Abstract
Chicken heterophils generate reactive oxygen species (ROS) molecules to defend against invading pathogens. The present study examined effects of quercetin on chicken heterophils. Heterophils were stimulated with PBS, 50 µM quercetin (QH), PMA or Escherichia coli (EC) and the resulting intracellular ROS molecules were determined. Flow cytometry results showed that cells stimulated with QH, PMA and EC had a higher ROS production. Increases in intracellular ROS molecules were identified in all treatment groups by fluorescence microscopy. Determination of the ability of quercetin to manipulate mRNA expression of ROS subunits was assessed using real-time RT-PCR. Quercetin and other stimulants up-regulated the majority of genes involved in ROS production: CYBB (NOX2), NCF1 (p47phox), NCF2 (p67phox), NOX1 and RAC2. The antioxidant property of QH was explored by measuring mRNA expression of CAT and SOD1. The data indicate increased levels of CAT with all treatments; however, only QH attenuated the expression of the SOD1 gene. To further investigate the effects of ROS-driven inflammation or cell death, IL6, CASP8 and MCL1 genes were preferentially tested. The inflammatory gene (IL6) was profoundly down-regulated in the QH- and PMA-treated groups while EC induced a strikingly high IL6 expression level. Investigation of the known apoptotic (CASP8) and anti-apoptotic (MCL1) genes found down-regulation of CASP8 in the QH- and PMA-treated groups which were contradicted to the MCL1 gene. In conclusion, quercetin can enhance ROS production by regulating the expression of genes involved in ROS production as well as in subsequent processes.
Keywords: chicken, gene expression, heterophil, quercetin, reactive oxygen species
Killing of pathogens in phagosomes by chicken heterophils is mediated by enzymatic granules harbored within that structure along with reactive oxygen species (ROS). In this regard, these cell types utilize the formation of intermediate oxygen (ROS) during the process of oxidative burst, such as superoxide (O2•-), hydrogen peroxide (H2O2), hydroxyl radicals (•OH) and hypochlorous acid (HOCl) [28]. Chicken heterophils exploit their ability to generate ROS by themselves; however, the amount generated is limited compared with the mammalian counterpart, neutrophils [5, 33]. The lack of functional myeloperoxidase (MPO) genes in the chicken genome prevents MPO production, a vital type of granule which contributes to the formation of HOCl from H2O2 to destroy bacteria [5, 33].
A group of multi-subunit enzymes named “the NOX family” or “NADPH oxidases” is the major source of ROS molecules in phagocytes, including chicken heterophils [28, 33, 48]. NOX protein subunits are composed primarily of p22phox, gp91phox, p40phox, p47phox and p67phox. These subunits interact with the small GTPase Rac1 or Rac2 to form competent protein complexes [16, 28, 43]. The proper stimuli, such as PMA, LPS or viable bacteria, allow the assembling of protein subunits found in the NOX family and aid in ROS generation [22]. Accumulating evidence indicates that ROS initiates key intracellular signals that dictate cellular responses to a variety of effector functions such as phagocytosis and extracellular traps (ETs) in neutrophils and heterophils [9, 25, 36, 47]. The downstream signaling pathways of ROS in heterophils are believed to mediate via protein kinase C (PKC), p38 MAP kinase, NF-κB, AP-1 and p53 [29, 45]. The subsequent events induce the expression of genes required in overall cellular functions in heterophils [3, 22, 41]. Intracellular antioxidants such as glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD), vitamin E, and vitamin C, are always functioning within the cells. These antioxidants help control the balance of free radicals in the body. When ROS production level increases in cells, the antioxidants awake and help stop the signal transduction induced by ROS molecules [44]. Hydrogen peroxide (H2O2), one of potent ROS molecules, will be dismutated by the action of catalase to produce harmless H2O and O2 molecules.
It has been proposed that the endogenous oxidant and anti-oxidant activities can be controlled by the action of some dietary flavonoids [23, 46]. Flavonoids, one of the most extensively studied plant compounds, are ubiquitous in foods including various types of vegetables, fruits, seeds, grains, and beverages including wine. Biological activities of flavonoids have been extensively reviewed in the literature, resulting ultimately in a proposed role in anti-oxidation [4, 18]. Of the flavonoids, quercetin is categorized within the flavonol group with myricetin and kaempherol [12, 18, 51]. Several lines of evidence indicate that quercetin possesses anti-inflammatory, anti-oxidant, antibacterial, antiviral, anti-hypertensive, anti-proliferative properties as well as other properties [4, 18]. Quercetin is also known to have beneficial roles in ROS scavenging [4]. In poultry, quercetin and its derivatives have been used to promote growth as well as to reduce heat stress in chickens [20, 37].
Although studies of quercetin in poultry production have been conducted, understanding of some aspects of chicken immunology remains very limited. In a previous study, we demonstrated the in vitro effects of quercetin on heterophil oxidative burst [36], but details of how intracellular ROS molecules of quercetin-treated heterophils can be manipulated is incompletely determined at the molecular level. The ROS gene expression patterns of quercetin in modulating ROS production in heterophils have not yet been fully elucidated. To further understanding of the immunomodulatory action of quercetin, this study investigated how quercetin modulates expression of NADPH oxidase (NOX) subunit genes, including CYBB (gp91phox), NCF1 (p47phox), NCF2 (p67phox), NOX1 and RAC2, in heterophils. We also determined the genes involved in anti-oxidants (CAT and SOD1). In addition, we investigated gene expression of CASP8, IL6 and MCL1 resulting from the ROS process including cell death and the inflammatory process.
MATERIALS AND METHODS
Chemicals purchased from Sigma-Aldrich, St. Louis, MO, U.S.A., included quercetin hydrate (QH), Hanks’ balanced salt solution (HBSS), Histopaque, Escherichia coli LPS O111: B4, Fluoroshield mounting medium with DAPI, and Trypan blue. Phorbol 12-myristate 13-acetate (PMA) was obtained from Calbiochem, EMD Millipore, Billerica, MA, U.S.A. Cell culture media RPMI 1640, Fetal bovine serum (FBS), fluorescent probe H2DCF-DA, and RNAlater were purchased from Life Technologies, Thermo Fisher Scientific, Waltham, MA, U.S.A.
Laboratory animals
Pathogen-free broiler chicks aged from 3 to 7 days (Gallus domesticus), n=20 chicks in each of 3 independent experiments were obtained from a local hatchery (Betagro Northern Agro Industries Ltd., Chiang Mai, Thailand). No statistical calculation was used to predetermine the appropriate sample size; rather, sample sizes were selected based on experience with variation within the immune system. The chicks were euthanized in order to collect heterophils from bone marrow. These animal experiments were approved by the Animal Care and Use Committee (FVM-ACUC) Ref. No. S7/2557.
Chicken heterophil isolation from bone marrow
Femurs and tibias were dissected, cleaned, and rinsed with sterile HBSS. Bone marrow from the bones was repeatedly flushed with HBSS supplemented with 1% FBS using a 21G needle attached to a 3-ml syringe until the solution came out clean [36]. The cell suspension culture was overlaid on a discontinuous gradient using Histopaque 1.077/1.119 as previously reported [10]. Cell viability was checked with 0.4% Trypan blue and the number of cells was adjusted to 1 × 106 cells per ml. The approximate proportion of viable cells was over 95% and the purity of the cell population, based on cytospin, was over 90%.
Preparation of quercetin hydrate
Quercetin hydrate (QH) powder was dissolved in 95% ethanol to make a 5 mM stock solution. Batches of freshly prepared working solution (50 µM, the optimal concentration of dose-dependent quercetin as determined in a preliminary study) were diluted with HBSS, wrapped with foil, and stored at room temperature until used.
Escherichia coli (E. coli) propagation
Fresh live Escherichia coli (EC) was prepared using the colony picking method and propagated in Luria-Bertani broth (LB broth, Caisson Laboratories, North Logan, UT, U.S.A.) at 37°C and 120 rpm in a shaking incubator until the log phase was reached. The bacterial number was adjusted to approximately 108 cfu/ml prior to use in the experiments.
Measurement of intracellular reactive oxygen species (ROS) by flow cytometry
To examine intracellular ROS generation, 1 × 105 heterophils were seeded into duplicate 96-well flat tissue culture plates. The cells were stimulated with PBS, 50 µM QH, 100 nM PMA, or 1 × 106 cfu EC for 30 min (41°C, 5% CO2). Following incubation, 10 µM H2DCF-DA was added to each well to stain the intracellular H2O2 [7, 8]. H2DCF-DA readily diffuses into cells, where it is oxidized to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) [2]. Cells were incubated in the dark for another 5 min, then washed with cold PBS, and sample acquisition (10,000 events) was performed on ROS-containing cells using a CyAnTM ADP Flow Cytometer (Beckman Coulter, Brea, CA, U.S.A.).
Fluorescence imaging of ROS-containing cells
Heterophils (2.5 × 104 cells) were seeded onto 15 mm diameter circular glass coverslips coated with 0.001% poly-L-lysine and placed onto 24-well plates. The plates were incubated for 15 min at room temperature (RT) to allow cell adhesion [38]. Heterophils were stimulated with either PBS (serving as a control), quercetin (50 µM), PMA (100 nM), or LPS from E. coli (100 ng) for 30 min (41°C, 5% CO2). Then 10 µM H2DCF-DA was added and cells were incubated for an additional 15 min in the dark [32]. Coverslips were then carefully rinsed with cold PBS (3 times) and fixed with 4% paraformaldehyde (PFA) for 25 min at RT. After fixing, cell nuclei were counterstained with DAPI prior to visualization and image capture with an Axio Scope A1 Fluorescence Microscope (Carl Zeiss, Thornwood, NY, U.S.A.) [6, 38]. The average percentage of cells double stained with both DCF and DAPI was determined manually in 3 random fields (100 cells per field) by three well-trained observers using blinded technique [49].
ROS gene expression profiles in chicken heterophils by real-time RT-PCR
To explore the effects of quercetin on ROS gene expression, heterophils (1 × 106 cells) were seeded into duplicate 24-well plates. Cells were stimulated with either quercetin (50 µM), PMA (100 nM), or EC (1 × 107 cfu), or were left untreated (PBS). Plates were incubated at 41°C, 5% CO2 for 1 hr. After incubation, samples from each well were combined to achieve a cell density of 2 × 106, then RNAlater® solution was added to preserve the total RNA [34]. RNA extraction was performed using a NucleoSpin RNA kit (Macherey-Nagel, Bethlehem, PA, U.S.A.) following the manufacturer’s instructions. RNA yields and concentrations were measured using a DU 730 nanoVette UV/Vis Spectrophotometer (Beckman Coulter). Two microgram (2 µg) of total RNA were used as a starter for cDNA synthesis using a Tetro cDNA Synthesis Kit (Bioline, Taunton, MA, U.S.A.) according to the manufacturer’s instructions.
One hundred nanogram (100 ng) samples of cDNA from chicken heterophils were quantitatively analyzed for the mRNA transcripts of CYBB (gp91phox or NOX2), NCF1 (p47phox), NCF2 (p67phox), NOX1, CAT, SOD1, RAC2, IL6, CASP8, MCL1 and GAPDH (as reference gene) with real-time RT-PCR (qPCR) using a SensiFAST SYBR Hi-ROX Kit (Bioline) following the protocol described by the manufacturer and using an ABI Prism 7300 real-time PCR (Applied Biosystems, Thermo Fisher Scientific). The genes used in this study were selected based on data mining of KEGG pathways (entry no. gga04145). The information regarding primer pairs used in this study is provided in Suppl. Table S1. The sequences of primers were designed by Primer3plus and primer synthesis was performed by MacroGen, South Korea. After completion of running of the qPCR cycles, dissociation curves were analyzed to confirm that the PCR product obtained by qPCR included the correct products. Expecting a single curve at high temperature (Tm) was validated (Suppl. Fig. S1). The specificity of qPCR on specified genes was confirmed by 2% agarose gel electrophoresis (Suppl. Fig. S1). Analysis of relative gene expression was calculated from the Ct of the gene of interest (target) and GAPDH. The expression levels (fold difference) were reported using the 2−ΔΔCt method [31].
Data analysis
The data from all three experiments were combined and analyzed for outliers using robust regression and outlier removal (ROUT) methods. The normality test was done using the D’Agostino-Pearson omnibus test prior to performing statistical analysis of each experiment. Statistical analyses were performed using either one-way ANOVA or the Kruskal-Wallis Test. Results of statistical analyses were considered significant in all experiments when P<0.05. Multiple comparison of each pair was done using the Tukey-Kramer Honest Significant Difference test (HSD). Results are reported as mean plus standard error (SE) or median value. Gene expression profiling of individual data points is presented in the form of heat maps and unsupervised hierarchical clustering by an average linkage method with Pearson correlation [15] generated by Genesis 1.7.6 [42]. Samples of selected genes from differential expression data were created and analysis of networks of genes involved in the ROS processes using CytoScape with a GeneMANIA plugin was conducted [35].
RESULTS
ROS mean fluorescence intensities (MFIs) increased with quercetin treatment
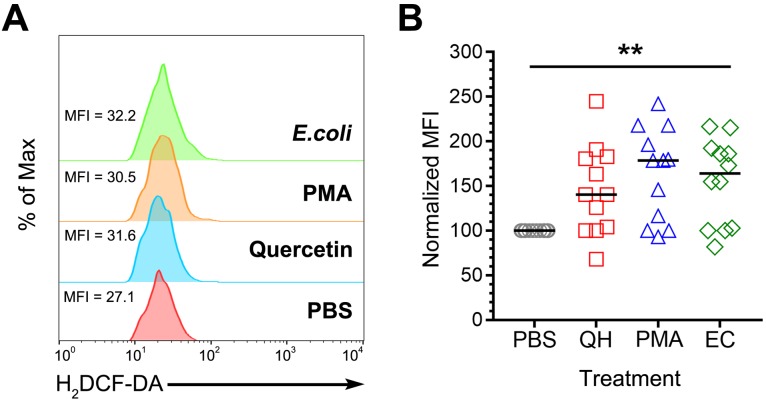
To determine the effects of quercetin on chicken heterophils in ROS generation, we fluorescently stained cells with H2DCF-DA after stimulation with various stimulants including PBS, quercetin (QH), PMA, and live bacteria (E. coli). Analysis of positive ROS-containing cells by flow cytometry showed a significant difference between the various treatments (Fig. 1). For quercetin, PMA and E. coli groups, there was a higher average mean fluorescence intensity (MFI) compared with the PBS group (P=0.005, Fig. 1B).
Fig. 1.
ROS generation in heterophil stimulated with quercetin and other stimulants by flow cytometry. (A) Representative offset histograms showing mean fluorescence intensity (MFI) values of ROS-positive cells from each treatment (PBS, quercetin (QH), PMA & Escherichia coli, EC). The height of histograms correlates to % of max. (B) Inclusive scatter plots generated from normalized MFIs to PBS to reduce inter-experimental variability. Each data point corresponds to an individual chick (n=12 each treatment). Data are the medians of two separate experiments, Kruskal-Wallis Test, **P<0.01.
Fluorescence microscopy showed a significant increase in H2DCF-DA positive staining cells with quercetin, PMA and LPS treatments
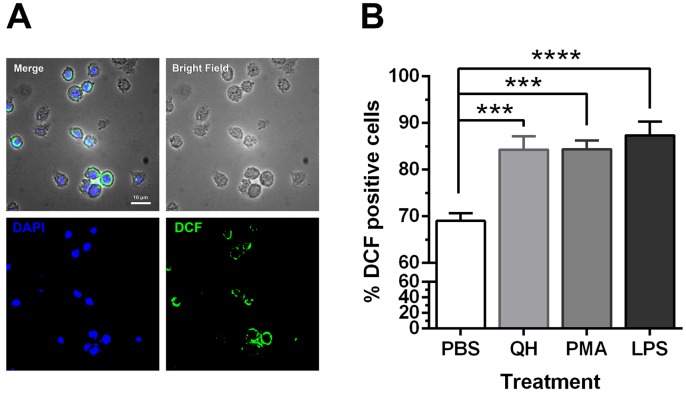
To visualize the intracellular ROS molecules in heterophils, we fluorescently labeled ROS and its derivatives with a specific fluorescent dye (H2DCF-DA). The ROS molecules generated by heterophils with PBS, quercetin, PMA or lipopolysaccharides (LPS) stimulations were clearly identifiable (Fig. 2A). Stimulated heterophils showed different percentages of ROS molecule staining (P<0.0001, Fig. 2B). Quercetin and other stimulants (PMA and LPS) had higher percentages of positive staining cells with DCF. The percentages of ROS-positive cells in the PBS, quercetin, PMA, and LPS groups were 69.05 ± 1.58, 84.32 ± 2.83, 84.40 ± 1.83 and 87.36 ± 2.99, respectively (Fig. 2B).
Fig. 2.
Characteristics of ROS-containing heterophils visualized by fluorescence microscope. (A) Representative set of fluorescence micrographs of quercetin-stimulated heterophils depicting intracellular stained ROS molecules (DCF) counter-stained with DAPI to indicate cell nuclei. The images were captured at 400 × magnification after a 45-min stimulation period; scale bar=10 µm. (B) Bar graphs represent percentage of DCF positive cells. Results are inclusive of three separate experiments. Data is represented as mean ± SE (n=13−15 each treatment), one-way ANOVA, ***P<0.001, ****P<0.0001.
Quercetin up-regulated ROS subunit genes
Validation of primers used in real-time PCRs in this study to detect the expression of genes involved in ROS and downstream processes is reported in Suppl. Fig. S1. The real-time PCRs also produced specific PCR products and dissociation curves (melting curves) as depicted in Suppl. Fig. S1.
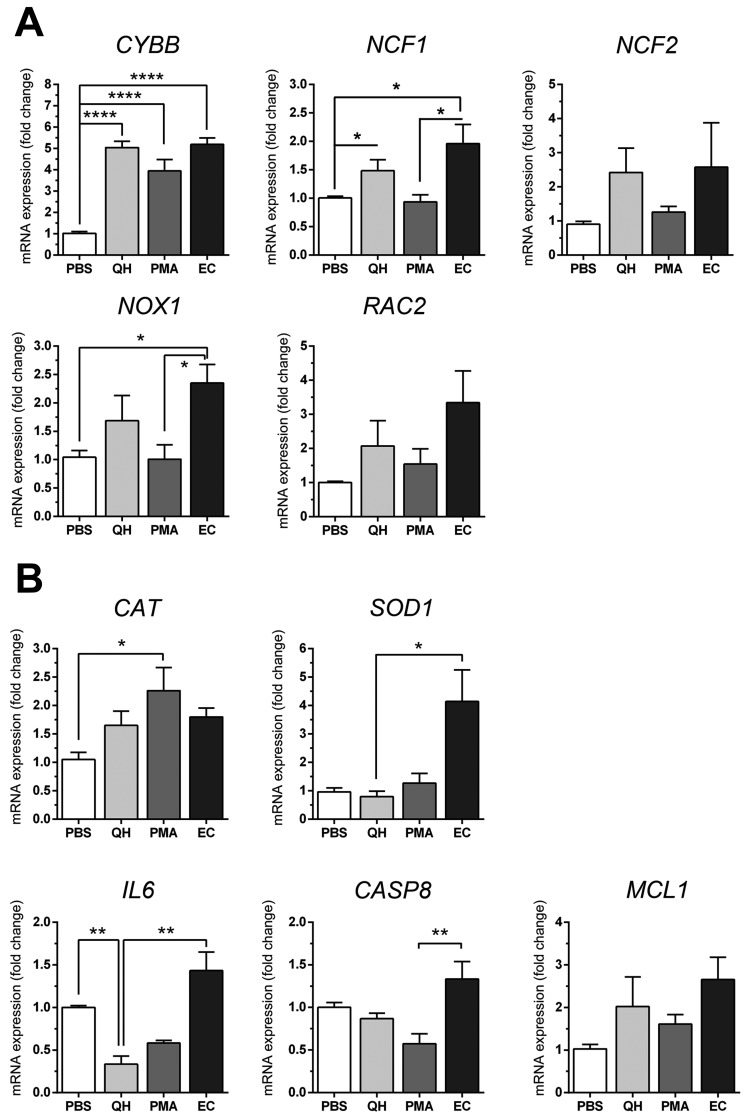
Gene expression in QH-stimulated heterophils overall was significantly more up-regulated compared with PBS, especially in ROS subunit genes (CYBB, NCF1, NCF2, NOX1 and RAC2) (Fig. 3A). Genes involved in anti-oxidation were found to have increased expression, e.g., CAT, whereas other important anti-oxidant genes, e.g., SOD1, exhibited decreased expression (Fig. 3B). Quercetin mitigated both pro-inflammatory gene (IL6) and apoptotic (CASP8) gene expression, while increasing expression of an anti-apoptotic (MCL1) gene (Fig. 3B).
Fig. 3.
Real-time RT-PCR analyses of chicken heterophil genes involved in ROS generation and biologically important downstream genes. (A) Bar graphs showing relative CYBB, NCF1, NCF2, NOX1 and RAC2 expression levels after normalization to GAPDH expression in PBS, quercetin (QH), PMA, and E. coli (EC) groups. (B) Relative CAT, SOD1, IL6, CASP8 and MCL1 expression levels of different treatment groups. The results are inclusive of three separate experiments. Data is represented as mean ± SE (n=6−8 each treatment), one-way ANOVA or Kruskal-Wallis test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
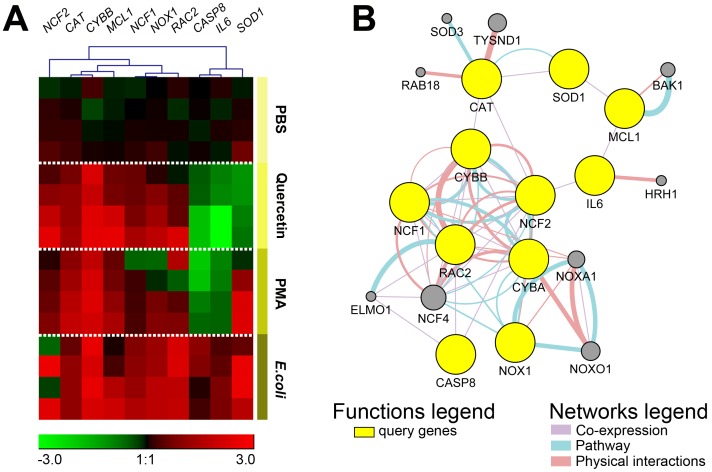
The expression levels of ROS-related genes in E. coli and PMA-stimulated heterophils were also similar to that of quercetin (Fig. 3). The expression of anti-oxidant genes (CAT and SOD1) was also increased in the PMA and E. coli groups. In contrast, SOD1 gene expression in quercetin was slightly reduced. The level of gene expression in downstream ROS processes were also altered. The expression of a gene in the inflammatory process (IL6) was reduced in quercetin and PMA, whereas the expression of this gene in E. coli was elevated. The expressions of CASP8 and MCL1, which are involved in cell death, were in opposite directions. The CASP8 gene, which is involved in extrinsic cell apoptosis, was decreased in QH- and PMA-stimulated cells, except for E. coli stimulation. The anti-apoptotic gene MCL1 showed increased expression in all treatments compared with PBS (Fig. 3B). The heat map created by log transformation of the real-time PCR data summarizes the expression of all genes in the study classified by stimulant (Fig. 4A). Overall gene expression in quercetin, PMA, and E. coli was increased in CAT, CYBB, NCF1, NCF2, NOX1, MCL1 and RAC2 (Fig. 4A, red). For CASP8, IL6 and SOD1, an overall reduction in expression in quercetin and PMA was found (green).
Fig. 4.
(A) Unsupervised clustering analysis (Pearson correlation, average linkage method) of gene expression in chicken heterophil ROS generation. Heat map was generated by a log transformation of 2-ΔΔCt values and is presented as individual data points. Columns represent specific ROS subunits, antioxidants, and downstream genes and the rows represent individual samples in the PBS, quercetin, PMA, and E. coli groups (n=4 per group). Expression values ranged from −3 to +3. Red indicates increased expression, green indicates decreased expression, and black indicates no change in expression. Expression levels are according to the color scale of the log2 values. (B) GeneMANIA Cytoscape interaction network of known gene co-expression, physical interactions, and genes involved in the ROS pathway. Yellow nodes represent query gene lists. Additional genes are predicted to be involved in the network (gray nodes).
Data on gene expression levels, both up- and down-regulation, were used to analyze the network of relationships using Cytoscape with GeneMANIA plug-ins. The results revealed evidence of a relationship among query genes (CYBB, NCF1, NCF2, NOX1, RAC2, CAT, SOD1, IL6, CASP8 and MCL1) and genes in the public dataset functional association networks. Prediction of known gene co-expression and physical interaction in the ROS pathway with query genes (yellow nodes) can be defined by additional ROS genes (gray nodes) that are related to individual query genes (Fig. 4B). It is worth noting that there are a number of genes associated with the ROS network, e.g., NOXO1, NOXA1, CYBA and SOD3 (Fig. 4B). In addition, there are some genes that indirectly present in the network, including IL6, CASP8 and MCL1, but these genes are expressed together by the genes NCF2, RAC2 and SOD1, which are already in network relationships (Fig. 4B).
DISCUSSION
Studies of the effect of quercetin on chicken heterophil effector functions suggest that this substance can manipulate reactive oxygen species (ROS) generation in vitro [36]. Information regarding the use of quercetin in promoting functions and gene expression in poultry is very limited, especially in heterophils. Representative information in reports that provides a perspective for comparing similarities and differences are mainly derived from animal species other than chickens. To the best of our knowledge, this is the first report in which quercetin was applied to chicken heterophils to evaluate ROS at both the cellular and the molecular levels. Gene expression associated with both NOX subunits and anti-oxidant genes are initially unraveling. Our findings suggest that quercetin universally effects most subunits of NADPH oxidase. On the other hand, quercetin suppresses the activation of the pro-inflammatory cytokine gene IL-6 and the cell death gene CASP8, whereas it specifically increases gene expression associated with anti-apoptosis (MCL1) resulting from respiratory oxidation.
The ability of quercetin to stimulate production of ROS molecules can be implied from this study and has been reported elsewhere [13, 17]. Those reports found that quercetin resulted in an increased ROS production in a dose-dependent manner. Quercetin also serves as a pro-oxidant as well as performing as a robust antioxidant [17]. Flavonoids play a pivotal role in either promoting or suppressing ROS production. The proposed mode of action of the earlier stated natural compound may result from the higher number of OH groups contained within the molecule which confers a stronger effect [7, 39]. The ability of quercetin and other flavonols to scavenge hypochlorous acid (HOCl) and chloramines but not H2O2 depends on the degree of hydroxylation of the flavonol B-ring [39]. The structure of quercetin is similar to that of luteolin in the flavones group. Luteolin and other similar substances have features to that enhance ROS production as measured by flow cytometry [7]. Over a longer period of supplementation, quercetin may result in an inhibition of ROS molecules instead of enhancement. In the present study, we used a concentration of quercetin of 50 µM, but, no toxicity to treated cells was detected [36, 40, 50, 51].
Gene expression patterns in the ROS process of chicken heterophils under quercetin supplementation have never been reported. The results of this study show that when heterophils receive quercetin, cells respond to the substance at both the cellular and molecular level as indicated by the accumulation of intracellular ROS molecules (Fig. 2) and gene expression to which is part of the ROS metabolic process (including CYBB, NOX1, NCF1 (p47phox), NCF2 and RAC2) (Fig. 3). The NOX complex, on the other hand, when assembled into viable protein subunit complexes, transmit signals via the protein kinase C (PKC), MAP Kinase and PKA, resulting in superoxide (SO) production and oxidative burst in phagocytes [21, 45]. p47phox is an essential NOX subunit which translocates from cytosol to the phagosomal membrane which could serve as a critical step in ROS production [48]. It has been well documented that the effect of quercetin may be exerted via the function of genes involved in other processes pertaining to ROS production. A group of genes that neutralizes free radicals, such as CAT and SOD, is an example of genes in which quercetin action can be observed [14, 19, 24].
We report here that the expression level of the CAT gene is essentially increased, but the level of SOD1 is reduced or unchanged. The results of the present study are consistent with a previous report [1] in which the authors found the expression of Cu/Zn superoxide dismutase and catalase to be increased when the hepatoma cell line (HepG2) was enriched with quercetin at 50 to 100 µM. In addition, other studies have shown that quercetin and other compounds hinder ROS generation and reduce tissue damage caused by free radicals [23, 40]. However, little research has focused on the regulatory effects of quercetin on NADPH oxidase subunit expression, in particular on terminally differentiated phagocytes like neutrophils and heterophils.
Results of the current experiment suggest a possible mechanism: heterophils generate harmful molecules in response to E. coli by increasing the expression of genes involved in the production of ROS. In reaction to noxious oxygen molecules, E. coli has its own mechanism for manipulating the expression of SOD1, acting as anti-oxidant in the host which neutralizes the effectiveness of quercetin modulation of ROS. The pro-inflammatory cytokine genes in chicken heterophils, e.g., IL-1, IL-6 and IL-8, after being triggered with ligands or pathogens may vigorously up-regulate [26, 27]. In this study, the gene expression pattern of the IL-6 gene was disrupted by quercetin, while the expression level of the same gene was increased when the cells were exposed to E. coli. This finding confirms that quercetin has the effect of suppressing the inflammatory mediator produced by pathogen-mediated signaling as previously reported [11, 30]. Therefore, we assume that quercetin keeps the level of inflammation in check by inhibition of inflammatory cytokine in avian species, just as has been postulated in mammals.
In the present study, we focused primarily on the effects and possible linkages of genes whose expression could be manipulated by the action of quercetin. The other subunits, i.e., p22phox (CYBA), p40phox (NCF4) and NOXO1 (p41NOX), remain unexplored. Aspects of other processes involved in the chicken heterophils ROS generation are also reported. Our analysis of gene networks suggests there are additional genes involved in production of ROS and accompanying antioxidants, but that information confirmation is beyond the scope of the present study.
Our findings support reports of benefits of quercetin supplementation in chicken feed. Given on a regular basis, it is able to act as a prophylaxis and to prevent detrimental microbial infections. Quercetin should achieve a balance between oxidative and anti-oxidative activity in phagocytes in the process attenuating the pathological severity of invading microbes or mitigating oxidative tissue damage by ROS generation. This concept suggests potential therapeutic targets of ROS generation, illustrating another facet of the properties of quercetin. Taken together, this study provides evidence that quercetin’s effect on ROS generation helps improve chicken phagocyte function, specifically, it helps heterophils to perform more effective and efficient oxidative killing of microbes. These findings fill a knowledge gap in chicken innate immune response related to modulation of the oxidative capacity of heterophils. This study also suggests potential directions for future studies of genes or group of genes which have a role in the production of ROS. In the future, both in vivo and in vitro studies will be needed to elucidate the mechanisms of quercetin on the other subunits of NADPH oxidase in promoting innate immune defense in chickens.
CONFLICTING OF INTERESTS.
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This study was financially supported in part by funding from Chiang Mai University (Grant no. R000012003) and the Excellent Center in Veterinary Biosciences (ECVB). We would like to sincerely thank the Medical Science Research Equipment Center at the CMU Medical Center for performing the flow cytometric analysis. The authors would also like to thank Dr. Chongchit Sripun Robert and Dr. G. Lamar Robert for proofreading the manuscript.
REFERENCES
- 1.Alía M., Mateos R., Ramos S., Lecumberri E., Bravo L., Goya L.2006. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur. J. Nutr. 45: 19–28. doi: 10.1007/s00394-005-0558-7 [DOI] [PubMed] [Google Scholar]
- 2.Bae Y. S., Kang S. W., Seo M. S., Baines I. C., Tekle E., Chock P. B., Rhee S. G.1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272: 217–221. doi: 10.1074/jbc.272.1.217 [DOI] [PubMed] [Google Scholar]
- 3.Bedard K., Krause K. H.2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87: 245–313. doi: 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- 4.Boots A. W., Haenen G. R., Bast A.2008. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 585: 325–337. doi: 10.1016/j.ejphar.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 5.Brune K., Leffell M. S., Spitznagel J. K.1972. Microbicidal activity of peroxidaseless chicken heterophile leukocytes. Infect. Immun. 5: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruns S., Kniemeyer O., Hasenberg M., Aimanianda V., Nietzsche S., Thywissen A., Jeron A., Latgé J. P., Brakhage A. A., Gunzer M.2010. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 6: e1000873. doi: 10.1371/journal.ppat.1000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang H., Mi M., Ling W., Zhu J., Zhang Q., Wei N., Zhou Y., Tang Y., Yu X., Zhang T.2010. Structurally related anticancer activity of flavonoids: involvement of reactive oxygen species generation. J. Food Biochem. 34: 1–14. doi: 10.1111/j.1745-4514.2009.00282.x [DOI] [Google Scholar]
- 8.Chen X., Zhong Z., Xu Z., Chen L., Wang Y.2010. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 44: 587–604. doi: 10.3109/10715761003709802 [DOI] [PubMed] [Google Scholar]
- 9.Chuammitri P., Ostojić J., Andreasen C. B., Redmond S. B., Lamont S. J., Palić D.2009. Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet. Immunol. Immunopathol. 129: 126–131. doi: 10.1016/j.vetimm.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 10.Chuammitri P., Redmond S. B., Kimura K., Andreasen C. B., Lamont S. J., Palić D.2011. Heterophil functional responses to dietary immunomodulators vary in genetically distinct chicken lines. Vet. Immunol. Immunopathol. 142: 219–227. doi: 10.1016/j.vetimm.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 11.Chuammitri P., Amphaiphan C., Nojit P.2015. In vitro modulatory effects of quercetin on bovine neutrophil effector functions. Thai J. Vet. Med. 45: 63–72. [Google Scholar]
- 12.Cushnie T. P., Lamb A. J.2005. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26: 343–356. doi: 10.1016/j.ijantimicag.2005.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Marchi U., Biasutto L., Garbisa S., Toninello A., Zoratti M.2009. Quercetin can act either as an inhibitor or an inducer of the mitochondrial permeability transition pore: A demonstration of the ambivalent redox character of polyphenols. Biochim. Biophys. Acta 1787: 1425–1432. doi: 10.1016/j.bbabio.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 14.Dupré-Crochet S., Erard M., Nüβe O.2013. ROS production in phagocytes: why, when, and where? J. Leukoc. Biol. 94: 657–670. doi: 10.1189/jlb.1012544 [DOI] [PubMed] [Google Scholar]
- 15.Eisen M. B., Spellman P. T., Brown P. O., Botstein D.1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U.S.A. 95: 14863–14868. doi: 10.1073/pnas.95.25.14863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkel T.2001. Reactive oxygen species and signal transduction. IUBMB Life 52: 3–6. doi: 10.1080/15216540252774694 [DOI] [PubMed] [Google Scholar]
- 17.Fonseca-Silva F., Inacio J. D., Canto-Cavalheiro M. M., Almeida-Amaral E. E.2011. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS One 6: e14666. doi: 10.1371/journal.pone.0014666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formica J. V., Regelson W.1995. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 33: 1061–1080. doi: 10.1016/0278-6915(95)00077-1 [DOI] [PubMed] [Google Scholar]
- 19.Gerin F., Sener U., Erman H., Yilmaz A., Aydin B., Armutcu F., Gurel A.2016. The effects of quercetin on acute lung injury and biomarkers of inflammation and oxidative stress in the rat model of sepsis. Inflammation 39: 700–705. doi: 10.1007/s10753-015-0296-9 [DOI] [PubMed] [Google Scholar]
- 20.Goliomytis M., Tsoureki D., Simitzis P. E., Charismiadou M. A., Hager-Theodorides A. L., Deligeorgis S. G.2014. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult. Sci. 93: 1957–1962. doi: 10.3382/ps.2013-03585 [DOI] [PubMed] [Google Scholar]
- 21.Görlach A., Dimova E. Y., Petry A., Martínez-Ruiz A., Hernansanz-Agustín P., Rolo A. P., Palmeira C. M., Kietzmann T.2015. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 6: 372–385. doi: 10.1016/j.redox.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He H., Farnell M. B., Kogut M. H.2003. Inflammatory agonist stimulation and signal pathway of oxidative burst in neonatal chicken heterophils. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 135: 177–184. doi: 10.1016/S1095-6433(03)00049-7 [DOI] [PubMed] [Google Scholar]
- 23.Hu X. T., Ding C., Zhou N., Xu C.2015. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur. J. Pharmacol. 754: 115–124. doi: 10.1016/j.ejphar.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 24.Huang R., Zhong T., Wu H.2015. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch. Med. Sci. 11: 427–432. doi: 10.5114/aoms.2015.50975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchner T., Möller S., Klinger M., Solbach W., Laskay T., Behnen M.2012. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm. 2012: 849136. doi: 10.1155/2012/849136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kogut M. H., Rothwell L., Kaiser P.2005. IFN-γ priming of chicken heterophils upregulates the expression of proinflammatory and Th1 cytokine mRNA following receptor-mediated phagocytosis of Salmonella enterica serovar enteritidis. J. Interferon Cytokine Res. 25: 73–81. doi: 10.1089/jir.2005.25.73 [DOI] [PubMed] [Google Scholar]
- 27.Kogut M. H., Swaggerty C., He H., Pevzner I., Kaiser P.2006. Toll-like receptor agonists stimulate differential functional activation and cytokine and chemokine gene expression in heterophils isolated from chickens with differential innate responses. Microbes Infect. 8: 1866–1874. doi: 10.1016/j.micinf.2006.02.026 [DOI] [PubMed] [Google Scholar]
- 28.Lambeth J. D.2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4: 181–189. doi: 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- 29.Li C., Zhang W. J., Frei B.2016. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 9: 104–113. doi: 10.1016/j.redox.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Li X., Yue Y., Li J., He T., He Y.2005. The inhibitory effect of quercetin on IL-6 production by LPS-stimulated neutrophils. Cell. Mol. Immunol. 2: 455–460. [PubMed] [Google Scholar]
- 31.Livak K. J., Schmittgen T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ CT) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32.Luehong N., Khaowmek J., Wongsawan K., Chuammitri P.2017. Preferential pattern of mouse neutrophil cell death in response to various stimulants. In Vitro Cell. Dev. Biol. Anim. 53: 513–524. doi: 10.1007/s11626-016-0129-7 [DOI] [PubMed] [Google Scholar]
- 33.Maxwell M., Robertson G.1998. The avian heterophil leucocyte: a review. Worlds Poult. Sci. J. 54: 155–178. doi: 10.1079/WPS19980012 [DOI] [Google Scholar]
- 34.Miao E. A., Leaf I. A., Treuting P. M., Mao D. P., Dors M., Sarkar A., Warren S. E., Wewers M. D., Aderem A.2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11: 1136–1142. doi: 10.1038/ni.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montojo J., Zuberi K., Rodriguez H., Kazi F., Wright G., Donaldson S. L., Morris Q., Bader G. D.2010. GeneMANIA Cytoscape plugin: fast gene function predictions on the desktop. Bioinformatics 26: 2927–2928. doi: 10.1093/bioinformatics/btq562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nojit P., Ampaipun J., Chuammitri P.2014. Effects of quercetin on chicken heterophil cellular functions. Chiang Mai Vet. J. 12: 167–178. [Google Scholar]
- 37.Peña J., Vieira S., López J., Reis R., Barros R., Furtado F., Silva P.2008. Ascorbic acid and citric flavonoids for broilers under heat stress: effects on performance and meat quality. Rev. Bras. Cienc. Avic. 10: 125–130. doi: 10.1590/S1516-635X2008000200008 [DOI] [Google Scholar]
- 38.Remijsen Q., Vanden Berghe T., Wirawan E., Asselbergh B., Parthoens E., De Rycke R., Noppen S., Delforge M., Willems J., Vandenabeele P.2011. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 21: 290–304. doi: 10.1038/cr.2010.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos E. O., Kabeya L. M., Figueiredo-Rinhel A. S., Marchi L. F., Andrade M. F., Piatesi F., Paoliello-Paschoalato A. B., Azzolini A. E. C., Lucisano-Valim Y. M.2014. Flavonols modulate the effector functions of healthy individuals’ immune complex-stimulated neutrophils: a therapeutic perspective for rheumatoid arthritis. Int. Immunopharmacol. 21: 102–111. doi: 10.1016/j.intimp.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 40.Saw C. L. L., Guo Y., Yang A. Y., Paredes-Gonzalez X., Ramirez C., Pung D., Kong A. N. T.2014. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 72: 303–311. doi: 10.1016/j.fct.2014.07.038 [DOI] [PubMed] [Google Scholar]
- 41.Simon H. U., Haj-Yehia A., Levi-Schaffer F.2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5: 415–418. doi: 10.1023/A:1009616228304 [DOI] [PubMed] [Google Scholar]
- 42.Sturn A., Quackenbush J., Trajanoski Z.2002. Genesis: cluster analysis of microarray data. Bioinformatics 18: 207–208. doi: 10.1093/bioinformatics/18.1.207 [DOI] [PubMed] [Google Scholar]
- 43.Sumimoto H.2008. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 275: 3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x [DOI] [PubMed] [Google Scholar]
- 44.Thannickal V. J., Fanburg B. L.2000. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279: L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005 [DOI] [PubMed] [Google Scholar]
- 45.Touyz R. M., Briones A. M., Sedeek M., Burger D., Montezano A. C.2011. NOX isoforms and reactive oxygen species in vascular health. Mol. Interv. 11: 27–35. doi: 10.1124/mi.11.1.5 [DOI] [PubMed] [Google Scholar]
- 46.Vellosa J. C. R., Regasini L. O., Belló C., Schemberger J. A., Khalil N. M., de Araújo Morandim-Giannetti A., da Silva Bolzani V., Brunetti I. L., de Faria Oliveira O. M. M.2015. Preliminary in vitro and ex vivo evaluation of afzelin, kaempferitrin and pterogynoside action over free radicals and reactive oxygen species. Arch. Pharm. Res. 38: 1168–1177. doi: 10.1007/s12272-014-0487-1 [DOI] [PubMed] [Google Scholar]
- 47.Vernon P. J., Tang D.2013. Eat-me: autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid. Redox Signal. 18: 677–691. doi: 10.1089/ars.2012.4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao L., Liu L., Guo X., Zhang S., Wang J., Zhou F., Liu L., Tang Y., Yao P.2017. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 105: 22–33. doi: 10.1016/j.fct.2017.03.048 [DOI] [PubMed] [Google Scholar]
- 49.Yan J., Meng X., Wancket L. M., Lintner K., Nelin L. D., Chen B., Francis K. P., Smith C. V., Rogers L. K., Liu Y.2012. Glutathione reductase facilitates host defense by sustaining phagocytic oxidative burst and promoting the development of neutrophil extracellular traps. J. Immunol. 188: 2316–2327. doi: 10.4049/jimmunol.1102683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W. S., Jeong D., Yi Y. S., Lee B. H., Kim T. W., Htwe K. M., Kim Y. D., Yoon K. D., Hong S., Lee W. S., Cho J. Y.2014. Myrsine seguinii ethanolic extract and its active component quercetin inhibit macrophage activation and peritonitis induced by LPS by targeting to Syk/Src/IRAK-1. J. Ethnopharmacol. 151: 1165–1174. doi: 10.1016/j.jep.2013.12.033 [DOI] [PubMed] [Google Scholar]
- 51.Yi L., Chen C. Y., Jin X., Zhang T., Zhou Y., Zhang Q. Y., Zhu J. D., Mi M. T.2012. Differential suppression of intracellular reactive oxygen species-mediated signaling pathway in vascular endothelial cells by several subclasses of flavonoids. Biochimie 94: 2035–2044. doi: 10.1016/j.biochi.2012.05.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.