Abstract
A functional region of left fusiform gyrus termed “the visual word form area” (VWFA) develops during reading acquisition to respond more strongly to printed words than to other visual stimuli. Here, we examined responses to letters among 5- and 6-year-old early Kindergarten children (N=48) with little or no school-based reading instruction who varied in their reading ability. We used functional magnetic resonance imaging (fMRI) to measure responses to individual letters, false fonts, and faces in left and right fusiform gyri. We then evaluated whether signal change and size (spatial extent) of letter-sensitive cortex (greater activation for letters versus faces) and letter-specific cortex (greater activation for letters versus false fonts) in these regions related to (a) standardized measures of word-reading ability and (b) signal change and size of face-sensitive cortex (fusiform face area or FFA; greater activation for faces versus letters). Greater letter specificity, but not letter sensitivity, in left fusiform gyrus correlated positively with word reading scores. Across children, in the left fusiform gyrus, greater size of letter-sensitive cortex correlated with lesser size of FFA. These findings are the first to suggest that in beginning readers, development of letter responsivity in left fusiform cortex is associated with both better reading ability and also a reduction of the size of left FFA that may result in right-hemisphere dominance for face perception.
Keywords: VWFA, print, development, reading, fMRI, specialization
Introduction
Reading is a cultural invention dating back only a few thousand years and therefore, the human brain has not yet evolved an inherent reading network to accomplish this task (Wolf, 2008). Rather, a print-specific reading network develops with literacy skills through childhood and adolescence (Blackburne et al., 2014; Centanni, King, Eddy, Whitfield-Gabrieli, & Gabrieli, 2017; Dundas, Plaut, & Behrmann, 2013; Olulade, Flowers, Napoliello, & Eden, 2013; Saygin et al., 2016; Ventura et al., 2013). One component of this reading network is the putative visual word form area (VWFA), a functionally defined region that is sensitive and specific to print and is located in the left fusiform gyrus near the temporo-occipital sulcus (Cohen et al., 2002; Dehaene & Cohen, 2011; McCandliss, Cohen, & Dehaene, 2003). The VWFA is considered an important component of the reading network, as it exhibits a specialized response to printed words as a result of reading acquisition (Cai et al., 2010; Cohen et al., 2002; Jobard, Crivello, & Tzourio-Mazoyer, 2003).
In adult typical readers, the VWFA shows stronger responses to printed words as compared to a variety of non-linguistic stimuli, such as checkerboards (Cohen et al., 2002), faces (Centanni et al., 2017; Dehaene et al., 2010), objects (Baker, Liu, & Wald, 2007; Centanni et al., 2017; Szwed, Dehaene, & Kleinschmidt, 2011), false fonts (Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003; Vinckier, Dehaene, Jobert, & Dubus, 2007), and unpronounceable consonant strings (Baker et al., 2007; Cohen et al., 2002; Vinckier et al., 2007). The importance of the VWFA for the reading network is supported by consistent evidence of VWFA hypoactivation or atypical patterns of activation in children and adults with developmental dyslexia (Olulade et al., 2015; van der Mark et al., 2009; also see meta-analyses: Maisog et al., 2008; Martin, Kronbichler, & Richlan, 2016; Richlan, Kronbichler, & Wimmer, 2011). Further, lesions to this region of left fusiform gyrus lead to alexia (Damasio & Damasio, 1983; Gaillaird et al., 2006).
The adult fusiform gyri include separate regions with preferential responses to multiple visual categories beyond print. The fusiform face area (FFA) responds primarily to face stimuli (Kanwisher, McDermott, & Chun, 1997), and in literate adults is typically larger in the right than in the left fusiform gyrus (Kim et al., 2014; McCarthy et al., 1997). As letters become familiar (most often in childhood, but also in previously illiterate adults learning to read), letter-selective regions develop in left fusiform gyri in a location that is often at least partially separate from the FFA (Cantlon et al., 2011; Dehaene et al., 2010; Saygin et al., 2016). There is some evidence in illiterate adults learning to read that the intensity of activation to faces in the left FFA decreases as activation to letters in the left VWFA increases (Dehaene et al., 2010), suggesting that experience-dependent specialization of neural tissue in the left fusiform for the perception of print is associated with a reduction of left-hemisphere specialization for faces. It is currently unknown as to whether such an association between a larger VWFA and a smaller FFA in the left fusiform cortex is characteristic in the brains of young children in the early stages of learning to read.
The little that is known about the early development of the VWFA can be considered in a framework that distinguishes between sensitivity versus specificity in response to print (Centanni et al., 2017). Sensitivity refers to the differential response to print versus stimuli that are unrelated to print or language, such as fixation, checkerboards, or unknown faces. Specificity refers to the differential response to print versus stimuli that are perceptually print-like, such as digits, false fonts, or letters from a different language (e.g., Hebrew) that share visual features, such as straight and curved lines. In this framework, there is evidence for early VWFA sensitivity (letters versus faces) but not specificity (letters versus digits) in prereaders at age 4 (Cantlon et al., 2011). Thus, sensitivity and specificity for print reflect different levels of specialization for print.
There is evidence for early developing sensitivity but slowly evolving specificity for letters and words in the VWFA. Children ages 7–14 have adult-like sensitivity to print, but immature specificity for print (Centanni et al., 2017). Slowly maturing specificity in response to print is also indicated by the finding that children ages 5–12 show no evidence for discriminating between normal and mirror-reversed letters in the VWFA, whereas adults show a marked difference (Blackburne et al., 2014). Explicit reading training increases word-specific responses and decreases false-font responses in a region near the VWFA in 6-year old children (Brem et al., 2010), but it is unknown how typical variation prior to school influences such development before children receive formal reading instruction.
No study has directly compared sensitivity versus specificity in left fusiform response and reading performance in pre- and beginning readers. In order to discover how the VWFA might typically reflect early stages of literacy development, we examined print-specific responses in the fusiform gyri in 5- and 6-year-old children who were in the final weeks of pre-kindergarten or the first weeks of kindergarten and had received little or no structured reading instruction in school.
We examined relations among activations in the left and right fusiform gyrus to individual letters, false-font letters, and faces and how these related to individual differences in kindergarten children’s word reading abilities. Because of evidence that the relatively small and variably located VWFA is better characterized in individual than group-averaged brains (Glezer and Riesenhuber, 2013), analyses were conducted on individually-defined regions of interest (ROIs). In order to identify in each child a region sensitive for print relative to other unrelated visual stimuli, we first defined a region with a letters > faces contrast. We also identified in each child a region sensitive for faces relative to print (faces > letters contrast). Then, in order to characterize the specificity of the area sensitive to letters actual letters encountered in reading, we compared activation for real letters to activation of visually matched false-font letters that are never encountered in reading but share basic perceptual features. In each case, we quantified both the intensity of response (percent signal change) in the ROI and the spatial extent (volume, in number of voxels) of the ROI.
We tested two hypotheses about the early development of print specialization in the left fusiform gyrus. First, we tested the hypothesis that specificity for print, and not sensitivity for print, would be related to reading (word identification ability) at this earliest stage of learning to read. This hypothesis was motivated by evidence that letter sensitivity is present in young children even before reading instruction, and that such letter sensitivity was unrelated to letter knowledge in prereaders (Cantlon et al., 2011). Conversely, letter specificity has been related to reading experience (e.g., Blackburne et al., 2014; Centanni et al., 2017). Second, we tested the hypotheses that a larger region (size) of VWFA (letter-sensitive cortex) would be associated with a smaller FFA (face-sensitive cortex) in left fusiform cortex. Adults learning to read for the first time exhibit a trade-off between a growing response to letter and a diminishing response to faces in left fusiform cortex (Dehaene et al., 2010), and we hypothesized that a similar trade-off between VWFA and FFA responses occurs in typical early reading development.
Materials and Methods
Participants
Kindergarten children were recruited through schools as part of a larger longitudinal study of reading and literacy development (The READ Study; see also Ozernov-Palchik et al., 2016; Saygin et al., 2013, 2016). In order to examine only children with typical reading development, a subset of 48 children were selected for analysis (mean age 66.5 months old, range 62–74 months, 30 females) on the basis of scoring in the average or above average range for their age on a measure of passage reading accuracy at the end of 1st grade (scaled score ≥ 9, equal to 37th percentile, on the Gray Oral Reading Test, 5th Edition/GORT-5; Wiederholt & Bryant, 2012). Children completed a short battery of standardized psycho-educational assessments administered individually by trained researchers during the spring of pre-kindergarten or fall of their kindergarten year. All children met the eligibility criteria including: being a native speaker of American English; born after at least 36 weeks gestation; no sensory or perceptual difficulties other than corrected vision; no history of head or brain injury or trauma; no neurological, neuropsychological, or developmental diagnoses; no medications affecting the nervous system; standard scores > 80 on measures of nonverbal IQ and vocabulary in kindergarten (Kaufman Brief Intelligence Test/KBIT-2 Matrices subtest; Kaufman & Kaufman, 1990, and the Peabody Picture Vocabulary Test/PPVT-4; Dunn & Dunn, 2007). This study was approved by the institutional review boards at the Massachusetts Institute of Technology and Boston Children’s Hospital. Parents gave written consent and children provided verbal assent to participate.
Assessments were audio recorded and checked for accuracy of administration and scoring. Behavioral assessment scores and in-scanner performance for this sample are reported in Table 1. Single word reading ability was assessed using the Word ID subtest of the Woodcock Reading Mastery Test-Revised/Normative Update (WRMT-R/NU Word ID subtest; Woodcock, 1998). In this test, children read aloud single regular and irregular real words of increasing difficulty. Initial words are high-frequency sight words (e.g., “you”). Letter knowledge was measured using the Letter ID subtest from the Woodcock Reading Mastery Test (WRMT-R/NU; Woodcock, 1998). In this test, letters are presented in a variety of cases and fonts (including in cursive for more difficult items) and the child is asked to say the name of the letter. The Matrices subtest of the Kaufman Brief Intelligence Test (KBIT-2) was administered to assess nonverbal cognitive ability. In this test, children are shown a matrix of pictures or symbols and asked to select from a series of choices the item that completes the matrix.
Table 1. Behavioral measures and in-scanner task performance.
Socio-economic status was quantified per the Barratt Simplified Measure of Social Status (BSMSS), which assigns codes for number of years of maternal education (18 = completed college, possible scores range from 3 to 21). Nonverbal IQ was assessed using the Kaufman Brief Intelligence Test (KBIT-2 Matrices); Letter ID and Word ID are subtests of the Woodcock Reading Mastery Test (WRMT-R/NU). Task performance accuracy (percent correct) on the one-back tasks was measured by number of (hits + correct rejections) / total stimuli.
| Mean ± SD | Range | |
|---|---|---|
| Age (months) | 66.46 ± 3.29 | 62 – 74 |
|
| ||
| Mother’s Education (BSMSS score) | 18.91 ± 2.35 | 12 – 21 |
|
| ||
| Nonverbal IQ | ||
| Standard score | 103.96 ± 7.38 | 90 – 118 |
|
| ||
| Letter ID | ||
| Raw score | 32.04 ± 5.72 | 6 – 41 |
| Standard score | 110.63 ± 8.40 | 88 – 130 |
|
| ||
| Word ID | ||
| Raw score | 13.52 ± 18.89 | 0 – 67 |
| Standard score | 112.92 ± 29.09 | 80 – 175 |
|
| ||
| In-scanner task accuracy (percent correct) | ||
| Letters | 94.83 ± 3.87 | 80 – 100 |
| False fonts | 94.53 ± 3.77 | 83.34 – 100 |
| Faces | 96.18 ± 2.87 | 88.34 – 100 |
Raw scores and standard scores and other descriptive statistics are presented in Table 1. Because we were interested in children’s ability to identify words relative to others in the sample and because these participants were within a narrow age range, we used raw scores rather than standard scores for Letter and Word ID in our analyses.
fMRI Task and Imaging Acquisition
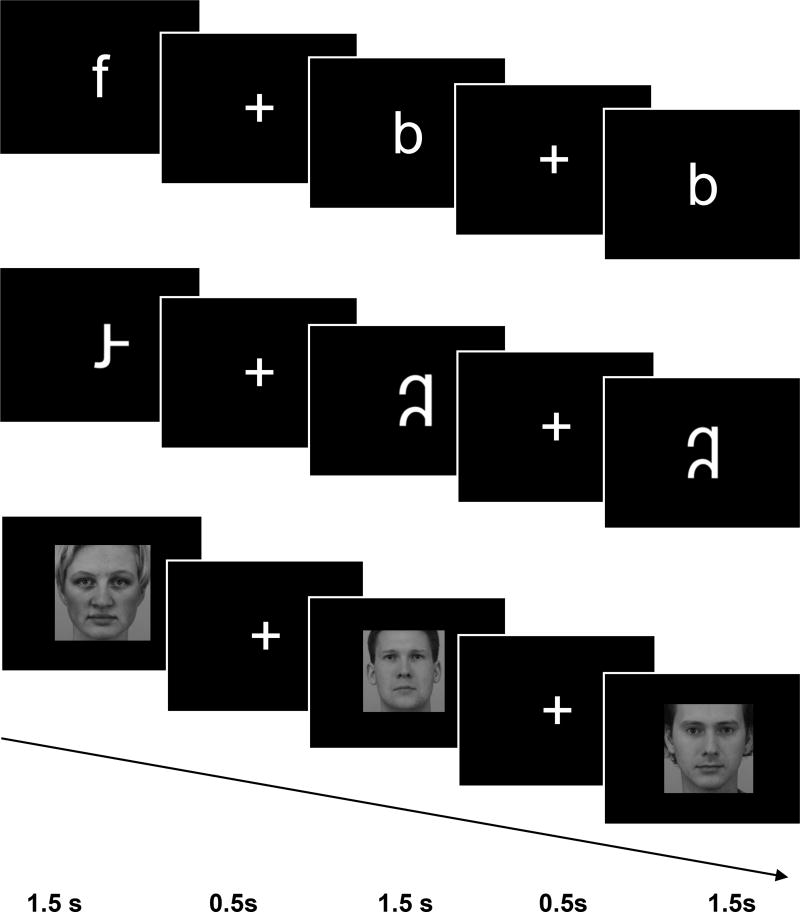
Participants completed a visual processing task in the scanner with three conditions: letters, false fonts, and faces (Figure 1). In each condition, participants were asked to watch stimuli presented one at a time in the middle of the screen, and press a button if any stimulus was repeated twice in a row (i.e., a one-back task). Ten unique stimuli were used in each condition. Letter stimuli included lowercase English letters (b, c, f, k, m, p, r, s, t, y) presented in bold Arial font. Letter stimuli were used instead of word stimuli to ensure that the print stimuli were recognizable to all children in this age range. In support of this choice, many children in our sample could not read any words, but all children had good knowledge of letters (Table 1). In order to control for visual complexity, false font stimuli were created by rearranging the components of the same 10 individual letter stimuli. False font letters followed general conventions of English (Roman alphabet) letters, e.g., no more than 1 ascending or descending portion. Faces were all of a neutral expression and forward gaze, half male and half female, all Caucasian (from Karolinska Directed Emotional Faces; Lundqvist, Flykt, & Ohman, 1998). Blocks of 10 trials of the same stimulus type (condition) and resting fixation blocks were presented. Each stimulus was presented for 1.5 seconds, then a fixation cross appeared for 0.5 seconds in between each stimulus, resulting in a 20-second block. Repeated stimuli occurred randomly 3 times in each block, and stimulus order was counterbalanced within the blocks and across runs. Order of the runs and the hand used to respond during the task were each counterbalanced across participants. Participants completed 6 blocks of each condition and 6 blocks of resting fixation, with the order of blocks pseudo-randomized so that no condition was presented twice in a row. In order to optimize performance in children, the task was divided into two runs lasting 4 minutes and 8 seconds each.
Figure 1. Task design and stimuli used.
Participants were presented with letters (top row), false fonts (middle row), and faces (bottom row). Each stimulus was presented for 1500 ms with a 500 ms fixation between trials. Children pressed a button to indicate when a stimulus was repeated immediately (one-back task).
Imaging was performed using a Siemens 3T MAGNETOM Trio, A Tim System, (Siemens Medical Solutions, Erlangen, Germany) and a commercial Siemens 32 channel head coil. Functional data were collected with 3×3×4mm resolution, 2000ms TR, 30ms TE, 90° flip, 64×64 base resolution, and 32 slices approximately parallel to the AC/PC line with coverage of the entire cortex. Prior to each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium. PACE, an online prospective motion correction algorithm (Thesen, Heid, Mueller, & Schad, 2000), was implemented to reduce the effect of motion artifacts on functional data.
A critical issue in developmental neuroimaging is the observation that head motion during fMRI is frequently correlated with age and other characteristics (Satterthwaite, Wolf, & Loughead, 2012) and this increased motion is especially troublesome when scanning young children. Therefore, proper care needs to be taken such that fMRI differences are neither manufactured nor masked by differences in head motion (Chai, Ofen, Gabrieli, & Whitfield-Gabrieli, 2014). In the current study, extreme care was taken to acclimate participants to the scanner environment prior to the actual fMRI session. This practice session consisted of the experimenter describing the parts of the scanner, introducing the participants to the sight, sound, and feel of the scanner using a custom built mock scanner setup, and practicing staying as still as possible during the scan. Additionally, children practiced a shortened run with the same experimental task using different stimuli, and experimenters monitored performance during practice to ensure that children understood and could complete the task during fMRI. We also accounted for head motion during the scans in our analyses, see below.
fMRI Preprocessing and Analysis
Preprocessing and data analyses were performed using Nipype, a Python-based framework for integrating neuroimaging analysis packages (Gorgolewski et al., 2011). The software packages used in this analysis pipeline included FMRIB Software Library (FSL 5.0.8), FreeSurfer (5.1.0), Advanced Normalization Tools (ANTS), and Nipype’s implementation of Artifact Detection Tools (ART).
FreeSurfer was used to generate cortical surfaces and subcortical segmentations from each participant’s anatomical image; surfaces were visually inspected for quality and manually edited. Functional images were realigned using FSL’s MCFLIRT, with the first volume of the first run used as the reference volume. We spatially smoothed the functional data with a 6mm FWHM Gaussian kernel, and applied a high-pass filter of 1/128 Hz. ART was used to identify outlier volumes based on motion (greater than 2mm of composite volume-to-volume motion) and to calculate the number of motion outliers that coincided with stimulus presentation (reported as the correlation coefficient). The median functional image for each run was averaged across the two runs for each participant. This average median was then coregistered to the structural scan using FreeSurfer’s bbregister. ANTS was used to register the structural image to MNI space (Oasis-30 Atropos template in MNI152, 2mm version). We chose to use an adult template for registration for a number of reasons. First, the VWFA was originally defined and extensively studied in the adult brain and previous studies have used the adult template to study children for this reason (Blackburne et al., 2014; Centanni et al., 2017; Olulade et al., 2015). Second, voxels in fMRI are relatively large and we chose to use a sufficiently broad search space (the entire fusiform gyrus) to account for any age-related variance. Finally, cross-sectional studies of structural MRIs in a number of age ranges (from 4 to 21 years) demonstrate negligible differences in localization over development (Ghosh et al., 2010) and that inferior brain regions show early maturation (Gogtay et al., 2004), suggesting that for an accurate comparison of an adult-defined functional region, such as the VWFA, in children, an adult template is appropriate.
First-level analyses were performed using a general linear model approach. Regressors in the design matrix included the three stimulus conditions (letters, false fonts, and faces) convolved with a double gamma hemodynamic response function. The six rigid-body realignment parameters (3 translations, 3 rotations) and the motion outliers detected by ART were included in the model as nuisance regressors to account for any degree of motion during the scan. Outliers were defined as any image where head placement deviated from the previous image by more than 1 mm or whose average signal intensity differed from the series average by more than 3 standard deviations. No participants had more than 20% of the acquired images flagged as outliers. Stimulus-correlated motion was calculated as the coefficient between the motion parameters and stimulus onset times (0.09 ± 0.03), indicating that children did not move significantly in response to stimulus presentations. A fixed effects analysis was performed to combine contrast images across runs, and a composite transform (bbregister and ANTS transformations) was used to normalize the resulting contrast images to MNI space in a single interpolation step.
The VWFA is usually defined as an average activation in an area of normalized space (Cohen et al., 2002; Dehaene et al., 2010; Olulade et al., 2013) or as an individualized activation in native space (Baker et al., 2007; Ben-Shachar et al., 2011; Glezer & Riesenhuber, 2013; Saygin et al., 2016). There is some evidence in adults that individually defined VWFAs are more sensitive for defining that region than group averages or a location defined by the literature because the VWFA is a relatively small functional region and its precise location within the left fusiform region varies somewhat across individuals (Glezer & Riesenhuber, 2013). If the VWFA develops with reading experience, then its size may be smaller in children who are beginning readers. Therefore, we analyzed findings using individually defined regions of interest (ROIs).
We used a combination of functional contrasts and anatomical landmarks to define each participant’s region of interest (ROI). Analyses were anatomically limited to left and right fusiform gyrus using a mask image created in WFU pickatlas (http://fmri.wfubmc.edu; see Figure 2A). We used this broad search space to accommodate any age-related anatomical differences between our participants and the MRI template used.
Figure 2. Search area and regions of interest for individual participants in left hemisphere.
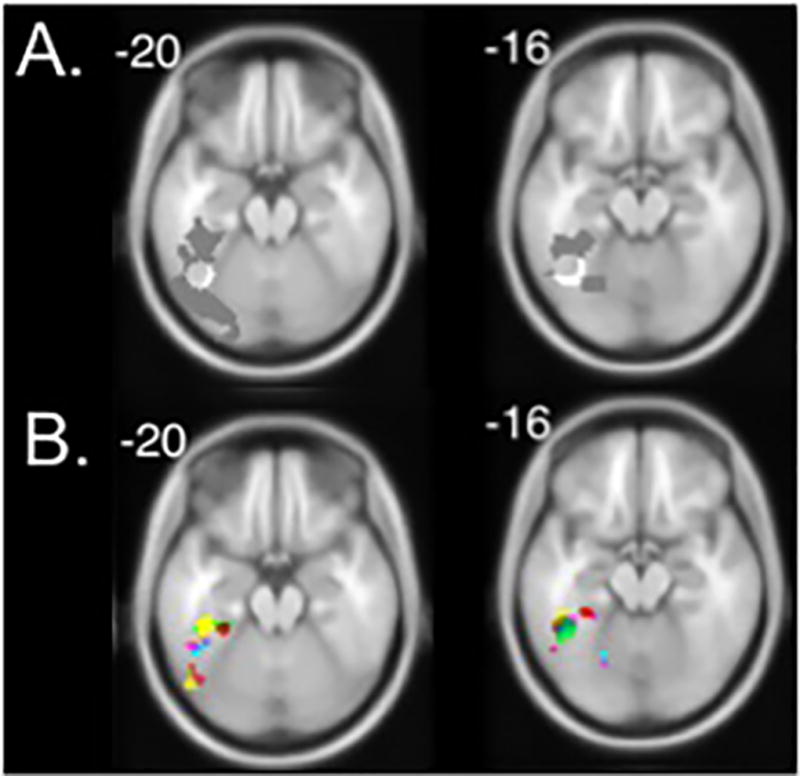
Numbers indicate z-coordinate of axial slices (MNI space) and are presented on a template brain. A. The search area for letter-selective clusters was restricted to the fusiform gyrus, shown in gray. White circle represents the average location of VWFA in adults. B. Functional regions of interest were identified individually for each participant. The locations of ten random individuals’ VWFA ROIs are shown, colors represent different participants. The same process occurred for face-selective ROIs in the left fusiform, and for both letter-selective and face-selective ROIs in the right fusiform.
To identify a letter-sensitive region, each participant’s response to the letters > faces contrast was thresholded to include only voxels with a z-value > 2 (p < 0.0455; Figure 2B). To identify a face-sensitive region, each participant’s response to the faces > letters contrast was thresholded to include only voxels with a z-value > 2 (to include voxels with a p < 0.0455). Letter and face sensitive regions were not constrained in terms of proximity to each other (i.e., they did not have to be adjacent), but contained no overlapping voxels and all existed within the boundaries of fusiform gyrus. In total, four ROIs were defined per participant; two face-sensitive ROIs (FFA, one left- and one right-hemisphere) and two letter-sensitive ROIs (VWFA, one left- and one right-hemisphere). Mean activation (intensity) values were then extracted for each participant’s VWFA and FFA ROIs. In the FFA ROI, we extracted intensity values for a sensitivity contrast (faces > letters). In the VWFA ROI, we extracted intensity values for a sensitivity contrast (letters > faces) and a specificity contrast (letters > false fonts). Spatial extent (number of voxels) of letter sensitive and face sensitive ROIs in each hemisphere were calculated.
Repeated measures ANOVA and post hoc t-tests were used to compare activation across hemispheres and stimuli. Due to the skew in scores on the Word ID measures, Spearman’s rho correlation coefficient (rs) was used to evaluate the relationship between brain responses and these behavioral measures. Correlations not involving this behavioral measure and correlations between brain region sizes or brain region activation intensities were evaluated using Pearson’s correlation coefficient (r). Other t-tests were paired or unpaired as appropriate and were two-tailed.
Results
MRI Data Quality and Behavioral Scores
The mean percentage of outlier frames across all participants was 3.10% ± 2.73% (mean ± standard deviation). The mean stimulus-correlated motion was 0.09 ± 0.03. To evaluate the relation between motion and reading performance, we examined the correlations between head motion and Word ID (raw scores used in all analyses). The number of motion outlier frames was not related to performance on Word ID (Spearman’s correlation, rs = −0.14, p = 0.36). There was also no relationship between stimulus-correlated motion and Word ID score (Spearman’s rs = 0.08, p = 0.61).
Children had high accuracy on Letter ID (M = 32.04 ± 5.72 of 41 total items, raw score range 6-41, all standard scores ≥ 88), demonstrating that in spite of the range of word reading scores (Word ID raw scores from 0 – 68), all children in this group had some letter knowledge (raw scores ≥ 6, standard scores ≥ 88). There was a significant positive relationship between Letter ID and Word ID (rs = 0.59, p < 0.0001). Age was not related significantly to Letter ID (rs = 0.02, p = 0.88) or Word ID (rs = 0.13, p = 0.36) raw scores. Additionally, there was no relationship between nonverbal IQ and Word ID scores (rs = 0.12, p = 0.43) or Letter ID scores (rs = 0.10, p = 0.50).
Identification of VWFA and FFA
VWFA (letters > faces) and FFA (faces > letters) were identified bilaterally in most of the individual children’s brains. In the left fusiform gyrus, 44 out of 48 children (91.7%) had a definable VWFA (>0 voxels) and all children had a definable FFA. In the right fusiform gyrus, 42 out of 48 children (87.5%) had a definable VWFA and 47 out of 48 (97.9%) had a definable FFA.
Relationship between Size of Face- and Letter-Selective Brain Regions
In a repeated measures ANOVA with factors of hemisphere (left vs. right) and region of interest (VWFA as defined by letters > faces vs. FFA as defined by faces > letters), there were significant main effects of both hemisphere (F (1, 94) = 5.75, p = 0.02) and ROI (F (1, 94) = 62.08, p < 0.0001) on the size of the ROI, and a significant interaction between hemisphere and ROI (F (1, 94) = 7.08, p = 0.001). Planned comparisons revealed that right-hemisphere ROIs were significantly larger than left-hemisphere ROIs (t (95) = 2.5, p = 0.01) and FFA was significantly larger than VWFA (t (95) = 7.64, p = 1.7e-11). The interaction reflected the finding that while the left VWFA was not significantly larger than the right VWFA (average size of left VWFA was 88.83 voxels vs. 65.21 voxels in right VWFA; paired t-test; t (47) = 1.35, p = 0.18), the right FFA was significantly larger than the left FFA (average size of left FFA was 222.94 ± 171.54 voxels vs. 336.04 ± 219.28 voxels in the right FFA; paired t-test; t (47) = 4.04, p = 0.0001).
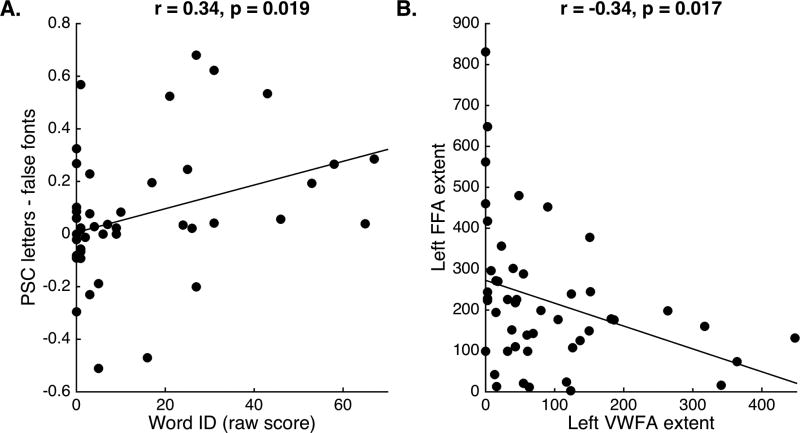
There was a significant and negative correlation between the size of each child’s left-hemisphere VWFA (defined using the letter sensitivity contrast of letters > faces) and their left-hemisphere FFA (defined using the face sensitivity contrast of faces > letters; r = −0.34, p = 0.017, Figure 3B), such that children with larger left-hemisphere VWFA regions had smaller left-hemisphere FFA regions; this relationship did not change when accounting for age. There was no significant correlation between the size of letter-sensitive ROIs in left-hemisphere and the size of face-sensitive ROIs in right-hemisphere (r = −0.04, p = 0.81). There was, however, a significant and negative correlation between ROI size of letter-sensitive regions in right hemisphere and face-sensitive regions in right hemisphere (r = −0.44, p = 0.002) (i.e., a larger FFA was associated with a smaller VWFA in right fusiform cortex). In addition, there was a trend in the positive relationship between the size of left VWFA and right VWFA (r = 0.23, p = 0.10), and a significant positive relationship between the size of left FFA and right FFA (r = 0.53, p = 0.001).
Figure 3. Left-fusiform letter specificity correlated with word reading ability, and left hemisphere VWFA and FFA extent are negatively correlated.
Statistics based on Spearman’s rho (A) and Pearson’s (B) correlations. A. Letter specificity (percent signal change/PSC of letters – false fonts) in left fusiform correlated with word reading ability (raw score on the Word ID subtest of the WRMT-R/NU). B. Greater extent (number of voxels) of left-hemisphere VWFA was significantly and negatively correlated with the extent of left-hemisphere FFA.
Despite the negative correlation between size of left VWFA and size of left FFA, there was no significant relationship between the intensity (percent signal change) of letter responses (> faces) in left VWFA and the intensity of face responses (> letters) in left FFA (r = 0.10, p = 0.48). There was also no significant correlation between intensity of left letter responses in VWFA and face responses in right FFA (r = 0.18, p = 0.21). There was a trend for a positive correlation between letter and face response intensity in the right VWFA and right FFA, respectively (r = 0.26, p = 0.07), such that increased letter responses in right VWFA were associated with increased face responses in right FFA.
Relationships Between Left-Hemisphere Activation or ROI Extent and Reading Ability
We ran several models to determine whether left-hemisphere activation to letters or ROI-size related to individual differences in reading ability. First, there were no relationships between the size of any ROI (left or right, VWFA or FFA) and word reading performance (ps > 0.39). We next characterized the responses of the letter-sensitive left-hemisphere ROI (VWFA ROI) by evaluating whether sensitivity (letters > faces) or specificity (letters > false fonts) related to individual Word ID scores. There was no significant relationship between letter sensitivity and Word ID raw scores (rs = 0.23, p = 0.12). We next evaluated whether letter specificity (percent signal change to letters > false fonts) was related to word reading ability. There was a significant and positive correlation between greater left-hemisphere letter specificity in each individual’s defined VWFA and Word ID raw scores (rs = 0.34, p = 0.02; Figure 3A). This relationship remained significant after controlling for the number of motion outlier frames (rs = 0.38, p = 0.008) and the amount of stimulus-correlated motion (rs = 0.41, p = 0.004).
To ensure that the relation between letter specificity and word reading was due to letter-specific knowledge and not to nonverbal IQ, age, or Letter ID performance, we calculated partial correlations to account for each of these metrics. The positive relation between letter specificity and Word ID raw scores remained significant when nonverbal IQ was added to this model as a covariate (rs = 0.37, p = 0.01), suggesting that the association between letter specificity and Word ID scores was not related to more broad cognitive abilities. The relationship remained significant after the addition of age to the model (r = 0.32, p = 0.03). Finally, this relationship was also not mediated by Letter ID performance, as the relationship between specificity and Word ID survived the addition of Letter ID as a second covariate (r = 0.39, p = 0.009). In addition, Letter ID scores were not significantly related to letter specificity (r = −0.16, p = 0.29).
Discussion
In beginning readers ages 5–6 years who had not yet received formal reading instruction in school, letter specialization in left fusiform cortex was related both to reading ability and response to faces in left fusiform cortex. Better reading ability was associated in particular with greater specificity of activation for letters (letters > false fonts), but not greater sensitivity for letters (letters > faces). Across children, larger extent of left-fusiform letter-sensitive cortex was associated with smaller extent of left-fusiform face sensitive cortex. Specifically, the findings support the hypotheses about left fusiform cortex that in beginning readers (1) letters specificity is associated with word identification, and (2) more extensive cortical letter sensitivity is associated with less extensive cortical face sensitivity. These findings offer new insights into the early growth of letter specialization and the VWFA in the left hemisphere and how that may relate to the development of face specialization in the right hemisphere.
Left Fusiform Letter-Sensitive and Face-Sensitive Cortices in Young Readers
Findings from the present study support the view that an expansion of cortex sensitive to print (VWFA) is associated with a reduction in the cortex sensitive to faces (FFA) in left fusiform cortex. We found a significant and negative relationship between the size of left-hemisphere letter-sensitive cortex and the size of left-hemisphere face-sensitive cortex in children at the beginning stages of learning to read. Neuroimaging evidence from adults suggests that the growth of left-fusiform specialization for print comes at the expense of left-fusiform specialization for faces and results in a right-hemisphere specialization for faces. In literate adults, the FFA exhibits a right-hemisphere lateralization that mirrors the left-hemisphere lateralization of the VWFA, although bilateral loci for the FFA are often apparent (Kanwisher, McDermott, & Chun, 1997). Illiterate adults who learned to read, however, developed a left-lateralized VWFA in tissue that had been responsive to faces (Dehaene et al., 2010). The present findings with children indicate that a similar process of hemispheric specialization occurs developmentally in children as they learn to read.
Two prior findings are consistent with the idea that the growth of print knowledge in children is associated with reduced specialized activation for faces in the left fusiform. One study of 4 year-olds found that better knowledge of letters was associated with reduced responses to faces in the left fusiform cortex (Cantlon et al., 2011). An ERP study examining the N170 response found that adults had left-lateralization for words and right-lateralization for faces, whereas children ages 7–12 exhibited left-lateralization for words and bilateral responses for faces (Dundas, Plaut, & Behrmann, 2014). These findings were interpreted as indicating that word lateralization in the left hemisphere precedes and drives face lateralization in the right hemisphere.
Across children, a smaller VWFA was associated with a larger FFA in the right fusiform gyrus. This finding suggests that as letter sensitivity contracts in the right fusiform gyrus, face-sensitivity expands in the right fusiform gyrus. This expansion may be related to the growing development of right-hemisphere dominance for face perception.
The present findings provide direct, albeit cross-sectional, anatomical evidence in favor of the idea that specialization for print comes at the expense of specialization for faces in left fusiform cortex in typical reading development. These and other findings suggest that there are not parallel developmental specializations for letters in left visual cortex and for faces in right visual cortex. Instead, it appears that bilateral specialization for faces is altered by the growth of left fusiform specialization for print that reduces extent of both right fusiform response to letters and left fusiform specialization for faces. This results in asymmetric right fusiform specialization for faces. This effective competition between print and face specialization for left fusiform tissue occurred in illiterate adults learning to read (Dehaene et al., 2010), and in older children by ERP measures (Dundas, Plaut, Behrmann, 2015), and the present study provides direct evidence in favor of such competition in typical development. Although the present findings have limitations that can only be clarified in longitudinal studies, they are consistent with the idea that the growth of print specialization in left fusiform cortex that is associated with the growth of reading ability drives the development of face specialization in the right fusiform cortex by reducing extent of face specialization in the left hemisphere (Dundas, Plaut, & Behrmann, 2013; Dundas, Behrmann, & Plaut, 2015).
Sensitivity versus Specificity for Letter Responses in Left Fusiform Gyrus
In this study, most children exhibited a region of sensitivity to letters > faces in the left fusiform cortex at our a priori threshold, but neither the spatial extent nor the magnitude of letter-sensitive activation correlated with children’s performance on reading (Word ID). The finding that this sensitivity exists in children at this young age, but that the intensity of activation in the sensitive region does not relate to reading ability is consistent with previous research showing significantly greater activation to letters versus faces in left fusiform, but no relation between activation in such regions with letter naming among 4 year olds who were mostly prereaders (Cantlon et al., 2011).
Some specificity for print (that is, activation for print as compared to print-like stimuli such as false fonts and consonant strings) has been demonstrated in typical readers as young as age 7 (Olulade et al., 2013, 2015; Vinckier et al., 2007), an age by which children in the US have had formal reading instruction. In the current study, children at age 5 with higher Word ID scores also exhibited greater specificity for print in VWFA, which supports the hypothesis that specificity is related to the development of early reading ability (Lochy, Reybroeck, Rossion, 2016; Maurer et al., 2005). The relation between VWFA specificity and reading ability is further supported by evidence that greater specificity for print relative to nameable objects was related to novel word decoding abilities in children age 7–14 (Centanni et al., 2017) and that a growth of specificity for words (compared to consonant strings) has been observed in previously illiterate adults learning to read compared to adults who had not yet learned to read (Dehaene et al., 2010).
Alhough we observed a significant relationship between VWFA specificity for print and Word ID scores, we did not observe any relationship between such specificity and Letter ID scores, even though Letter ID and Word ID scores are highly correlated. This could be due to a ceiling effect on Letter ID as nearly every child knew most of their letters. This finding could suggest that the acquisition of specificity provides additional gains in reading that are not accounted for by letter identification ability.
Early Sensitivity and Specificity for Print in Beginning Readers
The distinction between sensitivity versus specificity in responses to print in the left fusiform gyrus provides a framework for understanding two related, but distinct, developmental processes in the initial stages of learning to read. Sensitivity (e.g., for letters relative to faces) may reflect domain-specific perceptual development. Such perceptual development may foster the growth of orthographic specialization in the same left-hemisphere that hosts language cortex, and thus reduces left-hemisphere specialization for face perception (which in turn may promote right-hemisphere specialization and dominance for face perception). Specificity (e.g., for letters relative to perceptually matched false fonts) may reflect a visual form of linguistic development that associates print with meaning (e.g., reading) following the leftward lateralization of the reading network.
Initially, processing of letters is bilateral, because letters are visual objects requiring right-hemisphere processing of spatial information and relationships between lines and curves. In the current study, we found no significant differences, on average, between the sizes of the VWFA ROI in left vs. right hemisphere, although the left VWFA was, on average, slightly larger than the right VWFA. This suggests that letter-selective cortex may exist in both hemispheres early in reading development to support letter processing. Bilateral processing of letters was also observed in kindergarten children practicing letter recognition in a grapheme-centered game (Brem et al., 2010). Over the course of training, sensitivity to letters in these systems increased, but no lateralization of print processing had yet emerged. Further, the N1 response to words compared to symbol strings and pseudowords is lateralized in adults, but no lateralization was observed in kindergarten children (Maurer et al., 2005).
Lateralization for known print likely occurs later in the developmental trajectory for reading. In children as old as 12, left VWFA does not yet differentiate between letters and their mirror-reversals (Blackburne et al., 2014) and adult-like specialization for print is not yet present (Centanni et al., 2017), suggesting a long trajectory for the lateralization and specialization of the VWFA.
Study Design Considerations and Limitations
Several limitations of the present study can be considered. First, both the functional and structural localizations reported here reflect particular analytic strategies. In regards to function, we used constant a priori threshold of z > 2 (or p < 0.0455) to create individual first-level maps, which were then used across all analyses, but findings could vary with more or less conservative thresholds. Second, because we were interested in the earliest stages of reading acquisition during kindergarten, children varied in the degree of parental and pre-school reading instruction they received prior to starting kindergarten. Due to this variability, we were unable to determine whether the degree of specificity observed was due to instruction or general exposure to letters and words during development. Although some children could read well already and all eventually became typical readers by the end of second grade, many children could not read any words on the Word ID measure at the time they were assessed. Therefore, a sizable portion of the Word ID scores were clustered around zero. To account for this skew in the distribution, we used Spearman’s rho correlations rather than Pearson’s correlations. Third, all our analyses are correlational, thus designs such as training studies (e.g., Brem et al., 2010), will be needed to confirm these associations. Fourth, longitudinal designs will be more determinative about the cross-sectional pattern of activations that we found, including direct evidence about related fusiform lateralizations for print and faces.
Finally, we measured fMRI responses to individual letters rather than words, whereas the VWFA has most often been studied in relation to words. We used single letters because so many children at this age can read very few words, and findings with words as stimuli may have been difficult to interpret when comparing children with substantially varying reading abilities (in contrast, all the children were familiar with individual letters). The precise relation between brain responses to individual letters versus words is, however, complex. In adults, responses to individual letters and letter strings appear to occur in separable regions of the left fusiform cortex (James et al., 2005). There is evidence, however, that there is not a single VWFA, but rather a gradient exists in the fusiform gyrus such that selective activation for print increases in a posterior to anterior direction (Flowers et al., 2004; James et al., 2005; Vinckier et al., 2007). The fact that letter specificity in left fusiform cortex was associated with word reading ability in the present study indicates that we were measuring print-specific responses important for word reading, but future studies will be needed to directly relate responses to single letters and responses to words in beginning readers.
Highlights.
-
-
Compared brain activation to letters, false fonts, and faces in 5-year-old children
-
-
Greater specificity for letters in left fusiform correlated with better reading
-
-
Left fusiform face area was inversely related to size of left visual word form area
-
-
Left fusiform gyrus is selective to letters prior to school reading instruction
Acknowledgments
The authors thank Abigail Cyr, Keri-Lee Garel, Candice Coulter, and Andrew Peach for assistance with assessment and MRI data collection. We thank the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT and its staff. We also thank our READ Study research testers, school coordinators and principals, and participating families. Participating schools are listed at http://gablab.mit.edu/index.php/READstudy. This work was supported by a grant from NIH/NICHD (R01HD067312) to JDEG and NG.
References
- Baker C, Liu J, Wald L, Kwong KK, Benner T, Kanwisher N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc. Natl. Acad. Sci. 2007;104:9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt W. Barratt Simplified Measure of Social Status (BSMSS) Terre Haute, IN: Indiana State University; 2006. [Google Scholar]
- Ben-Shachar M, Dougherty R, Deutsch G, Wandell B. The development of cortical sensitivity to visual word forms. J. Cogn. Neurosci. 2011;23:2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburne LK, Eddy MD, Kalra P, Yee D, Sinha P, Gabrieli JDE. Neural correlates of letter reversal in children and adults. PLoS One. 2014;9:e98386. doi: 10.1371/journal.pone.0098386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst G, Cachia A, Vidal J, Simon G, Fischer C, Pineau A, Poirel N, Mangin JF, Houdé O. Folding of the anterior cingulate cortex partially explains inhibitory control during childhood: A longitudinal study. Dev. Cogn. Neurosci. 2014;9:126–135. doi: 10.1016/j.dcn.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. Tuning of the visual word processing system: distinct developmental ERP and fMRI effects. Hum. Brain Mapp. 2009;30:1833–1844. doi: 10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlgger BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cai Q, Lavidor M, Brysbaert M, Paulignan Y, Nazir TA. Cerebral lateralization of frontal lobe language processes and lateralization of the posterior visual word processing system. J. Cogn. Neurosci. 2008;20:672–681. doi: 10.1162/jocn.2008.20043. [DOI] [PubMed] [Google Scholar]
- Cai Q, Paulignan Y, Brysbaert M, Ibarrola D, Nazir TA. The left ventral occipito-temporal response to words depends on language lateralization but not on visual familiarity. Cereb. Cortex. 2010;20:1153–1163. doi: 10.1093/cercor/bhp175. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Pinel P, Dehaene S, Pelphrey KA. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb. Cortex. 2011;21:191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, King LW, Eddy MD, Whitfield-Gabrieli S, Gabrieli JDE. Development of sensitivity versus specificity for print in the visual word form area. Brain and Language. 2017;170:62–70. doi: 10.1016/j.bandl.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga L, Ventura P, Nunes Filho G, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;330:1359–64. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Dundas EM, Plaut DC, Behrmann M. Variable left-hemisphere language and orthographic lateralization reduces right-hemisphere face lateralization. J. Cogn. Neurosci. 2015;27:913–925. doi: 10.1162/jocn_a_00757. doi.org/10.1162/jocn_a_00757. [DOI] [PubMed] [Google Scholar]
- Dundas EM, Plaut DC, Behrmann M. The joint development of hemispheric lateralization for words and faces. J. Exp. Psychol. Gen. 2013;142:348–358. doi: 10.1037/a0029503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody Picture Vocabulary Test (PPVT-4) Pearson Assessments 2007 [Google Scholar]
- Flowers DL, Jones K, Noble K, VanMeter J, Zeffiro TA, Wood FB, Eden GF. Attention to single letters activates left extrastriate cortex. Neuroimage. 2004;21:829–839. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clemenceau S, Volle E, Hasboun D, Cohen L. Direct intracranial, fMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50(2):191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Kakunoori S, Augustinack J, Nieto-Castanon A, Kovelman I, Gaab N, Christodoulou JA, Triantafyllou C, Gabrieli JDE, Fischl B. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage. 2010;53:85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer L, Riesenhuber M. Individual variability in location impacts orthographic selectivity in the “visual word form area”. J. Neurosci. 2013;33:11221–11226. doi: 10.1523/JNEUROSCI.5002-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Rapoport JL. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National academy of the United States of America. 2004;101(210):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns C, Madison C, Clark D, Halchenko Y, Waskom M, Ghosh S. Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in Python. Front. Neuroinform. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Grant PE, Gaab N. Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cereb. Cortex. 2016;26:1138–48. doi: 10.1093/cercor/bhu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong AC, Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cogn Affect Behav Neurosci. 2005;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. K-BIT 2: Kaufman brief intelligence test. (Second) 2004 [Google Scholar]
- Kim NY, Lee SM, Erlendsdottir MC, McCarthy G. Discriminable spatial patterns of activation for faces and bodies in the fusiform gyrus. Front Hum Neurosci. 2014;8:632. doi: 10.3389/fnhum.2014.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochy A, Reybroeck M, Van Rossion B. Left cortical specialization for visual letter strings predicts rudimentary knowledge of letter-sound association in preschoolers. Proc Natl Acad Sci U S A. 2016;113:8544–8549. doi: 10.1073/pnas.1520366113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. Department of Clinical Neuroscience, Psychology section, Karolinska Institutet. 1998. The Karolinska Directed Emotional Faces – KDEF. [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N.Y. Acad. Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Martin A, Kronbichler M, Richlan F. Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Hum. Brain Mapp. 2016;37:2676–2699. doi: 10.1002/hbm.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Brandeis D. Emerging neurophysiological specialization for letter strings. J. Cogn. Neurosci. 2005;17:1532–1552. doi: 10.1162/089892905774597218. [DOI] [PubMed] [Google Scholar]
- Maurer U, Zevin JD, McCandliss BD. Left-lateralized N170 effects of visual expertise in reading: evidence from Japanese syllabic and logographic scripts. J. Cogn. Neurosci. 2008;20:1878–1891. doi: 10.1162/jocn.2008.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss B, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore J, Allison T. Face-specific processing in the human fusiform gyrus. J. Cogn. Neurosci. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- Olulade O, Flowers DL, Napoliello EM, Eden GF. Dyslexic children lack word selectivity gradients in occipito-temporal and inferior frontal cortex. NeuroImage. Clin. 2015;7:742–54. doi: 10.1016/j.nicl.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade O, Flowers D, Napoliello E, Eden G. Developmental differences for word processing in the ventral stream. Brain Lang. 2013;125:134–145. doi: 10.1016/j.bandl.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov-Palchik O, Norton ES, Sideridis G, Beach SD, Wolf M, Gabrieli JD, Gaab N. Longitudinal stability of pre-reading skill profiles of kindergarten children: implications for early screening and theories of reading. Developmental Science. 2017;20(5):e12471. doi: 10.1111/desc.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C, Devlin J. The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T, Wolf D, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z, Norton ES, Osher DE, Beach SD, Cyr AB, Ozernov-Palchik O, Yendiki A, Fischl B, Gaab N, Gabrieli J. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013;33:13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z, Osher D, Norton ES, Youssoufian D, Beach SD, Feather J, Gaab N, Gabrieli JD, Kanwisher N. Connectivity precedes function in the development of the visual word form area. Nat. Neurosci. 2016 doi: 10.1038/nn.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B, McCandliss B. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Szwed M, Dehaene S, Kleinschmidt A, Eger E, Valabrègue R, Amadon A, Cohen L. Specialization for written words over objects in the visual cortex. Neuroimage. 2011;56:330–344. doi: 10.1016/j.neuroimage.2011.01.073. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn. Reson. Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers D, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, Kronbichler M, Loenneker T, Klaver P, Martin E, Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Ventura P, Fernandes T, Cohen L, Morais J, Kolinsky R, Dehaene S. Literacy acquisition reduces the influence of automatic holistic processing of faces and houses. Neurosci. Lett. 2013;554:105–9. doi: 10.1016/j.neulet.2013.08.068. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus J, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wiederholt J, Bryant B. Gray Oral Reading Test- Fifth Edition (GORT-5) Pro-Ed; Austin, TX: 2012. [Google Scholar]
- Wolf M. Proust and the squid: the story and science of the reading brain. Harper, New York: 2008. [Google Scholar]
- Woodcock RW. Woodcock reading mastery tests, revised. American Guidance Service; Circle Pines, MN: 1998. [Google Scholar]