Abstract
The development of spontaneous autoimmune diabetes in nonobese diabetic (NOD) mice provides for their use as a model of human type 1 diabetes. To test the feasibility of muscle-directed gene therapy to prevent type 1 diabetes, we developed recombinant adeno-associated virus (rAAV) vectors containing murine cDNAs for immunomodulatory cytokines IL-4 or IL-10. Skeletal muscle transduction of female NOD mice with IL-10, but not IL-4, completely abrogated diabetes. rAAV-IL-10 transduction attenuated the production of insulin autoantibodies, quantitatively reduced pancreatic insulitis, maintained islet insulin content, and altered splenocyte cytokine responses to mitogenic stimulation. The beneficial effects were host specific, as adoptive transfer of splenocytes from rAAV IL-10-treated animals rapidly imparted diabetes in naive hosts, and the cells contained no protective immunomodulatory capacity, as defined through adoptive cotransfer analyses. These results indicate the utility for rAAV, a vector with advantages for therapeutic gene delivery, to transfer immunoregulatory cytokines capable of preventing type 1 diabetes. In addition, these studies provide foundational support for the concept of using immunoregulatory agents delivered by rAAV to modulate a variety of disorders associated with deleterious immune responses, including allergic reactions, transplantation rejection, immunodeficiencies, and autoimmune disorders.
The etiology of type 1 diabetes in NOD mice is both complex and multifactorial (1, 2). Both CD4+ and CD8+ T cells comprise the effector arm, with underlying functional defects in bone marrow-derived antigen-presenting cells (macrophages, dendritic cells, B lymphocytes) shown to be essential components in the selection and activation of the autoimmune repertoire (3, 4). The destruction of β cells apparently entails both necrotic and apoptotic events in response to invasion of the islets by leukocytes (5, 6). Autoreactive T cells are targeted against multiple autoantigens, including insulin and glutamic acid decarboxylase (7, 8).
Previous studies indicate that the pathogenic facet of the β cell destructive immune response in nonobese diabetic (NOD) mice is biased toward T helper 1-like immunities (4, 7). Depending on the time and mode of administration (early versus late, systemic versus local), treatment with the immunoregulatory cytokines IL-4 or IL-10 can inhibit the development of type 1 diabetes in NOD mice as well as prevent the recurrence of disease, either alloimmune and/or autoimmune, in mice receiving islet transplants (9–13). However, given their relatively short half-lives, the practicality of using these cytokines for initiation of immune deviation would be currently limited because of the need for repeated administration. We and others have demonstrated that sustained and stable production of secreted proteins can be achieved in vivo through recombinant adeno-associated virus (rAAV)-mediated gene delivery into skeletal muscle (14, 15). rAAV vectors have become increasingly recognized as having some superiority to other viral and nonviral gene delivery systems with regard to their safety, efficiency, lack of need for repeated viral administration, duration of action without known pathology, and only the occasional induction of modest immune responses (16, 17). A cellular modification toward the in vivo production of cytokines, achievable by rAAV gene transfer, could be exploited for developing novel intervention protocols for type 1 diabetes and other immune system-based disorders (18).
Materials and Methods
Plasmid Construction, Viral Packaging, and Production, Cellular Transfection, and Transduction.
The rAAV (serotype 2) vector plasmids are depicted diagrammatically (Fig. 1). Murine cDNAs for the cytokines IL-4 and IL-10 were cloned into the p43.2 plasmid. rAAV2 production, titer determination, and infectivity were performed as described (19). Transfection (5 μg DNA, Superfect; Qiagen, Chatsworth, CA) and transduction of myoblast C2C12 cells were performed as described (15). For studies using adenovirus as a helper virus, myoblasts were treated with adenovirus type 5 at a multiplicity of infection of 5 for 2 h (37°C, 5% CO2) before coinfection with rAAV.
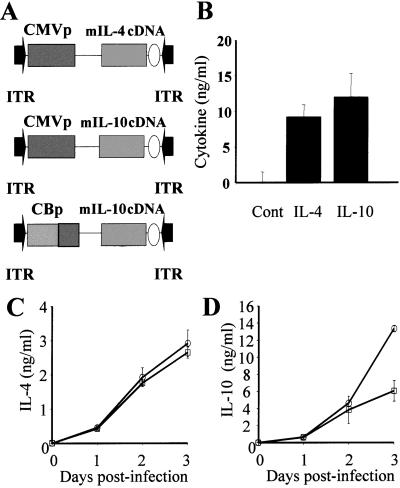
Figure 1.
rAAV-CMV-IL-4, rAAV-CMV-IL-10, and rAAV-CB-IL-10 constructs and expression in mouse myoblasts. (A) Vector cassette map where ITR, rAAV inverted terminal repeat, CMVp, CMV immediate early promoter, and CBp, CMV enhancer and chicken-β actin promoter with a hybrid chicken–rabbit β-globin intron. The circle after the gene is the simian virus 40 poly(A) signal. (B) The concentrations of IL-4 and -10 48 h after plasmid (pCMV–green fluorescent protein, pCMV-IL-4, pCMV-IL-10) transfection of C2C12 cells (performed in triplicate). (C and D) The concentrations of (C) IL-4 and (D) IL-10 0–3 days after viral (rAAV-CMV-IL-4, rAAV-CB-IL-10) transduction of C2C12 cells (performed in triplicate). Transductions with rAAV alone (□, multiplicity of infection 2,000) or under coinfection with rAAV (○, multiplicity of infection 2,000) and Ad5 (multiplicity of infection 5).
Mice.
Specific pathogen-free NOD.MarTac mice (Taconic Farms) were housed in a BSL-2 barrier facility. Blood glucose levels were determined weekly/biweekly, with animals considered type 1 diabetic when levels exceeded 240 mg/dl on two consecutive occasions, greater than 24 h apart.
rAAV Vector Administration.
Four-week-old female NOD mice were injected intramuscularly into the caudal muscle of the pelvic limb. These injections used 100 μl of saline, saline containing 1 × 1010 units of either rAAV-IL4 or rAAV-IL-10, or saline containing the latter two in combination (1 × 1010 units of rAAV-IL-4 and 1 × 1010 units of rAAV-IL-10) per mouse.
Cytokine, Serum IgE, and Insulin Autoantibody Analysis.
Supernatant cytokine levels for IL-2, -4, -10, and IFN-γ as well as serum IgE were measured with the use of OPTEIA kits (PharMingen) (20), with serum IL-10 assessed by microbead cytokine assay (Upstate Biotechnology, Lake Placid, NY). Insulin autoantibodies (IAAs) were measured by RIA with radiolabeled insulin (Amersham Pharmacia) and protein A Sepharose (Sigma) (20). An index was calculated as [(unknown cpm − negative control cpm)/(positive control cpm − negative control cpm)] × 100. The cutoff of 12.2 was chosen on the basis of the mean index + 3 SD of 30 C57/BL6 mice.
Histological Analysis.
Skeletal muscle samples were paraformaldehyde (4%) fixed, paraffin embedded, and hematoxylin/eosin stained. Insulitis was evaluated on hematoxylin/eosin-stained frozen sections of pancreas and scored on a blind basis with a standardized scoring system described by others (21). Pancreata were also stained for insulin with the use of antiporcine insulin (Dako) and intercellular adhesion molecule-1 (PharMingen) on frozen and paraffin sections, respectively.
Reverse Transcription–PCR for Detecting Transgene Expression.
Total RNA from the injection site or cells transduced with rAAV vector was purified and treated with RNase-free DNase (RNAqueous-4PCR; Ambion, Austin, TX). First-strand cDNA synthesis was performed with Maloney murine leukemia virus reverse transcriptase and random cecamer primers (RETROscript; Ambion). The cDNA was amplified by nested PCR. For detection of transcript from rAAV-IL-4, the first PCR reaction was performed with primers, P1, 5′-CAGTCTCGAACTTAAGCTGC-3′, and P2, 5′-GGACTTGGACTCATTCATGG-3′, for 35 cycles. Two percent of the reaction was used for the second PCR with primers, P3, 5′-CAGAAGTTGGTCGTGAGGCA-3′, and P4, 5′-GCAGCTCCATGAGAACACTA-3′, for 35 cycles. The final PCR product was cloned into a TA-cloning vector and sequenced to confirm that the transcript was from rAAV-cytomegalovirus (CMV)-IL-4.
Splenocyte Studies.
Splenocytes were cultured at 5 × 105 cells per well in 200 μl of RPMI 1640 medium (10% FBS) in 96-well round-bottom plates. Supernatants were collected at 24 and 48 h for cytokine analysis in response to Con A. For studies of in vivo activity, 8-week-old male NOD mice were irradiated (700 rads) and injected via the tail vein with splenic lymphocytes (2 × 107) obtained from 20-week-old newly diagnosed diabetic NOD mice or 32-week-old rAAV-IL-10-treated NOD mice under conditions of either adoptive transfer or at a 1:1 combination (adoptive cotransfer) (22).
Statistical Analysis.
Data are presented as the mean ± SEM. Student's t tests and ANOVA testing were used for analyses comparing the different groups, with statistical significance considered if P < 0.05.
Results
Effect of rAAV-Delivered Immunomodulatory Cytokines on Type 1 Diabetes.
To validate function, mouse myoblasts were either transfected with plasmids or transduced with packaged rAAV virions expressing IL-4 and -10 (Fig. 1A). Specifically, C2C12 myoblast cells were transfected with CMV-IL-4 or CMV-IL-10 plasmids or virally transduced with rAAV-CMV-IL-4 or rAAV-CB-IL-10. The transduction studies were performed in the presence and absence of adenovirus, a helper virus that aids in the conversion of rAAV from single-stranded to double-stranded DNA (16, 17). At 48 h, plasmid-transfected cells readily expressed either IL-4 or -10 (Fig. 1B), whereas control cells transfected with control green fluorescent protein failed to produce these cytokines. Similarly, within 24 h, production of IL-4 and -10 was observed in supernatants from rAAV-CMV-IL-4- and rAAV-CB-IL-10-transduced cells (Fig. 1 C and D) and did not depend on coinfection with adenovirus.
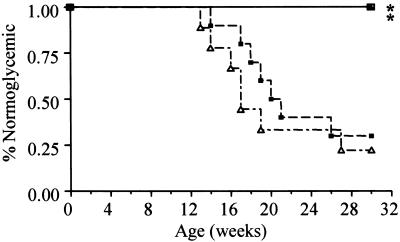
To observe the effects of skeletal muscle production of these cytokines on the development of type 1 diabetes in vivo, we intramuscularly injected female NOD mice at 4 weeks of age with purified vector preparations of rAAV-IL-4 and/or rAAV-IL-10. rAAV-IL-10 transduction completely abrogated the development of diabetes (0/10; 0% incidence at 30 weeks) (Fig. 2). Additionally, mice receiving the combination therapy of both rAAV-IL-10 and rAAV-IL-4 were also protected from the disease (0/10; 0%). This protection was associated with rAAV-IL-10, only as rAAV-IL-4-treated animals did not display a significant delay in the kinetics of disease development (Fig. 2) nor did they demonstrate long-term differences in disease frequency (8/10; 80%) when compared with control animals (7/10; 70%).
Figure 2.
Type 1 diabetes in NOD mice undergoing various treatment modalities. These life-table analyses demonstrate the percentage of mice (n =10 for each group) remaining normoglycemic after injection with saline (– ⋅ – ▵), rAAV-IL-4 (– – – ■), rAAV-IL-10 (—— ▴), or the combination of rAAV-IL-4 and rAAV-IL-10 (—— □). *, P < 0.005 versus the control group and the rAAV-IL-4-treated group.
Confirmation of Functional rAAV Transduction of Skeletal Muscle.
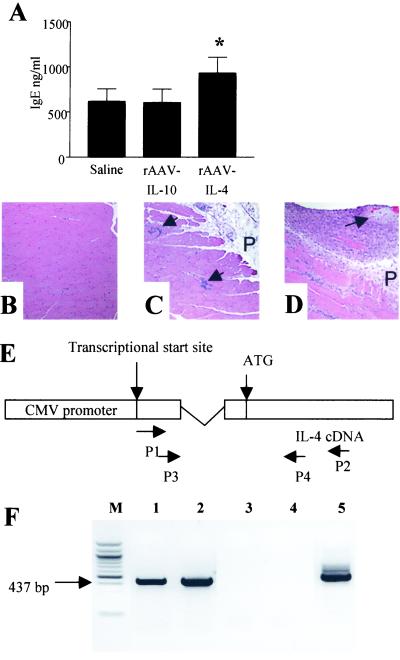
To assess transgene function, serum cytokine levels were determined in a separate series of similarly treated animals injected with rAAV vectors and killed at 10–12 weeks of age; studies identified elevated IL-10 levels in rAAV-IL-10-transduced animals (67.5 ± 14.9 pg/ml; n = 4) that were not detectable in saline controls (below assay detection limits of 15.6 pg/ml; n = 4). In contrast, serum IL-4 levels were not elevated/undetectable in rAAV-IL-4-transduced animals in comparison with controls. However, as an indirect indicator of biological activity in transduced animals, total serum IgE was elevated in rAAV-IL-4-treated animals (Fig. 3A), consistent with the known actions of IL-4 on IgE production and the difficulty of measuring serum cytokines (23–25). The site of injection was examined to observe the local effects of transgene expression. Whereas normal muscle histology was observed in saline-injected animals (Fig. 3B), the introduction of rAAV-IL-10 into muscle induced a mild degree of lymphocytic accumulation (Fig. 3C), and rAAV-IL-4 induced a mild to severe degree of lymphocytic accumulation (Fig. 3D), observations consistent with the action of these cytokines on immunological recruitment and proliferation (26). Furthermore, this lymphocytic accumulation appeared transgene-specific, as injections of NOD mice with rAAV-α-1-antitrypsin failed to induce abnormal muscle pathology and was similar to that of saline controls (data not shown), a finding consistent with the “nonimmunogenic” property often ascribed to rAAV. Additional evidence of skeletal muscle transduction was obtained by reverse transcription–PCR analysis of muscle, taken from injection sites of 16- to 30-week-old animals, with the use of cytokine-specific primers and subsequent sequencing of products. These studies confirmed the specific presence of IL-4 in rAAV-IL-4-injected (Fig. 3 E and F) and IL-10 in rAAV IL-10-treated (data not shown) animals as well as the lack of these two cytokine genes in saline-treated animals. Furthermore, transgene retention was suggested by the presence of reverse transcription–PCR products in animals at 30 weeks of age. Longitudinal analysis of control, rAAV-IL-4-transduced, and rAAV-IL-10-transduced animals in the period before the onset of diabetes would be expected (4–12 weeks) revealed no differences in blood glucose values, suggesting that the systemic introduction of rAAV-expressed transgenes also did not interfere with β cell function (data not shown).
Figure 3.
Analyses of the efficacy and function of viral transduction in vivo. (A) Total serum IgE from 10- to 14-week-old saline-, rAAV-IL-4-, and rAAV-IL-10-treated animals (n = 8 per group). *, P < 0.008 for rAAV-IL-4-treated animals versus control or rAAV-IL-10-treated animals. (B–D) Representative longitudinal cross sections of muscle (hematoxylin/eosin stained) from mice injected with (B) saline-injected, normal histology; (C) rAAV-IL-10-treated, focal myositis (arrows); or (D) rAAV-IL-4-treated, moderate myositis and perimyositis in surrounding fascia and perimysium (P; arrow denotes peripheral nerve). (E) Diagram of nested PCR strategy. Specifically, the first PCR was performed with primers P1 (downstream of the transcription start site) and P2 (within the IL-4 coding region). The second PCR was performed with primers P3 (between P1 and the site of the splicing donor) and P4 (between ATG and P2). (F) Reverse transcription–PCR products with total RNA from the muscle injection site. Lane m, 100-bp marker; lanes 1 and 2, mice injected with of rAAV-CMV-IL-4 (n = 2); lanes 3 and 4, saline-injected mice (n = 2); lane 5, C2C12 cells transduced with rAAV-CMV-IL-4. The transgene-specific product (437 bp) was confirmed by TA cloning and sequencing.
Mechanisms by Which rAAV-IL-10 Confers Protection.
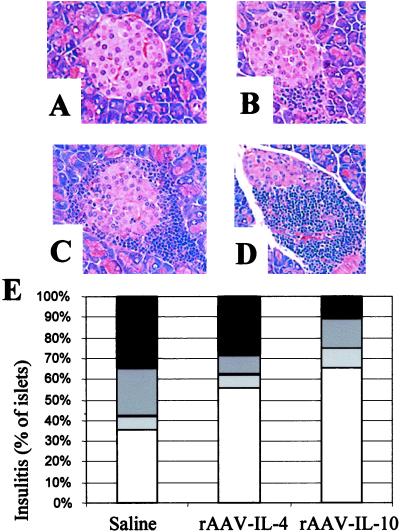
A key feature of type 1 diabetes in NOD mice is the infiltration of the endocrine pancreas with inflammatory cells. In contrast to a normal islet (stage 0 insulitis; Fig. 4A), the mildest form of inflammation is infiltration with inflammatory cells (dendritic cells, macrophages, T and B cells) around the perivascular duct and peri-islet regions of the islets of Langerhans (“peri-insulitis,” stage 1 insulitis; Fig. 4B). This peri-insulitis process in NOD mice normally begins when the animals are 4–6 weeks of age and is followed by an increase in the number of affected islets, a progressive increase in the quantity of intra-islet inflammatory cell accumulation (stages 2 and 3; Fig. 4 C and D), and the selective destruction of insulin-producing islet β cells (loss of insulin content). To examine the immunomodulatory effect of rAAV-cytokine gene therapy on the insulitis lesion before the period of developing overt clinical disease, we monitored insulitis in a separate series of animals injected with rAAV vectors and killed at 10–14 weeks of age. Cytokine transduction of NOD mice with rAAV-IL-10 and, to a lesser extent, with rAAV-IL-4 effectively reduced the quantitative parameters of insulitis in recipient animals (Fig. 4E). Specifically, pancreatic sections from all mice contained islets free of inflammation as well as islets that demonstrated moderate to severe insulitis. However, the percentage of islets affected by severe insulitis was far less in the pancreata from rAAV-IL-10 NOD mice than in control animals, with rAAV-IL-4-treated mice forming an intermediate group. Furthermore, although not subject to the same quantitative evaluation, insulin content appeared to be retained and at higher levels in islets of rAAV-IL-10-transduced mice compared with those from the rAAV-IL-4 or control group mice (data not shown). Finally, previous studies have suggested that the expression of intercellular adhesion molecule-1 in islets, as influenced by the systemic or localized production of IL-10, is associated with diabetogenesis (27). However, our studies analyzing four pancreatic structural components representing extra- and intra-islet vasculature did not reveal substantial differences between intercellular adhesion molecule-1 staining among animals from the three subject groups (data not shown). These findings indicate that rAAV-IL-10 gene therapy in part inhibits diabetes by reducing the severity of insulitis. However, they do not completely abrogate the recruitment of mononuclear cells to the islets.
Figure 4.
The effect of rAAV cytokine gene delivery on pancreatic islets. (A–D) Representative hematoxylin/eosin-stained pancreas sections obtained from various NOD mice used in these experiments, displayed to illustrate scoring categories shown in (E) (×400). (A) Normal islet architecture, devoid of lymphocytes (stage 0); (B) peri-insulitis only (stage 1); (C) insulitis involving <50% of the islet in cross section (stage 3); (D) insulitis involving >50% of the islet (stage 4); (E) histogram depicting percentage of normal islets (stage 1, unfilled bar), peri-insulitits (stage 2, light gray bar), insulitis involving <50% of the islet in cross section (stage 3, dark gray bar), or insulitis involving >50% of the islet (stage 4, black bar). A total of 171 islets obtained from 17 animals (n = 5–6 per group; 10-, 12-, and 14-week-old animals; average 10 islets per animal) were scored. The frequency of stage 0 insulitis was significantly lower in the PBS than in the IL-10 group (P = 0.01), and, conversely, stage 3 insulitis was significantly higher in PBS- than in IL-10-treated animals (P = 0.025).
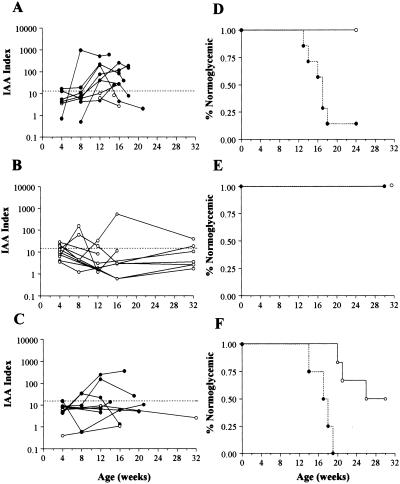
A recent report suggested that IAAs provide an excellent predictor of future development of type 1 diabetes in NOD mice, inasmuch as a majority of animals developing disease possessed this marker by 12 weeks of age (28). Hence, we performed longitudinal analysis of IAA in rAAV-IL-10, rAAV-IL-4, and control animals. As expected, serums from a minority of animals at 4 weeks of age were IAA positive (Fig. 5 A–C). In saline controls, both longitudinal (Fig. 5A) and life table analysis (Fig. 5D) of individual animals suggested a strong association between IAA development and formation of type 1 diabetes. Indeed, all saline-treated animals developing type 1 diabetes developed IAA by 16 weeks of age. Interestingly, the effect of rAAV-IL-10 treatment appeared to involve a reduction in IAA index (Fig. 5B) in the period beyond 8 weeks of age in nearly all animals. The observed effect of rAAV-IL-4 was less clear, with no specific pattern associated with protection from disease (Fig. 5 C and F). Hence, it is possible that the protection from type 1 diabetes observed in rAAV-IL-10-treated mice resulted to some degree from the attenuation of islet autoantigen specific immunity in vivo.
Figure 5.
rAAV cytokine gene delivery and the natural history of insulin autoantibodies in NOD mice. Longitudinal analysis of animals followed from 4 until 16 weeks or later. (A) Saline; (B) rAAV-IL-10; (C) rAAV-IL-4 (developed diabetes, ●; no diabetes, ○). The dashed line represents the definition for positive IAA responses. Life-table presentation of animals as a function of treatment group: (D) saline; (E) rAAV-IL-10; (F) rAAV-IL-4 (ever IAA positive, ●; never IAA positive, ○). P < 0.03 for IL-10 versus saline controls based on the frequency of IAA-positive animals at 12 or 16 weeks.
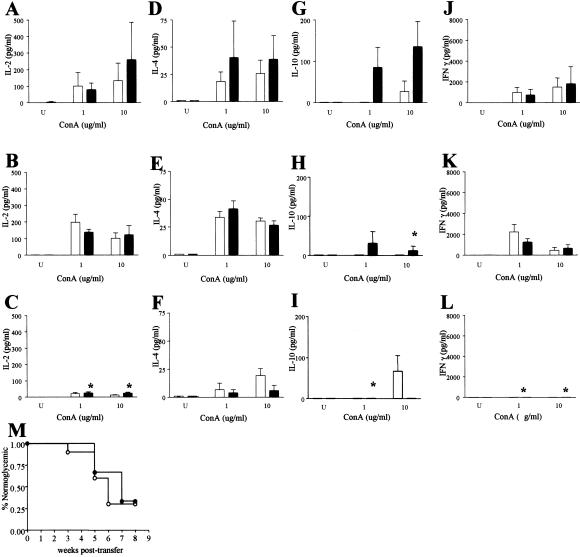
To learn whether protection from type 1 diabetes afforded by rAAV-IL-10 could have resulted, in part, from the induction of a shift in systemic cytokine production, we analyzed the levels of IL-2, -4, -10, and IFN-γ produced by splenocytes (Fig. 6) in response to mitogenic stimulation with Con A. In comparison with saline-treated animals (Fig. 6 A, D, G, and J), rAAV-IL-4-transduced mice (Fig. 6 B, E, H, and K) produced equivalent levels of IL-2, -4, and IFN-γ, whereas IL-10 production was markedly diminished. Quite strikingly, the introduction of rAAV-IL-10 into skeletal muscle resulted in markedly diminished production of IL-2, -4, -10, and IFN-γ (Fig. 6 C, F, I, and L). These results suggest that of the cytokines tested, a reduced production of splenocyte-derived IL-2 and IFN-γ may have been more closely associated with protection than IL-10 and possibly IL-4, as only the rAAV-IL-10-treated animals displayed diminished disease.
Figure 6.
The effect of rAAV cytokine gene delivery in skeletal muscle on splenocyte function. Splenocyte responses in the absence of Con A (U; untreated) or at two different Con A concentrations (1 and 10 μg/ml) at 24 (clear bar) and 48 h (solid bar) after stimulation are shown. (A–C) IL-2 production in (A) saline-treated, (B) rAAV-IL-4-treated, and (C) rAAV-IL-10-treated mice. (D–F) IL-4 production in (D) saline-treated, (E) rAAV-IL-4-treated, and (F) rAAV-IL-10-treated mice. (G–I) IL-10 production in (G) saline-treated, (H) rAAV-IL-4-treated, and (I) rAAV-IL-10-treated mice. (J–L) IFN-γ production in (J) saline-treated, (K) rAAV-IL-4-treated, and (L) rAAV-IL-10-treated mice. *, P < 0.01 versus control group. Note that statistical comparisons were made with the use of “peak” concentrations (1 or 10 μg/ml) at 24 and 48 h only. (M) Life-table analysis of incidence of hyperglycemia in irradiated male NOD mice adoptively transferred with splenocytes from NOD mice recently diagnosed with type 1 diabetes (●) or 30-week-old rAAV-IL-10-treated NOD mice.
Finally, to learn whether rAAV-IL-10 transduction modulates type 1 diabetes by altering the β cell destructive capacity and/or inducing immunoregulatory cells in vivo, we performed both adoptive transfer and adoptive cotransfer studies. Specifically, young (nondiabetic) irradiated male NOD mice were injected via the tail vein with splenocytes from either rAAV-IL-10-treated mice or from newly diabetic NOD mice. In addition, a third group of recipients was injected with a 1:1 mixture of splenocytes from rAAV-IL-10-treated animals and from newly diabetic NOD mice. Interestingly, type 1 diabetes developed in 50% of the rAAV-IL-10 transferred animals by 4 weeks posttransfer, in a time frame similar to that of newly diagnosed animals (Fig. 6M). Furthermore, recipient mice injected with equal mixtures of splenocytes from the rAAV-IL-10-protected animals and newly diagnosed NOD mice developed diabetes in an accelerated time frame (50% by 3 weeks after transfer), whereas control irradiated males not subject to splenocyte transfer failed to develop diabetes within 8 weeks after transfer (data not shown). These studies suggest that rAAV-IL-10 transduction did not induce immunoregulatory cells in vivo and that the mechanism of prevention is host specific. This conclusion further implies that the beneficial effects require the continuous expression of the IL-10 transgene, an important feature of rAAV vectors.
Discussion
These studies support the utility of rAAV-mediated gene delivery, specifically that involving IL-10, as a method of preventing type 1 diabetes. In addition to primary disease prevention, we and others have demonstrated the ability of rAAV to transduce islet cells (29, 30). The delivery to islets of anti-inflammatory cytokines, cytoprotective antioxidant, and anti-inflammatory enzymes, and/or anti-apoptotic molecules by rAAV may delay/prevent the recurrence of type 1 diabetes in islet transplantation and may offer a promising form of immunotherapy.
Previous studies of IL-10 in NOD mice have been described as “paradoxical” (31). Transgenic BALB/c mice expressing IL-10 in the pancreas exhibited peri-insulitis but not insulitis or diabetes (32). However, backcrossing of these transgenic mice onto the NOD background, rather than leading to protection, leads to disease acceleration, suggesting a potential pathogenic role for IL-10 in type 1 diabetes development (33). In contrast, administration of IL-10 to adult NOD mice attenuated type 1 diabetes, a finding consistent with disease prevention (34). One potential means for this variance may be the contrasting effects of local (islet) versus systemic production (35). Clearly, more investigation is needed to better define the mechanisms associated with the form of delivery and location of expression.
In terms of the mechanism(s) for prevention in this study, immunoregulation involving alterations in β cell-specific immunity was suggested by diminished insulitis scores and a reduction in index values for IAA. Although qualitative measures of insulitis composition have been shown to be of importance (“destructive” versus “nondestructive”), additional studies suggest that quantitative reductions of insulitis can also be associated with protection from disease (2). Aside from β cell-specific effects, we recognized the potential of skeletal muscle production of IL-4 and -10 for influencing systemic cytokine gene regulation and immune responsiveness. Interestingly, in terms of the effects of IL-4 or -10 on specific cytokine production, nearly all of the mitogen-induced cytokine responses in our studies were consistent with the published literature, and, more importantly, they provided additional clues to the potential mechanisms of cytokine action in terms of diabetes prevention (24). Studies suggest that IL-10 inhibits the synthesis of a number of cytokines, including IFN-γ, IL-2, and TNF-β. In macrophages, IL-10 can promote the degradation of cytokine mRNA, inhibit antigen presentation, and impede activation. In monocytes, IL-10 antagonizes the production and action of IFN-γ. IL-10 also inhibits mitogen-induced proliferation of T cells in the presence of accessory cells and reduces the production of IFN-γ and IL-2. It is clear that the exact mechanisms of disease prevention as well as expanded studies of other cytokines (e.g., IL-12) must be subject to further investigation. However, these results allow us to speculate that systemic elevations in IL-10 may have (among many things) imparted a state of functional anergy in T cells, interrupted the maturation of T cells from a naive state, and/or potentially delayed the maturation of antigen-presenting cells (macrophages, dendritic cells) to a state capable of antigen presentation to T cells, allowing for normal function.
Despite the observed success of rAAV-mediated delivery, further experimentation is necessary to bring practical reality to this form of therapy. One such activity could include examination of viral-IL-10, a molecule that possesses only a subset of cellular IL-10 activities, predominantly its suppression of cytokine synthesis by T helper type 1 cells (36). It has yet to be observed whether beneficial therapeutic function can be achieved in the absence of imparting intramuscular lymphocytic accumulation through gene delivery analysis with the use of rAAV-vIL-10. Furthermore, the doses used in these studies (1 × 1010 units per animal) are high by historical standards. The effect of therapeutic dose and, perhaps even more important, the time of vector administration (6–16 weeks) on the aforementioned intramuscular inflammation, insulitis, and development of type 1 diabetes are crucial subjects. Furthermore, contrary to published studies, rAAV-mediated IL-4 expression in this investigation did not result in attenuation of type 1 diabetes (9, 13). The results of this discrepancy are unclear and may be related to the dose, route, timing, and/or choice of vector used for administration, factors that have yet to be investigated.
In summary, we believe these studies represent an important step to validating the utility of cytokine gene therapy, afforded by rAAV-mediated gene delivery, on altering the immune response. These studies provide support for the concept of using immunoregulatory agents delivered by rAAV to modulate disorders associated with immune responses, including allergic reactions, transplantation rejection, immunodeficiencies, and autoimmune disorders.
Acknowledgments
We thank Anupam Agarwal and Harry Nick for their continual advice and Nora Sarvetnick (The Scripps Institute, La Jolla, CA) for her provision of murine cytokine cDNAs. This work was supported in part by grants from the Juvenile Diabetes Research Foundation International, the National Institutes of Health, and the Sebastian Family/American Diabetes Association Research Professorship.
Abbreviations
- rAAV
recombinant adeno-associated virus
- NOD
nonobese diabetic
- IAA
insulin autoantibody
- CMV
cytomegalovirus
References
- 1.Bach J F. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson M A, Leiter E H. Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 3.Serreze D V. FASEB J. 1993;7:1092–1096. doi: 10.1096/fasebj.7.11.8370480. [DOI] [PubMed] [Google Scholar]
- 4.Wong F S, Janeway C A. J Autoimmun. 1999;13:290–295. doi: 10.1006/jaut.1999.0322. [DOI] [PubMed] [Google Scholar]
- 5.Yoon J W, Jun H S, Santamaria P. Autoimmunity. 1998;27:109–122. doi: 10.3109/08916939809008041. [DOI] [PubMed] [Google Scholar]
- 6.Trudeau J D, Dutz J P, Arany E, Hill D J, Fieldus W E, Finegood D T. Diabetes. 2000;49:1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Tian J, Olcott A P, Hanssen L R, Zekzer D, Middleton B, Kaufman D L. Immunol Rev. 1998;164:119–127. doi: 10.1111/j.1600-065x.1998.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 8.Wegmann D R, Eisenbarth G S. J Autoimmun. 2000;15:286–291. doi: 10.1006/jaut.2000.0444. [DOI] [PubMed] [Google Scholar]
- 9.Rapoport M J, Jaramillo A, Zipris D, Lazarus A H, Serreze D V, Leiter E H, Cyopick P, Danska J S, Delovitch T L. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wogensen L, Lee M S, Sarvetnick N. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennline K J, Roque-Gaffney E, Monahan M. Clin Immunol Immunopathol. 1994;74:169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovitch A, Suarez-Pinzon W L, Sorensen O, Bleackley R C, Power R F, Rajotte R V. Transplantation. 1995;60:368–374. doi: 10.1097/00007890-199508270-00012. [DOI] [PubMed] [Google Scholar]
- 13.Cameron M J, Strathdee C A, Holmes K D, Arreaza G A, Dekaban G A, Delovitch T L. Hum Gene Ther. 2000;11:1647–1656. doi: 10.1089/10430340050111304. [DOI] [PubMed] [Google Scholar]
- 14.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song S, Morgan M, Ellis T, Poirier A, Chestnut K, Wang J, Brantly M, Muzyczka N, Byrne B, Atkinson M, Flotte T. Proc Natl Acad Sci USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muzyczka N. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 17.Rabinowitz J E, Samulski J. Curr Opin Biotechnol. 1998;9:470–475. doi: 10.1016/s0958-1669(98)80031-1. [DOI] [PubMed] [Google Scholar]
- 18.Kapturczak M H, Flotte T R, Atkinson M A. Curr Mol Med. 2001;1:245–258. doi: 10.2174/1566524013363979. [DOI] [PubMed] [Google Scholar]
- 19.Hauswirth W W, Lewin A S, Zolotukhin S, Muzyczka N. Methods Enzymol. 2000;316:743–761. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- 20.She J-X, Ellis T M, Wilson S B, Wasserfall C H, Marron M, Reimsneider S, Kent S C, Hafler D A, Neuberg D S, Muir A, et al. Proc Natl Acad Sci USA. 1999;96:8116–8119. doi: 10.1073/pnas.96.14.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arreaza G, Cameron M, Jaramillo A, Gill B, Hardy D, Laupland K, Rapoport M, Zucker P, Chakrabarti S, Chensue S, et al. J Clin Invest. 1997;100:2243–2253. doi: 10.1172/JCI119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowman M A, Campbell L, Darrow B L, Ellis T M, Suresh A, Atkinson M A. Diabetes. 1996;45:205–208. doi: 10.2337/diab.45.2.205. [DOI] [PubMed] [Google Scholar]
- 23.Fellowes R, Etheridge C J, Coade S, Cooper R G, Stewart L, Miller A D, Woo P. Gene Ther. 2000;7:967–977. doi: 10.1038/sj.gt.3301165. [DOI] [PubMed] [Google Scholar]
- 24.Chang Y, Prud'homme G J. J Gene Med. 1999;1:415–423. doi: 10.1002/(SICI)1521-2254(199911/12)1:6<415::AID-JGM66>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Shelburne C P, Ryan J J. Immunol Rev. 2001;179:82–93. doi: 10.1034/j.1600-065x.2001.790109.x. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitch A. Diabetes Metab Rev. 1998;14:129–151. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Balasa B, La Cava A, Van Gunst K, Mocnik L, Balakrishna D, Nguyen N, Tucker L, Sarvetnick N. J Immunol. 2000;165:7330–7337. doi: 10.4049/jimmunol.165.12.7330. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Robles D T, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth G S. Proc Natl Acad Sci USA. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. . (First published February 4, 2000; 10.1073/pnas.040556697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad K M, Yang Z, Bleich D, Nadler J L. Gene Ther. 2000;7:1553–1561. doi: 10.1038/sj.gt.3301279. [DOI] [PubMed] [Google Scholar]
- 30.Flotte T, Agarwal A, Wang J, Song S, Fenjves E, Inverardi L, Chestnut K, Afione S, Loiler S, Wasserfall C, et al. Diabetes. 2001;50:515–520. doi: 10.2337/diabetes.50.3.515. [DOI] [PubMed] [Google Scholar]
- 31.Balaji B, Sarvetnick N. J Autoimmun. 1996;9:283–286. doi: 10.1006/jaut.1996.0036. [DOI] [PubMed] [Google Scholar]
- 32.Wogensen L, Less M S, Sarvetnick N. J Exp Med. 1993;178:175–185. doi: 10.1084/jem.178.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moritani M, Yoshimoto K, Tashiro F, Hashimoto C, Miyazaki J, Li S, Kudo E, Iwahana H, Hayashi Y, Sano T E A. Int Immunol. 1994;6:1927–1936. doi: 10.1093/intimm/6.12.1927. [DOI] [PubMed] [Google Scholar]
- 34.Nitta Y, Tashiro F, Tokui M, Shimada A, Takei I, Tabayashi K, Miyazaki J I. Hum Gene Ther. 1998;9:1701–1707. doi: 10.1089/hum.1998.9.12-1701. [DOI] [PubMed] [Google Scholar]
- 35.Balasa B, Van Gunst K, Jung N, Balakrishna D, Santamaria P, Hanafusa T, Itoh N, Sarvetnick N. J Immunol. 2000;165:2841–2847. doi: 10.4049/jimmunol.165.5.2841. [DOI] [PubMed] [Google Scholar]
- 36.Ding Y, Quin L, Kotenko S V, Pestka S, Bromberg J S. J Exp Med. 2000;191:213–223. doi: 10.1084/jem.191.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]