Abstract
The popularity of fermented foods and beverages is due to their enhanced shelf-life, safety, functionality, sensory, and nutritional properties. The latter includes the presence of bioactive molecules, vitamins, and other constituents with increased availability due to the process of fermentation. Many fermented foods also contain live microorganisms that may improve gastrointestinal health and provide other health benefits, including lowering the risk of type two diabetes and cardiovascular diseases. The number of organisms in fermented foods can vary significantly, depending on how products were manufactured and processed, as well as conditions and duration of storage. In this review, we surveyed published studies in which lactic acid and other relevant bacteria were enumerated from the most commonly consumed fermented foods, including cultured dairy products, cheese, fermented sausage, fermented vegetables, soy-fermented foods, and fermented cereal products. Most of the reported data were based on retail food samples, rather than experimentally produced products made on a laboratory scale. Results indicated that many of these fermented foods contained 105−7 lactic acid bacteria per mL or gram, although there was considerable variation based on geographical region and sampling time. In general, cultured dairy products consistently contained higher levels, up to 109/mL or g. Although few specific recommendations and claim legislations for what constitutes a relevant dose exist, the findings from this survey revealed that many fermented foods are a good source of live lactic acid bacteria, including species that reportedly provide human health benefits.
Keywords: fermented foods, live microbes, lactic acid bacteria, health benefits, probiotics
Introduction
Fermentation has long been used to preserve and enhance the shelf-life, flavor, texture, and functional properties of food (Hutkins, 2018). More recently, the consumption of fermented foods containing live microorganisms has emerged as an important dietary strategy for improving human health (Marco et al., 2017). In general, lactic acid bacteria (LAB) from several genera, including Lactobacillus, Streptococcus, and Leuconostoc are predominant in fermented foods, but other bacteria as well as yeast and fungi also contribute to food fermentations. Commercially-produced fermented foods also frequently serve as carriers for probiotic bacteria. Despite this interest and the potential public health benefits of these foods, there is still considerable confusion about which fermented foods actually contain live microorganisms, as well as understanding the role of these microbes on the gut microbiome (Slashinski et al., 2012).
Nonetheless, yogurt and other cultured dairy products are generally perceived by consumers as good sources of live and health-promoting organisms (Panahi et al., 2016). Moreover, in a survey of 335 adults, yogurt was the main food associated with probiotic bacteria (Stanczak and Heuberger, 2009). However, the actual concept of fermentation is evidently not so familiar—a survey of 233 college students attending Brescia University College in London, Ontario revealed that nearly two-thirds were unfamiliar with the term “fermented dairy products,” and about the same percent were unsure that several cultured dairy products were fermented (Hekmat and Koba, 2006).
That a particular food or beverage is produced by fermentation does not necessarily indicate that it contains live microorganisms. Bread, beer, wine, and distilled alcoholic beverages require yeasts for fermentation, but the production organisms are either inactivated by heat (in the case of bread and some beers) or are physically removed by filtration or other means (in the case of wine and beer). Moreover, many fermented foods are heat-treated after fermentation to enhance food safety or to extend shelf-life. Thus, fermented sausages are often cooked after fermentation, and soy sauce and sauerkraut and other fermented vegetables are made shelf-stable by thermal processing. Some products, such as many of the commercial pickles and olives, are not fermented at all, but rather are placed into brines containing salt and organic acids. Even non-thermally processed fermented foods may yet contain low levels of live or viable organisms simply due to inhospitable environmental conditions that reduce microbial populations over time. It is important to note, however, that the absence of live microbes in the final product does not preclude a positive functional role. For example, food fermentation microbes may produce vitamins or other bioactive molecules in situ or inactivate anti-nutritional factors and yet be absent at the time of consumption.
Labeling live microbes in fermented foods and beverages
Yogurt, kefir, and other cultured dairy product manufacturers have long promoted the presence of live cultures. Indeed, the “live and active” seal was created by the National Yogurt Association (NYA), for yogurt products in the United States containing at least 100 million cells or cfu per gram at the time of manufacture (Frye and Kilara, 2016). According to the NYA, the “live and active” seal refers only to yogurt cultures, and specifically to the two species that comprise such cultures, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. However, frozen yogurt, kefir and other cultured dairy products also claim the presence of live and active cultures, even though the microorganisms may be different than those found in yogurt. In the U.S., there is no regulatory requirement to state microbial levels, thus these label declarations are strictly voluntary.
In contrast, in other regions, the number of live microbes present in yogurt and other cultured dairy products must satisfy regulatory requirements. For example, according to the CODEX standards for fermented milk products, the minimum number of starter culture bacteria in yogurt is 107 cfu per g (CODEX STAN 243-2003). If other organisms are indicated on the label, they must be present at 106 cfu per g. Nonetheless, in Europe, to make a claim for yogurt containing live cultures for improving lactose digestion, the European Food Safety Agency requires a minimum of 108 cfu per g of live bacteria (EFSA Panel on Dietetic Products, Nutrition and Allergies, 2010). In contrast, in Australia and New Zealand, a minimum of only 106 cfu per g is required (Commonwealth of Australia Gazette, 2015).
For many years, cultured dairy products were the only fermented foods that included label declarations regarding the presence of live microorganisms. Label declarations on sauerkraut or kimchi or miso, had, until recently, been rare. The popularity of artisan-style fermented foods (Johnson, 2016) and interest in their health properties (Marco et al., 2017) has led more manufacturers to inform consumers, via food labels, that their products contain live microorganisms. In some cases, the species in these types of foods have been identified and then compared to label claims (Yeung et al., 2002; Scourboutakos et al., 2017). However, to our knowledge, data on the actual levels of live microorganisms in most fermented retail products has not readily been reported or summarized in an organized form. Therefore, consumers, despite their interest in probiotics and functional fermented foods (Linares et al., 2017), have had little access to this useful information.
Survey design
The purpose of this study, therefore, was to survey the scientific literature and identify published papers in which the number of live microorganisms in a range of fermented foods was reported. Included were so-called western-fermented foods such as yogurt, cheese, and sausage, as well as soy-based and cereal-based fermented foods that are widely consumed in other regions (Tamang et al., 2016). We then organized and summarized the quantitative data from those reports. Our interest was focused on those reports in which foods were obtained from retail locations or were made under manufacturing conditions. Thus, reports describing results from experimentally-produced fermented foods on a laboratory or pilot scale were excluded, in part because they do not reflect commercial processing, distribution, and storage conditions as do retail products. A large number of the reports in the literature in which levels of microbes in fermented foods were described were of this sort. In addition, many reports have analyzed the importance of microbial food safety and hygienic conditions of fermented food products and have reported the presence of spoilage microorganisms or food pathogens. However, the organisms responsible for fermentation and that are commonly present in the finished products were the focus of this current study.
Search criteria
Scientific articles were chosen that satisfied specific parameters relevant to our stated goals. Specifically, our database search (Google Scholar, WorldCat, Scopus, and PubMed) focused on those studies that enumerated microorganisms exclusively in fermented food products. Keywords for these searches included, but were not limited to, the type of fermented food analyzed and, “commercially produced,” “commercial product,” “enumerated,” “lactic acid bacteria,” “microbial characterization,” “probiotic,” and “culture.” Food products that served only as vehicles for delivery of probiotic microorganisms were not included. Thus, studies that reported counts for frozen yogurt were included, but studies on ice cream containing probiotic microorganisms were not. In general, results were only included for commercial products, bought at retail locations, or those experimentally-produced under industrial manufacturing conditions. Thus, strictly experimental products (e.g., made in a laboratory or under small experimental-scale conditions) were not considered. The only exceptions were for products for which little or no data from retail or industrially manufactured sources was available. In those cases, lab- or pilot-scale-produced products were included, provided they were made using traditional manufacturing methods. No restrictions for date, location, or language were applied.
Data reporting
For most products, quantitative data relied on cultural methods using well-established types of differential, selective, and general purpose media, as well as appropriate incubation conditions. LAB were the main group described, although other bacterial groups were occasionally reported. Some studies reported single microbial counts, whereas other reported ranges. Although papers reported counts either as log or as actual values, all of the data described in this review are shown as logs. For some products, values were estimated from graphs or figures. When products were held for shelf-life or aging studies, the counts from multiple times points are shown. Otherwise, single time-point data was reported. The region or origin of product manufacture was also noted.
General survey results
Approximately 400 published studies were reviewed in which fermented foods were characterized for the presence of live microorganisms. However, about three-fourths were excluded and not used in our results. Several excluded studies focused on development of selective methods for distinguishing between different species of LAB, determining ratios (e.g., cocci-to-rods in yogurt), or for enumerating only probiotics organisms. Although most studies reported data based on traditional plating methods, many of the more recent studies reported abundance data (i.e., 16S rRNA-based community sequencing). Because the latter 16S-based methods also detect non-viable cells, these studies were excluded unless total counts were also reported. Ultimately, more than 140 studies were included in our survey. Although the literature from which the results were assembled covers a 50 year period and a range of different regions and methodologies, the results are remarkably consistent. As summarized below, nine groups of fermented foods were reviewed in this survey. These included yogurt and other cultured dairy products, cheese, fermented meats, fermented vegetables, traditional fermented Asian products, fermented cereals, beer, and fermented tea (Kombucha).
Yogurt and other cultured dairy products
Studies were conducted for retail or commercially manufactured yogurts and other cultured dairy products obtained in the U.S., Australia, Spain, France, Norway, Greece, Argentina, and South Africa (Table 1). All of the yogurts examined contained the yogurt culture organisms, S. thermophilus and L. delbrueckii subsp. bulgaricus, at levels ranging from < 104 to 109 cfu/g or ml. In general, counts for S. thermophilus were somewhat higher than for L. delbrueckii subsp. bulgaricus. In several studies, other microorganisms, including Bifidobacterium spp. and Lactobacillus spp., were also enumerated. Levels of the latter ranged from undetectable (< 10 cfu/g) to 108 cfu/g. The addition of these probiotic bacteria did not appear to have any effect on levels of the yogurt culture organisms. Although most studies reported counts at only a single time point, other studies reported initial counts as well as at a second time point, usually considered end-of-shelf-life. In such cases, counts were generally similar at both time points (>106 cfu/g), provided samples were stored at refrigeration temperatures (Hamann and Marth, 1984).
Table 1.
Organisms in commercial yogurt products by region.
| Region | Type | Source | Analyzed microorganisms | Initial Count (log cfu/mL or g) | Final Count (log cfu/mL or g) | Age | CP* | References |
|---|---|---|---|---|---|---|---|---|
| Argentina | Full and reduced fat yogurt | Retail | S. thermophilus | 8.87–9.46 | – | Within shelf life | 6 | Vinderola and Reinheimer, 2000 |
| L. bulgaricusa | 5.58–7.95 | – | ||||||
| Bifidobacteriaa | 2.60–8.71 | – | ||||||
| L. acidophilusa | 4.62–8.39 | – | ||||||
| L. caseib | 8.02–8.33 | – | ||||||
| Set, skimmed set, drinking, and set with “dulce de leche” yogurtc | Industrially manufactured | Total LAB | 7.54–8.62 | – | Within shelf life | 25 | Birollo et al., 2000 | |
| S. thermophilus | 7.72–8.58 | – | ||||||
| L. bulgaricus | 7.29–7.38 | – | ||||||
| Australia | Full and reduced fat yogurtd | Commercially Manufactured | Streptococci | 9.15–9.6 | 8.79–9.15 | After manufacture and by expiration | 4 | Micanel et al., 1997 |
| L. bulgaricus | 9.08 | 8.36 | ||||||
| L. acidophilus | 6.66–8.08 | 6.38–8.04 | ||||||
| Bifidobacteria | 5.81 | 7.54 | ||||||
| Skim milk and regular yogurte | Did not specify | L. casei | – | 3.41–7.49 | Did not specify | 2 | Ravula and Shah, 1998 | |
| Variety of flavored, natural, and skinny yogurtf | Retail | S. thermophilus | 8.62–9.17 | – | After purchase | 5 | Tharmaraj and Shah, 2003 | |
| L. bulgaricus | 4.92–7.68 | – | ||||||
| L. rhamnosus | 7.36–7.72 | – | ||||||
| L. casei | 4.01–5.53 | – | ||||||
| B. lactis | 6.36–7.4 | – | ||||||
| L. acidophilus | 5.23–7.83 | – | ||||||
| Variety of flavored yogurtsg | Retail | L. acidophilus | < 2–8.34 | < 2–8 | After purchase (around 20–30 days before expiration) and at expiration | 26 CP from 14 companies | Shah et al., 2000 | |
| Bifidobacteria | < 2–6.86 | < 2–6.18 | ||||||
| L. casei | 5.65–8.18 | < 2–8.08 | ||||||
| Yogurth | Did not specify | L. acidophilus | – | 6.56 | – | 18 | Talwalkar and Kailasapathy, 2004 | |
| Bifidobacteria | – | 6.54 | ||||||
| L. casei | – | 6.38 | ||||||
| Yogurtij | Obtained from manufacturer | L. acidophilus | 4–8.5 | NVO−7.7 | After manufacture and 30 days | 5 | Shah et al., 1995 | |
| B. bifidum | 3.3–7 | NVO−2.5 | ||||||
| China | Yogurt | Retail | S. thermophilus + Lactobacillus | – | 4.0–8.18 | End of shelf life | 31 | Dong et al., 2014 |
| England | Yogurtk | Retail | Bifidobacteria | – | 4.9–7.62 | Does not specify | 8 | Iwana et al., 1993 |
| Greecei | Greek type yogurt | Obtained from manufacturer | S. thermophilus | 9.1 | 8.5 | 50 days (product shelf life) | 1 | Alexopoulos et al., 2017 |
| L. delbrueckii subsp. bulgaricus | 8.8 | 7.9 | ||||||
| Italy | Plain stirred style yogurt | Retail | S. thermophilus | 7.71–8.9 | – | 10 days after manufacture | 11 | De Noni et al., 2004 |
| L. bulgaricus | 5.48–8.41 | – | ||||||
| Sweetened stirred style yogurt | Retail | S. thermophilus | 8.3–9.59 | – | 10 days after manufacture | 11 | De Noni et al., 2004 | |
| L. bulgaricus | < 4–8.18 | – | ||||||
| South Africa | Low fat, fruit flavoredi | Obtained from manufacturer | S. thermophilus | 8.7–9.5 | 7.9−9.5 | Directly after production, and at expiration date | 3 | Lourens-Hattingh and Viljoen, 2002 |
| L. bulgaricus | 7–8.6 | 5.5–7 | ||||||
| L. acidophilus | 7–8.7 | 4.9–7 | ||||||
| B. bifidum | 2–5.2 | 2.2–4.9 | ||||||
| United States | Custard style yogurt—plain and flavoredi | Retail | Total LAB | 9.1 | – | 15 days after manufacture | 2 CP from 1 manufacturer | Hamann and Marth, 1984 |
| S. thermophilus | 9.1 | – | ||||||
| L. bulgaricus | 8.1 | – | ||||||
| Dannon, Breyers, Yoplait, YoBaby, Wal-Mart, and Kroger varieties | Retail | Lactobacillus | – | 7.68–8.98 | before expiration | 10 | Dunlap et al., 2009 | |
| Flavored yogurt | Retail | L. bulgaricus | 5.2–8.87 | 6.15–8.69 | 0 and 4 weeks after purchase | 58 CP/7 brands | Ibrahim and Carr, 2006 | |
| S. thermophilus | 7.51–8.94 | 7.9–8.99 | ||||||
| Bifidobacteria | < 1–4.7 | NVO** | ||||||
| Plain nonfat yogurt | Retail | S. thermophilus | 8.14–9.83 | – | After manufacture | 3 | Laye et al., 1993 | |
| L. bulgaricus | 9.04–9.33 | – | ||||||
| Stirred style yogurt–flavoredi | Retail | Total LAB | 8.6 | – | 6 days after manufacture | 1 | Hamann and Marth, 1984 | |
| S. thermophilus | 8.6 | – | ||||||
| L. bulgaricus | 7.3 | – | ||||||
| Yogurt | Retail | Total LAB | - | 7.2–8.1 | At expiration date | 2 | Shin et al., 2000 | |
| Bifidobacteria | – | 6.5–7.1 |
CP, commercial products.
NVO, No viable organisms.
Only viable in 4 of 6 CP.
Only viable in 3 of 6 CP.
Reported as average on duplicate agar plates.
L. delbrueckii spp. bulgaricus—reported in only one product. L. acidophilus −1 of 4 CP had NVO. Bifidobacteria−1 of 4 CP had NVO and 1 product had no detectable counts at initial enumeration (week 0).1
Lower end of range are microbial counts for skim milk yogurt and higher end are for regular yogurt. Both products claimed to contain L. casei.
S. thermophilus —Seen in 5 of 5 CP, “yogurt culture” claimed in all 5 CP. L. bulgaricus—Seen in 2 of 5 CP, “yogurt culture” claimed in all 5 CP. L. rhamnous—Claimed in 2 of 5 CP. L. casei—Claimed in 2 of 5 CP. B. lactis—Claimed in 4 of 5 CP. L. acidophilus—Claimed in 4 of 5 CP.
L. acidophilus—Claimed in 24 CP. Bifidobacteria—Claimed in 18 CP. L. casei—Claimed in 8 CP.
L. acidophilus—9 of 18 CP. Bifidobacteria-−8 of 18 CP. L. casei—6 of 18 CP.
Interpreted from graph.
L. acidophilus—2 of 5 CP had NVO. B. bifidum—4 of 5 CP had NVO.
Observed in 5 of 8 CP, claimed in all products.
In addition to fresh yogurt, frozen yogurt was also examined for bacteria. Results from several studies indicates that when these products were assessed for the relevant yogurt LAB, levels were generally similar to fresh yogurt, with counts ranging from 104 to 109 cfu/g. The stability of lactic cultures in frozen yogurt during long-term storage at freezer temperature (-23 C) has also been studied (Lopez et al., 1998). In general, LAB (S. thermophilus and L. delbrueckii subsp. bulgaricus) survived beyond the designated shelf-life period (1 year), with less than a 0.5 log reduction for most samples.
The number and type of live microorganisms in other cultured dairy products have also been reported (Table 2). These include kefir, cultured buttermilk and simply “fermented milk.” As for other cultured dairy products, populations of LAB were in the 105–109 cfu/g range.
Table 2.
Organisms in commercial cultured dairy products separated by product.
| Dairy product | Region | Source | Analyzed microorganisms | Initial Count (log cfu/mL or g) | Final Count (log cfu/mL or g) | Age | CP* | References |
|---|---|---|---|---|---|---|---|---|
| Amasi | South Africa | Retail | LAB | 5.1–6.29 | – | Did not specify | 5 | Moyane and Jideani, 2013 |
| Total bacteria count | 3.62–4.96 | – | ||||||
| Cultured Buttermilk | Ethiopiaa | Dairy farms and processing units | Lactococci | 6.07–9.25 | – | Does not specify | 16 | Gebreselassie et al., 2016 |
| Lactobacilli | 6.07–8.61 | – | ||||||
| India | Restaurant | Total viable count | 6 | – | Does not specify | 1 | Jayashree et al., 2013 | |
| United States | Retail | Total bacteria count | 7.3–8.64 | 6.08–7.24 | After purchase and 7 days after | 8 | Vasavada and White, 1979 | |
| Fermented Milk | Argentina | Retail | S. thermophilus | 9.11–9.49 | – | Within shelf life | 2 | Vinderola and Reinheimer, 2000 |
| L. acidophilus | 4.62–6.60 | – | ||||||
| Spain | Commercially Manufactured | S. thermophilus | 8.42 | 8.37 | After manufacture and at shelf life (24 days) | 50 | Medina and Jordano, 1994 | |
| L. bulgaricus | 7.71 | 6.87 | ||||||
| Bifidobacteria | 6.87 | 6.62 | ||||||
| Spainb | Retail | S. thermophilus | 9 | 7 | 30 days | 10 | Gueimonde et al., 2004 | |
| Lactobacillus | 7–7.3 | 5.1–6.8 | ||||||
| Bifidobacteria | 5.6–7.5 | 4.1–7.6 | ||||||
| Spain | Retail | S. thermophilus | 9.27 | – | Within shelf life (28 days) | 1 | García-Cayuela et al., 2009 | |
| L. bulgaricus | 7.64 | – | ||||||
| L. acidophilus | 6.65 | – | ||||||
| L. casei | 6.79 | – | ||||||
| B. lactis | 8.2 | – | ||||||
| Frozen Yogurt | France | Obtained from manufacturerc | S. thermophilus | 8.19 | – | 5 weeks after manufacture | 1 | Lopez et al., 1998 |
| Spain | Obtained from manufacturer | S. thermophilus | 7.57–7.58 | – | 1 week after manufacture | 2 | Lopez et al., 1998 | |
| L. bulgaricus | 4.29–6.79 | – | ||||||
| United States | Variety of flavors soft/hard from retail and the manufacturerd | Total bacteria | < 5.52–8.81 | – | Does not specify | 34 | Kosikowski, 1981 | |
| Vanilla flavors from retaile | LAB | 6.11–9.32 | – | Does not specify | 10 | Schmidt et al., 1997 | ||
| Variety of flavors from retail | Total viable bacteria | 2.30–8.53 | – | Within shelf life | 19 | Tieszen and Baer, 1989 | ||
| Kefir | Greecef | Retail | Yeast | 5 | – | 15 days before expiration | 9 | Kalamaki and Angelidis, 2017 |
| Korea | Manufactured with commercial grain | LAB | 9.62 | – | After fermentation | –g | Kim et al., 2015 | |
| Acetic acid bacteria | 9.52 | – | ||||||
| Yeast | 7.67 | – | ||||||
| Norway bh | Obtained from TINE Meieret dairy company | Leuconostoc | 7.1 | 6.3 | After production and at expiration | 5 | Grønnevik et al., 2011 | |
| Lactobacillus | 8.1 | 6.4 | ||||||
| Lactococcus | 8.1 | 5.8 | ||||||
| Yeast | 3.3 | 3.9 | ||||||
| Turkey | Retail | Lactobacillus | 6.51–8.01 | – | Does not specify | 4 | Kesmen and Kacmaz, 2011 | |
| Lactococcus | 7.53–8.30 | – | ||||||
| United Statesi | Manufactured with commercial starter culture | Lactobacillus | 9.15 | – | After fermentation | –g | OBrien et al., 2016 | |
| Lactococcus | 9 | – | ||||||
| Yeast | 7.2 | – |
CP, Commercial Products.
Analyzed sour cream buttermilk and sour milk buttermilk.
Interpreted from graph.
No significant decrease in S. thermophilus over time. L. bulgaricus was absent in this CP.
Only 23 CP of 34 CP had viable organisms.
NVO in 6 CPs (< 1 log).
Only viable counts seen in 8 of the 9 CPs.
Lab-scale fermentation with commercial kefir grain/starter
Presumptive (95:5 ratio) for lactobacillus and lactococcus.
Reported as average from triplicate agar plates.
Cheese
Although considerable microbiological data for cheese exists, most of these reports are concerned with microorganisms having public health or cheese quality implications. Still, levels of lactic acid and related bacteria were reported for more than 30 types of cheese from 18 countries including the United States, Italy, France, Germany, Mexico, Ireland, and South Africa (Table 3). Many papers reported the microorganisms as mesophilic streptococci, lactococci, and lactobacilli or as thermophilic streptococci and lactobacilli. Others reported total microorganisms and total LAB. For most products, only one time period was recorded (usually the most aged sample). Microbial counts ranged from undetectable (< 103 cfu/g) to 109 cfu/g, with the highest levels found in Tilsit cheese (typically aged 2–4 months). In contrast, Grana Padano aged 1 year, Parmesan aged greater than 1 year, and Swiss Gruyere aged greater than 1 year all showed no detectable microorganisms (< 103 cfu/g). As noted for other products, the methods used by the investigators may have influenced the reported data. Thus, enumeration of selected organisms (e.g., S. thermophilus) was only possible if the appropriate medium and growth conditions were used.
Table 3.
Organisms in commercial cheese separated by product.
| Cheese | Region (Type) | Source | Analyzed microorganisms | Count (log CFU/g) | Age | CP* | References |
|---|---|---|---|---|---|---|---|
| Afuega'l Pitu | Spain | Traditionally manufactured | Total viable bacteria count | 8.06 | 60 days | 2 | Cuesta et al., 1996 |
| Lactococci | 6.77 | ||||||
| Leuconostocs | 6.76 | ||||||
| Lactobacilli | 8.01 | ||||||
| Armada* | Spain | Traditionally manufactured | Aerobic Mesophiles | 4.39–8.14 | 16 weeks | 2 | Tornadijo et al., 1995 |
| Lactococci | 4.17–6.38 | ||||||
| Lactobacilli | 4.19–8.09 | ||||||
| Leuconostocs | 3.38–7.58 | ||||||
| Asiago | Italy (Asiago Allevo) | Commercial sample | Meso. streptococci | 5.7 | 3–10 months | 1 | Gatti et al., 1999 |
| Therm. streptococci | 8.9 | ||||||
| Meso. lactobacilli | 4.5 | ||||||
| Therm. lactobacilli | 7.2 | ||||||
| Blue Cheeseb | United States | Retail | Total plate count | 7.32 | Within shelf life | 1 | Genigeorgis et al., 1991 |
| Brie | Italy | Commercial samples | Meso. streptococci | 5.3 | 1–2 months | 1 | Gatti et al., 1999 |
| Therm. streptococci | <3 | ||||||
| Meso. lactobacilli | n.d.** | ||||||
| Therm. lactobacilli | <3 | ||||||
| South Africaa | Commercially manufactured | LAB | 7–8.8 | 8 weeks | 8 | Viljoen et al., 2003 | |
| Burgos | Spain | Retail | LAB | 4.6–8.8 | Time of purchase | 36 | Garcia et al., 1987 |
| Cabrales | Spain | Obtained from manufacturers | Aerobic mesophiles | 7.45–8.36 | 90 days | 2 | Flórez et al., 2006 |
| Lactococci | 7.44–8.12 | ||||||
| Lactobacilli | 5.85–7.15 | ||||||
| Leuconostoc spp. | 5.40–6.14 | ||||||
| Obtained from manufacturersc, d | Total viable count | 6.8–7.9 | 120 days | 2 | Nuñez, 1978 | ||
| Streptococci | 3.5–5.9 | ||||||
| Leuconostocs | 3–3.8 | ||||||
| Lactobacilli | 3.2–6.5 | ||||||
| Yeast+Molds | 4.1–7.2 | ||||||
| Camemberta | South Africa | Commercially manufactured | LAB | 7.6–8.5 | 8 weeks | 8 | Viljoen et al., 2003 |
| Cheddar | Ireland | Commercially manufactured | L. paracasei | 8 | 39 weeks | 3 | Fitzsimons et al., 2001 |
| Irelandc | Obtained from manufacturer | NSLAB*** | 1.70–6.90 | 8 weeks | 8 | Jordan and Cogan, 1993 | |
| NSLAB | 6.15 | 52 weeks | 2 | ||||
| U.S.e | Traditionally manufactured with commercial starter culture | Lactobacillus | 5.1 | 180 days | – | Madkor et al., 2000 | |
| Colbyb | United States | Retail | Total plate count | 7.6 | Within shelf life | 1 | Genigeorgis et al., 1991 |
| Comte | Francef | Obtained from manufacturer | Lb. paracasei | 6.28–7.59 | 168–280 days | 4 | Depouilly et al., 2004 |
| Lb. rhamnosus | 5.37–6.9 | ||||||
| Switzerlandc g | Commercially manufactured | Thermophilic streptococci | 6.75 | 24 weeks | 3 | Bouton et al., 1998 | |
| Thermophilic lactobacilli | 7 | ||||||
| Facultative heterofermentative lactobacilli | 7.5 | ||||||
| Propionibacteria | 7.75 | ||||||
| Danbo | Denmark | Industrially manufactured | Lactococcus | 5.76 | 6 weeks | 1 | Gori et al., 2013 |
| Lactobacillus | 5.82–5.87 | ||||||
| Edam | Egypt (Edam-like cheese)h | Manufactured with commercial starter culture | Total viable bacteria count | 7.76 | 15 weeks | 1 | Ayana and El-Deeb, 2016 |
| Italy | Commercial samples | Meso. streptococci | 2.9 | 1–2 months | 1 | Gatti et al., 1999 | |
| Therm. streptococci | 4.3 | ||||||
| Meso. lactobacilli | 5.8 | ||||||
| Therm. lactobacilli | 5.3 | ||||||
| Feta | Greece | Obtained from manufacturerb | LAB | 6.1 | 60 days | 1 | Alexopoulos et al., 2017 |
| Retaili | Lactobacillus | 5.95–7.19 | >60 days | 4 | Rantsiou et al., 2008 | ||
| Lactococcus | 4.18– < 5 | ||||||
| Iran (Probiotic feta) | Commercially manufactured | Lactobacillus acidophilus | 6.7 | Did not specify | 1 | Mohammadmoradi et al., 2015 | |
| Bifidobacterium lactis | 6.7 | ||||||
| Fontina | Italy | Commercial sample | Meso. streptococci | 8.3 | 3–10 months | 1 | Gatti et al., 1999 |
| Therm. streptococci | 8.3 | ||||||
| Meso. lactobacilli | 4.6 | ||||||
| Therm. lactobacilli | 8.6 | ||||||
| Italy (Fontal) | Commercial samples | Meso. streptococci | <3 | 1–2 months | 1 | Gatti et al., 1999 | |
| Therm. streptococci | 5.2 | ||||||
| Meso. lactobacilli | <3 | ||||||
| Therm. lactobacilli | 4.4 | ||||||
| Galotyrih | Greece | Retail | Total viable count | 8.03 | Time of purchase | 1 | Samelis and Kakouri, 2007 |
| Lactobacilli | 7.55 | ||||||
| Lactococci | 8.11 | ||||||
| Gorgonzola | Italy | Commercial sample | Meso. streptococci | 3.5 | 3–10 months | 1 | Gatti et al., 1999 |
| Therm. streptococci | 7.4 | ||||||
| Meso. lactobacilli | 3.1 | ||||||
| Therm. lactobacilli | 6.4 | ||||||
| Obtained from manufacturerd | Total mesophilic bacteria | 7.36–7.56 | 86 days | 1 | Gobbetti et al., 1997 | ||
| S. thermophilus | 7.85–7.92 | ||||||
| Lb. delbrueckii subsp. bulgaricus | 3.67–5.77 | ||||||
| Mesophilic lactobacilli | 5.57–5.69 | ||||||
| Lactococci | 7.73–7.87 | ||||||
| Mold | 6.81–7.44 | ||||||
| Gouda | Belgiumk | Commercially manufactured | Total microflora count | 5.8 | 42 days | 1 | Messens et al., 1999 |
| LAB | 7.1 | ||||||
| Lactococcus lactis | 6.1 | ||||||
| Belgium (Bellie)c | Commercial starter culture | Enterococcus | 6.45–6.90 | 12 weeks | 1 | Van Hoorde et al., 2008 | |
| Lactobacillus | 6.3–7.3 | ||||||
| Lactococcus | 7.2–7.7 | ||||||
| Leuconostoc | 7.4–7.6 | ||||||
| Belgium (Dulses)c | Commercial starter culture | Enterococcus | 6.40–6.55 | 12 weeks | 1 | Van Hoorde et al., 2008 | |
| Lactobacillus | 6.90–7.20 | ||||||
| Lactococcus | 7.50–7.70 | ||||||
| Leuconostoc | 7.60–7.90 | ||||||
| South Africa | Commercially manufactured | Lactobacillus | 8.96 | 32 days | 1 | Welthagen and Viljoen, 1998 | |
| Lactococcus | 9.1 | ||||||
| Total plate count | 9.04 | ||||||
| Gubbeenl | Germanym | Traditionally manufactured with commercial starter culture | Total bacterial count | 7.3 | 16 days | 1 | Mounier et al., 2006 |
| Grana Padano | Italyn | Commercially manufactured | Lactobacillus | 4.94–6.22 | 9 months | 1 | Monfredini et al., 2012 |
| Lactococcus | 3.15–6.05 | ||||||
| Italy | Commercial samples | Meso. streptococci | <3 | >1 year | 3 | Gatti et al., 1999 | |
| Therm. streptococci | <3 | ||||||
| Meso. lactobacilli | <3 | ||||||
| Therm. lactobacilli | <3 | ||||||
| Italy | Commercial samples | Meso. streptococci | <3 | 3 days ripened | 1 | Gatti et al., 1999 | |
| Therm. streptococci | <3 | ||||||
| Meso. lactobacilli | 4.4 | ||||||
| Therm. lactobacilli | 7 | ||||||
| Italyf | Obtained from manufacturer | Lactobacillus | 4.53 | 13 months | 6 | Santarelli et al., 2013 | |
| Total viable count | 7.11 | ||||||
| Havarti | Denmark (Pasteurized milk havarti) | Traditionally manufactured | Lactococcus | 5.69 | 12 weeks | 1 | Gori et al., 2013 |
| Lactobacillus | 3.65–5.54 | ||||||
| Denmark (Raw milk Havarti) | Traditionally manufactured | Lactococcus | 7.56 | 12 weeks | 1 | Gori et al., 2013 | |
| Lactobacillus | 6.45–7.75 | ||||||
| Livarot | France | Retail | Total bacteria count | 8.58 | Does not specify | 1 | Mounier et al., 2009 |
| Yeast | 6.38 | ||||||
| Limburgerb | United States | Retail | Total plate count | 7.98 | Within shelf life | 1 | Genigeorgis et al., 1991 |
| Manchego | Spain | Retail | LAB | 4.6–10.03 | Time of purchase | 36 | Garcia et al., 1987 |
| Manufactured with commercial starter culturec | Lactococcus | 5.9 | 150 days | 1 | Poveda et al., 2003 | ||
| Lactobacillus | 5.5 | ||||||
| Monterey Jackb | United States | Retail | Total plate count | >6.0–8.62 | Within shelf life | 4 | Genigeorgis et al., 1991 |
| Mozzarella | Italy | Commercial Samples | Meso. streptococci | 6.3 | < 20 days | 1 | Gatti et al., 1999 |
| Therm. streptococci | 7.6 | ||||||
| Meso. lactobacilli | 4.3 | ||||||
| Therm. lactobacilli | <3 | ||||||
| Italy (Buffalo milk) | Retail | LAB | 4.82 | Within expiration date | 18 | Pisano et al., 2016 | |
| Italy (Mozzarella Bufala) | Commercial samples | Meso. streptococci | 5.6 | < 20 days | 1 | Gatti et al., 1999 | |
| Therm. streptococci | 5.6 | ||||||
| Meso. lactobacilli | 4.8 | ||||||
| Italy (Mozzarella Bufala Campana) | Local markets | LAB | 4.0–7.8 | Within shelf life | 3 | Devirgiliis et al., 2008 | |
| Italy (Cow milk) | Commercially manufactured with commercial starter | Therm. lactobacilli | 4.6 | 15 days | 1 | De Angelis et al., 2008 | |
| Meso. lactobacilli | 4.81 | ||||||
| Streptococcus | 7.85 | ||||||
| Enterococcus | 3.87 | ||||||
| Italy (Cow milk) | Retail | LAB | 7.08 | Within expiration date | 14 | Pisano et al., 2016 | |
| Muensterb | United States | Retail | Total plate count | 4.53 | Within shelf life | 1 | Genigeorgis et al., 1991 |
| Parmesan | Italy (Parmigiano Reggiano) | Obtained from manufacturer | LAB | 7.52 | 150 days | 15 | Coppola R. et al., 2000 |
| Italy (Parmigiano Reggiano) | Commercially manufactured | LAB | 6.18 | 2 months | 1 | Gatti et al., 2008 | |
| LAB | 2.3 | 24 months | |||||
| Italy (Parmigiano Reggiano) | Commercial samples | Meso. streptococci | <3 | >1 year | 1 | Gatti et al., 1999 | |
| Therm. streptococci | <3 | ||||||
| Meso. lactobacilli | <3 | ||||||
| Therm. lactobacilli | <3 | ||||||
| Puzzone di Moenao | Italy | Traditionally manufactured | Lactobacillus | 7.1–7.7 | 3 months | 2 | Franciosi et al., 2008 |
| Lactococcus | 7.5–7.7 | ||||||
| Pecorino Romano | Italy | Commercial sample | Meso. streptococci | 3.5 | 3–10 months | 1 | Gatti et al., 1999 |
| Therm. streptococci | 5.5 | ||||||
| Meso. lactobacilli | 3.7 | ||||||
| Therm. lactobacilli | 3 | ||||||
| Provolone | Italy (Piquant provolone) | Commercial sample | Meso. streptococci | 2.5–3.4 | 3–10 months | 2 | Gatti et al., 1999 |
| Therm. streptococci | 5.4–8.3 | ||||||
| Meso. lactobacilli | 2.8– < 3 | ||||||
| Therm. lactobacilli | 5.5–7.2 | ||||||
| Italy (Sweet provolone) | Commercial sample | Meso. streptococci | < 3–4.3 | 3–10 months | 2 | Gatti et al., 1999 | |
| Therm. streptococci | 4.5–7.1 | ||||||
| Meso. lactobacilli | <3 | ||||||
| Therm. lactobacilli | < 3–7.1 | ||||||
| Queso Frescop | Mexico | Obtained from manufacturer | Mesophilic streptococci | 6.85–9.07 | Within 5 days of manufacturer | 6 | Renye et al., 2008 |
| Thermophilic streptococci | 5.04–9.02 | ||||||
| Mesophilic lactobacilli | 7.13–8.99 | ||||||
| Thermophilic lactobacilli | 5.01–9.01 | ||||||
| Leuconostoc | 5.86–9.23 | ||||||
| Enterococcus | 5.05–7.91 | ||||||
| Serranol | Brazil | Retail | Lactococcus | 8.60–9.10 | Within shelf life | 10 | Delamare et al., 2012 |
| Lactobacillus | 7.95–9.10 | ||||||
| Stilton | United Kingdomq | Retail | Mesophilic LAB | 8.87 | Within shelf life | 16 | Ercolini et al., 2003 |
| Lactobacillus | 7.76 | ||||||
| Mesophilic streptococci | 8.97 | ||||||
| Mesophilic, anaerobic LAB | 8.85 | ||||||
| United Kingdom (blue-veined raw milk cheese)d | Obtained from manufacturer | LAB | 6.90–7.41 | After aging (12 weeks) | 1 | Yunita and Dodd, 2018 | |
| Lactobacillus | 4.85–6.18 | ||||||
| Lactococcus | 7.83–8.65 | ||||||
| Swissc, r | France | Traditionally manufactured | Propionibacteria | 7.5–7.6 | 24 weeks | 2 | Demarigny et al., 1996 |
| Facultatively heterofermentative Lactobacillus | 7.4–7.9 | ||||||
| Thermophilic streptococci | 3.0–5.6 | ||||||
| Thermophilic lactobacilli | 2.6–5.9 | ||||||
| Swiss Gruyere | Italy | Commercial sample | Mesophilic streptococci | <3 | >1 year | 1 | Gatti et al., 1999 |
| Thermophilic streptococci | <3 | ||||||
| Mesophilic lactobacilli | <3 | ||||||
| Thermophilic lactobacilli | <3 | ||||||
| Tilsit | Austria | Obtained from manufacturer | Total bacterial count | 8.4–9.7 | 21 days | 13 | Eliskases-Lechner and Ginzinger, 1995 |
CP, Commercial Products.
n.d., not determined.
NSLAB, non-starter LAB count.
Winter and summer cheese analyzed on surface and in center.
Did not support L. monocytogenes surface growth when enumerated.
Interpreted from graph.
Surface and interior of cheese was analyzed.
Lactobacillus count of control cheese (not adjunct culture added).
Lb. rhamnosus and Lb. paracasei were the only microorganisms enumerated in all 4 CP.
Average of CP.
The control from an Edam-like cheese experiment of goat's diet.
3 of 4 CP reported “not applicable” (< 5 log cfu/g).
jIndustrial Cheese with commercial starter cultures
Pressure treatment of 0.1 MPa.
Only licensed cheeses analyzed.
Measurement of bacterial growth on cheese surface.
Grana Trentino cheese; Measurements from middle section and core.
Winter and summer cheese at 30°C.
Raw and pasteurized milk cheese.
Reported as average of triplicate agar plates.
rRaw and microfiltered milk reported.
Fermented meats
Microbial counts for fermented sausages are shown in Table 4. In general, samples were either obtained from retail, directly from manufacturers, or were produced via industrial conditions. Most samples were from the United States, Spain, Portugal, and Italy and were composed of pork and/or beef. The levels of microorganisms (LAB and total) ranged from undetectable (< 102 cfu/g) to 1010 cfu/g. Data were reported as either within the product shelf life or after ripening or maturation of the sausage. Counts of viable microorganisms in sausages from the United States were generally lower (< 107 cfu/g) compared to sausages from other countries. In particular, LAB levels were all < 106 cfu/g. In contrast, several of the European sausages contained high levels of LAB (>108 cfu/g.). European sausages were more often artisan sausages from smaller manufacturers, although similar microorganisms are used in comparison to sausages from the United States.
Table 4.
Organisms in commercial sausage products by region.
| Country | Type | Source | Analyzed microorganisms | Count (log CFU/g) | Age | CP | References |
|---|---|---|---|---|---|---|---|
| France | Dry fermented sausage | Obtained from manufacturer | LAB | 6.50–7.74 | End of drying (9 weeks) | 1 | Chevallier et al., 2006 |
| Greece | Dry fermented sausage | Obtained from manufacturer | LAB | 7.63–8.20 | 28 days after formulation | 1 | Samelis et al., 1994 |
| Commercially produceda | LAB | 8.1–8.2 | End of curing period | 2 | Papamanoli et al., 2003 | ||
| Italy | Ciauscolo salami | Commercially produceda | LAB | 7.5 | End of ripening (45 days) | 1 | Aquilanti et al., 2007 |
| Yeast | 5.5 | ||||||
| Obtained from manufacturer | Total bacteria | 2.7–5.95 | End of ripening | 22 | Silvestri et al., 2007 | ||
| LAB | 6.77–8.65 | ||||||
| Fermented Sausage, Friuli Venezia Giulia region | Commercially produceda | Total bacteria | 6.1 | End of ripening (45 days) | 1 | Cocolin et al., 2001 | |
| LAB | 8.3 | ||||||
| Commercially producedb | Total aerobic count | 6.62–9.11 | End of ripening (21 days) | 3 | Comi et al., 2005 | ||
| LAB | 8.39–8.47 | ||||||
| Obtained from manufacturer | Total bacteria | 4.19–9.11 | End of maturation | 3 | Rantsiou et al., 2005 | ||
| LAB | 8.34–8.78 | ||||||
| Salami bergamasco | Obtained from manufacturer | Total bacteria | 6–7.17 | After maturation of 60 days | 2 | Cocolin et al., 2009 | |
| LAB | 9–9.14 | ||||||
| Salami Brianza | Local markets | Mesophilic lactobacilli | 8.6 | After purchase | 1 | Di Cagno et al., 2008 | |
| Salami cremonese | Obtained from manufacturer | Total bacteria | 5.17–6.69 | After maturation of 60 days | 5 | Capita et al., 2006 | |
| LAB | 7.54–9.38 | ||||||
| Salami Mantovano | Obtained from manufacturer | Total bacteria | 4.23–9.87 | After maturation of 60 days | 4 | Capita et al., 2006 | |
| LAB | 7.6–9.38 | ||||||
| Commercially producedc | Lactobacilli | 8.01–8.73 | End of ripening (60 days) | 2 | Pisacane et al., 2015 | ||
| Salami Milano | Obtained from manufacturer | LAB | 8.0 | End of ripening (60 days) | 1 | Rebecchi et al., 1998 | |
| Salami Napoli | Obtained from manufacturera | Mesophilic lactobacilli | 6.7 | End of ripening (30 days) | 1 | Coppola et al., 1995 | |
| Commercially producedd | Mesophilic LAB | 5.5 | End of ripening (41 days) | 1 | Coppola S. et al., 2000 | ||
| Salami Piacentino | Local markets | Mesophilic lactobacilli | 8.3 | After purchase | 1 | Di Cagno et al., 2008 | |
| Obtained from manufacturere | LAB | 8.02–8.84 | End of ripening (63 days) | 6 | Połka et al., 2015 | ||
| Salami Piedmontese | Commercially produced | LAB | 7.84 | End of ripening (45 days) | 1 | Greppi et al., 2015 | |
| Salami Varzi | Local markets | Mesophilic lactobacilli | 8.6 | After purchase | 1 | Di Cagno et al., 2008 | |
| Salsiccia Basilicataa | Commercially produced | LAB | 4–7.23 | End of ripening (40 days) | 10 | Parente et al., 2001 | |
| Yeast | 6–6.6 | ||||||
| Soppressata Basilicataa | Commercially produced | LAB | 8–8.4 | End of ripening (40 days) | 9 | Parente et al., 2001 | |
| Yeast | 5.2–7 | ||||||
| Soppressata Molisanaa | Commercially produced | LAB | 8.4 | End of ripening (28 days) | 2 | Coppola et al., 1998 | |
| Spain and Portugal | Alheiras | Retail | LAB | 5.9–10.5 | Within shelf life | 12 | Capita et al., 2006; Ferreira et al., 2006 |
| Androlla | Obtained from manufacturer | Total aerobic mesophilic bacteria | 7.81–9.52 | After 20–30 days of ripening | 20 | García Fontán et al., 2007b | |
| LAB | 8.78–9.36 | ||||||
| Botillo | Obtained from manufacturer | Total aerobic mesophilic bacteria | 7.63–9.37 | After 15–20 days of ripening | 15 | García Fontán et al., 2007a | |
| LAB | 8.34–9.56 | ||||||
| Chorizo Ostrich | Retail | Total bacteria | 7.3 | Within shelf life | 8 | Capita et al., 2006 | |
| LAB | 6.23 | ||||||
| Chorizo Deer | Retail | Total bacteria | 5.46 | Within shelf life | 6 | ||
| LAB | 5.15 | ||||||
| Chorizo Pork | Retail | Total bacteria | 8.25 | Within shelf life | 18 | ||
| LAB | 8.46 | ||||||
| Salchicón Ostrich | Retail | Total bacteria | 6.09 | Within shelf life | 22 | ||
| LAB | 5.61 | ||||||
| Salchicón Deer | Retail | Total bacteria | 6.28 | Within shelf life | 8 | ||
| LAB | 6.26 | ||||||
| Salchicón Pork | Retail | Total bacteria | 8.09 | Within shelf life | 19 | ||
| LAB | 7.5 | ||||||
| United States | Dry salami | Retail | Total bacteria | 3–6 | Does not specify | 11 | Acton and Dick, 1976 |
| LAB | 3–5 | ||||||
| Genoa salami | Retail | Total bacteria | 3–7 | Does not specify | 8 | ||
| LAB | 2–6 | ||||||
| Lebanon bologna | Retail | Total bacteria | 7–8 | Does not specify | 5 | ||
| LAB | < 3 | ||||||
| Pepperoni | Retail | Total bacteria | 4–7 | Does not specify | 14 | ||
| LAB | 2–6 | ||||||
| San Francisco dry salami | Retail | Total bacteria | 6–7 | Does not specify | 4 | ||
| LAB | 3–6 | ||||||
| Semidry salami | Retail | Total bacteria | 3–4 | Does not specify | 8 | ||
| LAB | < 2 | ||||||
| Summer sausage | Retail | Total bacteria | 3–4 | Does not specify | 19 | ||
| LAB | 4 | ||||||
| Thuringer | Retail | Total bacteria | 3–7 | Does not specify | 13 | ||
| LAB | 5–6 |
Interpreted from graph.
Three seasons were analyzed.
Crespone casings and Gentile casings were used.
Core and edge data reported.
With and without commercial starter cultures.
Fermented vegetables
Microbial counts for fermented vegetables, including sauerkraut, olives, mustard pickles, pickles, and kimchi are summarized in Table 5. Fermented cucumbers products were also considered (listed as pickles). Laboratory-manufactured products, using industrial or traditional practices, were included due to the lack of literature on fermented vegetables from retail sources.
Table 5.
Organisms in fermented vegetables separated by product.
| Product | Region (Type) | Source/Fermentation style | Analyzed microorganisms | Count (log cfu/mL or g) | Age | References |
|---|---|---|---|---|---|---|
| Kimchi | Taiwana | Supermarkets | Aerobic bacteria | 1–7.2 | Within shelf life | Tsai et al., 2005 |
| South Korea | Industrially produced with a spontaneous fermentationb, c | Leuconostoc citreum | 7.4 | 90 days | Cho et al., 2006 | |
| Leuconostoc gasicomitatum | 8 | |||||
| Weissella koreensis | 8 | |||||
| Lactobacillus sakei | 7.4 | |||||
| Retail (online and markets) with starter cultures and spontaneous fermentations | LAB | 7.14–9.23 | 5 days after purchase | Kim et al., 2016 | ||
| Obtained from commercial distributorsb,d | Total viable bacteria | 7.9–8.3 | 4 weeks of fermentation | Lee et al., 2018 | ||
| LAB | 7.8–8.3 | |||||
| Obtained from commercial distributorsb, e | Total viable bacteria | 7.9 | 4 weeks of fermentation | Lee M. et al., 2017 | ||
| LAB | 7.8 | |||||
| Mustard Pickles | Taiwanf | Supermarkets | Aerobic bacteria | < 1.0–4.2 | Within shelf life | Kung et al., 2006a |
| Olives | Greece (Conservolea naturally black olives) | Laboratory manufactured with a spontaneous fermentation | LAB count | 7.9 | 30 days | Panagou et al., 2008 |
| Laboratory manufactured with a commercial starter culture | LAB count | 8 | 30 days | Panagou et al., 2008 | ||
| Italy (Bella Di Cerignola -Debittered green table olives)b, g | Commercially manufactured with a spontaneous fermentation | LAB count | 5.5 | 90 days | De Bellis et al., 2010 | |
| Italy (Nocellara del Belice–Spanish-style green olives)h | Industrially manufactured with a spontaneous fermentation | Viable cell count | 6.58–7.40 | 131 days | Aponte et al., 2012 | |
| Lactobacillus | 7.21–7.35 | |||||
| Lactic streptococci | 6.49–6.95 | |||||
| Italy (Nocellara del Belice–green table olives) | Obtained from commercial manufacturer with spontaneous fermentation | LAB | 4.53 | 7–10 months | Romeo et al., 2012 | |
| Portugal (Galega and Cordovil)b | Laboratory manufactured with a spontaneous fermentation | Viable LAB count | 4.9 | 150 days | Silva et al., 2011 | |
| Southern Spain (Spanish-style green olives)b | Industrially manufactured with a spontaneous fermentation | Lactobacillus | 5.5 | 120 days | Ruiz-Barba and Jiménez-Díaz, 2012 | |
| Lactic cocci | NVO* | 120 days | ||||
| Industrially manufactured with commercial starter cultureb | Lactobacillus | 5.9 | 120 days | Ruiz-Barba and Jiménez-Díaz, 2012 | ||
| Lactic cocci | 4 | 120 days | ||||
| United States (Sicilian-style green olive–colossal Sevillano olives)b | Commercially manufactured with a spontaneous fermentation | LAB count | 7.4 | 200 days | Golomb et al., 2013 | |
| Pickles | Indiab, i | Laboratory manufactured with a spontaneous fermentation | LAB | 7.1 | 3 days | Singh and Ramesh, 2008 |
| United Statesb, j | Laboratory manufactured with a pure culture fermentation | P. cerevisiae | 8.26–8.77 | Did not specify | Etchells et al., 1964 | |
| L. plantarum | 8.72–8.96 | |||||
| L. brevis | 7.79–8.45 | |||||
| Sauerkraut | United Statesb | Commercially manufactured with starter culture | LAB | 8.3 | 10 days | Johanningsmeier et al., 2004 |
| Heterofermentative LAB | 2.7 | |||||
| United Statesb | Commercially manufactured with a spontaneous fermentation | Total microbial count | 7 | 60 days | Lu et al., 2003 | |
| LAB | 7 | |||||
| Croatiak | Laboratory manufactured with a spontaneous fermentation | Total microbial count | 6.04 | 42 days | Beganović et al., 2011 | |
| LAB | 3.79 | 42 days | ||||
| Finlandl | Large-scale manufacturing with a spontaneous fermentation | LAB | 7.3 | 15 days | Viander et al., 2003 |
NVO, No viable organisms.
20 commercial products.
Interpreted from graph.
Incubation of microorganisms were at 15°C.
Three seasons were analyzed.
19 out of 44 Chinese cabbage samples (88 total samples using other vegetables) were provided by commercial suppliers.
14 CP (Commercial Products).
Data from control set (no inoculation) with 8% NaCl.
Olive from both irrigated and not irrigated fields.
30 cucumber samples were used.
Individual fermentations of each microorganism.
Fermentations with 4% NaCl.
Fermentations with 1.2% NaCl.
Microbial counts for sauerkraut were generally reported as LAB with counts ranging from 103 to 108 cfu/g. Reported samples were for sauerkraut originating from the United States, Finland, and Croatia. Levels of LAB and Lactobacillus were reported for olives produced in Italy, Greece, Portugal, Spain, and the United States. These products contained 104 to 108 cfu/g and were between 30 and 200 days.
Other products for which quantitative data were reported included mustard pickles and kimchi from Taiwan and pickled cucumbers from China, India, and the United States. Microbial counts ranged from undetectable (< 101) to 108 cfu/g. For several of these products, levels of species (e.g., Lactobacillus plantarum, Lactobacillus brevis, and Pediococcus cerevisiae) were reported. Species of Leuconostoc, Weissella and Lactobacillus were also reported for Korean kimchi, where they were generally present between 107 and 108 cfu/g.
Traditional asian fermented products
Another group of fermented foods that contain lactic acid bacteria and other bacteria are those products traditionally manufactured in Asia and that rely on grain or legume substrates. One important difference in the fermentation of these food products compared to other fermented foods is the reliance on fungal enzymes to convert complex carbohydrates to simple sugars. Aerobic conditions are another unique characteristic used in various parts of the fermentation process. Data were collected for several products, including miso, tempeh, fish sauce, and fermented fish (Table 6). Similar to the fermented vegetables, there were few reports on products from retail sources. Therefore, laboratory manufactured products made using industrial or traditional practices were included. In general, aerobic bacteria counts of miso ranged from 102 to 107 cfu/g. Similar bacterial counts were reported for fish sauce. LAB counts for tempeh and fermented fish were between 103 to 107 cfu/g with fermented fish being at the lower end of the range.
Table 6.
Organisms present in traditional Asian fermented products separated by product.
| Product | Region (Type) | Source | Analyzed microorganism | Count (log cfu/g) | Age | References |
|---|---|---|---|---|---|---|
| Fermented Fish | Japan (Funazushi—fermented sushi) | Obtained from commercial manufacturer | LAB | 3.48–5.43 | Does not specify | Tsuda et al., 2012 |
| Fish Sauce | Malaysia (anchovy)a | Obtained from commercial manufacturer | Aerobic bacteria | 4.92–5.53 | 6–12 months | Zaman et al., 2010 |
| Miso | Taiwanb | Supermarkets | Aerobic bacteria | 2.1–7.1 | Within shelf life | Kung et al., 2006b |
| Japan | Laboratory manufactured with a spontaneous fermentation | Aerobic bacteria | 4.3 | 15 weeks | Onda et al., 2003 | |
| Tempeh | Netherlands | Laboratory manufactured with industrial processes and a spontaneous fermentationc | LAB | 7.01 | Does not specify | Nout et al., 1987 |
| Shops, production places, and restaurantsd, e | LAB | 3–9 | 24 h after purchase | Samson et al., 1987 |
5 CP.
27 CP (Commercial Products).
Measure of tempeh and not the soak.
81% of samples >107 CFU/g.
110 samples were analyzed.
Fermented cereals
Fermented porridges and gruels are widely consumed in many African countries. Here, studies were reported from Burkina Faso, Uganda, Ghana, Benin, Tanzania, and Mexico (Table 7). These cereals were made using pearl millet, millet, sorghum, and maize as starting grains. In general, the cereals contained LAB and mesophilic aerobic bacteria with a range of 105 to 109 cfu/g.
Table 7.
Organisms in commercial fermented cereals from Africa and Mexico.
| Product (Region) | Source | Analyzed microorganisms | Count (log CFU/g) | Grain | CP | References |
|---|---|---|---|---|---|---|
| Ben-saalga (Burkina Faso) | Obtained from manufacturer | Total aerobic mesophiles | 7.1 | Pearl millet | 12 | Tou et al., 2006 |
| LAB | 7 | |||||
| Yeast | 5.5 | |||||
| Bushera (Uganda) | Markets | LAB | 8.1–8.4 | Millet | 5 | Muyanja et al., 2003 |
| LAB | 8.4 | Sorghum | 5 | |||
| LAB | 8.9–9 | Millet and Sorghum | 5 | |||
| Fura (Ghana) | Obtained from manufacturer | LAB | 6.6–8 | Does not specify | 8 | Owusu-Kwarteng et al., 2012 |
| Koko Sour Water (Ghana)a | Obtained from manufacturer | LAB | 8 | Does not specify | 3 | Lei and Jakobsen, 2004 |
| Mawè (Benin) | Market and manufacturer | Total aerobic mesophiles | 8.8 | Does not specify | 30 | Hounhouigan et al., 1993 |
| LAB | 8.9 | |||||
| Yeast | 6.4–6.9 | |||||
| Pozol (Mexico)b | Market | Total bacteria | 9.5 | Does not specify | 1 | Omar and Ampe, 2000 |
| LAB | 9 | |||||
| Togwa (Tanzania)c | Obtained from manufacturer | LAB | 9 | Sorghum, maize, millet, and maize | 36 | Mugula et al., 2003 |
| Yeast | 7 |
Koko is porridge that have been heat treated. Koko sour water is the edible untreated water byproduct.
Interpreted from graph. Measured outside and inside of sample in triplicate.
Samples were obtained from manufacturer before fermentation.
Beer
Several sour beer products from Belgium, such as lambic and gueuze, were included in the survey (Table 8). LAB counts were reported for these products, ranging from 102 to 105 cfu/g. The age of the products reported in the table refers to the longest time the beer was left to age. This maximum aging time was found to range from 40 days to 5 years across the different products.
Table 8.
Organisms in commercial sour beer products.
| Product | Region | Source | Analyzed microorganisms | Count (log CFU/g) | Age | References |
|---|---|---|---|---|---|---|
| Gueuze | Belgium | Obtained from a traditional brewery | LAB | 5.25–5.31 | 2 years | Spitaels et al., 2015a |
| LAB | 3.87–3.88 | 4 years | ||||
| LAB | 3.49–3.96 | 5 years | ||||
| Lambic | Belgium | Obtained from a traditional brewerya | LAB | 3.08–4.26 | 24 months | Spitaels et al., 2014 |
| Obtained from industrial brewery | LAB | 4.33–4.38 | 12 months | Spitaels et al., 2015b | ||
| Obtained from two breweriesb | LAB | 2.3–2.75 | 40 days | Martens et al., 1991 |
Incubated at 28°C aerobically or 20°C anaerobically on MRS agar.
Interpreted from graph.
Fermented tea (kombucha)
Kombucha is a fermented beverage made from sweetened tea to which a specialized culture is added. The latter is comprised of a symbiotic culture of bacteria and yeast or SCOBY, normally within a cellulose-type membrane. Bacteria commonly found in kombucha include the acetic acid bacteria belonging to the genera, Acetobacter, Gluconacetobacter, and Gluconobacter, as well as LAB. Most of the yeasts associated with kombucha are species of Saccharomyces, although other yeast genera may also be present (Teoh et al., 2004; Coton et al., 2017). While this product is now widely consumed, and manufacturers promote the presence of live microorganisms on product labels, there are few published data on the levels of microbes present in retail products. One recent study reported both bacterial and yeast counts for two kombucha products that were produced under industrial manufacturing conditions (Coton et al., 2017). In general, acetic acid bacteria levels ranged from 106 to 107 cfu/mL at the end of the fermentation, and similar counts were reported for LAB and total aerobic bacteria. Total yeast counts of about 107 cfu/mL were also reported.
Discussion
Food-associated microbes travel and interact in the gut
The human gastrointestinal tract is home to more than 1012 microbes. This diverse ecosystem provides protection against pathogens, extracts nutrients from dietary components, and modulates the immune system (Lozupone et al., 2013). The gut microbiota is also very stable, although several factors, including exposure to antibiotics, stress, and disease can disrupt this community, leading to dysbiosis (Sommer et al., 2017). The ability of diet and dietary components to modulate the gastrointestinal microbiota, redress dysbiosis, and enhance human health is now well- established (David et al., 2014; Graf et al., 2015; Sonnenburg and Bäckhed, 2016).
Among the food components known to influence the composition of the microbiota are fermentable fibers and prebiotics that enrich for particular members of the gut microbiota. Another route by which the gastrointestinal microbiota may be modulated is via consumption of probiotics—live microbes consumed at a dose sufficient to provide beneficial effects (Hill et al., 2014). Probiotics, however, are temporary members of the microbiome and rarely persist more than a few days (Tannock, 2003; Derrien and van Hylckama Vlieg, 2015; Zhang et al., 2016).
Perhaps the easiest and most common way to introduce potentially beneficial microbes to the gastrointestinal tract is via consumption of microbe-containing foods, and fermented foods and beverages, in particular. Like many probiotics, many microbes associated with fermented foods may also have the capacity to survive digestion, reach the gastrointestinal tract, and ultimately provide similar health benefits (Marco et al., 2017). When consumed regularly, these fermentation-associated microbes form what some researchers have called the “transient microbiome” (Derrien and van Hylckama Vlieg, 2015).
In general, the microorganisms present in fermented foods and beverages originate via one of two ways. For so-called natural or spontaneous fermented foods, the microorganisms are autochthonous and are naturally present in the raw material or manufacturing environment. To survive fermentation and processing, the LAB, yeasts, and any other microorganisms present in the finished product must manage a range of selective and competitive pressures, including salt, organic acids, ethanol, anaerobiosis, and low pH. Many of the fermented foods reviewed in this survey, including fermented cereals, sauerkraut, kimchi, and other fermented vegetables, and fermented soy-based products are made by natural fermentation. In addition, many wines and even some fermented sausages and beers are made in this manner.
Other fermented foods rely on the addition of a starter cultures. Cultured dairy products, cheese, and fermented sausages are commonly made using starter cultures. When cultures are used, their selection is based on the performance characteristics specific to the product. In addition, the incubation temperature during fermentation and the nutrient content are usually well-suited to the needs of the microorganisms. In many cases, the culture is added at such high inoculum levels, there would be little competition from other organisms. Collectively, most food fermentation microorganisms are well-adapted to the food environment.
In contrast, once the organisms present in fermented foods are consumed, they become foreign or allochthonous to the gastrointestinal tract. In most cases, they lack the physiological and biochemical resources to compete in this ecological niche. If they survive transit, they do not become stable members of this community (Zhang et al., 2016). Nonetheless, the presence of food fermentation-associated microorganisms in the GI tract, even if they are just “passing through,” is now well-documented (Lee et al., 1996; Walter et al., 2001; Dal Bello et al., 2003; David et al., 2014; Derrien and van Hylckama Vlieg, 2015; Zhang et al., 2016; Lisko et al., 2017).
Evidence of health benefits associated with fermented foods
The evidence for the potential health benefits of fermented foods is based on numerous epidemiological as well as clinical reports (reviewed in Marco and Golomb, 2016; Kok and Hutkins, in press). In general, epidemiological studies have shown that consumption of fermented foods is associated with improvements of health status or reductions in disease risk. For example, yogurt-rich diets were associated with a reduced risk of metabolic syndrome in older Mediterranean adults (Babio et al., 2015). A similar finding was reported in another large cohort study that showed cultured milk consumption reduced the risk of bladder cancer (Larsson et al., 2008). Yogurt consumption has also been associated with reduced weight gain (Mozaffarian et al., 2011). Epidemiological data also suggests that consumption of other fermented foods may be correlated to beneficial health outcomes. Consumption of kimchi and other fermented vegetables, for example, correlated with reduced incidence of asthma and atopic dermatitis in Korean adults (Park and Bae, 2016; Kim et al., 2017). Reduced risks of type 2 diabetes and high blood pressure among Japanese adults was associated with consumption of fermented soybean foods rich in phytoestrogens and bioactive peptides (Kwon et al., 2010; Nozue et al., 2017). In contrast, the large European Prospective Investigation into Cancer and Nutrition cohort study from the Netherlands reported no association between fermented foods consumption and overall mortality (Praagman et al., 2015).
Although many human clinical studies have assessed the effects of probiotic-containing fermented foods on health biomarkers, fewer randomized controlled trials (RCT) have considered fermented foods alone. Nonetheless, several reports provide evidence that fermented foods, such as kimchi, fermented soy products, and yogurt, can improve relevant biomarkers. For example, kimchi consumption improved fasting blood glucose and other metabolic syndrome symptoms in overweight and obese adults (Kim et al., 2011), and similar improvements were observed in healthy adults (Choi et al., 2013). Consumption of a fermented soybean paste also improved plasma triglyceride levels in obese adults (Lee Y. et al., 2017). Perhaps the strongest evidence is for yogurt and improved lactose tolerance, due to in vivo expression and release of β-galactosidase by the yogurt culture microbes, S. thermophilus and L. delbrueckii subsp. bulgaricus (Kolars et al., 1984; Martini et al., 1987; Pelletier et al., 2001; Savaiano, 2014). This is the only approved health claim approved by the European Food Safety Authority (EFSA Panel on Dietetic Products, Nutrition and Allergies, 2010).
As noted previously, some fermented foods could impart health benefits even in the absence of live microorganisms in the finished products. For example, in sour dough bread manufacture, LAB may express phytase enzymes that degrade phytates and therefore enhance mineral absorption (Nuobariene et al., 2015). In the manufacture of red wine, ethanol produced early in the fermentation enhances extraction of polyphenolic compounds from the grape skins. Fermented foods may also contain vitamins and other bioactive molecules produced in situ from microbial metabolism that are not present in the original food. Recently, Saubade et al. (2017) noted that folic acid deficiency is a global health problem and suggested that fermented foods could be a food-based alternative for delivering folic acid to at-risk populations. Although some LAB are able to produce modest levels of folate (Leblanc et al., 2011), amounts produced in foods may be too low to be reach required levels (Saubade et al., 2017). Thus, selection of over-producing strains, as well as combining strains with non-LAB may be necessary to enhance production of this vitamin in foods.
If present, fermentation-derived microorganisms, despite their transient nature, may yet have the potential to influence gut microbiota diversity, structure, and function (Zhang et al., 2016). Notably, they may also affect health due to their ability to out-compete pathogens for resources, produce short chain fatty acids from available carbohydrates, secrete anti-microbial agents, contribute to immune homeostasis, and produce vitamins, in situ (Derrien and van Hylckama Vlieg, 2015).
The number of fermentation-associated microbes depends on region and product age
In this survey, we reviewed the literature for studies that included quantitative data on microorganisms present in commercial fermented food products. To our knowledge, this is the first time that there has been a compilation of the hundreds of previous studies that enumerated microbes in fermented foods from retail samples or commercial products. In general, most of the products for which data were available contained at least 106 cells/mL or g. However, there was considerable variation depending on product age and region, and several relevant bacterial species or groups were present at less than that amount.
Although regular consumption of yogurt is often included in dietary guidelines (Smug et al., 2014), recommendations for other fermented foods rarely exist (Chilton et al., 2015). Likewise, to our knowledge, there are few guidelines for what constitutes a minimum dose of live microorganisms. The one exception is the yogurt health claim for “improved lactose tolerance” that was approved in 2010 by the European Food Safety Authority (EFSA Panel on Dietetic Products, Nutrition and Allergies, 2010). The claim states that yogurt should contain at least 108 cfu live starter microorganisms per gram- the same count the NYA requires for the “live and active” seal, as noted above.
Even in the absence of a seal or stamp, many commercial yogurt products, as well as kefir, fermented vegetables, and miso, also provide numerical information on their labels. Recently, Derrien and van Hylckama Vlieg (2015) suggested that consumption of 1010 cells would be necessary to induce an effect on the microbiota and host health. This could be achieved by consuming 100 g of fermented food containing 108 cells/g.
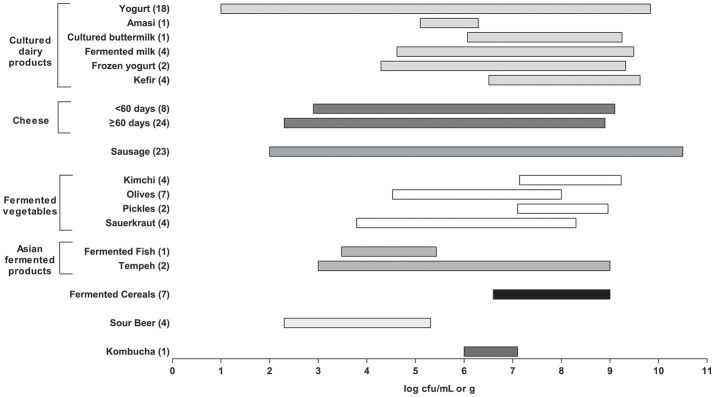
According to the results reported in this survey, many commercial fermented food products would be close to meeting this requirement (Figure 1). However, several caveats are relevant. First, there was a wide range of reported microbial counts (over several logs) within the various product groups. Some products also reported total LAB, whereas other reported specific genera or species or as thermophilic or mesophilic. Second, for most products, enumeration relied on standard cultural methods for LAB (including medium and incubation conditions), which may have under-estimated more fastidious species. This can be attributed to the high stress conditions of fermented products that can occasionally lead to injured microorganisms that are viable but not culturable.
Figure 1.
Summary of lactic acid bacteria (LAB) counts in all fermented foods as reported in Tables 1–8. The bar plots represents a range (minimum to maximum) of counts found across the studies surveyed. The number of studies used here for each fermented food is shown in brackets. Products were excluded if they had no viable counts or when LAB counts were not reported. For yogurt, initial counts were used for products that had counts for more than one timepoint. For cheese, the products were divided by aging time (60 days) and were excluded if aging time was not reported.
Finally, the age or time at which the products were analyzed also varied considerably. In general, “fresher” products had higher numbers. These would include yogurt and cultured dairy products, as well as kimchi, sauerkraut, and other fermented vegetables. The counts from the cheeses also varied widely, with the longer aged cheeses (e.g., Parmesan, Grana) consistently having the lowest counts.
Recommendation of fermented foods as part of dietary guidelines
In many cultures, fermented foods containing live microorganisms are consumed on a regular or even daily basis (Hutkins, 2018). Based on the data reported in this survey, consumption of fermented foods would not only provide important macronutrients, they could also deliver large numbers of potentially beneficial microorganisms to the gastrointestinal tract. For example, if Korean kimchi contains 108 lactic acid bacteria per g (Table 5), and given per capita consumption of kimchi is estimated at 100 g per person per day, then the daily consumption of live microbes from kimchi alone would be 1010. Likewise, in the Netherlands, where yogurt consumption is also around 100 g per day, similar levels of microbes (i.e., 1010 cfu per day) would be ingested. These are the doses noted above that can influence the gut microbiota and provide a potential health benefit (Derrien and van Hylckama Vlieg, 2015).
Recently, the concept of “shared core benefits” was introduced to explain how and why phylogenetically related organisms could deliver similar health benefits (Sanders et al., 2018). Thus, although the microbes in fermented foods cannot, by definition, be considered probiotic, many of them are evolutionarily highly related to probiotic organisms, and they often share the same molecular mechanisms responsible for health-promoting properties in probiotic organisms. The application of various omic approaches to understand functional properties of fermentation-derived microbes will also likely reveal new attributes relevant to the health benefits these microbes may provide (Macori and Cotter, 2018).
In part, this is why several prominent groups have recommended that health care professionals should promote fermented foods containing live microbes as part of public health policy (Ebner et al., 2014; Sanders et al., 2014; Chilton et al., 2015; Bell et al., 2017; Hill et al., 2017). In particular, including fermented foods in dietary guidelines for specific populations has also been recommended. For example, Bell et al. (2018) recently suggested fermented foods should be introduced to children early in life and incorporated into their everyday meal plans. In addition, regular consumption of fermented foods could be especially important for low income, resource-challenged communities that are disproportionally susceptible to gastrointestinal infections (Kort et al., 2015).
Author contributions
SR, CK, and RH each contributed 30% to data collection. MH contributed 10% to data collection. SR, CK, and RH wrote the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was funded by the National Dairy Council and facilitated by the International Scientific Association for Probiotics and Prebiotics. We thank Mary Ellen Sanders for her helpful comments.
References
- Acton J. C., Dick R. L. (1976). Composition of some commercial dry sausages. J. Food Sci. 41, 971–972. 10.1111/j.1365-2621.1976.tb00768_41_4.x [DOI] [Google Scholar]
- Alexopoulos A., Plessas S., Kourkoutas Y., Stefanis C., Vavias S., Voidarou C., et al. (2017). Experimental effect of ozone upon the microbial flora of commercially produced dairy fermented products. Int. J. Food Microbiol. 246, 5–11. 10.1016/j.ijfoodmicro.2017.01.018 [DOI] [PubMed] [Google Scholar]
- Aponte M., Blaiotta G., La Croce F., Mazzaglia A., Farina V., Settanni L., et al. (2012). Use of selected autochthonous lactic acid bacteria for Spanish-style table olive fermentation. Food Microbiol. 30, 8–16. 10.1016/j.fm.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Aquilanti L., Santarelli S., Silvestri G., Osimani A., Petruzzelli A., Clementi F. (2007). The microbial ecology of a typical Italian salami during its natural fermentation. Int. J. Food Microbiol. 120, 136–145. 10.1016/j.ijfoodmicro.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Ayana I. A. A. A., El-Deeb A. M. (2016). Quality enhancement of Edam-like cheese made from goat's milk. Am. J. Food Technol. 11, 44–53. 10.3923/ajft.2016.44.53 [DOI] [Google Scholar]
- Babio N., Becerra-Tomas N., Martinez-Gonzalez M. A., Corella D., Estruch R., Ros E., et al. (2015). Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J. Nutr. 145, 2308–2316. 10.3945/jn.115.214593 [DOI] [PubMed] [Google Scholar]
- Beganović J., Pavunc A. L., Gjuračić K., Špoljarec M., Šušković J., Kos B. (2011). Improved sauerkraut production with probiotic strain Lactobacillus plantarum L4 and Leuconostoc mesenteroides LMG 7954. J. Food Sci. 76, 124–129. 10.1111/j.1750-3841.2010.02030.x [DOI] [PubMed] [Google Scholar]
- Bell V., Ferrão J., Fernandes T. (2017). Nutritional guidelines and fermented food frameworks. Foods 6, 1–17. 10.3390/foods6080065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell V., Ferrão J., Fernandes T. (2018). Fermented food guidelines for children. J. Pediatr. Pediatr. Med. 2, 1–4. [Google Scholar]
- Birollo G. A., Reinheimer J. A., Vinderola C. G. (2000). Viability of lactic acid microflora in different types of yoghurt. Food Res. Int. 33, 799–805. 10.1016/S0963-9969(00)00101-0 [DOI] [Google Scholar]
- Bouton Y., Guyot P., Grappin R. (1998). Preliminary characterization of microflora of Comté cheese. J. Appl. Microbiol. 85, 123–131. 10.1046/j.1365-2672.1998.00476.x [DOI] [PubMed] [Google Scholar]
- Capita R., Llorente-Marigómez S., Prieto M., Alonso-Calleja C. (2006). Microbiological profiles, pH, and titratable acidity of chorizo and salchichón (two Spanish dry fermented sausages) manufactured with ostrich, deer, or pork meat. J. Food Prot. 69, 1183–1189. 10.4315/0362-028X-69.5.1183 [DOI] [PubMed] [Google Scholar]
- Chevallier I., Ammor S., Laguet A., Labayle S., Castanet V., Dufour E., et al. (2006). Microbial ecology of a small-scale facility producing traditional dry sausage. Food Control 17, 446–453. 10.1016/j.foodcont.2005.02.005 [DOI] [Google Scholar]
- Chilton S. N., Burton J. P., Reid G. (2015). Inclusion of fermented foods in food guides around the world. Nutrients 7, 390–404. 10.3390/nu7010390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Lee D., Yang C., Jeon J., Kim J., Han H. (2006). Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol. Lett. 257, 262–267. 10.1111/j.1574-6968.2006.00186.x [DOI] [PubMed] [Google Scholar]
- Choi I. H., Noh J. S., Han J.-S., Kim H. J., Han E.-S., Song Y. O. (2013). Kimchi, a fermented vegetable, improves serum lipid profiles in healthy young adults: randomized clinical trial. J. Med. Food 16, 223–229. 10.1089/jmf.2012.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocolin L., Dolci P., Rantsiou K., Urso R., Cantoni C., Comi G. (2009). Lactic acid bacteria ecology of three traditional fermented sausages produced in the North of Italy as determined by molecular methods. Meat Sci. 82, 125–132. 10.1016/j.meatsci.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Cocolin L., Manzano M., Cantoni C., Comi G. (2001). Denaturing gradient gel electrophoresis analysis of the 16S rRNA gene V1 region to monitor dynamic changes in the bacterial population during fermentation of Italian sausages. Appl. Environ. Microbiol. 67, 5113–5121. 10.1128/AEM.67.11.5113-5121.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi G., Urso R., Iacumin L., Rantsiou K., Cattaneo P., Cantoni C., et al. (2005). Characterisation of naturally fermented sausages produced in the North East of Italy. Meat Sci. 69, 381–382. 10.1016/j.meatsci.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Commonwealth of Australia Gazette (2015). Australia New Zealand Food Standards Code, Amendment No. 154-2015.Commonwealth of Australia Gazette No. FSC 96.
- Coppola R., Giagnacovo B., lorizzo M., Grazia L. (1998). Characterization of lactobacilli involved in the ripening of soppressata molisana, a typical southern Italy fermented sausage. Food Microbiol. 15, 347–353. 10.1006/fmic.1997.0179 [DOI] [Google Scholar]
- Coppola R., Marconi E., Rossi F., Dellaglio F. (1995). Artisanal production of Naples-type salami: chemical and microbiological aspects. Ital. J. Food Sci. 1, 57–61. [Google Scholar]
- Coppola R., Nanni M., Iorizzo M., Sorrentino A., Sorrentino E., Chiavari C., et al. (2000). Microbiological characteristics of Parmigiano Reggiano cheese during the cheesemaking and the first months of the ripening. Lait 80, 479–490. 10.1051/lait:2000139 [DOI] [Google Scholar]
- Coppola S., Mauriello G., Aponte M., Moschetti G., Villani F. (2000). Microbial succession during ripening of Naples-type salami, a southern Italian fermented sausage. Meat Sci. 56, 321–329. 10.1016/S0309-1740(00)00046-2 [DOI] [PubMed] [Google Scholar]
- Coton M., Pawtowski A., Taminiau B., Burgaud G., Deniel F., Coulloumme-Labarthe L., et al. (2017). Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 93:fix048. 10.1093/femsec/fix048 [DOI] [PubMed] [Google Scholar]
- Cuesta P., Fernández-Garcia E., González de Llano D., Montilla A., Rodriguez A. (1996). Evolution of the microbiological and biochemical characteristics of Afuega'l Pitu cheese during ripening. J. Dairy Sci. 79, 1693–1698. [Google Scholar]
- Dal Bello F., Walter J., Hammes W. P., Hertel C. (2003). Increased complexity of the species composition of lactic acid bacteria in human feces revealed by alternative incubation condition. Microb. Ecol. 45, 455–463. 10.1007/s00248-003-2001-z [DOI] [PubMed] [Google Scholar]
- David L. A., Maurice C. F., Carmody R. N., Gootenberg D. B., Button J. E., Wolfe B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M., de Candia S., Calasso M. P., Faccia M., Guinee T. P., Simonetti M. C., et al. (2008). Selection and use of autochthonous multiple strain cultures for the manufacture of high-moisture traditional Mozzarella cheese. Int. J. Food Microbiol. 125, 123–132. 10.1016/j.ijfoodmicro.2008.03.043 [DOI] [PubMed] [Google Scholar]
- De Bellis P., Valerio F., Sisto A., Lonigro S. L., Lavermicocca P. (2010). Probiotic table olives: microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus paracasei IMPC2.1 in an industrial plant. Int. J. Food Microbiol. 140, 6–13. 10.1016/j.ijfoodmicro.2010.02.024 [DOI] [PubMed] [Google Scholar]
- De Noni I., Pellegrino L., Masotti F. (2004). Survey of selected chemical and microbiological characteristics of (plain or sweetened) natural yoghurts from the Italian market. Lait 84, 421–433. 10.1051/lait:2004020 [DOI] [Google Scholar]
- Delamare A. P. L., de Andrade C. C. P., Mandelli F., Chequeller De Almeida R., Echeverrigaray S. (2012). Microbiological, physico-chemical and sensorial characteristics of Serrano, an artisanal Brazilian cheese. Food Nutr. Sci. 3, 1068–1075. 10.4236/fns.2012.38142 [DOI] [Google Scholar]
- Demarigny Y., Beuvier E., Dasen A., Duboz G. (1996). Influence of raw milk microflora on the characteristics of Swiss-type cheeses. I. Evolution of microflora during ripening and characterization of facultatively heterofermentative lactobacilli. Lait 76, 371–387. 10.1051/lait:1996428 [DOI] [Google Scholar]
- Depouilly A., Dufrene F., Beuvier É., Berthier F. (2004). Genotypic characterisation of the dynamics of the lactic acid bacterial population of Comté cheese. Lait 84, 155–167. 10.1051/lait:2003036 [DOI] [Google Scholar]
- Derrien M., van Hylckama Vlieg J. E. T. (2015). Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 23, 354–366. 10.1016/j.tim.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Devirgiliis C., Caravelli A., Coppola D., Barile S., Perozzi G. (2008). Antibiotic resistance and microbial composition along the manufacturing process of Mozzarella di Bufala Campana. Int. J. Food Microbiol. 128, 378–384. 10.1016/j.ijfoodmicro.2008.09.021 [DOI] [PubMed] [Google Scholar]
- Di Cagno R., Chaves Lòpez C., Tofalo R., Gallo G., De Angelis M., Paparella A., et al. (2008). Comparison of the compositional, microbiological, biochemical and volatile profile characteristics of three Italian PDO fermented sausages. Meat Sci. 79, 223–235. 10.1016/j.meatsci.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Dong Y. P., Chen Q., Cui S. H., Li F. Q. (2014). Enumeration, Genetic characterization and antimicrobial susceptibility of lactobacillus and streptococcus isolates from retail yoghurt in Beijing, China. Biomed. Env. Sci 27, 740–748. 10.3967/bes2014.109 [DOI] [PubMed] [Google Scholar]
- Dunlap B. S., Yu H., Elitsur Y. (2009). The probiotic content of commercial yogurts in West Virginia. Clin. Pediatr. 48, 522–527. 10.1177/0009922809331802 [DOI] [PubMed] [Google Scholar]
- Ebner S., Smug L. N., Kneifel W., Salminen S. J., Sanders M. E. (2014). Probiotics in dietary guidelines and clinical recommendations outside the European Union. World J. Gastroenterol. 20, 16095–16100. 10.3748/wjg.v20.i43.16095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergies (2010). Scientific opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion. EFSA J. 8:1763 10.2903/j.efsa.2010.1763 [DOI] [Google Scholar]
- Eliskases-Lechner F., Ginzinger W. (1995). The bacterial flora of surface-ripened cheeses with special regard to coryneforms. Lait 75, 571–584. 10.1051/lait:1995644 [DOI] [Google Scholar]
- Ercolini D., Hill P. J., Dodd C. E. R. (2003). Bacterial community structure and location in Stilton cheese. Appl. Environ. Microbiol. 69, 3540–3548. 10.1128/AEM.69.6.3540-3548.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells J. L., Costilow R. N., Anderson T. E., Bell T. A. (1964). Pure culture fermentation of brined cucumbers. Appl. Microbiol. 12, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V., Barbosa J., Vendeiro S., Mota A., Silva F., Monteiro M. J., et al. (2006). Chemical and microbiological characterization of alheira: a typical Portuguese fermented sausage with particular reference to factors relating to food safety. Meat Sci. 73, 570–575. 10.1016/j.meatsci.2006.02.011 [DOI] [PubMed] [Google Scholar]
- Fitzsimons N. A., Cogan T. M., Condon S., Beresford T. (2001). Spatial and temporal distribution of non-starter lactic acid bacteria in Cheddar cheese. J. Appl. Microbiol. 90, 600–608. 10.1046/j.1365-2672.2001.01285.x [DOI] [PubMed] [Google Scholar]
- Flórez A. B., María López-Díaz T., Alvarez-Martín P., Mayo B. (2006). Microbial characterisation of the traditional Spanish blue-veined Cabrales cheese: identification of dominant lactic acid bacteria. Eur. Food Res. Technol 223, 503–508. 10.1007/s00217-005-0230-8 [DOI] [Google Scholar]
- Franciosi E., Settanni L., Carlin S., Cavazza A., Poznanski E. (2008). A factory-scale application of secondary adjunct cultures selected from lactic acid bacteria during Puzzone di Moena cheese ripening. J. Dairy Sci. 91, 2981–2991. 10.3168/jds.2007-0764 [DOI] [PubMed] [Google Scholar]
- Frye C. P., Kilara A. (2016). Regulations for product standards and labeling, in Dairy Processing and Quality Assurance, eds Chandan R. C., Kilara A., Shah N. P. (Chichester: John Wiley & Sons, Ltd; ), 152–177. [Google Scholar]
- García Fontán M. C., Lorenzo J. M., Martínez S., Franco I., Carballo J. (2007a). Microbiological characteristics of Botillo, a Spanish traditional pork sausage. LWT Food Sci. Technol. 40, 1610–1622. 10.1016/j.lwt.2006.10.007 [DOI] [PubMed] [Google Scholar]
- García Fontán M. C., Lorenzo J. M., Parada A., Franco I., Carballo J. (2007b). Microbiological characteristics of androlla, a Spanish traditional pork sausage. Food Microbiol. 24, 52–58. 10.1016/j.fm.2006.03.007 [DOI] [PubMed] [Google Scholar]
- García-Cayuela T., Tabasco R., Peláez C., Requena T. (2009). Simultaneous detection and enumeration of viable lactic acid bacteria and bifidobacteria in fermented milk by using propidium monoazide and real-time PCR. Int. Dairy J. 19, 405–409. 10.1016/j.idairyj.2009.02.001 [DOI] [Google Scholar]
- Garcia M. C., Otero A., Garcia M. L., Moreno B. (1987). Microbiological quality and composition of two types of Spanish sheep's milk cheeses (Manchego and Burgos varieties). J. Dairy Res. 54, 551–557. [Google Scholar]
- Gatti M., Fornasari M. E., Mucchetti G., Addeo F., Neviani E. (1999). Presence of peptidase activities in different varieties of cheese. Lett. Appl. Microbiol. 28, 368–372. 10.1046/j.1365-2672.1999.00541.x [DOI] [PubMed] [Google Scholar]
- Gatti M., Lindner J. D. D., De Lorentiis A., Bottari B., Santarelli M., Bernini V., et al. (2008). Dynamics of whole and lysed bacterial cells during Parmigiano-Reggiano cheese production and ripening. Appl. Environ. Microbiol. 74, 6161–6167. 10.1128/AEM.00871-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreselassie N., Abay F., Beyene F. (2016). Biochemical and molecular identification and characterization of lactic acid bacteria and yeasts isolated from Ethiopian naturally fermented buttermilk. J. Food Sci. Technol. 53, 184–196. 10.1007/s13197-015-2049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genigeorgis C., Carniciu M., Dutulescu D., Farver T. B. (1991). Growth and survival of Listeria monocytogenes in market cheeses stored at 4 to 30°C. J. Food Prot. 54, 662–668. 10.4315/0362-028X-54.9.662 [DOI] [PubMed] [Google Scholar]
- Gobbetti M., Burzigotti R., Smacchi E., Corsetti A., De Angelis M. (1997). Microbiology and Biochemistry of Gorgonzola cheese during ripening. Int. Dairy J. 7, 519–529. 10.1016/S0958-6946(97)00047-2 [DOI] [Google Scholar]
- Golomb B. L., Morales V., Jung A., Yau B., Boundy-Mills K. L., Marco M. L. (2013). Effects of pectinolytic yeast on the microbial composition and spoilage of olive fermentations. Food Microbiol. 33, 97–106. 10.1016/j.fm.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Gori K., Ryssel M., Arneborg N., Jespersen L. (2013). Isolation and identification of the microbiota of Danish farmhouse and industrially produced surface-ripened cheeses. Microb. Ecol. 65, 602–615. 10.1007/s00248-012-0138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf D., Di Cagno R., Fåk F., Flint H. J., Nyman M., Saarela M., et al. (2015). Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 26:26164. 10.3402/mehd.v26.26164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greppi A., Ferrocino I., La Storia A., Rantsiou K., Ercolini D., Cocolin L. (2015). Monitoring of the microbiota of fermented sausages by culture independent rRNA-based approaches. Int. J. Food Microbiol. 212, 67–75. 10.1016/j.ijfoodmicro.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Grønnevik H., Falstad M., Narvhus J. A. (2011). Microbiological and chemical properties of Norwegian kefir during storage. Int. Dairy J. 21, 601–606. 10.1016/j.idairyj.2011.01.001 [DOI] [Google Scholar]
- Gueimonde M., Delgado S., Mayo B., Ruas-Madiedo P., Margolles A., De Los Reyes-Gavil C. G. (2004). Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res. Int. 37, 839–850. 10.1016/j.foodres.2004.04.006 [DOI] [Google Scholar]
- Hamann W. T., Marth E. H. (1984). Survival of Streptococcus thermophilus and Lactobacillus bulgaricus in commercial and experimental yogurts. J. Food Prot. 47, 781–786. 10.4315/0362-028X-47.10.781 [DOI] [PubMed] [Google Scholar]
- Hekmat S., Koba L. (2006). Fermented dairy products: knowledge and consumption. Can. J. Diet. Pract. Res. 67, 199–201. 10.3148/67.4.2006.199 [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G. R., Merenstein D. J., Pot B., et al. (2014). Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Hill D., Sugrue I., Arendt E., Hill C., Stanton C., Ross R. P. (2017). Recent advances in microbial fermentation for dairy and health. F1000Res. 6:751. 10.12688/f1000research.10896.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounhouigan D. J., Nout M. J. R., Nago C. M., Houben J. H., Rombouts F. M. (1993). Composition and microbiological and physical attributes of mawè a fermented dough from Benin. Int. J. Food Sci. Technol. 28, 513–517. 10.1111/j.1365-2621.1993.tb01300.x [DOI] [Google Scholar]
- Hutkins R. W. (2018). Microbiology and Technology of Fermented Foods, 2nd Edn Hoboken, NJ: Wiley. [Google Scholar]
- Ibrahim S. A., Carr J. P. (2006). Viability of bifidobacteria in commercial yogurt products in North Carolina during refrigerated storage. Int. J. Dairy Technol. 59, 272–277. 10.1111/j.1471-0307.2006.00282.x [DOI] [Google Scholar]
- Iwana H., Masuda H., Fujisawa T., Suzuki H., Mitsuoka T. (1993). Isolation and identification of Bifidobacterium spp. in commercial yogurts sold in Europe. Bifidobact. Microflora 12, 39–45. 10.12938/bifidus1982.12.1_39 [DOI] [Google Scholar]
- Jayashree S., Pushpanathan M., Rajendhran J., Gunasekaran P. (2013). Microbial diversity and phylogeny analysis of buttermilk, a fermented milk product, employing 16S rRNA-based pyrosequencing. Food Biotechnol. 27, 213–221. 10.1080/08905436.2013.811084 [DOI] [Google Scholar]
- Johanningsmeier S. D., Fleming H. P., Breidt F., Jr. (2004). Malolactic activity of lactic acid bacteria during sauerkraut fermentation. J. Food Sci. 69, M222–M227. 10.1111/j.1365-2621.2004.tb09891.x [DOI] [Google Scholar]
- Johnson A. J. (2016). Artisanal food microbiology. Nat. Microbiol. 1:16039. 10.1038/nmicrobiol.2016.39 [DOI] [PubMed] [Google Scholar]
- Jordan K. N., Cogan T. M. (1993). Identification and growth of non-starter lactic acid bacteria in Irish Cheddar cheese. Irish J. Agric. Food Res. 32, 47–55. [Google Scholar]
- Kalamaki M. S., Angelidis A. S. (2017). Isolation and molecular identification of yeasts in Greek kefir. Int. J. Dairy Technol. 70, 261–268. 10.1111/1471-0307.12329 [DOI] [Google Scholar]
- Kesmen Z., Kacmaz N. (2011). Determination of lactic microflora of kefir grains and kefir beverage by using culture-dependent and culture-independent methods. J. Food Sci. 76, M276–M283. 10.1111/j.1750-3841.2011.02191.x [DOI] [PubMed] [Google Scholar]
- Kim D. H., Chon J. W., Kim H., Kim H. S., Choi D., Hwang D. G., et al. (2015). Detection and enumeration of lactic acid bacteria, acetic acid bacteria and yeast in kefir grain and milk using quantitative real-time PCR. J. Food Saf. 35, 102–107. 10.1111/jfs.12153 [DOI] [Google Scholar]
- Kim E. K., An S. Y., Lee M. S., Kim T. H., Lee H. K., Hwang W. S., et al. (2011). Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr. Res. 31, 436–443. 10.1016/j.nutres.2011.05.011 [DOI] [PubMed] [Google Scholar]
- Kim H.-Y., Bong Y.-J., Jeong J.-K., Lee S., Kim B.-Y., Park K.-Y. (2016). Heterofermentative lactic acid bacteria dominate in Korean commercial kimchi. Food Sci. Biotechnol. 25, 541–545. 10.1007/s10068-016-0075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Ju S., Park Y. K. (2017). Kimchi intake and atopic dermatitis in Korean aged 19-49 years: The Korea National Health and Nutrition Examination Survey 2010-2012. Asia Pac. J. Clin. Nutr. 26, 914–922. 10.6133/apjcn.022017 [DOI] [PubMed] [Google Scholar]
- Kok C., Hutkins R. W. (in press). Yogurt other fermented foods as a source of health-promoting bacteria. Nutr. Rev. [DOI] [PubMed] [Google Scholar]
- Kolars J. C., Michael D. L., Aouji M., Savaiano D. A. (1984). Yogurt - an autodigesting source of lactose. N. Engl. J. Med. 310, 1–3. 10.1056/NEJM198401053100101 [DOI] [PubMed] [Google Scholar]
- Kort R., Westerik N., Mariela Serrano L., Douillard F. P., Gottstein W., Mukisa I. M., et al. (2015). A novel consortium of Lactobacillus rhamnosus and Streptococcus thermophilus for increased access to functional fermented foods. Microb. Cell Fact. 14:195. 10.1186/s12934-015-0370-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosikowski F. V. (1981). Properties of commercial flavored frozen yogurts. J. Food Prot. 44, 853–856. 10.4315/0362-028X-44.11.853 [DOI] [PubMed] [Google Scholar]
- Kung H. F., Lee Y. H., Teng D. F., Hsieh P. C., Wei C. I., Tsai Y. H. (2006a). Histamine formation by histamine-forming bacteria and yeast in mustard pickle products in Taiwan. Food Chem. 99, 579–585. 10.1016/j.foodchem.2005.08.025 [DOI] [Google Scholar]
- Kung H. F., Tsai Y. H., Wei C. I. (2006b). Histamine and other biogenic amines and histamine-forming bacteria in miso products. Food Chem. 101, 351–356. 10.1016/j.foodchem.2005.12.057 [DOI] [Google Scholar]
- Kwon D. Y., Daily J. W., III., Kim H. J., Park S. (2010). Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 30, 1–13. 10.1016/j.nutres.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Larsson S. C., Andersson S., Johansson J. E., Wolk A. (2008). Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am. J. Clin. Nutr. 88, 1083–1087. 10.1093/ajcn/88.4.1083 [DOI] [PubMed] [Google Scholar]
- Laye I., Karleskind D., Morr C. V. (1993). Chemical, microbiological and sensory properties of plain nonfat yogurt. J. Food Sci. 58, 991–995. 10.1111/j.1365-2621.1993.tb06096.x [DOI] [Google Scholar]
- Leblanc J. G., Laiño J. E., del Valle M. J., Vannini V., van Sinderen D., Taranto M. P., et al. (2011). B-group vitamin production by lactic acid bacteria - current knowledge and potential applications. J. Appl. Microbiol. 111, 1297–1309. 10.1111/j.1365-2672.2011.05157.x [DOI] [PubMed] [Google Scholar]
- Lee K. E., Cho U. H., Ji G. E. (1996). Effect of kimchi intake on the composition of human large intestinal bacteria. Korean J. Food Sci. Technol. 28, 981–986. [Google Scholar]
- Lee M., Song J. H., Jung M. Y., Lee S. H., Chang J. Y. (2017). Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiol. 66, 173–183. 10.1016/j.fm.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Lee M., Song J. H., Lee S. H., Jung M. Y., Chang J. Y. (2018). Effect of seasonal production on bacterial communities in Korean industrial kimchi fermentation. Food Control 91, 381–389. 10.1016/j.foodcont.2018.04.023 [DOI] [Google Scholar]
- Lee Y., Cha Y. S., Park Y., Lee M. (2017). PPARγ2 C1431T polymorphism interacts with the antiobesogenic effects of Kochujang, a Korean fermented, soybean-based red pepper paste, in overweight/obese subjects: a 12-week, double-blind randomized clinical trial. J. Med. Food 20, 610–617. 10.1089/jmf.2016.3911 [DOI] [PubMed] [Google Scholar]
- Lei V., Jakobsen M. (2004). Microbiological characterization and probiotic potential of koko and koko sour water, African spontaneously fermented millet porridge and drink. J. Appl. Microbiol. 96, 384–397. 10.1046/j.1365-2672.2004.02162.x [DOI] [PubMed] [Google Scholar]
- Linares D. M., Gómez C., Renes E., Fresno J. M., Tornadijo M. E., Ross R. P., et al. (2017). Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 8:846. 10.3389/fmicb.2017.00846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko D., Johnston G., Johnston C. (2017). Effects of dietary yogurt on the healthy human gastrointestinal (GI) microbiome. Microorganisms 5:E6. 10.3390/microorganisms5010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M. C., Medina L. M., Jordano R. (1998). Survival of lactic acid bacteria in commercial frozen yogurt. J. Food Sci. 63, 706–708. 10.1111/j.1365-2621.1998.tb15818.x [DOI] [Google Scholar]
- Lourens-Hattingh A., Viljoen B. C. (2002). Survival of probiotic bacteria in South African commercial bio-yogurt. S. Afr. J. Sci. 98, 298–300. Available online at: http://hdl.handle.net/10520/EJC97483 [Google Scholar]
- Lozupone C. A., Li M., Campbell T. B., Flores S. C., Linderman D., Gebert M. J., et al. (2013). Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14, 329–339. 10.1016/j.chom.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Breidt F., Plengvidhya V., Fleming H. P. (2003). Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69, 3192–3202. 10.1128/AEM.69.6.3192-3202.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macori G., Cotter P. D. (2018). Novel insights into the microbiology of fermented dairy foods. Curr. Opin. Biotechnol. 49, 172–178. 10.1016/j.copbio.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Madkor S. A., Tong P. S., El Soda M. (2000). Ripening of Cheddar cheese with added attenuated adjunct cultures of lactobacilli. J. Dairy Sci. 83, 1684–1691. 10.3168/jds.S0022-0302(00)75037-5 [DOI] [PubMed] [Google Scholar]
- Marco M. L., Golomb B. L. (2016). Fermented foods, Lactobacillus, and health. Microbe 11, 349–354. 10.1128/microbe.11.349.1 [DOI] [Google Scholar]
- Marco M. L., Heeney D., Binda S., Cifelli C. J., Cotter P. D., Foligné B., et al. (2017). Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 44, 94–102. 10.1016/j.copbio.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Martens H., Dawoud E., Verachtert H. (1991). Wort enterobacteria and other microbial populations involved during the first month of lambic fermentation. J. Insitute Brew. 97, 435–439. 10.1002/j.2050-0416.1991.tb01082.x [DOI] [Google Scholar]
- Martini M. C., Bollweg G. L., Levitt M. D., Savaiano D. A. (1987). Lactose digestion by yogurt beta-galactosidase: influence of pH and microbial cell integrity. Am. J. Clin. Nutr. 45, 432–436. 10.1093/ajcn/45.2.432 [DOI] [PubMed] [Google Scholar]
- Medina L. M., Jordano R. (1994). Survival of constitutive microflora in commercially fermented milk containing bifidobacteria during refrigerated storage. J. Food Prot. 57, 731–733. 10.4315/0362-028X-57.8.731 [DOI] [PubMed] [Google Scholar]
- Messens W., Estepar-Garcia J., Dewettinck K., Huyghebaert A. (1999). Proteolysis of high-pressure-treated Gouda cheese. Int. Dairy J. 9, 775–782. 10.1016/S0958-6946(99)00152-1 [DOI] [Google Scholar]
- Micanel N., Haynes I. N., Playne M. J. (1997). Viability of probiotic cultures in commercial Australian yogurts. Aust. J. Dairy Technol. 52, 24–27. [Google Scholar]
- Mohammadmoradi S., Javidan A., Kordi J., Goudarzi M. H. (2015). Comparing the effect of ultra-filtered feta cheese and yoghurt as probiotic carriers on lipid profile: a double blinded randomized controlled trial. Med. J. Nutr. Metab. 8, 27–36. 10.3233/MNM-140026 [DOI] [Google Scholar]
- Monfredini L., Settanni L., Poznanski E., Cavazza A., Franciosi E. (2012). The spatial distribution of bacteria in Grana-cheese during ripening. Syst. Appl. Microbiol. 35, 54–63. 10.1016/j.syapm.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Mounier J., Goerges S., Gelsomino R., Vancanneyt M., Vandemeulebroecke K., Hoste B., et al. (2006). Sources of the adventitious microflora of a smear-ripened cheese. J. Appl. Microbiol. 101, 668–681. 10.1111/j.1365-2672.2006.02922.x [DOI] [PubMed] [Google Scholar]
- Mounier J., Monnet C., Jacques N., Antoinette A., Irlinger F. (2009). Assessment of the microbial diversity at the surface of Livarot cheese using culture-dependent and independent approaches. Int. J. Food Microbiol. 133, 31–37. 10.1016/j.ijfoodmicro.2009.04.020 [DOI] [PubMed] [Google Scholar]
- Moyane J. N., Jideani A. I. O. (2013). The physicochemical and sensory evaluation of commercial sour milk (amasi) products. Afr. J. Food Sci. 7, 56–62. 10.5897/AJFS12.089 [DOI] [Google Scholar]
- Mozaffarian D., Hao T., Rimm E. B., Willett W. C., Hu F. B. (2011). Changes in diet and lifestyle and long- term weight gain in women and men. N. Engl. J. Med. 364, 2392–2404. 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugula J. K., Nnko S. A. M., Narvhus J. A., Sørhaug T. (2003). Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 80, 187–199. 10.1016/S0168-1605(02)00141-1 [DOI] [PubMed] [Google Scholar]
- Muyanja C. M. B. K., Narvhus J. A., Treimo J., Langsrud T. (2003). Isolation, characterisation and identification of lactic acid bacteria from bushera: a Ugandan traditional fermented beverage. Int. J. Food Microbiol. 80, 201–210. 10.1016/S0168-1605(02)00148-4 [DOI] [PubMed] [Google Scholar]
- Nout M. J. R., de Dreu M. A., Zuurbier A. M., Bonants-van Laarhoven T. M. G. (1987). Ecology of controlled soyabean acidification for tempe manufacture. Food Microbiol. 4, 165–172. 10.1016/0740-0020(87)90032-3 [DOI] [Google Scholar]
- Nozue M., Shimazu T., Sasazuki S., Charvat H., Mori N., Mutoh M., et al. (2017). Fermented soy product intake is inversely associated with the development of high blood pressure: the Japan public health center-based prospective study. J. Nutr. 147, 1749–1756. 10.3945/jn.117.250282 [DOI] [PubMed] [Google Scholar]
- Nuñez M. (1978). Microflora of cabrales cheese: changes during maturation. J. Dairy Res. 45, 501–508. 10.1017/S0022029900016721 [DOI] [Google Scholar]
- Nuobariene L., Cizeikiene D., Gradzeviciute E., Hansen Å. S., Rasmussen S. K., Juodeikiene G., et al. (2015). Phytase-active lactic acid bacteria from sourdoughs: isolation and identification. LWT Food Sci. Technol. 63, 766–772. 10.1016/j.lwt.2015.03.018 [DOI] [Google Scholar]
- OBrien K., Aryana K., Prinyawiwatkul W., Carabante Ordonez K., Boeneke C. (2016). Short communication: the effects of frozen storage on the survival of probiotic microorganisms found in traditionally and commercially manufactured kefir. J. Dairy Sci. 99, 7043–7048. 10.3168/jds.2015-10284 [DOI] [PubMed] [Google Scholar]
- Omar N. B., Ampe F. (2000). Microbial community dynamics during production of the Mexican fermented maize dough pozol. Appl. Environ. Microbiol. 66, 3664–3673. 10.1128/AEM.66.9.3664-3673.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T., Yanagida F., Tsuji M., Shinohara T., Yokotsuka K. (2003). Time series analysis of aerobic bacterial flora during miso fermentation. Lett. Appl. Microbiol. 37, 162–168. 10.1046/j.1472-765X.2003.01371.x [DOI] [PubMed] [Google Scholar]
- Owusu-Kwarteng J., Akabanda F., Nielsen D. S., Tano-Debrah K., Glover R. L. K., Jespersen L. (2012). Identification of lactic acid bacteria isolated during traditional fura processing in Ghana. Food Microbiol. 32, 72–78. 10.1016/j.fm.2012.04.010 [DOI] [PubMed] [Google Scholar]
- Panagou E. Z., Schillinger U., Franz C. M. A. P., Nychas G. J. E. (2008). Microbiological and biochemical profile of cv. conservolea naturally black olives during controlled fermentation with selected strains of lactic acid bacteria. Food Microbiol. 25, 348–358. 10.1016/j.fm.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Panahi S., Fernandez M., Marette A., Tremblay A. (2016). Yogurt, diet quality and lifestyle factors. Eur. J. Clin. Nutr. 71, 573–579. 10.1038/ejcn.2016.214 [DOI] [PubMed] [Google Scholar]
- Papamanoli E., Tzanetakis N., Litopoulou-Tzanetaki E., Kotzekidou P. (2003). Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 65, 859–867. 10.1016/S0309-1740(02)00292-9 [DOI] [PubMed] [Google Scholar]
- Parente E., Martuscelli M., Gardini F. (2001). Evolution of microbial populations and biogenic amine production in dry sausages produced in Southern Italy. J. Appl. Microbiol. 90, 882–891. 10.1046/j.1365-2672.2001.01322.x [DOI] [PubMed] [Google Scholar]
- Park S., Bae J. H. (2016). Fermented food intake is associated with a reduced likelihood of atopic dermatitis in an adult population (Korean National Health and Nutrition Examination Survey 2012-2013). Nutr. Res. 36, 125–133. 10.1016/j.nutres.2015.11.011 [DOI] [PubMed] [Google Scholar]
- Pelletier X., Laure-Boussuge S., Donazzolo Y. (2001). Hydrogen excretion upon ingestion of dairy products in lactose- intolerant male subjects : importance of the live flora. Eur. J. Clin. Nutr. 55, 509–512. 10.1038/sj.ejcn.1601169 [DOI] [PubMed] [Google Scholar]
- Pisacane V., Callegari M. L., Puglisi E., Dallolio G., Rebecchi A. (2015). Microbial analyses of traditional Italian salami reveal microorganisms transfer from the natural casing to the meat matrix. Int. J. Food Microbiol. 207, 57–65. 10.1016/j.ijfoodmicro.2015.04.029 [DOI] [PubMed] [Google Scholar]
- Pisano M. B., Scano P., Murgia A., Cosentino S., Caboni P. (2016). Metabolomics and microbiological profile of Italian mozzarella cheese produced with buffalo and cow milk. Food Chem. 192, 618–624. 10.1016/j.foodchem.2015.07.061 [DOI] [PubMed] [Google Scholar]
- Połka J., Rebecchi A., Pisacane V., Morelli L., Puglisi E. (2015). Bacterial diversity in typical Italian salami at different ripening stages as revealed by high-throughput sequencing of 16S rRNA amplicons. Food Microbiol. 46, 342–356. 10.1016/j.fm.2014.08.023 [DOI] [PubMed] [Google Scholar]
- Poveda J. M., Sousa M. J., Cabezas L., McSweeney P. L. H. (2003). Preliminary observations on proteolysis in Manchego cheese made with a defined-strain starter culture and adjunct starter (Lactobacillus plantarum) or a commercial starter. Int. Dairy J. 13, 169–178. 10.1016/S0958-6946(02)00150-4 [DOI] [Google Scholar]
- Praagman J., Dalmeijer G. W., van der Schouw Y. T., Soedamah-Muthu S. S., Monique Verschuren W. M., Bas Bueno-de-Mesquita H., et al. (2015). The relationship between fermented food intake and mortality risk in the European prospective investigation into cancer and nutrition-Netherlands cohort. Br. J. Nutr. 113, 498–506. 10.1017/S0007114514003766 [DOI] [PubMed] [Google Scholar]
- Rantsiou K., Urso R., Dolci P., Comi G., Cocolin L. (2008). Microflora of feta cheese from four Greek manufacturers. Int. J. Food Microbiol. 126, 36–42. 10.1016/j.ijfoodmicro.2008.04.031 [DOI] [PubMed] [Google Scholar]
- Rantsiou K., Urso R., Iacumin L., Cantoni C., Cattaneo P., Comi G., et al. (2005). Culture-dependent and -independent methods to investigate the microbial ecology of Italian fermented sausages. Appl. Environ. Microbiol. 71, 1977–1986. 10.1128/AEM.71.4.1977-1986.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravula R. R., Shah N. P. (1998). Selective enumeration of Lactobacillus casei from yogurts and fermented milk drinks. Biotechnol. Tech. 12, 819–822. 10.1023/A:1008829004888 [DOI] [Google Scholar]
- Rebecchi A., Crivori S., Sarra P. G., Cocconcelli P. S. (1998). Physiological and molecular techniques for the study of bacterial community development in sausage fermentation. J. Appl. Microbiol. 84, 1043–1049. 10.1046/j.1365-2672.1998.00442.x [DOI] [PubMed] [Google Scholar]
- Renye J. A., Somkuti G. A., Vallejo-Cordoba B., Van Hekken D. L., Gonzalez-Cordova A. F. (2008). Characterization of the microflora isolated from Queso fresco made from raw and pasteurized milk. J. Food Saf. 28, 59–75. 10.1111/j.1745-4565.2007.00095.x [DOI] [Google Scholar]
- Romeo F. V., Piscopo A., Mincione A., Poiana M. (2012). Quality evaluation of different typical table olive preparations (cv Nocellara del Belice). Grasas y Aceites 63, 19–25. 10.3989/gya.058511 [DOI] [Google Scholar]
- Ruiz-Barba J. L., Jiménez-Díaz R. (2012). A novel Lactobacillus pentosus-paired starter culture for Spanish-style green olive fermentation. Food Microbiol. 30, 253–259. 10.1016/j.fm.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Samelis J., Kakouri A. (2007). Microbial and safety qualities of PDO Galotyri cheese manufactured at the industrial or artisan scale in Epirus, Greece. Ital. J. Food Sci. 19, 81–90. [Google Scholar]
- Samelis J., Stavropoulos S., Kakouri A., Metaxopoulos J. (1994). Quantification and characterization of microbial populations associated with naturally fermented Greek dry salami. Food Microbiol. 11, 447–460. 10.1006/fmic.1994.1050 [DOI] [PubMed] [Google Scholar]
- Samson R. A., Van Kooij J. A., De Boer E. (1987). Microbiological quality of commercial tempeh in the Netherlands. J. Food Prot. 50, 92–94. 10.4315/0362-028X-50.2.92 [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Benson A., Lebeer S., Merenstein D. J., Klaenhammer T. R. (2018). Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr. Opin. Biotechnol. 49, 207–216. 10.1016/j.copbio.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Lenoir-Wijnkoop I., Salminen S., Merenstein D. J., Gibson G. R., Petschow B. W., et al. (2014). Probiotics and prebiotics: prospects for public health and nutritional recommendations. Ann. N. Y. Acad. Sci. 1309, 19–29. 10.1111/nyas.12377 [DOI] [PubMed] [Google Scholar]
- Santarelli M., Bottari B., Lazzi C., Neviani E., Gatti M. (2013). Survey on the community and dynamics of lactic acid bacteria in Grana Padano cheese. Syst. Appl. Microbiol. 36, 593–600. 10.1016/j.syapm.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Saubade F., Hemery Y. M., Guyot J. P., Humblot C. (2017). Lactic acid fermentation as a tool for increasing the folate content of foods. Crit. Rev. Food Sci. Nutr. 57, 3894–3910. 10.1080/10408398.2016.1192986 [DOI] [PubMed] [Google Scholar]
- Savaiano D. A. (2014). Lactose digestion from yogurt: mechanism and relevance. Am. J. Clin. Nutr. 99, 1251S−1255S. 10.3945/ajcn.113.073023 [DOI] [PubMed] [Google Scholar]
- Schmidt K. A., Kim J., Jeon I. J. (1997). Composition of carbohydrates and concentration of β-galactosidase of commercial frozen yogurt. J. Food Qual. 20, 349–358. 10.1111/j.1745-4557.1997.tb00478.x [DOI] [Google Scholar]
- Scourboutakos M. J., Franco-Arellano B., Murphy S. A., Norsen S., Comelli E. M., L'Abbé M. R. (2017). Mismatch between probiotic benefits in trials versus food products. Nutrients 9:E400 10.3390/nu9040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N. P., Ali J. F., Ravula R. R. (2000). Populations of Lactobacillus acidophilus, Bifidobacterium spp., and Lactobacillus casei in commercial fermented milk products. Biosci. Microflora 19, 35–39. 10.12938/bifidus1996.19.35 [DOI] [Google Scholar]
- Shah N. P., Lankaputhra W. E. V., Britzb M. L., Kyle W. S. A. (1995). Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in commercial yoghurt during refrigerated storage. Int. Dairy J. 5, 515–521. 10.1016/0958-6946(95)00028-2 [DOI] [Google Scholar]
- Shin H. S., Lee J. H., Pestka J. J., Ustunol Z. (2000). Viability of bifidobacteria in commercial dairy products during refrigerated storage. J. Food Prot. 63, 327–331. 10.4315/0362-028X-63.3.327 [DOI] [PubMed] [Google Scholar]
- Silva T., Reto M., Sol M., Peito A., Peres C. M., Peres C., et al. (2011). Characterization of yeasts from Portuguese brined olives, with a focus on their potentially probiotic behavior. LWT Food Sci. Technol. 44, 1349–1354. 10.1016/j.lwt.2011.01.029 [DOI] [Google Scholar]
- Silvestri G., Santarelli S., Aquilanti L., Beccaceci A., Osimani A., Tonucci F., et al. (2007). Investigation of the microbial ecology of Ciauscolo, a traditional Italian salami, by culture-dependent techniques and PCR-DGGE. Meat Sci. 77, 413–423. 10.1016/j.meatsci.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Singh A. K., Ramesh A. (2008). Succession of dominant and antagonistic lactic acid bacteria in fermented cucumber: insights from a PCR-based approach. Food Microbiol. 25, 278–287. 10.1016/j.fm.2007.10.010 [DOI] [PubMed] [Google Scholar]
- Slashinski M. J., McCurdy S. A., Achenbaum L. S., Whitney S. N., McGuire A. L. (2012). Snake-oil,” “quack medicine,” and “industrially cultured organisms:” biovalue and the commercialization of human microbiome research. BMC Med. Ethics 13:28 10.1186/1472-6939-13-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smug L. N., Salminen S., Sanders M. E., Ebner S. (2014). Yoghurt and probiotic bacteria in dietary guidelines of the member states of the European Union. Benef. Microbes 5, 61–66. 10.3920/BM2013.0050 [DOI] [PubMed] [Google Scholar]
- Sommer F., Anderson J. M., Bharti R., Raes J., Rosenstiel P. (2017). The resilience of the intestinal microbiota influences health and disease. Nat. Microbiol. 15, 630–638. 10.1038/nrmicro.2017.58 [DOI] [PubMed] [Google Scholar]
- Sonnenburg J. L., Bäckhed F. (2016). Diet–microbiota interactions as moderators of human metabolism. Nature 535, 56–64. 10.1038/nature18846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaels F., Kerrebroeck S., Snauwaert I., Aerts M., Landschoot A., et al. (2015a). Microbiota and metabolites of aged bottled gueuze beers converge to the same composition. Food Microbiol. 47, 1–11. 10.1016/j.fm.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Spitaels F., Wieme A. D., Janssens M., Aerts M., Daniel H. M., Van Landschoot A., et al. (2014). The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 9:e95384. 10.1371/journal.pone.0095384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaels F., Wieme A. D., Janssens M., Aerts M., Van Landschoot A., De Vuyst L., et al. (2015b). The microbial diversity of an industrially produced lambic beer shares members of a traditionally produced one and reveals a core microbiota for lambic beer fermentation. Food Microbiol. 49, 23–32. 10.1016/j.fm.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Stanczak M., Heuberger R. (2009). Assessment of the knowledge and beliefs regarding probiotic use. Am. J. Heal. Educ. 40, 207–211. 10.1080/19325037.2009.10599095 [DOI] [Google Scholar]
- Talwalkar A., Kailasapathy K. (2004). Comparison of selective and differential media for the accurate enumeration of strains of Lactobacillus acidophilus, Bifidobacterium spp. and Lactobacillus casei complex from commercial yoghurts. Int. Dairy J. 14, 143–149. 10.1016/S0958-6946(03)00172-9 [DOI] [Google Scholar]
- Tamang J. P., Watanabe K., Holzapfel W. H. (2016). Review: diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 7:377. 10.3389/fmicb.2016.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W. (2003). Probiotics: time for a dose of realism. Curr. Issues Intest. Microbiol. 4, 33–42. 10.3920/BM2016.0140 [DOI] [PubMed] [Google Scholar]
- Teoh A. L., Heard G., Cox J. (2004). Yeast ecology of Kombucha fermentation. Int. J. Food Microbiol. 95, 119–126. 10.1016/j.ijfoodmicro.2003.12.020 [DOI] [PubMed] [Google Scholar]
- Tharmaraj N., Shah N. P. (2003). Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and Propionibacteria. J. Dairy Sci. 86, 2288–2296. 10.3168/jds.S0022-0302(03)73821-1 [DOI] [PubMed] [Google Scholar]
- Tieszen K. M., Baer R. J. (1989). Composition and microbiological quality of frozen yogurts. Cult. Dairy Prod. J. 24, 11–14. [Google Scholar]
- Tornadijo M., Fresno J., Bernardo A., Martin Sarmiento R., Carballo J. (1995). Microbiological changes throughout the manufacturing and ripening of a Spanish goat's raw milk cheese (Armada variety). Lait 75, 551–570. [Google Scholar]
- Tou E. H., Guyot J. P., Mouquet-Rivier C., Rochette I., Counil E., Traoré A. S., et al. (2006). Study through surveys and fermentation kinetics of the traditional processing of pearl millet (Pennisetum glaucum) into ben-saalga, a fermented gruel from Burkina Faso. Int. J. Food Microbiol. 106, 52–60. 10.1016/j.ijfoodmicro.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Tsai Y. H., Kung H. F., Lin Q. L., Hwang J. H., Cheng S. H., Wei C. I., et al. (2005). Occurrence of histamine and histamine-forming bacteria in kimchi products in Taiwan. Food Chem. 90, 635–641. 10.1016/j.foodchem.2004.04.024 [DOI] [Google Scholar]
- Tsuda H., Kubota K., Matsumoto T., Ishimi Y. (2012). Isolation and identification of Lactic Acid Bacteria in traditional fermented sushi, Funazushi, from Japan. Food Sci. Technol. Res. 18, 77–82. 10.3136/fstr.18.77 [DOI] [Google Scholar]
- Van Hoorde K., Verstraete T., Vandamme P., Huys G. (2008). Diversity of lactic acid bacteria in two Flemish artisan raw milk Gouda-type cheeses. Food Microbiol. 25, 929–935. 10.1016/j.fm.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Vasavada P. C., White C. H. (1979). Quality of commercial buttermilk. J. Dairy Sci. 62, 802–806. 10.3168/jds.S0022-0302(79)83329-9 [DOI] [Google Scholar]
- Viander B., Aki M. M., Palva A. (2003). Impact of low salt concentration, salt quality on natural large-scale sauerkraut fermentation. Food Microbiol. 20, 391–395. 10.1016/S0740-0020(02)00150-8 [DOI] [Google Scholar]
- Viljoen B. C., Khoury A. R., Hattingh A. (2003). Seasonal diversity of yeasts associated with white-surface mould-ripened cheeses. Food Res. Int. 36, 275–283. 10.1016/S0963-9969(02)00169-2 [DOI] [Google Scholar]
- Vinderola C. G., Reinheimer J. A. (2000). Enumeration of Lactobacillus casei in the presence of L. acidophilus bifidobacteria and lactic starter bacteria in fermented dairy products. Int. Dairy, J. 10, 271–275. 10.1016/S0958-6946(00)00045-5 [DOI] [Google Scholar]
- Walter J., Hertel C., Tannock G. W., Lis C. M., Munro K., Hammes W. P. (2001). Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67, 2578–2585. 10.1128/AEM.67.6.2578-2585.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welthagen J. J., Viljoen B. C. (1998). Yeast profile in Gouda cheese during processing and ripening. Int. J. Food Microbiol. 41, 185–194. 10.1016/S0168-1605(98)00042-7 [DOI] [PubMed] [Google Scholar]
- Yeung P. S. M., Sanders M. E., Kitts C. L., Cano R., Tong P. S. (2002). Species-specific identification of commercial probiotic strains. J. Dairy Sci. 85, 1039–1051. 10.3168/jds.S0022-0302(02)74164-7 [DOI] [PubMed] [Google Scholar]
- Yunita D., Dodd C. E. R. (2018). Microbial community dynamics of a blue-veined raw milk cheese from the United Kingdom. J. Dairy Sci. 101, 4923–4935. 10.3168/jds.2017-14104 [DOI] [PubMed] [Google Scholar]
- Zaman M. Z., Bakar F. A., Selamat J., Bakar J. (2010). Occurrence of biogenic amines and amines degrading bacteria in fish sauce. Czech J. Food Sci. 28, 440–449. 10.17221/312/2009-CJFS [DOI] [Google Scholar]
- Zhang C., Derrien M., Levenez F., Brazeilles R., Ballal S. A., Kim J., et al. (2016). Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 10, 2235–2245. 10.1038/ismej.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]