Abstract
The CFTR gene encodes a transmembrane conductance regulator, which is dysfunctional in patients with cystic fibrosis (CF). The mechanism by which defective CFTR (CF transmembrane conductance regulator) leads to undersialylation of plasma membrane glycoconjugates, which in turn promote lung pathology and colonization with Pseudomonas aeruginosa causing lethal bacterial infections in CF, is not known. Here we show by ratiometric imaging with lumenally exposed pH-sensitive green fluorescent protein that dysfunctional CFTR leads to hyperacidification of the trans-Golgi network (TGN) in CF lung epithelial cells. The hyperacidification of TGN, glycosylation defect of plasma membrane glycoconjugates, and increased P. aeruginosa adherence were corrected by incubating CF respiratory epithelial cells with weak bases. Studies with pharmacological agents indicated a role for sodium conductance, modulated by CFTR regulatory function, in determining the pH of TGN. These studies demonstrate the molecular basis for defective glycosylation of lung epithelial cells and bacterial pathogenesis in CF, and suggest a cure by normalizing the pH of intracellular compartments.
Keywords: CFTR‖Pseudomonas aeruginosa‖pH-sensitive GFP‖sialyltransferases
Cystic fibrosis (CF) is the most common lethal disorder in Caucasians (1). The high morbidity and mortality in CF is due to chronic respiratory infections, culminating with chronic colonization with the bacterium Pseudomonas aeruginosa (2). CF is caused by mutations in the gene encoding a protein termed cystic fibrosis transmembrane conductance regulator (CFTR). The exact connection between CFTR mutations, their physiological effects, and P. aeruginosa infections has not been fully established, as reflected in the multitude of proposals explaining the predilection to bacterial infection in CF (3–16).
The disease-causing CFTR mutations affect the processing, intracellular localization, and activity of this protein (1). CFTR functions as a chloride channel (1), but also has pleiotropic effects on the activity of other ion transporters including the amiloride-sensitive epithelial sodium channel (ENaC; refs. 17 and 18). In attempts to explain the lung pathology in CF, several models have been proposed. It has been reported that defensins are impaired by high salt in CF lung secretions (4). However, the electrolyte composition of the airway surface fluid may not to differ between normal and CF lungs (6, 19). In contrast to the high-salt hypothesis, a low-volume proposal has been put forward in which uninhibited ENaC leads to hyperabsorption of sodium and water, leading to low airway surface liquid volume, which in turn impedes mucociliary clearance (7). However, mucociliary clearance alone also cannot explain the predilection for P. aeruginosa, as there are substantial differences between CF and diseases such as primary ciliary dyskinesia (8). Thus, the high-salt or low-volume proposals remain controversial (9). In a separate line of study, reduced inducible nitric oxide synthase (iNOS) production and NO output have been suggested to play a role in susceptibility to P. aeruginosa (10). However, infection models in iNOS transgenic mice support only a limited role for NO in innate defenses against P. aeruginosa (11). Recently, it has been proposed (14) that CFTR itself acts as a receptor for P. aeruginosa in bacterial uptake by the epithelial cells. If one assumes that epithelial uptake of the bacterium could be a part of the clearance process, the lack of CFTR could then translate into less efficient elimination of P. aeruginosa. However, a role for bacterial uptake by respiratory epithelial cells remains to be established.
It has been demonstrated that glycoproteins and glycolipids on the plasma membrane of CF cells display altered sialylation, and that undersialylated glycoconjugates act as adhesion receptors for bacterial pathogens in CF (15, 16). Because adhesion of a pathogen to host tissues occurs at the very initial stages of infection, the increased bacterial association with CF respiratory epithelial cells most likely represents a critical point in the colonization of the respiratory tract in CF. As the major CF pathogens P. aeruginosa and Staphylococcus aureus preferentially adhere to unsialylated glycoconjugates such as aGM1 (16, 20), it is possible that the undersialylation of surface molecules on CF epithelial cells promotes bacterial colonization (15, 21). However, the connection between CFTR defect and altered sialylation remains elusive (22). In one model, it has been proposed that CFTR plays a role in facilitating acidification of intracellular compartments, such as the trans-Golgi network (TGN), by allowing entry of anions (Cl−), thus maintaining charge neutrality as protons are pumped into the lumen of these organelles (3). According to this proposal, a loss of CFTR and chloride conductance would result in an increased pH of TGN (3). This model could have explained reduced sialylation of glycoconjugates, as sialyltransferase would not be fully active under suboptimal pH conditions in the TGN of CF cells. However, subsequent studies have indicated that TGN may not be alkalinized in CF (23, 24). Prompted by these conflicting observations and seeking to understand the glycosylation defect in CF, we hypothesized that TGN in CFTR mutant cells may still have abnormal pH, but on the acidic side. Here we present data that TGN is indeed hyperacidified in the CF lung epithelial cells, and that correction of hyperacidification can reverse the undersialylation defect in CF and reduce P. aeruginosa adhesion, thus linking the defect in CFTR and lung pathology via abnormally low organellar pH, and suggest a relatively simple cure by normalizing pH homeostasis in CF lung epithelia.
Materials and Methods
Cells and Constructs.
IB3–1 is a human bronchial epithelial cell line derived from a patient with CF with a ΔF508/W1282X CFTR mutant genotype (25). C38 and S9 are stably transfected derivatives of IB3–1 cells corrected for chloride conductance by introduction of a functional CFTR (26). The physiological levels of expression of CFTR and its functionality have been established for C38 cells (26). CFT1 (27, 28) is derived from the tracheal epithelium of a patient with CF homozygous for the CFTR ΔF508 mutation. Stably transfected derivatives of CFT1 included CFT1-LCFSN, expressing the wild-type CFTR gene, CFT1-Δ508, transfected with ΔF508 mutant CFTR gene, and CFT1-LC3, the vector-transfected control cells. TGN38-pHluorin green fluorescent protein (GFP) and glycosylphosphatidylinositol (GPI)-pHluorin GFP DNA constructs were from J. Rothman (29). The expression construct for α2,6-sialyltransferase (EC 2.4.99.1) Sttyr isoform carrying a c-myc tag at the C terminus was from K. Colley (30).
Fluorescence Microscopy, pH Measurements Using Ratiometric pH-Sensitive GFP, and Lectin-Binding Studies.
Immunofluorescence localization studies, pH determination by the ratio of fluorescence at 508 nm on excitation at 410 vs. 470 nm (29), and lectin-binding studies (22, 31) are described in Supporting Information, which is published on the PNAS web site, www.pnas.org.
Inhibition Studies and pH Normalization.
For H+-ATPase inhibition, TGN38-pHluorin GFP-transfected cells were incubated with 100 nM bafilomycin A1 (Sigma) in buffer A (25 mM Hepes, pH 7.4/119 mM NaCl/2.5 mM KCl/2 mM CaCl2/2 mM MgCl2/30 mM glucose) for 2.5 h at 37°C. For Na+/K+-ATPase inhibition, TGN38-pHluorin GFP-transfected cells were treated with 1 μM acetylstrophanthidin (Sigma). For pH normalization, cells were grown for 48 h in complete LHC-8 media in the presence of 0.1–1.0 mM NH4Cl, chloroquine, or lansoprazole (Sigma). For enhancement of CFTR folding and trafficking, cells were grown for 48 h in the presence of 4-phenylbutyric acid (4-PBA; gift from Triple Crown America, Perkasie, PA).
Bacterial Adhesion Assay.
IB3–1 cells were seeded onto glass slides and incubated for 24 h with 10 mCi [3H]leucine per ml, followed by 48 h of 0.1 mM NH4Cl treatment, as indicated. P. aeruginosa PAO1 were labeled for 80 min with [35S]methionine and used at a multiplicity of infection of 20. Slides with monolayers were inverted onto drops with P. aeruginosa (2 × 107 bacteria per slide). Adhesion was determined by counting 3H and 35S radioactivity.
Results
Hyperacidification of Trans-Golgi Network in CF Cells.
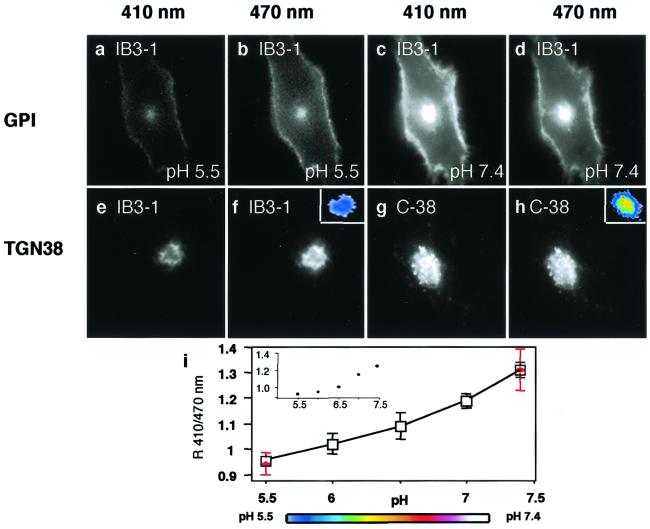
For determination of organellar pH in live bronchial epithelial cells, we used the recently developed pH-sensitive GFP (pHluorin GFP) system for ratiometric determination of the luminal pH in intracellular organelles (29). Two fusion constructs, TGN38-pHluorin GFP, with lumenally exposed pHluorin GFP, and GPI-pHluorin GFP, with the pHluorin GFP exposed on the plasma membrane to the extracellular fluid, were used (29). The TGN38- and plasma membrane-targeted pHluorin GFP probes were transfected into the well characterized human bronchial epithelial cells IB3–1, C38, and S9 (25, 26), which have been used as standard cell lines to model the effects of CFTR mutations (25, 26, 32). IB3–1 is derived from a patient with CF with a ΔF508/W1282X CFTR mutant genotype (25). C38 and S9 are stably transfected and fully characterized derivatives of IB3–1 cells corrected for chloride conductance by introduction of a functional CFTR gene (26). C38 cells contain a functional CFTR with a fortuitous deletion in the first extracellular loop, whereas S9 cells have been corrected with a full-size complete CFTR gene. Fig. 1 a–d displays the fluorescence appearance of GPI-pHluorin GFP at pH 7.4 and pH 5.5. A standard curve was generated within the working range of pHluorin GFP (pH 7.4–5.5; Fig. 1i; ref. 29) by using IB3–1, C38, and S9 cells. All cells showed identical fluorescence dependence of the GPI-pHluorin GFP on pH of the external buffer and did not differ between transfections. In addition, TGN38-pHluorin GFP-transfected cells were treated at the end of each experiment with monensin and nigericin to generate internal standards for pH calibration (Fig. 1i, red points).
Figure 1.
Fluorescence images of live CF and CFTR-corrected human bronchial epithelial cells expressing GPI and TGN38 fused to pH-sensitive GFP. Cells were excited at 410 or 470 nm as indicated above each column, and fluorescence emission at 508 nm was recorded by fluorescence microscopy. (a–d) IB3–1 transfected with GPI-pHluorin GFP at indicated pH applied externally. (e–h) TGN38-GFP pHluorin transfected CF IB3–1 cells (e and f) and CFTR-corrected C38 (g and h). The external buffer had a pH of 7.4. (Color Insets) pH values according to the color look-up table in i. (i) pH calibration curve was generated two ways: by using GPI-pHluorin GFP and changing the pH of the externally applied buffer, and by generating internal standards (red). The internal standards were obtained with TGN38-pHluorin after data collection by treating cells with 10 μM monensin and 10 μM nigericin at the end of the experiment and changing the pH of the external buffer. The data points are normalized to match the pH of the ratio obtained by using GPI-pHluorin GFP as external standard at 7.4. Dots represent an example of individual data sets.
The pH of TGN was probed with TGN38-pHluorin GFP (29). Fig. 1 e–h illustrates fluorescence of TGN38-pHluorin GFP transfected IB3–1 and C38 cells on excitation at 410 nm vs. 470 nm. The apparent pH of TGN38-pHluorin GFP compartment in C38 cells was 6.6 ± 0.1 (mean ± SE, n = 18) and in S9 cells was 6.7 ± 0.1 (mean ± SE, n = 24), whereas the corresponding apparent pH in IB3–1 CFTR mutant cells was 6.0 ± 0.1 (mean ± SE, n = 17; Table 1). Thus, the internal pH values of the TGN in IB3–1 cells were 0.7 and 0.6 pH units lower than in C38 and S9 cells, respectively (P = 0.0001), indicating that the TGN is hyperacidified in CF bronchial respiratory epithelial cells. In addition, confluent cells also displayed hyperacidification in IB3–1 CFTR mutant cells (pH 6.3 ± 0.0, mean ± range) compared with S9 CFTR-corrected cells (pH 6.7 ± 0.0, mean ± range). Furthermore, treatment of IB3–1 cells with 4-phenylbutyric acid (4-PBA), a chemical chaperone which restores Δ508-CFTR folding and normalizes its intracellular trafficking to the plasma membrane (33), corrected the hyperacidification of IB3–1 mutant cells to the levels matching those in CFTR-transfected C38 cells (4-PBA-treated IB3–1 vs. C38, P = 0.9796; Fig. 2a).
Table 1.
Hyperacidification of TGN in CF
| Cell line | TGN pH |
|---|---|
| IB3-1 (mutant) | 6.0 ± 0.1 (n = 17)* |
| C38 (corrected) | 6.6 ± 0.1 (n = 18) |
| S9 (corrected) | 6.7 ± 0.1 (n = 24) |
| CFT1 (mutant) | 6.2 ± 0.1 (n = 10)* |
| CFT1-LCFSN (corrected) | 6.7 ± 0.1 (n = 9) |
| CFT1-LC3 (mutant) | 6.3 ± 0.1 (n = 10) |
| CFT1-ΔF508 (mutant) | 6.2 ± 0.1 (n = 19) |
IB3-1 is a human bronchial cell line derived from a patient with a ΔF508/W1282X CFTR (25). C38 cells are IB3-1 cells corrected with a functional CFTR containing a fortuitous N-terminus alteration. S9 cells express a functional full-size CFTR (26). CFT1 (27) is a tracheal cell line derived from a ΔF508/ΔF508 patient with CF. CFT1-LCFSN (wild-type CFTR), CFT1-Δ508 (Δ508 CFTR), and CFT1-LC3 (vector control) are stably transfected derivatives of CFT1. Note that CFTR mutant cells have close to 0.5 units lower pH in their TGN than the CFTR-corrected cells.
, P = 0.0001.
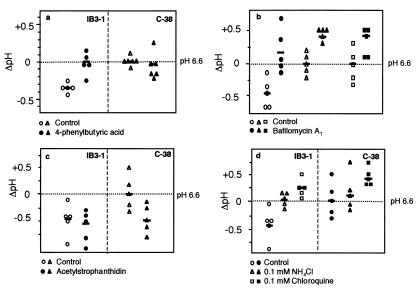
Figure 2.
Sodium-dependent hyperacidification of TGN in CF. Changes in pH (ΔpH) in IB3–1 (circles), C38 (triangles), and S9 (squares) cells expressing TGN38-pHluorin GFP (TGN38) were determined in five distinct cells for each treatment as indicated [Bar = mean value.] (a) Effect of 48-h treatment with 2.5 mM 4-phenylbutyric acid (4-PBA) (filled symbols) (control vs. treated): P = 0.005 (IB3–1), P = 0.629 (C38), and IB3–1-treated vs. C38 control P = 0.9796. (b) effect of bafilomycin A (filled symbols) (control vs. treated): P ≤ 0.0001 (IB3–1), P = 0.0029 (C38), P = 0.0087 (S9). (c) Effect of acetylstrophanthidin (control vs. treated): P = 0.4508 (IB3–1), P = 0.0111 (C38). (d) Effect of 48-h treatment with 0.1 mM ammonia and chloroquine on normalization of TGN pH (○, ▵, and □ = IB3–1 cells; and ●, ▴, and ■ = C38) (control vs. treatment). IB3–1 ammonia (P = 0.0269), IB3–1 chloroquine (P = 0.0004), C38 ammonia (P = 0.5873), and C38 chloroquine (P = 0.0172).
The observation that TGN is hyperacidified in CF cells was confirmed by using another well characterized CF cell line, CFT1 (27, 28), derived from the tracheal epithelium of a patient with CF homozygous for the ΔF508 mutation in CFTR. CFT1 and its stably transfected derivatives, CFT1-LCFSN, expressing the wild-type CFTR cDNA, CFT1-Δ508, expressing the ΔF508 mutant CFTR cDNA, and CFT1-LC3, serving as a vector control, were transiently transfected with TGN38-pHluorin GFP. The CFTR-corrected variant CFT1-LCFSN had an apparent pH of 6.7 ± 0.1 in TGN compared with the corresponding values in CFTR-mutant cells of 6.3 ± 0.1 for CFT1 (parental ΔF508/ΔF508 cell line), 6.2 ± 0.1 for CFT1-Δ508 (ΔF508 CFTR-transfected control), and 6.3 ± 0.1 for CFT1-LC3 (vector-transfected control; Table 1). Thus, the TGN38 showed hyperacidification of 0.4–0.5 pH units in CFTR-mutant tracheal epithelial cells.
Hyperacidification of TGN in CF Cells Is Affected by Inhibitors of H+ and Na+/K+ ATPases.
In contrast to previous models (3) relying on the chloride channel activity of CFTR, reduced Cl− influx into the organelle cannot readily explain the TGN hyperacidification in CF cells observed in our experiments. Instead, we considered an alternative model in which Na+ efflux from the organelle with a net effect similar to the influx of Cl− could play a role in luminal charge neutralization. In CF bronchial epithelial cells, sodium transport is under negative regulation by CFTR. In the absence of CFTR, sodium channels are relieved from CFTR inhibition, leading to an increase in Na+ transport (17, 18, 34, 35). To test the model in which altered sodium transport could play a role in TGN hyperacidification by means of effects on H+-ATPase activity in CF, we first examined whether H+-ATPase played a role in acidification of TGN in CF cells. Treatment with bafilomycin A1 abrogated hyperacidification of TGN in CFTR mutant cells (Fig. 2b). Treatment of cells with the Na+/K+-ATPase inhibitor acetylstrophanthidin reduced the pH of CFTR-corrected cells to the levels in CF cells (P = 0.0111). In contrast, and as predicted by the proposal of increased Na+ efflux in the absence of CFTR, acetylstrophanthidin had no effect on TGN acidification in CFTR mutant cells (P = 0.5170; Fig. 2c). These observations are consistent with a model in which Na+/K+-ATPase is a major source of luminal Na+ that causes differential TGN acidification in normal and CF cells.
Colocalization of TGN38-pHluorin GFP and Sialyltransferase and Correction of Undersialylation Defect and P. aeruginosa Adhesion with Weak Bases in CF Cells.
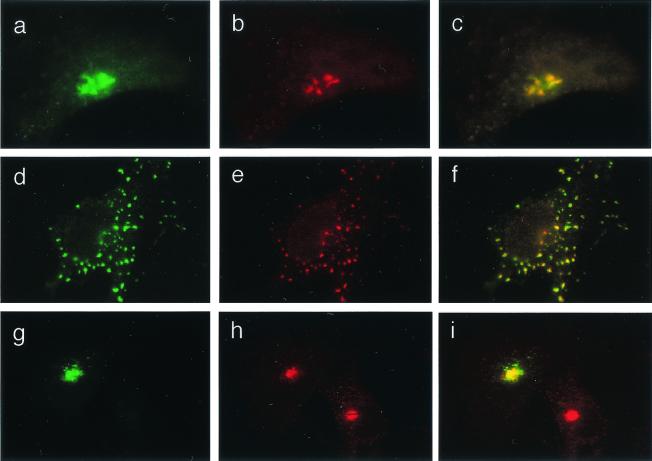
The increased acidification of TGN in CF cells may have significant consequences on glycosylation patterns. Similar to the alkalinization hypothesis (3), a hyperacidified TGN can lead to altered protein and lipid glycosylation, manifested as decreased sialylation, by generating a suboptimal pH for sialyltransferases. To address this possibility, we first determined whether the TGN38-pHluorin GFP pH probe was present in a compartment relevant for sialyltransferases. The colocalization studies of TGN38-pHluorin GFP and myc-tagged α-2,6-sialyltransferase Sttyr (30) in cotransfected cells showed identical overall organellar distribution and colocalization of the two markers (Fig. 3 a–c). Treatment with nocodazole disrupted the TGN, but TGN38 and myc-tagged α-2,6-sialyltransferase Sttyr colocalization was not affected (Fig. 3 d–f). The results of these experiments demonstrated a nearly complete overlap of our pH-sensitive probe and sialyltransferase in CF- and CFTR-corrected cells. Colocalization of TGN38-pHluorin GFP with the TGN SNARE syntaxin-6 further confirmed that the pH-sensitive GPF probe localized properly in the TGN (Fig. 3 g–i).
Figure 3.
Colocalization of TGN38-pHluorin GFP with TGN markers. Bronchial epithelial cells were cotransfected with TGN38-pHluorin GFP and myc-tagged α2,6-sialyltransferase Sttyr. (a–c) IB3–1 cell. (a) GFP fluorescence. (b) Immunofluorescent visualization of myc-tagged α2,6-sialyltransferase Sttyr by using anti-myc Ab and secondary Alexa 568-conjugated Ab (red fluorescence). (c) Merged images a and b. (d–f) IB3–1 cell treated as in a–c with the addition of 20 μg/ml of nocodazole for 1 h. (d) GFP fluorescence as in a. (e) Myc-tagged α2,6-sialyltransferase Sttyr (red fluorescence). (f) Merged images d and e. (g–i) TGN-38-pHluorin GFP colocalization with human syntaxin-6 in IB3–1 cell. (g) GFP fluorescence. (h) Immunofluorescent visualization of anti-human syntaxin-6 (primary Ab) by using secondary Alexa 568-conjugated Ab (red fluorescence). (i) Merged images g and h. Appearance of C38 and S9 cell was identical to IB3–1 cells.
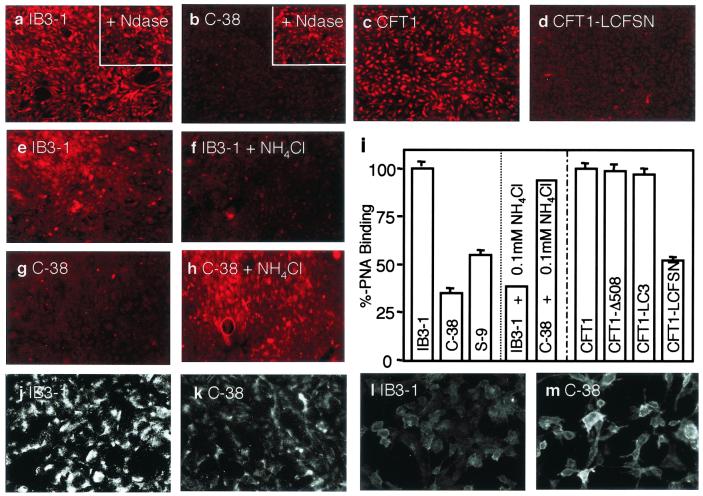
Next we examined sialylation of surface glycoconjugates. Fluorescently labeled peanut agglutinin (PNA), which recognizes unsialylated galactosyl (β-1,3) N-acetylgalactosamine, bound better to IB3–1 (100 ± 3%, mean ± SE, n = 30), CFT1 (100 ± 4%, mean ± SE, n = 30), CFT1-Δ508 (99 ± 3%, mean ± SE, n = 30), and CFT1-LC3 (97 ± 4%, mean ± SE, n = 30) cells than to the CFTR-corrected C38 (35 ± 3%, mean ± SE, n = 30), S9 (55 ± 4%, mean ± SE, n = 30), or CFT1-LCFSN (52 ± 3%, mean ± SE, n = 30) cells (P = 0.0001, CFTR mutants vs. CFTR-corrected cells) (Fig. 4 a–d and i) in a process that was neuraminindase-sensitive (Fig. 4 a and b Insets). The decreased sialylation of glycoconjugates on CF cells was also confirmed by anti-aGM1 Ab, which binds to aGM1 (Fig. 4 j and k), and reverse staining with fluorescently labeled cholera toxin B subunit, which binds sialylated GM1 (Fig. 4 l and m).
Figure 4.
Treatment with weak base corrects undersialylation of membrane glycoconjugates in CF. Tetramethylrhodamine B isothiocyanate (TRITC)-labeled PNA [binding preferentially to nonsialylated galactosyl (β-1,3) N-acetylgalactosamine residues] was incubated with respiratory epithelial cell monolayers, fluorescence images were captured, and intensity was quantitated. (a) IB3–1 (mutant). (b) C38 (corrected). Insets a and b show corresponding cells treated with neuraminidase type X (Ndase). (c) CFT1 (mutant). (d) CFT1-LCFSN (corrected). (e) IB3–1. (f) IB3–1 cells treated with 0.1 mM NH4Cl. (g) C38. (h) C38 cells treated with 0.1 mM NH4Cl. (i) Bar graph showing %-binding (converted from relative fluorescence intensity) calculated from 30 individual cells (IB3–1 and CFT1 mutant cells as 100% binding) (P = 0.0001, CFTR mutants vs. CFTR-corrected cells, IB3–1 controls vs. IB3–1 0.1 mM NH4Cl-treated, and C38 controls vs. C38 0.1 mM NH4Cl-treated). Decreased sialylation of glycoconjugates on CF cells was also confirmed by anti-aGM1 Ab, which binds to aGM1, (j) IB3–1, (k) C38, and reverse staining with fluorescently labeled cholera toxin B subunit, which binds sialylated GM1 (l) IB3–1 and (m) C38.
If hyperacidification of TGN is responsible or contributing to the undersialylation of glycoconjugates in CF, we reasoned that normalization of pH using membrane-permeant weak bases (e.g., ammonia) may correct this defect. We titrated ammonia concentrations and found that when IB3–1 cells were treated for 48 h with low concentrations of ammonium chloride (0.1 mM), the pH was corrected and matched the normal levels in CFTR-corrected C38 cells (ammonia-treated IB3–1 vs. C38, P = 0.7440; Fig. 2d). Furthermore, treatment of IB3–1 cells for 48 h with another weak base, chloroquine, also corrected the pH of TGN to normal levels (chloroquine-treated IB3–1 vs. C38, P = 0.1066; Fig. 2d). In contrast, treatment of IB3–1 cells with the gastric proton pump inhibitor lansoprazole did not have an effect on the pH of TGN (P = 0.7976).
Treatment for 48 h with ammonia at concentrations repairing the hyperacidification defect (i.e., 0.1 mM) also restored normal patterns of lectin binding in IB3–1 CFTR mutant cells, similar to those seen in CFTR-corrected cells (Fig. 4 e, f, and i). The levels of PNA binding in NH4Cl-treated IB3–1 cells were equal to those in CFTR-corrected C38 cells. Although the changes in pH on alkalinization of C38 cells were not as pronounced as in IB3–1 cells, treatment of C38 cells with 0.1 mM NH4Cl increased PNA binding, indicating a reduced sialylation in normal cells. These observations are consistent with an existence of a pH optimum for sialyltransferase activity (36) or its microlocalization. The levels of PNA binding in ammonia-treated C38 cells were similar to the levels seen in untreated and aberrantly undersialylated IB3–1 cells. These observations are in keeping with a requirement for an optimal pH in TGN in order for sialyltransferases to work properly, as noted by others (3). However, our findings differ in an essential way from the previous proposals, as we find that the TGN is hyperacidified rather than alkalinized in CF. Our experiments show that this optimum can be disturbed by conditions that are either too acidic (as in CF cells) or too alkaline (normal cells treated with ammonia) (Fig. 4 g–i). Furthermore, our findings indicate that pH correction of TGN in CFTR mutant cells can lead to a restoration of normal sialylation.
Correction of the sialylation defect should also lead to a reduction of bacterial adherence, because it has been shown that P. aeruginosa preferentially binds to undersialylated glycoconjugates (3, 15, 20, 22). We therefore treated IB3–1 cells with 0.1 mM NH4Cl for 48 h, before a 1-h incubation with P. aeruginosa. In keeping with the correction of the sialylation, P. aeruginosa adhered less to the 0.1 mM NH4Cl-treated cells (sialylation-corrected) compared with the untreated cells (P = 0.0433; Table 2).
Table 2.
Reduced adhesion of P. aeruginosa to IB3-1 CFTR mutant cells on normalization of TGN pH
| Cell line and treatment | % P. aeruginosa adhesion |
|---|---|
| IB3-1 control | 100 ± 7 |
| IB3-1 + 0.1 mM NH4Cl | 81 ± 5 |
IB3-1 cells (labeled with [3H]leucine) were treated with 0.1 mM NH4Cl for 48 h. P. aeruginosa (labeled with [35S]methionine) was added at a multiplicity of infection of 20. After a 1-h incubation, adhesion was quantitated based on 3H and 35S radioactivity. IB3-1 control (n = 6) vs. IB3-1 + 0.1 mM NH4Cl (n = 7). P = 0.0433.
Discussion
The application of pH-sensitive ratiometric GFP technology (29) and targeting of the pH-sensitive GFP to the TGN lumen in live cells permitted us to detect hyperacidification of this compartment in CF cells. Our work was inspired by the previous models where alkalinization of this normally mildly acidified organelle was predicted in CF on the basis of CFTR-dependent chloride conductance (3). It is important to note that the proposed action of CFTR as a chloride channel, with chloride ions acting to maintain electroneutrality by compensating for the positive luminal charge generated by protons pumped by the H+-ATPase (3), is not compatible with our observations. Instead, regulatory functions of CFTR, e.g., CFTR-dependent inhibition of sodium transport in human respiratory epithelial cells (17, 34, 35, 37), play a role. In our model, excess positive charge, caused by accumulation of H+ in the lumen of this organelle, may be compensated by Na+ efflux into the cytosol, thus dissipating the electrogenic charge differential (38) and allowing the development of a greater transmembrane pH gradient. If one considers the fact that the exit of Na+ is equivalent to the influx of Cl−, our data are consistent with the interpretation that it is the efflux of Na+ which plays the dominant role in relieving the proton pump from the inhibition associated with the membrane potential build up. In normal cells, inactive sodium channel and active Na+/K+-ATPase increase the interior positive membrane potential and thus counteract acidification. In CF cells, in the absence of CFTR-dependent inhibition (17, 18, 34, 35), the sodium channel is open, and Na+-efflux compensates for the H+-associated positive charge build up, thus neutralizing the membrane potential and facilitating excessive H+-ATPase action and TGN acidification.
Our observation that TGN compartments are hyperacidified in mutant CFTR IB3–1 and CFT1 cells may seem to be in variance with the published values reporting slight alkalinization of the TGN in CF nasal polyp cells compared with normal cells (ΔpH 0.3) (3). Others have reported that TGN in CFTR-transfected Swiss 3T3 cells is not alkalinized (23). The discrepancies between these studies and our findings may be explained (i) by differences in CFTR–sodium channel interactions, which can be either negative or positive, in a fashion that strongly depends on the cell type (17, 34, 35, 37); and (ii) by differences in these relationships in heterologous cell types investigated in the previous reports (e.g., fibroblasts or genetically unmatched epithelial cells). We emphasize that in the present work, human bronchial and tracheal epithelial cells and their genetically matched CFTR-rescued clones were compared.
The colocalization of sialyltransferases with TGN38-pHluorin GFP in IB3–1, C38, and S9 cells, observed in cotransfections with TGN38-pHluorin and myc-tagged α2,6-sialyltransferase Sttyr, indicates that our pH determinations were in a compartment relevant for sialyltransferase activity and thus could explain undersialylation of glycoconjugates in CF cells. The data with ammonia used to neutralize pH in acidic intracellular compartments indicate that hyperacidification is responsible for undersialylation of glycoconjugates in CF. Treatment of CF cells with a low concentration of ammonium chloride decreased binding of PNA to CF cells and P. aeruginosa adherence most likely by normalizing sialylation in IB3–1 CFTR mutant cells and reversing this CF defect. Although the effect on Pseudomonas adhesion may seem relatively modest, it was nevertheless reproducible and it is likely that it could improve with further optimization and use of additional drugs.
Others have attempted to address the problem of altered pH in CF by using ammonia (39). In such studies, addition of high concentrations (10 mM) of ammonium chloride did not correct the defect, but instead decreased sialylation and increased PNA binding to both normal and CF cells. These reports are nevertheless consistent with our observation that changes in sialylation are exquisitely pH-sensitive; the addition of even low concentrations of ammonium chloride to normal (CFTR-corrected C38) cells caused defective sialylation and increased PNA binding (Fig. 4), whereas a similar treatment of CFTR-mutant cells reversed the hyperacidification and undersialylation defects. Recently (40), it was shown that some clinical isolates of P. aeruginosa did not exhibit an enhanced binding to MDCK cells treated with aGM1 dissolved in DMSO. However, using the same model, P. aeruginosa strain PA103 was found to attach at higher levels to MDCK cells, thus supporting reported findings (3, 15, 20, 22, 41) while indicating strain variability. It is important to keep in mind that CF isolates are not best-suited to explore initial colonization stages, as these strains usually undergo massive mutations and other adaptation changes, making them better for modeling of chronic infection events that occur many months or years after the initial association and colonization (2). Moreover, although MDCK cells are an excellent model system for fundamental cell biology processes, the full validity of their use to extrapolate precise events involving highly specialized respiratory epithelial cells is not clear at present.
As demonstrated (3, 15, 20, 22), the decreased sialylation in CF leads to increased bacterial adhesion. The phenomena described here provide a physiological link between the CFTR defect via organellar hyperacidification and the downstream effects including respiratory pathogenesis in CF. The correction of the undersialylation defect and bacterial adherence by using low concentrations of weak bases has strong therapeutic implications as pH-normalizing drugs may prove beneficial in the treatment of CF.
Supplementary Material
Acknowledgments
We thank J. Rothman for pHluorin GFP constructs; P. Zeitlin for IB3–1, C38, and S9 cells; J. R. Yankaskas for CTF1 cells and derivatives; and K. Colley for myc-tagged α2,6-sialyltransferase expression clone. This work was supported by National Institutes of Health Grant AI31139 and by Cystic Fibrosis Foundation Grant 9680 (to V.D.). J.F.P. was a Cystic Fibrosis Foundation Fellow.
Abbreviations
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- GPI
glycosylphosphatidylinositol
- GFP
green fluorescent protein
- PNA
peanut agglutinin
- TGN
trans-Golgi network
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Schwiebert E M, Benos D J, Egan M E, Stutts M J, Guggino W B. Physiol Rev. 1999;79:S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- 2.Govan J R, Deretic V. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al-Awqati Q. Nature (London) 1991;352:70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- 4.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 5.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 6.Knowles M R, Robinson J M, Wood R E, Pue C A, Mentz W M, Wager G C, Gatzy J T, Boucher R C. J Clin Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui H, Grubb B R, Tarran R, Randell S H, Gatzy J T, Davis C W, Boucher R C. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 8.Levison H, Mindorff C M, Chao J, Turner J A, Sturgess J M, Stringer D A. Eur J Respir Dis Suppl. 1983;127:102–117. [PubMed] [Google Scholar]
- 9.Guggino W B. Cell. 1999;96:607–610. doi: 10.1016/s0092-8674(00)80570-x. [DOI] [PubMed] [Google Scholar]
- 10.Kelley T J, Drumm M L. J Clin Invest. 1998;102:1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H, Nasr S Z, Deretic V. Infect Immun. 2000;68:2142–2147. doi: 10.1128/iai.68.4.2142-2147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pier G B, Grout M, Zaidi T S. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pier G B. Proc Natl Acad Sci USA. 2000;97:8822–8828. doi: 10.1073/pnas.97.16.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imundo L, Barasch J, Prince A, Al-Awqati Q. Proc Natl Acad Sci USA. 1995;92:3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryan R, Kube D, Perez A, Davis P, Prince A. Am J Respir Cell Mol Biol. 1998;19:269–277. doi: 10.1165/ajrcmb.19.2.2889. [DOI] [PubMed] [Google Scholar]
- 17.Stutts M J, Canessa C M, Olsen J C, Hamrick M, Cohn J A, Rossier B C, Boucher R C. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber R, Hopf A, Mall M, Greger R, Kunzelmann K. Proc Natl Acad Sci USA. 1999;96:5310–5315. doi: 10.1073/pnas.96.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman A S. J Clin Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krivan H C, Roberts D D, Ginsburg V. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kube D, Adams L, Perez A, Davis P B. Am J Physiol. 2001;280:L482–L492. doi: 10.1152/ajplung.2001.280.3.L482. [DOI] [PubMed] [Google Scholar]
- 22.Dosanjh A, Lencer W, Brown D, Ausiello D A, Stow J L. Am J Physiol. 1994;266:C360–C366. doi: 10.1152/ajpcell.1994.266.2.C360. [DOI] [PubMed] [Google Scholar]
- 23.Seksek O, Biwersi J, Verkman A S. J Biol Chem. 1996;271:15542–15548. doi: 10.1074/jbc.271.26.15542. [DOI] [PubMed] [Google Scholar]
- 24.Gibson G A, Hill W G, Weisz O A. Am J Physiol. 2000;279:C1088–C1099. doi: 10.1152/ajpcell.2000.279.4.C1088. [DOI] [PubMed] [Google Scholar]
- 25.Zeitlin P L, Lu L, Rhim J, Cutting G, Stetten G, Kieffer K A, Craig R, Guggino W B. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 26.Egan M, Flotte T, Afione S, Solow R, Zeitlin P L, Carter B J, Guggino W B. Nature (London) 1992;358:581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- 27.Olsen J C, Johnson L G, Stutts M J, Sarkadi B, Yankaskas J R, Swanstrom R, Boucher R C. Hum Gene Ther. 1992;3:253–266. doi: 10.1089/hum.1992.3.3-253. [DOI] [PubMed] [Google Scholar]
- 28.Lee A, Chow D, Haus B, Tseng W, Evans D, Fleiszig S, Chandy G, Machen T. Am J Physiol. 1999;277:L204–L217. doi: 10.1152/ajplung.1999.277.1.L204. [DOI] [PubMed] [Google Scholar]
- 29.Miesenbock G, De Angelis D A, Rothman J E. Nature (London) 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Qian R, Rausa F M, 3rd, Colley K J. J Biol Chem. 1997;272:672–679. doi: 10.1074/jbc.272.1.672. [DOI] [PubMed] [Google Scholar]
- 31.Poschet J F, Boucher J C, Firoved A M, Deretic V. Methods Enzymol. 2001;336:65–76. doi: 10.1016/s0076-6879(01)36579-5. [DOI] [PubMed] [Google Scholar]
- 32.Schwiebert E M, Egan M E, Hwang T H, Fulmer S B, Allen S S, Cutting G R, Guggino W B. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 33.Rubenstein R C, Zeitlin P L. Am J Physiol. 2000;278:C259–C267. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- 34.Ismailov I I, Awayda M S, Jovov B, Berdiev B K, Fuller C M, Dedman J R, Kaetzel M, Benos D J. J Biol Chem. 1996;271:4725–4732. doi: 10.1074/jbc.271.9.4725. [DOI] [PubMed] [Google Scholar]
- 35.Stutts M J, Rossier B C, Boucher R C. J Biol Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- 36.Busam K, Decker K. Eur J Biochem. 1986;160:23–30. doi: 10.1111/j.1432-1033.1986.tb09934.x. [DOI] [PubMed] [Google Scholar]
- 37.Reddy M M, Light M J, Quinton P M. Nature (London) 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- 38.Zen K, Biwersi J, Periasamy N, Verkman A S. J Cell Biol. 1992;119:99–110. doi: 10.1083/jcb.119.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang X, Hill W G, Pilewski J M, Weisz O A. Am J Physiol. 1997;273:L913–L920. doi: 10.1152/ajplung.1997.273.5.L913. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder T H, Zaidi T, Pier G B. Infect Immun. 2001;69:719–729. doi: 10.1128/IAI.69.2.719-729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zar H, Saiman L, Quittell L, Prince A. J Pediatr. 1995;126:230–233. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.