Abstract
Mesenchymal stem cells (MSCs) have shown promise as therapeutic agents in treating morbidities associated with premature birth. MSCs derived from human umbilical cords are easy to isolate, have low immunogenicity, and a robust ability to secrete paracrine factors. To date, there are no studies evaluating preterm versus term umbilical cord tissue-derived MSCs. Therefore, our aim was twofold: 1) To compare stem cell properties in preterm versus term MSCs, and 2) Examine the impact of oxygen tension on stem cell behavior. Umbilical cord tissue was obtained from 5 preterm and 5 term neonates. The cells were isolated and characterized as MSCs in accordance to The International Society for Cellular Therapy. We exposed MSCs to differing oxygen tensions to examine the impact of environmental factors on cell performance. We studied the following stem cell properties: i) motility, ii) proliferation, iii) senescence, iv) cell viability, v) colony forming unit efficiency, and vi) inflammatory cytokine expression. Under normoxia (21% O2), the cells from preterm and term infants had similar properties. Under hypoxic conditions (1% O2), term MSCs had better cell proliferation; however, cells exposed to hyperoxia (90% O2) had the slowest motility and lowest cell viability (p<0.05). There was no difference in the expression of senescence or cytokine expression between the groups. The term cells demonstrated more colony forming efficiency compared to the preterm cells. In sum, our preliminary findings suggest that MSCs derived from term and preterm umbilical cords have similar characteristics, offering the potential of future autologous/allogeneic MSC transplants in neonates.
Keywords: Mesenchymal stromal cells, umbilical cord, regenerative medicine, neonatology, hyperoxia, hypoxia
1. INTRODUCTION
Human umbilical cord derived mesenchymal stem cells (MSCs) have recently shown promise as potential therapeutic agents in treating medical conditions in preterm infants [Chang et al., 2014; Cotten et al., 2014]. These cells are attractive for clinical use due to their ease of isolation, lack of ethical constraints, and non-invasive retrieval [Forraz, and McGuckin, 2011]. Unlike the cord blood, the umbilical cord is considered medical waste and typically discarded after birth. Wharton’s jelly-derived MSCs (WJ-MSCs) are therefore a novel opportunity to create a therapeutic product from an unused resource. Additionally, studies have found that umbilical cord-derived MSCs have greater proliferative capacity when compared to other adult stem cell sources and have a decreased risk of immune rejection [Weiss, and Troyer, 2006; Hass et al., 2011].
Understanding the factors that affect stem cell growth and performance is critical to maximizing their utility for regenerative medicine. In this respect, significant gaps remain before applying cord-derived MSCs to clinical practice. For example, current evidence suggests that there may be a correlation between stem cell age and ability to elicit a therapeutic response [Duscher et al., 2014; Li et al., 2011]. Researchers have shown that aged (or senescent) stem cells have reduced capacities for migration, modulation of anti-inflammatory responses, and impaired differentiation potential compared to young stem cells [Bustos et al., 2014; Turinetto et al., 2016]. In contrast, Markel et al found that bone marrow stem cells derived from adults or aged subjects improved post-ischemic myocardial recovery, while neonatal stem cells did not [Markel et al., 2009].
Clearly, the relationship between stem cell source age and regenerative properties is not well understood, and to date mostly neonatal and adult stem cells have been compared [Duscher et al., 2014; Li et al., 2011; Bustos et al., 2014; Naaldijk et al., 2015]. Messerli and associates showed that WJ-MSCs from preterm births can differentiate into neural progenitors in much the same capacity as full term stem cells [Messerli et al., 2013]. Additionally, studies on hematopoietic progenitor cells (HPCs) show that cord blood from preterm deliveries contain a higher percentage of HPCs and that the preterm HPCs display a higher clonogenic capacity compared to full term cord blood HPCs [Podestà et al., 2015; Wisgrill et al., 2014a].
In 2013, Lim et al compared the ability of term and preterm human amnion epithelial cells to reduce inflammation and fibrosis in a rodent model of lung injury [Lim et al., 2013]. Although they found that preterm cells have improved cell yield, viability, and a higher proliferative rate compared to term cells, their ability to lessen inflammation and fibrosis did not reach those found in the term amnion cells. A more recent study evaluated the efficacy of term versus preterm-derived umbilical cord blood cells in a large animal model of white matter injury [Li et al., 2017]. The authors found that both preterm and term cord blood cells normalized white matter density and decreased cell death in sheep that underwent a hypoxic-ischemic brain injury. However, the mechanisms by which the cells alleviated injury differed: preterm cells decreased tumor necrosis factor α, while term cells increased the regulation of interleukin-10 and abated oxidative stress.
Traditionally, MSCs are grown in normoxia (21% oxygen); however, investigators are now expanding their culture conditions in efforts to optimize their restorative ability [Krinner et al., 2009; Mohyeldin et al., 2010; Bader et al., 2015]. For instance, preconditioning MSCs in hypoxia (1–10% oxygen) has shown improved regenerative/reparative properties in animal models of heart, brain, and lung injury [Lan et al., 2015; Xu et al., 2016; Wakai et al., 2016; Cruz, and Rocco, 2015]. These findings build on the logic that in vivo MSCs survive in a “hypoxic” niche where oxygen tensions are usually below 10%. On the other hand, hyperoxia is an important mediator of the most common lung injury that develops in preterm infants who require mechanical ventilation for survival. Therefore, studying umbilical cord-derived MSC properties when exposed to hyperoxia and hypoxia examines the potential impact of environmental factors on cell behavior. Furthermore, it allows further elucidation into the advantages/disadvantages of treating preterm and/or term morbidities with autologous versus allogeneic cell-based products.
In this project, we examined differences in the properties of WJ-MSCs derived from preterm infants and term infants. We defined preterm babies as those delivered before 37 completed weeks’ gestation. Compared to term WJ-MSCs, we hypothesized that preterm WJ-MSCs: i) would display higher proliferative capacity, increased viability, improved motility, and decreased senescence when grown under normoxia, ii) demonstrate similar proliferative capacity, viability, motility, and senescence after subjection to hyperoxia and hypoxia, and iii) have a distinct inflammatory cytokine profile.
2. MATERIALS and METHODS
To investigate the differences between preterm and full term WJ-MSCs, cells were isolated from fresh human umbilical cords. Once cultured, cells were analyzed for surface antigen markers and differentiated into osteogenic, chondrogenic, and adipogenic lineages. Institutional review board approval was sought at University of Texas Health San Antonio and University Health System; however, since the research did not involve human subjects and just leftover de-identified specimens the board did not require patient/family consent.
2.1 Primary isolation and expansion of umbilical cord Wharton’s jelly cells
Human umbilical cords were collected after preterm (n=5) or full term (n=5) deliveries. A segment of 5–10 cm was aseptically cut by newborn nurses prior to routine disposal of umbilical cord. Segments were conserved on ice in phosphate buffered saline (PBS) solution (Sigma-Aldrich, Saint Louis, MO) supplemented with antibiotics (Gibco, Waltham, MA) until use in the laboratory (within 24 hours).
Cells were isolated from the umbilical cord Wharton’s jelly using an enzyme digestion method [Bruyn et al., 2011; Azandeh et al., 2012; Rostamzadeh et al., 2015]. The enzymatic method for extraction of cells was chosen over explant technique for several reasons, including: high popularity of this method among other researchers, concern of umbilical tissue detaching and thereby reducing cell migration onto culture dish, potentially higher risk for plate contamination from intact tissue pieces, cell yield is comparable, and excellent success with this method in our laboratory [Hua et al., 2014; Arutyunyan et al., 2016a; Salehinejad et al., 2012; Han et al., 2013].
Briefly, the cords were washed in PBS and antibiotic solution to remove residual blood from the vein and arteries. The cords were then rinsed in 70% ethanol followed by three more PBS rinses. The cord was longitudinally cut, and the Wharton’s jelly was dissected from the cord lining with a surgical blade. The tissue fragments were placed into a 60mm culture dish (Falcon, Corning, NY) with Eagle’s Minimum Essential Medium, alpha modification (αMEM) (Sigma-Aldrich) supplemented with 1% antibiotic and antimycotic (Gibco) solution. The tissue was digested in 1% collagenase (Sigma-Aldrich) at 37º C in humidified air with 5% carbon dioxide for 3 hours, at which time 0.15% hyaluronidase (Sigma-Aldrich) was added and the mixture incubated for another hour.
After digestion, the tissue remnants and media were passed through a 40 µm cell strainer and placed into a 15-ml conical tube and centrifuged at 500g for 5 minutes. The supernatant was discarded, and the remaining cell pellet was plated into a T-25 culture flask (Corning, Corning, NY) with MSC growth media (αMEM completed with lglutamine (Gibco) and supplemented with 20% fetal bovine serum (Sigma-Aldrich) and 1% antibiotic and antimycotic solution).
The cultures were then expanded with media changes every 2–3 days until the cells were harvested and used for experiments. All experiments were conducted at passages 3–6. We adhered to passages 3–6 since previous studies have not shown significant alterations in MSC behavior within this range [Smith et al., 2016; Zhuang et al., 2015; Arutyunyan et al., 2016b].
2.2 Differentiation of umbilical cord derived cells
Differentiation of cord cells was achieved by culturing cells in lineage specific media for 21–28 days. Chondrogenesis was achieved using the StemPro chondrogenesis kit (Thermo Fisher, Waltham, MA) (Thermo Fisher) per the manufacturer’s instructions. Chondrogenesis was verified with Alcian blue staining (Sigma-Aldrich). Osteogenesis was achieved using the StemXVivo osteogenesis kit (R&D Systems, Minneapolis, MN) per the manufacturer’s instructions. Osteogenic differentiation was confirmed by Alizarin Red-S staining (Sigma-Aldrich) for calcium deposits. Adipogenesis was achieved by seeding the cells in 0.5 mM isobutylmethylxanthine, 200 μM indomethacin, 1 μM dexamethasone, and 10 μg/ml of insulin in low-glucose DMEM (Invitrogen) with 10% FBS [Scott et al., 2011]. Adipogenesis was confirmed by Oil Red-O staining (Sigma-Aldrich) for lipid droplets.
2.3 Surface antigen expression of cord cells
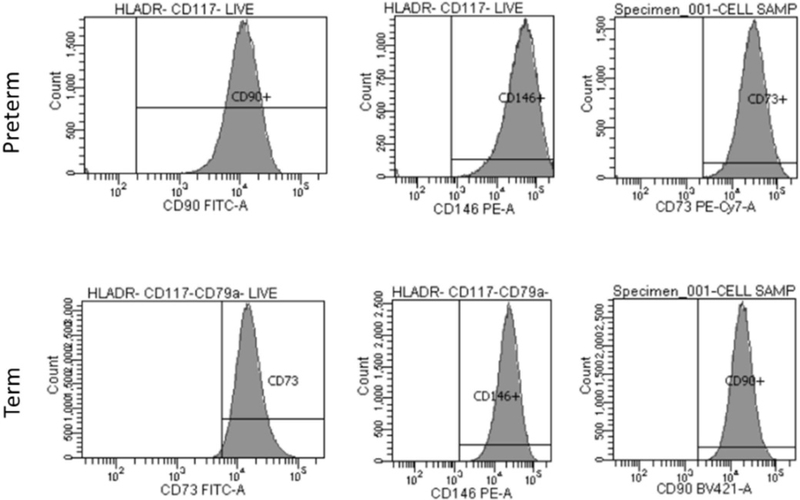
Flow cytometry was performed with the assistance of the flow cytometric core facilities at UT Health-San Antonio. The following monoclonal antibodies (mouse anti-human) were used for MSC cell-surface antigen phenotyping: 7AAD PerCP-Cy5.5 (Phycoerythrin-cyanine 5.5), CD 90 BV421 (Brilliant Violet 421), CD 73 FITC (Fluorescein isothiocyanate), CD 146 PE (Phycoerythrin), HLA-DR APC (Allophycocyanin), CD 117 APC, CD 79a APC. This combination of surface antigens has been reported in the literature to identify MSCs from hematopoietic stem cells [Wu et al., 2016; Huang et al., 2014; Hermida-Gómez et al., 2011; Mafi, 2011].
Briefly, cells were cultured in regular media, trypsinized, aliquoted and resuspended at a concentration of 1 × 106 cells/mL. They were incubated for 30 min with either conjugated specific antibodies or isotype-matched control mouse IgG at recommended concentrations at 4°C. Labeled cells were washed, resuspended in PBS, and analyzed on a Beckton Dickinson LSR II Flow Cytometer. The gating was done by using Forward and Side Scatter doublet discrimination to remove debris. Afterwards, gating of doublets was performed to negatively selected dead cells and HLA-DR, CD 117 and CD 79A. From this population, we analyzed percent of positive cells for each of the following individual markers: CD 90, CD 73 and CD 146. Results were expressed as the mean percentage of positive cells.
2.4 Motility of WJ-MSCs
Motility of WJ-MSCS was analyzed using an in vitro scratch assay modified from Liang et al [Liang et al., 2007]. In brief, cells were plated into twelve-well plates and allowed to form a confluent monolayer for 24 hours. Cells were then scratched across the wells with a P-200 pipet tip to form an area free of cells. The wells were then rinsed with PBS and the growth media replaced. Images of the scratches were acquired under light microscopy at 0, 4, 8, and 24-hours post disturbance. Scratch size was analyzed using ImageJ software (National Institutes of Health, MD, USA). Three trials were conducted for each cell line.
2.5 Proliferation of WJ-MSCs
Proliferation of WJ-MSCs was analyzed using Abcam’s Quick cell proliferation kit (Abcam; Cambrigde, MA catalog #ab65473) according to manufacturer’s instructions. WJ-MSCs were plated at a density of 4 ×104 into each well (repeated in triplicate) of a 96 well flat bottom tissue culture plate (Falcon) for 24 hours. WST-1/ECS solution was added to each well and incubated in standard culture conditions for 2 hours. The absorbance was then measured using a microplate reader, BioTek Instruments, Inc. (Winooski, VT, USA), at 450 nanometers with a reference wavelength of 650 nm. The results were expressed as mean absorbance in nanometers.
2.6 Senescence
Senescence is considered an underlying cause of aging and represents an arrested state of cells. Senescent cells display increase in senescence-associated expression of β-galactosidase (SA-β-Gal) activity.
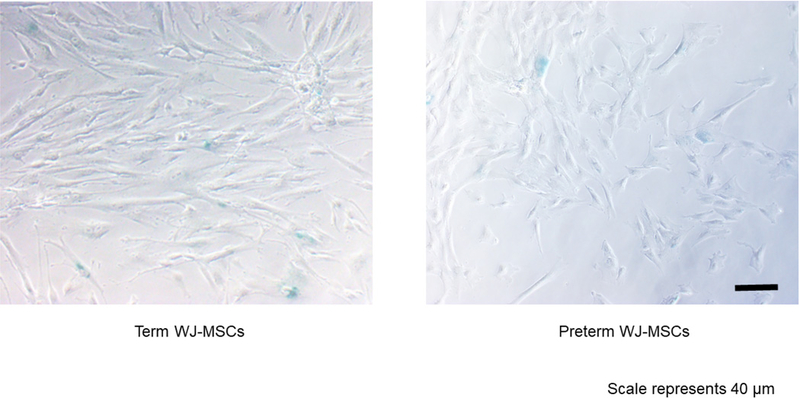
Senescence of the cells was analyzed using Abcam’s Senescence Detection Kit (Abcam: Catalog#ab65351). This kit is designed to histochemically detect SA-β-Gal activity in cultured cells and tissue sections. Specifically, WJ-MSCs were plated at a density of 2 ×105 cells into each well of a twelve-well plate (replicated in duplicate). The cells were then fixed with 0.5 mL of Fixative Solution for 10–15 min at room temperature. Half an ml of the Staining Solution Mix was then added to each well and it was incubated at 37°C overnight. Cells were then observed under a microscope (Olympus IX50, 10x magnification) for development of blue color (Figure 6). Two random images were obtained per well and were analyzed using Image J software.
Figure 6.
Senescence expression in preterm and term WJ-MSC. Scale bar= 40 micrometers
2.7 Cell viability
Cell viability was assessed by calculating the percentage of live cells at 21% oxygen. Cells were plated at a density of 150 cells per well of a six-well plate (repeated in duplicate). Cells were then trypsinized with 1ml of trypsin (TrypLE Express Enzyme, Gibco) per well. After deactivation of trypsin with 1ml of growth media, the cell suspension was centrifuged at 500g for five minutes. Cell numbers were automated using a Countess Cell counter (Countess II FL Automated Cell counter, ThermoFisher Scientific). WJ-MSCs were then re-plated and re-counted after 24 hours of 90% or 1% oxygen concentration.
2.8 Colony forming efficiency of WJ-MSCs
Colony forming efficiency of WJMSCs was analyzed using a modified CFU assay from Alt et al [Alt et al., 2011]. One hundred fifty WJ-MSCs were plated into a 60-mm culture dish and maintained for 14 days under standard culture conditions with media changes every 2–3 days. After 14 days, cultures were stained with crystal violet (Sigma Aldrich) and colonies were counted. CFU efficiency was calculated by dividing the number of colonies per dish by the number of cells (150) seeded per dish multiplied by 100%. Cultures were repeated three times for each cell line.
2.9 Exposure to hyperoxia (90% O2) and hypoxia (1% O2)
After isolation and characterization of preterm and term WJ-MSCs, studies were also conducted in oxygen tensions of 90% and 1%. An airtight modular incubator chamber was used to maintain oxygen tension (Billups-Rothenberg, Del Mar, CA, USA) and a digital oxygen analyzer (Hudson RCI, Teleflex, Morrrisville, NC, USA) was used to monitor the concentration of O2 inside the chamber. The 24-hour time point was chosen based on viability testing (in triplicate) of the cells at different time points in hyperoxia (refer to Supplementary Figure 1).
2.10 Cytokine secretion profiling by cytokine antibody array
Cytokine profiling of the cells was performed using the Human Inflammation Antibody Array- Membrane (Abcam product#ab134003). This array analyses human protein samples for 40 different inflammatory cytokines. Array membranes, were incubated for 30 min in 2 ml of blocking buffer supplied with the kit. Following this, they were further incubated for 60 min at room temperature with 200µg of protein from each cell sample. One ml of Biotin-conjugated Anti-Cytokines were then added to each membrane after a thorough wash. This was succeeded by an overnight incubation at 4°C and then incubated with a 1:2000 dilution of HRP-Conjugated Streptavidin for 60 min at room temperature. The proteins were detected by chemiluminescence western blot and signals were captured on x-ray films (Denville Scientific, MA, USA), scanned at high resolution, and quantified using ImageJ software.
2.11 Statistical analysis
Data was analyzed using GNU PSPP Statistical Analysis Software Release 0.10.2. Student’s T-tests was performed were applicable. P-values of less than 0.05 were considered significant. Data is presented as mean ± standard error of the mean.
3. RESULTS
3.1 Characteristics of the preterm and term WJ-MSCs
The range of gestational ages for the preterm umbilical cords was between 25–30 weeks’ gestation while term cords ranged between 37–42 weeks (data summarized in Table 1). The mean gestational age for the preterm cohort was 28 weeks ± 2 weeks (70% term equivalent). Average cell viability for preterm and term WJ-MSCs was similar.
Table 1.
Gestational age of the preterm and term WJ-MSCs.
| Group | Gestation (weeks) | % Mature | Viability (%) |
|---|---|---|---|
| Preterm 1 | 25 | 62 | 96 |
| Preterm 2 | 27 | 67 | 95 |
| Preterm 3 | 28 | 70 | 96 |
| Preterm 4 | 28 | 70 | 97 |
| Preterm 5 | 30 | 75 | 96 |
| Term 1 | 37–42 | 90–100 | 92 |
| Term 2 | 37–42 | 90–100 | 94 |
| Term 3 | 37–42 | 90–100 | 96 |
| Term 4 | 37–42 | 90–100 | 99 |
| Term 5 | 37–42 | 90–100 | 97 |
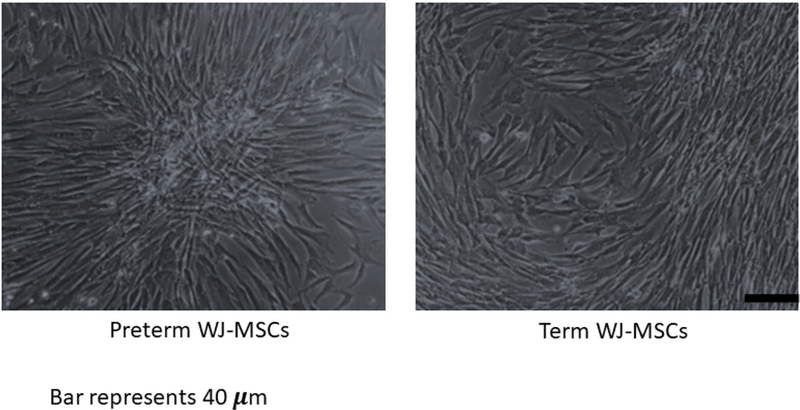
3.2 Cell morphology and adherence to plastic dish
Figure 1 depicts the spindle-shaped morphology characteristically observed in preterm and term WJ-MSCs as well as the adherence to plastic.
Figure 1.
Classic fibroblast-like appearance of MSCs in preterm (27 week gestational age) and term (37–42 weeks) umbilical cord Wharton’s jelly tissue. Magnification at 10x. Scale bar= 40 micrometers.
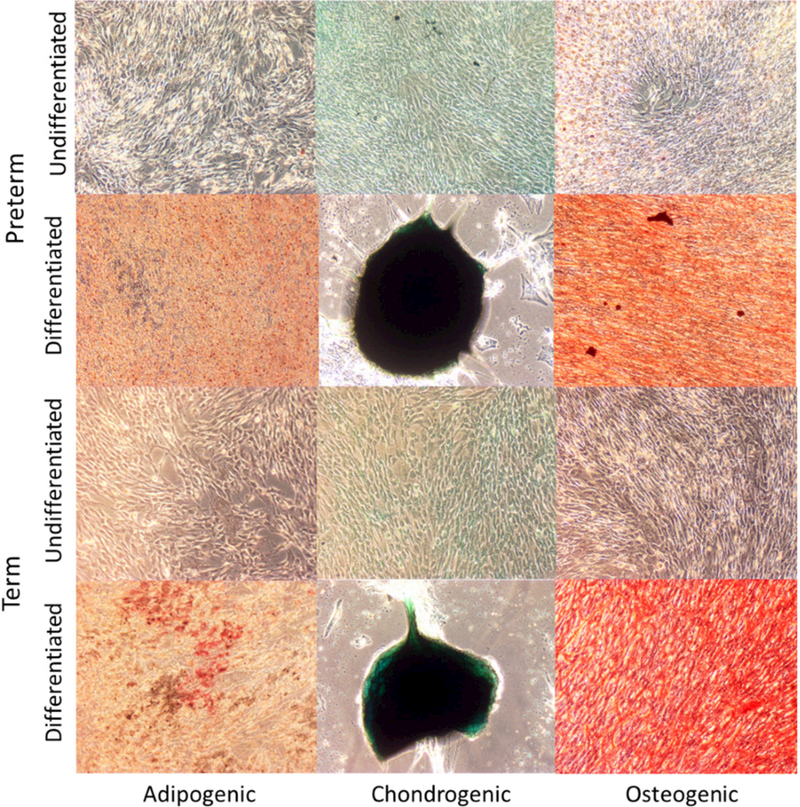
3.3 Differentiation
Both preterm and term WJ-MSCs demonstrated the ability to differentiate into osteocytes, chondrocytes, and adipocytes upon in vitro stimulation (Figure 2).
Figure 2.
Multi-lineage differentiation of preterm and term WJ-MSCs into i) Adipogenic (Oil Red O stain), ii) Chondrogenic (Alcian blue), and iii) Osteogenic (Alizarin Red stain) cells. Magnification at 10x. Scale bar= 40 micrometers.
3.4 Flow cytometry
Preterm and term WJ-MSCs expressed CD 73 above 95%, CD 90 above 95%, and CD 146 above 90%. Neither cell type expressed CD 79, CD 117, or HLADR above 5% (as shown in Figure 3).
Figure 3.
Flow cytometry of surface antigen markers on sample preterm and term WJ cells.
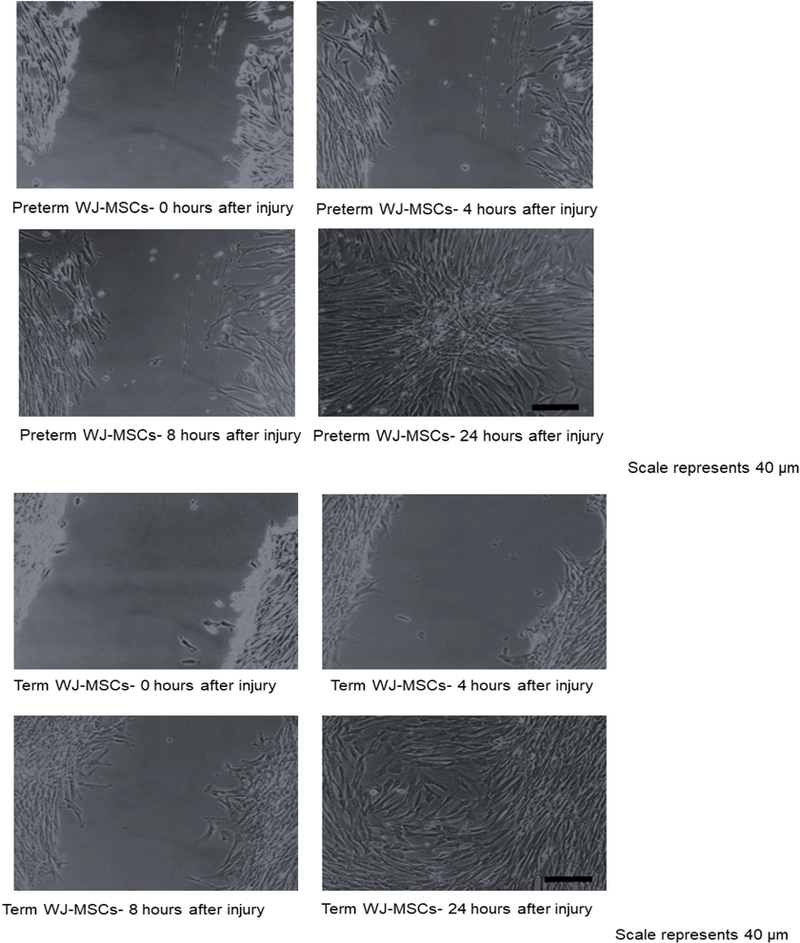
3.5 Motility
Table 2 summarizes the results of the motility test, while Figure 4 shows representative images of the test in progress.
Table 2.
Motility studies comparing preterm and term WJ-MSCs. Comparison of the % wound closed (average ± SEM) between preterm and term WJMCs at 21%, 90% and 1% oxygen, at the 4-hour and 8-hour time points after scratch.
| Preterm (n=5) | Term (n=5) | |
|---|---|---|
|
Normoxia 4 hours 8 hours |
41 ± 11 67 ± 14 |
31 ± 5 62 ± 7 |
|
Hyperoxia 4 hours 8 hours |
17 ± 5 38 ± 12 |
12 ± 3 28 ± 6 |
|
Hypoxia 4 hours 8 hours |
23 ± 5 37 ± 7 |
25 ± 8 56 ± 12 |
Figure 4.
Motility of preterm(4a) vs term(4b) WJ-MSCs after in vitro scratch wound at 0, 4, 8 and 24-hour time points. Magnification at 10x. Scale bar= 40 micrometers
There were no significant differences in cell motility between the term and the preterm cells in normoxia, hyperoxia, or hypoxia at 4 and 8 hours after the scratch injury. Both cell types closed most of the scratch area by 24-hours post disturbance, although small areas remained free of cells in a few of the trials.
There were significant differences within the term cell lines when cultured in normoxia versus hyperoxia. At 4 hours, the term normoxic group had scratch closure of 31.0 ± 5.5% vs. 12.8 ± 3.9% closure after hyperoxic injury (p=0.03). Eight hours after the scratch, the term normoxic group had 62.6 ± 7.4% closure while hyperoxic injury only 28.4 ± 6.0% wound closure (p=0.01).
A trend was appreciated between the term hyperoxic and hypoxic exposures at 8 hours, but it did not reach significance (28.4 ± 6.0% vs 56.0 ± 12.0, respectively, p=0.07).
3.6 Proliferation
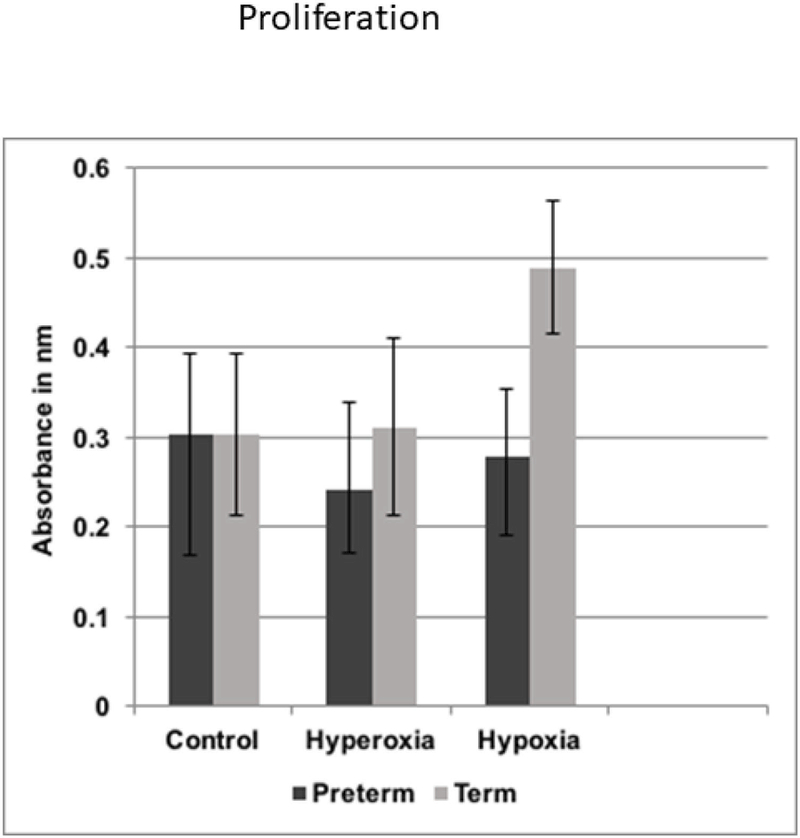
Figure 5 summarizes the results of the proliferation test.
Figure 5.
Cell proliferation of preterm and term WJ-MSCs.
Preterm and term WJ-MSCs had very similar proliferation rates in normoxic and hyperoxic conditions.
However, the highest proliferation was in term cells exposed to hypoxia (p<0.05 compared to both groups in normoxia and hyperoxia). The preterm WJ-MSCs also had improved cell proliferation in hypoxia, but the changes did not reach significance.
3.7 Senescence
Figure 7 demonstrates no differences between the groups in SA-β-gal staining in normoxia, hyperoxia, nor hypoxia.
Figure 7.
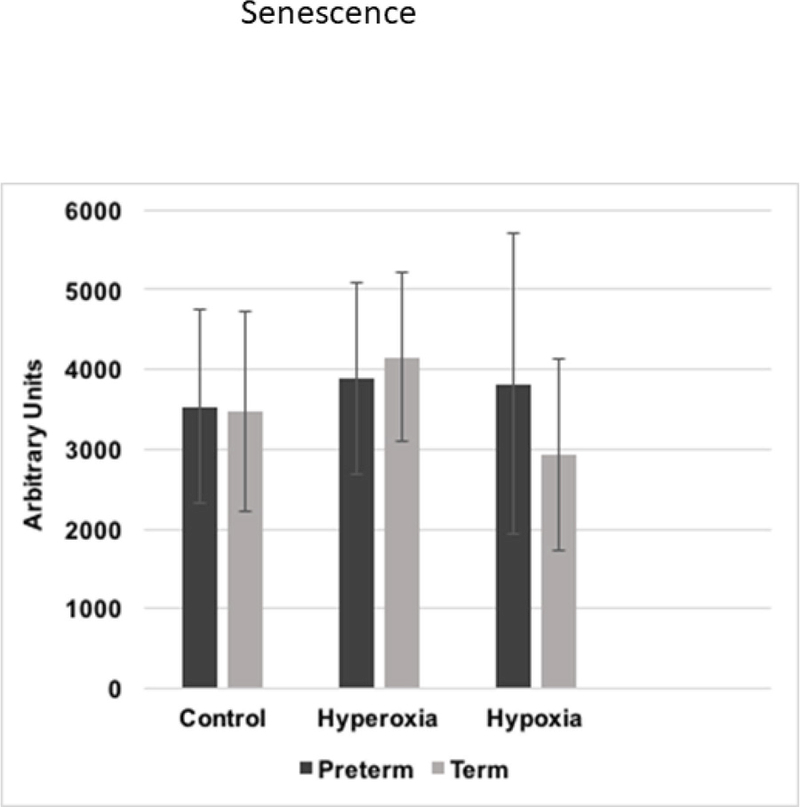
Senescence of preterm and term WJ-MSCs after 21%, 90% and 1% oxygen exposure. Magnification at 10x. Scale bar= 40 micrometers
3.8 Cell viability after hyperoxia and hypoxia
Preterm and term WJ-MSCs had similar viability in normoxia, hyperoxia, and hypoxia. Yet when both preterm and term cells were grouped (n=10), hyperoxia showed a reduction in viability (94.9 ± 1.92% vs. normoxia 88.6 ± 4.0%, p=0.03). Data shown in Table 3.
Table 3.
Cell viability in preterm and term WJ-MSCs. Comparison of the % live cells (average ± SEM) between preterm and term WJMCs before and after exposure to 21%, 90% and 1% oxygen
| Preterm (n=5) | Term (n=5) | |
|
Normoxia Before After |
95 ± 0.5 94 ± 2.3 |
95 ± 1.2 95 ± 2.0 |
|
Hyperoxia Before After |
95 ± 0.5 89 ± 1.8 |
95 ± 1.2 87 ± 5.6 |
|
Hypoxia Before After |
95 ± 0.5 87 ± 6.7 |
95 ± 1.2 89 ± 9.8 |
3.9 Colony forming efficiency
Colony forming efficiency was higher in the term cells (24.7 ± 3.2%) when compared to the preterm cells (15.2 ± 3.0%; p<0.05). Data depicted in Figure 8.
Figure 8.
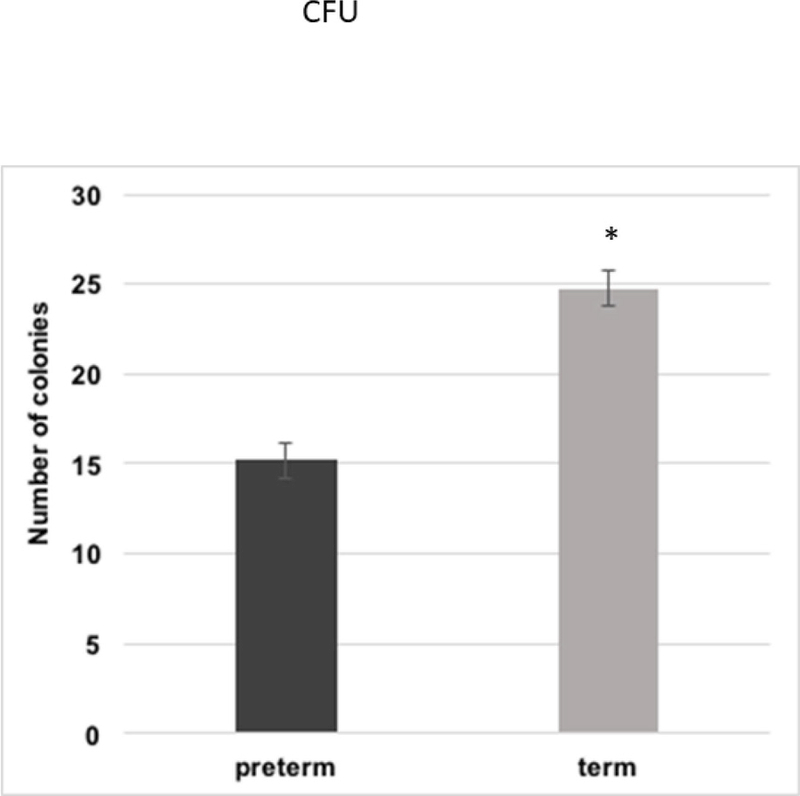
Colony forming unit in preterm versus term WJ-MSCs. Asterisk signifies a p value of ≤0.05.
3.10 Inflammatory cytokines
There were no significant differences in the inflammatory cytokine profile expression of the two populations in normoxia. Of note, 3 cytokines were strongly expressed in the array for WJ-MSCs: interferon gamma-induced protein 10 (IP-10), macrophage inflammatory protein 1β (MIP-1b), and monocyte chemotactic protein 1 (MCP-1). None of the 10 samples expressed the following cytokines: interleukins (4, 7, 10, 11, 13, 17), granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, monokine induced by interferon gamma, T-lymphocyte-secreted protein I-309, and macrophage inflammatory protein-1 delta. Data is summarized in Figure 9.
Figure 9.
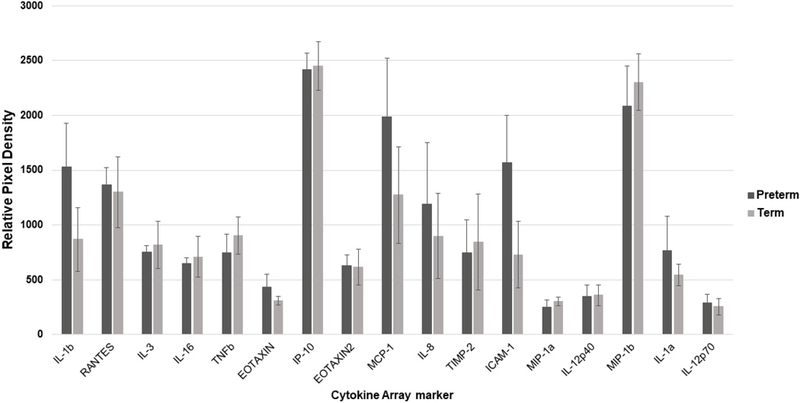
Inflammatory cytokine expression of the preterm and term WJ-MSCs. Data is presented as the mean with the standard error of the mean.(IL-1b-Interleukin 1 beta), RANTES- regulated upon activation normal T cell expressed and secreted, IL-3-Interleukin 3, IL-16-Interleukin 16, TNFb-Tumor necrosis factor beta, EOTAXIN, IP-10- interferon gamma-induced protein 10, EOTAXIN2, MCP-1-Monocyte chemoattractant protein 1, IL-8-Interleukin 8, TIMP-2-Tissue inhibitor of metalloproteinases 2, ICAM-1-Intercellular adhesion molecule 1, MIP-1a-Macrophage inflammatory protein 1 alpha, IL-12p40-Interleukin 12 subunit p40, MIP-1b- Macrophage inflammatory protein 1 beta, IL-1a-Interleukin 1 alpha, IL-12p70- Interleukin 12 subunit p70).
4. DISCUSSION
The perinatal period offers an opportune time to collect tissues, in a non-invasive approach, that are rich in hematopoietic and mesenchymal stem cells [Weiss, and Troyer, 2006; Messerli et al., 2013; Yousefifard et al., 2016; Moreira et al., 2017]. In this study, we sought to evaluate characteristics in umbilical cord-derived cells isolated from preterm and term infants. Our results suggest that MSCs derived from term and preterm neonatal umbilical cords have similar morphologies, adhere to plastic, express similar surface antigen markers, and can multi-differentiate.
Similar to findings by Duscher et al, age did not play a significant role in MSC proliferative capacity [Duscher et al., 2014]. Their study examined the replicative ability of adipose-derived MSCs in young adult wild-type mice versus an aged cohort. Interestingly, we found hypoxia stimulated proliferation in term WJ-MSCs. These findings are substantiated in studies involving hematopoietic and neural stem cells grown in hypoxic niches. The investigators speculate lower oxygen tensions reduce oxidative stress and thus DNA injury [Hubbi, and Semenza, 2015].
Another theory explaining improved cell replication of term cells in hypoxia may be a result of the higher number of mitochondria observed in relation to preterm MSCs [Panfoli et al., 2015]. More mitochondria allows for a greater efficiency of the electron transport chain and therefore increase cell proliferation [Antico Arciuch et al., 2012]. Furthermore, hypoxia promotes mitochondrial enzymes known to decrease oxygen consumption [Ghafourifar, and Cadenas, 2005].
Studies by Naaldijk and associates demonstrated comparable migration rates in neonatal and adult MSCs [Naaldijk et al., 2015]. Our migration studies had similar findings, except we appreciated a reduction in motility in term hyperoxic MSCs. Investigations using endothelial cells grown under hyperoxia led to a decrease in motility that was attributed to a lower expression of CXCR4 [Uno et al.; do Carmo et al., 2010]. In contrast, Pendyala et al showed an increase in cell motility with hyperoxia; however, their experiments employed different cells, duration of hyperoxia, and at baseline their cells appeared less motile than MSCs [Pendyala et al., 2009].
The colony forming units were deceased in preterm WJ-MSCs. Although Wyrsch’s study found a higher proliferative ability in preterm umbilical cord blood cells, term cord blood cells had a slightly greater colony formation when grown in media without growth factors [Wyrsch et al., 1999]. On the other hand, Podesta and Wisgrill observed better clonogenicity in preterm umbilical cord blood progenitor cells [Podestà et al., 2015; Wisgrill et al., 2014b]. A plausible explanation for the decreased reproductive potential in term MSCs may be that they differentiated quicker [Franken et al., 2006; Boyette et al., 2014]. Also, since the current study isolated MSCs from the cord tissue rather than the blood, there could be site-specific differences explaining the clonogenic potential of MSCs across gestational ages.
There was no difference in senescence in both groups. Changes in senescence could have potentially hindered other cell processes, including cell growth, migration, and differentiation [Turinetto et al., 2016]. Bustos et al reported no influence of hyperoxia on senescence in preterm and term MSCs [Bustos et al., 2014]. There are various other ways for assessing senescence in cells besides β-galactosidase. They include studying cyclin- dependent kinase inhibitors (p16, p21), cytoskeleton markers, and retinoblastoma protein [Turinetto et al., 2016]. Cells are also more prone to senescence under higher production of reactive oxygen species and/or lower antioxidant enzymes. Studies measuring oxidative stress in preterm versus term WJ-MSCs showed that preterm MSCs have higher production of reactive oxygen species, that may be due to mitochondrial immaturity [Ravera et al., 2017]. However, the study did not measure markers of senescence. Future studies should also incorporate apoptosis as a measure in injured preterm and term MSCs.
Exposure to hyperoxia caused a decrease in viability when we grouped all cells. Masalunga et al describe an increase in necroptosis and apoptosis in progenitor cells treated with chronic elevated oxygen levels [Masalunga et al., 2007]. Analogous to our study, Aly et al, found comparable viability rates of MSCs isolated from preterm and term cord cells under normal culture conditions [Aly et al., 2012].
The inflammatory profile of WJ-MSCs from preterm and term populations were alike. Sullivan et al hypothesized that the serum concentrations of leukocyte chemokines eotaxin, RANTES (regulated upon activation normal T cell expressed and secreted), and macrophage inflammatory protein-1α (MIP-1α) would be lower in preterm infants. They concluded that concentrations of alpha and beta chemokines tested from preterm infants were equivalent to those measured in term neonates. Additionally, in a study involving 34 healthy preterm or term newborns, investigators examined serum cytokines during the first week of life. No difference between the groups was detected in the concentration of RANTES, interleukin-1β, eotaxin, and MIP-1β. Contrasting to umbilical cord blood monocytes, cells from our study did not show a decrease in interleukin-12 subunits in the preterm samples [Pérez et al., 2010; Lavoie et al., 2010].
Interestingly, there was a remarkable expression of MIP-1β, MCP-1 (monocyte chemoattractant protein-1), and IP-10 (interferon gamma-induced protein 10) in both preterm and term WJ-MSCs. In human patients with critical limb ischemia, MIP-1β measurements were significantly higher in patients who responded to MSC treatment. The investigators ascribe the higher recruitment of macrophages and endothelial cells (by MIP-1β) as an explanation for ischemic healing [Altaner et al., 2013]. Boomsma et al revealed that the paracrine expression of MCP-1 and MIP-1β from MSCs led to improved wound healing by stimulating cell migration and inhibiting apoptosis [Boomsma, and Geenen, 2012]. A comparative analysis study of WJ-MSCs showed IP-10 suppressed inflammation after induction with interferon-γ via regulation of T-cells and natural killer cells [Mao et al., 2010].
Limitations to this study include the small sample size of preterm and term umbilical cords. A larger population may provide stronger arguments in phenotypic and functional differences. Another drawback is that the gestational age in the preterm umbilical cords approached 30 weeks and the neonatal population that would gain the most benefit from future stem cell therapies are those with the highest risk for morbidity and mortality, infants less than 28 weeks’ gestation. Furthermore, data on maternal characteristics would have benefitted this study, being that maternal health can impact umbilical cord cell behavior [Kim et al., 2015].
The umbilical cord Wharton’s jelly is a promising and emerging tissue source for future opportunities in regenerative medicine. Overall, the present study demonstrated that preterm and term isolated WJ-MSCs are comparable when cultured at 21% O2. However, exposure to hyperoxia was noted to impair the motility and cell viability of both populations. Moreover, term WJ-MSCs had higher proliferative capacity after exposure to hypoxia. Translationally, these results suggest that autologous administration of MSCs to treat diseases of the neonate remains feasible regardless of the gestational age, when the cells are cultured under normal culture conditions (21% O2). Preconditioning term MSCs might increase their turnover time in situations where a rapid yield of cells is needed for mitigating injury/inflammation in a short timeframe (e.g. hypoxic ischemic injury, necrotizing enterocolitis). Future investigations should compare the reparative capability of WJ-MSCs after injuries that are commonly observed in the clinical setting (i.e., hyperoxia and mechanical stress from the ventilator, inflammation, and infection).
Supplementary Material
ACKNOWLEDGEMENTS:
Data was generated in the Flow Cytometry Shared Resource Facility which is supported by UTHSCSA, NIH-NCI P30 CA054174–20 (CTRC at UTHSCSA) and UL1 TR001120 (CTSA grant).
FUNDING
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR001118. This study was also supported by The University of Texas Health San Antonio School of Medicine Clinical Investigator Kickstart Pilot Grant.
LIST OF ABBREVIATIONS
- MSC
Mesenchymal stem cell
- WJ-MSC
Wharton’s jelly-derived mesenchymal stem cell
- HPC
Hematopoietic progenitor cell
- PBS
Phosphate buffered saline
- MEM
Modified eagle medium
- PCR
Polymerase chain reaction
- O2
Oxygen
- PBS
Phosphate buffered saline
- FBS
Fetal bovine serum
- DMEM
Dulbecco’s modified eagle medium
- SA-β-Gal
β-galactosidase
- CFU
Colony forming unit
- IP-10
interferon gamma-induced protein 10
- MIP-1b
macrophage inflammatory protein 1β
- MCP-1
monocyte chemotactic protein 1
- RANTES
regulated upon activation normal T cell expressed and secreted
Footnotes
DISCLOSURES:
The authors declare no conflicts of interest and have nothing to disclose
REFERENCES
- Azandeh S, Orazizadeh M, Hashemitabar M, Khodadadi A, Shayesteh AA, Nejad DB, et al. (2012) : Mixed enzymatic-explant protocol for isolation of mesenchymal stem cells from Wharton’s jelly and encapsulation in 3D culture system. J Biomed Sci Eng 2012;5:580–586. [Google Scholar]
- Arutyunyan I, Fatkhudinov T, Kananykhina E, Usman N, Elchaninov A, Makarov A, et al. (2016): Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro study. Stem Cell Res Ther 2016a Dec 22;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T (2016) : Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int 2016b;2016. DOI: 10.1155/2016/6901286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt E, Yan Y, Gehmert S, Song Y-H, Altman A, Gehmert S, et al. (2011): Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 2011 Apr;103:197–208. [DOI] [PubMed] [Google Scholar]
- Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC (2012) : Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 2012 May 15;16:1150–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly H, Mohsen L, Badrawi N, Gabr H, Ali Z, Akmal D (2012) : Viability and neural differentiation of mesenchymal stem cells derived from the umbilical cord following perinatal asphyxia. J Perinatol 2012 Sep 1;32:671–676. [DOI] [PubMed] [Google Scholar]
- Yachie A, Takano N, Ohta K, Uehara T, Fujita S, Miyawaki T, and Taniguchi N (1992): Defective production of interleukin-6 in very small premature infants in response to bacterial pathogens. Infect Immun 1992 Mar; 60(3): 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, et al. (2014): Aging Mesenchymal Stem Cells Fail to Protect Because of Impaired Migration and Antiinflammatory Response. Am J Respir Crit Care Med 2014 Apr 1;189:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, et al. (2015): Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS One 2015;10:e0138477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyn D, Najar M, Raicevic G, Meuleman N, Pieters K, Stamatopoulos B, et al. (2011): A Rapid , Simple , and Reproducible Method for the Isolation of Mesenchymal Stromal Cells from Wharton ‘ s Jelly Without Enzymatic Treatment 2011;20. [DOI] [PubMed] [Google Scholar]
- Boyette LB, Creasey OA, Guzik L, Lozito T, Tuan RS (2014) : Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med 2014 Feb;3:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma RA, Geenen DL (2012): Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLOS ONE 7: e35685. doi: 10.1371/journal.pone.0035685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FF, Rocco PRM (2015) : Hypoxic preconditioning enhances mesenchymal stromal cell lung repair capacity. Stem Cell Res Ther 2015 Jul 14;6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh W Il, et al. (2014) : Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr 2014 May;164:966–972.e6. [DOI] [PubMed] [Google Scholar]
- Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, et al. (2014) : Feasibility of autologous cord blood cells for infants with hypoxicischemic encephalopathy. J Pediatr 2014 May;164:973–979.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaner Cestmir, Altanerova Veronika, Cihova Marina, Hunakova Lubica, Kaiserova Katarina, Klepanec Andrej, Vulev Ivan, Madaric Juraj (2013): Characterization of Mesenchymal Stem Cells of “No-Options” Patients with Critical Limb Ischemia Treated by Autologous Bone Marrow Mononuclear Cells. PLOS ONE September 2013 Volume 8 Issue 9 e73722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duscher D, Rennert RC, Januszyk M, Anghel E, Maan ZN, Whittam AJ, et al. (2014): Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 2014 Nov 21;4:7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo A, Patrico I, Curz MT, Carvalheiro H, Oliveira CR, Lopes MC (2010): CXCL12/CXCR4 promotes motility and proliferation of glioma cells: Cancer Biology & Therapy 2010; 9: 56–65. Ann Neurosci 2010 Apr;17:85–6.19923906 [Google Scholar]
- Forraz N, McGuckin CP (2011): The umbilical cord: a rich and ethical stem cell source to advance regenerative medicine. Cell Prolif 2011 Apr;44:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C (2006): Clonogenic assay of cells in vitro. Nat Protoc 2006 Dec 21;1:2315–2319. [DOI] [PubMed] [Google Scholar]
- Mao Fei, Xu Wen-Rong, Qian Hui, Zhu Wei, Yan Yong-Min, Shao Qi-Xiang, Xu Hua-Xi (2010): Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm. Res 59:219–225 DOI 10.1007/s00011-009-0090-y [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Cadenas E(2005): Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 2005 Apr;26:190–195. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FK et al. (2013): Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev 2013 Nov 1;22(21):2825–35. doi: 10.1089/scd.2013.0193. Epub 2013 Aug 9. [DOI] [PubMed] [Google Scholar]
- Hua J, Gong J, Meng H, Xu B, Yao L, Qian M, et al. (2014): Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: P proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol Int 2014 Feb 7;38:198–210. [DOI] [PubMed] [Google Scholar]
- Han Y-F, Tao R, Sun T-J, Chai J-K, Xu G, Liu J (2013) : Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology 2013 Oct 11;65:819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Wu D, Yuan Y, Li X, Holm R, Trope CG, et al. (2014): CD117 Expression in Fibroblasts-Like Stromal Cells Indicates Unfavorable Clinical Outcomes in Ovarian Carcinoma Patients. PLoS One 2014 Nov 7;9:e112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida-Gómez T, Fuentes-Boquete I, Gimeno-Longas MJ, Muiños-López E, Díaz-Prado S, de Toro FJ, et al. (2011) : Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J Rheumatol 2011 Feb 1;38:339–49. [DOI] [PubMed] [Google Scholar]
- Hass R, Kasper C, Böhm S, Jacobs R (2011): Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011 May 14;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbi ME, Semenza GL (2015) : Regulation of cell proliferation by hypoxia-inducible factors. Am J Physiol Cell Physiol 2015 Dec 15;309:C775-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Piao Y, Pak YK, Chung D, Han YM, Hong JS, et al. : Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev 2015;24:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinner A, Zscharnack M, Bader A, Drasdo D, Galle J: Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif 2009. Aug;42:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdzíková Lucia Machová, Růžička Jiří, Michael LaBagnara, et al. : Human Mesenchymal Stem Cells Modulate Inflammatory Cytokines after Spinal Cord Injury in Rat. Int. J. Mol. Sci 2014, 15, 11275–11293; doi: 10.3390/ijms150711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu C, Xie Z, Song P, Zhao RCH, Guo L, et al. : Epigenetic Dysregulation in Mesenchymal Stem Cell Aging and Spontaneous Differentiation. PLoS One 2011. Jun 9;6:e20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Chan ST, Tan JL, Mockler JC, Murphy SV., Wallace EM: Preterm human amnion epithelial cells have limited reparative potential. Placenta 2013;34:486–492. [DOI] [PubMed] [Google Scholar]
- Li J, Yawno T, Sutherland A, Loose J, Nitsos I, Allison BJ, et al. : Term vs. preterm cord blood cells for the prevention of preterm brain injury 2017;0:1–9. [DOI] [PubMed] [Google Scholar]
- Lan Y-W, Choo K-B, Chen C-M, Hung T-H, Chen Y-B, Hsieh C-H, et al. : Hypoxiapreconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther 2015. Dec 20;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y-W, Choo K-B, Chen C-M, Hung T-H, Chen Y-B, Hsieh C-H, et al. : Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther 2015. Dec 20;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C-C, Park AY, Guan J-L: In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007. Feb;2:329–333. [DOI] [PubMed] [Google Scholar]
- Liang C-C, Park AY, Guan J-L: In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007. Feb;2:329–333. [DOI] [PubMed] [Google Scholar]
- Masalunga C, Rozycki HJ, Mainali ES: The Impact of Hyperoxia on the Neonatal and Adult Developing Dendritic Cell. Pediatr Res 2007. Jul 1;62:78–82. [DOI] [PubMed] [Google Scholar]
- Markel TA, Crisostomo PR, Manukyan MC, Al-Azzawi D, Herring CM, Lahm T, et al. : Are neonatal stem cells as effective as adult stem cells in providing ischemic protection? J Surg Res 2009. Apr;152:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli M, Wagner A, Sager R, Mueller M, Baumann M, Surbek D V., et al. : Stem Cells From Umbilical Cord Wharton’s Jelly From Preterm Birth Have Neuroglial Differentiation Potential. Reprod Sci 2013. Dec 1;20:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafi P: Adult Mesenchymal Stem Cells and Cell Surface Characterization - A Systematic Review of the Literature. Open Orthop J 2011. Jul 28;5:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzó N-Muvdi TS, Quiñ Ones-Hinojosa A: Cell Stem Cell Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Stem Cell 2010;7:150–161. [DOI] [PubMed] [Google Scholar]
- Moreira A, Alayli Y, Balgi S, Winter C, Kahlenberg S, Mustafa S, et al. : Upcycling umbilical cords: bridging regenerative medicine with neonatology. J Matern Neonatal Med 2017. Nov 13;1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaldijk Y, Johnson AA, Ishak S, Meisel HJ, Hohaus C, Stolzing A: Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp Cell Res 2015;338:97–104. [DOI] [PubMed] [Google Scholar]
- Pendyala S, Gorshkova IA, Usatyuk P V, He D, Pennathur A, Lambeth JD, et al. : Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 2009. Apr;11:747–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podestà M, Bruschettini M, Cossu C, Sabatini F, Dagnino M, Romantsik O, et al. : Preterm Cord Blood Contains a Higher Proportion of Immature Hematopoietic Progenitors Compared to Term Samples. PLoS One 2015. Sep 29;10:e0138680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfoli I, Ravera S, Podestà M, Cossu C, Santucci L, Bartolucci M, et al. : Exosomes from human mesenchymal stem cells conduct aerobic metabolism in term and preterm newborn infants. FASEB J 2015;30 DOI: 10.1096/fj.15-279679 [DOI] [PubMed] [Google Scholar]
- Ravera S, Podestà M, Sabatini F, Fresia C, Columbaro M, Bruno S, et al. : Mesenchymal stem cells from preterm to term newborns undergo a significant switch from anaerobic glycolysis to the oxidative phosphorylation. Cell Mol Life Sci 2017. Oct 3;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostamzadeh A, Anjomshoa M, Kurd S, Chai J- K, Jahangiri F, Nilforoushzadeh MA, et al. : The Role of Wharton’s Jelly Mesenchymal Stem Cells in Skin Reconstruction. J Ski Stem Cell 2015. Jun 23;2 DOI: 10.17795/jssc30347 [DOI] [Google Scholar]
- Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, et al. : Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. Vitr Cell Dev Biol - Anim 2012. Feb 25;48:75–83. [DOI] [PubMed] [Google Scholar]
- Smith JR, Pfeifer K, Petry F, Powell N, Delzeit J, Weiss ML: Standardizing Umbilical Cord Mesenchymal Stromal Cells for Translation to Clinical Use: Selection of GMP-Compliant Medium and a Simplified Isolation Method. Stem Cells Int 2016. Feb 4;2016:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MA, Nguyen VT, Levi B, James AW: Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev 2011. Oct;20:1793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinetto V, Vitale E, Giachino C: Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int J Mol Sci 2016. Jul 19;17 DOI: 10.3390/ijms17071164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatad AM, Nesin M, Peoples J, Cheung S et al. : Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes. Neonatology 2008;94(1):8–15. Epub 2007 Dec 19. [DOI] [PubMed] [Google Scholar]
- Uno K, Merges CA, Grebe R, Lutty GA, Prow TW: Hyperoxia Inhibits Several Critical Aspects of Vascular Development DOI: 10.1002/dvdy.21122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ML, Troyer DL: Stem cells in the umbilical cord. Stem Cell Rev 2006;2:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisgrill L, Schüller S, Bammer M, Berger A, Pollak A, Radke TF, et al. : Hematopoietic stem cells in neonates: any differences between very preterm and term neonates? PLoS One 2014a;9:e106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Narasimhan P, Sakata H, Wang E, Yoshioka H, Kinouchi H, et al. : Hypoxic preconditioning enhances neural stem cell transplantation therapy after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab 2016. Dec 22;36:2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C- C, Liu F- L, Sytwu H- K, Tsai C- Y, Chang D- M: CD146+ mesenchymal stem cells display greater therapeutic potential than CD146– cells for treating collagen-induced arthritis in mice. Stem Cell Res Ther 2016. Dec 3;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrsch A, dalle Carbonare V, Jansen W, Chklovskaia E, Nissen C, Surbek D, et al. : Umbilical cord blood from preterm human fetuses is rich in committed and primitive hematopoietic progenitors with high proliferative and self-renewal capacity. [Internet]. . Exp Hematol 1999. Aug [cited 2017 Nov 21];27:1338–45. [DOI] [PubMed] [Google Scholar]
- Wisgrill L, Schüller S, Bammer M, Berger A, Pollak A, Radke TF, et al. : Hematopoietic Stem Cells in Neonates: Any Differences between Very Preterm and Term Neonates? PLoS One 2014b. Sep 2;9:e106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Sun Y, Chen Z, Yao Y, Ma G: Hypoxic Preconditioning Inhibits Hypoxia-induced Apoptosis of Cardiac Progenitor Cells via the PI3K/Akt-DNMT1-p53 Pathway. Sci Rep 2016. Nov 4;6:30922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefifard M, Nasirinezhad F, Shardi Manaheji H, Janzadeh A, Hosseini M, Keshavarz M, et al. : Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther 2016. Dec 8;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X: Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep 2015. Jan;11:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


