Abstract
Background
During intensive BP lowering, acute declines in renal function are common, thought to be hemodynamic, and potentially reversible. We previously showed that acute declines in renal function ≥20% during intensive BP lowering were associated with higher risk of ESRD. Here, we determined whether acute declines in renal function during intensive BP lowering were associated with mortality risk among 1660 participants of the African American Study of Kidney Disease and Hypertension and the Modification of Diet in Renal Disease Trial.
Methods
We used Cox models to examine the association between percentage decline in eGFR (<5%, 5% to <20%, or ≥20%) between randomization and months 3–4 of the trials (period of therapy intensification) and death.
Results
In adjusted analyses, compared with a <5% eGFR decline in the usual BP arm (reference), a 5% to <20% eGFR decline in the intensive BP arm was associated with a survival benefit (hazard ratio [HR], 0.77; 95% confidence interval [95% CI], 0.62 to 0.96), but a 5% to <20% eGFR decline in the usual BP arm was not (HR, 1.01; 95% CI, 0.81 to 1.26; P<0.05 for the interaction between intensive and usual BP arms for mortality risk). A ≥20% eGFR decline was not associated with risk of death in the intensive BP arm (HR, 1.18; 95% CI, 0.86 to 1.62), but it was associated with a higher risk of death in the usual BP arm (HR, 1.40; 95% CI, 1.04 to 1.89) compared with the reference group.
Conclusions
Intensive BP lowering was associated with a mortality benefit only if declines in eGFR were <20%.
Keywords: AASK (African American Study of Kidney Disease and Hypertension), chronic renal disease, hypertension, mortality risk
Acute declines in renal function occur frequently during intensive BP lowering.1–4 These effects of BP lowering have typically been thought to be hemodynamic in nature and a harbinger of potential benefits of BP treatment, especially in the setting of renin-angiotensin-aldosterone system inhibition.5–8 However, it is possible that patients who receive intensive BP lowering may experience a higher incidence of AKI or be at higher risk for chronic renal hypoperfusion, which may, in turn, lead to accelerated progression of CKD.9–13
Some recent studies have raised concerns that adverse kidney outcomes may be more likely to occur with an intensive BP lowering strategy. For example, in the Systolic Blood Pressure Intervention Trial (SPRINT), there was a higher prevalence of AKI among participants assigned to the lower BP target, although the vast majority of these participants recovered from these AKI events.12,14 In the Secondary Prevention of Small Subcortical Strokes Trial, participants in the intensive BP control arm were observed to have more rapid decline in renal function (>30% decline in eGFR) within the first year of follow-up, but rapid decline was only associated with higher risk for adverse events in the usual, but not the intensive treatment, arm.15 In our prior work, we showed that acute declines in renal function ≥20% during the period of intensive BP lowering in the African American Study of Kidney Disease and Hypertension (AASK) and the Modification of Diet in Renal Disease (MDRD) Trial were associated with a higher long-term risk of ESRD in the intensive (versus usual) BP treatment arm (compared with those with a <5% decline in the usual BP arm).10
If intensive BP lowering is associated with a higher risk of AKI or ESRD among the subset of patients with greater declines in renal function during intensification of therapy, it is possible that these patients would also have a higher (rather than lower) risk of death due to the morbidity and mortality associated with events, such as AKI or ESRD.16 The objective of this study was to examine the association between acute changes in renal function and long-term risk of death in two completed trials of intensive BP lowering in CKD: the AASK and the MDRD Trial.
Methods
Study Populations
The AASK was a large 2×3 factorial randomized trial that assessed the effect of intensive BP control and different antihypertensive agents on the progression of CKD in black participants with hypertension-attributed CKD.17,18 Between 1995 and 2001, 1094 participants between 18 and 70 years of age with GFR of 20–65 ml/min per 1.73 m2 were randomized to either intensive (mean arterial pressure [MAP] ≤92 mm Hg) or usual (MAP=102–107 mm Hg) BP control. Patients were also simultaneously randomized to an angiotensin-converting enzyme (ACE) inhibitor (ramipril), β-blocker (metoprolol), or calcium channel blocker (amlodipine) as their first-line antihypertensive agent in 2:2:1 allocation. At trial closure, 691 participants (87% of eligible participants) who had not developed ESRD or died continued in the cohort phase of the study, which began in 2002 and ended in 2007.17–19 All AASK cohort participants were switched to an ACE inhibitor as first-line therapy or an angiotensin receptor blocker if an ACE inhibitor could not be tolerated. During the AASK cohort study, all participants were treated to a BP target of <140/90 mm Hg, and this target was changed to <130/80 mm Hg after 2004.18,20
The MDRD Trial randomized 840 participants with nondiabetic CKD between 18 and 70 years of age with GFR of 13–55 ml/min per 1.73 m2 to either intensive (MAP<92 or <98 mm Hg depending on age) or usual (MAP<107 or <113 mm Hg) BP control between 1989 and 1993.21 ACE inhibitors were encouraged as first-line antihypertensive agents.21 We combined the AASK and the MDRD Trial in this study to enhance power and because we found similar associations between the magnitude of decline in renal function during intensive BP lowering and long-term risk of ESRD in our prior study.10 Additionally, we tested for but did not find the presence of effect modification by trial (the AASK versus the MDRD Trial) or BP agent use (ACE inhibitor versus other agents) when we examined the association between the exposure and outcome of interest in this study (all P>0.05 for interaction), and hence, trial data were pooled for analyses.
Exposure
The main exposure of interest was the acute change in eGFR over the period of time that reflects active titration of antihypertensive regimens to achieve MAP goals in the AASK and the MDRD Trial. Change in renal function was determined as the percentage change in eGFR between baseline and visit 3 (approximately month 3) in the AASK and between baseline and visit 4 (approximately month 4) in the MDRD Trial as previously described.10
We categorized the changes in renal function that occurred during intensification of antihypertensive therapy as <5% (small), 5% to <20% (moderate), or ≥20% (large) change and further subdivided participants according to their randomized assignment to either intensive or usual BP control as previously described.10 These cutoffs were chosen a priori on the basis of results from our prior study,10 which showed a renal benefit to intensive BP lowering when the acute declines in eGFR were <20% but a higher risk of ESRD with declines in eGFR≥20% in the intensive BP arm.
In sensitivity analyses, we also used lower (<5%, 5% to <15%, or ≥15%) and higher thresholds (<5%, 5% to <25%, or ≥25%) to categorize the changes in renal function that occurred during intensification of antihypertensive therapy.
Finally, to further explore whether the threshold of decline in renal function that associates with increased mortality risk was similar to the threshold for increased ESRD (as was observed in our prior study),10 we also took a data-driven approach to examining this issue. We divided the percentage change in renal function into 5% increments ranging from a 5% to a 40% decline in renal function during intensification of antihypertensive therapy separately for the intensive and usual BP treatment arms. We then examined the association between these 5% incremental declines in renal function and mortality risk (using a <5% decline in the usual BP arm as the reference group). We were specifically interested in the threshold of decline in renal function in the intensive BP treatment arm where the benefit of intensive BP lowering (hazard ratio [HR] <1) would no longer be observed (HR>1) for the risk of death.
As previously described, 195 AASK participants were excluded from our study due to missing central laboratory serum creatinine (n=151), missing long-term follow-up data (n=27), or deaths (n=17) at or before visit 3.10 In the MDRD Trial, we excluded 77 participants from the MDRD Trial analyses due to missing creatinine at the month 4 visit, and two additional patients were excluded who died before the month 4 visit.10
Outcome Ascertainment
The primary outcome of interest was all-cause mortality. Long-term ascertainment of vital status through national death indices in the AASK and the MDRD Trial participants has been previously described. Participants were censored from our analyses if they died or censored administratively on June 30, 2012 in the AASK and December 31, 2010 in the MDRD Trial. We included deaths that occurred both before and after onset of ESRD, because we were interested in mortality regardless of ESRD status. We performed linkage of former AASK and MDRD Trial participants to the US Renal Data System for ascertainment of ESRD status as previously described.22–24 We defined ESRD as receipt of chronic dialysis or kidney transplant.
Statistical Analyses
We first examined baseline characteristics of participants by categories of decline in renal function separately in the intensive and usual BP treatment arms.
Next, we assessed the risk for death according to our six-level primary predictor (<5%, 5% to <20%, and ≥20% declines in the strict or usual BP arms) using unadjusted Cox models in the AASK and the MDRD Trial adjusted for data source. We subsequently adjusted these models for baseline age, race, sex, heart disease (defined as the presence of atherosclerotic disease or heart failure by a combination of self-report, chart review, and baseline electrocardiogram data in the AASK and defined as the presence of coronary artery disease in the MDRD Trial), baseline MAP, baseline eGFR (categorized by CKD stages 2, 3, or 4 for non-normality), baseline ACE inhibitor use (yes/no), baseline proteinuria (<150 or ≥150 mg/g, which was the median in this population and categorized for non-normality), and trial data source (the AASK versus the MDRD Trial). We considered this adjusted model our primary analysis. We tested for effect modification in the association between categories of decline in renal function and mortality risk by randomized BP arm assignment. In sensitivity analyses, we also repeated our primary analysis adjusting for proteinuria as a continuous variable.
To confirm the threshold at which acute declines in renal function would be associated with higher risk of death, in sensitivity analyses, we repeated our unadjusted and adjusted Cox models using a 15% or 25% threshold of decline in eGFR (in lieu of the 20% threshold used in our primary analysis) for risk of death.
Finally, to further explore the threshold at which acute declines in renal function would be associated with higher risk of death, we repeated our adjusted Cox models using 5% increments of decline in renal function as the predictor of interest for mortality risk in adjusted models as described above. We then examined the threshold of decline in renal function that was associated with a switch from potential benefit (HR<1) to potential harm (HR>1) from an intensive BP lowering strategy.
We used Stata 14 (StataCorp LP) for all statistical analyses, and our main analyses were verified by a separate analyst using SAS Version 9 (SAS Institute). Institutional review board approval was obtained at the University of California, San Francisco and the Cleveland Clinic, and deidentified National Institute of Digestive and Diabetes and Kidney Diseases Central Repository datasets were used for analyses.
Results
The baseline characteristics of the 1660 pooled AASK and MDRD Trial participants included for study and stratified by the magnitude of renal function decline during the period of intensification of antihypertensive therapy are shown in Table 1. Participants with larger acute declines in renal function had higher baseline proteinuria and larger declines in MAP in the intensive compared with usual BP arm in both the AASK and the MDRD Trial (Table 1). Overall, the incidence of death was 3.3 per 100 person-years in the intensive BP treatment arm and 3.8 per 100 person-years in the usual treatment arm.
Table 1.
Characteristics of the African American Study of Kidney Disease and Hypertension and the Modification of Diet in Renal Disease Trial participants according to treatment arm and percent renal function decline from the time of randomization until month 3–4
| AASK and MDRD Trial Baseline Characteristics, n=1660 | Intensive BP Control | Usual BP Control | ||||
|---|---|---|---|---|---|---|
| <5% Decline, n=461 | 5% to <20% Decline, n=289 | ≥20% Decline, n=86 | <5% Decline, n=501 | 5% to <20% Decline, n=234 | ≥20% Decline, n=89 | |
| Mean age ± SD, yr | 53.8±10.8 | 52.4±12.4 | 51.8±12.7 | 53.4±11.3 | 53.2±10.7 | 52.8±12.5 |
| Men, N (%) | 277 (60.1) | 196a (67.8) | 51 (59.3) | 329 (65.7) | 129a (55.1) | 51 (57.3) |
| Baseline MAP ± SD, mm Hg | 106.3±16.6 | 108.0±15.4 | 108.9±13.2 | 105.8±15.5 | 106.3±13.8 | 107.7±13.5 |
| Difference between baseline and achieved MAP ± SD between baseline and 3-mo visit, mm Hg | 6.5±18.6a | 10.8±16.4a | 10.9±16.4a | 0.78±15.4a | 3.7±13.7a | 5.2±15.2a |
| Baseline median proteinuria [IQR], g/d | 0.12 [0.04–0.48] | 0.22a [0.06–1.09] | 1.11 [0.12–2.95] | 0.09 [0.04–0.43] | 0.42a [0.10–1.46] | 1.47 [0.18–3.1] |
| Baseline median serum creatinine [25th–75th percentile], mg/dl | 1.9 [1.5–2.5] | 2.0 [1.6–2.6] | 2.2 [1.6–3.1] | 1.9 [1.6–2.4] | 1.9 [1.6–2.6] | 2.4 [1.8–3.2] |
| Baseline mean eGFR ± SD, ml/min per 1.73 m2 | 39.6±14.0 | 38.6±14.5 | 35.0±15.3 | 40.1±13.7 | 36.7±14.2 | 31.2±13.0 |
| Baseline heart disease, N (%) | 169 (36.7) | 95a (32.9) | 22 (25.6) | 178 (35.5) | 57a (24.4) | 24 (27.0) |
| Assignment to ACE inhibitor use, N (%) | 174 (37.7) | 118 (40.8) | 27 (31.4) | 179 (35.7) | 85 (36.3) | 33 (37.1) |
AASK, African American Study of Kidney Disease and Hypertension; MDRD, Modification of Diet in Renal Disease; MAP, mean arterial pressure; IQR, interquartile range; ACE, angiotensin-converting enzyme.
P<0.05 comparing intensive with usual BP arm.
A total of 86 participants in the intensive BP treatment arm and 89 participants in the usual BP treatment arm experienced a 20% or greater decline in renal function during the period of intensification of antihypertensive therapy. A total of 800 deaths (417 in the usual BP arm) and 912 cases of ESRD (466 in the usual BP arm) occurred in the AASK and the MDRD Trial during median follow-up of 9.9 (interquartile range, 4.0–11.0) years.
A small (<5%) change in renal function in the intensive BP arm was not associated with a statistically significantly difference in risk of death compared with a small change (<5%) in renal function in the usual BP arm (where declines are presumed to be secondary to natural CKD progression) in either unadjusted or adjusted analyses (Table 2). Moderate declines (5% to <20%) in renal function in the intensive BP arm were associated with a lower risk of death (adjusted HR, 0.77; 95% confidence interval [95% CI], 0.62 to 0.96) compared with the reference group (<5% in the usual BP arm), but renal function declines of the same magnitude (5% to <20%) in the usual BP arm were not (adjusted HR, 1.01; 95% CI, 0.81 to 1.26). There was a statistically significant difference comparing the risk of death in the intensive (HR, 0.77; 95% CI, 0.62 to 0.96) BP arm with that in the usual (HR, 1.01; 95% CI, 0.81 to 1.26) BP arm among participants who exhibited a 5% to <20% decline in renal function (P=0.04).
Table 2.
Association between percentage decline in renal function in the African American Study of Kidney Disease and Hypertension and the Modification of Diet in Renal Disease Trial participants from time of randomization until months 3–4 and risk of death
| Renal Function Decline, % | Intensive BP Arm Hazard ratio (95% CI) | Usual BP Arm Hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Death Ratea | Unadjusted Model | Adjusted Modelb | N | Death Ratea | Unadjusted Model | Adjusted Modelb | |
| <5 | 461 | 3.2 | 0.93 (0.78 to 1.13) | 0.87 (0.72 to 1.05) | 501 | 3.4 | 1.0 (Reference) | 1.0 (Reference) |
| 5 to <20 | 289 | 3.0 | 0.88c (0.71 to 1.10) | 0.77c (0.62 to 0.96) | 234 | 3.9 | 1.16c (0.93 to 1.44) | 1.01c (0.81 to 1.26) |
| ≥20 | 86 | 4.3 | 1.31 (0.97 to 1.79) | 1.18 (0.86 to 1.62) | 89 | 5.5 | 1.74 (1.30 to 2.32) | 1.40 (1.04 to 1.89) |
95% CI, 95% confidence interval.
Per 100 person-years.
Adjusted for age, sex, race, baseline heart disease, baseline angiotensin-converting enzyme inhibitor use, baseline eGFR category, baseline proteinuria category, baseline mean arterial pressure, and stratified by trial data source (the Modification of Diet in Renal Disease Trial or the African American Study of Kidney Disease and Hypertension).
Hazard ratios are statistically significantly different comparing intensive and usual BP arms (P<0.05).
A large (≥20%) decline in renal function was not associated with a higher risk of death in the intensive BP arm, but it was associated with a statistically significantly higher risk of death in the usual BP arm compared with the reference group (<5% decline in the usual BP arm) (Table 2). There was no statistically significant difference when comparing the risk of death in the intensive versus usual BP arm among participants with a ≥20% decline in renal function (P=0.36 for interaction). Similar findings were noted when we repeated our primary analysis accounting for proteinuria as a continuous variable (Supplemental Table 1).
When we changed the threshold to define a moderate decline in renal function as ≥15% (with moderate decline thus 5% to <15%) in sensitivity analysis, the data were similar to those of our primary analysis. The AASK participants assigned to strict BP control who had a 5% to <15% decline in renal function had a statistically significantly lower risk of death (Supplemental Table 2). However, the AASK participants assigned to usual BP control with a 5% to <15% decline in eGFR did not have a lower risk of death (P=0.03 for interaction).
When we changed the threshold to define a moderate decline in renal function to ≥25% (with moderate decline thus 5% to <25%) in sensitivity analysis, there was not a statistically significantly lower risk of death in the AASK participants assigned to strict BP control with a 5% to <25% decline in renal function in either the strict BP control arm or the usual BP control arm (Supplemental Table 2). There was also no longer a statistically significant interaction in the risk of death of the strict and usual BP control arm participants who experienced a 5% to <25% decline in renal function during intensification of antihypertensive therapy.
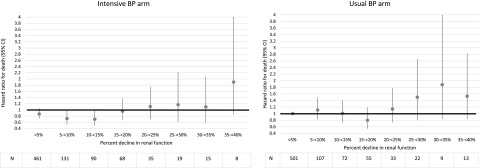
When we repeated our adjusted models using smaller increments of change in renal function (5%) as the predictor of death in exploratory analysis, we found that there was a change in the point estimate for the HR from <1 to >1 around a 20% decline in renal function in the intensive BP control arm (Figure 1). When declines in renal function were in the 5%–15% range, there was a statistically significant survival benefit from an intensive BP lowering strategy (compared with the reference group of <5% decline in the usual BP arm). However, with incremental increases in the magnitude of renal function decline beyond 15%, intensive BP lowering became progressively associated with a higher risk of death in the strict BP arm (versus the reference group).
Figure 1.
Acute declines in renal function of <15% in the intensive BP arm is associated with lower mortality risk compared to the reference group (<5% decline in the usual BP arm). Association between 5% increments in renal function decline and risk of death in adjusted Cox models using a <5% decline in renal function in the usual BP arm as the reference group. All hazard ratios are adjusted for age, sex, race, baseline heart disease, baseline angiotensin-converting enzyme inhibitor use, baseline eGFR category, baseline proteinuria category, baseline mean arterial pressure, and stratified by trial data source. 95% CI, 95% confidence interval.
In contrast, the point estimate for the risk of death was almost always >1 for participants in the usual BP arm after the decline in renal function exceeded >5% compared with those with declines in renal function that were <5% in the usual BP arm.
Discussion
We previously showed that a subset of the AASK and the MDRD Trial participants who had 20% or greater decline in renal function during the period of intensification of antihypertensive therapy had a higher risk of ESRD compared with participants with small eGFR changes receiving usual BP control.10 In this follow-up study, we found that declines in eGFR of <20% during intensive BP lowering were associated with a survival benefit compared with a <5% decline in eGFR in the usual BP arm. The magnitude of mortality benefit (approximately a 25% reduction in risk of death) associated with intensive BP control in the 5% to <20% decline in eGFR was similar to that observed in the SPRINT.26 However, among individuals with a ≥20% decline in renal function, intensive BP control was not associated with risk of death compared with in those with a <5% decline in eGFR in the usual BP arm.
The American Heart Association recently released updated BP guidelines and changed the systolic BP target for patients with CKD from <140 to <130 mm Hg.27 This change was primarily on the basis of results of the SPRINT, which showed a cardiovascular (CV) and mortality benefit to intensively lowering systolic BP among patients with and without CKD.14 However, most clinical trials of BP control only provide a mean follow-up duration of approximately 3 years, and whether these targets apply to patients with more advanced CKD and those who have higher levels of proteinuria remains unclear.14,21,26,28 We believe that our data are complementary to those of the SPRINT, and they provide reassurance that declines in eGFR up to 20% in the intensive BP arm seem be associated with lower mortality risk during long-term follow-up and no increased risk of ESRD.10 Furthermore, a large proportion (more than one third) of the AASK and the MDRD Trial participants who were assigned to intensive BP control experienced declines in eGFR in the range (5% to <20%) that was associated with this mortality benefit. Although both the MDRD Trial and the AASK targeted MAPs in the <92- to 98-mm Hg range and did not target systolic BP, these MAP levels are comparable with systolic BP values in the range of 120–130 mm Hg. We believe that our data are additive to the SPRINT results given that the AASK and the MDRD Trial participants had higher levels of proteinuria and lower levels of renal function at baseline, and our study provides significantly longer follow-up of participants.14,18,21
Intensive BP lowering has been shown to be cost effective in simulation models, regardless of whether the CV benefits from this treatment strategy are sustained lifelong or reduced after 5 years.25 Yet, at the same time, the risk of serious adverse events, including increased risk of AKI and worsening of renal function, has been a subject of concern with an intensive BP control strategy.12,14,30 In the SPRINT, the episodes of AKI associated with intensive BP lowering seemed reversible in the majority of participants and without long-term sequelae, but the follow-up duration in the SPRINT was limited, and the level of baseline renal dysfunction among participants with CKD was only mild to moderate.12 In our study, the participants who benefited the most from intensive BP lowering (either from an ESRD10 or mortality standpoint) had small to moderate acute declines in renal function (e.g., up to 20%). Interestingly, the patients who benefited the most from intensive BP control did not seem to be the participants who had no changes to their renal function (e.g., <5% decline) in response to antihypertensive therapy intensification. We hypothesize that this range of acute eGFR decline may signal successful reduction of glomerular pressure and prevention of further renal injury, which may reduce the risk of ESRD and death.6,7 Thus, if our results are confirmed, it would be important for providers not to deintensify therapy in response to these moderate acute changes in renal function during intensive BP lowering (e.g., <20%). However, we speculate that, when larger hemodynamic declines in renal function (e.g., in the range of ≥20%) occur in response to intensive BP lowering, the reversibility of such declines may diminish over time in the setting of worsening renal autoregulation with CKD progression and exposure to AKI, thereby accelerating progression to ESRD and increasing the risk of death.16,30
Because of the long duration that is needed for the progression of CKD to ESRD as opposed to the development of CV outcomes, it remains a challenge to balance the tension between kidney and CV or mortality benefits when the risk-to-benefit ratio is not always concordant.29,31 We believe that our data may help inform practice as lower BP targets are more frequently implemented in patients with CKD and provide reassurance of the long-term safety of a <20% decline in renal function that may occur during intensification of antihypertensive therapy. We do note, however, that we are underpowered to pinpoint the exact threshold of decline in renal function that is associated with a higher risk of death, and the difference in the HRs for the risk of death between the intensive and usual BP treatment arms may be more pronounced at higher thresholds of decline (e.g., >25%). Until further studies confirm or refute our findings, in the setting of larger acute declines in renal function during intensification of antihypertensive therapy, an individualized approach may be prudent, because the burden of additional medications and provider visits that may be needed to achieve a lower BP target may not associate with substantial renal or mortality benefit during the long term.
The strengths of our study include the large number of ESRD events and deaths and the availability of nearly two decades of follow-up in the AASK and the MDRD Trial participants. In addition, we provide data from two separate trials with a heterogeneous group of participants. However, we acknowledge that our study is post hoc and observational in its design, and our findings do not imply causation. We also note that our study does not preserve the original randomization schema of the intervention (and therefore, may be subject to unmeasured residual confounding), and the reference group used could predispose to bias, because it was selected for absence of substantial CKD progression. We emphasize that there were differences in baseline characteristics of those who sustained a large decline in GFR during BP lowering compared with those who did not (e.g., differences in baseline proteinuria), and although we have adjusted for these differences, residual confounding may still be present. We also acknowledge that it is difficult to determine from these observational analyses if relaxation of the blood targets in those with moderate decline in the intensive group would have resulted in improved outcomes, which needs to be evaluated in additional studies or trials. Although we took a data-driven approach to the determination of the threshold of decline in renal function that may signal potential risk versus benefit, we are limited in power to determine this exact threshold, and it is also possible that the acute declines in eGFR observed in our study could be related to other unmeasured confounders. Larger studies are needed to confirm our findings.
In conclusion, we show that an acute decline in renal function up to 20% during the lowering of BP levels to the 125/75-mm Hg range was associated with a lower risk of death compared with similar or lower rates of declines in renal function during treatment to a 140/90-mm Hg target BP range. In contrast, a ≥20% decline in renal function over the first 3–4 months of intensive BP lowering was not associated with risk of mortality. Further trials are needed to assess the long-term risk-to-benefit ratio of intensive BP lowering when large declines in renal function occur.
Disclosures
None.
Supplementary Material
Acknowledgments
The data from the African American Study of Kidney Disease and Hypertension (AASK) and the Modification of Diet in Renal Disease (MDRD) Trial reported here were supplied in part by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repositories.
This work was supported by National Institutes of Health (NIH) grant K23 HL131023 (to E.K.) and K24 DK 110427 (to J.H.I.). The AASK and the MDRD Trial were conducted by the AASK investigators and the MDRD Trial investigators and supported by the NIDDK.
The funding source did not have any role in manuscript preparation. This manuscript does not necessarily reflect the opinions or views of the AASK and the MDRD Trial and cohort studies, the NIDDK Central Repositories, or NIDDK grants 000182, UL1TR000124, and P30AG021684. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018040365/-/DCSupplemental.
References
- 1.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al.: An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Christensen PK, Hansen HP, Parving HH: Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney Int 52: 1369–1374, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Anonymous: Short-term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease study. J Am Soc Nephrol 7: 2097–2109, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Palmer BF: Renal dysfunction complicating the treatment of hypertension. N Engl J Med 347: 1256–1261, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Anderson S, Brenner BM: Intraglomerular hypertension: Implications and drug treatment. Annu Rev Med 39: 243–253, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Apperloo AJ, de Zeeuw D, de Jong PE: A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int 51: 793–797, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Neuringer JR, Brenner BM: Hemodynamic theory of progressive renal disease: A 10-year update in brief review. Am J Kidney Dis 22: 98–104, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Anderson S, Rennke HG, Brenner BM: Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest 77: 1993–2000, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaumont M, Pourcelet A, van Nuffelen M, Racapé J, Leeman M, Hougardy JM: Acute kidney injury in elderly patients with chronic kidney disease: Do angiotensin-converting enzyme inhibitors carry a risk? J Clin Hypertens (Greenwich) 18: 514–521, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ku E, Bakris G, Johansen KL, Lin F, Sarnak MJ, Campese VM, et al.: Acute declines in renal function during intensive BP lowering: Implications for future ESRD risk. J Am Soc Nephrol 28: 2794–2801, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palevsky PM, Zhang JH, Seliger SL, Emanuele N, Fried LF; VA NEPHRON-D Study : Incidence, severity, and outcomes of AKI associated with dual renin-angiotensin system blockade. Clin J Am Soc Nephrol 11: 1944–1953, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, et al.: Effects of intensive blood pressure treatment on acute kidney injury events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 71: 352–361, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weir MR: Acute changes in glomerular filtration rate with renin-angiotensin system (RAS) inhibition: Clinical implications. Kidney Int 91: 529–531, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al.; SPRINT Research Group : A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, McClure LA, Scherzer R, Odden MC, White CL, Shlipak M, et al.: Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: A post hoc analysis of the Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial. Circulation 133: 584–591, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel LJ, Middleton J, Miller ER 3rd, Lipkowitz M, Norris K, Agodoa LY, et al.: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al.; AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sika M, Lewis J, Douglas J, Erlinger T, Dowie D, Lipkowitz M, et al. : Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Clinical Trial and Cohort study. Am J Kidney Dis 50: 78–89, 89.e1, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al.; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee : The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al.; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Ku E, Gassman J, Appel LJ, Smogorzewski M, Sarnak MJ, Glidden DV, et al.: BP control and long-term risk of ESRD and mortality. J Am Soc Nephrol 28: 671–677, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku E, Glidden DV, Johansen KL, et al.: Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney international 87: 1055–1060, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ku E, Lipkowitz MS, Appel LJ, Parsa A, Gassman J, Glidden DV, et al.: Strict blood pressure control associates with decreased mortality risk by APOL1 genotype. Kidney Int 91: 443–450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bress AP, Kramer H, Khatib R, et al. : Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey). Circulation 135: 1617–1628, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al.; SPRINT Research Group : Effects of intensive BP control in CKD. J Am Soc Nephrol 28: 2812–2823, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. : 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 71: 2199–2269, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, et al.; REIN-2 Study Group : Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): Multicentre, randomised controlled trial. Lancet 365: 939–946, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Obi Y, Kalantar-Zadeh K, Shintani A, Kovesdy CP, Hamano T: Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: A post hoc analysis of a randomized clinical trial. J Intern Med 283: 314–327, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Palmer BF: Disturbances in renal autoregulation and the susceptibility to hypertension-induced chronic kidney disease. Am J Med Sci 328: 330–343, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Chertow GM, Beddhu S, Lewis JB, Toto RD, Cheung AK: Managing hypertension in patients with CKD: A marathon, not a SPRINT. J Am Soc Nephrol 27: 40–43, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.