Abstract
Self-regulation of brain activation using real-time functional magnetic resonance imaging neurofeedback (rtfMRI-nf) is an emerging approach for treating mood and anxiety disorders. The effect of neurofeedback training on resting-state functional connectivity warrants investigation as changes in spontaneous brain activation could reflect the association between sustained symptom relief and brain alteration. We investigated the effect of amygdala-focused rtfMRI-nf training on resting-state functional connectivity in combat veterans with and without posttraumatic stress disorder (PTSD) who were trained to increase a feedback signal reflecting left amygdala activity while recalling positive autobiographical memories (Zotev et al., 2018). The analysis was performed in three stages: i) first, we investigated the connectivity in the left amygdala region; ii) next, we focused on the abnormal resting-state functional connectivity identified in our previous analysis of this data (Misaki et al., 2018); and iii) finally, we performed a novel data-driven longitudinal connectome-wide analysis. We introduced a longitudinal multivariate distance matrix regression (MDMR) analysis to comprehensively examine neurofeedback training effects beyond those associated with abnormal baseline connectivity.
These comprehensive exploratory analyses suggested that abnormal resting-state connectivity for combat veterans with PTSD was partly normalized after the training. This included hypoconnectivities between the left amygdala and the left ventrolateral prefrontal cortex (vlPFC) and between the supplementary motor area (SMA) and the dorsal anterior cingulate cortex (dACC). The increase of SMA-dACC connectivity was associated with PTSD symptom reduction. Longitudinal MDMR analysis found a connectivity change between the precuneus and the left superior frontal cortex. The connectivity increase was associated with a decrease in hyperarousal symptoms. The abnormal connectivity for combat veterans without PTSD - such as hypoconnectivity in the precuneus with a superior frontal region and hyperconnectivity in the posterior insula with several regions - could also be normalized after the training. These results suggested that the rtfMRI-nf training effect was not limited to a feedback target region and symptom relief could be mediated by brain modulation in several regions other than in a feedback target area. While further confirmatory research is needed, the results may provide valuable insight into treatment effects on the whole brain resting-state connectivity.
Keywords: combat veterans, neurofeedback, amygdala, positive memories, prefrontal cortex, precuneus
Highlights
-
•
fMRI neurofeedback training effect on resting-state connectivity was examined
-
•
Left amygdala activity was trained to increase with positive memory
-
•
Neurofeedback normalized altered connectivity in veterans with and without PTSD
-
•
PTSD symptom reductions were significant but not specific to group (exp/ctrl)
-
•
Connectivity-symptom association was seen in mPFC and precuneus
1. Introduction
Neurofeedback training with real-time functional magnetic resonance imaging (rtfMRI-nf) enables self-regulation of brain activation by presenting ongoing brain activation measured with the blood oxygenation level dependent (BOLD) signal (Weiskopf, 2012). Emerging evidence suggests clinical utility of self-regulation of brain activation with rtfMRI-nf training. This includes, but is not limited to, rtfMRI-nf training to major depressive disorder (MDD) patients (Hamilton et al., 2016; Linden et al., 2012; Young et al., 2017a; Young et al., 2017b; Young et al., 2014; Zotev et al., 2016) and posttraumatic stress disorder (PTSD) patients (Gerin et al., 2016; Nicholson et al., 2017). These studies demonstrated that participants can learn to self-regulate feedback target regions as a result of rtfMRI-nf training. Symptom reduction effects, however, have not been consistent and the associations between therapeutic and neurobiological effects are not clear. As our knowledge of the neurofeedback treatment effect on whole brain activation is still limited, more exploratory research on how neurofeedback brain modulation impacts psychiatric symptoms is necessary.
Resting-state fMRI functional connectivity (Biswal et al., 1995) has potential for elucidating brain changes underlying rtfMRI-nf therapeutic effects. This measure evaluates correlations among BOLD signals during rest. Examining the effects of rtfMRI-nf on this spontaneous brain activation could yield insight into if the effect of neurofeedback training extends beyond a training context and results in sustained treatment effects. In fact, the effects of neurofeedback training with fMRI and/or EEG are not limited to a training task but have been observed on resting-state functional connectivity as well (Kluetsch et al., 2014; Kopel et al., 2017; Nicholson et al., 2016; Scheinost et al., 2013; Yuan et al., 2014).
Studies also showed that neurofeedback training effects are not restricted to a neurofeedback target region. Scheinost et al. (2013) examined the effect of rtfMRI-nf training to reduce orbitofrontal cortex (OFC) activity and found changes in connectivity in many brain regions including reduced connectivity with limbic structures and increased connectivity in the dorsolateral prefrontal cortex. Emmert et al. (2016) also reported a broad effect of the training in a meta-analysis of rtfMRI-nf training studies. These results suggest symptom reduction may be due to neurobiological changes beyond a targeted region of neurofeedback. The effect of training therefore needs to be examined across the whole-brain to elucidate the neurobiological basis of the therapeutic effect.
For whole-brain comprehensive functional connectivity analysis, a connectome-wide association approach has been proposed (Shehzad et al., 2014). This approach uses a nonparametric multivariate analysis of variance called multivariate distance matrix regression (MDMR). This enables a comprehensive search in whole-brain voxel-by-voxel connectivity without an a priori definition of a seed region (Anderson, 2001; Satterthwaite et al., 2016). Our previous study (Misaki et al., 2018) investigated the connectome-wide alteration in resting-state functional connectivity between the groups of combat veterans with and without PTSD and non-trauma exposed healthy controls (NC). The study identified altered connectivities for veterans with PTSD compared to NC, including decreased connectivities between the left parahippocampal region and the visual cortex and between multiple left lateral prefrontal regions and salience network (SN) regions. The study also identified altered connectivities for veterans without PTSD compared to NC including decreased connectivities between the left superior frontal region and the posterior default mode network areas, between the precuneus and the right transverse temporal area as well as the left superior temporal area, and increased connectivity in bilateral posterior insula regions. The analysis did not find a significant connectivity difference between the veterans with and without PTSD.
In the current study, we extended our previous analysis of resting-state functional connectivity (Misaki et al., 2018) by examining the effect of the rtfMRI-nf training on resting-state functional connectivity within the same participants. Details regarding the neurofeedback procedure and the effects of the procedure on brain activation during the task have been described in a prior report (Zotev et al., 2018). Participants were trained to increase the feedback signal from the left amygdala by recalling a positive autobiographical memory. Pathological hyperactivity of the amygdala is consistently observed for PTSD patients, both in response to the presentation of a negative stimulus (Etkin and Wager, 2007; Fonzo et al., 2010; Hayes et al., 2012; Patel et al., 2012; Pitman et al., 2012; Rauch et al., 2000; Shin et al., 2006; Simmons et al., 2011; St Jacques et al., 2011) and at rest (Koch et al., 2016; Wang et al., 2016). A meta-analysis demonstrated that the amygdala response is valence-general; it responds to both positive and negative stimulus presentations (Lindquist et al., 2016). Young et al. (2017a) indicated that the training to increase left amygdala activity with positive memory decreased amygdala response to negative stimuli. This training approach also demonstrated significant depression symptom reduction resulting from the procedure (Young et al., 2017b). Given the links between PTSD and abnormal functioning of the amygdala, along with evidence suggesting amygdala-focused neurofeedback reduces depressive symptoms among people with MDD, where abnormal amygdala activity has been observed, it stands to reason that the same rtfMRI-nf training approach has a potential for reducing PTSD symptoms. Indeed, veterans with PTSD showed symptom reduction after the training and the responder rate was larger for the experimental group who received left amygdala neurofeedback than for the control group who received a neurofeedback from a control region (parietal area putatively not involved in emotion regulation) (Zotev et al., 2018). However, there was no significant difference in symptom change between the groups. This suggests that both amygdala neurofeedback and a non-specific effect to the neurofeedback region could have contributed to symptom reduction.
The present report focused on changes in resting-state functional connectivity as a result of the training and how such changes were related to PTSD symptom reduction. We also examined resting-state functional connectivity for combat veterans without PTSD. Combat exposure could leave subclinical alterations in their resting-state brain activation (Misaki et al., 2018). The present study investigated how those alterations were affected by the neurofeedback training. A three-step approach to the analysis of resting-state data was employed to yield a comprehensive exploration of connectivity change. The first analysis investigated resting-state connectivity for the feedback target region, the left amygdala region of interest (ROI). An ordinary seed-based connectivity analysis from the ROI to the whole brain was done to examine altered connectivity for veterans and how that connectivity was affected by the training. The second analysis focused on changes in the abnormal resting-state functional connectivity at baseline that was identified in our previous connectome-wide analysis conducted within the same participants (Misaki et al., 2018). The third analysis investigated a connectome-wide training effect to identify the effect outside of abnormal connectivity. For this analysis, we developed a longitudinal MDMR analysis. The analysis enabled comprehensive examination of training effects and associations with symptom change in whole brain voxel-by-voxel connectivity.
2. Materials and methods
2.1. Participants
The participants included in the current analysis were drawn from the same participants described previously by Misaki et al. (2018). Forty male U.S. military combat veterans with PTSD and 22 male U.S. military combat veterans without PTSD (veteran control, VC) participated in the baseline resting-state fMRI scan session. Exclusion criteria included a clinically significant or unstable cardiovascular, pulmonary, endocrine, neurological, or gastrointestinal illness or unstable medical disorder, meeting DSM-IV criteria for substance abuse or substance dependence (other than nicotine) within 3 months prior to screening, endorsing suicidal intent or a suicide attempt within the preceding three months, current or past history of schizophrenia, schizoaffective disorder, bipolar disorder, or dementia, moderate to severe traumatic brain injury, and an inability to complete an MRI scan due to claustrophobia or general MRI exclusions (e.g. shrapnel inside body). Participants with vision and/or hearing loss severe enough to interfere with testing and participants not fluent in English were also excluded. See supplementary material “Veteran participants” section for more details regarding recruitment and inclusion/exclusion criteria. The study was approved by the Western Institutional Review Board (IRB), Puyallup, WA. All procedures were conducted according to the code of ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Participants provided written informed consent as approved by the IRB.
PTSD participants were randomly assigned to the experimental group (PTSD-exp, N = 25) where they received left amygdala rtfMRI-nf or to the control group (PTSD-ctrl, N = 11) where they received rtfMRI-nf from the left horizontal segment of the intraparietal sulcus, a region not involved in emotion regulation. Participants were blind to which group they were assigned. All VC participants were in the experimental group (VC-exp). The study consisted of seven visits. Several participants quit voluntarily before completing all the sessions. Four PTSD and one VC did not participate in the training sessions. Two PTSD-exp, one PTSD-ctrl, and one VC-exp quit before the second training session (Visit 4), two PTSD-exp, one PTSD-ctrl, and two VC quit before the third training session (Visit 5), and one PTSD-ctrl quit before the post-training resting-state session (Visit 6). As a result, 30 PTSD (21 PTSD-exp and 9 PTSD-ctrl) and 17 VC participants completed 3 sessions of rtfMRI-nf training and the post-training resting-state scan session.
Participants with excessive head motion (>40 censored volumes, see MRI measurement and image processing section below for details) were excluded from the analysis. Five PTSD and four VC participants in the baseline session and an additional three PTSD and three VC participants who completed the post-training session were excluded from the analysis. If a participant completed the study but baseline data was not available due to excessive head motion, his data was also excluded from the analysis. One VC participant did not complete the last neurofeedback session but performed the post-training resting-state scan. This participant was included in the analysis because the training effect on resting-state functional connectivity for this participant was not significantly different from others.
The current study only included participants who completed both the baseline and the post-training sessions. The final number of samples analyzed in the present study and their mean and standard deviation of age were as follows: N = 16 (30 ± 6 years-old) for PTSD-exp; N = 6 (31±9) for PTSD-ctrl; and N = 11 (36±1) for VC. Groups did not significantly differ in regards to age (F[2,30] = 1.791, p = .184). Motion size (a mean L2-norm of frame-wise displacement) was not different between groups in either session (Baseline: F[2,78] = 1.292, p = .281, Post-training: F[2,30] = 0.404, p = .671).
Of these participants, 8 PTSD participants (8 PTSD-exp) endorsed current MDD comorbidity, 5 PTSD participants (2 PTSD-exp, 3 PTSD-ctrl) endorsed partial remitted MDD comorbidity, and 3 PTSD participants (3 PTSD-exp) endorsed a history of MDD that was fully remitted at study time by the baseline assessment. Two VC subjects who participated only in the pre-training session endorsed a history of MDD that was fully remitted by the baseline assessment. A prior analysis indicated that remitted comorbidity was not related to the baseline connectivity difference for the VC group (Misaki et al., 2018).
2.2. RtfMRI-nf training schedule
Veteran groups participated in three days of rtfMRI-nf training sessions. Details of the training schedule and the training procedures at each visit are provided in the “Schedules of real-time fMRI neurofeedback (rtfMRI-nf) training” section of the supplementary material and Zotev et al. (2018). Supplementary fig. S1 shows mean feedback signals across training sessions for the participants who completed the post-training session. The participants could keep feedback signal increased during the training although the PTSD-ctrl group had difficulty in increasing the signal at several runs.
Resting-state scans at the 2nd and 6th visits were analyzed as the baseline and the post-training scans, respectively. No rtfMRI-nf training was performed at these visits and the resting-state scan was performed before any other task runs to avoid contamination from another task. The mean intervals between each visit (and its standard deviation) were 14 ± 13, 11 ± 6, 10 ± 7, 13 ± 8, and 11 ± 6 days for visits 1–2, 2–3, 3–4, 4–5 and 5–6, respectively. The mean interval between the last training session (Visit 5) and the follow-up assessment (Visit 7) was 15±7 days. There was no significant difference in the intervals between the groups.
The Clinician-Administered PTSD Scale (CAPS) for DSM-IV (Blake et al., 1995) and the PTSD Checklist - Military Version (PCL-M) (Weathers et al., 1993) were used to identify PTSD diagnosis and to measure symptom levels. The CAPS was administered at the first and the last visits by a research staff member trained to mastery in administration of the interview. The staff was blind to which group the participants were assigned. The PCL-M was administered at the 2nd to 6th visits. Depression and anxiety symptoms were also measured at the 2nd to 6th visits by the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Åsberg, 1979) and the Hamilton Anxiety Scale (HAM-A) (Hamilton et al., 1976), respectively.
2.3. MRI measurement and image processing
The same resting-state fMRI measurement and image processing procedure as described in Misaki et al. (2018) were used in both the baseline and the post-training sessions, which is summarized here. A single-shot gradient-recalled echo-planner imaging (EPI) sequence with sensitivity encoding (SENSE) was used for fMRI with imaging parameters of TR = 2000 ms, TE = 30 ms, FA = 90°, FOV = 240 mm, 34 axial slices with 2.9 mm thickness with 0.5 mm gap, matrix = 96 × 96, SENSE acceleration factor R = 2. The EPI images were reconstructed into a 128 × 128 matrix resulting 1.875 × 1.875 × 3.4 mm3 voxel volume. The resting fMRI run time was 6 min 50s (205 volumes). T1-weighted MRI images were acquired for anatomical reference with magnetization-prepared rapid gradient-echo (MPRAGE) sequence.
Analysis of Functional NeuroImages (AFNI) software (http://afni.nimh.nih.gov/afni/) was used for image processing. The process included despike, RETROICOR (Glover et al., 2000) and respiration volume per time (RVT) correction (Birn et al., 2008), slice-timing and motion corrections, nonlinear warping to the MNI template brain with resampling to 2mm3 voxels using the Advanced Normalization Tools (ANTs) software (Avants et al., 2008) (http://stnava.github.io/ANTs/). Further noise reduction was applied by regressing out three principal components of the ventricle signal, local white matter average signal (ANATICOR) (Jo et al., 2010), 12 motion parameters (3 shift and 3 rotation parameters with their temporal derivatives), and low-frequency fluctuation (3rd-order Legendre polynomial model) from the signal time course. Any fMRI time point with large motion (>0.25 mm frame-wise displacement (FD)) along with the following point was censored within the regression (Power et al., 2015).
2.4. ROI analysis in the neurofeedback target region
Baseline resting-state connectivity difference between the groups in the neurofeedback target region, LA and HIPS, were examined to complement the previous connectome-wide analysis (Misaki et al., 2018). LA region of interest (ROI) was anatomically defined using the Jülich histological atlas on MNI template brain (Eickhoff et al., 2005) provided with the FSL package. Voxels with larger than 50% probability of the left amygdala region were extracted. HIPS ROI was defined as 7-mm-radius sphere centered at x, y, z = −42, −51, 53 mm in the MNI template brain. The first principal component of voxel resting-state signal time-course was used as a seed time-course. The sign of the principal component signal was adjusted to make its correlation with the mean signal positive. The signal was extracted from a fully processed image (after regressing out noise components). Pearson's correlations between the seed time-course and time-courses in all other brain voxels were calculated and applied Fisher's z-transform (z = arctanh(r), where r is correlation coefficient) to make a connectivity map. This map was subject to the following group analyses.
The baseline abnormality of the ROI connectivity was examined by general linear model analysis with group (PTSD, VC, NC), age, and motion size as predictor variables. For this baseline analysis, we included participants who did not complete the rtfMRI-nf training akin to what we had done previously (Misaki et al., 2018) (see supplementary table S3 for the number of participants in the baseline). The statistical parametric map for the pairwise group contrast was thresholded by p < .005 voxel-wise and family-wise error correction by cluster-extent p < .016 (= 0.05/3 for Bonferroni correction of three group comparisons). A cluster-extent threshold was evaluated by permutation test with 10,000 repetitions (Eklund et al., 2016). An abnormal resting-state functional connectivity found in this analysis was examined its change after the training sessions using a longitudinal analysis described below.
2.5. Longitudinal analysis for the training effect on abnormal connectivity
The previous study (Misaki et al., 2018) identified altered connectivity across the PTSD, VC, and NC groups at baseline. The current study examined the effect of rtfMRI-nf training on these abnormal connectivities as well as on the LA connectivity. The training effect was examined by linear mixed-effect (LME) model analysis for longitudinal design. The LME model included fixed effects of session (baseline, post-training), group (PTSD-exp, PTSD-ctrl, VC-exp), session by group interaction, age, and motion size and a random effect of the subject on intercept. The LME analysis was performed with R language and environment for statistical computing (R Core Team, 2017) with nlme package (Pinheiro et al., 2017). Pairwise comparison of the groups was done within the LME fitted model by Tukey's multiple comparison method corrected by critical values from multivariate t-distribution with lsmeans package in R (Lenth, 2016). We also examined an LME model with additional regressors of symptom change and its interaction with session and group as fixed effects to search for a connectivity change that was associated with symptom change. The analysis for symptom association was done separately for each symptom measure only with the PTSD groups. The symptom association in each group within the LME fitted model was also tested with multiple comparison correction using critical values from multivariate t-distribution with lsmeans package in R (Lenth, 2016). All reported p-values for pairwise comparisons and the group-wise test were applied multiple testing correction.
The training effect on the neurofeedback target ROI was also examined in whole-brain voxels. The LME analysis was performed for all connectivities between the ROI and whole-brain voxels. The statistical parametric maps for the main effect of session and the interaction between session and group was thresholded by p < .005 voxel-wise and family-wise error correction by cluster-extent p < .05. Since a permutation test for the LME analysis was computationally too expensive, we used an improved cluster-size simulation with 3dClustSim in AFNI (Cox et al., 2017). The new approach used an improved spatial autocorrelation function to simulate the null distribution of cluster size that remedies the false positive problem (Cox et al., 2017).
2.6. Longitudinal MDMR for the connectome-wide training effect
A longitudinal MDMR analysis was performed for a comprehensive investigation of the training effect that was not limited to the abnormal connectivity at baseline. The longitudinal MDMR included the connectivity maps before and after the training for each subject. The distance matrix of these maps was the dependent variable in the MDMR. The design matrix was made following a longitudinal design example in Winkler et al. (2014) (example 6 in the appendix of Winkler et al. (2014)). This design matrix included session, group (PTSD-exp, PTSD-ctrl, VC-exp), session by group interaction, age, and motion size. In addition, subject-wise factor variables were included in the design matrix. These regressors had 1 at a pair of a same subject's samples and 0 for the others. This could regress out subject-wise average effect, so that the longitudinal analysis could find the session and the group effect on within-subject connectivity difference. Exchangeability block of permutation test in the MDMR was defined for each subject. That is, permutation was performed within a subject to randomize session order and then subject blocks were randomly permuted. This permutation randomized the order of the sessions as well as the subject-group correspondence.
We found this design matrix was rank-deficient due to collinearity between the subject-wise regressors and age and motion regressors, which made the MDMR estimation unstable. We solved this problem by orthogonalizing the design matrix using singular value decomposition (SVD) (Mandel, 1982). The design matrix was decomposed to X = USVT using SVD. VT is a transpose of V. MDMR analysis can be described as G = Xβ = USVTβ = Uα, where G is a centered negative distance matrix (G = CAC, where , , n is the number of subjects, I is the n × n identity matrix and 1 is a vector of n 1 s (Shehzad et al., 2014)) and α = SVTβ. This transformation improved the stability of the analysis because columns of U are orthogonal to each other. Pseudo-F value can be evaluated by , where H = UUT. SVD does not change the total amount of variance in the design matrix and covariance between the design matrix and distance matrix (Mandel, 1982) so that this operation does not change the F value except by improving the stability of computation with avoiding a singular matrix. MDMR evaluates an individual effect of regressor using a partial design matrix, XN, which is a design matrix excluding effects of interest columns. Pseudo-F value of the effect of interest is obtained by , where HI = H − HN, , UNSNVNT = SVD(XN), and mI is the number of effects of interest regressors. We note that this procedure with SVD is equivalent to the original MDMR when the design matrix X is full-rank.
The processed resting-state fMRI image was down-sampled to 4mm3 voxels to apply longitudinal MDMR. Significance of the pseudo-F value was evaluated by permutation test with 10,000 repeats and thresholded by p < .005 voxel-wise and family-wise error correction by cluster-extent p < .05. Cluster-extent threshold was evaluated by permutation test. The regions with a significant main effect of interest (sum of the effects of session, group, and the session by group interaction) in the MDMR were used as seed regions for post-hoc connectivity analysis.
A seed-based post-hoc analysis for the significant regions with the MDMR was done in the original resolution images. Seed regions were placed at a peak location of the significant cluster in the MDMR statistical map for the main effect of interest. Peak coordinates in each significant cluster separated by at least 30 mm were extracted. Seed area was a 6 mm-radius sphere centered at the peak coordinates of the MDMR statistical map. Mean signal time-course of the seed area was used as a reference signal to calculate correlations with other voxels. The statistical test of the post-hoc analysis was the same as in the LA ROI analysis using LME and 3dClustSim. We also performed a longitudinal MDMR analysis with additional regressors of symptom change and its interaction with session and group to examine an association between connectivity change and symptom change. The analysis for symptom association was done separately for each symptom measure only with the PTSD groups.
3. Results
3.1. Symptom measure
Symptom changes for PTSD patients have been reported in Zotev et al. (2018). The present analysis complemented the previous report by checking to ensure that the rtfMRI-nf procedure did not increase PTSD symptoms for veterans without PTSD (VC group) as well as examining the influence of MDD comorbidity and training intervals.
Table 1 shows symptom levels at the baseline and the post-training sessions for the veterans who completed post-training resting-state session. Post-training CAPS scores were not available for five PTSD-exp, one PTSD-ctrl, and one VC participants. Supplementary fig. S2 shows symptom change across sessions. At baseline, significant differences were seen in between the VC and the PTSD groups (p < .01 for all measures in pairwise t-test) but there was no significant difference between the PTSD-exp and the PTSD-ctrl groups in any symptom scores.
Table 1.
Symptom measures at baseline and post-training sessions.
| Baseline |
Post-training |
|||||
|---|---|---|---|---|---|---|
| PTSD-exp | PTSD-ctrl | VC-exp | PTSD-exp | PTSD-ctrl | VC-exp | |
| CAPS (total) | 51.7±16.7 | 59.2±21.0 | 2.6±2.6 | 38.2±19.8*** | 50.8±30.1 | 2.0±2.3 |
| CAPS (sub-B) | 9.9±6.9 | 15.2±7.1 | 0.0±0.0 | 7.5±6.1 | 11.2±10.3 | 0.2±0.6 |
| CAPS (sub-C) | 19.5±8.5 | 22.0±11.1 | 0.0±0.0 | 14.7±11.0** | 17.6±18.1 | 0.8±1.7 |
| CAPS (sub-D) | 22.1±5.9 | 22.0±5.0 | 2.6±2.6 | 15.9±7.6** | 22.0±6.0 | 1.0±2.2 |
| PCL-M | 42.2±10.6 | 49.3±16.8 | 19.8±3.0 | 36.0±12.8* | 45.0±23.5 | 18.1±1.4 |
| MADRS | 19.8±8.7 | 15.3±13.2 | 1.8±2.1 | 12.8±8.4** | 14.0±9.7 | 0.7±1.0 |
| HAM-A | 14.9±6.5 | 18.3±10.5 | 2.2±1.5 | 10.4±6.0** | 14.5±6.7 | 1.1±1.4 |
Means and standard deviations of symptom measures at the baseline and the post-training sessions. CAPS: Clinician-Administered PTSD Scale; CAPS (sub-B): CAPS Criterion B subscale, re-experiencing symptoms; CAPS (sub-C): CAPS Criterion C subscale, avoidance and numbing symptoms; CAPS (sub-D): CAPS Criterion D subscale, hyperarousal symptoms; PCL-M: MADRS: Montgomery-Åsberg Depression Scale; HAM-A: Hamilton Anxiety Rating Scale. * (p < .05), ** (p < .005), and *** (p < .001) indicate significant (corrected p-values) symptom change between the baseline and the post-training sessions.
LME longitudinal analysis showed a significant main effect of session on CAPS total scores (χ2[1] = 10.257, p = .001 with type II analysis of deviance test (Fox and Weisberg, 2011)), CAPS Criterion C subscale (sub-C; χ2[1] = 6.226, p = .013), CAPS Criterion D subscale (sub-D; χ2[1] = 9.938, p = .002), PCL-M total symptom scores (χ2[1] = 9.467, p = .002), MADRS (χ2[1] = 7.150, p = .007), and HAM-A (χ2[1] = 15.393, p < .001). Significant interaction between session and group was seen on CAPS total scores (χ2[2] = 7.673, p = .022), CAPS sub-C (χ2[2] = 7.350, p = .025), and CAPS sub-D (χ2[2] = 7.795, p = .020). However, a follow-up pairwise comparison on the session effect showed no significant difference between the PTSD-exp and PTSD-ctrl groups in any symptom scores. This was consistent with the previous report that found no difference in average symptom change between exp. and ctrl groups for PTSD patients (Zotev et al., 2018).
The effect of MDD comorbidity (not including partial remitted MDD) on symptom change for the PTSD-exp group was also examined because the PTSD-exp group included participants with current MDD comorbidity while the PTSD-ctrl did not (see supplementary table S1). Significant interaction between session and MDD comorbidity was seen only on CAPS sub-D (χ2[1] = 7.364, p = .007). Analysis of session effect for each group of PTSD with and without MDD comorbidity indicated that significant symptom reduction was seen for PTSD patients without MDD (t[9] = −4.808, p = .002) but not for PTSD patients with MDD (t[9] = −1.242, p = .418).
The effect of training intervals on symptom change was also examined by LME analysis with fixed effects of the session, the group, days between the last training session and the post assessment, their interaction as well as a random effect of participants on intercept. The analysis found no significant effect of interval length on symptom changes.
3.2. Connectivity in the neurofeedback target region
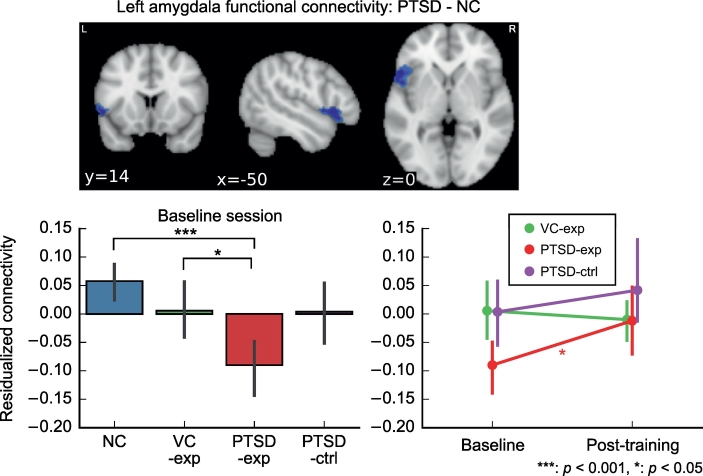
The analysis showed that the PTSD group had significantly lower connectivity between the LA and the left ventrolateral prefrontal cortex (vlPFC) than the NC group. Fig. 1 shows the baseline group difference in the mean connectivity between the LA and the voxels in the left vlPFC cluster for the current study group who complete the post-training session. This connectivity was significantly lower for PTSD-exp compared to both NC (t[55] = −5.154, p < .001) and VC (t[55] = −2.639, p = .050). MDD comorbidity did not associated with this connectivity alteration in the PTSD-exp group (t[31] = 0.5001, p = .620). No other significant difference of LA connectivity between the groups was found in the baseline analysis. The same analysis for HIPS connectivity at baseline did not find significant difference between the groups.
Fig. 1.
The region with significantly lower functional connectivity from the left amygdala ROI at the baseline session and its change between the sessions. Graphs show the group means and its 95% confidence intervals. Connectivity values are z-transformed correlation coefficients residualized with regard to age and motion.
Longitudinal LME analysis for the mean connectivity between the LA and the left vlPFC cluster showed tendency of main effect of session (χ2[1] = 3.459, p = .063) but no significant interaction between session and group (χ2[2] = 3.879, p = .144). Analysis for the session effect in each group showed a significant session effect only for the PTSD-exp group (t[29] = 2.554, p = .047). The connectivity was increased after the training for the PTSD-exp group (Fig. 1). This connectivity change, however, was not significantly associated with symptom change. Fig. 1 also indicated that the PTSD-exp group had lower baseline connectivity than the PTSD-ctrl group although the difference was not significant (t[55] = −2.152, p = .145). A whole-brain longitudinal analysis of the LA and HIPS connectivity with or without an effect of symptom change found no connectivity that was significantly associated with session and symptom change.
3.3. Training effects on abnormal resting-state functional connectivity
Supplementary material tables S4 and S5 show the altered connectivity for PTSD and VC compared to NC, respectively, at baseline that was identified in the previous study (Misaki et al., 2018). No significant difference between PTSD and VC was found in this study.
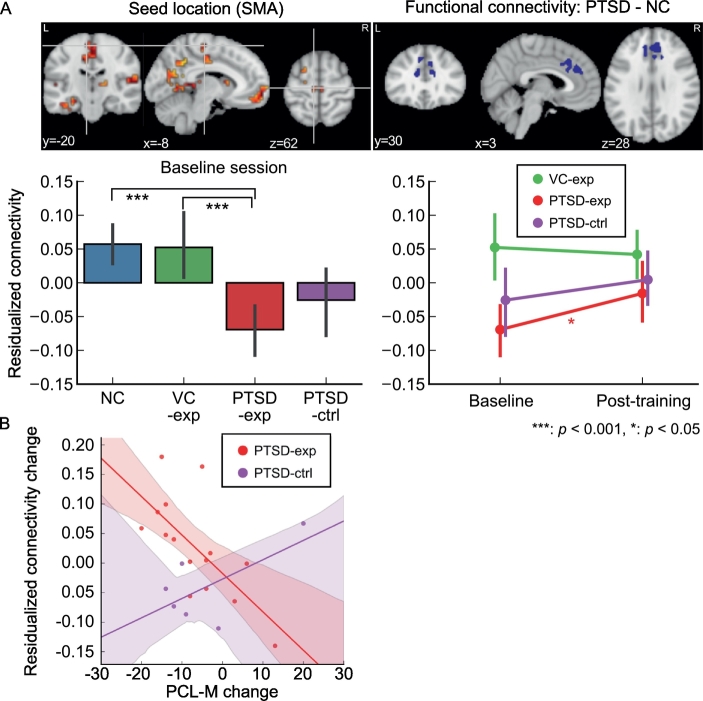
Fig. 2A shows connectivity that had been altered in the PTSD group at baseline (Misaki et al., 2018) and showed a significant change after training. Connectivity between the supplementary motor area (SMA) and the dorsal anterior cingulate cortex (dACC) was significantly lower in the PTSD group compared to the NC group at baseline. Baseline analysis excluding the participants who did not complete the post-training session indicated that this connectivity was significantly low for PTSD-exp group compared to NC (t[55] = −5.196, p < .001) and VC-exp (t[55] = −4.203, p < .001) but there was no significant difference between PTSD-exp and PTSD-ctrl (t[55] = −1.272, p = .577) and between PTSD-ctrl and VC-exp (t[55] = −2.120, p = .155). Longitudinal LME analysis for the mean connectivity between the seed (SMA) and the voxels in the altered connectivity cluster at dACC showed significant main effect of session (χ2[1] = 4.394, p = .036) but interaction between session and group was not significant (χ2[2] = 4.328, p = .115). Analysis of the session effect for each group showed a significant increase of connectivity only for the PTSD-exp group (t[29] = 2.758, p = .029). This connectivity increase was significantly associated with a decrease of PCL-M in the PTSD-exp group (Fig. 2B, t[15] = −3.092, p = .007) but not in the PTSD-ctrl group (t[15] = 1.262, p = .226). MDD comorbidity did not associate with the connectivity at baseline and its change after training.
Fig. 2.
Training effect on abnormal connectivity for PTSD. A. Upper panels show seed location identified with MDMR (upper left) and abnormal connectivity region found in a post-hoc analysis of PTSD–NC contrast (upper right). Lower panels show mean connectivity between the seed and the voxels in the cluster of significantly altered connectivity at the baseline. The connectivity value was a z-transformed correlation with regressing out age and motion effects. Error bars show 95% confidence interval of the mean value. B. Association between the PCL-M score change and the connectivity change for PTSD participants are shown with fitted lines. Shadow around the line indicates the 95% confidence intervals of a fitted line. The connectivity change is the change in z-transformed correlations with regressing out age and motion effects.
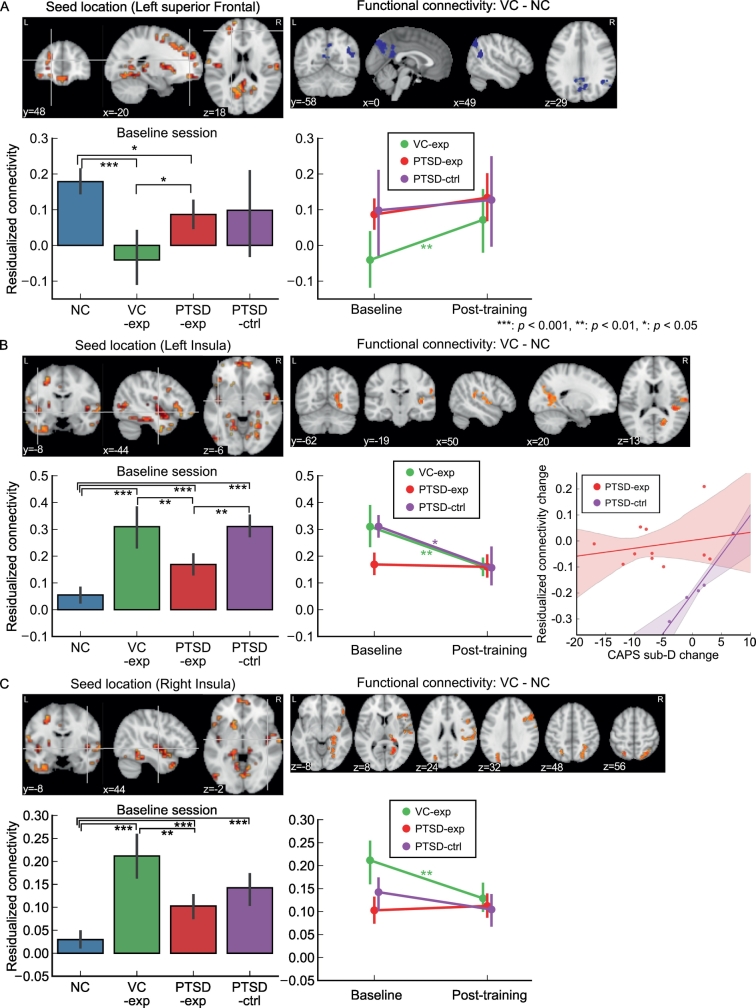
Fig. 3 shows connectivity that had been altered for the VC group at baseline (Misaki et al., 2018) and showed a significant change after training. Connectivity between the left superior frontal region and the precuneus and the supramarginal gyrus was significantly low for the VC group compared to the NC group at baseline (Fig. 3A). Baseline analysis excluding the participants who did not complete the post-training session indicated that this connectivity was significantly low for VC-exp compared to NC (t[55] = −5.443, p < .001) and PTSD-exp (t[55] = −2.904, p = .026). PTSD-exp also had lowered connectivity compared to NC (t[55] = −2.714, p = .041). Longitudinal LME analysis for the mean connectivity between the seed (left superior frontal region) and the voxels in the clusters of significantly altered connectivity showed significant main effect of session (χ2[1] = 12.031, p < .001) but interaction between session and group was not significant (χ2[2] = 3.66, p = .160). Analysis of the session effect in each group showed a significant session effect only for the VC-exp group (t[29] = 3.518, p = .004). This connectivity was increased after training for the VC-exp group (Fig. 3A). All significant clusters for the left superior frontal seed connectivity had a similar connectivity pattern at baseline and its change after training (supplementary fig. S3).
Fig. 3.
Training effect on abnormal connectivity for VC. For each panel A, B, and C, seed location (upper left) was identified with MDMR analysis and altered connectivity region (upper right) found in a post-hoc analysis of VC–NC contrast. Bar and line plots show mean connectivity between the seed and the voxels in the cluster of significantly altered connectivity at baseline. The connectivity value was a z-transformed correlation with regressing out age and motion effects. Error bars show the 95% confidence interval of the mean value.
The hyperconnectivity between the left insula seed and several brain areas for VC compared to NC has been identified in Misaki et al. (2018) at the baseline session (Fig. 3B). Baseline analysis excluding the participants who did not complete the post-training session indicated that this connectivity was significantly high for VC-exp compared to NC (t[55] = 6.317, p < .001) and PTSD-exp (t[55] = 3.982, p = .001). PTSD-exp and PTSD-ctrl also had increased connectivity compared to NC (t[55] = 4.039, p < .001 and t[55] = 6.317, p < .001, respectively). This connectivity was significantly higher for PTSD-ctrl than PTSD-exp (t[55] = 3.304, p = .009), despite random assignment of the groups. Longitudinal LME analysis for the mean connectivity between the left insula seed and the voxels in the clusters of significantly altered connectivity showed significant main effect of session (χ2[1] = 11.194, p = .004) and interaction between session and group (χ2[2] = 11.354, p = .003). Analysis for the session effect in each group showed a significant session effect for the VC-exp group (t[29] = −3.990, p = .001) and for the PTSD-ctrl group (t[29] = −3.156, p = .011). For the PTSD-ctrl group, the decrease in this connectivity was associated with a decrease in CAPS sub-D (hyperarousal) symptoms (Fig. 3B, t[10] = 2.754, p = .020). All significant clusters for the left insula seed connectivity had a similar connectivity pattern at baseline and its change after training (supplementary fig. S4).
The hyperconnectivity between the right insula seed and several brain areas for VC compared to NC has also been identified in Misaki et al. (2018) at the baseline session (Fig. 3C). Baseline analysis excluding the participants who did not complete the post-training session indicated that this connectivity was significantly high for VC-exp compared to NC (t[55] = 8.388, p < .001) and PTSD-exp (t[55] = 4.606, p < .001). PTSD-exp and PTSD-ctrl also had increased connectivity compared to NC (t[55] = 4.015, p = .001 and t[55] = 4.290, p < .001, respectively). Longitudinal LME analysis for the mean connectivity between the right insula seed and the voxels in the clusters of significantly altered connectivity showed the significant main effect of session (χ2[1] = 4.637, p = .031) and significant interaction between session and group (χ2[2] = 9.466, p = .009). Analysis for the session effect in each group showed significant session effect only for VC-exp (t[29] = −3.485, p = .005). All significant clusters for the right insula seed connectivity had a similar connectivity pattern at baseline and its change after training (supplementary fig. S5).
3.4. Longitudinal MDMR for connectome-wide training effect
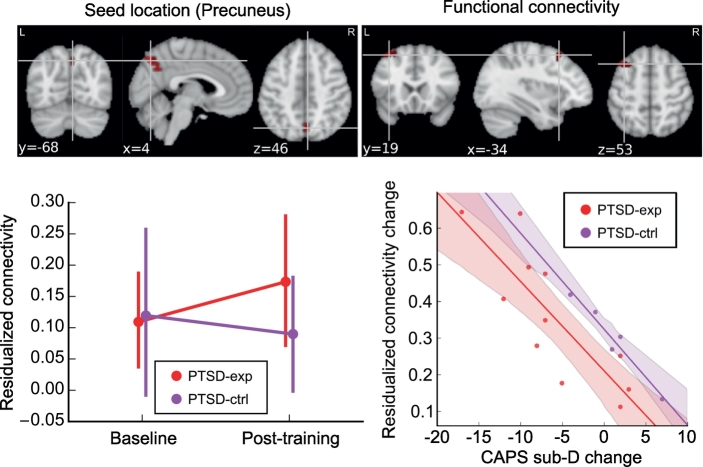
The connectome-wide training effect that was not limited to baseline abnormality was examined using longitudinal MDMR analysis. The analysis, however, found no significant effect of session or interaction between session and group when no symptom change was included in the model. When the change in CAPS sub-D (hyperarousal) score was included in the analysis, longitudinal MDMR found a significant cluster in the right precuneus region for the sum of the effect of interest (Fig. 4). The LME analysis for the functional connectivity from this region revealed a significant interaction between session and CAPS sub-D score change (χ2[1] = 14.150, p < .001) in the connectivity between the precuneus and the left superior frontal region (Fig. 4). The interaction between a change in CAPS sub-D score and group was not significant (χ2[1] = 0.017, p = .897). The increase in this connectivity was significantly associated with a decrease in CAPS sub-D (hyperarousal) symptoms for the PTSD-exp group (t[10] = −4.192, p = .002). The PTSD-ctrl group also showed a similar trend (t[10] = −2.015, p = .072).
Fig. 4.
Training effect on resting-state functional connectivity identified with longitudinal MDMR. Upper panels show seed location (upper left) identified with longitudinal MDMR and abnormal connectivity region (upper right) found in a post-hoc LME analysis for the interaction between the CAPS sub-D score change and the session. The bottom left panel shows mean connectivity between the seed and the voxels in a significant cluster. The connectivity value was z-transformed correlation with regressing out age and motion effects. Error bars show 95% confidence interval of the mean value. The bottom right panel shows the association between the CAPS sub-D change and the connectivity change for PTSD participants. The shadow around the line indicates the 95% confidence interval of a fitted line. The connectivity change is the difference in the z-transformed correlations with regressing out age and motion effects.
4. Discussion
A comprehensive exploratory investigation of training effects on resting-state functional connectivity showed that changes in connectivity could be observed both in left amygdala connectivity as well as in the SMA, ACC, insula, precuneus, and prefrontal regions. These changes were in the direction of normalizing abnormal connectivity. Connectivity increases between the SMA and the dACC and between the precuneus and the left superior frontal gyrus were associated with a decrease in PTSD symptoms measured by PCL-M and CAPS sub-D (hyperarousal) symptoms, respectively. MDD comorbidity was only associated with changes in the CAPS hyperarousal symptoms. Reduction of this symptom cluster was significant for PTSD patients without MDD comorbidity but not for PTSD patients with MDD comorbidity. These results suggest that the current rtfMRI-nf training approach, which has demonstrated effects in reducing MDD symptoms (Young et al., 2017b), could be effective in normalizing PTSD-related neurobiological functional alterations. The current results also suggested that the effect on resting-state functional connectivity could not be limited to the feedback target region and the training effect on those regions might be associated with symptom decreases.
There are, however, several open questions in this study such as: the observed baseline difference in functional connectivity between the PTSD-exp and PTSD-ctrl groups, the MDMR analysis found no significant difference between the PTSD-exp and the VC-exp groups, and the association between the connectivity normalization and symptom change. We also note that comprehensive exploratory analyses could increase the false positive rate. We discuss these issues focusing on the changes in resting-state functional connectivity and its association with symptom change.
4.1. Connectivity change in the neurofeedback target regions
The PTSD group evidenced hypoconnectivity between the LA and the vlPFC region compared to the NC group at baseline. Amygdala hyperactivity both at rest (Koch et al., 2016; Wang et al., 2016; Yan et al., 2013) and during negative emotion-inducing tasks (Etkin and Wager, 2007; Fonzo et al., 2010; Hayes et al., 2012; Patel et al., 2012; Pitman et al., 2012; Rauch et al., 2000; Shin et al., 2006; Simmons et al., 2011; St Jacques et al., 2011) has been consistently reported for PTSD. This hyperactivity suggests a failure of emotion regulation that could be instantiated by hypoconnectivity between the amygdala and the prefrontal emotion-regulation regions, including the ventromedial PFC (vmPFC) (Hayes et al., 2012; Patel et al., 2012) and medial prefrontal cortex (mPFC) (Jin et al., 2014). Brown et al. (2014) also reported decreased resting-state functional connectivity between the right basolateral amygdala (BLA) complex and the left inferior frontal gyrus for PTSD compared to trauma-exposed controls. The vlPFC has been linked to emotion regulation in many studies (Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014; Ochsner and Gross, 2005; Zilverstand et al., 2017). Taken together, the hypoconnectivity between the amygdala and the vlPFC was consistent with prior research, suggesting dysfunction of emotion regulation in PTSD related to deficient prefrontal activity and its hypoconnectivity with the amygdala.
The hypoconnectivity between the LA and left vlPFC was recovered after the training for the PTSD-exp group, while the HIPS region had no altered connectivity at baseline and no change of connectivity after training in any group. The connectivity change between the LA and left vlPFC may be more pronounced in emotion enhancement training than in emotion suppression training. Ellard et al. (2017) indicated that amygdala-vlPFC connectivity was increased during emotion acceptance and decreased during emotion suppression among people with anxiety disorders. Fonzo et al. (2017) investigated a difference between responders and non-responders to exposure therapy for PTSD in baseline brain response to emotion reactivity and emotion reappraisal tasks. They found that treatment response was associated with high brain activation during an emotion-reactivity task but not associated with an emotion-reappraisal task. This suggests that the treatment effect was not mediated by a reappraisal of emotion, but by accepting and habituating to emotion. Our approach that trained subjects to enhance positive emotion did not involve efforts to suppress or reappraise emotion and, indeed, significant symptom reduction was observed. Taken together, these results suggest that neurofeedback procedures that involve emotion enhancement might be more effective for PTSD treatment than those that involve emotion suppression or reappraisal training.
Although the connectivity increase was significant only for the PTSD-exp group, we could not conclude that the training effect was distinct to the LA neurofeedback. The PTSD-ctrl group had less hypoconnectivity than the PTSD-exp group at baseline that could affect the size of the training effect. This baseline difference was unfortunately emerged despite the use of random assignment to the group and could not be controlled, as we could not predict which participants would complete all sessions.
The LA connectivity change was not associated with PTSD symptom change. This indicated that symptom reduction effects of the rtfMRI-nf training may not be mediated by the modulation of the neurofeedback target region. In fact, we observed several resting-state connectivity changes in regions other than the LA, and some of those changes were associated with symptom reduction. This suggests that rtfMRI-nf training reduced symptoms not because of its localized effect in a target region, but because of the whole-brain co-modulating effect during the training. While the LA feedback signal could be useful for self-monitoring how well an emotional state is induced, there might be a better region to use for feedback in the treatment of PTSD, where activation or connectivity change is directly associated with symptom reduction.
4.2. Connectome-wide training effect
Several abnormal connectivities that had been identified in a connectome-wide analysis at baseline (Misaki et al., 2018) could be normalized after training. These include the increased connectivity between the SMA and the dACC, which showed hypoconnectivity for PTSD compared to NC at baseline (Fig. 2A). The increase in this connectivity was significantly correlated with a decrease in PCL-M scores (Fig. 2B). SMA and premotor regions have been consistently implicated as central nodes of the emotion regulatory network (Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014). Etkin et al. (2011) suggested that the dorsal-caudal part of the ACC and medial PFC are involved in both expression and reappraisal of negative emotion. Bonini et al. (2014) used intracerebral electroencephalography to investigate SMA function in an action monitoring task. They indicated that SMA is a center of performance monitoring that detects errors in action and sends signals to other mPFC and ACC regions to drive action correction. Ellard et al. (2017) also showed that dACC activation was increased during emotion acceptance. Taken together, the lowered connectivity between the SMA and dACC at baseline for PTSD might be associated with a deficit in emotion representation and emotion monitoring. The current training approach, which encourages subjects to repeatedly recall memories that elicit positive emotion, may normalize neurobiological mechanisms involved in emotion representation. As a correlated symptom change was seen for the self-reporting PCL-M score - and not for clinician-evaluated CAPS - this connectivity change might be particularly associated with subjective symptom evaluation. Normalized emotion representation, especially for positive aspects of an emotional response, might help to improve a subjective view of the symptom state.
Another connectivity change that could be associated with symptom relief was between the precuneus and the left superior frontal gyrus. The increase in this connectivity was associated with the decrease in CAPS-measured hyperarousal symptoms. This association was discovered in the longitudinal MDMR analysis (Fig. 4) and no significant abnormality was found at baseline. This effect was found only with an analysis including the CAPS hyperarousal symptoms. This indicated that the connectivity change was not common for all PTSD participants but seen only for the participants with hyperarousal symptom reduction. Considering that both the PTSD-exp and the PTSD-ctrl groups showed the same association between the changes in connectivity and symptom, this connectivity change was not specific to the LA neurofeedback signal but might be associated with symptom reduction in general, regardless of the training procedure. We should, however, note that this result did not indicate that either feedback signal had the same therapeutic effect because the reduction in hyperarousal symptoms was significant only for PTSD-exp but not for PTSD-ctrl group (Table 1).
The precuneus has been implicated in memory retrieval, mental imagery, and self-related processing (Brewin et al., 2010; Zhang and Li, 2012). A meta-analysis (Ochsner et al., 2012) indicated consistent left superior frontal activity in emotion regulation tasks. The increase of this connectivity, therefore, might be due to repetitive positive memory retrieval during the training. Interestingly, the increase in this connectivity was associated with a decrease in PTSD symptoms, while previous studies indicated abnormal hyperactivity in the precuneus among people with PTSD (Etkin and Wager, 2007; Lanius et al., 2006; Morey et al., 2008; Patel et al., 2012; Rabellino et al., 2015). Cwik et al. (2016) reported a positive correlation between the precuneus response to trauma-related pictures and subsequent PTSD symptom severity in acute stress disorder patients. Also, paroxetine treatment for PTSD has been shown to decrease resting-state amplitude of low-frequency fluctuation in the precuneus (Zhu et al., 2015). Collectively, these results suggest that elevated precuneus activity in PTSD is a pathological brain alteration. Although the increased connectivity between the precuneus and the superior frontal region could potentially enhance precuneus activity, this connectivity change was not associated with symptom increases, but rather decreases. This increase in connectivity might be associated with better control of precuneus activity or the pathological hyperactivity of the precuneus was specific to negative memories so that connectivity enhancement with positive memory retrieval may not have enhanced the abnormal activity.
It also warrants comment that the correlated symptom change was seen in CAPS-measured hyperarousal symptoms. This symptom measure consists of ‘difficulty in falling or staying asleep’, ‘irritability or outbursts of anger’, ‘difficulty in concentrating’, ‘hypervigilance’, and ‘exaggerated startle response.’ These symptoms might be associated with precuneus malfunction in memory retrieval, mental imagery, and self-related processing (Brewin et al., 2010; Zhang and Li, 2012). Abrupt happenings of memory retrieval or self-related thought could disturb sleep and concentration and disrupted mental imagery could be associated with irritability, hypervigilance, and startle response. The increased connectivity between the precuneus and the left superior frontal gyrus might help better control on such abnormal activity.
The current results also showed that reduction in CAPS hyperarousal symptoms was significant specifically for PTSD patients without MDD comorbidity. MDD patients often show hyperconnectivity in the default mode network (DMN) (Hamilton et al., 2015), and the precuneus is a core part of the DMN (Utevsky et al., 2014). The DMN hyperconnectivity is considered to be related to maladaptive rumination in MDD (Hamilton et al., 2015). The DMN malfunction with depression comorbidity might interfere with the connectivity change at the precuneus that could result in less reduction of hyperarousal symptoms for PTSD patients with MDD comorbidity.
The increase of precuneus connectivity after training was also observed for the VC group (Fig. 3A). The VC group showed hypoconnectivity in the precuneus compared to NC at baseline (Misaki et al., 2018). It has been suggested that decreased precuneus activation is associated with efforts to terminate self-reflection of aversive sensations (Vogt and Laureys, 2005; Whalley et al., 2013). The decreased connectivity in the precuneus for the VC group, therefore, could be adaptive in that it promotes healthy recovery by effectively suppressing retrieval of traumatic memories. The recovery of this connectivity might be considered a side effect of repetitive memory retrieval training. Importantly, however, no symptom change was seen for the VC group after the training. This also suggests that the increase of the precuneus connectivity did not enhance pathological activation.
Connectivity normalization for the VC group was also seen in the bilateral insula regions (Fig. 3B, C). The VC group had hyperconnectivity in these regions compared to NC at baseline (Misaki et al., 2018). This hyperconnectivity was normalized after training. These insula regions were more posterior areas than the anterior insula, which has been consistently reported as hyperactive among people with PTSD (Pitman et al., 2012; Wang et al., 2016; Whalley et al., 2013). While the mid-to-posterior insula is a region implicated in proprioceptive sensation (Craig, 2003; Menon and Uddin, 2010), its abnormality among people with PTSD has also been reported (Tursich et al., 2015; Zhang et al., 2016). A connectivity change in the posterior insula region with neurofeedback training was also reported in another rtfMRI-nf study. Scheinost et al. (2013) performed rtfMRI-nf training for subjects with significant contamination anxiety to reduce orbitofrontal cortex activation while viewing a contamination-related image. They found reduced connectivity in the bilateral mid and posterior insula after training. These suggest that mid and posterior insula regions may also be responsive to emotional and cognitive tasks and a decreased insula connectivity might be a general effect of emotion regulation training.
Interestingly, the PTSD-ctrl group, who had insula hyperconnectivity at baseline, also showed a decrease in this connectivity after training and its change was positively correlated with CAPS hyperarousal symptom change (Fig. 3B). This also suggests that a change in insula connectivity might not be specifically associated with the neurofeedback signal. The association between the connectivity and symptom reduction, however, is perplexing because hyperconnectivity in both the VC and the PTSD-ctrl groups at baseline suggested this connectivity was not associated with PTSD symptom, while the positive correlation between the changes in connectivity and symptoms suggested an association. We note that we cannot draw definitive conclusions from this result because the number of participants in the PTSD-ctrl group was small. However, this might indicate that baseline abnormality is not necessarily associated with observed symptoms and symptom reduction effects could be seen in the connectivity that is not associated with a symptom levels at baseline. The increased insula connectivity would not be associated with PTSD symptoms as the VC group evidenced this pattern at baseline. A decrease of this connectivity, however, might reduce PTSD symptoms. How such connectivity change works for symptom reduction remains unknown, but the current results with longitudinal MDMR also indicated that a connectivity change that was associate with symptom reduction was observed in a connectivity that was not abnormal at baseline (Fig. 4). Although the implication of normalizing insula hyperconnectivity is not entirely clear, it is important that no exacerbation of PTSD symptoms was observed for the VC participants and even a correlated symptom reduction was seen for the PTSD-ctrl group.
4.3. Connectivity difference between veterans with and without PTSD
The connectome-wide analysis at baseline (Misaki et al., 2018) did not reveal significant connectivity differences between veterans with PTSD versus without PTSD (VC). The current longitudinal MDMR analysis also did not find significant differences between the PTSD and the VC groups. A similar non-significant difference in resting-state connectivity between veterans with and without PTSD has been reported by DiGangi et al. (2016). They found no significant difference in the DMN resting-state connectivity between veterans with PTSD and combat-exposed controls but found significant differences between veterans and never-traumatized healthy controls. This indicated that combat exposure could alter resting-state connectivity regardless of the presence of PTSD.
We, however, note that no statistically significant difference does not necessarily indicate group equivalence. We observed alterations of resting-state functional connectivity specifically to either the PTSD or VC groups compared to the NC (Misaki et al., 2018). In addition, abnormality of resting-state connectivity could have large variability within a group that limits the sensitivity to see group differences. In fact, the baseline difference between the PTSD-exp and PTSD-ctrl groups indicated that the abnormality in the group was not homogeneous. We also note that the non-significant difference could be due to limited sensitivity of MDMR analysis, which is discussed in the next section.
4.4. Limitations
The major limitation of the current study was the small number and biased sample of PTSD-ctrl participants who completed the training sessions. Despite random assignment, there were large baseline differences between the PTSD-exp and the PTSD-ctrl groups in functional connectivities (e.g. Fig. 3B). We could not control for this group difference because we could not predict which participants would complete the feedback sessions. Due to this limitation, inferences regarding the specificity of training effects resulting from left amygdala-focused neurofeedback remain tentative. Training effects may not be specific to the neurofeedback but rather due to positive autobiographical memory recall. However, prior work has documented that positive memory recall did not improve mood ratings for depressed individuals (Joormann et al., 2007), suggesting that in the current study positive memory recall alone would not likely reduce symptoms. It is possible that the neurofeedback signal could enhance induction of a positive emotional state, which would explain why more patients showed symptom reduction in the PTSD-exp group compared to the PTSD-ctrl group (Zotev et al., 2018).
We should also note a limited statistical significance due to a comprehensive exploratory approach in this research. Although the abnormal connectivity was identified with a stringent whole-brain correction in our previous study (Misaki et al., 2018), the present analysis for the longitudinal effect was not corrected for multiple testing among the connectivities. The longitudinal MDMR analysis, while applied a stringent whole-brain correction, was also performed for several symptom scales. The present results, therefore, should be considered tentative and need further confirmation in future work. Notwithstanding the limited significance, we think the results of this exploratory analysis merit considerations because they may point to novel hypothesis for future confirmatory research.
A limited sensitivity of the longitudinal MDMR analysis also merits comment. Indeed, while the ROI analysis for left amygdala connectivity detected significant hypoconnectivity for PTSD at baseline, this was not detected in the MDMR analysis (Misaki et al., 2018). Also, significant connectivity changes that were detected when we focused on abnormal connectivities at baseline were not detected by the longitudinal MDMR. These dissociations suggest limited sensitivity of the MDMR analysis. Limited sensitivity was also due to an MDMR analysis mechanism. The MDMR evaluates between-subject distance of connectivity maps and this distance measure could be relatively insensitive to connectivity change between small regions because it summarizes the differences in a large dimensional connectivity map into one measure. The distance measure is also insensitive to how connectivity maps differ so that the same distance could be derived from different changes in connectivity patterns. These limitations could explain the dissociation between the results of ROI-based analysis and MDMR analysis. We need to note that a non-significant result of MDMR should not be interpreted as strong evidence of a negative finding. As there is no perfect analysis to investigate changes in whole-brain connectivity, the present study performed analyses for the amygdala ROI, pre-identified abnormal connectivity, and whole-brain connectome-wide.
We also did not consider individual variability of training success in the training sessions for the resting-state analysis. Including such variability might improve the sensitivity of the analysis to detect the effect of training on resting-state connectivity. We note, however, that since the treatment effect could be associated with brain regions other than the neurofeedback target region, training success might not be defined only by the regulation of the feedback target region. We will require further comprehensive investigation to elucidate the association between the brain activation during the training and changes in symptom and resting-state connectivity.
5. Conclusions
Comprehensive investigations for the effect of rtfMRI-nf training to increase left amygdala activity with positive memory retrieval on resting-state functional connectivity suggested that the training effect was not restricted to the feedback target region but could be seen in multiple connectivities that were associated with emotion regulation and memory retrieval, including the lateral prefrontal cortex, SMA, dACC, precuneus, and posterior insula regions. A connectome-wide approach using MDMR enables exploratory analysis of connectivity. Despite the limited sensitivity of MDMR, this approach is promising for connectome-wide investigation of training effects on brain functional connectivity. Interestingly, the symptom reduction did not appear due to a connectivity change in the amygdala, but rather due to connectivities between the SMA and dACC and between the precuneus and the left anterior frontal region. This suggests a neurofeedback effect on symptoms may be due to a larger brain network than only the targeted region. While these results need to be confirmed by further confirmatory researches, they may provide valuable insight into the treatment effect on the whole brain resting-state connectivity.
The connectivity changes suggested in this study might involve correcting emotion representation and memory retrieval. Such an effect could be promoted by positive emotion enhancement training rather than suppressing or reappraising negative emotions. Many rtfMRI-nf treatment studies have focused on decreasing abnormal activity (Gerin et al., 2016; Hamilton et al., 2016; Linden et al., 2012; Nicholson et al., 2017; Paret et al., 2016; Scheinost et al., 2013; Zilverstand et al., 2015). However, promoting positive emotional experience might help correct abnormal emotion representation and could have the same, if not more of, a therapeutic effect in treating the biological underpinnings of dysregulated emotion and mood disorder symptoms (Young et al., 2017b). Future development of rtfMRI-nf training methods may benefit from further testing of training approaches that promote healthy emotional brain responses rather than suppress abnormal responses.
Acknowledgement
This research was supported by W81XWH-12-1-0697 award from the U.S. Department of Defense, the Laureate Institute for Brain Research (LIBR), and the William K. Warren Foundation. The data of non-trauma exposed healthy males were provided by NIMH/NIH grant R01 MH098099. The funding agencies were not involved in the design of experiment, data collection and analysis, interpretation of results and preparation and submission of the manuscript. The authors thank all LIBR staff members, Matthew Meyer, M.D., and William Yates, M.D. for conducting psychiatric interviews, Tim Collins, Lisa Kinyon, and Megan Cole for administering clinical interviews and assessments, and Julie Owen, Julie Crawford, Leslie Walker, and Tressia Lewis for helping with MRI scanning. A part of this study was presented at 2017 annual meeting of the International Society for Magnetic Resonance in Medicine and at 2017 annual meeting of the Organization of Human Brain Mapping. The authors declare that they have no conflict of financial interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.08.025.
Appendix A. Supplementary data
Supplementary material
References
- Anderson M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Smith M.A., Jones T.B., Bandettini P.A. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blake D., Weathers F., Nagy L., Kaloupek D., Charney D., Keane T. National Center for Posttraumatic Stress Disorder, Behavioral Science Division, Boston VA Medical Center; Boston, MA: 1995. Clinician-Administered PTSD Scale for DSM-IV (CAPS-DX) [Google Scholar]
- Bonini F., Burle B., Liegeois-Chauvel C., Regis J., Chauvel P., Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343:888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Brewin C.R., Gregory J.D., Lipton M., Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 2010;117:210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V.M., LaBar K.S., Haswell C.C., Gold A.L., McCarthy G., Morey R.A. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., Weber J., Ochsner K.N. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Cwik J.C., Sartory G., Nuyken M., Schürholt B., Seitz R.J. Posterior and prefrontal contributions to the development posttraumatic stress disorder symptom severity: an fMRI study of symptom provocation in acute stress disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2016:1–11. doi: 10.1007/s00406-016-0713-6. [DOI] [PubMed] [Google Scholar]
- DiGangi J.A., Tadayyon A., Fitzgerald D.A., Rabinak C.A., Kennedy A., Klumpp H., Rauch S.A., Phan K.L. Reduced default mode network connectivity following combat trauma. Neurosci. Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard K.K., Barlow D.H., Whitfield-Gabrieli S., Gabrieli J.D., Deckersbach T. Neural correlates of emotion acceptance versus worry or suppression in generalized anxiety disorder. Soc. Cogn. Affect. Neurosci. 2017;12:1009–1021. doi: 10.1093/scan/nsx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert K., Kopel R., Sulzer J., Brühl A.B., Berman B.D., Linden D.E.J., Horovitz S.G., Breimhorst M., Caria A., Frank S., Johnston S., Long Z., Paret C., Robineau F., Veit R., Bartsch A., Beckmann C.F., Van De Ville D., Haller S. Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: how is brain regulation mediated? NeuroImage. 2016;124:806–812. doi: 10.1016/j.neuroimage.2015.09.042. Part A. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G.A., Simmons A.N., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G.A., Goodkind M.S., Oathes D.J., Zaiko Y.V., Harvey M., Peng K.K., Weiss M.E., Thompson A.L., Zack S.E., Lindley S.E., Arnow B.A., Jo B., Gross J.J., Rothbaum B.O., Etkin A. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am. J. Psychiatry. 2017;174:1163–1174. doi: 10.1176/appi.ajp.2017.16091072. appiajp201716091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Weisberg H.S. 2011. An R Companion to Applied Regression. (Second Edition. Sage) [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., Schaeffer D.J., Ball B.H., Schwarz N.F., Hussein A.A., Smart L.M., Sabatinelli D. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Gerin M.I., Fichtenholtz H.P., Roy A., Walsh C.J., Krystal J.H., Southwick S., Hampson M.P. Real-time fMRI neurofeedback with war veterans with chronic PTSD: a feasibility study. Front. Psychiatry. 2016;7:111. doi: 10.3389/fpsyt.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G.H., Li T.Q., Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hamilton M., Schutte N., Malouff J. Sourcebook of Adult Assessment: Applied Clinical Psychology. 1976. Hamilton anxiety scale (HAMA) pp. 154–157. [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Glover G.H., Bagarinao E., Chang C., Mackey S., Sacchet M.D., Gotlib I.H. Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Res. 2016;249:91–96. doi: 10.1016/j.pscychresns.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2012;2:1–13. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Qi R., Yin Y., Hu X., Duan L., Xu Q., Zhang Z., Zhong Y., Feng B., Xiang H., Gong Q., Liu Y., Lu G., Li L. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol. Med. 2014;44:1927–1936. doi: 10.1017/S003329171300250X. [DOI] [PubMed] [Google Scholar]
- Jo H.J., Saad Z.S., Simmons W.K., Milbury L.A., Cox R.W. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage. 2010;52:571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Siemer M., Gotlib I.H. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. J. Abnorm. Psychol. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kluetsch R.C., Ros T., Theberge J., Frewen P.A., Calhoun V.D., Schmahl C., Jetly R., Lanius R.A. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr. Scand. 2014;130:123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016;33:592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopel R., Emmert K., Scharnowski F., Haller S., Ville D.V.D. Distributed patterns of brain activity underlying real-time fMRI neurofeedback training. IEEE Trans. Biomed. Eng. 2017;64:1228–1237. doi: 10.1109/TBME.2016.2598818. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R., Lanius U., Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J. Psychiatr. Res. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lenth R.V. Least-squares means: the R package lsmeans. J. Stat. Softw. 2016;69:1–33. [Google Scholar]
- Linden D.E.J., Habes I., Johnston S.J., Linden S., Tatineni R., Subramanian L., Sorger B., Healy D., Goebel R. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex. 2016;26:1910–1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J. Use of the singular value decomposition in regression analysis. Am. Stat. 1982;36:15–24. [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M., Phillips R., Zotev V., Wong C.K., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Connectome-wide investigation of altered resting-state functional connectivity in war veterans with and without posttraumatic stress disorder. NeuroImage: Clinical. 2018;17:285–296. doi: 10.1016/j.nicl.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Åsberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Petty C.M., Cooper D.A., Labar K.S., McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Ros T., Frewen P.A., Densmore M., Theberge J., Kluetsch R.C., Jetly R., Lanius R.A. Alpha oscillation neurofeedback modulates amygdala complex connectivity and arousal in posttraumatic stress disorder. Neuroimage Clin. 2016;12:506–516. doi: 10.1016/j.nicl.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Schmahl C., Theberge J., Neufeld R.W., McKinnon M.C., Reiss J., Jetly R., Lanius R.A. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 2017;38:541–560. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C., Ruf M., Gerchen M.F., Kluetsch R., Demirakca T., Jungkunz M., Bertsch K., Schmahl C., Ende G. fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal-limbic brain connectivity. NeuroImage. 2016;125:182–188. doi: 10.1016/j.neuroimage.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team . 2017. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-131. [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ URL. [Google Scholar]
- Rabellino D., Tursich M., Frewen P., Daniels J., Densmore M., Théberge J., Lanius R. Intrinsic connectivity networks in post-traumatic stress disorder during sub-and supraliminal processing of threat-related stimuli. Acta Psychiatr. Scand. 2015;132:365–378. doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Whalen P.J., Shin L.M., McInerney S.C., Macklin M.L., Lasko N.B., Orr S.P., Pitman R.K. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Cook P.A., Bruce S.E., Conway C., Mikkelsen E., Satchell E., Vandekar S.N., Durbin T., Shinohara R.T., Sheline Y.I. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol. Psychiatry. 2016;21:894–902. doi: 10.1038/mp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Stoica T., Saksa J., Papademetris X., Constable R.T., Pittenger C., Hampson M. Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl. Psychiatry. 2013;3:e250. doi: 10.1038/tp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z., Kelly C., Reiss P.T., Cameron Craddock R., Emerson J.W., McMahon K., Copland D.A., Castellanos F.X., Milham M.P. A multivariate distance-based analytic framework for connectome-wide association studies. NeuroImage. 2014;93(Pt 1):74–94. doi: 10.1016/j.neuroimage.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Simmons A.N., Matthews S.C., Strigo I.A., Baker D.G., Donovan H.K., Motezadi A., Stein M.B., Paulus M.P. Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biol. Mood Anxiety Disord. 2011;1:6. doi: 10.1186/2045-5380-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques P.L., Botzung A., Miles A., Rubin D.C. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J. Psychiatr. Res. 2011;45:630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich M., Ros T., Frewen P.A., Kluetsch R.C., Calhoun V.D., Lanius R.A. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr. Scand. 2015;132:29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- Utevsky A.V., Smith D.V., Huettel S.A. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A., Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu J., Zhang J., Zhan W., Li L., Wu M., Huang H., Zhu H., Kemp G.J., Gong Q. Altered resting-state functional activity in posttraumatic stress disorder: A quantitative meta-analysis. Sci. Rep. 2016;6 doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Herman D.S., Huska J.A., Keane T.M. Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX. 1993. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. [Google Scholar]
- Weiskopf N. Real-time fMRI and its application to neurofeedback. NeuroImage. 2012;62:682–692. doi: 10.1016/j.neuroimage.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Whalley M.G., Kroes M.C., Huntley Z., Rugg M.D., Davis S.W., Brewin C.R. An fMRI investigation of posttraumatic flashbacks. Brain Cogn. 2013;81:151–159. doi: 10.1016/j.bandc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Brown A.D., Lazar M., Cressman V.L., Henn-Haase C., Neylan T.C., Shalev A., Wolkowitz O.M., Hamilton S.P., Yehuda R. Spontaneous brain activity in combat related PTSD. Neurosci. Lett. 2013;547:1–5. doi: 10.1016/j.neulet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Young K.D., Zotev V., Phillips R., Misaki M., Yuan H., Drevets W.C., Bodurka J. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D., Misaki M., Harmer C.J., Victor T., Zotev V., Phillips R., Siegle G.J., Drevets W.C., Bodurka J. Real-time functional magnetic resonance imaging amygdala neurofeedback changes positive information processing in major depressive disorder. Biol. Psychiatry. 2017;82:578–586. doi: 10.1016/j.biopsych.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D., Siegle G.J., Zotev V., Phillips R., Misaki M., Yuan H., Drevets W.C., Bodurka J. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am. J. Psychiatry. 2017;174:748–755. doi: 10.1176/appi.ajp.2017.16060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Young K.D., Phillips R., Zotev V., Misaki M., Bodurka J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 2014;4:690–701. doi: 10.1089/brain.2014.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li C.S. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage. 2012;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xie B., Chen H., Li M., Guo X., Chen H. Disrupted resting-state insular subregions functional connectivity in post-traumatic stress disorder. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000004083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Qiu C., Meng Y., Cui H., Zhang Y., Huang X., Zhang J., Li T., Gong Q., Zhang W., Lui S. Altered spontaneous neuronal activity in chronic posttraumatic stress disorder patients before and after a 12-week paroxetine treatment. J. Affect. Disord. 2015;174:257–264. doi: 10.1016/j.jad.2014.11.053. [DOI] [PubMed] [Google Scholar]
- Zilverstand A., Sorger B., Sarkheil P., Goebel R. fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front. Behav. Neurosci. 2015;9:148. doi: 10.3389/fnbeh.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A., Parvaz M.A., Goldstein R.Z. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage. 2017;151:105–116. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Yuan H., Misaki M., Phillips R., Young K.D., Feldner M.T., Bodurka J. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. Neuroimage Clin. 2016;11:224–238. doi: 10.1016/j.nicl.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Misaki M., Wong C.K., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. NeuroImage: Clinical. 2018;19:106–121. doi: 10.1016/j.nicl.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material