Abstract
An imbalance between neuronal excitation and inhibition represents a core feature in multiple neuropsychiatry disorders, necessitating the development of novel strategies to calibrate the excitatory–inhibitory balance of therapeutics. Here we identify a natural compound quercetin that reduces prefrontal cortical GABAergic transmission and alleviates the hyperactivity induced by glutamatergic N-methyl-d-aspartate receptor antagonist MK-801. Quercetin markedly reduced the GABA-activated currents in a noncompetitive manner in cultured cortical neurons, and moderately inhibited spontaneous and electrically-evoked GABAergic inhibitory postsynaptic current in mouse prefrontal cortical slices. Notably, systemic and prefrontal-specific delivery of quercetin reduced basal locomotor activity in addition to alleviated the MK-801-induced hyperactivity. The effects of quercetin were not exclusively dependent on α5-subunit-containing A type GABA receptors (GABAARs), as viral-mediated, region-specific genetic knockdown of the α5-subunit in prefrontal cortex improved the MK-801-evoked psychotic symptom but reserved the pharmacological responsivity to quercetin. Both interventions together completely normalized the locomotor activity. Together, quercetin as a negative allosteric GABAAR modulator exerted antipsychotic activity, facilitating further therapeutic development for the excitatory–inhibitory imbalance disorders.
Keywords: Antipsychotic, Excitatory–inhibitory imbalance disorders, GABAAR, Prefrontal transmission, Quercetin
Abbreviations: AAV, Adeno-associated virus; ACSF, artificial cerebrospinal fluid; ANOVA, analysis of variance; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; DAPI, 4,6-diamidino-2-phenylindole dihydrochloride; D-APV, D-2-amino-5-phosphonopentanoic acid; DMSO, dimethyl sulfoxide; EGTA, ethylene glycol tetraacetic acid; eEPSC, evoked excitatory postsynaptic current; eIPSC, evoked inhibitory postsynaptic current; EPSC, excitatory postsynaptic current; EYFP, enhanced yellow fluorescent protein; GABA, γ-aminobutyric acid; GABAAR, A-type GABA receptor; GABACR, C-type GABA receptor; GFP, green fluorescence protein; HEPES, N-hydroxyethylpiperazine-N-2-ethanesulphonic acid; i.p., intraperitoneal; IPSC, inhibitory postsynaptic current; mPFC, medial prefrontal cortex; NC-Ctrl, negative control; nH, Hill coefficient; NMDA, N-methyl-d-aspartate; NMDAR, N-methyl-d-aspartate receptor; PBS, phosphate-buffered solution; sIPSC, spontaneous inhibitory postsynaptic current
1. Introduction
Accurate calibration of excitatory–inhibitory balance across the central nervous system is fundamental for the normal functioning of the brain, while an imbalance in neuronal excitation/inhibition is a core feature observed in neuropsychiatric disorders, but not restricted to schizophrenia. The finding [37] that the psychotomimetic drug phencyclidine noncompetitively blocked the N-methyl-d-aspartate receptor (NMDAR) gave rise to the glutamate theory of schizophrenia [10, 11, 26], according to which NMDAR hypofunction and disturbances in NMDAR-related gene expression and metabolic pathways confer the disease phenotypes [46, 62]. The NMDAR antagonist ketamine has been shown to induce significant psychosis [31] and exacerbated it further in individuals predisposed to schizophrenia symptomatology [32]. Consequently, the NMDAR antagonist such as MK-801 has been shown to induce hyperactivity using locomotor activity paradigm in rodents to model the part of the positive symptoms in psychosis [1, 23, 70, 71] for novel antipsychotic drug discovery [58] and therapeutic development [[42], [43], [44]]. Not surprisingly, agents potentiating glutamatergic transmission, by activating the glycine modulatory site on the NMDAR, have been reported to reduce some of the cognitive symptoms of schizophrenia [4, 14, 15]. Specifically, schizophrenia, particularly the cognitive symptoms of the disorder, may result from the low activity of NMDAR on the GABAergic inhibitory interneurons in the prefrontal cortex [[10], [11], [12], 26, 42, 43, 46, 71], as the postnatal ablation of NMDAR in this subtype of neurons conferred most schizophrenia-like phenotypes [5, 20]. The progress in the understanding of pathogenesis of psychosis is encouraging; however, unfortunately, current treatments for schizophrenia are far from satisfactory. Yet, these treatments have substantially improved outcomes for most patients with schizophrenia [26]. Therefore, continued efforts are necessary to develop or discover novel strategies for preventing and curing this type of disorder.
The γ-aminobutyric acid (GABA) system is pivotal for the orchestration of local networks and the functional interaction across different brain regions [64], which can act as an alternative target to calibrate the excitatory–inhibitory balance in the central nervous system. A-type GABA receptors (GABAARs) are pentameric Cl−-permeable ion channels activated by the GABA transmitter and widely distributed in the central nervous system, which primarily confer fast inhibitory control over neural activity [56]. To date, at least up to 19 known subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3) have been identified. Of note, many of functional GABAARs contain two α-subunits, two β-subunits, and one γ-subunit [41, 55]. Because GABAARs are responsible for inhibitory tone in the central nervous system, they are indispensable for controlling the neuronal balance between excitation and inhibition and thus participate in almost every physiological and pathophysiological brain function. Accordingly, GABAARs have long been considered as an important pharmaceutical target. They are positively modulated [59] by benzodiazepines [63], barbiturates [38], steroids [25], and anesthetics [45, 52, 65, 69]. Most of these drugs have been in clinical use for decades and are still among the most widely prescribed drugs for the treating insomnia and anxiety disorders.
Growing evidence also suggests that low doses of GABAAR antagonists show therapeutic potentials in a particular type of neurodevelopmental disorders such as in Down syndrome [18] and the antipsychotic efficacy [51, 60]. Of note, negative allosteric modulators selectively targeting α5-subunit-containing GABAARs consistently exhibit substantial pharmacological effects to restore cognitive deficits [2, 3, 30, 40, 53, 61], promote functional recovery after stroke [8], or exert the anti-depressant action [72]. It is worth noting that GABAAR-mediated inhibition can be cell type-specific [66], and targeting GABAARs would have different roles in the network dependent on the target neurons [17]. Inhibiting glutamatergic pyramidal neuron by GABAAR reduces network excitability while inhibiting GABAergic interneurons increases network excitability. Nevertheless, most of GABAergic agents do not distinguish between these two alternatives, providing an alternative explanation for the aforementioned GABAAR inhibitors, on occasion, capable of reducing network excitability for their beneficial efficacy. Collectively, therapeutic potentials by virtue of negative modulators of GABAARs for neuropsychiatric disorders [6, 29, 64], including schizophrenia, are far underestimated.
The current study took advantage of a natural flavonoid compound quercetin [2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one, Fig. 1A], which has been identified as a negative modulator for recombinant GABAARs and C-type GABA receptors (GABACRs, also designated to be ρ1-subunit-containing GABAARs) [21, 22, 28], to examine its pharmacological effects on brain activity. The present study examined the effects of quercetin on the endogenous GABAAR currents in cultured mouse cortical neurons, in addition to that on synaptic transmission in mouse prefrontal cortex slices (using patch-clamp electrophysiology), and MK-801-evokded locomotor hyperactivity (using behavioral assessment). The study found that quercetin, as an inhibitor of GABAARs, reduced GABAergic transmission in the prefrontal cortex and alleviated the hyperactivity caused by MK-801.
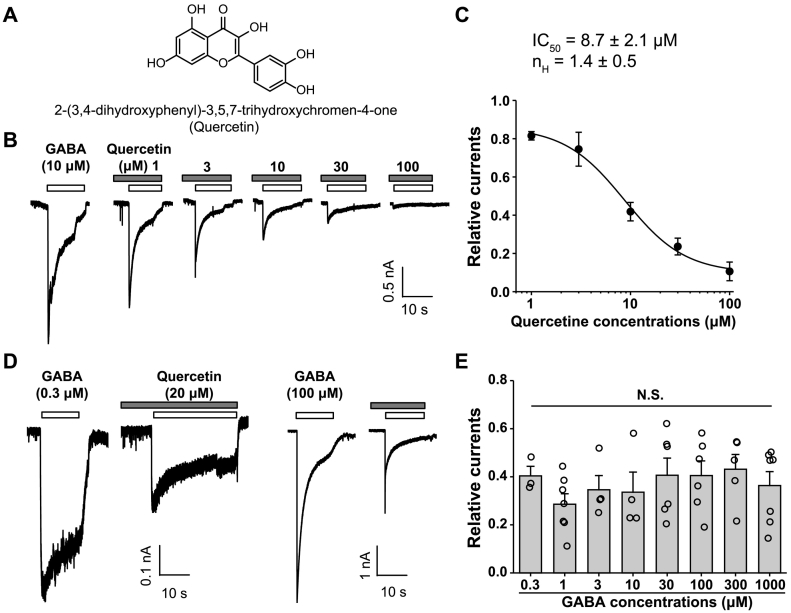
Fig. 1.
Modulation of GABA response by quercetin in cultured cortical neurons. (A) Chemical structures of quercetin. (B) Representative traces showing the currents evoked by GABA (10 μM) alone, or in the presence of various concentrations of quercetin as indicated. (C) Concentration–response curves of quercetin plus GABA-evoked induced currents. Current amplitudes were normalized to the GABA-induced response in the absence of quercetin. The IC50 and Hill coefficient values were 8.7 ± 2.1 μM and 1.4 ± 0.5, respectively. n = 4–10 for each group. (D, E) Representative traces (D) and pooled data (E) showing the relative inhibition induced by 20 μM quercetin of the response to various concentrations of GABA. n = 3–7 for each group. F(7,42) = 0.662, p = .702, one-way ANOVA.
2. Materials and Methods
2.1. Animals
All behavioral measurements were performed in adult unrestrained awake male C57BL/6J mice (8–12 weeks old), which were obtained from Shanghai Slac Laboratory Animal Company Limited (Shanghai, China). The Mice were subjected to a 12-h light/dark cycle, and the behavioral experiments were always performed during the light phase of the cycle. The mice had access to food and water ad libitum except during tests. All efforts were made to minimize animal suffering and reduce the number of animals used. All experimental protocols were approved by the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine, China. In all experiments, the investigators were blind to the drug treatment of mice. The experiments were performed on the mice in a randomized order.
2.2. Drugs
Primary cultures of mouse cortical neurons were prepared according to previously described techniques [36]. In brief, 15-day-old embryonic C57BL/6 J mice were isolated using a standard enzyme treatment protocol. Brains were removed rapidly and placed in ice-cold Ca2+- and Mg2+-free phosphate-buffered solution (PBS). Tissues were dissected and incubated with 0.05% trypsin-EDTA for 10 min at 37 °C, followed by trituration with fire-polished glass pipettes, and plated on poly-d-lysine-coated 35 mm culture dishes (Corning, USA) at a density of 1 × 106 cells per dish. Neurons were cultured using Neurobasal medium (Thermo Fisher Scientific, USA) supplemented with B27 (Thermo Fisher Scientific, USA) and maintained at 37 °C in a humidified 5% CO2 atmosphere incubator. Cultures were fed twice a week and used for electrophysiological recording 10–20 days after plating.
The cDNA of mouse GABAAR α5-subunit (GenBank accession: NM_176942.4) or γ2-subunit (GenBank accession: NM_008073.4) was expressed in human embryonic kidney (HEK)-293T cells by transient transfection as reported in a previous study [36]. The HEK-293T cells were cultured in the Dulbecco's modified Eagle's medium supplemented with 1 mM l-glutamine, 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin (all from Thermo Fisher Scientific, USA), at 37 °C in a humidified atmosphere of 5% (v/v) CO2 and 95% O2 (v/v) and passaged twice a week. Transient transfection of HEK-293T cells was performed using HilyMax liposome transfection reagent (Dojindo Laboratories, Japan).
2.3. Chemicals
All drugs were purchased from Sigma-Aldrich (Merck Millipore, USA) except otherwise indicated. In the electrophysiological experiment, the final concentration of dimethyl sulfoxide (DMSO) was lower than 0.1% and verified to be ineffective alone at the same concentration in control experiment. Other drugs were first dissolved in ion-free water and then diluted to the final concentrations in the standard external solution just before use or dissolved directly in the standard external solution.
2.4. Electrophysiological Recording in Cultured Cells
Whole-cell recordings were made using an Axon 200B patch-clamp amplifier (Axon Instruments, USA). Membrane currents were sampled and analyzed using a Digidata 1440 interface and a personal computer running Clampex and Clampfit software (Version 10, Axon Instruments). The membrane potential was held at −60 mV throughout the experiment under voltage clamp conditions. All the experiments were carried out at room temperature (23 ± 2 °C).
The standard external solution contained (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 N-hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES), and 10 glucose (pH 7.4 with Tris-base, 325–330 mOsm). The pipette solution was composed of (in mM): 120 KCl, 30 NaCl, 1 MgCl2, 0.5 CaCl2, 5 ethylene glycol tetraacetic acid (EGTA), 2 Mg-ATP, 10 HEPES, pH 7.2 adjusted with Tris-base. For the majority of electrophysiological recordings, drugs were applied using the “Y-tube” method, which allowed a complete exchange of external solution surrounding the cell within 20 ms [35, 48]. Throughout the experiment, the bath was superfused continuously with a standard external solution.
2.5. Brain Slice Preparation and Electrophysiological Recording
Experiments were performed on 300-μm transverse medial prefrontal cortical slices from male C57BL/6J mice (6–10 weeks old), as described in a previous study [33] with minor modifications. Briefly, after decapitation, the mouse brains were quickly removed and placed in a well-oxygenated (95% O2/5% CO2) ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 2.5 KCl, 12.5 d-glucose, 1 MgCl2, 2 CaCl2, 1.25 NaH2PO4, and 25 NaHCO3 (pH 7.35–7.45). Two to three coronal prefrontal cortical slices were cut with a vibratome (Leica VT 1000S, Germany) and incubated at 30 ± 1 °C in oxygenated ACSF at least 1 h before transferring to a recording chamber. Whole-cell patch-clamp recordings were made from neurons in medial prefrontal cortex (mPFC) using an infrared-differential interference contrast video microscope (Olympus, BX51WI, Japan). The placement of individual slices was observed using an infrared-differential interference contrast video monitor. The slices were continuously perfused with well-oxygenated ACSF at 35 ± 1 °C during all electrophysiological studies. Electrophysiological indexes were measured from the superficial layer (layer II–III) pyramidal neurons using an Axon 200B amplifier (Axon Instruments, USA). Membrane currents were sampled and analyzed using a Digidata 1440 interface and a personal computer running Clampex and Clampfit software (version 10, Axon Instruments). For recordings of spontaneous inhibitory postsynaptic currents (sIPSCs), the holding potential was clamped at −70 mV (Fig. 2). Patch pipettes had open-tip resistances of 3–5 MΩ when filled with an intracellular solution that contained (in mM): 140 CsCl, 10 HEPES, 1 MgCl2, 0.1 EGTA, 4 NaCl, 2 Mg-ATP (pH 7.3). The D-2-amino-5-phosphonopentanoic acid (D-APV, 50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM) were added to the bath through a gravity-driven perfusion system. The MiniAnalysis 6.0.1 program (Synaptosoft Inc., USA) was used to analyze sIPSCs. The amplitude threshold for event detection was set to 10 pA while other parameters were the default values. Every single event in each recorded cell was fully characterized using the following parameters: amplitude, rise time, decay time, and area constants, and calculated using the MiniAnalysis 6.0.1 program.
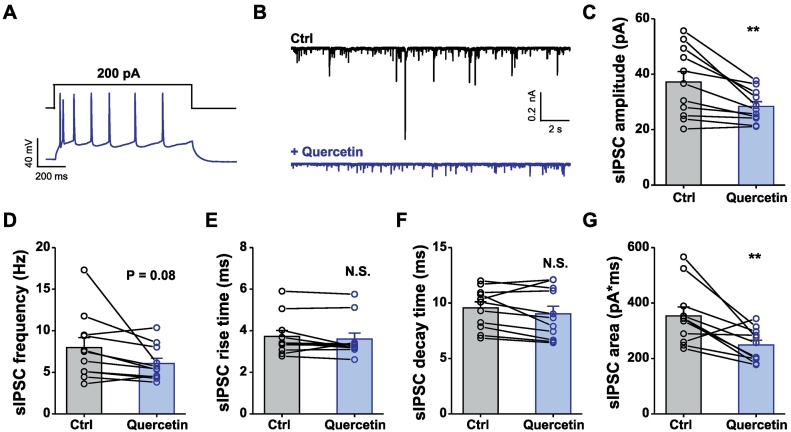
Fig. 2.
Quercetin reduces the spontaneous GABAergic inhibitory postsynaptic current in prefrontal cortical slices. (A) Representative traces showing voltage responses of neurons in layer II–III of prefrontal cortex to 500-ms injection of the current of +200 pA. (B) Representative traces showing GABAergic sIPSCs in the absence (Ctrl, black) or presence (+quercetin, blue) of 100 μM quercetin. (C–G) Summary data showing the amplitude (C), frequency (D), kinetics (E–G) of GABAergic sIPSCs in the absence or presence of 100 μM quercetin. Data are means ± S.E.M. n = 11 cells from 7 mice. N.S., not significant difference; ⁎p < .05, ⁎⁎p < .01, paired Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The electrically evoked inhibitory postsynaptic currents (IPSCs, Fig. 3) were recorded from superficial layer (layer II–III) pyramidal neurons, and the stimulation was delivered with a bipolar tungsten stimulating electrode placed in the deep layer (layer V–VI). The electrical stimulation was delivered repetitively every 20 s, with the neuron voltage-clamped at 0 mV. The recording pipettes (3–5 MΩ) were filled with a solution containing (in mM): 132.5 cesium gluconate, 17.5 CsCl, 2 MgCl2, 0.5 EGTA, 10 HEPES, 4 Mg-ATP, 5 QX-314 chloride (280–300 mOsm, pH 7.2 with CsOH).
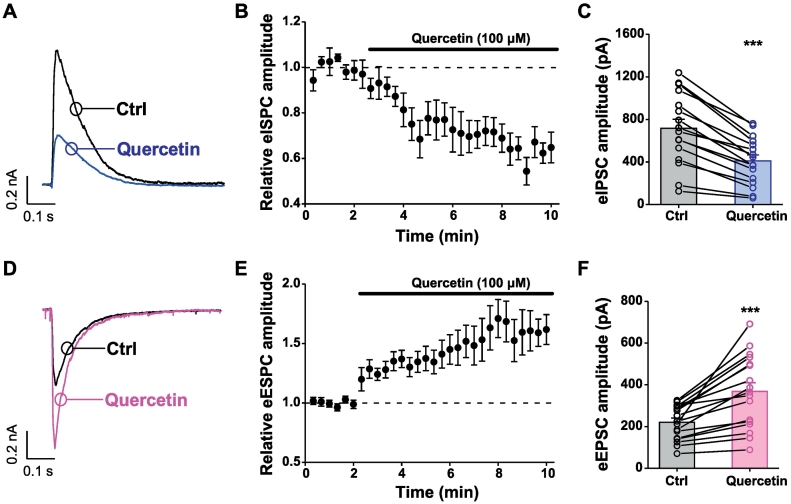
Fig. 3.
Effects of quercetin on electrically-evoked IPSC and EPSC in prefrontal cortical slices. (A) Representative traces showing that quercetin strongly inhibited eIPSCs in mPFC neurons (layer II–III) in mouse brain slice. (B) Pooled data showing the time course of quercetin-induced inhibition of eIPSCs. eIPSC amplitudes were normalized to the average of eIPSC amplitudes (dashed line) during the first 2 min of the recording. (C) Summary data showing the amplitude of eIPSCs in the absence or presence of 100 μM quercetin. n = 16 cells from 9 mice. ⁎⁎⁎p < .001, paired Student's t-test. (D) Representative traces showing that quercetin strongly inhibited eEPSCs in mPFC neurons (layer II–III) in mouse brain slice. (E) Pooled data showing the time course of quercetin-induced inhibition of eEPSCs. eEPSC amplitudes were normalized to the average of eEPSC amplitudes (dashed line) during the first 2 min of the recording. (F) Summary data showing the amplitude of eEPSCs in the absence or presence of 100 μM quercetin. n = 17 cells from 11 mice. ⁎⁎⁎p < .001, paired Student's t-test.
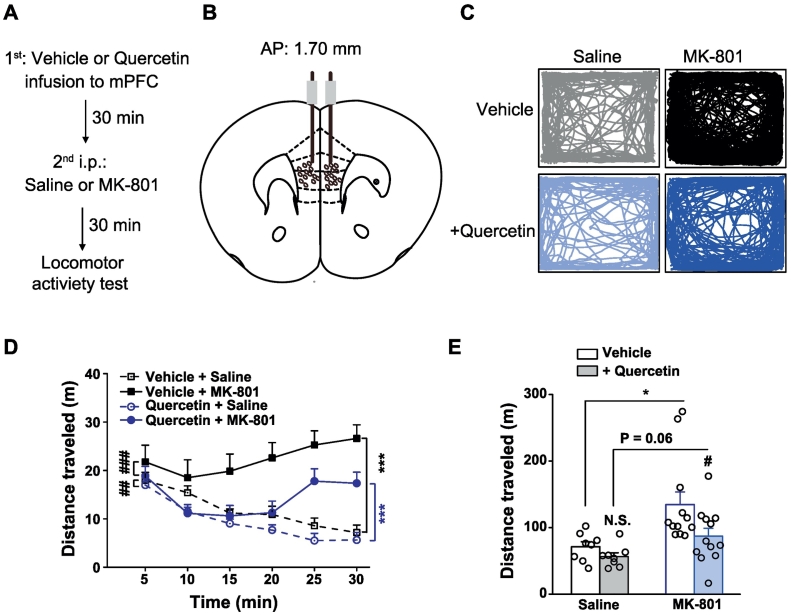
2.6. Open Field Test
The mice were brought to the testing room to acclimatize to the environment prior to the experiment. They were placed in the center of a square Plexiglas open field apparatus (40 × 40 × 35 cm) and allowed to freely explore according to differential protocols shown in Fig. 4, Fig. 5, Fig. 6, Fig. 7. The first protocol shown in Fig. 4 was consisted of a 30-min habituation session in which each mouse was allowed to explore freely in the open field, a 30-min test session in which the effects of quercetin on basal locomotor activity were determined and following a 30-min challenge session evoked by MK-801. During the test session, the treated mice were administered quercetin [50 mg in 10 ml volume per kg mice; intraperitoneally (i.p.)], which was dissolved in a vehicle solution containing 0.5% carboxymethyl cellulose, while the control mice were administered vehicle only. During the irritation session, the treated mice were administered MK-801 (0.2 mg in 10 ml volume per kg mice; i.p.), which was dissolved in saline, whereas the control mice were administered saline only. Another protocol shown in Fig. 5, Fig. 6, Fig. 7 contained only a 30-min irritation session using the mice not acclimated to the open field but started with the 30-min session after bilaterally prefrontal cortex-specific infusion (Fig. 5) of quercetin (100 mM, in DMSO, 0.5 μl each side) or vehicle (DMSO only, 0.5 μl each side), or i.p. treatment (Fig. 6, Fig. 7) of quercetin or vehicle only. During the irritation session, the treated mice were administered MK-801 (i.p.) or saline only. The total distance traveled was quantified using the Ethovision video-tracking system (Noldus Information Technology, Netherlands).
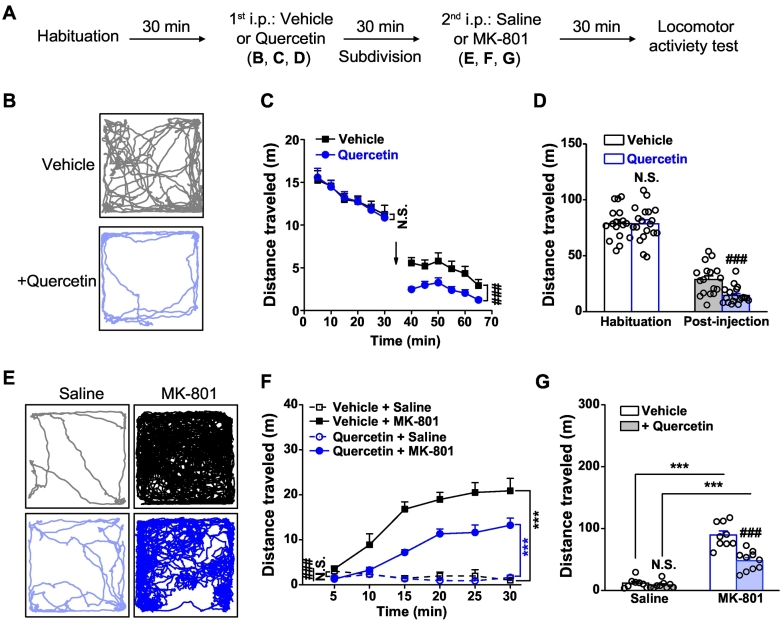
Fig. 4.
Effects of systemic treatment of quercetin on baseline locomotor activity and MK-801-induced locomotor hyperactivity. (A) Behavioral protocol. (B) Sample locomotion traces of mice after injection (i.p.) with control vehicle (grey) or quercetin (50 mg/10 ml per kg, light blue), following a 30-min habituation in the open field. (C) The total distance traveled during each 5-min interval. n = 17–19 for each group. N.S., not significant difference, ###p < .001, vehicle vs. quercetin, two way repeated-measures ANOVA. (D) The total distance of vehicle- and quercetin-treated mice during the first 30-min habituation session and the following 30-min test session, respectively. n = 17–19 for each group. N.S., not significant difference, ###p < .001, vehicle vs. quercetin, unpaired Student's t-test. (E) Sample locomotion traces of vehicle- or quercetin-treated mice, each of which were then subdivided into two groups and treated with saline or MK-801 (in saline, 0.2 mg/10 ml per kg mice), respectively. (F) The total distance of vehicle- or quercetin-treated mice followed by injection (i.p.) of saline or MK-801 traveled during each 5-min interval. n = 8–10 for each group. ⁎⁎⁎p < .001, saline vs. MK-801; N.S., not significant difference, ###p < .001, vehicle vs. quercetin, two way repeated-measures ANOVA. (G) The total distance in the 30-min session of mice followed by saline or MK-801 injection. n = 8–10 for each group. ⁎⁎⁎p < .001, saline vs. MK-801; N.S., not significant difference, ###p < .001, vehicle vs. quercetin, unpaired Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Effects of prefrontal cortex–specific delivery of quercetin on MK-801-induced locomotor hyperactivity. (A) Behavioral protocol. (B) A diagram depicting drug infusion to the mPFC. (C) Sample locomotion traces of vehicle- or quercetin (100 mM, 0.5 μl each side)-infused mice to mPFC following injection (i.p.) with saline or MK-801 (0.2 mg/10 ml per kg) and a 30 min test session in the open field. (D) The total distance of mice during each 5-min interval. n = 8–12 for each group. ⁎⁎⁎p < .001, saline vs. MK-801; ##p < .01, ###p < .001, vehicle vs. quercetin, two way repeated-measures ANOVA. (E) The total distance in the 30-min session of mice followed by saline or MK-801 injection. n = 8–12 for each group. ⁎p < .05, saline vs. MK-801; N.S., not significant difference, #p < .05, vehicle vs. quercetin, unpaired Student's t-test.
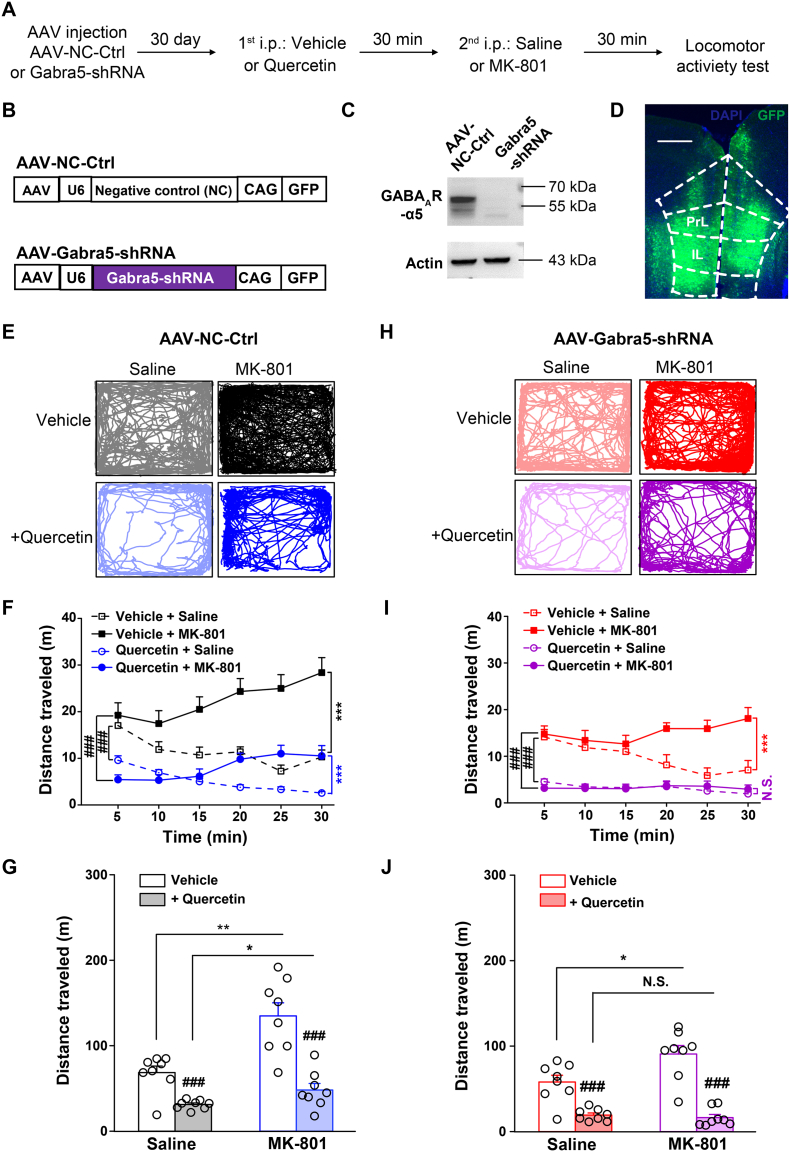
Fig. 6.
Effects of quercetin on MK-801-induced locomotor hyperactivity in mice with mPFC-specific genetic knockdown of α5-GABAAR (Gabra5) subunit. (A) Behavioral protocol. (B) Schematics of AAV vector constructs. (C) Representative images of Western blotting showing the efficacy of AAV-Gabra5-shRNA compared with that of AAV-NC-Ctrl. (D) Diagram illustrating the AAV infection to the mPFC. (E) Sample locomotion traces of AAV-NC-Ctrl-infected mice to mPFC with vehicle- or quercetin (50 mg/10 ml per kg)-treatment (i.p.) and then following injection (i.p.) with saline or MK-801 (0.2 mg/10 ml per kg) in addition to a 30 min test session in the open field. (F) The total distance of AAV-NC-Ctrl-infected mice to mPFC during each 5-min interval. n = 8 for each group. ⁎⁎⁎p < .001, saline vs. MK-801; ###p < .001, vehicle vs. quercetin, two way repeated-measures ANOVA. (G) The total distance in the 30-min session of AAV-NC-Ctrl-infected mice followed by saline or MK-801 injection. n = 8 for each group. ⁎p < .05, ⁎⁎p < .01, saline vs. MK-801; ###p < .001, vehicle vs. quercetin, unpaired Student's t-test. (H) Sample locomotion traces of AAV-Gabra5-shRNA-infected mice to mPFC with vehicle- or quercetin (50 mg/10 ml per kg)-treatment (i.p.), followed by injection (i.p.) with saline or MK-801 (0.2 mg/10 ml per kg) in addition to a 30 min test session in the open field. (I) The total distance of AAV-Gabra5-shRNA-infected mice to mPFC during each 5-min interval. n = 8 for each group. N.S., not significant difference, ⁎⁎⁎p < .001, saline vs. MK-801; ###p < .001, vehicle vs. quercetin, two way repeated-measures ANOVA. (J) The total distance in the 30-min session of AAV-Gabra5-shRNA-infected mice followed by saline or MK-801 injection. n = 8 for each group. N.S., not significant difference, ⁎p < .05, saline vs. MK-801; ###p < .001, vehicle vs. quercetin, unpaired Student's t-test.
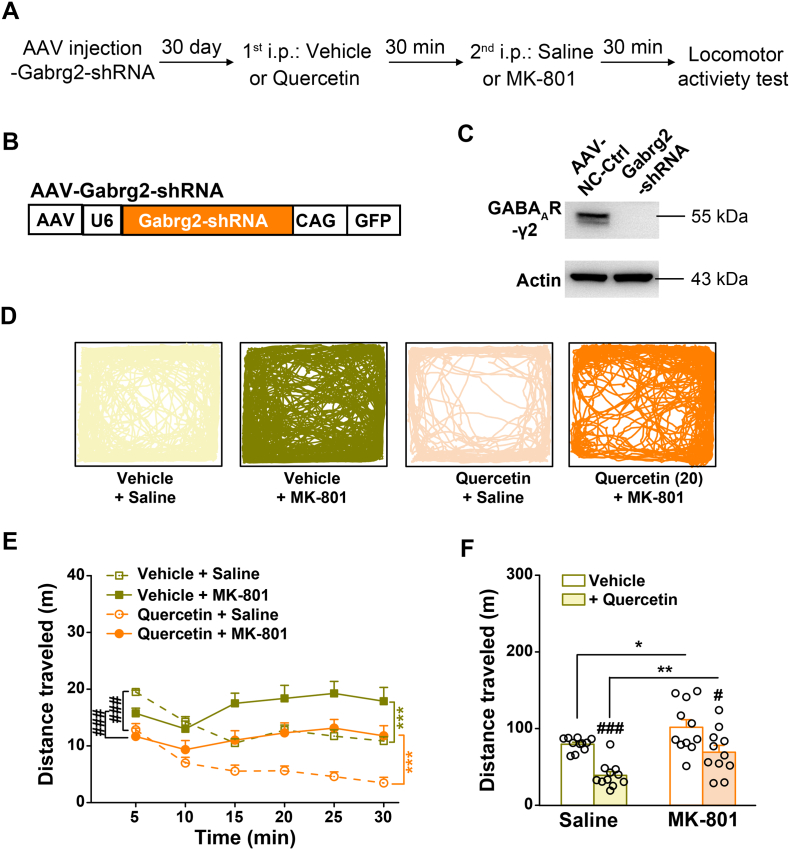
Fig. 7.
Effects of quercetin on MK-801-induced locomotor hyperactivity in mice with mPFC-specific genetic knockdown of γ2-GABAAR (Gabrg5) subunit. (A) Behavioral protocol. (B) Schematics of AAV vector constructs. (C) Representative images of Western blotting showing the efficacy of AAV-Gabrg2-shRNA compared with that of AAV-NC-Ctrl. (D) Sample locomotion traces of AAV-Gabrg2-shRNA infected mice to mPFC with vehicle- or quercetin (50 mg/10 ml per kg)-treatment (i.p.) and then following injection (i.p.) with saline or MK-801 (0.2 mg/10 ml per kg) in addition to a 30 min test session in the open field. (E) The total distance of AAV-NC-Ctrl-infected mice to mPFC during each 5-min interval. n = 11 for each group. ⁎⁎⁎p < .001, saline vs. MK-801; ###p < .001, vehicle vs. quercetin, two way repeated-measures ANOVA. (F) The total distance in the 30-min session of AAV-Gabrg2-shRNA-infected mice followed by saline or MK-801 injection. n = 11 for each group. ⁎p < .05, ⁎⁎p < .01, saline vs. MK-801; #p < .05, ###p < .001, vehicle vs. quercetin, unpaired Student's t-test.
2.7. Generation of Adeno-Associated Virus Vectors
To construct shRNA, oligonucleotides that contained 21-base sense and antisense sequences targeting mouse Gabra5 (GenBank accession: NM_176942.4, sense sequence, 5′-GTCCATTGCACACAACATGAC-3′) or Gabrg2 (GenBank accession: NM_008073.4, sense sequence, 5′-CTCCGGTTGAATAGCAATATG-3′) were connected with a hairpin loop followed by a poly (T) termination signal. For initial testing of the efficacy of the shRNA, the full-length Gabra5 or Gabrg2 cDNA was transfected into HEK-293T cells together with the negative control (NC-Ctrl, with sense sequence: 5′-GTTCTCCGAACGTGTCACGT-3′) or shRNA plasmid using a vector AAV-CAG-GFP-U6-shRNA (provided by Shanghai SunBio Biomedical technology, China), which contained a CAG promoter driving green fluorescence protein (GFP) and a U6 promoter driving shRNA expression. The levels of mouse α5-GABAAR or γ2-GABAAR protein expression was then assessed using Western blotting. The cells were washed with PBS at 48 h after co-transfection and lysed in the lysis buffer to prepare protein samples from HEK-293T cells. The resuspended lysates were incubated on ice for 30 min and centrifuged at 13,000 g at 4 °C for 15 min. Then, the supernatants were collected for Western blotting. Finally, the AAV vectors engineered to express the validated NC-Ctrl and Gabra5-shRNA or Gabrg2-shRNA were constructed under the promoter control of U6, a Pol III promoter that selectively drives the expression of short hairpin RNAs (Figs. 6B and 7B).
2.8. Surgical Procedures and Virus or Drug Microinjection
Surgeries were performed in mice as described in a previous study [33]. For virus injection, mice at the age of 8 weeks were anesthetized with 5% chloral hydrate and gently placed in a stereotaxic frame (RWD Life Science, China). Virus preparations (all vector titers were >1.0 × 1012 viral genome-containing particles per ml) were injected bilaterally into the mPFC. The stereotactic coordinates according to the mouse brain atlas [57] were as follows: anteroposterior, +1.78 mm; lateral, ±0.35 mm; and dorsoventral, −2.65 mm. One injection (0.5 μl) was performed on each side of the mPFC using microelectrodes connected with a microinjector pump (KDS 310, KD Scientific, USA) at a rate of 0.1 μl/min. The microelectrodes were left in situ for an additional 10 min to allow the viruses to diffuse. The mice were allowed to recover for 4 weeks before behavioral analysis, and the injection sites were examined at the end of the experiment. Brain slices from animals treated with the viruses were examined directly by fluorescent microscopy, and mice with incorrect diffusion scope were excluded from the data analysis.
For drug microinjection, mice were anesthetized with 5% chloral hydrate and placed in a stereotactic frame (RWD Life Science, China). Then, a 26-gauge guide cannula was implanted bilaterally and aimed at 1.00 mm above the targeted region with the following coordinates [57]: anteroposterior, +1.78 mm; lateral, ±0.35 mm; and dorsoventral, −2.65 mm. The cannulas were angled at 30° and positioned in place using acrylic dental cement and secured with skull screws. A stylus was placed in the guide cannula to prevent clogging. The mice were allowed to recover from surgery for a week before experimental manipulations. The infusion cannula was connected via PE20 tubing to a microsyringe driven by a microinfusion pump (KDS 310, KD Scientific, USA). The injection sites were examined at the end of the experiments, and mice with incorrect diffusion scope were excluded from the data analysis.
2.9. Western Blotting
Protein samples from cultured HEK-293T cells were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride filters. The filters were incubated overnight at 4 °C with appropriate antibodies. Secondary antibodies conjugated to horseradish peroxidase were added to the filters and then visualized in the enhanced chemiluminescence solution (Thermo Scientific, USA). The visualization was performed via the ImageQuant LAS 4000 mini Molecular Imaging System (GE Healthcare Life Sciences, USA), and the Image J software (NIH, USA) was used for the analysis of band intensity. The antibodies used were as follows: β-actin (1:1000; Chemicon, Cat # MAB1501), FLAG (1:4000; Sigma-Aldrich/Merck Millipore, Cat # F1804), GABAAR-α5 (1: 1000; Chemicon, Cat # AB9678), and GABAAR-γ2 (1:100; Santa Cruz, Cat # sc-101963).
2.10. Immunohistochemistry
Mice were deeply anesthetized with 5% chloral hydrate and transcardially perfused with 40 ml of prewired 1 × PBS. The brains were dissected, fixed in 4% paraformaldehyde at 4 °C overnight, and dehydrated in 30% sucrose (in 1 × PBS) at 4 °C overnight. On the day of the experiment, the frozen brains were transferred to −20 °C for 2 h to allow the temperature of the frozen tissue block to equilibrate to the temperature of the cryostat, cut in desired thickness (30 μm) sections, and mounted on glass slides. After incubating it for 5 min in 100 μl 4,6-Diamidino-2-phenylindole dihydrochloride hydrate (DAPI) solution (1: 1000), the sections were washed for 3 min with 1 × PBS for three times. Slides were mounted in the dark with glass coverslips using the mounting media. The coverslips were sealed to the slide using nail polish. Stained slides were ready for microscopy. The GFP and DAPI signals in mPFC were observed using a fluorescence microscope.
2.11. Statistics
No statistical methods were used to predetermine the sample size, but the sample sizes used were similar to those generally employed in the field. The data were collected and processed randomly. All behavioral tests and analysis were blindly conducted. The variance was similar between groups, and data were found to be normally distributed using parametric statistics. Data were analyzed using the Student's t-test or one-way analysis of variance (ANOVA) or two-way repeated-measures ANOVA, followed by Fisher's least significant difference post hoc comparisons, where appropriate. A p values < .05, represented significant differences; N.S. represented no significant difference; and ⁎p < .05, ⁎⁎p < .01, and ⁎⁎⁎p < .001 were considered statistically significant. The smooth concentration–response curve of quercetin on inhibition of the GABA response in cultured cortical neurons was fitted with the following equation: I = Imax(IC50)nH/[CnH + (IC50)nH], where I is the normalized value of the current, Imax is the maximal response, C is the drug concentration, IC50 represents the antagonist concentration producing a half-maximal inhibitory effect, and nH is the apparent Hill coefficient.
3. Results
3.1. Quercetin Inhibited GABA-Induced Response in Cultured Cortical Neurons
Previous studies [21, 22, 28] identified that quercetin (Fig. 1A) as a natural flavonoid compound inhibited the ionic currents mediated by the α1-β2-γ2 or ρ1-containing GABAARs (the latter previously denoted as the GABACR) expressed in Xenopus laevis oocytes; however, to more physiologically relevant extent, its pharmacological action on endogenous GABAARs together with the functional output remains to be determined. To this end, this study recorded GABA-evoked currents in the presence and absence of quercetin in cultured cortical neurons. As shown in Fig. 1B, quercetin alone at the concentrations up to 100 μM induced no significant current in cortical neurons, but significantly inhibited the GABA-induced current with an IC50 of 8.1 ± 2.1 μM and a Hill coefficient (nH) of 1.4 ± 0.5 (Fig. 1C). Next, the effect of 20 μM quercetin on the current induced by a wide range of GABA concentrations (0.3–1000 μM) was measured. Quercetin was found to effectively reduce GABA-evoked current at various GABA concentrations, including subsaturating and saturating concentrations (Fig. 1D and E). Additionally, quercetin suppression of GABA-induced current was independent of GABA concentrations (close to 60% inhibition at all concentrations; Fig. 1D and E), suggesting that quercetin inhibited the GABA-induced currents in cortical neurons probably in a noncompetitive manner.
3.2. Quercetin Reduced Spontaneous GABAergic Inhibitory Postsynaptic Current in Prefrontal Cortical Slices
The aforementioned experiments demonstrated that quercetin inhibited the GABA-induced response noncompetitively in cortical neurons similar to Xenopus laevis oocytes in recombinantly expressing GABAARs [21, 22, 28]. Spontaneous inhibitory postsynaptic currents (sIPSCs) were measured in prefrontal cortical slices in the absence and presence of quercetin (100 μM) to achieve a better understanding of the influence of quercetin on GABAergic inhibition at the synaptic level. The study first investigated whether quercetin inhibits spontaneous GABAergic transmission in the pyramidal neurons at the layer II–III of mPFC, which were largely identified based on their ability to exhibit spike frequency adaptation in response to prolonged depolarizing current injection (Fig. 2A). As shown in Fig. 2B, quercetin at a concentration of 100 μM substantially reduced the amplitude (for quantification, see Fig. 2C) of sIPSC. Moreover, quercetin also demonstrated a trend to decrease the frequency of sIPSCs (Fig. 2D). However, the kinetics of sIPSCs, including the rise time (Fig. 2E) and decay time (Fig. 2F), was not substantially affected. The area (Fig. 2G) of the sIPSCs was consequently reduced largely due to the effects of quercetin on the sIPSC amplitude, indicating a potent inhibitory effect of quercetin on GABAergic inhibition at the synaptic level.
3.3. Quercetin Reduced Electrically Evoked GABAergic Inhibitory Postsynaptic Current in Prefrontal Cortical Slices
Next, the effects of quercetin on the electrically evoked GABAergic IPSCs of pyramidal neurons at the layer II–III were assessed by stimulation in the deep layers (V and VI) of the mPFC. Pyramidal neurons were clamped at 0 mV, the reversal potential of glutamate receptor-mediated cationic currents, to record for the electrically evoked IPSCs (eIPSCs). As shown in Fig. 3A–C, the amplitudes of evoked GABAergic IPSCs were reduced to 60% of control by 100 μM quercetin. Together with the pharmacological efficacy on the sIPSCs, quercetin as a GABAAR inhibitor [21, 22, 28] (Fig. 1) was capable of inhibiting the GABAergic transmission regardless of the ways to generate.
3.4. Quercetin Potentiated Prefrontal Cortical Glutamatergic Transmission
Inhibition of GABAergic transmission was expected to increase the overall network activity and facilitate the glutamatergic excitatory transmission. Therefore, the effects of quercetin on the EPSC were examined. Pyramidal neurons at the layer II–III by stimulation in the deep layers (V and VI) of the prefrontal cortex were clamped at −70 mV, the reversal potential of GABAAR receptor-mediated Cl− currents, to record the electrically evoked EPSCs (eEPSCs). As expected, quercetin at a concentration of 100 μM significantly enhanced the amplitudes of evoked glutamatergic EPSCs to about 150% of the control (Fig. 3D–F). These results suggested that by inhibiting GABAergic transmission, quercetin relieved the inhibitory tone on the neurons (leading to enhanced neuronal activity) and potentiated the glutamatergic transmission as a result to shape the neuronal activity.
3.5. Systemic Treatment of Quercetin Alleviates MK-801-Induced Hyperactivity
Considering the significant impact of quercetin on GABAergic inhibition and consequent enhancement of glutamatergic transmission, the present study further examined the effects of quercetin on animal activity in vivo. An open-field behavioral assessment of locomotor activity using mice was conducted with and without the administration of quercetin. Moreover, the potential therapeutic efficacy of quercetin on the locomotor hyperactivity induced by the NMDAR antagonist MK-801 was also examined, a rodent model that was used to mimic the part of the positive symptoms in psychosis [1, 23, 70, 71] for novel antipsychotic drug discovery [58] and therapeutic development [[42], [43], [44]]. To gauge the effect of quercetin on mice, we systematically administered (i.p.) quercetin at the doses of 5 and 20 mg/kg (Supplementary Fig. 1A). As expected, systemic injection of MK-801 (0.2 mg/kg) evoked a significant locomotor hyperactivity (Supplementary Fig. 1B) in the vehicle-pretreated mice (each 5-min bin, F(1,120) = 65.144, p < .001, Supplementary Fig. 1C; total, p = .001, Supplementary Fig. 1C, vehicle + saline vs. vehicle + MK-801) within the 30 min time window. In the presence of quercetin, the MK-801-induced locomotor hyperactivity was moderately alleviated (5 mg/kg: each 5-min bin, F(1,120) = 8.087, p = .005, Supplementary Fig. 1C; total, p = .1926, Supplementary Fig. 1D, vehicle + MK-801 vs. quercetin + MK-801; 20 mg/kg: each 5-min bin, F(1,120) = 20.174, p < .001, Supplementary Fig. 1C; total, p = .0484, Supplementary Fig. 1D, vehicle + MK-801 vs. quercetin + MK-801). To make a more obvious and significant effect of quercetin, we thus chose 50 mg/kg as the effective dosage in the following study.
Upon the systemic injection of quercetin (50 mg/kg) in a habituation-test protocol (Fig. 4A), the mice displayed greatly reduced basal locomotor activity compared with the vehicle solution (habituation: each 5-min bin, F(1,216) = 0.047, p = .949, Fig. 4C; total, p = .9719, Fig. 4D; post-injection: each 5-min bin, F(1,216) = 45.58, p < .001, Fig. 4C; total, p = 3.525E − 04, Fig. 4D, vehicle vs. quercetin). Also, treatment of MK-801 (0.2 mg/kg) evoked a significant locomotor hyperactivity (Fig. 4E) in this cohort of vehicle-pretreated animals (each 5-min bin, F(1,102) = 197.067, p < .001, Fig. 4F; total, p = 3.390E − 08, Fig. 4G, vehicle + saline vs. vehicle + MK-801), consistent with the results shown in Supplementary Fig. 1. Notably, the reaction to MK-801 (Fig. 4E) was more strongly reduced (each 5-min bin, F(1,114) = 58.933, p < .001, Fig. 4F; total, p = 8.670E − 05, Fig. 4G, vehicle + MK-801 vs. quercetin + MK-801) but not totally lost (each 5-min bin, F(1,114) = 170.81, p < .001, Fig. 4F; total, p = 1.798E − 08, Fig. 4G, quercetin + saline vs. quercetin + MK-801) in the quercetin-pretreated mice. Thus, these results implied a noticeable inhibitory effect of quercetin on the psychotic hyperactivity in addition to the basal locomotor activity.
3.6. Prefrontal Cortex–Specific Delivery of Quercetin Alleviated MK-801-Induced Hyperactivity
The study then determined whether quercetin in mPFC specifically was sufficient to counteract MK-801-induced hyperlocomotor activity likely via regulation of synaptic transmission (Fig. 2, Fig. 3). To this end, quercetin (100 mM, 0.50 μl per side) or vehicle was injected bilaterally into mPFC (Fig. 5A and B) 30 min before the irritation using a systemic treatment of MK-801. Consistently, in the vehicle-delivered mice, systemic injection of MK-801 (0.2 mg/kg) evoked a significant locomotor hyperactivity (Fig. 5C) compared with that of saline only (each 5-min bin, F(1,120) = 37.271, p < .001, Fig. 5D; total, p = .01747, Fig. 5E, vehicle + saline vs. vehicle + MK-801) within the 30-min time window. Notably, MK-801-induced hyperactivity was largely attenuated by the mPFC–specific administration of quercetin compared with that of vehicle only (each 5-min bin, F(1,144) = 24.007, p < .001, Fig. 5D; total, p = .04538, Fig. 5E, vehicle + MK-801 vs. quercetin + MK-801). Notably, quercetin pretreatment did not absolutely abolish the MK-induced hyperactivity (each 5-min bin, F(1,120) = 18.828, p < .001, Fig. 5D; total, p = .06278, Fig. 5E, quercetin + saline vs. quercetin + MK-801), arguing for more complex mechanisms beyond quercetin regulation. The finding that prefrontal cortex–specific delivery of quercetin was sufficient to counteract the NMDAR antagonism-induced hyperactivity strengthened the importance of the effect of quercetin on prefrontal cortical activity for the control of psychosis.
3.7. Effects of Quercetin Were Associated with the GABAAR Activity
Next, we assessed whether the effects of quercetin on the locomotor activity are associated with the activity of GABAARs, aiming to match these behavioral effects of quercetin to its reduction on GABA-induced response and GABAergic transmission observed at the cellular and synaptic levels (Fig. 1, Fig. 2, Fig. 3). To this end, we systematically administered (Supplementary Fig. 2A) an antagonist of GABAARs, picrotoxin (PTX), alongside with quercetin for the locomotor activity test. Application of the PTX (i.p., 1 mg/10 ml per kg mice) alone did not significantly affect basal locomotor activity (each 5-min bin, F(1,120) = 2.999, p = .086, Supplementary Figs. 1C and 2C; total, p = .4360, Supplementary Figs. 1D and 2D, total distance = 52.4 ± 9.6 vs. 44.3 ± 3.3 m, for vehicle + saline vs. PTX + saline, respectively). However, PTX produced a significant impact on the MK-801-induced locomotor hyperactivity (each 5-min bin, F(1,120) = 52.188, p < .001, Supplementary Figs. 1C and 2C; total, p = .0026, Supplementary Figs. 1D and 2D, total distance = 119.4 ± 15.0 vs. 64.4 ± 4.8 m, for vehicle + MK-801 vs. PTX + MK-801, respectively). Notably, PTX did not abolish the effects of quercetin, as that quercetin was still capable of reducing the basal locomotor activity (each 5-min bin, F(1,120) = 74.080, p < .001, Supplementary Fig. 2C; total, p = 5.326E − 05, Supplementary Fig. 2D, PTX + saline vs. PTX/Quercetin + saline) and alleviating the MK-801-evoked hyperactivity (each 5-min bin, F(1,120) = 67.483, p < .001, Supplementary Fig. 2C; total, p = 8.672E − 05, Supplementary Fig. 2D, PTX + MK-801 vs. PTX/Quercetin + MK-801). These observations implied an incomplete inhibition of GABAAR in vivo by systemic delivery of either PTX or quercetin in a safe dose range. Together, inhibition of GABAAR activity could constitute a valid mechanism to counteract the NMDAR antagonism-induced locomotor hyperactivity, while the pharmacological effects of quercetin more likely depends on suppressing the activity of GABAARs.
3.8. Effects of Quercetin Were Not Exclusively Dependent on α5-Subunit-Containing GABAARs in the Prefrontal Cortex
Τhe molecular substrates were then determined using quercetin in the mPFC to antagonize the psychotic hyperactivity. The negative allosteric modulators of α5-subunit-containing GABAARs were consistently found to exhibit substantial pharmacological effects on cognitive enhancement [2, 3, 30, 40, 53, 61]. The necessity of α5-subunit-containing GABAAR in mediating the effects of quercetin on counteracting MK-801-induced hyperactivity was thus evaluated (Fig. 6A). An AAV construct was generated that expressed a short hairpin RNA (shRNA, Fig. 6B and C) targeting the α5 subunit of GABAARs, which were present in the cortical pyramidal neurons [7, 68], driven by U6 promoter (Fig. 6B). The AAV-Gabra5-shRNA or a negative control virus (AAV-NC-Ctrl) was injected into the mPFC (Fig. 6D) of mice.
In the AAC-NC-Ctrl-injected mice (Fig. 6E), systemic injection of MK-801 evoked a strong hyperactivity (NC-Ctrl: each 5-min bin, F(1,96) = 66.589, p < .001, Fig. 6F; total, p = .0017, Fig. 6G, vehicle + saline vs. vehicle + MK-801), which was substantially inhibited by treatment of quercetin (NC-Ctrl: each 5-min bin, F(1,96) = 118.547, p < .001, Fig. 6F; total, p = 1.743E − 04, Fig. 6G, vehicle + MK-801 vs. quercetin + MK-801). However, in the AAV-Gabra5-shRNA-injected animals (Fig. 6H), MK-801-induced hyperactivity (vehicle + MK-801: each 5-min bin, F(1,96) = 27.836, p < .001, Fig. 6F and I; total, p = .0302, Fig. 6G and J, NC-Ctrl vs. Gabra5-shRNA) over basal locomotor activity (vehicle + saline: each 5-min bin, F(1,96) = 3.128, p = .081, Fig. 6F and I; total, p = .3541 Fig. 6G and J, NC-Ctrl vs. Gabra5-shRNA) were substantially reduced. Of note, quercetin was still effective in the AAV-Gabra5-shRNA-injected mice, and reduced MK-801-induced hyperactivity (Gabra5-shRNA: each 5-min bin, F(1,96) = 196.608, p < .001, Fig. 6I; total, p = 6.539E − 07, Fig. 6J, vehicle + MK-801 vs. quercetin + MK-801), implicating the not completely overlapping mechanisms between genetic reduction of α5-GABAAR expression and quercetin inhibition of GABAergic transmission. As supporting evidence, MK-801-evoked psychotic symptom was totally lost under both of these interventions (Gabra5-shRNA: each 5-min bin, F(1,96) = 0.006, p = .94, Fig. 6I; total, p = .52038, Fig. 6J, quercetin + saline vs. quercetin + MK-801). These results suggested that both α5-subunit-dependent and α5-subunit-independent mechanisms were involved in the regulation of MK-801-evoked psychotic symptom by quercetin.
3.9. Effects of Quercetin Were Partially Mediated by γ2-Subunit-Containing GABAARs in the Prefrontal Cortex
Finally, we examined the roles of the γ2 subunit of GABAARs, which is present in approximately 90% of GABAARs [63], in conferring the pharmacological effects of quercetin. The AAV-Gabrg2-shRNA virus (Fig. 7A–C), targeting the γ2-subunit of GABAARs, was injected into the mPFC of mice. In the AAV-Gabrg2-shRNA-injected animals, compared with the AAV-NC group shown in Fig. 6, MK-801-induced hyperactivity was substantially reduced (vehicle + MK-801: each 5-min bin, F(1,114) = 6.119, p = .015, Figs. 6F and 7E; total, p = .0735, Figs. 6G and 7F, total distance = 134.9 ± 15.3 vs. 101.7 ± 9.7 m, for NC-Ctrl vs. Gabrg2-shRNA, respectively). Instead, the basal locomotor activity was increased in the AAV-Gabrg2-shRNA- than the AAV-NC-injected animals (vehicle + saline: each 5-min bin, F(1,114) = 6.739, p = .011, Figs. 6F and 7E; total, p = .1400, Figs. 6G and 7F, total distance = 68.5 ± 7.7 vs. 79.6 ± 2.6 m, for NC-Ctrl vs. Gabrg2-shRNA, respectively), supporting a specific engagement of prefrontal GABAAR activity in control of psychotic over basal locomotor activity. Strikingly, for the pharmacological treatment of MK-801-induced hyperactivity by quercetin, genetic knockdown of the γ2 subunit of GABAARs in mPFC failed to further improve the psychotic activity, but canceled out a proportion of therapeutic efficacy of quercetin (quercetin + MK-801: each 5-min bin, F(1,114) = 14.331, p < .001, Figs. 6F and 7E; total, p = .1063, Figs. 6G and 7F, total distance = 48.2 ± 7.6 vs. 69.2 ± 8.9 m, for NC-Ctrl vs. Gabrg2-shRNA, respectively). This observation was in agreement with a notion that the effects of quercetin were partially mediated by the prefrontal γ2-subunit-containing GABAARs. Supportingly, AAV-Gabrg2-shRNA-injected mice were still responsive to quercetin (Gabrg2-shRNA: each 5-min bin, F(1,132) = 27.971, p < .001, Fig. 7E; total, p = .0233, Fig. 7F, vehicle + MK-801 vs. quercetin + MK-801). These results thus suggested that the actions of quercetin require complex mechanisms indeed including the regulation of GABAAR activity in prefrontal cortex. Taken together, being a negative allosteric GABAAR modulator, quercetin represents a promising leading compound holding a strong antipsychotic activity (Fig. 8), shedding more lights on therapeutic development for excitatory–inhibitory imbalance disorders.
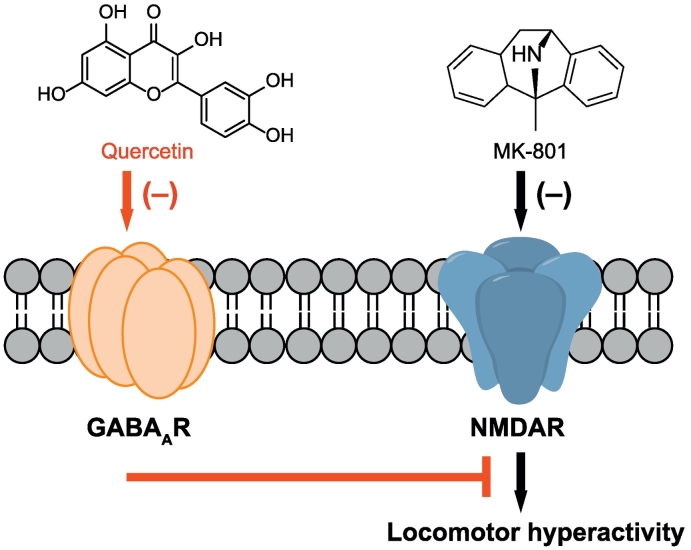
Fig. 8.
A proposed scheme for quercetin-mediated suppression of cortical GABAergic transmission and its involvement in alleviating the MK-801-induced hyperactivity. Please see text for more details.
4. Discussion
The present study showed that quercetin, a natural flavonoid compound identified as the inhibitor of α1-β2-γ2 or ρ1-containing GABAARs [21, 22, 28], concentration dependently reduced GABA-induced currents in the cultured cortical neurons. Moreover, on the prefrontal cortex slice, quercetin moderately inhibited the spontaneous and electrically evoked GABAergic transmission and potentiated glutamatergic transmission as a result. Remarkably, systemic treatment of quercetin decreased the basal locomotor activity and MK-801-induced hyperactivity in vivo. Notably, the prefrontal cortex–specific delivery of quercetin recapitulated its pharmacologic efficacy as the systemic administration. Interestingly, viral-mediated genetic knockdown of α5-subunit of GABAARs selectively in the mPFC combined with the quercetin treatment, but not each alone, totally abolished the MK-801-induced hyperactivity. Finally, genetic knockdown of γ2-subunit failed to further improve the therapeutic effects of quercetin, implying an involvement of the γ2-containing GABAARs in prefrontal cortex for the compound's action. Taken together, these results identified quercetin as a novel antipsychotic leading agent targeting against the GABAergic inhibition in the prefrontal cortex.
Quercetin as a bioactive compound has diverse pharmacologic effects [39, 47, 54], including antioxidant, anti-inflammatory, anti-proliferative, anti-angiogenic, and asthma-relieving properties. On the central nervous system [13, 67], quercetin exerts several beneficial effects such as neuroprotective [9, 16, 34, 67], regulator of sleep–wake cycle [27], anti-nociceptive [19], and anticonvulsant [49]. Here we identified a novel pharmacological efficacy of quercetin for the treatment of psychotic hyperactivity. First, this study demonstrated that systemic administration of quercetin significantly decreased the baseline locomotor activity (Fig. 4A–D). Second, under MK-801-induced psychosis model, quercetin treatment dramatically counteracted the hyperactivity (Fig. 4E–G). Third, mPFC region-specific delivery of quercetin was still effective to antagonize the MK-801-evoked hyperactivity (Fig. 5), underscoring the importance of prefrontal cortical activity for psychotic control. Overall, quercetin was capable of targeting the prefrontal cortex to downregulate the physiological and pathological locomotor activity.
It seems unusual to ascribe the quercetin-induced behavioral phenotypes to its inhibition of GABAARs at the molecular level because of the proconvulsant nature of GABAAR inhibitors, such as PTX [50] and pentylenetetrazol [24]. This disparity could be at least partially explained by the dosage of GABAAR inhibitors used for therapeutics. First, the present finding that quercetin repressed the GABAAR-mediated synaptic response and alleviated MK-801-caused psychotic symptoms was reminiscent of previous studies showing that low doses of PTX gave rise to substantial therapeutic effects in Down syndrome [18]. Consistently, both mPFC-specific delivery (Fig. 5) and the systemic treatment (Fig. 4) of GABAAR antagonist quercetin were indeed effective in antagonizing the MK-801-evoked hyperactivity in the present study. Second, PTX at the dosage of 1 mg/kg in mice alone did not significantly affect basal locomotor activity but produced a significant impact on the MK-801-induced locomotor hyperactivity (Supplementary Fig. 2), arguing for a more valid strategy of repressing GABAAR activity for correction of the NMDAR antagonism-induced psychotic hyperactivity. Third, genetic reduction of either α5 or γ2 subunits of GABAAR alone alleviated the MK-801-induced locomotor hyperactivity, validating the targetable molecular mechanisms of GABAAR inhibition or downregulation for anti-psychotic therapeutics. Fourth, combined treatments of quercetin together with PTX or genetic manipulation of GABAAR subunits resulted either overlapping or additive effects, suggesting that GABAAR regulation, likely in a specific way, would underlie the pharmacological effects of quercetin on psychotics.
Quercetin, a GABAAR inhibitor, possesses several properties distinguishing it from PTX or other known inhibitors. Different from the classic benzodiazepine modulation, quercetin is able to antagonize both the α1-β2-γ2 and ρ1 subtypes of GABAARs, and the modulation was insensitive to the benzodiazepine antagonist flumazenil [22]. Moreover, for the ρ1-containing GABAARs, while the effects of picrotoxin were use dependent, strongly relied on the agonist concentration and had a slow onset and offset, the effects of quercetin were use independent, had relatively fast onset and offset, and resulted in a slowed time course of the GABA-evoked currents [21]. As supporting evidence, this study showed that quercetin reduced GABA-induced currents in cultured cortical neurons in a noncompetitive manner (Fig. 1D and E). Moreover, quercetin displayed a moderately and partially inhibitory efficacy against the GABA-mediated response compared with picrotoxin. This study demonstrated that quercetin inhibited the GABA (10 μM)-induced currents at the IC50 of 8.7 ± 2.1 μM in the cultured cortical neurons. Notably, at the slice level, quercetin at the concentrations up to 100 μM (Fig. 2, Fig. 3) meaningfully reduced the GABAergic transmission but could not achieve the complete inhibition. Based on the moderate and uncompetitive antagonism of quercetin, its effects on the GABAergic tonic inhibition, primarily by ambient extracellular GABA acting on extrasynaptic high-affinity GABAARs [7], was not so evident (data not shown) as the phasic (i.e. synaptic) inhibition (Fig. 2, Fig. 3) that resulted from high-level GABA transients associated with evoked release of GABA and subsequent synaptic GABAAR activation. In this regard, the pharmacological efficacy of quercetin would not be covered by the cognitive enhancement effects of negative allosteric modulators selectively targeting α5-subunit-containing GABAARs [2, 3, 30, 40, 53, 61], which were present in the cortical pyramidal neurons [7, 68] and considerably constituted the main molecular substrate for tonic inhibition [7]. Consistently, this study demonstrated that although viral-mediated, region-specific genetic knockdown of the α5-subunit in prefrontal cortex improved the MK-801-evoked psychotic symptom, it reserved the pharmacological responsivity to quercetin, and both interventions together completely normalized the hyperactivity (Fig. 6). By contrast, genetic knockdown of γ2-subunit failed to further improve the therapeutic effects of quercetin (Fig. 7), implying at least a partial involvement of the γ2-containing GABAARs in prefrontal cortex for the compound's action. Together, quercetin as the negative allosteric GABAAR modulator indeed exerts antipsychotic activity (Fig. 8), although its specific molecular substrates such as the particular GABAAR subtypes targeted by quercetin remain to be further clarified in the future studies.
5. Conclusion
In conclusion, the present study showed that quercetin decreased the GABA-induced currents in cultured cortical neurons, and reduced GABAergic synaptic transmission, leading to a strong inhibition of baseline locomotor activity and MK-801-induced psychotic activity in vivo. Thus, the present study suggested a novel pharmacological efficacy of quercetin depending on the regulation of GABAergic transmission in the prefrontal cortex.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (2014CB910300), from the National Natural Science Foundation of China (81571031, 91632304, 81701334, 81730095, 81771214, 81761128035, and 81781220701), the Shanghai Committee of Science and Technology (17XD1403200, 18DZ2313505, and 14DJ1400204), the Shanghai Municipal Education Commission (Research Physician Project: 20152234), the Shanghai Municipal Commission of Health and Family Planning (2017ZZ02026, 2017EKHWYX-02, and GDEK201709), the Shanghai Shenkang Hospital Development Center (16CR2025B), and was sponsored by Shanghai Rising-Star Program (18QA1402500).
Author Contributions
Hui-Ran Fan: Research design, data collection, data analysis, and contribution to the writing.
Wei-Feng Du: Research design.
Tao Zhu: Data collection.
Yan-Jiao Wu: Data collection and data analysis.
Yan-Mei Liu: Data collection.
Qi Wang: Data collection.
Qin Wang: Data collection.
Xue Gu: Data collection.
Xingyue Shan: Data collection.
Shining Deng: Data collection.
Tailin Zhu: Data collection.
Tian-Le Xu: Research design.
Wei-Hong Ge: Research design.
Wei-Guang Li: Research design, data collection, data analysis, and contribution to the writing.
Fei Li: Research design ad contribution to the writing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.07.031.
Contributor Information
Wei-Guang Li, Email: wgli@shsmu.edu.cn.
Fei Li, Email: feili@shsmu.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Andine P., Widermark N., Axelsson R., Nyberg G., Olofsson U., Martensson E., Sandberg M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- 2.Atack J.R. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Ballard T.M., Knoflach F., Prinssen E., Borroni E., Vivian J.A., Basile J., Gasser R., Moreau J.L., Wettstein J.G., Buettelmann B., Knust H., Thomas A.W., Trube G., Hernandez M.C. RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–223. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- 4.Basurto E., Flores O.G., Hoffman K.L. Glycinamide prevents MK-801-induced hyperactivity and deficits in object recognition memory in an animal model of positive and cognitive symptoms of schizophrenia. Schizophr Res. 2015;166:349–350. doi: 10.1016/j.schres.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Belforte J.E., Zsiros V., Sklar E.R., Jiang Z., Yu G., Li Y., Quinlan E.M., Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braat S., Kooy R.F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron. 2015;86:1119–1130. doi: 10.1016/j.neuron.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Brickley S.G., Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarkson A.N., Huang B.S., Macisaac S.E., Mody I., Carmichael S.T. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa L.G., Garrick J.M., Roque P.J., Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. 2016;2016:2986796. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyle J.T. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 11.Coyle J.T. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 12.Coyle J.T. NMDA receptor and schizophrenia: a brief history. Schizophr Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dajas F., Abin-Carriquiry J.A., Arredondo F., Blasina F., Echeverry C., Martinez M., Rivera F., Vaamonde L. Quercetin in brain diseases: potential and limits. Neurochem Int. 2015;89:140–148. doi: 10.1016/j.neuint.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Depoortere R., Dargazanli G., Estenne-Bouhtou G., Coste A., Lanneau C., Desvignes C., Poncelet M., Heaulme M., Santucci V., Decobert M., Cudennec A., Voltz C., Boulay D., Terranova J.P., Stemmelin J., Roger P., Marabout B., Sevrin M., Vige X., Biton B., Steinberg R., Francon D., Alonso R., Avenet P., Oury-Donat F., Perrault G., Griebel G., George P., Soubrie P., Scatton B. Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology. 2005;30:1963–1985. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch S.I., Mastropaolo J., Schwartz B.L., Rosse R.B., Morihisa J.M. A “glutamatergic hypothesis” of schizophrenia. Rationale for pharmacotherapy with glycine. Clin Neuropharmacol. 1989;12:1–13. [PubMed] [Google Scholar]
- 16.Elumalai P., Lakshmi S. Role of quercetin benefits in neurodegeneration. Adv Neurobiol. 2016;12:229–245. doi: 10.1007/978-3-319-28383-8_12. [DOI] [PubMed] [Google Scholar]
- 17.Ferando I., Mody I. Interneuronal GABAA receptors inside and outside of synapses. Curr Opin Neurobiol. 2014;26:57–63. doi: 10.1016/j.conb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez F., Morishita W., Zuniga E., Nguyen J., Blank M., Malenka R.C., Garner C.C. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 19.Filho A.W., Filho V.C., Olinger L., de Souza M.M. Quercetin: further investigation of its antinociceptive properties and mechanisms of action. Arch Pharm Res. 2008;31:713–721. doi: 10.1007/s12272-001-1217-2. [DOI] [PubMed] [Google Scholar]
- 20.Gordon J.A. Testing the glutamate hypothesis of schizophrenia. Nat Neurosci. 2010;13:2–4. doi: 10.1038/nn0110-2. [DOI] [PubMed] [Google Scholar]
- 21.Goutman J.D., Calvo D.J. Studies on the mechanisms of action of picrotoxin, quercetin and pregnanolone at the GABA rho 1 receptor. Br J Pharmacol. 2004;141:717–727. doi: 10.1038/sj.bjp.0705657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goutman J.D., Waxemberg M.D., Donate-Oliver F., Pomata P.E., Calvo D.J. Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur J Pharmacol. 2003;461:79–87. doi: 10.1016/s0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]
- 23.Halberstadt A.L., Geyer M.A. Effect of hallucinogens on unconditioned nehavior. Curr Top Behav Neurosci. 2018;36:159–199. doi: 10.1007/7854_2016_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen S.L., Sperling B.B., Sanchez C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:105–113. doi: 10.1016/j.pnpbp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Hosie A.M., Wilkins M.E., da Silva H.M., Smart T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 26.Insel T.R. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 27.Kambe D., Kotani M., Yoshimoto M., Kaku S., Chaki S., Honda K. Effects of quercetin on the sleep-wake cycle in rats: involvement of gamma-aminobutyric acid receptor type A in regulation of rapid eye movement sleep. Brain Res. 2010;1330:83–88. doi: 10.1016/j.brainres.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.J., Lee B.H., Choi S.H., Jung S.W., Kim H.S., Lee J.H., Hwang S.H., Pyo M.K., Kim H.C., Nah S.Y. Differential effects of quercetin glycosides on GABAC receptor channel activity. Arch Pharm Res. 2015;38:108–114. doi: 10.1007/s12272-014-0409-2. [DOI] [PubMed] [Google Scholar]
- 29.Knabl J., Witschi R., Hosl K., Reinold H., Zeilhofer U.B., Ahmadi S., Brockhaus J., Sergejeva M., Hess A., Brune K., Fritschy J.M., Rudolph U., Mohler H., Zeilhofer H.U. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 30.Knust H., Achermann G., Ballard T., Buettelmann B., Gasser R., Fischer H., Hernandez M.C., Knoflach F., Koblet A., Stadler H., Thomas A.W., Trube G., Waldmeier P. The discovery and unique pharmacological profile of RO4938581 and RO4882224 as potent and selective GABAA alpha5 inverse agonists for the treatment of cognitive dysfunction. Bioorg Med Chem Lett. 2009;19:5940–5944. doi: 10.1016/j.bmcl.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 31.Krystal J.H., Karper L.P., Seibyl J.P., Freeman G.K., Delaney R., Bremner J.D., Heninger G.R., Bowers M.B., Jr., Charney D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 32.Lahti A.C., Koffel B., Laporte D., Tamminga C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 33.Li W.G., Liu M.G., Deng S., Liu Y.M., Shang L., Ding J., Hsu T.T., Jiang Q., Li Y., Li F., Zhu M.X., Xu T.L. ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion. Nat Commun. 2016;7:13770. doi: 10.1038/ncomms13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Wang H., Wen G., Li L., Gao Y., Zhuang Z., Zhou M., Mao L., Fan Y. Neuroprotection by quercetin via mitochondrial function adaptation in traumatic brain injury: PGC-1alpha pathway as a potential mechanism. J Cell Mol Med. 2018;22:883–891. doi: 10.1111/jcmm.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y.F., Wu L.J., Li Y., Xu L., Xu T.L. Mechanisms of H+ modulation of glycinergic response in rat sacral dorsal commissural neurons. J Physiol. 2003;552:73–87. doi: 10.1113/jphysiol.2003.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y.M., Fan H.R., Ding J., Huang C., Deng S., Zhu T., Xu T.L., Ge W.H., Li W.G., Li F. Curcumol allosterically modulates GABA(A) receptors in a manner distinct from benzodiazepines. Sci Rep. 2017;7:46654. doi: 10.1038/srep46654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodge D., Anis N.A. Effects of phencyclidine on excitatory amino acid activation of spinal interneurones in the cat. Eur J Pharmacol. 1982;77:203–204. doi: 10.1016/0014-2999(82)90022-x. [DOI] [PubMed] [Google Scholar]
- 38.MacDonald R.L., Olsen R.W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 39.Manthey J.A., Grohmann K., Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001;8:135–153. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Cue C., Martinez P., Rueda N., Vidal R., Garcia S., Vidal V., Corrales A., Montero J.A., Pazos A., Florez J., Gasser R., Thomas A.W., Honer M., Knoflach F., Trejo J.L., Wettstein J.G., Hernandez M.C. Reducing GABAA alpha5 receptor-mediated inhibition rescues functional and neuromorphological deficits in a mouse model of down syndrome. J Neurosci. 2013;33:3953–3966. doi: 10.1523/JNEUROSCI.1203-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKernan R.M., Whiting P.J. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 42.Meltzer H.Y. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 43.Meltzer H.Y. New trends in the treatment of schizophrenia. CNS Neurol Disord Drug Targets. 2017;16:900–906. doi: 10.2174/1871527316666170728165355. [DOI] [PubMed] [Google Scholar]
- 44.Meltzer H.Y., Rajagopal L., Huang M., Oyamada Y., Kwon S., Horiguchi M. Translating the N-methyl-d-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2013;16:2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- 45.Mihic S.J., Ye Q., Wick M.J., Koltchine V.V., Krasowski M.D., Finn S.E., Mascia M.P., Valenzuela C.F., Hanson K.K., Greenblatt E.P., Harris R.A., Harrison N.L. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 46.Moghaddam B., Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moskaug J.O., Carlsen H., Myhrstad M., Blomhoff R. Molecular imaging of the biological effects of quercetin and quercetin-rich foods. Mech Ageing Dev. 2004;125:315–324. doi: 10.1016/j.mad.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Murase K., Randic M., Shirasaki T., Nakagawa T., Akaike N. Serotonin suppresses N-methyl-d-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res. 1990;525:84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- 49.Nassiri-Asl M., Hajiali F., Taghiloo M., Abbasi E., Mohseni F., Yousefi F. Comparison between the effects of quercetin on seizure threshold in acute and chronic seizure models. Toxicol Ind Health. 2016;32:936–944. doi: 10.1177/0748233713518603. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson E., Eyrich B. On treatment of barbiturate poisoning. Acta Med Scand. 1950;137:381–389. doi: 10.1111/j.0954-6820.1950.tb12129.x. [DOI] [PubMed] [Google Scholar]
- 51.Nomura T., Oyamada Y., Fernandes H.B., Remmers C.L., Xu J., Meltzer H.Y., Contractor A. Subchronic phencyclidine treatment in adult mice increases GABAergic transmission and LTP threshold in the hippocampus. Neuropharmacology. 2016;100:90–97. doi: 10.1016/j.neuropharm.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nury H., Van Renterghem C., Weng Y., Tran A., Baaden M., Dufresne V., Changeux J.P., Sonner J.M., Delarue M., Corringer P.J. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 53.Nutt D.J., Besson M., Wilson S.J., Dawson G.R., Lingford-Hughes A.R. Blockade of alcohol's amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology. 2007;53:810–820. doi: 10.1016/j.neuropharm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto T. Safety of quercetin for clinical application (review) Int J Mol Med. 2005;16:275–278. [PubMed] [Google Scholar]
- 55.Olsen R.W., Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsen R.W., Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paxinos G., Franklin K.B.J. Academic Press; San Diego, CA: 2001. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 58.Porsolt R.D., Moser P.C., Castagne V. Behavioral indices in antipsychotic drug discovery. J Pharmacol Exp Ther. 2010;333:632–638. doi: 10.1124/jpet.110.166710. [DOI] [PubMed] [Google Scholar]
- 59.Puthenkalam R., Hieckel M., Simeone X., Suwattanasophon C., Feldbauer R.V., Ecker G.F., Ernst M. Structural studies of GABAA receptor binding sites: which experimental structure tells us what? Front Mol Neurosci. 2016;9:44. doi: 10.3389/fnmol.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajagopal L., Soni D., Meltzer H.Y. Neurosteroid pregnenolone sulfate, alone, and as augmentation of lurasidone or tandospirone, rescues phencyclidine-induced deficits in cognitive function and social interaction. Behav Brain Res. 2018;350:31–43. doi: 10.1016/j.bbr.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Redrobe J.P., Elster L., Frederiksen K., Bundgaard C., de Jong I.E., Smith G.P., Bruun A.T., Larsen P.H., Didriksen M. Negative modulation of GABAA alpha5 receptors by RO4938581 attenuates discrete sub-chronic and early postnatal phencyclidine (PCP)-induced cognitive deficits in rats. Psychopharmacology (Berl) 2012;221:451–468. doi: 10.1007/s00213-011-2593-9. [DOI] [PubMed] [Google Scholar]
- 62.Ross C.A., Margolis R.L., Reading S.A., Pletnikov M., Coyle J.T. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Rudolph U., Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudolph U., Mohler H. GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sauguet L., Howard R.J., Malherbe L., Lee U.S., Corringer P.J., Harris R.A., Delarue M. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun. 2013;4:1697. doi: 10.1038/ncomms2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semyanov A., Walker M.C., Kullmann D.M. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- 67.Suganthy N., Devi K.P., Nabavi S.F., Braidy N., Nabavi S.M. Bioactive effects of quercetin in the central nervous system: focusing on the mechanisms of actions. Biomed Pharmacother. 2016;84:892–908. doi: 10.1016/j.biopha.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 68.Yamada J., Furukawa T., Ueno S., Yamamoto S., Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb Cortex. 2007;17:1782–1787. doi: 10.1093/cercor/bhl087. [DOI] [PubMed] [Google Scholar]
- 69.Yip G.M., Chen Z.W., Edge C.J., Smith E.H., Dickinson R., Hohenester E., Townsend R.R., Fuchs K., Sieghart W., Evers A.S., Franks N.P. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat Chem Biol. 2013;9:715–720. doi: 10.1038/nchembio.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young J.W., Henry B.L., Geyer M.A. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263–1284. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young J.W., Minassian A., Geyer M.A. Locomotor profiling from rodents to the clinic and back again. Curr Top Behav Neurosci. 2016;28:287–303. doi: 10.1007/7854_2015_5015. [DOI] [PubMed] [Google Scholar]
- 72.Zanos P., Nelson M.E., Highland J.N., Krimmel S.R., Georgiou P., Gould T.D., Thompson S.M. A negative allosteric modulator for alpha5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro. 2017;4 doi: 10.1523/ENEURO.0285-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material