circumstances are summarized in Box 1 [15].
Box 1. . Relative contraindications to resection or ablation of colorectal liver metastases.
Nontreatable primary tumor
Widespread pulmonary disease
Nonresectable locoregional recurrence
Uncontrollable peritoneal disease
Extensive nodal disease, such as retroperitoneal or mediastinal lymph nodes
Bone or CNS metastases
Based on NICE guidance presented in [15].
This shift to defining resectability based on what will remain, rather than by what is removed, has led to an increase in the number of patients eligible for resection at diagnosis from 10% in 1999 to approximately 25% currently [16].
▪ The adequate future liver remnant
Defining the adequate future liver remnant is one of the challenges of hepatic resection, as misestimation can result in posthepatectomy liver failure and, ultimately, death. Posthepatectomy liver failure is a major cause of morbidity and the leading cause of postoperative mortality [16].
A number of risk factors for the development of posthepatectomy liver failure have been identified. These include preoperative chemotherapy, diabetes, advancing age, obesity, pre-existing liver disease, portal hypertension, preoperative sepsis, smaller liver remnant, prolonged operative time, blood loss in excess of 1000 ml and the incidence of postoperative biliary leaks [16]. Following preoperative chemotherapy, posthepatectomy liver failure is more common, occurring in up to 16% overall, and almost universally following major hepatectomy [16].
Current standards suggest that in a patient with normal hepatic function, a minimum ‘safe’ volume of future liver remnant can be considered to be 20% [17]. Better prediction may be possible with adjustment for patient mass or body surface area [18]. However, this safe volume must still considered in conjunction with other intraoperative factors including the expected extent of hepatic ischemia and intraoperative blood loss. Unfortunately, this safe limit is increasingly difficult to utilize in clinical practice as patients undergo more complex treatment pathways, with multiagent chemotherapy and intrahepatic therapies. These treatments, in particular chemotherapy, can have negative effects on hepatic function.
Perioperative chemotherapy for colorectal liver metastases
In the last 10 years, overall survival (OS) in patients with metastatic colorectal cancer has improved significantly [19]. Given that only a minority of patients with liver-limited metastases are potentially treatable with curative intent surgery, much of this can be attributed to advances in chemotherapy. In patients with initially irresectable liver-limited metastases, the aim of treatment is to bring people to potentially curative surgery. This approach is often referred to as ‘induction’ or ‘conversion’ chemotherapy [20]. In patients with upfront resectable metastases, chemotherapy may be used to reduce the occult disease burden with the hope of preventing early metastases adjuvant (following) or neoadjuvant (preoperative) chemotherapy.
▪ Conversion chemotherapy
It has been established for over 15 years that excellent long-term outcomes are achievable in initially irresectable patients brought to resection by systemic chemotherapy [21]. Consequently, the primary aim of treatment for irresectable liver-limited metastatic colorectal cancer has been conversion to resectability. These patients, brought to secondary resection by systemic therapy, enjoy long-term survival that is comparable to patients with upfront resectable disease at the time of presentation (10-year survival 23 vs 30% [22]), and far superior to those receiving palliative systemic chemotherapy [23].
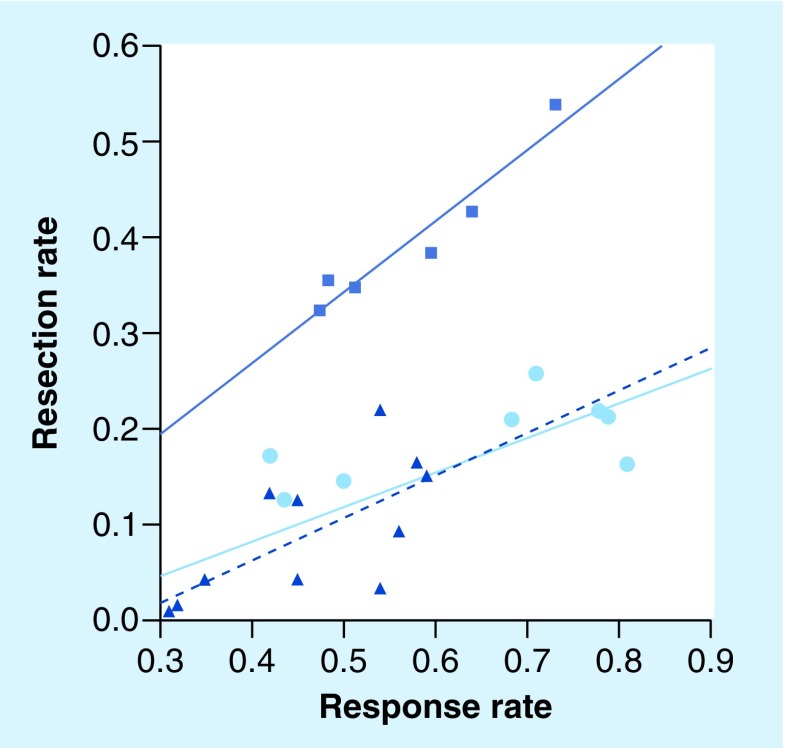
A variety of chemotherapy regimens have been used to bring patients with liver-limited disease to resection, with secondary resection rates of approaching 60% [24]. Folprecht et al. investigated the link between response rate and secondary resection rate, demonstrating a highly significant correlation between response and secondary resection. They suggested that if a tumor response rate of 70% can be achieved, up to 50% of patients may be bought to potentially curative secondary resection (Figure 1) [24].
Figure 1. Rate of liver resection following chemotherapy.
The squares represent patients in studies/retrospective analyses with nonresectable metastases confined to the liver (‘selected patients’, r = 0.96; p = 0.002; solid dark blue line). Studies with nonselected patients with colorectal cancer as shown as circles and triangles. Due to their high heterogeneity of these studies, the observed correlation is less strong (r = 0.74; p < 0.001; solid light blue line). A similar correlation was observed when the Phase III trials (triangles) were separately analyzed (r = 0.67; p = 0.024; dashed dark blue line).
Reproduced from [24] with permission from Oxford University Press.
Achieving response rates approaching 70% is a realistic goal of conversion chemotherapy, although studies demonstrating this degree of response typically require multiple chemotherapeutic agents [25]. Other strategies to achieve this may involve multiple systemic chemotherapy agents in combination with liver-directed chemotherapy, and possibly targeted radiotherapy.
▪ Neoadjuvant & adjuvant chemotherapy
While the number of patients being brought to surgery following conversion chemotherapy continues to increase, there is considerable debate about the role of adjuvant and neoadjuvant chemotherapy for colorectal liver metastases [26]. While it is considered as standard of care in patients with high-risk stage II and all stage III cancers [15], its role in patients with established liver-limited metastatic disease is less clear.
Recurrence is common following hepatic resection of colorectal liver metastases, with over two-thirds of patients developing recurrent metastases within 2 years of surgery [27]. Using chemotherapy either prior to or following resection aims to treat occult metastases, thereby reducing this early recurrence.
The evidence supporting adjuvant chemotherapy following hepatectomy for colorectal liver metastases is limited. Two studies randomized patients to liver resection with or without 5-fluorouracil (5-FU)/leucovorin, but failed to reach their end points of 5-year OS due to poor accrual [28,29]. Subsequent pooled analysis suggested a median survival of 62 months in the chemotherapy arm versus 47 months in the surgery-alone arm [28]. Subsequent studies utilizing FU/leucovorin versus combination 5-FU/irinotecan demonstrated no difference in survival between the arms, and 5-year survival rates were similar to resection alone [30,31]. As a result, the benefit of adjuvant chemotherapy is unclear and its role may be restricted to a poorly defined high-risk cohort.
Preoperative chemotherapy offers several theoretical benefits, which includes monitoring the effect of chemotherapy on measurable disease to aid selection of patients in whom adjuvant chemotherapy may be of benefit. It has also been shown that patients who progress on preoperative chemotherapy have poorer long-term survival [22]. This observation can aid in the assessment of risk and benefit, particularly in patients where the proposed surgical resection is deemed to be of high perioperative risk.
The largest study investigating the role of preoperative chemotherapy is the EORTC 40983 study, which randomized 364 patients with resectable colorectal liver metastases to either surgery alone or surgery with perioperative FOLFOX (5-FU, leucovorin and oxaliplatin) chemotherapy (six preoperative and six postoperative cycles) [32]. In the combined arm, less than 80% received the full six preoperative cycles, and less than 45% received the full preoperative and postoperative chemotherapy treatment, highlighting the challenges of chemotherapy in this population. A benefit in progression-free survival at 3 years was demonstrated in the chemotherapy arm in operated patients over surgery alone (36.2 vs 28.1%), which was the primary end point of the study, leading to neoadjuvant chemotherapy being accepted as standard of care in many countries [26,33]. Recently, however, long-term results of EORTC 4093 have demonstrated no benefit in OS in the chemotherapy arm; however, this is a secondary end point for which the study was not adequately powered to measure [26]. Reasons for this are unclear but it has been suggested that while progression-free survival can be a good indicator of overall cancer survival, OS is influenced by a number of other factors [26].
Currently, there remains considerable debate about the place of chemotherapy in patients with resectable liver metastases, but a recent paper from Jones et al. suggested considering these as two groups [26]. First, in those deemed to be at low risk of recurrence, resection should be undertaken without delay, and consideration given to adjuvant chemotherapy based on pathological analysis of resected specimen. In those patients deemed to be at higher risk for early recurrence, or where operative intervention is likely to be technically challenging, consideration of up to six cycles of neoadjuvant chemotherapy should be considered [26].
Given that the current evidence for adjuvant and neoadjuvant chemotherapy is conflicting, the use of chemotherapy should be at the discretion of the specialist multidisciplinary team (MDT). A MDT can balance the potential benefits against the risks of the various chemotherapeutic agents and regimens. In particular, the increased perioperative risk and the risk of developing chemotherapy-associated liver injury, a complication of chemotherapy that can prevent curative resection.
Liver-targeted therapies
In healthy liver tissue, the predominant blood supply is obtained through the portal venous system in contrast to metastatic disease, which predominantly relies on arterial supply [4]. This dual blood supply has led to the development of hepatic artery delivered therapies including chemotherapy and radiation.
▪ Liver-targeted chemotherapy
The two most common mechanisms for hepatic artery-delivered chemotherapy in colorectal liver metastases are hepatic arterial infusion (HAI) pumps and drug-eluting beads for transarterial chemoembolization (DEB-TACE).
HAI appears to be a biologically rational approach with significant advantages over systemic chemotherapy, capable of delivering high tumor response rates and complete pathological response [4,34]. HAI was initially trialed as a replacement for systemic adjuvant chemotherapy. A catheter is inserted at laparotomy into the umbilical vein remnant, through which a portable pump delivers an infusion of a chemotherapeutic agent. However, a meta-analysis by Mocellin et al. found no evidence to support its use over systemic chemotherapy in the treatment of irresectable colorectal metastases. Interest is now growing in the use of HAI alongside first-line systemic therapy [35].
A Phase III randomized trial considering floxuridine HAI as adjuvant treatment alongside systemic 5-FU after resection of colorectal liver metastases showed a significant improvement in 2-year recurrence-free survival of 90% compared with 60% for those receiving systemic chemotherapy alone [36]. These impressive results stimulated interest into whether the high response rates to HAI could be harnessed to bring more patients to resection, with a systematic review of neoadjuvant hepatic arterial infusion alongside systemic chemotherapy in irresectable colorectal liver metastases reporting secondary resection rates between 6–47% in an unselected series [37]. Although these results are promising, technical and toxic complications remain a concern with 16% of HAI pumps failing within 2 years of insertion [38].
DEB-TACE has been suggested as a possible solution to these delivery difficulties, offering a theoretical advantage over HAI, as it involves the delivery of eluting beads loaded with chemotherapy (irinotecan) via a percutaneous catheter placed under interventional radiology guidance [39]. This eliminates the associated morbidity of a laparotomy for surgical placement and the longer term complications of pump failure [40]. While DC Beads® (Biocompatibles UK Ltd, Surrey, UK) appear to be safe, and have yielded promising response rates in patients with both resectable and irresectable hepatic metastases, currently there is no evidence demonstrating survival advantage over systemic chemotherapy. Therefore, it seems likely that the future role of DEBIRI® (Biocompatibles UK Ltd) will be as an adjunct alongside systemic treatments for liver-dominant disease.
▪ Selective internal radiation therapy
Selective internal radiation therapy is delivered in a similar manner to DEB-TACE, but involves the delivery of yttrium-90 microspheres (SIR-Spheres®, Sirtex Medical Inc., NSW, Australia). This has been shown to lead to successful conversion of unresectable hepatic metastases with promising survival data [41]. A Phase III study is currently underway to evaluate its role in nonresectable patients with colorectal liver metastases [42].
Preoperative staging of colorectal liver metastases
The goal of preoperative staging is to identify all macroscopic disease so that surgical resection of all disease can be performed and, where this is not possible, patients can be prevented from undergoing futile laparotomy. In its earliest days, resection of surgical metastases was dependant on intraoperative findings, however, as methods of radiological assessment have advanced, this preoperative assessment has become increasingly complex.
Triple-phase contrast-enhanced computed tomography (CT) should be performed to assess all patients with colorectal liver metastases and it is now considered standard of care [15]. Additional radiological assessment with modalities, such as MRI and PET, may offer additional benefits.
MRI is a highly effective imaging modality for detecting and characterizing liver lesions, particularly when diffusion-weighted imaging is used [43]. The addition of liver-specific contrast media, such as superparamagnetic iron oxide, gadoxetic acid (Primovist®, Bayer Australia Ltd, NSW, Australia) and mangafodipir trisodium, may aid in the detection of colorectal liver metastasis [44,45]. Limitations of MRI include the longer time to perform, a large number of absolute contraindications and a low sensitivity for detecting extrahepatic disease [45].
PET is often used in combination with CT (PET–CT) as it is highly sensitive for colorectal cancer [46], often identifying occult irresectable extrahepatic disease that would render liver resection futile [47]. Limitations include the rate of false positives as it can be difficult to differentiate between malignant tissue and other metabolically active tissue, such as inflammatory tissue, due to infective or postsurgical causes [47]. Mucinous colorectal metastases may be falsely negative owing to their reduced glucose uptake [48]. Other disadvantages include high cost and limited sensitivity for lesions smaller than 1 cm [17].
Staging laparoscopy was purported to be of benefit in reducing the number of patients undergoing futile laparotomy before the availability of quality radiological assessment had developed. It has largely been abandoned and offers little value when patients are staged routinely with triple liver assessment (CT, MRI and PET–CT) [49]. If managed by this triple-assessment protocol, futile laparotomy occurs in just 4.4% of patients, meaning laparoscopy has a very limited role in highly selected patients where there is preoperative radiological concern of peritoneal disease [49].
▪ Assessment of preoperative fitness
Establishing whether it is feasible to macroscopically resect or ablate all a patient's disease, while preserving an adequate hepatic function and volume, is irrelevant without an appreciation of whether the patient is physiologically capable of surviving a proposed intervention. Consequently, the assessment of fitness should be seen as an integral part of the preoperative assessment process. Specifically targeted research defining fitness for hepatectomy is limited, but methods for defining preoperative fitness include scoring systems, questionnaires and quantitative fitness measures (Box 2).
Box 2. . Methods of assessing patient fitness.
The American Society of Anesthesiologists (ASA) scoring system is widely employed and has been shown to correlate with outcome [50], but ASA is deemed insufficient to adequately risk-stratify patients [9]. Other scoring systems and questionnaires have yet to be validated in patients undergoing hepatectomy, and are often seen as being open to bias and subjective interpretation [51].
Quantifying patient fitness in an independent fashion is an attractive concept, and two common methods that address this are the 6-Minute Walk Test and cardiopulmonary exercise testing [52,53]. The latter of these has been validated in patients undergoing liver resection, with evidence showing that patients with lower fitness are at higher risk of complications [54].
Multidisciplinary management of advanced colorectal cancer
Given the increasing complexity of managing patients with advanced colorectal cancer including hepatic metastases, MDT working has become integral to that management. Management of patients with advanced colorectal cancer outside of MDT working has been shown to be associated with poorer resection rates and survival [55]. MDT management is now standard practice within the UK, where a number of other benefits are now widely accepted (Box 3). Despite this routine MDT assessment, there remains wide variation in the rates of hepatic resection. One of the reasons suggested for this variation was the presence or absence of a liver surgeon within the MDT [30]. Studies examining this have suggested that early MDT involvement of a liver surgeon leads to better survival and outcomes for patients eventually undergoing hepatectomy [56]. A recent paper investigated patients given palliative chemotherapy for hepatic-only metastases without review by a liver surgeon. In these patients, nearly 75% had potentially operable disease, with 40% considered easily resectable [57]. This landmark study confirmed that these patients are best managed by specialist tertiary centers, and contributed to UK guidance mandating specialist hepato-pancreato-biliary surgical review for all patients with liver-limited colorectal metastases. It is hoped that this will translate into higher resection rates, better survival in resected patients and better OS. Many of these patients have other synchronous diseases including locally advanced and recurrent rectal cancer, lung metastases and peritoneal disease. All of these are potentially amenable to curative resection. In addition, involvement of specialists in these fields may yield further improvements in curative resection rates.
Box 3. . Accepted benefits of multidisciplinary team working.
Improved coordination and consistency of care
Improved clinical outcomes
Increased patient satisfaction and psychological wellbeing
Improved communication between health professionals
Educational opportunities for health professionals
Support from collegial environment
Opportunities to improve audit
Increased recruitment into clinical trials
Strategies to increase resection
Given that the main limitation to surgical resection is the preservation of an adequate future remnant liver, a number of strategies have been developed to help address this issue including ablation, portal vein embolization (PVE), two-stage hepatectomy, in situ liver splitting and advanced vascular reconstructive techniques.
▪ Ablation
Ablation has become a widely used method to treat colorectal liver metastases. This treatment destroys tumors to achieve local control in patients that are deemed surgically irresectable either due to insufficient future liver remnant or poor patient fitness. The most frequently used technology is radiofrequency ablation [58], more recently microwave ablation [59] has been increasingly used, and irreversible electroporation represents a new alternative modality [60]. These modalities all have specific uses and may be the method of choice in specific circumstances; irreversible electroporation in particular may be the only potentially curative option for patients with metastases involving major inflow or outflow vasculature [60].
The use of ablation (radiofrequency ablation) as an adjunct to resection has a demonstrable benefit in disease-free survival [61,62], but long-term survival data are lacking, partly owing to the rapidly changing view of what is ‘resectable’. When viewed just as a method to locally control disease, the evidence of survival benefit is greater, with local recurrence rates of just 4–11% [59].
▪ Portal vein embolization
PVE induces atrophy of the liver to be resected and hypertrophy of the liver that will remain (i.e., increases the future liver remnant) with the aim of avoiding posthepatectomy liver failure. A meta-analysis has confirmed that this technique significantly increases the volume of the future liver remnant [63]. PVE appears to be safe, even when combined with conversion chemotherapy [64].
▪ Two-stage hepatectomy
Two-stage hepatectomy is employed in patients when resection of all the metastases would leave insufficient functional liver and where the metastases are distributed across both liver lobes. Two-stage hepatectomy involves a first-stage nonanatomical resection of metastases from the future remnant with PVE (or ligation of the portal vein during surgery) of the future liver to be resected. This is followed by a period of liver regeneration and a second-stage resection some 4 weeks later.
A recent systematic review included 459 patients in whom a two-stage resection was attempted [65]. It was achieved in 76.6% (range: 69–92%) of patients who underwent the first stage. Median OS at 3 and 5 years was 59 and 42%, respectively, demonstrating its usefulness as a technique. The success of this technique relies on patients undergoing hepatic regeneration that is sufficient to allow a second stage, while maintaining their fitness, and not developing progressive disease.
▪ Associating liver partition with portal vein ligation for staged hepatectomy
A recently purported alternative to the staged two-stage liver resection is the associating liver partition with portal vein ligation for staged hepatectomy (also known as the ALPPS procedure), which involves an in situ splitting of the liver with portal vein ligation followed by a formal hepatectomy at a median of 9 days [66]. This demonstrated a median increase in the future remnant liver of 71%. This represents an interesting development in the field of liver surgery that may help to bring more patients to resection, however, the long-term results of this technique have yet to be validated.
▪ Advanced hepatic reconstruction techniques
Despite evolving definitions of resectability, there remain a group of patients in whom technical resectability is only possible with the use of advanced surgical techniques. Typically, cases include metastases involving the hepatic inflow, the hepatic outflow, the inferior vena cava, or all three of these structures. Techniques employed to achieve macroscopic resection in these cases have included portal vein resection and reconstruction, hepatic artery resection and reconstruction (or arterialization of the portal vein as an alternative), total hepatic vascular exclusion, in situ hypothermic perfusion, and ex vivo (bench) hepatic resection [67–70]. These techniques are at the limits of what is currently feasible and are associated with significant morbidity and mortality. Therefore, these techniques should be reserved for carefully selected individuals with an otherwise poor prognosis.
Postoperative morbidity & mortality
In early series, in-hospital mortality was approximately 5%, despite a highly selective approach to patient selection [71]. This has progressively diminished despite more complex resections being undertaken in patients with more extensive disease [72]. In recently reported large series of patients undergoing resection of colorectal liver metastases, mortality is now 1–3%. Unfortunately, despite a near halving in postoperative mortality, in large series, postoperative morbidity remains consistently high at approximately 41.6–45.0% [50,72,73].
Common causes of morbidity following hepatectomy include posthepatectomy liver failure (2.6–6.0%), biliary leak (4.8–8.9%), perihepatic collections (2.9–7.0%), pulmonary complications (6.1–21.9%), wound infections (3.4–5.9%) and cardiac complications (3.0–10%). The wide variation in complications may, in part, be due to the interplay between complications and the subjective interpretation of these data.
Studies have sought to elicit factors associated with poorer postoperative outcome in the hope of better clarifying the population at risk of complications. These can largely be considered as factors associated with an individual's premorbid state, factors associated with the disease and prior treatments and factors relating specifically to the intervention undertaken.
Patient factors identified as being associated with increased postoperative risk include increasing age, impaired hepatic function, cirrhosis, hepatic steatosis, renal failure and medical comorbidity.
The only treatment-related factor consistently identified as being associated with an increased incidence of perioperative morbidity is the use of neoadjuvant chemotherapy [74]. Repeat hepatectomy has been associated with increased bile leaks, but not overall morbidity and mortality. Although associated with a longer operating time and increased blood loss [75], the absence of an association with increased complication may reflect the selective nature of patients considered for repeat hepatectomy. The consequences of the newer liver-directed therapies, including DEBIRI-TACE and selective internal radiation therapy, on postoperative morbidity and mortality are unknown.
The perioperative factors associated with increased postoperative morbidity and mortality have been extensively studied. The overarching theme of these studies suggest, as we would expect, that with increasing surgical complexity and magnitude there is an increase in perioperative morbidity and mortality. The key factors identified as being associated with poorer postoperative outcome are summarized in Box 4.
Box 4. . Intraoperative factors associated with poorer perioperative outcome.
Repeat hepatectomy
Major hepatectomy
Longer operating time
Increased use of pringle maneuver
Blood loss
Transfusion
Additional extrahepatic resection
Vascular resection
Bile duct resection
Hepatico–jejunostomy construction
Diaphragm resection
▪ Improving perioperative outcomes
‘Enhanced recovery programs’ or ‘fast-track programs’ are established in other surgical disciplines as methods of improving perioperative outcomes and reducing costs [76]. The evidence for the use of enhanced recovery programs in liver surgery is more limited with just six small published series identified in a recent systematic review [77]. No large series of enhanced recovery in liver surgery has been published and no series has assessed patients with colorectal liver metastases only. Adoption of these practices following resection of colorectal liver metastases would appear to be beneficial, and recent data suggest it is feasible and associated with lower morbidity and hospital length of stay [78].
Laparoscopic surgery has been used for colorectal liver metastases with the aim of providing curative resection while reducing the trauma associated with open surgery. There are no published randomized trials of laparoscopic liver surgery but the ORANGE II trial investigating the advantage of laparoscopic liver surgery in patients undergoing left lateral sectionectomy within an ERAS® program is underway, and should enhance our understanding of the advantage of this technique [79]. In the absence of randomized trials, there a number of single-center or multicenter cohort studies [80–82]. Median operating time was reported to be 210–278 min for laparoscopic hepatectomy, with a low median blood loss of 200–300 ml and short median postoperative stay of 4 days. These studies conclude that laparoscopic hepatectomy performed by appropriately experienced surgeons is a safe and oncologically comparable approach to open hepatectomy in appropriately selected patients with colorectal liver metastases [80,81,83].
Survival
OS for patients following hepatectomy for colorectal liver metastases is reported to be between 36 and 50% with latter series tending to report better survival [84]. Survival to 10 years is between 23 and 25%, with this seen effectively as cure. A number of scoring systems have been developed to aid in the prediction of survival, but all were derived in an era before the use of modern chemotherapy/biological therapy regimens, and advances in our understanding of disease biology and better treatment mean that these are no longer as relevant in clinical practice. In a similar manner to perioperative outcomes, factors associated with survival can be considered as patient factors, disease factors and factors relating to the operative intervention.
Unlike postoperative outcome, age has not been shown to predict poorer survival, suggesting that if patients are appropriately managed through surgery, cancer-related outcomes are comparable [85]. Some factors suggested to be associated with poorer OS include patients with higher ASA scores, sarcopenia and fatty liver disease [84]. Fatty liver disease and sarcopenia appear to be related, but the mechanism by which they affect survival is unclear. It is increasingly difficult to interpret the role of fatty liver disease given that chemotherapy is often delivered to treat those with biologically worse disease and is a known cause of fatty liver disease [86]. Fatty liver disease also serves as a surrogate marker for other comorbid conditions, such as diabetes, and this increasing comorbidity and lower fitness may be a secondary mechanism. This would also link to the work suggesting that an ASA score of 3 and above is associated with worse long-term survival [84].
After the initial postoperative period, the major cause of death is related to the underlying disease biology. This has been a major focus of research to establish disease-related prognostication. A number of factors have been identified and are summarized in Box 5. These factors often serve as surrogate markers of aggressive tumor biology, but even for these patients survival is achievable, meaning further characterization of tumor biology remains a research priority.
Box 5. . Tumor-related factors for predicting survival.
Primary tumor
Node-positive primary tumor
Poorly differentiated primary tumor
KRAS mutation
Metastatic presentation
Extrahepatic metastatic disease
Increased number of metastases
Mucinous tumor
Invasion of surrounding hepatic structures by a metastases
Large metastases (>5 cm and >10 cm)
C-reactive protein >10
Elevated neutrophil-to-lymphocyte ratio
In the perioperative management of colorectal liver metastases, a number of factors have been identified that can affect long-term survival. While some, such as the preoperative use of chemotherapy, may reflect patients with poorer tumor biology, a number of factors in the perioperative period appear to have direct influence on survival, including the incidence of postoperative complications and the management of these patients outside an MDT environment [56,87]. Other key perioperative determinants of survival include a positive margin in a resected specimen, the degree of response to neoadjuvant chemotherapy, and perioperative bleeding and transfusion.
Conclusion
Outcomes for patients presenting with metastatic colorectal cancer to the liver are improving. This improvement in outcomes is multifactorial and includes an expanding chemotherapeutic armamentarium, better preoperative assessment and advancing surgical techniques. This is leading to increasingly complex treatment pathways involving a variety of specialists. As a result, management of these patients within a MDT is essential, and this has been shown to be associated with better outcomes. In patients with liver-limited hepatic metastases, review by a liver surgeon is associated with higher curative intent surgery and should now be seen as a standard of care.
Newer treatment modalities including DEB-TACE and selective internal radiation therapy are exciting developments that offer promise for improving outcomes, but the consequences for perioperative outcomes and long-term effects on hepatic function are unclear. Defining an adequate hepatic reserve and hepatic function itself are key challenges that, if better defined, could allow better tailoring of chemotherapy and surgical management. The advancing age of our patient population and the associated increase in comorbidities poses a challenge to maintaining this improvement in outcomes, and strategies to approach this should be developed.
Future perspective
Currently, only a minority of patients who develop metastatic disease are eligible for curative intent surgery. Evidence would suggest that even these are being suboptimally managed, with many patients being palliated rather than aggressively managed.
The authors are optimistic that, in the future, these patients will be appropriately treated and that many more patients that are currently deemed incurable will be brought to surgery by combination treatments. These treatment pathways are likely to become increasingly complex, and the management of these patients will necessitate multidisciplinary treatment. The authors believe that this may well lead to the development of MDTs specializing in the management of advanced colorectal cancer. This in turn should allow greater recruitment into clinical trials and ultimately an expansion of the treatments available. We are optimistic that, in the future, metastatic colorectal cancer will be increasingly manageable with treatment prolonging survival over many years.
Footnotes
Financial & competing interests disclosure
G Poston is on advisory boards for Merck Serono, Sanofi Aventis, BTG Biocompatibles and Bayer; and on speaker panels for Merck Serono, Sanofi Aventis and BTG Biocompatibles. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J. Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Bouchahda M, Lévi F, Adam R, Rougier P. Modern insights into hepatic arterial infusion for liver metastases from colorectal cancer. Eur. J. Cancer. 2011;47(18):2681–2690. doi: 10.1016/j.ejca.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C English Bowel Cancer Screening Evaluation Committee. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 2012;61(10):1439–1446. doi: 10.1136/gutjnl-2011-300843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anaya DA, Becker NS, Abraham NS. Global graying, colorectal cancer and liver metastasis: new implications for surgical management. Crit. Rev. Oncol. Hematol. 2011;77(2):100–108. doi: 10.1016/j.critrevonc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Quaglia A, Tavilla A, Shack L, et al. EUROCARE Working Group. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur. J. Cancer. 2009;45(6):1006–1016. doi: 10.1016/j.ejca.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann. Surg. 2007;246(2):215–221. doi: 10.1097/SLA.0b013e318070838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Department of Health. The Impact of Age on Clinical Decision Making in Oncology. 2012. [Google Scholar]
- 10.Grünhagen D, Jones RP, Treasure T, Vasilakis C, Poston GJ. The history of adoption of hepatic resection for metastatic colorectal cancer: 1984–1995. Crit. Rev. Oncol. Hematol. 2013;86(3):222–231. doi: 10.1016/j.critrevonc.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann. Surg. 2000;231(4):487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann. Surg. Oncol. 2006;13(10):1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 13.van Cutsem E, Nordlinger B, Adam R, et al. European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer. 2006;42(14):2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Malde DJ, Khan A, Prasad KR, Toogood GJ, Lodge JPA. Inferior vena cava resection with hepatectomy: challenging but justified. HPB (Oxford) 2011;13(11):802–810. doi: 10.1111/j.1477-2574.2011.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poston GJ, Tait D, O’Connell S, Bennett A, Berendse S Guideline Development Group. Diagnosis and management of colorectal cancer: summary of NICE guidance. BMJ. 2011;343:d6751. doi: 10.1136/bmj.d6751. [DOI] [PubMed] [Google Scholar]; ▪ Recent guidelines summarizing best practice within the UK.
- 16.Shindoh J, Tzeng CW, Aloia TA, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann. Surg. Oncol. 2013;20(8):2493–2500. doi: 10.1245/s10434-012-2864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garden OJ, Parks RW, editors. Hepatobiliary and Pancreatic Surgery: Companion to Specialist Surgical Practice Companion to Specialist Surgical Practice (5th Edition) Saunders Ltd; Philadelphia, PA, USA: 2013. [Google Scholar]
- 18.Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig. Surg. 2012;29(1):6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 19.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009;27(22):3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poston G, Adam R, Vauthey JN. Downstaging or downsizing: time for a new staging system in advanced colorectal cancer? J. Clin. Oncol. 2006;24(18):2702–2706. doi: 10.1200/JCO.2006.05.8404. [DOI] [PubMed] [Google Scholar]
- 21.Bismuth H, Adam R, Lévi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann. Surg. 1996;224(4):509–520. doi: 10.1097/00000658-199610000-00009. discussion 520–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann. Surg. 2004;240(4):644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam VW, Spiro C, Laurence JM, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann. Surg. Oncol. 2012;19(4):1292–1301. doi: 10.1245/s10434-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 24.Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann. Oncol. 2005;16(8):1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 25.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised Phase 2 trial. Lancet Oncol. 2010;11(1):38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 26.Jones RP, Malik HZ, Fenwick SW, Poston GJ. Perioperative chemotherapy for resectable colorectal liver metastases: where now? Eur. J. Surg. Oncol. 2013;39(8):807–811. doi: 10.1016/j.ejso.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Jones RP, Dunne D, Sutton P, et al. Segmental and lobar administration of drug-eluting beads delivering irinotecan leads to tumour destruction: a case–control series. HPB (Oxford) 2013;15(1):71–77. doi: 10.1111/j.1477-2574.2012.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J. Clin. Oncol. 2008;26(30):4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 29.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J. Clin. Oncol. 2006;24(31):4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 30.Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br. J. Surg. 2010;97(7):1110–1118. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- 31.Ychou M, Hohenberger W, Thezenas S, et al. A randomized Phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann. Oncol. 2009;20(12):1964–1970. doi: 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 32.Nordlinger B, Sorbye H, Glimelius B, et al. EORTC Gastro-Intestinal Tract Cancer Group, Cancer Research UK, Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO), Australasian Gastro-Intestinal Trials Group (AGITG), Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Key publication reporting on neoadjuvant chemotherapy in colorectal liver metastases.
- 33.Nordlinger B, Van Cutsem E, Gruenberger T, et al. European Colorectal Metastases Treatment Group, Sixth International Colorectal Liver Metastases Workshop. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann. Oncol. 2009;20(6):985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 34.Auer RC, White RR, Kemeny NE, et al. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer. 2010;116(6):1502–1509. doi: 10.1002/cncr.24912. [DOI] [PubMed] [Google Scholar]
- 35.Mocellin S, Pilati P, Lise M, Nitti D. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J. Clin. Oncol. 2007;25(35):5649–5654. doi: 10.1200/JCO.2007.12.1764. [DOI] [PubMed] [Google Scholar]
- 36.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N. Engl. J. Med. 1999;341(27):2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 37.Pwint TP, Midgley R, Kerr DJ. Regional hepatic chemotherapies in the treatment of colorectal cancer metastases to the liver. Semin. Oncol. 2010;37(2):149–159. doi: 10.1053/j.seminoncol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J. Am. Coll. Surg. 2005;201(1):57–65. doi: 10.1016/j.jamcollsurg.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Martin RC, Joshi J, Robbins K, Tomalty D, O’Hara R, Tatum C. Transarterial chemoembolization of metastatic colorectal carcinoma with Drug-Eluting Beads, Irinotecan (DEBIRI): multi-institutional registry. J. Oncol. 2009;2009:539795. doi: 10.1155/2009/539795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin RC, Howard J, Tomalty D, et al. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc. Intervent. Radiol. 2010;33(5):960–966. doi: 10.1007/s00270-010-9937-4. [DOI] [PubMed] [Google Scholar]
- 41.Van Hazel G, Blackwell A, Anderson J, et al. Randomised Phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J. Surg. Oncol. 2004;88(2):78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 42.Sharma RA, Wasan HS, Love SB, Dutton S, Stokes JC, Smith JL FOXFIRE Trial Management Group. FOXFIRE: a Phase III clinical trial of chemo-radio-embolisation as first-line treatment of liver metastases in patients with colorectal cancer. Clin. Oncol. (R. Coll. Radiol.) 2008;20(3):261–263. doi: 10.1016/j.clon.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Eiber M, Fingerle AA, Brügel M, Gaa J, Rummeny EJ, Holzapfel K. Detection and classification of focal liver lesions in patients with colorectal cancer: retrospective comparison of diffusion-weighted MR imaging and multi-slice CT. Eur. J. Radiol. 2012;81(4):683–691. doi: 10.1016/j.ejrad.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 44.Huppertz A, Balzer T, Blakeborough A, et al. European EOB Study Group. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004;230(1):266–275. doi: 10.1148/radiol.2301020269. [DOI] [PubMed] [Google Scholar]
- 45.Xu LH, Cai SJ, Cai GX, Peng WJ. Imaging diagnosis of colorectal liver metastases. World J. Gastroenterol. 2011;17(42):4654–4659. doi: 10.3748/wjg.v17.i42.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Israel O, Mor M, Gaitini D, et al. Combined functional and structural evaluation of cancer patients with a hybrid camera-based PET/CT system using (18)F-FDG. J. Nucl. Med. 2002;43(9):1129–1136. [PubMed] [Google Scholar]
- 47.Kochhar R, Liong S, Manoharan P. The role of FDG PET/CT in patients with colorectal cancer metastases. Cancer Biomark. 2010;7(4):235–248. doi: 10.3233/CBM-2010-0201. [DOI] [PubMed] [Google Scholar]
- 48.Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am. J. Roentgenol. 2000;174(4):1005–1008. doi: 10.2214/ajr.174.4.1741005. [DOI] [PubMed] [Google Scholar]
- 49.Dunne DF, Gaughran J, Jones RP, et al. Routine staging laparoscopy has no place in the management of colorectal liver metastases. Eur. J. Surg. Oncol. 2013;39(7):721–725. doi: 10.1016/j.ejso.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Reddy SK, Barbas AS, Turley RS, et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford) 2011;13(7):494–502. doi: 10.1111/j.1477-2574.2011.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moonesinghe SR, Mythen MG, Grocott MP. Patient-related risk factors for postoperative adverse events. Curr. Opin. Crit. Care. 2009;15(4):320–327. doi: 10.1097/MCC.0b013e32832e067c. [DOI] [PubMed] [Google Scholar]
- 52.Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16(12):1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 53.West M, Jack S, Grocott MP. Perioperative cardiopulmonary exercise testing in the elderly. Best Pract. Res. Clin. Anaesthesiol. 2011;25(3):427–437. doi: 10.1016/j.bpa.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Junejo MA, Mason JM, Sheen AJ, et al. Cardiopulmonary exercise testing for preoperative risk assessment before hepatic resection. Br. J. Surg. 2012;99(8):1097–1104. doi: 10.1002/bjs.8773. [DOI] [PubMed] [Google Scholar]
- 55.Segelman J, Singnomklao T, Hellborg H, Martling A. Differences in multidisciplinary team assessment and treatment between patients with stage IV colon and rectal cancer. Colorectal. Dis. 2009;11(7):768–774. doi: 10.1111/j.1463-1318.2008.01648.x. [DOI] [PubMed] [Google Scholar]
- 56.Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10-year study of outcome following hepatic resection for colorectal liver metastases – the effect of evaluation in a multidisciplinary team setting. Eur. J. Surg. Oncol. 2009;35(3):302–306. doi: 10.1016/j.ejso.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 57.Jones RP, Vauthey JN, Adam R, et al. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br. J. Surg. 2012;99(9):1263–1269. doi: 10.1002/bjs.8835. [DOI] [PubMed] [Google Scholar]; ▪ Highlights the importance of specialist input from the outset.
- 58.Pathak S, Jones R, Tang JM, et al. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal. Dis. 2011;13(9):e252–e265. doi: 10.1111/j.1463-1318.2011.02695.x. [DOI] [PubMed] [Google Scholar]
- 59.Stättner S, Jones RP, Yip VS, et al. Microwave ablation with or without resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2013;39(8):844–849. doi: 10.1016/j.ejso.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Kingham TP, Karkar AM, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J. Am. Coll. Surg. 2012;215(3):379–387. doi: 10.1016/j.jamcollsurg.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 61.Ruers T, Punt C, van Coevorden F, et al. EORTC Gastro-Intestinal Tract Cancer Group, Arbeitsgruppe Lebermetastasen und – tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO) and the National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG) Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup Phase II study (EORTC 40004) Ann. Oncol. 2012;23(10):2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evrard S, Rivoire M, Arnaud JP, et al. Unresectable colorectal cancer liver metastases treated by intraoperative radiofrequency ablation with or without resection. Br. J. Surg. 2012;99(4):558–565. doi: 10.1002/bjs.8724. [DOI] [PubMed] [Google Scholar]
- 63.Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann. Surg. 2008;247(1):49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 64.Covey AM, Brown KT, Jarnagin WR, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann. Surg. 2008;247(3):451–455. doi: 10.1097/SLA.0b013e31815ed693. [DOI] [PubMed] [Google Scholar]
- 65.Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 2013;15(7):483–491. doi: 10.1111/j.1477-2574.2012.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012;255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 67.Azoulay D, Andreani P, Maggi U, et al. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann. Surg. 2006;244(1):80–88. doi: 10.1097/01.sla.0000218092.83675.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hemming AW, Reed AI, Langham MR, Fujita S, Howard RJ. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann. Surg. 2004;239(5):712–719. doi: 10.1097/01.sla.0000124387.87757.eb. discussion 719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kondo S, Hirano S, Ambo Y, Tanaka E, Kubota T, Katoh H. Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br. J. Surg. 2004;91(2):248–251. doi: 10.1002/bjs.4428. [DOI] [PubMed] [Google Scholar]
- 70.Lodge JP, Ammori BJ, Prasad KR, Bellamy MC. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann. Surg. 2000;231(4):471–479. doi: 10.1097/00000658-200004000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nordlinger B, Quilichini MA, Parc R, Hannoun L, Delva E, Huguet C. Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann. Surg. 1987;205(3):256–263. doi: 10.1097/00000658-198703000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmitti G, Roses RE, Andreou A, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J. Gastrointest. Surg. 2013;17(1):57–64. doi: 10.1007/s11605-012-2000-9. discussion 64–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann. Surg. 2002;236(4):397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Narita M, Oussoultzoglou E, Fuchshuber P, et al. What is a safe future liver remnant size in patients undergoing major hepatectomy for colorectal liver metastases and treated by intensive preoperative chemotherapy? Ann. Surg. Oncol. 2012;19(8):2526–2538. doi: 10.1245/s10434-012-2274-x. [DOI] [PubMed] [Google Scholar]
- 75.Antoniou A, Lovegrove RE, Tilney HS, et al. Meta-analysis of clinical outcome after first and second liver resection for colorectal metastases. Surgery. 2007;141(1):9–18. doi: 10.1016/j.surg.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 76.Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149(6):830–840. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Hall TC, Dennison AR, Bilku DK, Metcalfe MS, Garcea G. Enhanced recovery programmes in hepatobiliary and pancreatic surgery: a systematic review. Ann. R. Coll. Surg. Engl. 2012;94(5):318–326. doi: 10.1308/003588412X13171221592410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schultz NA, Larsen PN, Klarskov B, et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br. J. Surg. 2013;100(1):138–143. doi: 10.1002/bjs.8996. [DOI] [PubMed] [Google Scholar]; ▪ Postoperative outcomes following hepatectomy with enhanced recovery principles.
- 79.van Dam RM, Wong-Lun-Hing EM, van Breukelen GJ, et al. ORANGE II Study Group. Open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery ERAS® programme (ORANGE II-trial): study protocol for a randomized controlled trial. Trials. 2012;13(1):54. doi: 10.1186/1745-6215-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen KT, Laurent A, Dagher I, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann. Surg. 2009;250(5):842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 81.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann. Surg. 2009;250(5):849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]; ▪ Multicenter study reporting on oncological outcomes for laparoscopic hepatectomy.
- 82.Abu Hilal M, Di Fabio F, Abu Salameh M, Pearce NW. Oncological efficiency analysis of laparoscopic liver resection for primary and metastatic cancer: a single-center UK experience. Arch. Surg. 2012;147(1):42–48. doi: 10.1001/archsurg.2011.856. [DOI] [PubMed] [Google Scholar]
- 83.Buell JF, Cherqui D, Geller DA, et al. World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann. Surg. 2009;250(5):825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 84.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann. Surg. 2008;247(1):125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 85.Adam R, Frilling A, Elias D, et al. Liver Met Survey Centres. Liver resection of colorectal metastases in elderly patients. Br. J. Surg. 2010;97(3):366–376. doi: 10.1002/bjs.6889. [DOI] [PubMed] [Google Scholar]
- 86.Pathak S, Tang JM, Terlizzo M, Poston GJ, Malik HZ. Hepatic steatosis, body mass index and long term outcome in patients undergoing hepatectomy for colorectal liver metastases. Eur. J. Surg. Oncol. 2010;36(1):52–57. doi: 10.1016/j.ejso.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Mavros MN, de Jong M, Dogeas E, Hyder O, Pawlik TM. Impact of complications on long-term survival after resection of colorectal liver metastases. Br. J. Surg. 2013;100(5):711–718. doi: 10.1002/bjs.9060. [DOI] [PubMed] [Google Scholar]
- 88.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2(3):281. [Google Scholar]
- 89.Epstein SK, Faling LJ, Daly BD, Celli BR. Predicting complications after pulmonary resection. Preoperative exercise testing vs a multifactorial cardiopulmonary risk index. Chest. 1993;104(3):694–700. doi: 10.1378/chest.104.3.694. [DOI] [PubMed] [Google Scholar]