Abstract
Background
Apoptosis plays a key role in the pathogenesis of Parkinson disease (PD). Active caspase-3, which is a proapoptotic factor, has been shown to reduce cardiac contractility, causing cardiac dysfunction in many pathological diseases. Reduced cardiac contractility and cardiac autonomic dysfunction have been reported in PD patients and PD mice treated with MPTP. The aim of this study was to show the impact of PD induction on the expression of the apoptotic mediators p53 and active caspase-3 in the heart.
Material/Methods
Equal control and PD groups were formed by 20 randomly selected normal albino mice. We used 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (25 mg/kg) and probenecid (250 mg/kg) (MPTP/p) to induce chronic Parkinsonism in the PD group. Immunohistochemistry was performed to investigate the expression of p53, active caspase-3, and β-adrenergic receptor in hearts from the 2 animal groups.
Results
P53 and active caspase-3 expression was significantly higher in PD hearts than in the control hearts (p value <0.01). β-adrenergic receptor expression was significantly lower in PD hearts than in control hearts (p value <0.01).
Conclusions
Our results show an association of PD with p53 and active caspase-3 overexpression and β-adrenergic receptor underexpression in the heart, potentially promoting the cardiac autonomic dysfunction frequently observed in PD.
MeSH Keywords: Caspase 3, Heart, Parkinsonian Disorders, Tumor Suppressor Protein p53
Background
Parkinson disease is among the most common neurodegenerative diseases [1]. It is caused by substantial depletion in dopamine due to the degeneration of dopaminergic neurons in the substantia nigra pars compacta [2]. Parkinson disease is characterized by motor and non-motor symptoms [3–5]. Motor symptoms include at-rest tremor, bradykinesia, and akinesia [6,7] and non-motor symptoms include cardiovascular dysfunction [8,9].
MPTP is a neurotoxin that crosses the blood-brain barrier to enter the CNS [10], where it is metabolized into the active metabolite 1-methyl-4-phenylpyridinium (MPP+), which selectively kills and destroys the dopaminergic neurons only causing PD induction [10]. An in vitro study has shown downregulation of the major isoform of parkin (PARK2) following treatment with MPP+ [11]. Parkin mutations are the most common cause of the autosomal recessive juvenile-onset form of PD, and they are involved in other pathological conditions, such as glioblastoma multiforme [12]. Parkin isoforms are expressed in human gliomas and have consequently been proposed as specific markers of malignancy and potential tools for diagnosing brain tumors [13].
Apoptosis is a programmed cell death that is involved in the pathogenesis of PD, causing the loss of dopaminergic neurons [14,15]. Apoptosis is mediated by the tumor suppressor protein p53 [16]. P53 causes the activation of caspases, which constitute a family of cysteine proteases [17]. Although caspases are synthesized in the cell as inactive zymogens, they are cleaved and consequently activated in response to apoptotic stimuli [18,19]. Initiator caspases, activated by the apoptotic stimuli, cleave and subsequently activate executioner caspases, which in turn cleave the various cellular substrates, resulting in the morphological characteristics of apoptosis [18–20].
The apoptotic mediators p53 and active caspase-3 are overexpressed in PD brains, proposing the implication of apoptosis in PD [21,22]. Increased levels of p53 and active caspase-3 have been illustrated in different cardiovascular diseases, including myocardial infarction, heart failure, and various types of cardiomyopathy [23,24]. Furthermore, apoptosis has been proposed to contribute to cardiomyocyte loss in cardiac pathologies, including sinoaortic denervation [25,26]. Hence, we hypothesized that p53 and active caspase-3 are involved in the pathogenesis of the cardiovascular dysfunction frequently seen in PD. We used immunohistochemistry and light microscopy to investigate any alterations in the cardiac expression of p53 and active caspase-3 subsequent to inducing PD by MPTP/p.
Material and Methods
Animals
Individual cages with standard chow and water were used to house 20 normal albino mice at 22±1°C and 12-h dark/light cycle. The mice were separated into 2 equal groups: control and PD. Animal-related protocols were carried out according to the recommendations of the Institutional Animal Care and Use Committee at Jordan University of Science and Technology. A previously described protocol was used to induce chronic Parkinson disease in the mice [27,28]. In summary, intraperitoneal doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (25 mg/kg) and subcutaneous doses of probenecid (250 mg/kg) were administered in PD mice every 3.5 days for 5 weeks. Simultaneous intraperitoneal saline (25 mg/kg) injections were administered in control mice. Using cervical dislocation, the mice were sacrificed 4 weeks after MPTP/p treatment, when considerable striatal dopamine depletion and cardiac autonomic dysfunction, characterizing PD, have been shown to have occurred [27,29].
Immunohistochemistry of β-adrenergic receptor, P53 and active caspase-3 in the cardiac tissue
Animals were sacrificed and their dissected hearts were fixed in 4% paraformaldehyde. Subsequently, the tissue specimens were processed and embedded in paraffin. Then, serial longitudinal 4-μm–thick paraffin-embedded sections were cut. The sections were processed via immunohistochemistry according to previously described protocols [28,30–36]. To summarize, the sections were firstly deparaffinized and rehydrated. Then, antigen retrieval was performed. After that, the sections were incubated in 3% hydrogen peroxidase in methanol and subsequently washed using phosphate-buffered saline (PBS). Next, some of the sections were incubated with anti-β-adrenergic receptor antibody (Cat #: ab3442, Abcam, Cambridge, MA, USA) and other sections were incubated with anti-p53 antibody (Cat #: sc-6243, Santa Cruz Biotechnology, Santa Cruz, CA, USA); the rest of the sections were incubated with anti-active caspase-3 antibody (Cat #: ab13847, Abcam, Cambridge, MA, USA) using the dilutions suggested by the manufacturer. Following that, the sections were rinsed in PBS before and after incubating them with biotinylated secondary antibody (LSAB kit, Dako Carpinteria, CA, USA). After that, streptavidin HRP (LSAB kit, Dako, Carpinteria, CA, USA) was used to incubate the sections, which were subsequently rinsed with PBS. Next, sections were incubated in 3′-Diaminobenzidine (0.05% DAB) until the desired intensity was visualized. Subsequently, the reaction was stopped under tap water. After that, hematoxylin was used to stain all the sections, which were then observed under the light microscope. Primary antibodies were omitted from the processing of the negative control slides. Sections of PD gastrocnemius skeletal muscle were processed as a positive control for p53 and active caspase-3.
Data collection and analysis
Data were collected as described previously [28,30–36]. Briefly, 10 sections from each animal in each group were tested microscopically and photographed using a digital camera. Ten randomly selected regions from each slice were evaluated for β-adrenergic receptor, p53, and active caspase-3 expression in the heart. Using Adobe Photoshop software, the entire pixels area occupied by positive staining was counted in each region and calculated as a proportion of the entire pixels region (Figures 1B, 1D, 2C, 3C). After that, the mean of the pixels region of positive staining relative to the entire pixels region was computed for every mouse in every group.
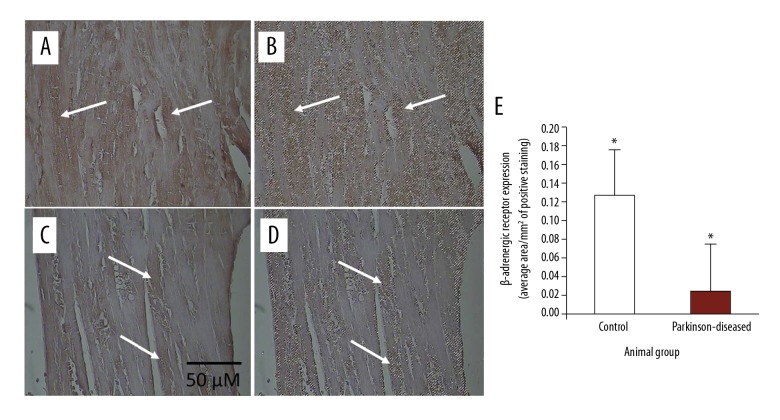
Figure 1.
Immunohistochemical staining of β-adrenergic receptor in longitudinal 4-μm-thick paraffin-embedded heart sections. (A) From control. (C) From Parkinson-diseased (PD). Scale bar shown in (C) applies to all images in the figure. β-adrenergic receptor immunostaining was very strongly observed in the control hearts. In contrast, β-adrenergic receptor immunoreactivity is weak in hearts from the PD group (such as those at the tips of the arrows). Areas of positive immunostaining of β-adrenergic receptor, shown in brown at the tips of the arrows in control (A) and PD (C) heart sections, are mapped as pixel areas at the tips of the arrows (B, D, respectively). (E) β-adrenergic receptor levels decreased significantly in the PD hearts compared to those in the control group (* P<0.01).
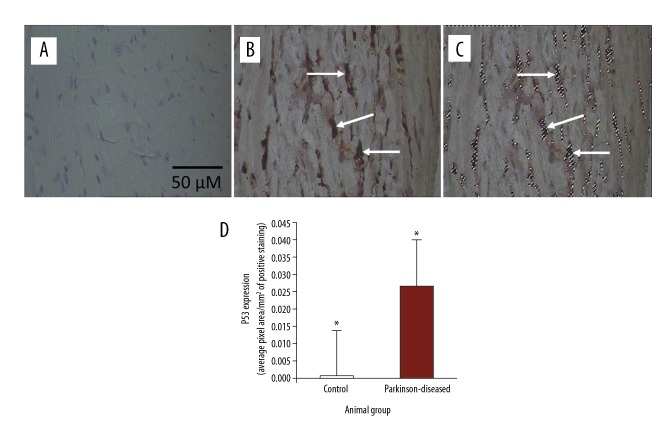
Figure 2.
Immunohistochemical staining of p53 in longitudinal 4-μm–thick paraffin-embedded heart sections. (A) From control. (B) From Parkinson-diseased (PD). Scale bar shown in (A) applies to all images in the figure. p53 immunostaining was weak in the control hearts. In contrast, p53 immunoreactivity is very strongly observed in hearts from the PD group (at the tip of the arrows). Areas of positive immunostaining of p53, shown in brown at the tips of the arrows in PD heart (B), are mapped as pixel areas at the tips of the arrows (C). (D) p53 levels increased significantly in the PD hearts compared to those in the control group (* P<0.01).
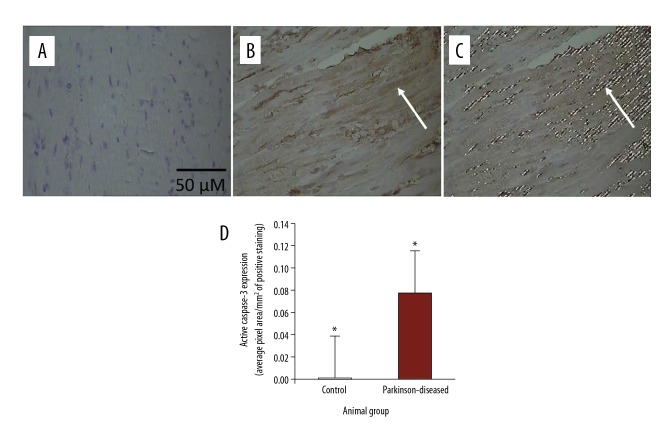
Figure 3.
Immunohistochemical staining of active caspase-3 in longitudinal 4-μm–thick paraffin-embedded heart sections. (A) From control. (B) From Parkinson-diseased (PD). Scale bar shown in (A) applies to all images in the figure. Active caspase-3 immunostaining was weak in the control hearts. In contrast, active caspase-3 immunoreactivity is very strongly observed in hearts from the PD group, such as that at the tip of the arrow. Areas of positive immunostaining of active caspase-3, shown in brown in PD hearts (B), are mapped as pixel areas, such as that at the tip of the arrow (C). (D) Active caspase-3 levels increased significantly in the PD hearts compared to those in the control group (* P<0.01).
Statistical analysis
The averages of the expression of β-adrenergic receptor, p53, and active caspase-3 in the heart for each animal in each group were calculated. Then, the independent-samples t test was performed to compare the expression of β-adrenergic receptor, p53, and active caspase-3 between PD hearts and control hearts (n=10 animals per group, 10 sections per animal) by SPSS software version 19.0, (SPSS Inc., USA). Differences in the expression of β-adrenergic receptor, P53, and active caspase-3 were regarded as statistically significant at p value <0.05.
Results
Immunohistochemistry showed highly abundant β-adrenergic receptor immunostaining in the heart sections from control mice (Figure 1A) but relatively weak immunostaining of β-adrenergic receptor was detected in heart sections from PD mice (Figure 1C). β-adrenergic receptor expression was significantly (p<0.01) reduced in the hearts following inducing PD by MPTP/p treatment (Figure 1E).
Immunohistochemistry revealed weak p53 immunostaining in heart sections from control mice (Figure 2A). In contrast, p53 immunostaining was very conspicuous in heart sections from PD mice (Figure 2B). p53 expression was significantly (p<0.01) upregulated in the hearts subsequent to inducing PD by MPTP/p (Figure 2D).
Active caspase-3 immunoreactivity was weak in the control hearts (Figure 3A). In comparison, active caspase-3 immunostaining was very abundant in heart sections from PD mice (Figure 3B). The expression of active caspase-3 was statistically significantly (p<0.01) elevated following PD induction (Figure 3D).
Discussion
Our study is the first to show the effect of inducing PD on the expression of apoptotic mediators – p53 and active caspase-3 – in the heart. We showed p53 and active caspase-3 overexpression in the cardiac muscle after inducing PD by MPTP/p.
Chronic parkinsonism has been demonstrated to be induced in mice by treating them with MPTP/p [27,37]. Significant dopamine depletion and concomitant dopaminergic neuronal loss, which are characteristic of PD, have been reported 3 weeks after MPTP/p treatment [29]. Cardiovascular autonomic dysfunction is a PD symptom that has been reported in mouse models of PD [38]. Cardiac dysfunction and depressed cardiac contractility have been reported in mice treated with MPTP [38,39]. Additionally, β-adrenergic receptor expression in myocardial membranes, as well as response to norepinephrine in myocytes, was reduced in MPTP-treated mice [39]. Consistent with the above evidence, our results revealed significant reduction in β-adrenergic receptor expression in the hearts of PD mice compared with control mice (Figure 1). Hence, we assessed the expression of p53 and active caspase-3 as a potential mechanism promoting cardiac dysfunction observed in PD at 4 weeks after MPTP/p treatment.
P53 is expressed at low levels in adult non-stressed tissues due to its rapid turnover [40,41]. This is in line with our results revealing weak p53 in the control hearts (Figure 2A). Apoptosis has been shown to be involved in the degeneration of the dopaminergic neurons causing PD [42]. In addition, increased p53 has been shown in the PD-diseased brain, mediating apoptosis [43–49]. Additionally, increased p53 has been reported in denervated skeletal muscle and in many cardiovascular diseases, such as heart failure [50,51]. P53 is involved in the development of end-stage cardiac disease, also known as heart failure, which results from various cardiovascular diseases [51]. Thus, our results (Figure 2B, 2D) revealing p53 overexpression in the heart subsequent to PD induction are in line with these previous reports [47–49,51,52].
The present findings are the first to illustrate alterations in p53 expression in cardiac tissue following PD induction. P53 has been demonstrated to stimulate proteolysis and consequent activation of caspase-3, leading to apoptosis [53–59]. Hence, we sought to examine the changes in active caspase-3 expression subsequent to inducing PD. Very low levels of active caspase-3 are reported in normal hearts [60]. This supports our result of weak active caspase-3 immunoreactivity in the control hearts (Figure 3A). Active caspase-3 has been reported to be involved in the pathogenesis of many heart diseases, including myocardial infarction, cardiac dysfunction, and heart failure [61–63]. Caspase activation has been shown to play an important role in myocardial cell dysfunction [64,65]. Consistent with this, our results (Figure 3B, 3D) revealed elevated levels of active caspase-3 in the cardiac tissue subsequent to inducing PD.
P53 and active caspase-3 overexpression has been proposed to mediate apoptosis, contributing to neuronal loss in the substantia nigra pars compacta, resulting in PD [66]. Apoptosis has been shown to be involved in the pathogenesis of many pathological conditions [67–71]. For instance, apoptosis of chondrocytes has been shown to cause degenerative processes in articular cartilage osteoarthritis [67,68]. In addition, aging-induced decline in hippocampal serotonin and related detrimental features, such as cognitive decline, have been associated with apoptosis in the hippocampus [69,70]. Indeed, hippocampal apoptosis was inhibited by enhanced tryptophan intake, which potentially increased serotonin neurotransmission [70]. Additionally, aging-induced degenerative processes and associated apoptosis in the brain were inactivated by low-fat diet [71]. Furthermore, apoptosis has been suggested to contribute to cardiomyocyte loss that causes myocardial dysfunction in many cardiovascular diseases, including myocardial infarction, and in various types of cardiomyopathy [72]. Consistently, our investigation found overexpression of p53 and active caspase-3 (Figures 2, 3) in PD hearts, which mediate apoptosis, consequent to inducing PD.
Conclusions
In summary, cardiac autonomic dysfunction is a non-motor symptom that frequently develops at an early stage of PD in PD patients and in MPTP-treated mice, and it has unmet medical needs. To the best of our knowledge, the present study is the first and earliest preclinical study identifying potential therapeutic targets in cardiovascular autonomic dysfunction. Our findings are the first to indicate a correlation of PD with the underexpression of β-adrenergic receptor and the overexpression of the apoptotic mediators – p53 and active caspase-3 – in the cardiac muscle, indicating the occurrence of apoptosis in the heart, potentially promoting the cardiac dysfunction frequently observed in PD. We propose considering the inhibition of p53 and/or active caspase-3 in treating the cardiac dysfunction frequently seen in PD. Additionally, we propose testing the effect of nutrition, such as enhanced phenylalanine and/or tyrosine intake and low-fat diet intake, on the expression of the apoptotic mediators in MPTP-treated mice.
Footnotes
Source of support: This study was financially supported by The Deanship of Research at Jordan University of Science and Technology, Irbid, Jordan. Grant #: 20130252
References
- 1.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115(6):1449–57. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stott SR, Barker RA. Time course of dopamine neuron loss and glial response in the 6-OHDA striatal mouse model of Parkinson’s disease. Eur J Neurosci. 2014;39(6):1042–56. doi: 10.1111/ejn.12459. [DOI] [PubMed] [Google Scholar]
- 3.Jellinger KA. Neuropathobiology of non-motor symptoms in Parkinson disease. J Neural Transm (Vienna) 2015;122(10):1429–40. doi: 10.1007/s00702-015-1405-5. [DOI] [PubMed] [Google Scholar]
- 4.Dewey RB, Jr, Taneja A, McClintock SM, et al. Motor symptoms at onset of Parkinson disease and risk for cognitive impairment and depression. Cogn Behav Neurol. 2012;25(3):115–20. doi: 10.1097/WNN.0b013e31826dfd62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Lloret S, Rascol O. Piribedil for the treatment of motor and non-motor symptoms of Parkinson Disease. CNS Drugs. 2016;30(8):703–17. doi: 10.1007/s40263-016-0360-5. [DOI] [PubMed] [Google Scholar]
- 6.Mazzoni P, Shabbott B, Cortes JC. Motor control abnormalities in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(6):a009282. doi: 10.1101/cshperspect.a009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magrinelli F, Picelli A, Tocco P, et al. Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinsons Dis. 2016;2016 doi: 10.1155/2016/9832839. 9832839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahina M, Mathias CJ, Katagiri A, et al. Sudomotor and cardiovascular dysfunction in patients with early untreated Parkinson’s disease. J Parkinsons Dis. 2014;4(3):385–93. doi: 10.3233/JPD-130326. [DOI] [PubMed] [Google Scholar]
- 9.Strano S, Fanciulli A, Rizzo M, et al. Cardiovascular dysfunction in untreated Parkinson’s disease: A multi-modality assessment. J Neurol Sci. 2016;370:251–55. doi: 10.1016/j.jns.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Choi SJ, Panhelainen A, Schmitz Y, et al. Changes in neuronal dopamine homeostasis following 1-methyl-4-phenylpyridinium (MPP+) exposure. J Biol Chem. 2015;290(11):6799–809. doi: 10.1074/jbc.M114.631556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Cognata V, Maugeri G, D’Amico AG, et al. Differential expression of PARK2 splice isoforms in an in vitro model of dopaminergic-like neurons exposed to toxic insults mimicking Parkinson’s disease. J Cell Biochem. 2018;119(1):1062–73. doi: 10.1002/jcb.26274. [DOI] [PubMed] [Google Scholar]
- 12.Maugeri G, D’Amico AG, Reitano R, et al. Parkin modulates expression of HIF-1alpha and HIF-3alpha during hypoxia in gliobastoma-derived cell lines in vitro. Cell Tissue Res. 2016;364(3):465–74. doi: 10.1007/s00441-015-2340-3. [DOI] [PubMed] [Google Scholar]
- 13.Maugeri G, D’Amico AG, Magro G, et al. Expression profile of parkin isoforms in human gliomas. Int J Oncol. 2015;47(4):1282–92. doi: 10.3892/ijo.2015.3105. [DOI] [PubMed] [Google Scholar]
- 14.Venderova K, Park DS. Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a009365. pii: a009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olanow CW. The pathogenesis of cell death in Parkinson’s disease – 2007. Mov Disord. 2007;22(Suppl 17):S335–42. doi: 10.1002/mds.21675. [DOI] [PubMed] [Google Scholar]
- 16.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–14. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 17.Soengas MS, Alarcón RM, Yoshida H, et al. Apaf-1 and Caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284(5411):156–59. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 18.Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. 2013;5(6) doi: 10.1101/cshperspect.a008672. pii: a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erekat NS. Cerebellar Purkinje cells die by apoptosis in the shaker mutant rat. Brain Res. 2017;1657:323–32. doi: 10.1016/j.brainres.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Erekat NS. Autophagy precedes apoptosis among at risk cerebellar Purkinje cells in the shaker mutant rat: An ultrastructural study. Ultrastruct Pathol. 2018;42(2):162–69. doi: 10.1080/01913123.2018.1424744. [DOI] [PubMed] [Google Scholar]
- 21.Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14(4):478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann A, Hunot S, Michel PP, et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA. 2000;97(6):2875–80. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim NH, Kang PM. Apoptosis in cardiovascular diseases: Mechanism and clinical implications. Korean Circ J. 2010;40(7):299–305. doi: 10.4070/kcj.2010.40.7.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang LP, Jiang YC, Yu XF, et al. Ginsenoside Rg3 improves cardiac function after myocardial ischemia/reperfusion via attenuating apoptosis and inflammation. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/6967853. 6967853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao X, Zhang S-H, Chu Z-X, Su D-F. Apoptosis is involved in the cardiac damage induced by sinoaortic denervation in rats. Clin Exp Pharmacol Physiol. 2003;30(5–6):362–68. doi: 10.1046/j.1440-1681.2003.03840.x. [DOI] [PubMed] [Google Scholar]
- 26.Tao X, Zhang S-H, Shen F-M, Su D-F. High-level apoptosis is persistent in myocardiocytes of sinoaortic-denervated rats. J Hypertens. 2004;22(3):557–63. doi: 10.1097/00004872-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ. Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord. 2008;14(Suppl 2):S112–15. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erekat NS. Apoptotic mediators are upregulated in the skeletal muscle of chronic/progressive mouse model of Parkinson’s disease. Anat Rec (Hoboken) 2015;298(8):1472–78. doi: 10.1002/ar.23124. [DOI] [PubMed] [Google Scholar]
- 29.Potashkin JA, Kang UJ, Loomis PA, et al. MPTP administration in mice changes the ratio of splice isoforms of fosB and rgs9. Brain Res. 2007;1182:1–10. doi: 10.1016/j.brainres.2007.08.080. [DOI] [PubMed] [Google Scholar]
- 30.Erekat N, Al-Khatib A, Al-Jarrah M. Heat shock protein 90 is a potential therapeutic target for ameliorating skeletal muscle abnormalities in Parkinson’s disease. Neural Regen Res. 2014;9(6):616–21. doi: 10.4103/1673-5374.130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erekat NS, Al-Jarrah MD, Al Khatib AJ. Treadmill exercise training improves vascular endothelial growth factor expression in the cardiac muscle of type I diabetic rats. Cardiol Res. 2014;5(1):23–29. doi: 10.14740/cr314w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Jarrah MD, Erekat NS. Parkinson disease-induced upregulation of apoptotic mediators could be attenuated in the skeletal muscle following chronic exercise training. NeuroRehabilitation. 2017;41(4):823–30. doi: 10.3233/NRE-172196. [DOI] [PubMed] [Google Scholar]
- 33.Erekat N, Al Khatib A, Al-Jarrah M. Endurance exercise training attenuates the up regulation of iNOS in the skeletal muscles of chronic/progressive mouse model of Parkinson’s disease. J Neurol Res. 2013;3:108–13. [Google Scholar]
- 34.Erekat NS, Al-Jarrah AA, Shotar AM, Al-Hourani ZA. Methyl. Methacrylate-induced increase in the hepatic expression of tumor necrosis factor alpha and activation of nuclear factor kappa beta in the rat. Int J Pharmacol. 2018 doi: 10.3923/ijp.2018. in Press. [DOI] [Google Scholar]
- 35.Erekat NS, Al-Jarrah MD. Interleukin-1 beta and tumor necrosis factor alpha upregulation and nuclear factor kappa B activation in the skeletal muscle from a mouse model of chronic/progressive Parkinson disease. Med Sci Monit. 2018 doi: 10.3923/ijp.2018. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Jarrah M, Erekat N, Al Khatib A. Upregulation of vascular endothelial growth factor expression in the kidney could be reversed following treadmill exercise training in type I diabetic rats. World J Nephrol Urol. 2014;3:25–29. [Google Scholar]
- 37.Petroske E, Meredith GE, Callen S, et al. Mouse model of Parkinsonism: A comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106(3):589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- 38.Fleming SM. Cardiovascular autonomic dysfunction in animal models of Parkinson’s disease. J Parkinsons Dis. 2011;1(4):321–17. doi: 10.3233/JPD-2011-11042. [DOI] [PubMed] [Google Scholar]
- 39.Ren J, Porter JE, Wold LE, et al. Depressed contractile function and adrenergic responsiveness of cardiac myocytes in an experimental model of Parkinson disease, the MPTP-treated mouse. Neurobiol Aging. 2004;25(1):131–38. doi: 10.1016/s0197-4580(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 40.Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53(1) Cancer Res. 1996;56(11):2649–54. [PubMed] [Google Scholar]
- 41.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351(6326):453–56. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 42.Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12(1):25–31. [PubMed] [Google Scholar]
- 43.Benchimol S. p53-dependent pathways of apoptosis. Cell Death Differ. 2001;8(11):1049–51. doi: 10.1038/sj.cdd.4400918. [DOI] [PubMed] [Google Scholar]
- 44.Shen Y, White E. p53-dependent apoptosis pathways. Adv Cancer Res. 2001;82:55–84. doi: 10.1016/s0065-230x(01)82002-9. [DOI] [PubMed] [Google Scholar]
- 45.Martin LJ, Kaiser A, Yu JW, et al. Injury-induced apoptosis of neurons in adult brain is mediated by p53-dependent and p53-independent pathways and requires Bax. J Comp Neurol. 2001;433(3):299–311. doi: 10.1002/cne.1141. [DOI] [PubMed] [Google Scholar]
- 46.Liu B, Behura SK, Clem RJ, et al. P53-mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster. PLoS Pathog. 2013;9(2):e1003137. doi: 10.1371/journal.ppat.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mogi M, Kondo T, Mizuno Y, Nagatsu T. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett. 2007;414(1):94–97. doi: 10.1016/j.neulet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 48.da Costa CA, Sunyach C, Giaime E, et al. Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson’s disease. Nat Cell Biol. 2009;11(11):1370–75. doi: 10.1038/ncb1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de la Monte SM, Sohn YK, Ganju N, Wands JR. P53- and CD95-associated apoptosis in neurodegenerative diseases. Lab Invest. 1998;78(4):401–11. [PubMed] [Google Scholar]
- 50.Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol. 2005;565(Pt 1):309–23. doi: 10.1113/jphysiol.2004.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das B, Young D, Vasanji A, et al. Influence of p53 in the transition of myotrophin-induced cardiac hypertrophy to heart failure. Cardiovasc Res. 2010;87(3):524–34. doi: 10.1093/cvr/cvq068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Chung L, Ng YC. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim Biophys Acta. 2006;1762(1):103–9. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Pratheeshkumar P, Sheeja K, Kuttan G. Andrographolide induces apoptosis in B16F-10 melanoma cells by inhibiting NF-kappaB-mediated bcl-2 activation and modulating p53-induced caspase-3 gene expression. Immunopharmacol Immunotoxicol. 2012;34(1):143–51. doi: 10.3109/08923973.2011.588233. [DOI] [PubMed] [Google Scholar]
- 54.Pratheeshkumar P, Thejass P, Kutan G. Diallyl disulfide induces caspase-dependent apoptosis via mitochondria-mediated intrinsic pathway in B16F-10 melanoma cells by up-regulating p53, caspase-3 and down-regulating pro-inflammatory cytokines and nuclear factor-kappabeta-mediated Bcl-2 activation. J Environ Pathol Toxicol Oncol. 2010;29(2):113–25. doi: 10.1615/jenvironpatholtoxicoloncol.v29.i2.50. [DOI] [PubMed] [Google Scholar]
- 55.Chan KM, Rajab NF, Siegel D, et al. Goniothalamin induces coronary artery smooth muscle cells apoptosis: The p53-dependent caspase-2 activation pathway. Toxicol Sci. 2010;116(2):533–48. doi: 10.1093/toxsci/kfq151. [DOI] [PubMed] [Google Scholar]
- 56.Son YO, Hitron JA, Wang X, et al. Cr(VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol Appl Pharmacol. 2010;245(2):226–35. doi: 10.1016/j.taap.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi HS, Cho MC, Lee HG, Yoon DY. Indole-3-carbinol induces apoptosis through p53 and activation of caspase-8 pathway in lung cancer A549 cells. Food Chem Toxicol. 2010;48(3):883–90. doi: 10.1016/j.fct.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 58.Lopergolo A, Pennati M, Gandellini P, et al. Apollon gene silencing induces apoptosis in breast cancer cells through p53 stabilisation and caspase-3 activation. Br J Cancer. 2009;100(5):739–46. doi: 10.1038/sj.bjc.6604927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nigam N, Prasad S, George J, Shukla Y. Lupeol induces p53 and cyclin-B-mediated G2/M arrest and targets apoptosis through activation of caspase in mouse skin. Biochem Biophys Res Commun. 2009;381(2):253–58. doi: 10.1016/j.bbrc.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 60.Todor A, Sharov VG, Tanhehco EJ, et al. Hypoxia-induced cleavage of caspase-3 and DFF45/ICAD in human failed cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;283(3):H990–95. doi: 10.1152/ajpheart.01003.2001. [DOI] [PubMed] [Google Scholar]
- 61.Chang J, Wei L, Otani T, et al. Inhibitory cardiac transcription factor, SRF-N, is generated by caspase 3 cleavage in human heart failure and attenuated by ventricular unloading. Circulation. 2003;108(4):407–13. doi: 10.1161/01.CIR.0000084502.02147.83. [DOI] [PubMed] [Google Scholar]
- 62.Narula J, Pandey P, Arbustini E, et al. Apoptosis in heart failure: Release of cytochrome C from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA. 1999;96(14):8144–49. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bott-Flugel L, Weig HJ, Uhlein H, et al. Quantitative analysis of apoptotic markers in human end-stage heart failure. Eur J Heart Fail. 2008;10(2):129–32. doi: 10.1016/j.ejheart.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Neviere R, Fauvel H, Chopin C, et al. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med. 2001;163(1):218–25. doi: 10.1164/ajrccm.163.1.2003109. [DOI] [PubMed] [Google Scholar]
- 65.Fauvel H, Marchetti P, Chopin C, et al. Differential effects of caspase inhibitors on endotoxin-induced myocardial dysfunction and heart apoptosis. Am J Physiol Heart Circ Physiol. 2001;280(4):H1608–14. doi: 10.1152/ajpheart.2001.280.4.H1608. [DOI] [PubMed] [Google Scholar]
- 66.Lev N, Melamed E, Offen D. Apoptosis and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):245–50. doi: 10.1016/S0278-5846(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 67.Musumeci G, Castrogiovanni P, Trovato FM, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16(9):20560–75. doi: 10.3390/ijms160920560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musumeci G, Castrogiovanni P, Mazzone V, et al. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur J Histochem. 2014;58(2):2371. doi: 10.4081/ejh.2014.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Musumeci G, Castrogiovanni P, Castorina S, et al. Changes in serotonin (5-HT) and brain-derived neurotrophic factor (BDFN) expression in frontal cortex and hippocampus of aged rat treated with high tryptophan diet. Brain Res Bull. 2015;119(Pt A):12–18. doi: 10.1016/j.brainresbull.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Musumeci G, Castrogiovanni P, Szychlinska MA, et al. Protective effects of high Tryptophan diet on aging-induced passive avoidance impairment and hippocampal apoptosis. Brain Res Bull. 2017;128:76–82. doi: 10.1016/j.brainresbull.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Castrogiovanni P, Li Volti G, Sanfilippo C, et al. Fasting and fast food diet play an opposite role in mice brain aging. Mol Neurobiol. :2018. doi: 10.1007/s12035-018-0891-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Zhao ZQ, Morris CD, Budde JM, et al. Inhibition of myocardial apoptosis reduces infarct size and improves regional contractile dysfunction during reperfusion. Cardiovasc Res. 2003;59(1):132–42. doi: 10.1016/s0008-6363(03)00344-4. [DOI] [PubMed] [Google Scholar]