Abstract
The intestinal microbiota, composed of pro- and anti-inflammatory microbes, has an essential role in maintaining gut homeostasis and functionality. An overly hygienic lifestyle, consumption of processed and fiber-poor foods, or antibiotics are major factors modulating the microbiota and possibly leading to longstanding dysbiosis. Dysbiotic microbiota is characterized to have altered composition, reduced diversity and stability, as well as increased levels of lipopolysaccharide-containing, proinflammatory bacteria. Specific commensal species as novel probiotics, so-called next-generation probiotics, could restore the intestinal health by means of attenuating inflammation and strengthening the epithelial barrier. In this review we summarize the latest findings considering the beneficial effects of the promising commensals across all major intestinal phyla. These include the already well-known bifidobacteria, which use extracellular structures or secreted substances to promote intestinal health. Faecalibacterium prausnitzii, Roseburia intestinalis, and Eubacterium hallii metabolize dietary fibers as major short-chain fatty acid producers providing energy sources for enterocytes and achieving anti-inflammatory effects in the gut. Akkermansia muciniphila exerts beneficial action in metabolic diseases and fortifies the barrier function. The health-promoting effects of Bacteroides species are relatively recently discovered with the findings of excreted immunomodulatory molecules. These promising, unconventional probiotics could be a part of biotherapeutic strategies in the future.
Keywords: commensal bacteria, intestinal health, next generation probiotics, dysbiosis, intestinal permeability, butyrate producing bacteria, anti-inflammatory
1. Introduction
The human gastrointestinal microbiota is a complex ecosystem consisting mainly of bacteria, but also viruses and eukaryotic organisms. It has been estimated that we harbor 1013 bacterial cells, which equals the number of eukaryotic cells in the human body [1]. In total, over 3000 bacterial species have been found in human feces to date [2], although only 1000 of these have been characterized by culturing, while the remaining have only been detected by sequencing [3]. Most individuals have around 200 different species in their digestive tract with highly variable abundance and a combination that makes the microbiota make-up very personal [4]. From this bacterial population about 30% are shared between subjects, creating the so-called common core microbiota [4,5].
The intestinal microbiota affects the human health in many ways and is considered a major contributing factor in a number of diseases. Microbiota plays a role in the maintenance of the intestinal barrier, which is essential for homeostasis and functionality of the gut. Impaired barrier function is associated with both intestinal and systemic diseases. In a balanced situation regarding the pro- and anti-inflammatory properties of the microbiota, it stimulates and challenges the host and its immune system at an appropriate level to keep the defense mechanisms alerted, while, at the same time, providing regulatory signals to induce tolerance towards commensal microbiota.
The digestive tract has two distinct microbial ecosystems, the luminal and the mucosal microbiota [6]. In the luminal compartment, over 90% of bacteria belong to Firmicutes and Bacteroidetes, and the minor phyla include Actinobacteria, Proteobacteria, and Verrucomicrobia. In the mucosal layer, the number of bacteria, as well as the diversity, is considerably lower, and the composition is clearly different as Firmicutes are generally higher in abundance compared to the Bacteroidetes in both humans [7] and mice [8].
Microbial composition is affected by a number of endogenous and exogenous factors, such as hosts’ physiology and immunity, diet, and medication. In contemporary societies, an overly hygienic lifestyle with few exposures to environmental microbes, consumption of processed and fiber-poor foods and frequent use antibiotics are considered as major modulators of human microbiota [9]. Strong perturbations like these may lead to a state of dysbiosis, which has been associated with a number of human diseases [10]. The causal links between microbiota dysbiosis and disease states are currently under intense investigation, as well as the mechanisms by which the microbiota could contribute to the pathological processes [10].
Microbiota dysbiosis is generally characterized by altered composition, as well as reduced diversity and stability [11]. This has also led to observations where the relative proportion of facultative anaerobic bacteria has been increased and the proportion of strictly anaerobic bacteria with protective functions, such as the production of short-chain fatty acids (SCFA) has been reduced [12]. Overall, a dysbiotic microbiota has not only lost the capacity to support intestinal homeostasis, but it may even exacerbate intestinal inflammation [11]. For example, an increase in the gamma-Proteobacteria levels with highly proinflammatory cell wall structures, i.e., lipopolysaccharide (LPS), will induce inflammation and the following oxidative stress, in turn, will exacerbate dysbiosis by promoting facultative anaerobic bacteria [13]. Furthermore, microbiota dysbiosis can impair the epithelial barrier leading to so-called leaky gut allowing the intestinal content to be in contact with the host periphery potentially inducing inflammatory responses, which is often observed in several human diseases [12]. While anti-inflammatory drugs provide different alternatives to dampen inflammation effectively, it would also be essential to treat the microbial dysbiosis and strengthen the epithelial integrity in order to restore intestinal homeostasis in the long-term. The growing knowledge of the importance of the human microbiota suggests that commensal species can be isolated and used for therapeutic purposes as so-called next-generation probiotics, alongside lactobacilli and bifidobacteria with a long history of use as probiotics. Next-generation probiotics are potential novel health-promoting commensal strains, which have not yet been used for nutritional and therapeutic purposes and could possibly be exploited in dietary and pharmaceutical applications. Emerging evidence shows that a large number of commensal species across all major intestinal phyla have the ability to strengthen the epithelial barrier and alleviate inflammation, as will be discussed in the following sections.
In this review we summarize the beneficial effects of well-known, promising commensal bacteria on the intestinal epithelial barrier and discuss their possible use as unconventional, so-called next-generation probiotics of the future. The importance of butyrate-producing bacteria to the intestinal health is well documented, but at the molecular level the mechanisms, e.g., effector molecules secreted by commensal bacteria, are still being investigated. Hence, this review summarizes the potential mechanisms known so far behind the health-promoting action of the commensals in the gut.
2. Intestinal Barrier Function
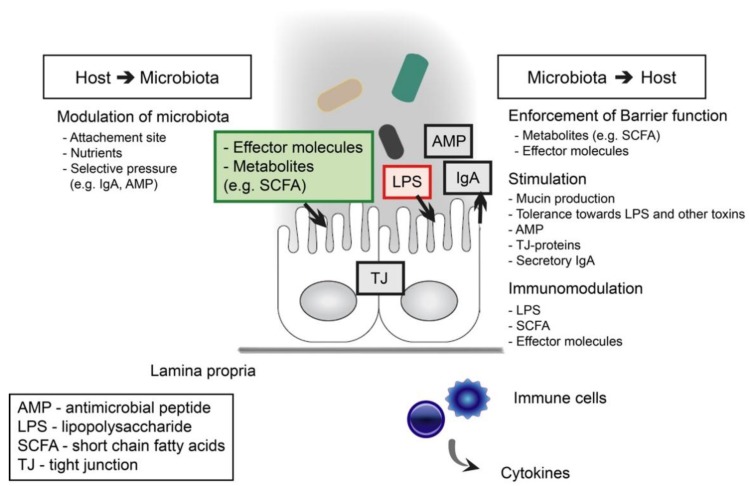
The intestinal epithelium acts as a barrier between the host and the luminal confinement of commensal bacteria, enabling the symbiotic relationship between the two (Figure 1). In addition to the physical barrier of epithelial cells and mucus layer, other host defense mechanisms include the secretion of antimicrobial peptides (AMPs) and secretory immunoglobulin A (sIgA), as well as the presence of immune cells like phagocytes and macrophages [14]. The most prominent antimicrobial peptides include defensins, cathelicidins, and C-type lectins, which are expressed as a result of pattern recognition receptor (PRR) activation in the intestinal epithelial cells [15]. In order to sustain intestinal integrity, the epithelial cells need to be renewed constantly by continuous differentiation from stem cells to goblet cells, Paneth cells, enteroendocrine cells, or enterocytes [16]. Enterocytes form the physical barrier by linking together with different cell junctions, including desmosomes, adherens junctions, and tight junctions. In turn, goblet cells secrete mucin, which forms a thick, protective mucus layer on the epithelial mucosa. Paneth cells are specialized producers of antimicrobial molecules mostly located in the small intestine. Endocrine cells secrete serotonin and various neuropeptides, which act as signals in the crosstalk between the enteroendocrine cells and the immune system. Gut-associated lymphoid tissue (GALT) is located under the epithelial layer, where submucosal dendritic cells (DCs) sample antigens from the lumen or luminal antigens are presented to DCs by microfold cells (M-cells) leading to the activation of IgA secretion [17]. Recently it was discovered that IgA, besides its defensive role, also generates host–microbe symbiosis by enabling mucosal colonization of bacteria [18]. Bacteroides fragilis modulates its surface structures to bind IgA, which is needed for the colonization of the mucosal niche in the gut.
Figure 1.
Host-microbiota interactions affecting the epithelial barrier function.
Tight junctions, such as claudins and occludin, are intercellular junctions connecting the epithelial cells tightly together and controlling the paracellular permeability [19]. Hence, the tight junction proteins also moderate the transepithelial transport. The intestinal barrier enhancement, for example, by probiotic bacteria, is associated with changes in the tight junction protein expression and distribution. Intestinal inflammation, commensal microbes, and dietary components are among the main factors affecting epithelial permeability [20]. Food factors, including amino acids like glutamine and tryptophan, as well as polyphenols, enhance the barrier integrity. Glutamine, a well-studied food component, is a primary energy source for enterocytes, which regulates tight junction expression and localization in Caco-2 intestinal cells [21]. On the other hand, there are also detrimental food factors, such as dietary saturated fat, which has been shown to increase intestinal permeability in vivo [22]. In general, more studies on the effect of nutrition on intestinal barrier function and the mechanisms are needed.
3. Proteobacteria as a Marker of Dysbiosis and Mediator of Inflammation
In a healthy intestine, Proteobacteria is numerically a minor phyla. Increased abundance of Proteobacteria, particularly the class gamma-Proteobacteria and the family Enterobacteriaceae within it, has been associated with a number of human diseases [23]. As Gram-negative bacteria their outer membrane surface is covered with LPS, which is a complex glycolipid and a major bacterial virulence factor [24,25]. The LPS molecule is composed of three domains; lipid A, core, and O-specific polysaccharides [26]. The lipid A structure, which determines the immunogenicity of LPS, differs among different bacterial species. Most of the Gram-negative bacteria express the hexaacylated LPS, which is one of the most potent agonists of the human innate immune system. LPS triggers a strong proinflammatory reaction and secretion of proinflammatory cytokines from the host cells [27]. Proinflammatory cytokines include interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), whereas anti-inflammatory cytokines IL-10 and IL-17 decrease and restore the intestinal permeability [20]. In addition of hexaacylation, tetraacylated and pentaacylated LPS also exist among Gram-negative bacteria. The number of lipid A acyl chains usually correlates well with its ability to induce proinflammatory cytokine production and the hexaacylated forms are the most proinflammatory ones [28]. Consequently, proinflammatory cytokines involved in the intestinal inflammation modify the tight junctions leading to disruption of the intestinal barrier, which plays a role in the pathogenesis of many intestinal diseases, such as inflammatory bowel disease (IBD).
Due to the highly proinflammatory cell wall structure, Proteobacteria can induce and maintain unspecific inflammation in the intestine without an actual infection if their abundance in the gut microbiota has been increased. The Proteobacteria phylum also includes several notable human pathogens. The high abundance of Proteobacteria has been associated with many disease conditions where intestinal or systemic inflammation or both of these are involved as a key pathoetiological factors. For example, the connection between LPS sustained low-grade inflammation and the development of metabolic disorders has been well established [29]. It has been shown that members of the Enterobacteriaceae family are more abundant in obese people when compared to healthy controls with normal weight. After weight loss, the Enterobacteriaceae population was the most affected family with the relative abundance decreasing from 13% to less than 1% [30]. Furthermore, it was shown that gamma-Proteobacteria were increased in the intestines of patients suffering from nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH) [31]. When comparing the microbiota composition of healthy and obese children, as well as children suffering from NASH, a gradual increase in Proteobacteria has been observed, respectively [32]. Proteobacteria have also been found to be increased in the microbiotas of IBD patients [33,34,35].
Despite the proinflammatory nature of Proteobacteria, it has been well established that there are strain-dependent variations in the immunomodulatory properties. A striking example of this is the Proteobacteria Escherichia coli Nissle 1917 (EcN), which is widely used as a probiotic strain [36]. EcN has a specific LPS form, due to a point mutation, which gives it an ability to withstand antibacterial defense mechanisms of blood serum [37]. EcN has been shown to have efficacy in reducing the severity of rotaviral diarrhea and modulating viral immunity in several randomized clinical studies and experimental studies in an animal models [38,39,40,41,42,43]. In addition, it has been shown to very efficiently maintain remission in ulcerative colitis (UC) [44,45,46]. Interestingly, the probiotic effect of EcN is, in part, due to its antimicrobial effect via the production of bacteriocins and inhibition of other E. coli strains [47]. Furthermore, EcN interacts with the gut epithelium by inducing the defensin production, strengthening the epithelial layer, decreasing the production of proinflammatory cytokines, and increasing the production of anti-inflammatory cytokines [47]. Thus, EcN seems to counteract the proinflammatory effects commonly induced by Proteobacteria.
4. Health-Associated Commensal Bacteria
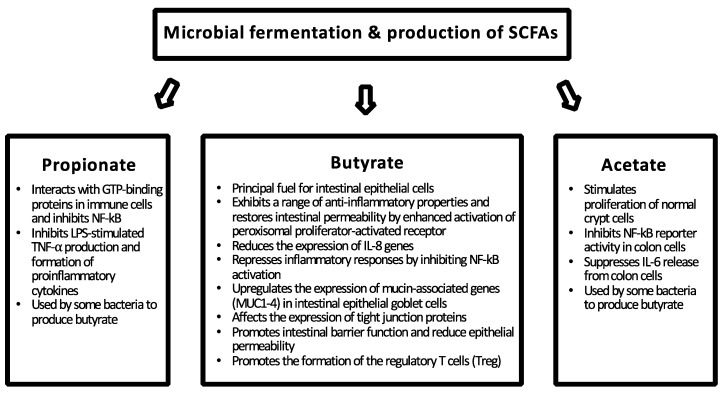
A dysbiotic microbiota can be ameliorated with the health-promoting action of next-generation probiotics or boosting the growth and metabolism of beneficial commensals in the colon with fiber-rich food components, especially targeting the butyrate production. The protective effect of commensal bacteria on barrier function is achieved by preventing the colonization of pathogens, interacting directly with the host enterocytes and metabolizing undigested carbohydrates to short-chain fatty acids (Figure 1). Commensal bacteria metabolize dietary fibers and resistant starch to SFCAs, such as butyrate, acetate, and propionate, which serve as a major energy source for the host enterocytes (Figure 2). Dietary fibers and SFCAs have been shown in vivo to suppress intestinal inflammation and colon cancer [48]. Microbial-derived butyrate increases mitochondrial-dependent oxygen consumption in enterocytes, stabilizes the hypoxia-inducible factor (HIF) involved in barrier protection, and induces expression of HIF-target genes that augment barrier function [49]. On the other hand, intestinal homeostasis is also maintained via direct interaction of the microbiota and the host epithelium and immune system, where the recognition of bacterial molecules, such as LPS, by host PRRs plays a major role (Figure 1) [50].
Figure 2.
The major short-chain fatty acids (SCFAs) produced by gut microbiota and their health-promoting effects on the gut epithelium [54,55].
In the following paragraphs, we summarize the recent research results from the promising, cultivable commensal bacteria with health-promoting and anti-inflammatory capacity in the gut. Probiotic characteristics are generally considered strain-dependent and, therefore, cannot be generalized to cover other than the studied strain of the species unless the mechanism of action is known and the strains in question are known to harbor or produce the effector molecules [51,52]. There is a strong evidence of strain-specificity, as well as disease-specificity of probiotic action, which makes it very challenging to authenticate the efficacy of probiotics in clinical trials [52]. Furthermore, it can be difficult to show the beneficial action of probiotics in healthy individuals by recording specific biomarkers rather than symptoms and if the study cohort is not a high-risk population with elevated levels of biomarkers [53]. Thus, all strains of a specific species cannot be expected to exert all described traits described below, but the purpose is rather to give an overview of the beneficial traits found within a species or genus.
4.1. Bifidobacterium spp.
The genus Bifidobacterium, belonging to the phylum Actinobacteria, contains 51 recognized species and 10 subspecies [56], the majority of which originate from human or animal (mammals and bees) digestive tracts, and also few species that have been originally isolated from other sources, such as milk or sewage [57]. For 100 years these bacteria have been considered health-promoting and bifidobacterial strains have been widely used as probiotics along with lactobacilli. The level of knowledge concerning the health-promoting mechanisms of action, as well as the efficacy of bacteria marketed as probiotics varies substantially between strains, which can complicate the comparison and selection of certain strains to specific indications [58]. However, some strains of Lactobacillus and Bifidobacterium spp. are extensively characterized, also in comparative genomics studies, which makes them good examples and forerunners in the search of next-generation probiotics targeted for specific therapeutic purposes [57,59].
In humans, bifidobacteria are among the founder-species of the infant gut and frequently one of the major taxa or even the most abundant genus of infant gut microbiota [60]. In vaginally born infants, bifidobacteria are found within three days after birth, and after one week they may compose as high as 96.4% of the overall microbiota in breast-fed infants [61,62]. Bifidobacteria seem to be transferred vertically from mother to infant as evidenced by both metagenomic studies and whole genome sequencing of isolated strains from mother-infant pairs [61,63,64]. Duranti et al. [61] showed that strains of B. longum, B. bifidum, B. breve, B. adolescentis, B. dentium, B. pseudocatenulatum, and B. catenulatum, i.e., representatives of all major human bifidobacterial species, were shared between the mothers and their infants. The relative proportion of bifidobacteria in children declines after weaning, but stays higher than in adults over the childhood and pre-adolescentis years [65,66]. The abundance of bifidobacteria in adults is estimated to be 1–2% in many countries around the world, and approximately 7% in the Japanese population [66]. The age-related decline in the proportion of bifidobacteria seem to continue until senescence.
Human milk oligosaccharides (HMOs) stimulate the growth of bifidobacteria, but different species and strains have their specific HMO preferences [67]. For example a positive correlation was found between sialylated HMOs and B. breve and non-fucosylated/non-sialylated HMOs and the B. longum group [67]. Likewise, bifidobacterial species and subspecies have different abilities to degrade glycans of the intestinal mucus layer and plant-derived polysaccharides, which makes some strains better adapted to infant gut, while some are better adapted to adults [68,69,70]. On the other hand, B. breve has capabilities to utilize both plant- and host-derived carbohydrates and could, therefore, be called a generalist [71]. Altogether, bifidobacterial genomes are better equipped with genes related to carbohydrate metabolism than average bacterial genomes, and by resource sharing and cross-feeding, bifidobacteria may have large effects on overall gut physiology [71]. In vitro co-culture studies have shown that bifidobacteria have the ability to degrade oligo-fructose and produce acetate, thereby promoting butyrate production of Faecalibacterium prausnitzii, Anaerostipes caccae, and Roseburia intestinalis, by means of cross-feeding [72,73,74]. Detailed knowledge on the metabolic capabilities and cross-feeding chains could open a possibility to modulate the composition and action of gut microbiota in a desired direction by premeditated dietary fiber consumption.
Epithelial adhesion of bifidobacteria is mediated by extracellular structures, e.g., pili or fimbriae and the extracellular polysaccharide (EPS) layer or capsule (Table 1) [57]. Most strains of bifidobacteria have pilus-encoding loci in their genomes. The role of pili in mediating bifidobacterial adhesion to enterocytes, as well as in autoaggregation, was first demonstrated for B. bifidum [75]. In a murine model, B. bifidum pili evoked TNF-α expression and a significantly lower IL-10 response in cecal mucosa as compared to nonpiliated cells [75]. Later, another type of pili, tight adherence (Tad) pili were discovered in B. breve [76]. Interestingly, the genes of B. breve Tad-pilus were found to be heavily up-regulated in vivo in a mouse model as compared to in vitro cultivation [76]. Experiments with pilusless mutant strains confirmed the importance of this structure in the colonization and persistence of B. breve in the gut. In addition to pili, other surface structures of bifidobacteria such as EPS, also have interesting functional properties. EPS provides acid and bile resistance to bacteria and can act as an immunological silencer by modulating B-cell trafficking and IL-12 production in the epithelium [77]. Furthermore, EPS may play a role in reducing initial colonization and burden of pathogens as evidenced by Citrobacter rodentium infection mouse model [77].
Table 1.
Known effector molecules of the selected commensal bacteria and their effect on intestinal health. All in vivo studies were mice studies.
| Organism | Effector Molecule | Mediated Effect | Study Type | Reference |
|---|---|---|---|---|
| B. breve UCC2003 | IVb tight adherence (Tad) pili | Host colonization and persistence mechanism | in silico, in vivo | [76] |
| B. breve UCC2003 | Surface exopolysaccharide (EPS) | Acid and bile tolerance, immunomodulation, protection against pathogen colonization and burden | in silico, in vitro, in vivo | [77] |
| Several bifidobacterial strains | Sortase-dependent pili | Adhesion to host mucus components | in silico, in vitro, in vivo | [82] |
| B. bifidum PRL2010 | Sortase-dependent pili | Autoaggregation, evoked TNF-α expression and a significantly lower IL-10 response | in vitro, in vivo | [75] |
| B. longum ssp. longum CCM 7952 | Ligands for NOD2 | Reduced clinical symptoms in a colitis mouse model, preserved expression of tight junction proteins | in vitro, in vivo | [81] |
| A. muciniphila MucT | Amuc_1100 (OM pili-like protein) | Induction of cytokine production, interactionwith TLR 2, improves gut barrier | in vitro | [83,84] |
| A. muciniphila ATCC BA-835 | Extracellular vesicles | Improves intestinal barrier integrity, improved body weight & glucose tolerance | in vitro, in vivo | [85] |
| F. prausnitzii A2-165 | Microbial anti-inflammatory molecule (MAM) | Peptides of MAM inhibit the activation of NF-κB, ameliorate colitis symptoms in mice | in vitro, in vivo | [86] |
| R. intestinalis DSMZ-14610 | Flagellin | Upregulation of IncRNA, alleviation of colonic inflammation | in vitro, in vivo | [87] |
| B. fragilis NCTC 9343 | Polysaccharide A | Immune activation, elicits cytokine production, protects and treats colitis in an animal model | in vitro, in vivo | [88] |
| B. fragilis 323-J-86 | Outer-membrane vesicle | Transports PSA molecule to immune cells | in vitro, in vivo | [89] |
In addition to extracellular structures, also secreted substances of bifidobacteria can mediate probiotic effects. Acetate, an end product of bifidobacterial fermentation, can give protection against E. coli O157 by affecting the regulation of certain genes involved in anti-inflammatory responses and preventing or alleviating damage to intestinal epithelium due to E. coli O157-induced cell death and reducing the translocation of toxins from gut to systemic circulation [78]. The resistance to infection was mediated through a higher amount of mouse fecal acetate produced by certain bifidobacterial strains, while the growth rate or the expression of virulence genes of E. coli O157 were not affected. The role of acetate was apparent also in the ability of a B. bifidum strain to strengthen tight junctions and prevent the disruption of epithelial barrier induced by TNF-α in vitro [79]. Antagonism of cytotoxic effects of Clostridium difficile toxins by certain strains of bifidobacteria has been demonstrated with HT29 intestinal epithelial monolayer, where effective strains were able to remove toxins from the medium and preserve the F-actin microstructure and tight junctions between epithelium [80].
Recently, the immunomodulatory ability of various bifidobacterial species was studied with naïve mouse spleen and dendritic cells and immunostimulatory patterns were found to be strain-specific (Table 1) [81]. Interestingly, a strain with a low stimulatory potential in vitro was able to prevent clinical symptoms in a mouse model of dextran sulfate sodium (DSS)-induced colitis, preserve the tight junction protein expression, and protect the epithelial barrier function, while another strain of the same species and with a higher in vitro immunomodulation capacity was not. These examples of bifidobacterial ability to protect the epithelial barrier and interact with the host immunology tells about strain-specific properties and highlights the need of a detailed characterization of selected bacteria and their effects on the molecular level in the search for specific therapeutic properties of the bacteria.
Due to the high proportion of bifidobacteria in the healthy infant gut and the significant reduction of it in the elderly, benefits of bifidobacterial probiotic strains, especially in those two age groups, has raised interest. The effects of probiotic bifidobacteria on the health of pre-term infants is widely studied and several systematic reviews and meta-analyses have found them effective in reducing the risk of necrotizing enterocolitis and sepsis, which are major causes of death among pre-term infants, and in reducing mortality and hospital stays [90,91,92]. In the elderly, bifidobacterial probiotics can improve constipation and enhance cellular immune activity [93,94].
4.2. Akkermansia muciniphila
Akkermansia muciniphila is a Gram-negative, anaerobic, mucin-degrading bacterium which belongs to the Planctomycetes-Verrucomicrobia-Chlamydia superphylum [95,96]. The type strain A. muciniphila MucT was originally isolated from a healthy adult by using gastric mucin as a sole carbon and nitrogen source [95,97]. Later, it was also found to tolerate low levels of oxygen [95,97]. By degrading the complex mucin glycoproteins and due to its ability to produce acetate, propionate and 1,2-propandiol, A. muciniphila contributes to the bacterial community cross-feeding [95,98]. Co-culturing of butyrate-producing bacteria with A. muciniphila revealed two types of trophic chains, metabolic and cofactor syntrophic interactions. [98]. First, A. muciniphila can metabolically support the growth of butyrate-producers by degrading mucin and providing acetate. Second, A. muciniphila can use pseudovitamin B12 produced by other bacteria for its propionate production. Additionally, A. muciniphila produces sulfatases and it may be able to use hydrogen sulfide for cysteine production and, as a result, limit the possible toxicity of sulfate-reducing bacteria [83,96].
The abundance of A. muciniphila has been found to be reduced in many disease states [99,100,101,102,103]. Studies on metabolic disorders, such as obesity and diabetes, have given the most promising evidence on the beneficial effect of A. muciniphila on health [96]. Everard et al. [104] noticed that a daily treatment with live A. muciniphila restored the mucus layer thickness in mice on a high-fat diet (HF diet) and reversed the HF diet-induced metabolic disorders. The bacterium also contributed to the production of antimicrobial peptides in the colon as it was found to affect RegIIIγ production [104]. Furthermore, administration of A. muciniphila increased the number of mucin-producing goblet cells and anti-inflammatory regulatory T cells (Treg) in mice fed with HF-diet [105]. More recently, the bacterium was shown to protect against atherosclerosis in an Apoe−/− mouse model by attenuating metabolic endotoxemia-induced inflammation through restoration of the gut barrier [106]. In vitro results showing that A. muciniphila adheres to intestinal epithelial cell lines and strengthens the cell monolayer support the idea that the bacterium has a special barrier fortifying function in the epithelium [107]. A. muciniphila also induces only a low-level proinflammatory stimulation (IL-8) in enterocytes as compared to E. coli, which may be beneficial to the gut health as it keeps the immune system alerted [107]. Extracellular vesicles of A. muciniphila were also found to have a positive effect on intestinal integrity, and due to their small size they may also reach gut epithelium in places where the mucus layer is firm and impermeable to bacteria [85,108].
Interactions of A. muciniphila outer membrane proteins with cells of the human immune system have been studied in vitro [83,109]. These outer membrane proteins are involved in the health-promoting effects of A. muciniphila towards the intestinal homeostasis and barrier function (Table 1). The 32 kDa pili-like protein (Amuc_1100), was found to induce IL-1β, IL-6, IL-8, IL-10, and TNF-α production in human-derived peripheral blood mononuclear cells (PBMCs) [83]. Toll-like receptor (TLR) 2 was induced by the purified recombinant Amuc_1100 protein. Furthermore, both A. muciniphila and purified Amuc_1100 enhanced trans-epithelial resistance. Interestingly, treatment with Amuc_1100 protein (or pasteurized A. muciniphila) lowered body weight and fat mass gain in HF-diet fed mice, corrected HF-diet-induced higher adipocyte diameter and hypercholesterolemia and improved glucose tolerance [84]. Furthermore, Amuc_1100 maintained its activity in stimulating TLR2 after pasteurization, as its melting temperature is 70°C. Thus, Amuc_1100 seems to be responsible for mediating at least part of the beneficial effects of A. muciniphila. The first clinical trial suggests that live or pasteurized A. muciniphila is safe for use for obese humans and it is a promising candidate for next-generation probiotics [84].
4.3. Faecalibacterium prausnitzii
F. prausnitzii is one of the most abundant bacterial species inhabiting the human intestine, representing 2–15% of the total bacterial load [110,111]. Taxonomically, F. prausnitzii belongs to the phylum Firmicutes, class Clostridia, and family Ruminococcaceae with the species currently being the only characterized representative within the genus [112]. The species itself comprises at least two distinct phylogroups based on 16S rRNA sequencing [113]. Metabolically, F. prausnitzii is an extremely oxygen-sensitive anaerobe [112]. The bacterium can use various simple sugars as its energy source, whereas the utilization of more complex carbohydrates is strain-specific [114]. The nutrients may be either host- or diet-derived, or they may result from cross-feeding by other gut microbes [114]. The major fermentation products from glucose and acetate by F. prausnitzii are formate, d-lactate, and butyrate [112].
F. prausnitzii promotes gut health in various ways [114,115]. For instance, it is involved in the feeding of colonocytes, the maintenance of immune homeostasis, and the strengthening of barrier function. It has also been noted that the abundance of F. prausnitzii is significantly decreased in several intestinal disorders, such as Crohn’s disease (CD), UC, and colorectal cancer [116,117,118], making it a potential biomarker in the diagnostics of various intestinal disorders. This depletion may result from changes in the environmental parameters, as the bacterium has been shown to be sensitive to environmental changes that accompany intestinal disorders [114]. Replenishment of the diseased gut with F. prausnitzii could improve intestinal health making this bacterium a vital part of commensal microbiota [114,115]. In healthy human subjects the consumption of prebiotics, such as inulin (10 g/day) or other dietary fiber (21 g/day), significantly increased the abundance of F. prausnitzii [119,120]. Thus, the health-promoting action of prebiotics and fiber could be, in part, mediated by the increase of F. prausnitzii and other beneficial commensals.
In respect to health, the substantial production of butyrate by F. prausnitzii is of special interest. Butyrate is a major energy source for colonocytes [55] and it also takes part in immune modulation by inhibiting nuclear factor kappa B (NF-κB) transcription factor activation, upregulating peroxisome proliferator-activated receptor gamma (PPARγ), and inhibiting IFN-γ, consequently reducing inflammation (Figure 2) [114]. These anti-inflammatory properties may protect the colon against inflammation and colorectal cancer. As a member of the intestinal ecosystem, F. prausnitzii is in constant association with its environment and, therefore, relies on other microbes in order to exhibit its health-promoting effects. It has been proposed that metabolically complementary F. prausnitzii and Bacteroides thetaiotaomicron modulate the intestinal mucus barrier by affecting the development of goblet cells and the production of mucus glycans [121].
It has been shown that F. prausnitzii interacts with the host epithelial cells and attenuates the inflammatory response. According to Sokol et al., F. prausnitzii affects the release of cytokines in vitro from PBMCs in a manner that favors the anti-inflammatory cytokine profile (high IL-10/IL-12 ratio) [117]. In the same study, the cell-free culture supernatant of F. prausnitzii was shown to reduce the secretion of IL-8 by CaCo-2 cells and to abolish NF-κB activation. Interestingly, the latter was not achieved with butyrate. The experiments on a murine model with acute chemical-induced inflammation showed that both F. prausnitzii cells and supernatant reduced the severity of acute colitis and induced increased secretion of anti-inflammatory IL-10 and decreased secretion of proinflammatory cytokines [117]. Furthermore, F. prausnitzii cells and supernatant were shown to decrease intestinal permeability by affecting apical junction proteins [122] and enhance the barrier function in murine model with chemical-induced colitis [123]. In addition to butyrate, F. prausnitzii produces salicylic acid, which blocks the activation of NF-κB, and, consequently, the production of IL-8 [124]. Furthermore, F. prausnitzii produces an anti-inflammatory protein called Microbial Anti-inflammatory Molecule (MAM), whose peptides inhibit the activation of NF-κB in vitro and in vivo (Table 1) [86,125]. The anti-inflammatory properties of MAM produced by F. prausnitzii were also shown to inhibit T helper 1 cells (Th1) and Th17 immune responses in chemically-induced colitis models in mice [86]. Although it is evident that F. prausnitzii or its components are able to modulate immune responses, many of the effector molecules still remain uncharacterized, emphasizing the need for further studies. Since all the F. prausnitzii strains isolated from healthy volunteers showed promising anti-inflammatory properties in vitro [126], the species is a promising target for therapeutic purposes. However, the development of therapeutic supplements utilizing strictly anaerobic bacteria, such as F. prausnitzii, is undoubtedly challenging due to the demand of anaerobic conditions and large-scale production. Possible solutions would be freeze-drying the microbial cell biomass, followed by encapsulation or isolating the commensal-derived effector molecules harboring the beneficial effect [127,128].
4.4. Roseburia intestinalis
R. intestinalis is a Gram-positive, anaerobic, butyrate-producing bacteria originally isolated from the human feces [129]. It belongs to the phylum Firmicutes and family Lachnospiraceae. The genus Roseburia includes five characterized species, R. intestinalis, R. hominis, R. inulinivorans, R. faecis, and R. cecicola [130,131]. The species of genus Roseburia are among the most abundant butyrate-producing bacteria accounting for 0.9 to 5.0% of the total microbial load [132]. The abundance of Roseburia has been found to be decreased in many intestinal disorders suggesting the bacterium has an important role in maintaining the gut homeostasis, e.g., by producing SFCAs [133]. Significant reduction in the abundance of Roseburia spp. and other butyrate and propionate-producing bacteria has been observed in patients with UC [101,134]. Similarly, the abundance of R. inulivorans and other butyrate producers has been shown to be decreased in patients with CD compared to healthy controls [135]. Geirnaert et al. studied the microbiota profile of CD patients after supplementation of butyrate-producing bacteria, including R. hominis and R. inulivorans, using an in vitro system [136]. The mix of six butyrate producers increased the level of butyrate in the dysbiosed bacterial community and improved the epithelial barrier function in an in vitro model, which suggests that butyrate-producing bacteria may have a therapeutic potential as probiotics.
R. intestinalis has also been shown to exert anti-inflammatory actions in in vivo and in vitro models. R. intestinalis exhibited an anti-inflammatory pattern regarding cytokines in mono-associated mice by inducing increased, anti-inflammatory cytokine IL-22 production and decreased the production of proinflammatory cytokines IFNγ and IL-17 [137]. Similar results were obtained in another study, which demonstrated the inhibited secretion of IL-17 in vivo and in vitro by R. intestinalis [138]. In the same study, the bacterium also promoted the differentiation of Treg cells in a colitis mouse model. Furthermore, R. intestinalis exerted anti-inflammatory action against LPS-triggered inflammation in enterocytes by promoting the secretion of thymic stromal lymphopoietin (TSLP) and transforming growth factor beta (TGF-β) [139]. R. intestinalis colonizes the gut epithelium possibly by using its flagella to penetrate the mucus layer [140]. The flagellin may act as an effector molecule as it has been shown to induce anti-inflammatory effects by upregulating certain IncRNA (Table 1) [87].
4.5. Eubacterium hallii
Eubacterium hallii is one the major butyrate producers found in the human gut [141]. Quantitatively, the species represents up to 3% of the total bacteria present in the feces of healthy individuals [110]. As for the taxonomy, this obligate anaerobe belongs to the phylum Firmicutes, class Clostridia, and family Eubacteriaceae [142]. The amount of Eubacterium spp. have been reported to decrease in patients with UC and CD as compared to healthy individuals, indicating its value in a healthy, balanced microbial community [135,143]. With respect to intestinal health, E. hallii could also be involved in detoxification of the most abundant dietary carcinogen 2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine by transforming the compound into glycerol-derived conjugate in the colon [144].
As E. hallii cannot use complex carbohydrates, it relies on simple sugars or metabolic intermediates as energy sources [145]. The production of butyrate by E. hallii has been shown to result from either utilization of glucose, or acetate and lactate [146]. In the human gut, where E. hallii is part of complex nutritional webs, this bacterium is presumably cross-fed by other microbes. Co-culture studies have revealed cross-feeding interactions between lactobacilli, bifidobacteria, and butyrate-producing colon bacteria [147,148]. As inulin-type fructans are metabolized into acetate and lactate by lactobacilli and bifidobacteria, E. hallii and other butyrate-producing bacteria can use the metabolites in the production of butyrate. The relationship between E. hallii and A. muciniphila is another example of cross-feeding as these two bacteria exhibit bidirectional syntropy. Belzer et al. [98] has showed that as A. muciniphila degrades mucin, it releases oligosaccharides and 1,2-propanediol which E. hallii can use for growth. In turn, E. hallii produces pseudovitamin B12, which A. muciniphila can then use to produce propionate. Additionally, other mucin-degrading bacteria, such as B. bifidum can provide mucin-derived monosaccharides to E. hallii, which is then able to utilize lactose, galactose, and N-acetylglucosamine (GlcNac). The co-culture of these two bacteria has been shown to result in the production of acetate, butyrate, propionate and formate [147,148]. Recently, E. hallii was shown to harbor the capability to produce propionate also independently and to stimulate its production in other microbes [149].
4.6. Bacteroides spp.
Bacteroides species belong to the major phylum Bacteroidetes. The genus Bacteroides contains the most predominant species of Bacteroidales order in the human intestinal tract [150]. Bacteroides species are adapted to colonize the harsh environment of the intestine with different mechanisms, such as oxygen toleration using cytochrome bd oxidase, metabolizing a variety of diet- and host-derived polysaccharides, and extensive expression of cell surface structures [151]. One of the most abundant species, Bacteroides fragilis, a Gram-negative obligate anaerobe, exhibits both beneficial and pathogenic actions for the host [152,153]. The health-promoting properties of the species within this genus have been recognized relatively recently with B. fragilis being the best studied representative.
B. fragilis produces an immunomodulatory molecule called polysaccharide A (PSA), which activates T-cell-dependent immune responses involved in the gut homeostasis (Table 1) [154]. The bacterium is capable of niche-specific mucosal colonization in the gut, which is PSA-mediated by activating Treg cells through TLR2 signaling to suppress Th17 responses and, thus, inducing mucosal tolerance [155]. Specifically, PSA has been shown to protect from Helicobacter hepaticus induced colitis in mice suggesting it has immunomodulatory capacity and is a potential therapeutic mechanism to the host [88]. Moreover, this symbiotic surface factor has been shown to decrease IL-1β-induced IL-8 cytokine levels in human fetal enterocytes, necrotizing enterocolitis enterocytes, and in fetal mouse small intestine through the stimulation of TLR2 and TLR4 receptors [156]. In the DSS-induced colitis mouse model, B. fragilis inhibits the expression of proinflammatory cytokines IL-6 and TNF-α and enhances the anti-inflammatory IL-10 expression, and thereby ameliorates colitis symptoms [88,157]. In another mouse model, B. fragilis enforces epithelial barrier by correcting intestinal permeability, tight-junction alterations and cytokine expression [158]. In addition, B. fragilis affects the host immune system by enhancing the phagocytic function of macrophages and polarizing them into the M1 phenotype, which promotes Th1 response [159].
B. fragilis communicates with the host immune system, e.g., via its PSA, which is delivered to dendritic cells packed in outer membrane vesicles (OMVs) released from the bacterial surface (Table 1) [89]. Secretion of these signaling molecules enables Bacteroides spp. to interact with the intestinal epithelium, which would be otherwise inaccessible due to the thick mucus layer [160]. Bacteroides vulgatus, which has been shown to protect against E. coli -induced colitis in monocolonized mice, and also produces OMVs and regulates immune responses in the host [160,161]. In addition to B. fragilis, other less studied Bacteroides spp. are also capable of producing multiple capsular polysaccharides making their surface structures more diverse [162]. Different Bacteroides spp. isolated from fecal microbiota transplantation (FMT)-donor were capable of attenuating LPS-induced inflammation in vitro, which indicates that they may exert anti-inflammatory action in the intestine [163]. However, the attenuation capacity is not adhesion-dependent suggesting the bacteria may secrete effector molecules responsible for the interaction with the enterocytes. The currently used probiotics on the market may not colonize the intestinal tract in the long-term. Many of the probiotic strains have been isolated originally from other niches than the human gut, which could explain their poor colonization capacity. For this reason, these next-generation probiotics are of interest as they could possibly colonize the gut more efficiently, being adapted to the intestinal conditions. In fact, results from FMT-studies suggest long-term colonization of commensal donor species, including Bacteroides spp., in the recipients [164,165].
5. Conclusions
Certain intestinal bacteria, like those discussed above, reinforce the host epithelium and give cross-tolerance to LPS, which counteracts or decreases the toxic effects of LPS in dysbiosis. Furthermore, these commensal gut colonizers produce immunoregulatory effects via secreted metabolites, such as SCFAs, especially butyrate. Roseburia, Faecalibacterium, and E. hallii-related bacteria are the most abundant butyrate-producers in the human gut [132]. Moreover, cross-feeding chains have been established between bifidobacteria, F. prausnitzii and R. intestinalis, which enhance the butyrate production in the colon. Additionally, A. muciniphila takes part in the cross-feeding by degrading complex mucin glycoproteins to oligosaccharides, which E. hallii and other butyrate producers, in turn, are able to use for growth and, thus, the production of butyrate. The importance of butyrate producers to gut health is well established, since these bacteria are significantly reduced in many intestinal diseases. Bacteroides species, on the other hand, exert different mechanisms to enhance the gut homeostasis, e.g., secreting immunomodulatory effector molecules. The B. fragilis-produced PSA molecule is an exciting discovery in this field. The mechanisms of action of the potential next-generation probiotics at the molecular level are still largely unknown, but the knowledge is increasing rapidly.
Dietary changes and the use of antibiotics associated with intestinal diseases also modulate the microbiota composition, which plays a part in the depletion of essential health-promoting commensals in the colon and, thus, contributes to unbalanced microbiota composition. The most drastic example of disturbed microbial ecosystem and function by antibiotics and, on the other hand, its restoration by bacteriotherapeutic supplementation, is recurrent Clostridium difficile infection and its treatment with fecal microbiota transplantation [164]. Considering the beneficial effects of the commensal species in fortifying intestinal barrier function and immunoregulation discussed above, it seems justified to consider strategies to promote their presence and activity in the human gut. This could be achieved by de novo introduction of the species into the gut in the form of probiotic or bacteriotherapeutic preparations or by stimulating the growth of the species in the gut by dietary means, or the combination of these two [128,166]. As a promising example, the treatment with A. muciniphila has been shown to counteract high fat diet -induced complications and gut barrier dysfunction in mice and the bacterium is tested safe for humans [84,104,105]. The dietary approaches include adequate dosages of fiber and prebiotics, whose beneficial effects are transmitted to the host particularly through bacterial fermentation and the health-promoting action of commensals that are stimulated by these compounds [167]. Interestingly, the microbiota composition and presence of certain key commensal species seem to affect the individual’s fiber fermentability capacity, as was discovered in a feeding study where individuals lacking Ruminococcus bromii had reduced fermentability (20–30% compared to 100%) of resistant starch [168]. Within prebiotics, inulin-type fructans and oligosaccharides mimicking HMOs have already shown promising efficacy in modulating the microbiota composition and metabolism, in which cross-feeding has a central role at the gut ecosystem level.
In the future, increased knowledge on the cross-feeding chains within microbiota combined with the affordable profiling of individual’s microbiota will open exciting and efficient possibilities in personalized nutrition to modulate the composition and action of gut microbiota in the desired direction.
Acknowledgments
The financial support from the Academy of Finland (grants 304490 for RS and 285632 for VK), Sigrid Juselius, Paulo and Päivikki, and Sakari Sohlberg Foundations (for RS) and University of Helsinki, Microbiology and Biotechnology Doctoral Program (for KH) is gratefully acknowledged.
Author Contributions
All authors participated in writing and revising of the manuscript and accepted the final version of the manuscript.
Funding
Academy of Finland grants 304490 and 285632.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sender R., Fuchs S., Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J., Jia H., Cai X., Zhong H., Feng Q., Sunagawa S., Arumugam M., Kultima J.R., Prifti E., Nielsen T., et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014;32:834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 3.Rajilic-Stojanovic M., de Vos W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faith J.J., Guruge J.L., Charbonneau M., Subramanian S., Seedorf H., Goodman A.L., Clemente J.C., Knight R., Heath A.C., Leibel R.L., et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salonen A., Salojarvi J., Lahti L., de Vos W.M. The adult intestinal core microbiota is determined by analysis depth and health status. Clin. Microbiol. Infect. 2012;18(Suppl. 4):16–20. doi: 10.1111/j.1469-0691.2012.03855.x. [DOI] [PubMed] [Google Scholar]
- 6.Ouwerkerk J.P., de Vos W.M., Belzer C. Glycobiome: Bacteria and mucus at the epithelial interface. Best Pract. Res. Clin. Gastroenterol. 2013;27:25–38. doi: 10.1016/j.bpg.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Willing B.P., Dicksved J., Halfvarson J., Andersson A.F., Lucio M., Zheng Z., Jarnerot G., Tysk C., Jansson J.K., Engstrand L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Nava G.M., Friedrichsen H.J., Stappenbeck T.S. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 2011;5:627–638. doi: 10.1038/ismej.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Hertzen L., Beutler B., Bienenstock J., Blaser M., Cani P.D., Eriksson J., Farkkila M., Haahtela T., Hanski I., Jenmalm M.C., et al. Helsinki alert of biodiversity and health. Ann. Med. 2015;47:218–225. doi: 10.3109/07853890.2015.1010226. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder B.O., Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 11.Satokari R. Contentious host-microbiota relationship in inflammatory bowel disease—Can foes become friends again? Scand. J. Gastroenterol. 2015;50:34–42. doi: 10.3109/00365521.2014.966320. [DOI] [PubMed] [Google Scholar]
- 12.Walker A.W., Lawley T.D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013;69:75–86. doi: 10.1016/j.phrs.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013;7:1256–1261. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L.C., Wang J.T., Wei S.C., Ni Y.H. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J. Gastrointest. Pathophysiol. 2012;3:27–43. doi: 10.4291/wjgp.v3.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muniz L.R., Knosp C., Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front. Immunol. 2012;3:310. doi: 10.3389/fimmu.2012.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goll R., van Beelen Granlund A. Intestinal barrier homeostasis in inflammatory bowel disease. Scand. J. Gastroenterol. 2015;50:3–12. doi: 10.3109/00365521.2014.971425. [DOI] [PubMed] [Google Scholar]
- 17.Ohno H. Intestinal M cells. J. Biochem. 2016;159:151–160. doi: 10.1093/jb/mvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson G.P., Ladinsky M.S., Yu K.B., Sanders J.G., Yoo B.B., Chou W.C., Conner M.E., Earl A.M., Knight R., Bjorkman P.J., et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science. 2018;360:795–800. doi: 10.1126/science.aaq0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N., Lewis P., Samuelson D., Liboni K., Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G726–G733. doi: 10.1152/ajpgi.00012.2004. [DOI] [PubMed] [Google Scholar]
- 22.Lam Y.Y., Ha C.W., Hoffmann J.M., Oscarsson J., Dinudom A., Mather T.J., Cook D.I., Hunt N.H., Caterson I.D., Holmes A.J., et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity. 2015;23:1429–1439. doi: 10.1002/oby.21122. [DOI] [PubMed] [Google Scholar]
- 23.Lupp C., Robertson M.L., Wickham M.E., Sekirov I., Champion O.L., Gaynor E.C., Finlay B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Rizzatti G., Lopetuso L.R., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heumann D., Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin. Chim. Acta. 2002;323:59–72. doi: 10.1016/S0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 26.Cochet F., Peri F. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int. J. Mol. Sci. 2017;18:2318. doi: 10.3390/ijms18112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backhed F., Normark S., Schweda E.K., Oscarson S., Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: A biological role for LPS modifications. Microbes Infect. 2003;5:1057–1063. doi: 10.1016/S1286-4579(03)00207-7. [DOI] [PubMed] [Google Scholar]
- 28.Teghanemt A., Zhang D., Levis E.N., Weiss J.P., Gioannini T.L. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 2005;175:4669–4676. doi: 10.4049/jimmunol.175.7.4669. [DOI] [PubMed] [Google Scholar]
- 29.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 30.Fei N., Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880–884. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michail S., Lin M., Frey M.R., Fanter R., Paliy O., Hilbush B., Reo N.V. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015;91:1–9. doi: 10.1093/femsec/fiu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D., Gill S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 33.Gophna U., Sommerfeld K., Gophna S., Doolittle W.F., Veldhuyzen van Zanten S.J. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J. Clin. Microbiol. 2006;44:4136–4141. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehman A., Lepage P., Nolte A., Hellmig S., Schreiber S., Ott S.J. Transcriptional activity of the dominant gut mucosal microbiota in chronic inflammatory bowel disease patients. J. Med. Microbiol. 2010;59:1114–1122. doi: 10.1099/jmm.0.021170-0. [DOI] [PubMed] [Google Scholar]
- 35.Sartor R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenborn U. Escherichia coli strain Nissle 1917-from bench to bedside and back: History of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 2016;363 doi: 10.1093/femsle/fnw212. [DOI] [PubMed] [Google Scholar]
- 37.Grozdanov L., Zahringer U., Blum-Oehler G., Brade L., Henne A., Knirel Y.A., Schombel U., Schulze J., Sonnenborn U., Gottschalk G., et al. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J. Bacteriol. 2002;184:5912–5925. doi: 10.1128/JB.184.21.5912-5925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henker J., Laass M., Blokhin B.M., Bolbot Y.K., Maydannik V.G., Elze M., Wolff C., Schulze J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur. J. Pediatr. 2007;166:311–318. doi: 10.1007/s00431-007-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kandasamy S., Vlasova A.N., Fischer D., Kumar A., Chattha K.S., Rauf A., Shao L., Langel S.N., Rajashekara G., Saif L.J. Differential Effects of Escherichia coli Nissle and Lactobacillus rhamnosus Strain GG on Human Rotavirus Binding, Infection, and B Cell Immunity. J. Immunol. 2016;196:1780–1789. doi: 10.4049/jimmunol.1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreuzer S., Machnowska P., Assmus J., Sieber M., Pieper R., Schmidt M.F., Brockmann G.A., Scharek-Tedin L., Johne R. Feeding of the probiotic bacterium Enterococcus faecium NCIMB 10415 differentially affects shedding of enteric viruses in pigs. Vet. Res. 2012;43:58. doi: 10.1186/1297-9716-43-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lodinova-Zadnikova R., Sonnenborn U. Effect of preventive administration of a nonpathogenic Escherichia coli strain on the colonization of the intestine with microbial pathogens in newborn infants. Biol. Neonate. 1997;71:224–232. doi: 10.1159/000244421. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder B., Duncker S., Barth S., Bauerfeind R., Gruber A.D., Deppenmeier S., Breves G. Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig. Dis. Sci. 2006;51:724–731. doi: 10.1007/s10620-006-3198-8. [DOI] [PubMed] [Google Scholar]
- 43.Splichalova A., Trebichavsky I., Rada V., Vlkova E., Sonnenborn U., Splichal I. Interference of Bifidobacterium choerinum or Escherichia coli Nissle 1917 with Salmonella Typhimurium in gnotobiotic piglets correlates with cytokine patterns in blood and intestine. Clin. Exp. Immunol. 2011;163:242–249. doi: 10.1111/j.1365-2249.2010.04283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kruis W., Fric P., Pokrotnieks J., Lukas M., Fixa B., Kascak M., Kamm M.A., Weismueller J., Beglinger C., Stolte M., et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthes H., Krummenerl T., Giensch M., Wolff C., Schulze J. Clinical trial: Probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN) BMC Complement. Altern. Med. 2010;10:13. doi: 10.1186/1472-6882-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rembacken B.J., Snelling A.M., Hawkey P.M., Chalmers D.M., Axon A.T. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/S0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 47.Scaldaferri F., Gerardi V., Mangiola F., Lopetuso L.R., Pizzoferrato M., Petito V., Papa A., Stojanovic J., Poscia A., Cammarota G., et al. Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: An update. World J. Gastroenterol. 2016;22:5505–5511. doi: 10.3748/wjg.v22.i24.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M., Friesen L., Park J., Kim H.M., Kim C.H. Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. Eur. J. Immunol. 2018;48:1235–1247. doi: 10.1002/eji.201747122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A., et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llewellyn A., Foey A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients. 2017;9:1156. doi: 10.3390/nu9101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 52.McFarland L.V., Evans C.T., Goldstein E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018;5:124. doi: 10.3389/fmed.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worthley D.L., Le Leu R.K., Whitehall V.L., Conlon M., Christophersen C., Belobrajdic D., Mallitt K.A., Hu Y., Irahara N., Ogino S., et al. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: Effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am. J. Clin. Nutr. 2009;90:578–586. doi: 10.3945/ajcn.2009.28106. [DOI] [PubMed] [Google Scholar]
- 54.Cushing K., Alvarado D.M., Ciorba M.A. Butyrate and Mucosal Inflammation: New Scientific Evidence Supports Clinical Observation. Clin. Transl. Gastroenterol. 2015;6:e108. doi: 10.1038/ctg.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macfarlane G.T., Macfarlane S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011;45:S120–S127. doi: 10.1097/MCG.0b013e31822fecfe. [DOI] [PubMed] [Google Scholar]
- 56.Biavati B., Mattarelli P. Bifidobacterium. In: Whitman W.B., Rainey F., Kämpfer P., Trujillo M., Chun J., DeVos P., Hedlund B., Dedysh S., editors. Bergley’s Manual of Systematics of Archaea and Bacteria. Wiley; New York, NY, USA: 2015. [DOI] [Google Scholar]
- 57.Bottacini F., van Sinderen D., Ventura M. Omics of bifidobacteria: Research and insights into their health-promoting activities. Biochem. J. 2017;474:4137–4152. doi: 10.1042/BCJ20160756. [DOI] [PubMed] [Google Scholar]
- 58.Douillard F.P., Mora D., Eijlander R.T., Wels M., de Vos W.M. Comparative genomic analysis of the multispecies probiotic-marketed product VSL#3. PLoS ONE. 2018;13:e0192452. doi: 10.1371/journal.pone.0192452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douillard F.P., Ribbera A., Kant R., Pietila T.E., Jarvinen H.M., Messing M., Randazzo C.L., Paulin L., Laine P., Ritari J., et al. Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet. 2013;9:e1003683. doi: 10.1371/journal.pgen.1003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turroni F., Peano C., Pass D.A., Foroni E., Severgnini M., Claesson M.J., Kerr C., Hourihane J., Murray D., Fuligni F., et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE. 2012;7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duranti S., Lugli G.A., Mancabelli L., Armanini F., Turroni F., James K., Ferretti P., Gorfer V., Ferrario C., Milani C., et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5:66. doi: 10.1186/s40168-017-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makino H., Kushiro A., Ishikawa E., Kubota H., Gawad A., Sakai T., Oishi K., Martin R., Ben-Amor K., Knol J., et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS ONE. 2013;8:e78331. doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milani C., Mancabelli L., Lugli G.A., Duranti S., Turroni F., Ferrario C., Mangifesta M., Viappiani A., Ferretti P., Gorfer V., et al. Exploring Vertical Transmission of Bifidobacteria from Mother to Child. Appl. Environ. Microbiol. 2015;81:7078–7087. doi: 10.1128/AEM.02037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korpela K., Costea P., Coelho L.P., Kandels-Lewis S., Willemsen G., Boomsma D.I., Segata N., Bork P. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 2018;28:561–568. doi: 10.1101/gr.233940.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollister E.B., Riehle K., Luna R.A., Weidler E.M., Rubio-Gonzales M., Mistretta T.A., Raza S., Doddapaneni H.V., Metcalf G.A., Muzny D.M., et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36. doi: 10.1186/s40168-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J.Z., Abe F., Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aakko J., Kumar H., Rautava S., Wise A., Autran C., Bode L., Isolauri E., Salminen S. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef. Microbes. 2017;8:563–567. doi: 10.3920/BM2016.0185. [DOI] [PubMed] [Google Scholar]
- 68.Arboleya S., Bottacini F., O’Connell-Motherway M., Ryan C.A., Ross R.P., van Sinderen D., Stanton C. Gene-trait matching across the Bifidobacterium longum pan-genome reveals considerable diversity in carbohydrate catabolism among human infant strains. BMC Genom. 2018;19:33. doi: 10.1186/s12864-017-4388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Callaghan A., Bottacini F., O’Connell Motherway M., van Sinderen D. Pangenome analysis of Bifidobacterium longum and site-directed mutagenesis through by-pass of restriction-modification systems. BMC Genom. 2015;16:832. doi: 10.1186/s12864-015-1968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sela D.A., Chapman J., Adeuya A., Kim J.H., Chen F., Whitehead T.R., Lapidus A., Rokhsar D.S., Lebrilla C.B., German J.B., et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA. 2008;105:18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milani C., Lugli G.A., Duranti S., Turroni F., Mancabelli L., Ferrario C., Mangifesta M., Hevia A., Viappiani A., Scholz M., et al. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Sci. Rep. 2015;5:15782. doi: 10.1038/srep15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falony G., Vlachou A., Verbrugghe K., De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Environ. Microbiol. 2006;72:7835–7841. doi: 10.1128/AEM.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moens F., Weckx S., De Vuyst L. Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between bifidobacteria and Faecalibacterium prausnitzii. Int. J. Food Microbiol. 2016;231:76–85. doi: 10.1016/j.ijfoodmicro.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Rios-Covian D., Gueimonde M., Duncan S.H., Flint H.J., de los Reyes-Gavilan C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015;362 doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 75.Turroni F., Serafini F., Foroni E., Duranti S., O’Connell Motherway M., Taverniti V., Mangifesta M., Milani C., Viappiani A., Roversi T., et al. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium-host interactions. Proc. Natl. Acad. Sci. USA. 2013;110:11151–11156. doi: 10.1073/pnas.1303897110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Connell Motherway M., Zomer A., Leahy S.C., Reunanen J., Bottacini F., Claesson M.J., O’Brien F., Flynn K., Casey P.G., Munoz J.A., et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. USA. 2011;108:11217–11222. doi: 10.1073/pnas.1105380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fanning S., Hall L.J., Cronin M., Zomer A., MacSharry J., Goulding D., Motherway M.O., Shanahan F., Nally K., Dougan G., et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc. Natl. Acad. Sci. USA. 2012;109:2108–2113. doi: 10.1073/pnas.1115621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 79.Hsieh C.Y., Osaka T., Moriyama E., Date Y., Kikuchi J., Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015;3:e12327. doi: 10.14814/phy2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valdes-Varela L., Alonso-Guervos M., Garcia-Suarez O., Gueimonde M., Ruas-Madiedo P. Screening of Bifidobacteria and Lactobacilli Able to Antagonize the Cytotoxic Effect of Clostridium difficile upon Intestinal Epithelial HT29 Monolayer. Front. Microbiol. 2016;7:577. doi: 10.3389/fmicb.2016.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Srutkova D., Schwarzer M., Hudcovic T., Zakostelska Z., Drab V., Spanova A., Rittich B., Kozakova H., Schabussova I. Bifidobacterium longum CCM 7952 Promotes Epithelial Barrier Function and Prevents Acute DSS-Induced Colitis in Strictly Strain-Specific Manner. PLoS ONE. 2015;10:e0134050. doi: 10.1371/journal.pone.0134050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milani C., Mangifesta M., Mancabelli L., Lugli G.A., Mancino W., Viappiani A., Faccini A., van Sinderen D., Ventura M., Turroni F. The Sortase-Dependent Fimbriome of the Genus Bifidobacterium: Extracellular Structures with Potential To Modulate Microbe-Host Dialogue. Appl. Environ. Microbiol. 2017;83:e01295-17. doi: 10.1128/AEM.01295-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ottman N., Reunanen J., Meijerink M., Pietila T.E., Kainulainen V., Klievink J., Huuskonen L., Aalvink S., Skurnik M., Boeren S., et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 85.Chelakkot C., Choi Y., Kim D.K., Park H.T., Ghim J., Kwon Y., Jeon J., Kim M.S., Jee Y.K., Gho Y.S., et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mole. Med. 2018;50:e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Breyner N.M., Michon C., de Sousa C.S., Vilas Boas P.B., Chain F., Azevedo V.A., Langella P., Chatel J.M. Microbial Anti-Inflammatory Molecule (MAM) from Faecalibacterium prausnitzii Shows a Protective Effect on DNBS and DSS-Induced Colitis Model in Mice through Inhibition of NF-kappaB Pathway. Front. Microbiol. 2017;8:114. doi: 10.3389/fmicb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quan Y., Song K., Zhang Y., Zhu C., Shen Z., Wu S., Luo W., Tan B., Yang Z., Wang X. Roseburia intestinalis-derived flagellin is a negative regulator of intestinal inflammation. Biochem. Biophys. Res. Commun. 2018;501:791–799. doi: 10.1016/j.bbrc.2018.05.075. [DOI] [PubMed] [Google Scholar]
- 88.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 89.Shen Y., Giardino Torchia M.L., Lawson G.W., Karp C.L., Ashwell J.D., Mazmanian S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.AlFaleh K., Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD005496.pub4. [DOI] [PubMed] [Google Scholar]
- 91.Deshpande G., Jape G., Rao S., Patole S. Benefits of probiotics in preterm neonates in low-income and medium-income countries: A systematic review of randomised controlled trials. BMJ Open. 2017;7:e017638. doi: 10.1136/bmjopen-2017-017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun J., Marwah G., Westgarth M., Buys N., Ellwood D., Gray P.H. Effects of Probiotics on Necrotizing Enterocolitis, Sepsis, Intraventricular Hemorrhage, Mortality, Length of Hospital Stay, and Weight Gain in Very Preterm Infants: A Meta-Analysis. Adv. Nutr. 2017;8:749–763. doi: 10.3945/an.116.014605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Martinez M.I., Calabuig-Tolsa R., Cauli O. The effect of probiotics as a treatment for constipation in elderly people: A systematic review. Arch. Gerontol. Geriatr. 2017;71:142–149. doi: 10.1016/j.archger.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Miller L.E., Lehtoranta L., Lehtinen M.J. The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis. Nutrients. 2017;9:E191. doi: 10.3390/nu9030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Derrien M., Vaughan E.E., Plugge C.M., de Vos W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evolut. Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 96.Ottman N., Geerlings S.Y., Aalvink S., de Vos W.M., Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017;31:637–642. doi: 10.1016/j.bpg.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Ouwerkerk J.P., van der Ark K.C., Davids M., Claassens N.J., Robert Finestra T., de Vos W.M., Belzer C. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl. Environ. Microbiol. 2016 doi: 10.1128/AEM.01641-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belzer C., Chia L.W., Aalvink S., Chamlagain B., Piironen V., Knol J., de Vos W.M. Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts. mBio. 2017;8:e000770-17. doi: 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karlsson C.L., Onnerfalt J., Xu J., Molin G., Ahrne S., Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20:2257–2261. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 100.Png C.W., Linden S.K., Gilshenan K.S., Zoetendal E.G., McSweeney C.S., Sly L.I., McGuckin M.A., Florin T.H. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 101.Rajilic-Stojanovic M., Shanahan F., Guarner F., de Vos W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel Dis. 2013;19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 102.Swidsinski A., Dorffel Y., Loening-Baucke V., Theissig F., Ruckert J.C., Ismail M., Rau W.A., Gaschler D., Weizenegger M., Kuhn S., et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut. 2011;60:34–40. doi: 10.1136/gut.2009.191320. [DOI] [PubMed] [Google Scholar]
- 103.Zhang X., Shen D., Fang Z., Jie Z., Qiu X., Zhang C., Chen Y., Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S., Bae J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 106.Li J., Lin S., Vanhoutte P.M., Woo C.W., Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation. 2016;133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 107.Reunanen J., Kainulainen V., Huuskonen L., Ottman N., Belzer C., Huhtinen H., de Vos W.M., Satokari R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang C.S., Ban M., Choi E.J., Moon H.G., Jeon J.S., Kim D.K., Park S.K., Jeon S.G., Roh T.Y., Myung S.J., et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE. 2013;8:e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ottman N., Huuskonen L., Reunanen J., Boeren S., Klievink J., Smidt H., Belzer C., de Vos W.M. Characterization of Outer Membrane Proteome of Akkermansia muciniphila Reveals Sets of Novel Proteins Exposed to the Human Intestine. Front. Microbiol. 2016;7:1157. doi: 10.3389/fmicb.2016.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Louis P., Flint H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 111.Tap J., Mondot S., Levenez F., Pelletier E., Caron C., Furet J.P., Ugarte E., Munoz-Tamayo R., Paslier D.L., Nalin R., et al. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 2009;11:2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 112.Duncan S.H., Flint H.J. Faecalibacterium. In: Vos P., Garrity G., Jones D., Krieg N.R., Ludwig W., Rainey F.A., Schleifer K.-H., Whitman W., editors. Bergey’s Manual of Systematics of Archaea and Bacteria. John Wiley & Sons; New York, NY, USA: 2015. [Google Scholar]
- 113.Lopez-Siles M., Khan T.M., Duncan S.H., Harmsen H.J., Garcia-Gil L.J., Flint H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012;78:420–428. doi: 10.1128/AEM.06858-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez-Siles M., Duncan S.H., Garcia-Gil L.J., Martinez-Medina M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017;11:841–852. doi: 10.1038/ismej.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferreira-Halder C.V., Faria A.V.S., Andrade S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 116.Lopez-Siles M., Martinez-Medina M., Suris-Valls R., Aldeguer X., Sabat-Mir M., Duncan S.H., Flint H.J., Garcia-Gil L.J. Changes in the Abundance of Faecalibacterium prausnitzii Phylogroups I and II in the Intestinal Mucosa of Inflammatory Bowel Disease and Patients with Colorectal Cancer. Inflamm. Bowel Dis. 2016;22:28–41. doi: 10.1097/MIB.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 117.Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermudez-Humaran L.G., Gratadoux J.J., Blugeon S., Bridonneau C., Furet J.P., Corthier G., et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sokol H., Seksik P., Furet J.P., Firmesse O., Nion-Larmurier I., Beaugerie L., Cosnes J., Corthier G., Marteau P., Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 119.Hooda S., Boler B.M., Serao M.C., Brulc J.M., Staeger M.A., Boileau T.W., Dowd S.E., Fahey G.C., Jr., Swanson K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012;142:1259–1265. doi: 10.3945/jn.112.158766. [DOI] [PubMed] [Google Scholar]
- 120.Ramirez-Farias C., Slezak K., Fuller Z., Duncan A., Holtrop G., Louis P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 121.Wrzosek L., Miquel S., Noordine M.L., Bouet S., Joncquel Chevalier-Curt M., Robert V., Philippe C., Bridonneau C., Cherbuy C., Robbe-Masselot C., et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martin R., Miquel S., Chain F., Natividad J.M., Jury J., Lu J., Sokol H., Theodorou V., Bercik P., Verdu E.F., et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15:67. doi: 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carlsson A.H., Yakymenko O., Olivier I., Hakansson F., Postma E., Keita A.V., Soderholm J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013;48:1136–1144. doi: 10.3109/00365521.2013.828773. [DOI] [PubMed] [Google Scholar]
- 124.Miquel S., Leclerc M., Martin R., Chain F., Lenoir M., Raguideau S., Hudault S., Bridonneau C., Northen T., Bowen B., et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio. 2015;6:e00300-15. doi: 10.1128/mBio.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quevrain E., Maubert M.A., Michon C., Chain F., Marquant R., Tailhades J., Miquel S., Carlier L., Bermudez-Humaran L.G., Pigneur B., et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin R., Miquel S., Benevides L., Bridonneau C., Robert V., Hudault S., Chain F., Berteau O., Azevedo V., Chatel J.M., et al. Functional Characterization of Novel Faecalibacterium prausnitzii Strains Isolated from Healthy Volunteers: A Step Forward in the Use of F. prausnitzii as a Next-Generation Probiotic. Front. Microbiol. 2017;8:1226. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bircher L., Geirnaert A., Hammes F., Lacroix C., Schwab C. Effect of cryopreservation and lyophilization on viability and growth of strict anaerobic human gut microbes. Microb. Biotechnol. 2018;11:721–733. doi: 10.1111/1751-7915.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olle B. Medicines from microbiota. Nat. Biotechnol. 2013;31:309–315. doi: 10.1038/nbt.2548. [DOI] [PubMed] [Google Scholar]
- 129.Duncan S.H., Hold G.L., Barcenilla A., Stewart C.S., Flint H.J. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evolut. Microbiol. 2002;52:1615–1620. doi: 10.1099/00207713-52-5-1615. [DOI] [PubMed] [Google Scholar]
- 130.Duncan S.H., Aminov R.I., Scott K.P., Louis P., Stanton T.B., Flint H.J. Proposal of Roseburia faecis sp. nov., Roseburia hominis sp. nov. and Roseburia inulinivorans sp. nov., based on isolates from human faeces. Int. J. Syst. Evolut. Microbiol. 2006;56:2437–2441. doi: 10.1099/ijs.0.64098-0. [DOI] [PubMed] [Google Scholar]
- 131.Stanton T.B., Savage D.C. Colonization of gnotobiotic mice by Roseburia cecicola, a motile, obligately anaerobic bacterium from murine ceca. Appl. Environ. Microbiol. 1983;45:1677–1684. doi: 10.1128/aem.45.5.1677-1684.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hold G.L., Schwiertz A., Aminov R.I., Blaut M., Flint H.J. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 2003;69:4320–4324. doi: 10.1128/AEM.69.7.4320-4324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tamanai-Shacoori Z., Smida I., Bousarghin L., Loreal O., Meuric V., Fong S.B., Bonnaure-Mallet M., Jolivet-Gougeon A. Roseburia spp.: A marker of health? Future Microbiol. 2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- 134.Machiels K., Joossens M., Sabino J., De Preter V., Arijs I., Eeckhaut V., Ballet V., Claes K., Van Immerseel F., Verbeke K., et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 135.Takahashi K., Nishida A., Fujimoto T., Fujii M., Shioya M., Imaeda H., Inatomi O., Bamba S., Sugimoto M., Andoh A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion. 2016;93:59–65. doi: 10.1159/000441768. [DOI] [PubMed] [Google Scholar]
- 136.Geirnaert A., Calatayud M., Grootaert C., Laukens D., Devriese S., Smagghe G., De Vos M., Boon N., Van de Wiele T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017;7:11450. doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoffmann T.W., Pham H.P., Bridonneau C., Aubry C., Lamas B., Martin-Gallausiaux C., Moroldo M., Rainteau D., Lapaque N., Six A., et al. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J. 2016;10:460–477. doi: 10.1038/ismej.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu C., Song K., Shen Z., Quan Y., Tan B., Luo W., Wu S., Tang K., Yang Z., Wang X. Roseburia intestinalis inhibits interleukin17 excretion and promotes regulatory T cells differentiation in colitis. Mol. Med. Rep. 2018;17:7567–7574. doi: 10.3892/mmr.2018.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shen Z., Zhu C., Quan Y., Yang J., Yuan W., Yang Z., Wu S., Luo W., Tan B., Wang X. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol. 2018 doi: 10.1111/jgh.14144. [DOI] [PubMed] [Google Scholar]
- 140.Van den Abbeele P., Belzer C., Goossens M., Kleerebezem M., De Vos W.M., Thas O., De Weirdt R., Kerckhof F.M., Van de Wiele T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7:949–961. doi: 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Louis P., Young P., Holtrop G., Flint H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 142.Wade W.G. Eubacterium. In: Vos P., Garrity G., Jones D., Krieg N.R., Ludwig W., Rainey F.A., Schleifer K.-H., Whitman W., editors. Bergey’s Manual of Systematics of Archaea and Bacteria. John Wiley and Sons; New York, NY, USA: 2015. [Google Scholar]
- 143.Hirano A., Umeno J., Okamoto Y., Shibata H., Ogura Y., Moriyama T., Torisu T., Fujioka S., Fuyuno Y., Kawarabayasi Y., et al. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2018;10 doi: 10.1111/jgh.14129. [DOI] [PubMed] [Google Scholar]
- 144.Fekry M.I., Engels C., Zhang J., Schwab C., Lacroix C., Sturla S.J., Chassard C. The strict anaerobic gut microbe Eubacterium hallii transforms the carcinogenic dietary heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Environ. Microbiol. Rep. 2016;8:201–209. doi: 10.1111/1758-2229.12369. [DOI] [PubMed] [Google Scholar]
- 145.Scott K.P., Martin J.C., Duncan S.H., Flint H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014;87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 146.Duncan S.H., Louis P., Flint H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moens F., Verce M., De Vuyst L. Lactate- and acetate-based cross-feeding interactions between selected strains of lactobacilli, bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int. J. Food Microbiol. 2017;241:225–236. doi: 10.1016/j.ijfoodmicro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 148.Bunesova V., Lacroix C., Schwab C. Mucin Cross-Feeding of Infant Bifidobacteria and Eubacterium hallii. Microb. Ecol. 2018;75:228–238. doi: 10.1007/s00248-017-1037-4. [DOI] [PubMed] [Google Scholar]
- 149.Engels C., Ruscheweyh H.J., Beerenwinkel N., Lacroix C., Schwab C. The Common Gut Microbe Eubacterium hallii also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016;7:713. doi: 10.3389/fmicb.2016.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zitomersky N.L., Coyne M.J., Comstock L.E. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order Bacteroidales in the human gut. Infect. Immun. 2011;79:2012–2020. doi: 10.1128/IAI.01348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wexler A.G., Goodman A.L. An insider’s perspective: Bacteroides as a window into the microbiome. Nature Microbiol. 2017;2:17026. doi: 10.1038/nmicrobiol.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wexler H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Erturk-Hasdemir D., Kasper D.L. Finding a needle in a haystack: Bacteroides fragilis polysaccharide A as the archetypical symbiosis factor. Ann. N. Y. Acad. Sci. 2018;1417:116–129. doi: 10.1111/nyas.13660. [DOI] [PubMed] [Google Scholar]
- 154.Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 155.Round J.L., Lee S.M., Li J., Tran G., Jabri B., Chatila T.A., Mazmanian S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang F., Meng D., Weng M., Zhu W., Wu W., Kasper D., Walker W.A. The symbiotic bacterial surface factor polysaccharide A on Bacteroides fragilis inhibits IL-1beta-induced inflammation in human fetal enterocytes via toll receptors 2 and 4. PLoS ONE. 2017;12:e0172738. doi: 10.1371/journal.pone.0172738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chang Y.C., Ching Y.H., Chiu C.C., Liu J.Y., Hung S.W., Huang W.C., Huang Y.T., Chuang H.L. TLR2 and interleukin-10 are involved in Bacteroides fragilis-mediated prevention of DSS-induced colitis in gnotobiotic mice. PLoS ONE. 2017;12:e0180025. doi: 10.1371/journal.pone.0180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deng H., Li Z., Tan Y., Guo Z., Liu Y., Wang Y., Yuan Y., Yang R., Bi Y., Bai Y., et al. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci. Rep. 2016;6:29401. doi: 10.1038/srep29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maerz J.K., Steimle A., Lange A., Bender A., Fehrenbacher B., Frick J.S. Outer membrane vesicles blebbing contributes to B. vulgatus mpk-mediated immune response silencing. Gut Microbes. 2018;9:1–12. doi: 10.1080/19490976.2017.1344810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Waidmann M., Bechtold O., Frick J.S., Lehr H.A., Schubert S., Dobrindt U., Loeffler J., Bohn E., Autenrieth I.B. Bacteroides vulgatus protects against Escherichia coli-induced colitis in gnotobiotic interleukin-2-deficient mice. Gastroenterology. 2003;125:162–177. doi: 10.1016/S0016-5085(03)00672-3. [DOI] [PubMed] [Google Scholar]
- 162.Xu J., Mahowald M.A., Ley R.E., Lozupone C.A., Hamady M., Martens E.C., Henrissat B., Coutinho P.M., Minx P., Latreille P., et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007;5:e156. doi: 10.1371/journal.pbio.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heini T., Kainulainen V., Hiippala K., Satokari R. Adhesion ja anti-inflammatory properties of selected bacteroidales isolates from healthy human microbiota; Proceedings of the Abstract and Poster in Nutrition Winter School: Breaking Barriers: Gut, Brain, Bugs—And Beyond; Levi, Finland. 22–26 January 2018. [Google Scholar]
- 164.Jalanka J., Mattila E., Jouhten H., Hartman J., de Vos W.M., Arkkila P., Satokari R. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med. 2016;14:155. doi: 10.1186/s12916-016-0698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li S.S., Zhu A., Benes V., Costea P.I., Hercog R., Hildebrand F., Huerta-Cepas J., Nieuwdorp M., Salojarvi J., Voigt A.Y., et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352:586–589. doi: 10.1126/science.aad8852. [DOI] [PubMed] [Google Scholar]
- 166.Patel R., DuPont H.L. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015;60(Suppl. 2):S108–S121. doi: 10.1093/cid/civ177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., Ze X., Brown D., Stares M.D., Scott P., Bergerat A., et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]