Abstract
Semaphorins SEMA3B and its homologue SEMA3F are 3p21.3 candidate tumor suppressor genes (TSGs), the expression of which is frequently lost in lung cancers. To test the TSG candidacy of SEMA3B and SEMA3F, we transfected them into lung cancer NCI-H1299 cells, which do not express either gene. Colony formation of H1299 cells was reduced 90% after transfection with wild-type SEMA3B compared with the control vector. By contrast, only 30–40% reduction in colony formation was seen after the transfection of SEMA3F or SEMA3B variants carrying lung cancer-associated single amino acid missense mutations. H1299 cells transfected with wild-type but not mutant SEMA3B underwent apoptosis. We found that lung cancers (n = 34) always express the neuropilin-1 receptor for secreted semaphorins, whereas 82% expressed the neuropilin-2 receptor. Because SEMA3B and SEMA3F are secreted proteins, we tested conditioned medium from COS-7 cells transfected with SEMA3B and SEMA3F and found that medium from wild-type SEMA3B transfectants reduced the growth of several lung cancer lines 30–90%, whereas SEMA3B mutants or SEMA3F had little effect in the same assay. Sequencing of sodium bisulfite-treated DNA showed dense methylation of CpG sites in the SEMA3B 5′ region of lung cancers not expressing SEMA3B but no methylation in SEMA3B-expressing tumors. These results are consistent with SEMA3B functioning as a TSG, the expression of which is inactivated frequently in lung cancers by allele loss and promoter region methylation.
Keywords: semaphorin‖neuropilin‖methylation
The semaphorin family comprised of secreted and membrane-associated proteins contributes to axonal path-finding during neural development by repulsing axons, inhibiting growth cone extension, and causing collapse of growth cones (1–3). The SEMA3 family members encode secreted proteins that signal through binding to neuropilin receptors (NP) interacting with plexins (1–3). Several semaphorins are expressed in adult nonneuronal tissues, suggesting other functions. For example, SEMA3A inhibited the motility of aortic endothelial cells expressing NP1, disrupted the formation of lamellipodia, induced depolymerization of F-actin (4), and inhibited branching morphogenesis in the fetal mouse lung (5). However, the roles of SEMA3B and SEMA3F in nonneuronal cells and human cancer are unknown.
The loss of heterozygosity of chromosome 3p sequences is a critical event in the pathogenesis of lung and other cancers and directed a tumor suppressor gene (TSG) search to this region. Multiple distinct 3p regions are involved in human lung cancer pathogenesis including one at 3p21.3 where we identified 19 candidate TSGs (6, 7). This defined 3p21.3 region undergoes allele loss in ≈80% of primary lung cancers and ≈40% of preneoplastic or normal epithelial samples of smoking-damaged lung, marking it as one of the first sites involved (6). Two of the 19 genes are semaphorin family members (SEMA3B and SEMA3F) lying ≈70 kb apart (7, 8). In assessing the TSG candidacy of SEMA3B and SEMA3F, we found only a few mutations but frequent loss of expression of SEMA3B mRNA (occurring in ≈80% of lung cancers). We and others found no SEMA3F mutations, and loss of SEMA3F expression occurred in 18% of these same lung cancers (8, 9). However, recent immunohistochemical studies of lung cancers, found reduction of SEMA3F expression in higher stages of lung cancer, and a change in SEMA3F localization from the membrane to the cytoplasm compared with normal lung epithelium (10). In addition, functional studies using a P1 clone containing SEMA3F (and potentially SEMA3B) showed a tumor-suppressive effect for mouse A9 fibrosarcoma cells (11). Recent studies have implicated tumor-acquired promoter hypermethylation as a mechanism of inactivation of mRNA expression of TSGs in the pathogenesis of several human cancers (12). In fact, we and others have found that one isoform at the RASSF1 locus, RASSF1A, located ≈60 kb centromeric of SEMA3B, underwent tumor-acquired promoter methylation, leading to inactivated expression in lung and breast tumors (13, 14). Because of the mutations and the functional studies, we also needed to study the semaphorin genes. Here we demonstrate that lung cancers express semaphorin receptors, exhibit 5′ CpG island SEMA3B methylation, and reexpression of exogenous wild-type SEMA3B but not SEMA3F or lung cancer-related SEMA3B mutations induce apoptosis in lung cancers. Furthermore, conditioned medium from COS-7 cells transfected with wild-type but not mutant SEMA3B genes also suppresses lung cancer growth. These results suggests that SEMA3B can function as an epigenetically inactivated potent suppressor of lung cancer growth.
Materials and Methods
Analysis of CpG Methylation of the SEMA3B 5′ Region.
Genomic DNAs from lung cancer cell lines not expressing SEMA3B (NCI-H209, H524, H1299, and H661) or expressing SEMA3B (H2009 and H1666; ref. 8) were modified by sodium bisulfite treatment as described (15). Treated DNAs were PCR-amplified with the primers M2AS (5′-TAACCCTAAAAATATACCCA-3′) and M1S (5′-TATTTTAGTAGTTTAGGGTG-3′) targeting a 269-bp sequence with multiple CpG sites immediately 5′ of the SEMA3B ATG. Note that primers M2AS and M1S are designed to amplify the opposite strand of sodium bisulfite-treated DNA promoter sequence shown in Fig. 1. PCR cycling conditions consisted of 12 min at 95°C followed by 40 cycles of 30 sec denaturation at 94°C, 30 sec of annealing at 50°C, 30 sec of extension at 72°C, with final extension at 72°C for 10 min. We reamplified and sequenced the PCR product to obtain CpG methylation levels (Fig. 1).
Figure 1.
Methylation analysis of SEMA3B. (A) Sequence of the region that was subjected to analysis. The positions of CpG sites are numbered 1–8. Primers used for amplification are indicated by arrows. (B) Methylation status of each CpG site (labeled 1–8 in A) determined by sequencing sodium bisulfite-treated genomic DNA from tumor cells. White and black squares represent unmethylated and methylated CpGs, respectively. Partially filled squares represent partially methylated CpG.
Analysis of Primary Lung Cancer Samples for SEMA3B Mutations.
Forty-six primary lung tumors [nine small cell lung cancers (SCLCs) and 37 non-SCLCs (NSCLCs) selected pathologically to contain ≥90% tumor tissue] and corresponding noncancerous tissues were obtained from the National Cancer Center Hospital (Tokyo, Japan), and genomic DNA was prepared (16). Seventeen genomic DNA fragments covering the entire coding region of SEMA3B were amplified by PCR with SEMA3B-specific oligonucleotide primers using exon/intron information from cDNA (U28369) and genomic (U73167) sequences and subjected to single-stranded conformation polymorphism analysis, and abnormal bands were sequenced. (Primers and conditions are available on request.)
Cell Lines.
Lung cancer cell lines (8, 17) were propagated in RPMI medium 1640 (Life Technologies GIBCO) supplemented with 10% fetal bovine serum. Normal human bronchial/tracheal epithelial cells (Clonetics, San Diego) were propagated in Clonetics growth medium.
Expression Plasmids.
The expression plasmids pcDNA3-SEMA3B (pCB11, pSEMA3B) and pcDNA3-SEMA3B antisense (pCB14, pSEMA3B-Antisense) (8) and site-directed mutagenesis (Stratagene) were used to make the mutant SEMA3B constructs (pSEMA3B-R348C, pSEMA3B-D397H, pSEMA3B-T415I, and pSEMA3B-D561N) containing lung cancer single amino acid missense mutations (8). A SEMA3F pcDNA3 expression construct (pSEMA3F) was also made. All constructs had their sequences confirmed through the PCR-manipulated regions, and all produced appropriately sized peptides detected in Western blotting by specific anti-SEMA3B or SEMA3F antibodies after transfection.
Transfection and Colony Formation Assays.
Transfections with DMRIE-C reagent (Life Technologies GIBCO) used 2 μg of each plasmid per 10-cm dish containing 5 × 105 cells seeded 24 h before transfection. Transfections were terminated at 5 h; 48 h after transfection, 5 × 104 transfected cells were seeded and maintained in RPMI medium 1640 10% fetal bovine serum supplemented with 800 μg/ml G418 (Life Technologies GIBCO). Surviving colonies were counted 12–21 days later after staining with methylene blue.
Antibodies and Western-Blot Analysis.
The peptides corresponding to amino acid residues Thr-732 to Trp-749 of human SEMA3B (U28369) and Pro-722 to Lys-742 of SEMA3F (U38276) were synthesized, and three rabbits were immunized with each peptide (Alpha Diagnostic, San Antonio, TX). SEMA3B antisera were purified on immunoaffinity columns in which the peptide was covalently linked to an Amino Link column (Pierce). Anti-NP1 and -NP2 rabbit polyclonal antibodies were provided by Dr. A. Kolodkin. Cellular proteins were extracted from 106 cells with 40 μl of lysis buffer (40 mM Hepes-NaOH, pH 7.4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, and 10 μg/ml aprotinin). Fifty μg of total protein per lane was separated on an 8% SDS/polyacrylamide gel and electroblotted to nitrocellulose membranes (Bio-Rad). After blocking with 5% nonfat dry milk and 0.1% Tween 20 in Tris-buffered saline, membranes were incubated at 37°C for 2 h with anti-NP1, anti-NP2 (18), anti-SEMA3B, or anti-SEMA3F antibodies. The membranes then were developed with peroxidase-labeled anti-rabbit IgG (Amersham Pharmacia) by Super Signal chemiluminescence substrate (Pierce). Equal loading of protein was confirmed after detection by staining the membrane with amido black 10B (Sigma).
Cell Cycle Analysis.
Cells were harvested 48 h after the transfection, fixed with 50% ethanol, treated with 5 mg/ml RNase A (Roche Molecular Biochemicals), stained with 50 μg/ml propidium iodide, and analyzed by flow cytometry for DNA synthesis and cell cycle status (FACSCaliber instrument, Becton Dickinson, with CELLQUEST software).
Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL) and Caspase-3 Apoptosis Assays.
Cells were fixed 24 h after the transfection with 4% paraformaldehyde (Sigma) solution in PBS for 1 h at room temperature, treated with 0.3% H2O2-methanol solution, and then permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate solution for 2 min on ice. The TUNEL assay (Roche Molecular Biochemicals) was carried out following the manufacturer's instruction. Caspase-3 activity was measured with an Apoalert Caspase-3 colorimetric kit (CLONTECH). Cells (5 × 105) were transfected with empty or SEMA3B plasmids by lipofection. After 20 h, cell lysates were prepared and preincubated with or without the caspase inhibitor DEVD-fmk (CLONTECH) for 30 min. Then, DEVD-pNA, the caspase-3 substrate, was added to the samples and incubated for 1 h, and the product was measured at 400 nm.
Results
Methylation Status of CpG Sites in SEMA3B Nonexpressing Lung Cancers.
We determined the CpG methylation status in the 5′ region of SEMA3B by sequencing sodium bisulfite-modified DNA from four lung cancer cell lines not expressing SEMA3B as well as two lung cancer lines expressing SEMA3B (Fig. 1). All the SEMA3B nonexpressing tumor cell lines exhibited methylation of almost all CpG dinucleotides in this region. The two tumor cell lines that did express SEMA3B were either not methylated at these CpG sites or else showed a single CpG site with a mixed methylation pattern.
Additional Mutations Found in SEMA3B in Primary Lung Tumors.
Genomic DNA from 46 primary lung tumors were examined for mutations in SEMA3B by PCR/single-strand conformational polymorphism analysis and direct DNA sequencing (data not shown). An acquired mutation in SEMA3B (nucleotide G1916A substitution leading to a D561N amino acid change in the semaphorin domain of SEMA3B) was detected in one of nine primary SCLCs (but not the normal tissue), and germline changes T415I, and a G-to-A nucleotide substitution at the intron side of the exon 15/intron boundary were found in one NSCLC each. All three were associated with a loss of the wild-type alleles in tumors. As discussed below, T415I and D561N lead to loss of SEMA3B growth-suppressing function.
Expression of Neuropilins in Lung Cancer Cell Lines.
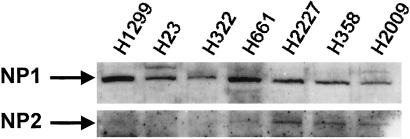
Because SEMA3 family members act through NPs (and plexin coreceptors; refs. 19 and 20), we examined the expression of NP1 and NP2 proteins in 18 SCLC, 14 NSCLC, and 2 mesothelioma cell lines in which the expression of SEMA3B and SEMA3F mRNAs were also known (Fig. 2; ref. 8). NP1 was expressed strongly in all 34 cancer cell lines, whereas NP2 was expressed to varying degrees in all SCLCs, nine NSCLCs, and one mesothelioma. We found no correlation of the expression patterns of SEMA3B, SEMA3F, and the neuropilins.
Figure 2.
Expression of neuropilin peptides in lung cancer cell lines by Western blotting (50 μg of total cell lysate per lane). NCI-H1299, H23, and H322 express NP1 but not NP2, whereas H661, H2227, H358, and H2009 express both NP1 and NP2. Not shown are: SCLC lines expressing NP1 and NP2 NCI-H187, H209, H345, H378, H524, H526, H740, H865, H889, H1045, H1092, H1238, H1514, H1618, H1672, H2141, H2171, and H2227; and NSCLC lines expressing NP1 and NP2 H358, H838, H1437, H1792, H2009, H2077, H661, H2106, and H28 (mesothelioma). Lung cancer lines expressing NP1 but not NP2 are H1666, H460, and H2052 (mesothelioma).
Inhibition of Colony Formation by SEMA3B in Lung Cancer Cell Lines.
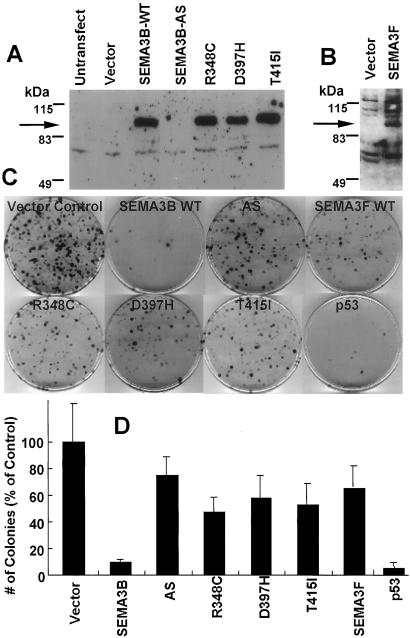
To test for the growth-suppressive effect of ectopically expressed semaphorins, we performed colony formation assays selecting for the neo gene carried by our expression plasmids. Plasmids containing the full open reading frame of SEMA3B, anti-sense SEMA3B, the full open reading frame of SEMA3F, and four SEMA3B constructs carrying lung cancer associated missense mutations were transfected into NSCLC NCI-H1299 cells, which do not express endogenous SEMA3B or SEMA3F (8). A positive control was wild-type p53, which is known to inhibit the growth and induce apoptosis of H1299 cells, which contain a homozygous deletion of p53 (21). The wild-type SEMA3B and SEMA3F and mutant SEMA3B expression constructs all produced equivalent amounts of protein after transient transfection detected by using affinity-purified anti-SEMA3B or anti-SEMA3F antibodies (Fig. 3 A and B). Forty-eight hours after transfection, cells were selected with geneticin (G418), and resistant colonies developing 12 days later were stained. As expected, the wild-type p53 control dramatically suppressed colony formation (Fig. 3 C and D). In addition, the number of G418-resistant colonies after transfection with wild-type SEMA3B was reduced 90% compared with transfection with the control vector in five independent experiments by using three independent plasmid DNA preparations (Fig. 3 C and D). By contrast, four separate SEMA3B missense mutations had lost most of this colony-suppressing activity despite robust protein expression (Fig. 3 C and D). As a further control, we moved SEMA3B from pcDNA3 to the pcDNA3.1 vector and still saw the same degree of suppression of colony formation. We also tested the growth-suppressive effect of SEMA3F and found the number of colonies after transfection with SEMA3F was only slightly different (70 ± 17%) than the vector control (Fig. 3 C and D). The numbers of colonies were reduced also after transfection with SEMA3B into many other NSCLC lines, whereas little reduction was found in NSCLC line NCI-H23 (Table 1). The growth-suppressive effect was seen in lung cancer lines both expressing and not expressing NP2 and in two lung cancers (H2009 and H358) expressing endogenous mRNA for SEMA3B (Table 1). In the case of H358, the expressed SEMA3B mRNA contains a D397H mutation that, as shown above, has very reduced growth-suppressing activity.
Figure 3.
The effect of exogenous expression of SEMA3B and SEMA3F on the colony formation of H1299 NSCLC cells. (A) Western-blot analysis of NSCLC H1299 cells transfected with various SEMA3B plasmids; (B) Western blot of H1299 cells transfected with SEMA3F; (C and D) H1299 colony formation after transfection and G418 selection. In A and B, plasmids were transfected into 5 × 105 H1299 cells by lipofection, cells were harvested 48 h later, and 50 μg of total lysate was Western-blotted with anti-SEMA3B (A) and anti-SEMA3F antisera (B). (C) colony formation after transfection and selection with G418 stained with methylene blue. Vector Control, pcDNA3; AS, pcDNA3 with wild-type SEMA3B in antisense direction; SEMA3BWT, SEMA3B wild type; SEMA3F, SEMA3F wild type; R348C, D397H, and T415I, SEMA3B constructs with indicated mutations introduced; p53, pcDNA3 with wild-type p53. (D) Quantitation of the number of G418-selected H1299 colonies. The vector control was set at 100%. The data represent the mean ± SD of five independent experiments, each done in triplicate plates. The D561N data are not shown.
Table 1.
G418-resistant colony formation after transfection of a SEMA3B-neo expression plasmid into lung cancer cell lines with various SEMA3B, NP1, and NP2 expression patterns
| NCI lung cancer cell lines | Expression of:
|
G418-resistant colony formation,* % of vector control transfections | ||
|---|---|---|---|---|
| SEMA3B | Npn1 | Npn2 | ||
| H23 (NSCLC) | − | + | − | 71 ± 5 |
| H1299 (NSCLC) | − | + | − | 10 ± 1 |
| H2227 (SCLC) | − | + | + | 10 ± 4 |
| H661 (NSCLC) | − | + | + | 4 ± 4 |
| H322 (NSCLC) | − | + | − | 3 ± 1 |
| H2009 (NSCLC) | ++++ | + | + | 1 ± 1 |
| H358 (NSCLC) | ++ | + | + | 0 ± 0 |
Values are the mean ± SD of three separate experiments, each calculated from counting colonies on triplicate plates. In each case the pcDNA3.1 (neo) vector control-transfected number of G418-resistant colonies was set at 100%. SEMA3B expression is from Sekido et al. (8). Note that H358 carries a D397H SEMA3B mutation.
Induction of Apoptosis by Exogenous Expression of SEMA3B.
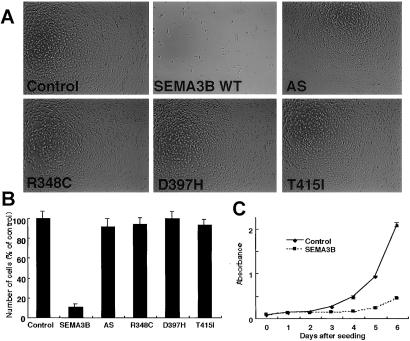
When the wild-type SEMA3B expression plasmid was transfected into H1299 cells, the number of surviving cells was decreased at 48 h after transfection compared with the control plasmid (Fig. 4A). The amount of this decrease was impressive because the transfection efficiency for the overall population was ≈30%. Thus, it is likely a “bystander” effect may be active, potentially mediated by secretion of SEMA3B from transfected cells (see below). This decrease in tumor cell number was associated with the appearance of TUNEL-positive cells (Fig. 4B) and a 10-fold increase (2–24%) of cells with sub-G1 content DNA (Fig. 4C) compared with the control vector, indicating that growth suppression by SEMA3B was caused by induction of apoptosis. Transfection of the mutant SEMA3B constructs did not result in apoptosis detected by the TUNEL assay (data not shown). We did not use an epitope-tagged version of SEMA3B in these experiments, because other studies showed that a SEMA3B C-terminal FLAG-tagged construct was inactive in tumor growth suppression despite conferring similar levels of SEMA3B protein on the transfected cells (data not shown). The caspase-3 activity was increased significantly (P < 0.05) after wild-type p53 and SEMA3B transfection, and the activity was blocked in both cases by the caspase-3 inhibitor DEVD-fmk, indicating caspase involvement in SEMA3B induced apoptosis (Fig. 4D).
Figure 4.
SEMA3B transfection induces apoptosis in H1299 NSCLC cells. (A) H1299 cells (1 × 104) were seeded in 35-mm dishes. After 24 h, empty vector control or SEMA3B expression plasmids were transfected (≈20–30% efficiency), and the number of cells was counted 48 h later. The data represent the mean ± SD of five independent experiments. (B) The TUNEL assay 24 h after H1299 cells were transfected with a SEMA3B plasmid. The TUNEL-positive cells (≈20%) are indicated by arrows. Vector control-transfected cells (TUNEL positive <2%) are not shown. (C) Fluorescence-activated cell-sorting profiles of H1299 transfected with empty vector (Control) or the SEMA3B expression plasmid. Cells were harvested 48 h later, stained with propidium iodide, and analyzed by using the flow cytometer. Horizontal and vertical axes represent DNA content and cell number, respectively. The percentage of sub-G1 cells undergoing apoptosis is indicated. (D) Caspase-3 activity in H1299 cells 20 h after transfection with SEMA3B or p53 (positive control) expression plasmids. The data represent the mean ± SD of three independent experiments. p53- and SEMA3B-transfected cells had significantly higher caspase-3 activity than vector controls, whereas DEVD-fmk-treated p53 and SEMA3B cells did not.
Growth Suppression by the Conditioned Medium from COS-7 Cells Transfected with SEMA3B.
Because SEMA3B is a secreted protein and lung cancers express NP receptors, we analyzed the effect of conditioned medium harvested from COS-7 cells transfected with SEMA3B on H1299 lung cancer cells. The growth rate of H1299 cells treated with conditioned medium from COS-7 cells transfected with SEMA3B was reduced compared with conditioned medium vector control-transfected COS-7 cells, conditioned medium harvested after transfection with SEMA3B-Antisense, or mutant SEMA3B constructs (Fig. 5). This lung cancer growth-suppressing effect was not found in conditioned medium from SEMA3F-transfected COS-7 cells (data not shown). To confirm the growth-suppressive effect of SEMA3B-transfected COS-7 conditioned medium, this assay was performed in several other lung cancer cell lines that have various expression patterns of SEMA3B, NP1, and NP2, and growth inhibition of 30–60% was seen compared with vector control-transfected COS-7 cell conditioned medium (Table 2). By contrast, the growth rate of normal human bronchial/tracheal epithelial cells treated with SEMA3B COS-7 conditioned medium was not significantly different from treatment with vector control-transfected COS-7 control medium (Table 2). We note that although NSCLC NCI-H23 colony formation was resistant to transfection-induced expression of SEMA3B, this tumor line did show growth inhibition to SEMA3B COS-7 cell conditioned medium (Table 2).
Figure 5.
The effect of COS-7 condition medium transfected with SEMA3B expression plasmids on H1299 NSCLC cells. (A) Each indicated plasmid was transfected into 5 × 105 COS-7 cells by lipofection. After 48 h of incubation, the conditioned medium was collected and applied to 5 × 103 H1299 cells seeded in each well of 6-well plates. After 4 days, the cells in each indicated conditioned medium were photographed (A; original magnification is ×40) or counted (B). The number of H1299 cells in the cultures treated with conditioned medium from empty vector controls were set at 100%. The data represent the mean ± SD of three independent experiments. SEMA3B-transfected COS-7 cell conditioned medium gave significantly fewer numbers of H1299 cells compared with treatment with condition medium after transfection with the SEMA3B mutants (R348C, D397H, T415I, and D561N; data not shown) or the SEMA3B antisense (AS) constructs. (C) Growth curve of H1299 NSCLC cells in the conditioned medium of COS-7 cells transfected with control or SEMA3B expression plasmids. At each indicated time point, cell viability was determined and represented as the degree of absorbance 540 nm using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The mean ± SD absorbance (triplicate wells) for each time point is plotted as a function of the number of days after seeding.
Table 2.
Lung cancer cell line growth after exposure to conditioned medium from COS-7 cells transfected with SEMA3B
| NCI lung cancer cell lines | Growth inhibition* |
|---|---|
| H1299 | 90 ± 3 |
| H358 | 62 ± 10 |
| H661 | 56 ± 13 |
| H23 | 55 ± 12 |
| H322 | 37 ± 16 |
| H2009 | 36 ± 12 |
| H2227 | 31 ± 6 |
| NHBE | 11 ± 10 |
The experiments were performed as described in the legend for Fig. 5B with cell counts performed at day 4. The values are the mean ± SD of three separate experiments with triplicate wells for cell counting in each experiment. Vector control-transfected COS-7 cell condition medium was applied to the replicate cell cultures, and cell numbers at day 4 were set as showing 0% growth inhibition. NHBE, normal human bronchial/tracheal epithelial cell cultures (Clonetics).
Discussion
Recently it has become clear that tumor-acquired promoter hypermethylation is a common mechanism for the inactivation of TSG expression in lung and other cancers (7, 12, 22), and the current study shows that the loss of SEMA3B expression is associated with methylation of CpGs in the putative SEMA3B promoter region. After transfection and expression, wild-type SEMA3B induces growth inhibition of lung cancer cells, whereas lung cancer-associated SEMA3B missense mutations or the addition of a C-terminal FLAG tag cause a loss of this tumor growth-suppressive function. In addition, exogenous expression of SEMA3F caused only a slight decrease in colony formation. We note that these SEMA3B mutant constructs differ in only a single amino acid from the wild-type sequence and were expressed at similar protein levels but were not toxic to lung cancer cells compared with wild-type SEMA3B. These mutants, tagged versions, and the resistant NCI-H23 tumor line provide important specificity controls. The regions of mutations in SEMA3B are located at codons 348, 397, 415, and 561. These sites are located in the semaphorin domain, which is necessary for semaphorin dimerization and biological activity (1–3). Thus, it is likely that the semaphorin domain in SEMA3B is important for the lung cancer growth-suppressive activity. Two lung cancers continuing to express SEMA3B mRNA (H2009 and H358) also showed inhibition of colony formation after forced expression of SEMA3B. In the case of H358 this result was expected, because the expressed SEMA3B mRNA contained a D397H mutation (8). However, the inhibition of colony formation of lung cancer H2009 by SEMA3B was unexpected and potentially could indicate haploinsufficiency of SEMA3B expression or post-translational mechanisms of inactivation of SEMA3B in this tumor.
In the Knudson recessive oncogene model, one defective copy of a gene is inherited in the germline with subsequent loss of the wild-type allele in tumors (23). Thus, it was of great interest to discover that the germline T415I alteration leads to a significant loss of wild-type SEMA3B lung cancer growth-suppressing activity. This suggests further studies to see whether germline T415I represents an inherited risk factor for lung cancer, because we found it associated with wild-type allele loss in tumors. Because many lung cancers undergo 3p21.3 allele loss and the large majority of lung cancers do not express SEMA3B, it seems that there is biallelic inactivation of SEMA3B in sporadic tumors also consistent with Knudson's model. Whether other events besides promoter methylation can lead to loss of SEMA3B expression requires further study.
The mechanism of SEMA3B-induced apoptosis is unknown. Another secreted semaphorin, SEMA3A, induced apoptosis in sympathetic (24) and sensory neurons (25). Semaphorins contain a short sequence of homology to tarantula hanatoxin, and the sequence is required to induce growth cone collapse in neuronal cells (26). However, we did not find growth suppression after transfection of mutant SEMA3B constructs retaining the hanatoxin sequence. The growth of several lung cancer cell lines but not normal bronchial epithelial cultures was suppressed by conditioned medium harvested from COS-7 cells transfected with wild-type SEMA3B. In contrast, conditioned medium from mutant SEMA3B-transfected COS-7 cells did not suppress growth. The difference between normal and cancer cells could exist because of the difference in expression for receptors for semaphorins, other components of the SEMA3B signaling pathway, or response to other products secreted by COS-7 cells after SEMA3B transfection. We need to consider whether secreted SEMA3B acts directly to inhibit tumor growth or indirectly by causing COS-7 cells to secrete other factors that inhibit growth. In preliminary experiments, anti-NP1 antibodies did not block SEMA3B-transfected COS-7 cell conditioned medium growth suppression (data not shown). In addition, NSCLC NCI-H23 was resistant to overexpression of SEMA3B but sensitive to SEMA3B-transfected COS-7 conditioned medium. These findings would seem to support an indirect mechanism. Bachelder et al. found that vascular endothelial growth factor (VEGF) was an autocrine survival factor for NP expressing breast cancer cells (27), whereas SEMA3A competed with VEGF165 for binding to NP1 receptors and inhibited vascular endothelial cell motility, suggesting a role in inhibiting angiogenesis (4). In fact, the different effects of SEMA3B on normal human bronchial/tracheal epithelial cells versus cancer cells may occur because normal cells do not depend on survival factors such as VEGF, whereas lung cancer cells may require these factor(s). NP1 was expressed in all the lung cancers, and overexpression of NP1 itself may contribute to oncogenesis by enhancing angiogenesis, whereas a soluble NP1 bound VEGF and inhibited tumorigenesis (28, 29). Thus, one model to consider is that SEMA3B acts to antagonize VEGF by acting through the NP1 receptors. Substantial data indicate that the complex of NP1 and Plexin 1 is the physiologic signal transducer for SEMA3A (1–3). Therefore, plexins also need to be studied to understand the growth suppression and apoptosis induced in lung cancer by SEMA3B.
SEMA3F is implicated also as a TSG by functional studies in mouse A9 cells and loss of expression or mislocalization in lung cancers, the latter correlating with high levels of lung tumor VEGF staining (9–11, ‡‡). Thus, although we did not find an in vitro growth-suppressing effect of SEMA3F, it is conceivable that both semaphorins play a role in tumor suppression in vivo. One model to consider is that the loss of SEMA3F function plays a role in loss of cell adhesion and altered motility response to tumor-produced VEGF, whereas SEMA3B induces apoptosis possibly also related to competition with tumor-produced VEGF, which in this case would be acting as a tumor-survival or growth factor.
Several other reports have noted correlations between semaphorins and growth control or cancer. In Caenorhabditis elegans, Sema2a as a null mutant led to errant epidermal cell migrations and affected epidermal enclosure of the embryo (30). In contrast to the tumor-suppressing function of SEMA3B, SEMA3C and SEMA3E are overexpressed in metastatic human and mouse tumors, and SEMA3C led to cancer therapy drug resistance (31–33). Thus, abnormalities of semaphorin family genes may play a cooperative role in carcinogenesis analogous to the role these genes play in the developing nervous system.
In summary, we have provided evidence that SEMA3B is a potent lung cancer growth suppressor, acting through apoptosis pathways, that is epigenetically inactivated in human lung cancer. The signaling pathway(s) mediating lung cancer cell growth suppression and apoptosis after the reexpression of SEMA3B is unclear, and indirect mechanisms must be considered. Further studies will be necessary to elucidate the mechanism of suppressive effect of SEMA3B in human tumors and to determine whether the neighboring homologue SEMA3F can function as a tumor suppressor in vivo.
Acknowledgments
We thank A. Kolodkin for advice and neuropilin antibodies. This work is supported by National Cancer Institute grants CA71618 and Lung Cancer SPORE P50 CA70907 and the G. Harold and Leila Y. Mathers Charitable Foundation. M.I.L. was funded by National Cancer Institute Contract N01-CO-56000. We are grateful for encouragement by Professor Masatomo Mori, First Department of Internal Medicine, Gunma University, School of Medicine, Japan.
Abbreviations
- NP
neuropilin receptor
- TSG
tumor suppressor gene
- SCLC
small cell lung cancer
- NSCLC
non-SCLC
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- VEGF
vascular endothelial growth factor
Footnotes
Nalor, S., Davalos, A., Hense, C. & Xiang, R. (1998) Am. J. Hum. Genet. 63, abstr. 431, 80.
References
- 1.Nakamura F, Kalb R G, Strittmatter S M. J Neurobiol. 2000;44:219–229. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Raper J A. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 3.Tamagnone L, Comoglio P M. Trends Cell Biol. 2000;10:377–383. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- 4.Miao H Q, Soker S, Feiner L, Alonso J L, Raper J A, Klagsbrun M. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito T, Kagoshima M, Sasaki Y, Li C, Udaka N, Kitsukawa T, Fujisawa H, Taniguchi M, Yagi T, Kitamura H, Goshima Y. Mech Dev. 2000;97:35–45. doi: 10.1016/s0925-4773(00)00401-9. [DOI] [PubMed] [Google Scholar]
- 6.Wistuba I I, Behrens C, Virmani A K, Mele G, Milchgrub S, Girard L, Fondon J W, III, Garner H R, McKay B, Latif F, et al. Cancer Res. 2000;60:1949–1960. [PubMed] [Google Scholar]
- 7.Lerman M, Minna J. Cancer Res. 2000;60:6116–6133. [PubMed] [Google Scholar]
- 8.Sekido Y, Bader S, Latif F, Chen J-Y, Duh F-M, Wei M-H, Albanesi J P, Lee C-C, Lerman M I, Minna J D. Proc Natl Acad Sci USA. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang R H, Hensel C H, Garcia D K, Carlson H C, Kok K, Daly M C, Kerbacher K, van den Berg A, Veldhuis P, Buys C H, Naylor S L. Genomics. 1996;32:39–48. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla E, Constantin B, Drabkin H, Roche J. Am J Pathol. 2000;156:939–950. doi: 10.1016/S0002-9440(10)64962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd M C, Xiang R H, Garcia D K, Kerbacher K E, Moore S L, Hensel C H, Liu P, Siciliano M J, Kok K, van den Berg A, et al. Oncogene. 1996;13:2387–2396. [PubMed] [Google Scholar]
- 12.Baylin S B, Herman J G, Graff J R, Vertino P M, Issa J P. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 13.Dammann R, Li C, Yoon J H, Chin P L, Bates S, Pfeifer G P. Nat Genet. 2000;25:315–319. doi: 10.1038/77083. [DOI] [PubMed] [Google Scholar]
- 14.Burbee D G, Forgacs E, Zöchbaüer-Müller S, Shivakumar L, Fong K, Gao B, Randle D, Kondo M, Virmani A, Bader S, et al. J Natl Cancer Inst. 2001;93:691–699. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark S J, Harrison J, Paul C L, Frommer M. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto H, Mori M, Taira M, Yoshida T, Matsukawa S, Shimizu K, Sekiguchi M, Terada M, Sugimura T. Proc Natl Acad Sci USA. 1986;83:3997–4001. doi: 10.1073/pnas.83.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phelps R M, Johnson B E, Ihde D C, Gazdar A F, Carbone D P, McClintock P R, Linnoila R I, Matthews M J, Bunn P A, Jr, Carney D, Minna J D, Mulshine J L. J Cell Biochem Suppl. 1996;24:32–91. doi: 10.1002/jcb.240630505. [DOI] [PubMed] [Google Scholar]
- 18.Giger R J, Urquhart E R, Gillespie S K, Levengood D V, Ginty D D, Kolodkin A L. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- 19.Tamagnone L, Artigiani S, Chen H, He Z, Ming G I, Song H, Chedotal A, Winberg M L, Goodman C S, Poo M, et al. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 20.Comoglio P M, Tamagnone L, Boccaccio C. Exp Cell Res. 1999;253:88–99. doi: 10.1006/excr.1999.4684. [DOI] [PubMed] [Google Scholar]
- 21.Chen J Y, Funk W D, Wright W E, Shay J W, Minna J D. Oncogene. 1993;8:2159–2166. [PubMed] [Google Scholar]
- 22.Zöchbauer-Müller S, Fong K, Virmani A, Geradts J, Gazdar A, Minna J. Cancer Res. 2001;61:249–255. [PubMed] [Google Scholar]
- 23.Knudson A G., Jr Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirvan A, Ziv I, Fleminger G, Shina R, He Z, Brudo I, Melamed E, Barzilai A. J Neurochem. 1999;73:961–971. doi: 10.1046/j.1471-4159.1999.0730961.x. [DOI] [PubMed] [Google Scholar]
- 25.Gagliardini V, Fankhauser C. Mol Cell Neurosci. 1999;14:301–316. doi: 10.1006/mcne.1999.0787. [DOI] [PubMed] [Google Scholar]
- 26.Behar O, Mizuno K, Badminton M, Woolf C J. Proc Natl Acad Sci USA. 1999;96:13501–13505. doi: 10.1073/pnas.96.23.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachelder R E, Crago A, Chung J, Wendt M A, Shaw L M, Robinson G, Mercurio A M. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 28.Miao H Q, Lee P, Lin H, Soker S, Klagsbrun M. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 29.Gagnon M L, Bielenberg D R, Gechtman Z, Miao H Q, Takashima S, Soker S, Klagsbrun M. Proc Natl Acad Sci USA. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. . (First Published February 25, 2000; 10.1073/pnas.040337597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy P J, Zheng H, Warren C E, Culotti J G. Development (Cambridge, UK) 2000;127:755–767. doi: 10.1242/dev.127.4.755. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Satue M, Blanco J. J Surg Oncol. 1999;72:18–23. doi: 10.1002/(sici)1096-9098(199909)72:1<18::aid-jso5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T, Endo R, Gotoh M, Hirohashi S. Proc Natl Acad Sci USA. 1997;94:14713–14718. doi: 10.1073/pnas.94.26.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen C R, Klingelhofer J, Tarabykina S, Hulgaard E F, Kramerov D, Lukanidin E. Cancer Res. 1998;58:1238–1244. [PubMed] [Google Scholar]