Abstract
All plants flower late in their life cycle. For example, in Arabidopsis, the shoot undergoes a transition and produces reproductive flowers after the adult phase of vegetative growth. Much is known about genetic and environmental processes that control flowering time in mature plants. However, little is understood about the mechanisms that prevent plants from flowering much earlier during embryo and seedling development. Arabidopsis embryonic flower (emf1 and emf2) mutants flower soon after germination, suggesting that a floral repression mechanism is established in wild-type plants that prevents flowering until maturity. Here, we show that polycomb group proteins play a central role in repressing flowering early in the plant life cycle. We found that mutations in the Fertilization Independent Endosperm (FIE) polycomb gene caused the seedling shoot to produce flower-like structures and organs. Flower-like structures were also generated from the hypocotyl and root, organs not associated with reproduction. Expression of floral induction and homeotic genes was derepressed in mutant embryos and seedlings. These results suggest that FIE-mediated polycomb complexes are an essential component of a floral repression mechanism established early during plant development.
Plants flower late in their life cycle, after embryogenesis, seedling development, and a period of vegetative growth (1). For example, during the Arabidopsis life cycle, double fertilization results in the formation of an embryo and endosperm, an organ that nourishes the embryo. The embryo develops within the seed and forms an axis, cotyledons, and shoot and root apical meristems (2). When the seed germinates, the seedling has two cotyledon leaves of embryonic origin. Subsequently, the shoot apical meristem generates a rosette of vegetative leaves. After a period of vegetative growth, in response to genetic and environmental signals, the shoot apical meristem of the adult plant undergoes a dramatic transition, and the inflorescence is initiated by internode elongation and the production of sessile cauline leaves and secondary inflorescences. Finally, in the late inflorescence phase, nodes give rise to solitary flowers comprised of whorls of sepals, petals, stamens, and carpels arranged in a crucifer phyllotaxis typical of Arabidopsis flowers (3).
A great deal is understood about the genes and environmental signals (e.g., day length, light quality, temperature) that control the transition to flowering in the mature Arabidopsis plant (4–6). This knowledge is due, in part, to the isolation and analysis of mutations in genes that accelerate or delay flowering time during the adult stage of Arabidopsis development after the formation of vegetative rosette leaves. By contrast, much less is known about processes that prevent the embryo or the seedling from initiating floral development. Seedlings with mutations in the Embryonic Flower (EMF1 and EMF2) genes skip the vegetative phase of growth and immediately initiate inflorescence development (7, 8). Thus, EMF genes are required for normal vegetative shoot development. It is possible that EMF genes represent part of a floral repression mechanism, established early in plant development, that prevents flowering until later in the vegetative stage of plant development (3, 7, 9). Recently, EMF1 was shown to encode a polypeptide that has no homology with any protein of known function (10). Thus, little is known about the molecular mechanisms responsible for early floral repression in the Arabidopsis seedling.
Arabidopsis Fertilization Independent Endosperm (FIE) is a Trp-Asp dipeptide (WD)-motif polycomb protein related to Drosophila Extra Sex Combs (ESC) and mouse Embryonic Ectoderm Development (EED) (11). The maternal FIE allele is required for proper development of the embryo and endosperm. Loss-of-function fie mutations cause precocious endosperm formation before fertilization and prolonged endosperm nuclear proliferation after fertilization, indicating that FIE functions as a suppressor of endosperm development (12–14). However, because embryos with a maternal null fie allele abort, it has not been possible to generate and examine the postembryonic phenotypes of homozygous fie mutant plants. Thus, the extent that FIE-mediated polycomb complexes might regulate postembryonic development is not known. We have addressed this issue by using a modified FIE transgene that specifically provides FIE protein for seed viability, revealing new functions for polycomb proteins during plant development. Here, we show that the shoot apical meristem of fie mutant seedlings skips vegetative development and produces flowers and floral organs. Floral-like shoots were also observed on the seedling hypocotyl and root. Moreover, fie mutant embryos and seedlings activate the expression of floral induction genes. These results indicate that FIE-mediated polycomb complexes constitute a floral repression mechanism that is established very early during plant development.
Materials and Methods
Plant Materials.
Seedlings were grown on hormone-free agar plates [0.5× MS salts (GIBCO/BRL)], 2% sucrose, Gamborg's B5 vitamins (GIBCO/BRL), and 0.8% agar (Difco). Plants were grown on soil under long day conditions (16 h light). LFY∷GUS (LFY promoter ligated to GUS cDNA) seed was provided by the Arabidopsis Biological Resource Center (CS6297). AG∷GUS (AG promoter ligated to GUS cDNA; KB9) and AP3∷GUS (AP3 promoter ligated to GUS cDNA; 890-7) seed were provided by D. Weigel and T. Jack.
Transgenic Plants.
To construct a FIE-green fluorescent protein (GFP) fusion protein, the FIE cDNA was amplified with primers FIE-Sal (5′-ATGTCGACGAGAGTCAGACAGAGAGAGAG-3′) and FIE-NcoI (5′-CACCATGGCTCCGCCACCTCCGCCACCCTTGGTAATCACGTCCCAGCG-3′), digested with SalI and NcoI, and inserted into the CaMV35S-sGFPS65T-Nos vector (15) to obtain the CaMV35S∷FIE-sGFPS65T-Nos plasmid. The GFP gene used in these experiments lacks subcellular localization sequences. To have transcription of the FIE-GFP fusion gene under the control of a FIE promoter, 1,639 base pairs of FIE 5′-flanking sequences were amplified with primers FIE-Sph (5′-TTCCTATAAGAGGCATGCGAGGAAGCGAGCAAGTACACA-3′) and FIE-SalRV (5′-TCTGACTCTCGTCGACTAATCTAAGCTCACAAGTCTCTCA-3′), digested with SalI and Sph, and inserted into the CaMV35S∷FIE-sGFPS65T-Nos plasmid to create the CaMV35S∷pFIE∷FIE-sGFPS65T-Nos plasmid. This plasmid was digested with PstI and HindIII to liberate the pFIE∷FIE-GFP (FIE promoter ligated to FIE and GFP cDNA sequences) transgene that was then inserted into pBI101.1 (16), replacing the β-glucuronidase (GUS) reporter, to create plasmid pBI(pFIE∷FIE-GFP) that was introduced into Agrobacterium GV3101. Arabidopsis plants (i.e., Columbia-0 ecotype) were transformed as described (11). Transgenic plants were crossed with heterozygous fie-1/FIE plants, and F1 progeny were self-pollinated to generate plants heterozygous for fie-1/FIE and homozygous for pFIE∷FIE-GFP. Expression of pFIE∷FIE-GFP was highly restricted compared with previously reported constructs (17), and this result may be due, at least in part, to the process of creating a SalI site in the FIE cDNA that changed nucleotides −38 to −36 (translation start site is position 1) in the 5′-untranslated region from GTG to CGA.
Plant Genotypes.
To amplify endogenous FIE gene sequences we used primers 579dXba (5′-CATTACTGCCATTGGTGTATCTCTTATTATCTA-3′) and 48S4 (5′-CACTGTTGACGTCAATGACTCGG-3′). Because the 579dXBa primer is located in the first intron of the FIE gene, it does not amplify any sequences associated with the pFIE∷FIE-GFP transgene. We distinguished fie-1 and wild-type FIE alleles by digesting the amplified products with XbaI restriction endonuclease followed by agarose gel electrophoresis. The PCR amplified product from the wild-type FIE allele is digested, whereas the fie-1 allele is not. To amplify pFIE∷FIE-GFP transgene sequences, we used a primer in the FIE cDNA region, FIE-RTf (5′-CTGTAATCAGGCAAACAGCC-3′) and a primer in the GFP cDNA region, GFP274r (5′-GCATGGCGGACTTGAAGA-3′). PCR reactions were performed as described (17). Seedlings with the pFIE∷FIE-GFP transgene were identified by growth on agar plates with 50 μg/ml kanamycin.
Gene Expression.
RNA (0.2 μg) was converted to cDNA as described (18). Amplification of cDNA by PCR involved incubation at 94°C for 2 min, followed by 30 cycles except when indicated at 94°C for 30 s, 55°C for 30 s, and 72°C for 10 s. Gene-specific primers were as follows: AP1 (AP1500f 5′-GATGATATAAGAACATCGAACATTTGCCA-3′ and AP1991r 5′-GATGATATAAGAACATCGAACATTTGCCA-3′), LFY (LFY4042f 5′-GCTAAAGACCGTGGCGAA-3′ and LFY5371r 5′-GCATCCACCACGTCCAGA-3′), AG (AG5523f 5′-GTTGATTTGCATAACGATAACCAGA-3′ and AG6116r 5′-TTCACTGATACAACATTCATGGGAT-3′), PI (PI1500f 5′-CACGCCATTGAACATGGCCT-3′ and PI2020r 5′-TCGATGATCAATCGATGACCAA-3′), actin (ACT.conf 5′-GATTTGGCATCACACTTTCTACAATG-3′ and ACT.conr 5′-GTTCCACCACTGAGCACAATG-3′), PK (PKf 5′-CTTCACCACATGGGTCACA-3′ and PKr2 5′-CTAAACCGGAAGGAATGGA-3′), APG (APGf 5′-CTTGTGTCTCTGGTTGATCA-3′ and APGr2 5′-CTCTGTGTTTGCTTGGAGGA-3′), FIE (FIE-RTr 5′-TCAAGGTCTCAGGGAGTAGCA-3′ and FIE-RTf), and FIE-GFP (FIE-RTf and GFP274r).
Results
Rescue of fie Seed Viability by the pFIE∷FIE-GFP Transgene.
To understand FIE polycomb function, and to visualize the pattern of recombinant FIE protein accumulation, we generated Arabidopsis lines bearing a transgene, pFIE∷FIE-GFP, with 1,639 bp of FIE 5′-flanking sequences (pFIE), a modified FIE 5′-untranslated region, a sequence that encodes the full-length FIE protein (FIE), a linker sequence encoding six glycine amino acids, and then a sequence encoding a green fluorescent protein (GFP; ref. 15). GFP fluorescence was detected in the progenitor of the endosperm, the central cell nucleus, before (Fig. 1A) and after (Fig. 1B) fertilization. By the eight-nuclei endosperm stage, GFP fluorescence was no longer detectable (Fig. 1C), and could not be detected at any later stage of plant development (data not shown). Nor could FIE-GFP RNA be detected in pFIE∷FIE-GFP transgenic seedlings when the endogenous FIE gene is expressed (Fig. 2). Thus, expression of the pFIE∷FIE-GFP transgene in the very early stages of seed development, observed in multiple independently transformed lines, was highly restricted when compared with the pattern of FIE RNA accumulation in the ovule, female gametophyte, embryo, endosperm, seedling (Fig. 2), leaf, stem, root, and flower (11, 19). Although pFIE∷FIE-GFP transgene expression did not recapitulate the wild-type pattern of FIE RNA accumulation, perhaps because of a requirement for additional regulatory sequences and/or the sensitivity of GFP fluorescence detection, it was able to complement the fie seed abortion phenotype. That is, self-pollinated heterozygous fie-1/FIE plants that are homozygous for the pFIE∷FIE-GFP transgene displayed siliques with no seed abortion (Fig. 1D). When germinated, we observed seedlings, all homozygous for the pFIE∷FIE-GFP transgene, with Mendelian 1:2:1 segregation (20:28:12, χ2 = 2.6, P = 0.35) of FIE/FIE:fie-1/FIE:fie-1/fie-1 genotypes. In contrast, siliques from control self-pollinated fie-1/FIE plants contain 50% nonviable seeds (Fig. 1E) and when germinated the seedlings segregate 1:1 for FIE/FIE:fie-1/FIE genotypes. Thus, expression of the pFIE∷FIE-GFP transgene in the central cell and early endosperm rescues embryo and seed abortion associated with inheritance of a maternal mutant fie-1 allele. This result shows that the FIE-GFP fusion protein is active and suggests that fie embryo and seed abortion may be due primarily to a defect in endosperm development.
Figure 1.
Expression of a pFIE∷FIE-GFP transgene rescues viability of seeds with a maternal mutant fie-1 allele. In A–C, stage 12 flowers (11) from homozygous pFIE∷FIE-GFP transgenic plants were emasculated and manually self-pollinated. Siliques were harvested at the indicated time and dissected, and isolated ovules were mounted on slides. GFP fluorescence was visualized by confocal laser microscopy. Excitation wave-length was 488 nm, GFP emission was 500 to 530 nm, and chlorophyll florescence was above 650 nm. GFP and chlorophyll florescences were converted to green and red, respectively. (A) Mature ovule before fertilization. (B) Seed 6 h after pollination. (C) Seed with an eight-nuclei endosperm 24 h after pollination. Arrows point to endosperm nuclei. (D) Silique that is heterozygous fie-1/FIE and homozygous for the pFIE∷FIE-GFP transgene. (E) Heterozygous fie-1/FIE silique. Arrows point to aborted seeds. In A–C, bars = 20 μm. In D and E, bars = 0.4 mm. CCN, central cell nucleus; PEN, primary endosperm nucleus.
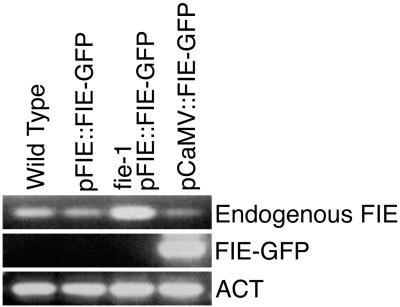
Figure 2.
Expression of the endogenous FIE gene, pFIE∷FIE-GFP, and CaMV∷FIE-GFP transgenes in seedlings. Lane designations refer to seedlings used for RNA isolation: pFIE∷FIE-GFP, homozygous for the wild-type FIE allele and the pFIE∷FIE-GFP transgene; fie-1 pFIE∷FIE-GFP, homozygous for the mutant fie-1 allele with at least one copy of the pFIE∷ FIE-GFP transgene; pCaMV∷FIE-GFP, homozygous for the wild-type FIE allele and the pCaMV∷FIE-GFP transgene. Total RNA from 7-day seedlings was isolated (18) and was amplified by reverse transcription–PCR as described in Materials and Methods, except that 40 cycles were used. ACT, actin RNA; Endogenous FIE, RNA from the endogenous FIE or fie-1 allele as indicated in the lane designation.
Mutant fie Seedlings Produce Flowers.
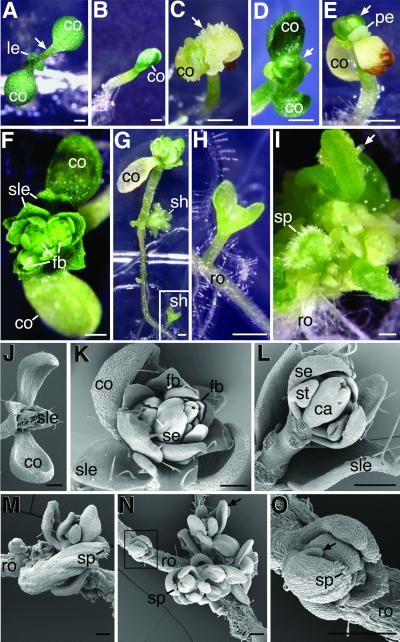
Plants homozygous for the pFIE∷FIE-GFP transgene that were either homozygous for the wild-type FIE allele, or heterozygous fie-1/FIE, developed normally (Fig. 3A and data not shown). Thus, in the presence of a wild-type FIE allele, the pFIE∷FIE-GFP transgene caused no detectable alteration in plant development. However, none of the seedlings tested that were homozygous for the fie-1 allele and the pFIE∷FIE-GFP transgene developed normally. These seedlings were smaller (Fig. 3B) and did not produce normal rosettes. In some cases, highly disorganized structures emerged from the shoot apical meristem (Fig. 3C). Seedlings often produced sessile leaves (Fig. 3D) with trichomes (Fig. 3 J and K) that resembled cauline leaves. We also observed white petal-like organs lacking trichomes (Fig. 3E). In some cases, floral buds emerged that were surrounded by sessile leaves (Fig. 3 F and K). Within the floral buds, outer whorl sepals could be distinguished by the appearance of unbranched trichomes on their abaxial surfaces (Fig. 3K) and by their highly elongated cells (Fig. 3L). Inner whorl organs whose distinctive shapes resembled immature stamens and carpels were also observed (Fig. 3L). Finally, floral organs within flower buds often displayed proper floral crucifer phyllotaxy (Fig. 3 F and L). The mutant phenotypes described above were observed in multiple independently isolated transgenic lines (data not shown) as well as in seedlings homozygous for the null fie-1 allele and for a FIEp∷FIE transgene that produces FIE protein without a GFP moiety (data not shown). Thus, the mutant phenotypes were not due to any effect of GFP on the structure or cellular location of FIE. Taken together, these results show that the transition from vegetative to inflorescence development has prematurely occurred in the shoot apical meristem of seedlings homozygous for the null fie-1 allele and the pFIE∷FIE-GFP transgene. This result suggests that one of the functions of the FIE gene is to repress the transition to flowering in the seedling shoot apical meristem.
Figure 3.
Early flowering phenotypes. Seedlings were analyzed by light microscopy (A–I) or scanning electron microscopy (J–O). (A) Control seedling homozygous for the wild-type FIE allele and the pFIE∷FIE-GFP transgene 6 days after germination. Arrow points to cotyledon petiole. (B–O) Seedlings homozygous for the null fie-1 allele and the transgene, pFIE∷FIE-GFP. (B) Mutant seedling 4 days after germination. (C) Three-week mutant seedling. Arrow points to region of disorganized growth. (D) Ten-day mutant seedling. Arrow indicates sessile leaf. (E) Three-week mutant seedling. Arrow indicates sessile leaf. (F) Three-week mutant seedling. (G) Four-week mutant seedling. The boxed region is magnified in H showing the shoot emerging from the root. (I) A portion of a root from a 1-week seedling was excised and cultured for 3 weeks on hormone-free agar media. Arrow points to an ovule-like primordium. (J) One-week mutant seedling. (K) Three-week mutant seedling. (L) Three-week mutant seedling. Cotyledons, sessile leaves, secondary flower buds, sepals, petals, and stamens have been removed to reveal sepal, stamen, and carpel organs in a single flower bud. (M) Root of a 4-week intact seedling. (N) Intact root of a 4-week seedling. Arrow points to organs arranged in a floral crucifer phyllotaxy. A second shoot in the boxed region magnified in O has an organ with stigmatic papillae and an ovule-like primordium (arrow). ca, carpel; co, cotyledon; fb, flower bud; le, vegetative rosette leaf; pe, petal; ro, root; se, sepal; sh, shoot; sle, sessile leaf; sp, stigmatic papillae; st, stamen. Bars in A–I = 0.5 mm. Bars in J–O = 0.25 mm.
Production of shoots and flower-like organs was not limited to the shoot apical meristem in seedlings homozygous for the fie-1 allele and the pFIE∷FIE-GFP transgene. Shoots frequently emerged from the hypocotyl and roots of intact seedlings (Fig. 3 G and H). Carpel-shaped organs with stigmatic papillae at their tips were present in such adventitious shoots (Fig. 2M). In addition, organs tipped with stigmatic papillae and with structures resembling ovule primordia on their edges were observed (Fig. 3 N and O). In some cases, shoots with organs arranged in a floral crucifer phyllotaxy were observed (Fig. 3N). When roots were cultured in hormone-free media, many additional organs were produced with stigmatic papillae and structures resembling ovule primordia (Fig. 3I). These results show that ectopic shoots and organs resembling flowers were produced in seedlings homozygous for the fie-1 allele and the pFIE∷FIE-GFP transgene. This result suggests that another function of the wild-type FIE allele is to repress the formation of shoots and floral organs outside of the seedling shoot apical meristem region.
Rescue of fie Vegetative Development by the pCaMV∷FIE-GFP Transgene.
The mutant phenotypes described above were observed in transgenic pFIE∷FIE-GFP seedlings that were also homozygous for the null fie-1 allele, and were never observed when a wild-type FIE allele was present. Thus, the pFIE∷FIE-GFP transgenic allele is recessive to the wild-type FIE allele. This fact, along with the restriction of pFIE∷FIE-GFP gene expression to very early seed development (Fig. 1 A–C), suggests that loss of FIE activity is responsible for early flowering and ectopic shoot formation in seedlings that are homozygous for the null fie-1 allele and have a pFIE∷FIE-GFP transgene. From this result, we reasoned that expanding the period of FIE activity during embryogenesis and seedling development with a different transgene might result in plants that develop more normally. To test this hypothesis, we generated pCaMV∷FIE-GFP transgenic plants where transcription of FIE-GFP is under the control of the cauliflower mosaic virus (pCaMV) promoter (20). In pCaMV∷FIE-GFP lines, we detected a broad pattern of GFP fluorescence throughout embryo development, including the later stages, that diminished in the germinating seedling (data not shown). Whereas pFIE∷FIE-GFP gene expression was not detected in seedlings, FIE-GFP RNA encoded by the pCaMV∷FIE-GFP gene was present (Fig. 2). By genetic crosses, we obtained plants that were homozygous for fie-1, pFIE∷FIE-GFP, and pCaMV∷FIE-GFP. These plants did not display any of the mutant phenotypes shown in Fig. 3 and could not be distinguished from wild-type plants. They produced rosette leaves, followed by inflorescences, fertile flowers, and siliques with viable seed (data not shown). Thus, we were able to adjust the level of FIE activity with the two transgenes to recapitulate the wild-type flowering time phenotype. These results verify that early flowering and ectopic shoot and floral organ formation is due to a critical lack of FIE activity in seedlings homozygous for fie-1 and the pFIE∷FIE-GFP transgene.
Expression of Floral Induction and Homeotic Genes in fie Embryos and Seedlings.
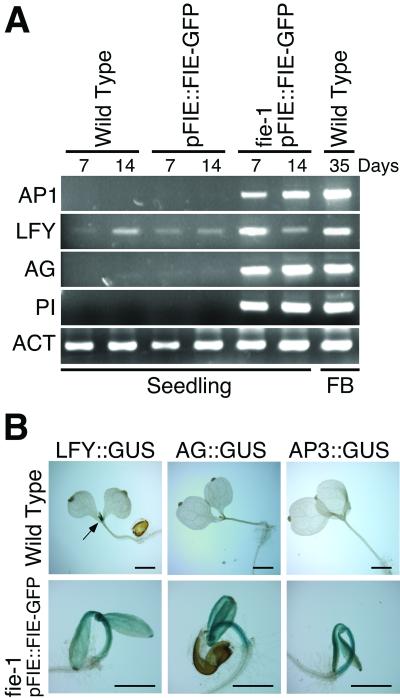
To understand the molecular basis for the early flowering phenotypes, we measured the expression of meristem and floral organ identity genes in mutant and control seedlings. LEAFY (LFY) and APETALA1 (AP1) encode transcription factors that promote floral meristem identity in Arabidopsis and, with the aid of other factors, activate transcription of floral meristem and organ identity genes such as AGAMOUS (AG), APETALA3 (AP3), and PISTILLATA (PI) (21). As shown in Fig. 4A, LFY RNA was present at a low level, and AP1 RNA was not detected in wild-type 7-day and 14-day seedlings. The same result was observed in 7- and 14-day seedlings containing the pFIE∷FIE-GFP transgene. However, in seedlings that were homozygous fie-1 with a pFIE∷FIE-GFP transgene, LFY RNA concentration was significantly elevated in 7-day seedlings, and AP1 RNA concentration was increased in both 7-day and 14-day seedlings. No effect on the expression of other genes that accelerate flowering time, CONSTANS (CO; ref. 22) or FLOWERING LOCUS T (FT; refs. 23 and 24), was observed (data not shown). Because ectopic expression of either LFY or AP1 is sufficient to convert the normally indeterminate shoot apex to a floral meristem that forms a terminal flower (25, 26), it is likely that aspects of the fie early flowering phenotype are due to their ectopic expression. Floral organ identity gene expression was also affected by the level of FIE activity. Whereas AG and PI RNAs were not detected in control wild-type seedlings, or control seedlings with the pFIE∷FIE-GFP transgene, both RNAs accumulated in homozygous fie-1, pFIE∷FIE-GFP seedlings (Fig. 4A). Taken together, these results suggest that FIE-mediated polycomb complexes, either directly or indirectly, repress expression of both floral meristem identity genes and floral organ identity genes during seedling development.
Figure 4.
Analysis of expression of floral genes in seedlings. (A) RNA analysis. Lane designations refer to seedlings used for RNA isolation: pFIE∷FIE-GFP, homozygous for the wild-type FIE allele and the pFIE∷FIE-GFP transgene; fie-1 pFIE∷FIE-GFP, homozygous for the mutant fie-1 allele with at least one copy of the pFIE∷FIE-GFP transgene. Total RNA from seedlings was isolated at the indicated day after germination, or from stage 1 to stage 12 (11) floral buds (FB) and was amplified by reverse transcription–PCR as described in Materials and Methods. (B) Analysis of floral promoter activity. β-glucuronidase enzyme activity was measured as described (16). Arrow points to GUS stained shoot apical meristem region in the LFY∷GUS seedling. fie-1 pFIE∷FIE-GFP, seedlings that are homozygous for the mutant fie-1 allele with at least one copy of the pFIE∷FIE-GFP and indicated reporter transgene. Bars = 1 mm. ACT, actin RNA.
To investigate the spatial regulation of gene transcription by FIE-mediated polycomb complexes, we determined the activity of LFY, AG, and AP3 promoters ligated to a β-glucuronidase (GUS) reporter gene (16) in transgenic seedlings. As shown in Fig. 4B, LFY∷GUS transcription was restricted to the wild-type seedling shoot apical meristem, whereas there was no detectable transcription of the AG∷GUS or AP3∷GUS transgenes in wild-type seedlings. In contrast, homozygous fie-1 seedlings with the pFIE∷FIE-GFP transgene showed elevated LFY∷GUS, AG∷GUS, and AP3∷GUS transcription in the shoot apical meristem, cotyledon, and hypocotyl regions. These results show that FIE-mediated polycomb complexes, either directly or indirectly, repress transcription of floral meristem identity and floral organ identity genes in the aerial seedling.
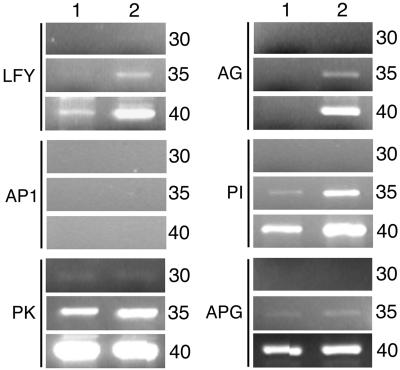
When does repression of flowering and floral-promoting gene transcription begin? One possibility is that aspects of floral repression initiate early in plant development during embryogenesis. To test this hypothesis, embryo RNA was isolated from self-pollinated plants heterozygous for fie-1/FIE and homozygous for the pFIE∷FIE-GFP transgene (Fig. 5, lane 2). Twenty-five percent of these embryos are homozygous for fie-1 and the pFIE∷FIE-GFP transgene. Control wild-type embryo RNA was also isolated (Fig. 5, lane 1). Semiquantitative reverse transcription–PCR analysis indicated that the level of LFY, AG, and PI RNA was elevated in the population that included homozygous fie-1, pFIE∷FIE-GFP embryos. In this experiment, we could not detect AP1 embryo RNA in either embryo population, perhaps because a factor not regulated by FIE is required for AP1 expression in the embryo. Finally, expression of control genes PK and APG was the same in both embryo populations. These results suggest that FIE-mediated polycomb complexes function during embryogenesis, either directly or indirectly, to repress the expression of specific floral meristem and organ identity genes.
Figure 5.
Analysis of expression of floral genes in embryos. Total RNA was isolated from walking stick stage embryos dissected from either self-pollinated wild-type siliques (lane 1) or from self-pollinated siliques that were heterozygous fie-1/FIE and homozygous for the pFIE∷FIE-GFP transgene (lane 2). Twenty-five percent of the embryos used for RNA isolation in lane 2 were predicted to be homozygous for fie-1 and for the pFIE∷FIE-GFP transgene. As controls, we measured the expression of two genes that flank the AG gene in the Arabidopsis genome: PK (protein kinase-like protein; CAB78897.1) and APG (proline rich protein; CAB78899.1). Cycles, number of PCR cycles.
Discussion
Polycomb proteins represent an evolutionarily highly conserved cellular memory system that maintains transcriptional repression of target genes (27). For example, during Drosophila embryogenesis, gap proteins repress homeotic gene transcription outside their appropriate expression domain. However, gap proteins are only transiently expressed, and it is the formation of polycomb complexes that ensure that the repressed state is maintained over many cell divisions. Failure to maintain repression because of mutations in polycomb genes can result in dramatic homeotic transformations of organ identity. Here, we show that the plant FIE polycomb protein, either directly or indirectly, represses homeotic gene transcription (e.g., LFY, AP1, AG, AP3, and PI) outside their appropriate temporal and spatial domains during embryo and seedling development (Figs. 4 and 5). Diminished FIE activity because of loss-of-function mutations in the FIE gene results in premature flowering by the seedling shoot apical meristem and inappropriate formation of shoots and flower-like structures along the root and hypocotyl (Fig. 3). Previously, we showed that the FIE polycomb protein prevents premature formation of an endosperm before fertilization (11). Taken together, these results demonstrate that, similar to animal systems (27), plant polycomb proteins function to repress inappropriate spatial and temporal programs of gene transcription and development very early in the plant life cycle.
Multiple genes have been shown to repress flowering during Arabidopsis development. Loss-of-function mutations in the cloned genes CLF, EBS, HY1, PHYB, ELF3, HY2, SPY, and TFL all result in accelerated flowering. However, these mutant plants flower only after the production of embryonic cotyledon leaves and multiple vegetative rosette leaves by the shoot apical meristem (28–33). By contrast, fie (Fig. 3) and emf (7, 8) mutant seedlings can skip the normal adult vegetative phase and flower. Thus, FIE and EMF genes function uniquely during the earliest stages of plant development to repress flowering.
WD motif polycomb proteins related to FIE in mammals and Drosophila function by assembling polycomb protein complexes (27). The WD motifs form surface loops that are used as scaffolds for the generation of protein complexes that include additional polycomb proteins (e.g., SET-domain polycombs) and histone deacetylase. These complexes remodel chromatin and repress gene transcription (27). With regard to its endosperm repression function, FIE is likely to be associated with a complex that includes the FIS2 zinc finger protein and the MEA SET-domain polycomb protein. Evidence for complex formation among these three proteins includes the similar mutant phenotypes of loss-of-function mutations in the FIE, MEA, and FIS2 genes, and experiments which show that FIE and MEA directly interact (17, 19, 34). Analogous to the Drosophila ESC polycomb, FIE may be recruited to specific chromatin sites by a zinc finger protein such as FIS2. However, in contrast to what is observed for fie seedlings, homozygous mea and fis2 mutant seedlings do not display early flowering phenotypes (14, 35), strongly suggesting that FIE represses flowering by associating with other protein partners. To repress flowering, it is possible that FIE interacts with additional SET-domain polycomb group proteins encoded in the Arabidopsis genome (36). One candidate is the SET-domain polycomb protein Curly Leaf (CLF), because mature clf mutant plants display somewhat early flowering and ectopic AG and AP3 expression in vegetative leaves (28). However, the fact that clf mutant seedlings do not flower suggests that FIE may interact with other SET-domain polycomb proteins that function to repress flowering during the early phases of plant development. FIE may also interact, directly or indirectly, with proteins encoded by EMF genes. This hypothesis is supported by the fact that emf1 and emf2 mutant seedlings bear a striking resemblance to fie seedlings and display derepressed AP1 and AG promoter activity (8, 37). FIE, encoded by a single-copy gene in the Arabidopsis genome (11), therefore might form complexes with distinct transcription factors and polycomb proteins in the embryo and seedling to repress transcription of floral induction and homeotic genes.
In animals, the formation of polycomb complexes ensures that the repressed state is mitotically stable and maintained over many cell divisions, perhaps over the lifetime of the organism. However, the repression of development by plant polycomb proteins, at least in some cases, appears to be reversible during the plant life cycle. For example, repression of endosperm development ends immediately after fertilization of the central cell, and flowering at the shoot apical meristem, initially repressed in the embryo and seedling, initiates when the plant reaches the appropriate stage of development. Recently, it has been shown in Drosophila that there is a discontinuity in polycomb silencing of a transgene between embryo and subsequent larval stages, suggesting that differences exist between the maintenance properties of polycomb complexes at different stages of development (38). It is possible that modulation of polycomb repression occurs during plant development as well.
A model has been proposed whereby flowering initiates when floral repression has decreased in response to genetic (i.e., autonomous and gibberellin pathways) and environmental (i.e., vernalization and photoperiod pathways) signals (3, 7–9). An alternative model states that the genetic and environmental promotion pathways are directly integrated at the promoters of the floral meristem identity genes and do not directly interact with a floral repression mechanism (4). These models are not mutually exclusive and it is possible that aspects of both contribute to the control of flowering. Results from this study reveal that, early during the plant life cycle, FIE-mediated polycomb protein complexes prevent flowering in both the shoot apical meristem and along the hypocotyl and root of the seedling. It is not known how the effect of polycomb repression is diminished in the shoot apical meristem where flowering occurs later in plant development. One possibility is that genetic and environmental promotion pathways produce transcriptional activators that can function in the presence of polycomb complexes. Alternatively, polycomb complex activity may diminish in the shoot apical meristem during plant development. This result might be due to reduced polycomb gene expression, modification of a key polycomb protein, failure to maintain the polycomb complex during mitosis, and/or antagonistic interactions between polycomb proteins and components of the genetic and environmental promotion pathways. Additional experiments are needed to determine whether there is a relationship between polycomb-mediated embryonic and seedling floral repression and the activation of floral promotion pathways later in plant development.
Acknowledgments
This research was supported by U.S. Department of Agriculture (USDA; 2000-01539), Ceres, Inc. (B970602), and Binational Agricultural Research and Development (USDA; IS-3158-99C) grants to R.L.F. and by a fellowship from the Japan Society for the Promotion of Science to T.K.
Abbreviations
- FIE
Fertilization Independent Endosperm
- GFP
Green Fluorescent Protein
- pFIE∷FIE-GFP
FIE promoter ligated to FIE and GFP cDNA sequences
- pCaMV∷FIE-GFP
cauliflower mosaic virus promoter ligated to FIE and GFP cDNA sequences
- GUS
β-glucuronidase
- LFY∷GUS
LFY promoter ligated to GUS cDNA
- AG∷GUS
AG promoter ligated to GUS cDNA
- AP3∷GUS
AP3 promoter ligated to GUS cDNA
- EMF
Embryonic Flower
References
- 1.Walbot V. Trends Genet. 1985;1:165–169. [Google Scholar]
- 2.Goldberg R B, de Paiva G, Yadegari R. Science. 1994;266:605–614. doi: 10.1126/science.266.5185.605. [DOI] [PubMed] [Google Scholar]
- 3.Haughn G W, Schultz E A, Martinez-Zapater J M. Can J Bot. 1995;73:959–981. [Google Scholar]
- 4.Araki T. Curr Opin Plant Biol. 2001;4:63–68. doi: 10.1016/s1369-5266(00)00137-0. [DOI] [PubMed] [Google Scholar]
- 5.Koornneef M, Alonso-Blanco C, Peeters A J M, Soppe W. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:345–370. doi: 10.1146/annurev.arplant.49.1.345. [DOI] [PubMed] [Google Scholar]
- 6.Levy Y Y, Dean C. Plant Cell. 1998;10:1973–1998. doi: 10.1105/tpc.10.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung Z R, Belachew A, Shunong B, Bertrand-Garcia R. Science. 1992;258:1645–1647. doi: 10.1126/science.258.5088.1645. [DOI] [PubMed] [Google Scholar]
- 8.Yang C-H, Chen L-J, Sung Z R. Dev Biol. 1995;169:421–435. doi: 10.1006/dbio.1995.1158. [DOI] [PubMed] [Google Scholar]
- 9.Weigel D. Annu Rev Genet. 1995;29:19–39. doi: 10.1146/annurev.ge.29.120195.000315. [DOI] [PubMed] [Google Scholar]
- 10.Aubert D, Chen L, Moon Y-H, Martin D, Castle L A, Yang C-H, Sung Z R. Plant Cell. 2001;13:1865–1875. doi: 10.1105/TPC.010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada J J, Goldberg R B, Fischer R L. Plant Cell. 1999;11:407–415. doi: 10.1105/tpc.11.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinkenoog R, Spielman M, Adams S, Fischer R L, Dickinson H G, Scott R J. Plant Cell. 2000;12:2271–2282. doi: 10.1105/tpc.12.11.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohad N, Margossian L, Hsu Y-C, Williams C, Repetti P, Fischer R L. Proc Natl Acad Sci USA. 1996;93:5319–5324. doi: 10.1073/pnas.93.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhury A M, Ming L, Miller C, Craig S, Dennis E S, Peacock W J. Proc Natl Acad Sci USA. 1997;94:4223–4228. doi: 10.1073/pnas.94.8.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H. Plant J. 1999;18:455–463. doi: 10.1046/j.1365-313x.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson R A, Kavanagh T A, Bevan M V. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadegari R, Kinoshita T, Lotan O, Cohen G, Katz A, Choi Y, Katz A, Nakashima K, Harada J J, Goldberg R B, et al. Plant Cell. 2000;12:2367–2381. doi: 10.1105/tpc.12.12.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita T, Yadegari R, Harada J J, Goldberg R B, Fischer R L. Plant Cell. 1999;11:1945–1952. doi: 10.1105/tpc.11.10.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spillane C, MacDougall C, Stock C, Kohler C, Vielle-Calzada J-P, Nunes S M, Grossniklaus U, Goodrich J. Curr Biol. 2000;10:1535–1538. doi: 10.1016/s0960-9822(00)00839-3. [DOI] [PubMed] [Google Scholar]
- 20.Rogers S G, Klee H J, Horsch R B, Fraley R T. Methods Enzymol. 1987;153:253–277. [Google Scholar]
- 21.Parcy G, Nilsson O, Busch M A, Weigel I L, Weigel D. Nature (London) 1998;395:561–566. doi: 10.1038/26903. [DOI] [PubMed] [Google Scholar]
- 22.Putterill J, Robson R, Lee K, Simon R, Coupland G. Cell Mol Life Sci. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 24.Kardailsky I, Shukla V K, Ahn J H, Dagenail N, Christensen S K, Nguyen J T, Chory J, Harrison M J, Weigel D. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 25.Weigel D, Nilsson O. Nature (London) 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- 26.Mandel M A, Yanofsky M F. Nature (London) 1995;377:522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- 27.Francis N J, Kingston R E. Nat Rev Mol Cell Biol. 2001;2:409–421. doi: 10.1038/35073039. [DOI] [PubMed] [Google Scholar]
- 28.Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz E M, Coupland G. Nature (London) 1997;386:44–51. doi: 10.1038/386044a0. [DOI] [PubMed] [Google Scholar]
- 29.Goto N, Kumagai T, Koornneef M. Physiol Plant. 1991;83:209–215. [Google Scholar]
- 30.Zagotta M T, Hicks K A, Jacobs C I, Young J C, Hangarter R P, Meeks-Wagner R. Plant J. 1996;10:691–702. doi: 10.1046/j.1365-313x.1996.10040691.x. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen S E, Olszewski N E. Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shannon S, Meeks-Wagner R. Plant Cell. 1991;3:877–892. doi: 10.1105/tpc.3.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Mena C, Pineiro M, Franco-Zorrilla J M, Salinas J, Coupland G, Martinez-Zapater J M. Plant Cell. 2001;13:1011–1024. [PMC free article] [PubMed] [Google Scholar]
- 34.Luo M, Bilodeau P, Koltunow A, Dennis E S, Peacock W J, Chaudhury A M. Proc Natl Acad Sci USA. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiyosue T, Ohad N, Yadegari R, Hannon M, Dinneny J, Wells D, Katz A, Margossian L, Harada J, Goldberg R B, et al. Proc Natl Acad Sci USA. 1999;96:4186–4191. doi: 10.1073/pnas.96.7.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Arabidopsis Genome Initiative. (2000) Nature (London)408, 796–815. [DOI] [PubMed]
- 37.Chen L, Cheng J-C, Castle L, Sung Z R. Plant Cell. 1997;9:2011–2024. doi: 10.1105/tpc.9.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poux S, McCabe D, Pirrotta V. Development (Cambridge, UK) 2001;128:75–85. doi: 10.1242/dev.128.1.75. [DOI] [PubMed] [Google Scholar]