Sickle cell disease (SCD) is caused by a point mutation in the adult β-globin gene and characterized by hemoglobin S polymerization which leads to the formation of sickle erythrocytes under deoxygenated conditions.1 Affected individuals suffer from chronic hemolysis, severe anemia, and other life-threatening complications. Elevation of fetal hemoglobin (HbF) production in adult erythrocytes reverses the clinical phenotypes associated with SCD,2 therefore diverse strategies to reactivate γ-globin gene expression are under investigation. Insights gained from rare genetic mutations in the human b-globin locus producing hereditary persistence of HbF expression have informed the experimental design of studies presented herein. Previous studies demonstrated that the T/G transversion at nucleotide -567 upstream of HBG2 alters GATA-1 binding to produce high HbF levels.3 This site, among others, provides molecular targets to reactivate γ-globin gene transcription during adult erythropoiesis. We previously demonstrated that a zinc finger DNA-binding domain (ZF-DBD) targeting the -567 GATA binding site in the Gγ-globin gene promoter (-567GγZF-DBD) elevated Gγ-globin expression when directly delivered to normal primary human erythroid cells.4 Here, we expanded these findings and show that direct delivery of the -567GγZF-DBD protein to erythroid progenitors generated from SCD patients increased F-cells and HbF production comparable to induction by hydroxyurea (HU). A negative control ZF-DBD did not induce HbF expression. We conclude that direct delivery of ZF-DBDs that interfere with the function of γ-globin repressors represents a promising strategy for the development of a protein therapy for SCD and b-thalassemia.
Our studies were conducted with the -567GγZF-DBD and a negative control ZF-DBD that was designed to target a neutral site upstream of the mouse locus control region (LCR) hypersensitive site 2 (NC-ZF-DBD). The binding characteristics for the NC-ZF-DBD are shown in Online Supplementary Figures S1 and S2. We delivered both proteins directly to erythroid progenitors derived from peripheral blood mononuclear cells (PBMCs) isolated from children with SCD who were never treated with hydroxyurea (HU), after obtaining informed consent (Online Supplementary Methods). The complete blood count, differential, and reticulocyte counts were similar for the 3 individuals in our study (Online Supplementary Table S1). Of note, the clinical HbF levels ranged from 4.5% to 15.5%. Methods for the generation of erythroid progenitor cells from PBMCs5 and protein delivery into these cells have been previously published4 and are summarized in the Online Supplementary Methods. Briefly, erythroid cells were treated with ZF-DBD or HU on day 8 of culture and harvested after 48 hours (h) (day 10) for the various studies.
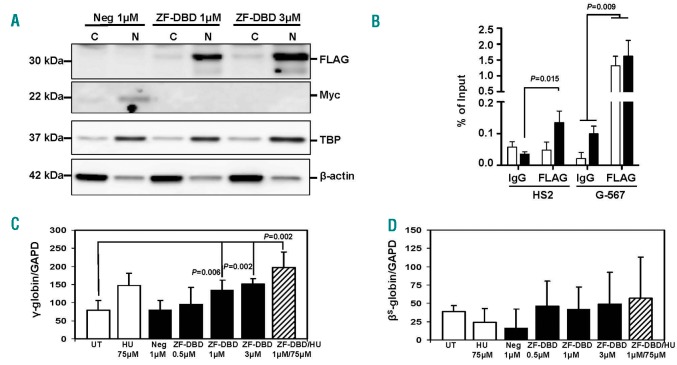
To demonstrate protein delivery, we used different ZF-DBD concentrations (1 or 3 µM). The -567GγZF-DBD (ZF-DBD) contained a FLAG-tag while the NC-ZF-DBD (Neg) contained a Myc-tag. After direct protein delivery, the ZF-DBDs co-localized with the TATA binding protein (TBP) primarily in the nucleus, while b-actin localized predominantly in the cytoplasm (Figure 1A). This shows that ZF-DBD delivery to primary cells derived from SCD patients is as efficient as delivery to cells derived from individuals with normal adult hemoglobin A, as described previously.4 Chromatin immunoprecipitation (ChIP) experiments revealed an enrichment of the -567GγZF-DBD at the -567Gγ-globin promoter region and no binding (with 1 µM) or little binding (with 3 µM) at the locus control region HS2 (Figure 1B). ChIP experiments in mouse erythroleukemia cells revealed that the NC-ZF-DBD interacted with the murine LCR HS2 but not with other regions in the murine globin gene locus (Online Supplementary Figure S2).
Figure 1.
Increased γ-globin gene transcription in sickle erythroid progenitors after delivery of the -567GγZF-DBD. Peripheral blood mononuclear cells isolated from sickle cell patients were used to induce differentiation along the erythroid lineage according to a previously published method.5 On day 8 of differentiation (see Online Supplementary Methods), cells were exposed for 48 hours (h) to HU, NC-ZF-DBD (Neg), or -567GγZF-DBD (ZF-DBD) at the concentrations shown. Experimental conditions were tested in triplicate for the 3 sickle cell patients (n=9) for each completed data analysis. (A) Localization of NC-ZF-DBD and ZF-DBD in the nucleus after protein delivery. Proteins were isolated from the cytoplasm (C) or nucleus (N) as previously published;4 western blot analysis was conducted as described previously15 using antibodies specific for the -567GγZF-DBD (aFLAG, F3165, Sigma, St. Louis, MO, USA), the NC-ZF-DBD (aMyc, MA121316, Thermo Fisher Scientific, Waltham, MA, USA), nuclear TATA-Binding Protein (aTBP, SC-204, Santa Cruz Biotechnology, Dallas, TX, USA) and b-actin (b-actin, A5316, Sigma, St. Louis, MO, USA). (B) Specific interaction of the -567GγZF-DBD with the Gγ-globin upstream promoter region. Cells were exposed to 1 µM (white bars) or 3 µM ZF-DBD (black bars), crosslinked with formaldehyde, and subjected to ChIP assay as described previously15 using -567GγZF-DBD (aFLAG) or IgG control (I8140, Sigma, St. Louis, MO, USA) antibodies. The purified DNA was analyzed by quantitative (q) PCR using primers specific for LCR HS2 and the Gγ-globin upstream promoter (G-567). (C and D) To measure gene transcription rates, reverse transcriptase-qPCR was conducted using RNA isolated from sickle erythroid progenitors after 48 h of treatment (day 10) in culture (see Online Supplementary Methods). Increased γ-globin (C) but not bS-globin (D) gene transcription was observed in cells exposed to hydroxyurea (HU), the -567GγZF-DBD (ZF-DBD), or both but not in cells treated with the NC-ZF-DBD (Neg) alone. RNA was extracted from sickle erythroid progenitors treated under the different conditions shown, reverse transcribed, and subjected to qPCR using primers specific for GAPDH, γ-globin, and bS-globin as described previously.15 The results in (B), (C), and (D) are from 3 independent experiments and error bars reflect the Standard Deviation (SD).
Exposure of the erythroid progenitors generated from each SCD patient to different concentrations of the -567GγZF-DBD (0.5, 1, and 3 µM; in triplicate, n=9), led to a dose-dependent 2-fold increase in γ-globin gene expression while the NC-ZF-DBD (Neg) at 1 µM had no effect (Figure 1C). The increase in γ-globin expression observed after treatment with 3 µM of the -567GγZF-DBD was similar to the increase produced by treatment with 75 µM HU. Interestingly, the combined treatment with 1µM -567GγZF-DBD and 75µM HU produced an additive 2.5-fold (P=0.002) increase in γ-globin gene expression. In contrast, exposure to HU, the -567GγZF-DBD, or the NC-ZF-DBD had no statistically significant effect on the expression of the adult βS-globin gene in these cells (Figure 1D).
In addition to the functional studies, we determined the effect of the ZF-DBDs on cell viability using trypan blue staining on day 8 and day 10 of culture. Treatment with the NC-ZF-DBD and both higher concentrations of the -567GγZF-DBD reduced cell viability from 92-93% to 70-81% (Online Supplementary Table S2). Toxicity may be due to the presence of bacterial endotoxins, which will be removed in future in vivo studies. To determine the impact of ZF-DBDs on cell differentiation, we examined expression of erythroid markers under the different treatment conditions by RT-qPCR (Online Supplementary Methods). Interestingly, treatment with the -567GγZF-DBD at all concentrations increased CD71, CD235a, and α-globin gene expression significantly compared to NC-ZF-DBD (Online Supplementary Figure S3); by contrast, HU had no effect on the expression of erythroid markers. These data suggest that despite mild cellular toxicity, treatment with the -567GγZF-DBD stimulated erythroid progenitor growth and differentiation while inducing HbF expression.
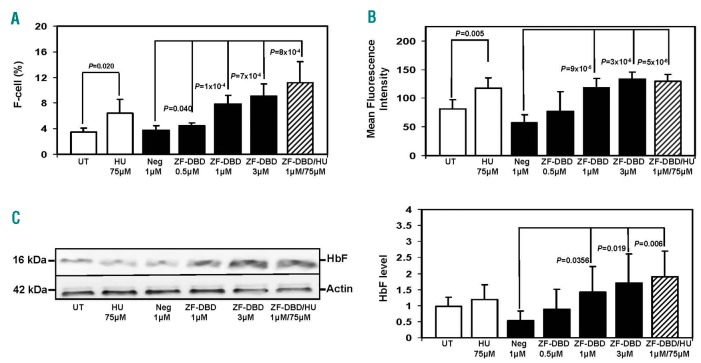
To achieve anti-sickling effects in SCD, enhanced HbF expression is required to inhibit hemoglobin S polymerization. Therefore, we quantified HbF levels by flow cytometry and western blot analysis. After the various treatments, erythroid progenitors stained with FITC-anti-HbF antibody were analyzed by flow cytometry. Representative flow cytometry histograms are shown in Online Supplementary Figure S4. The summary of the results from 3 independent experiments, analyzed in triplicate (n=9), is shown in Figure 2A. Overall, there was a 2-fold increase in the number of F-cells in the presence of 1 μM -567GγZF-DBD and a greater than 2-fold increase with 3 µM. Furthermore, treatment with HU and 1 µM -567GγZF-DBD produced an additive effect in the increase of F-cells, reaching an almost 3-fold increase compared to untreated control cells or cells treated with the NC-ZF-DBD. By mean fluorescent intensity quantification, we observed increased HbF concentrations after treatment with 1 µM and 3 µM -567GγZF-DBD (ZF-DBD) but not after treatment with the NC-ZF-DBD (Neg; Figure 2B). Western blotting experiments showed a consistent increase in HbF protein levels in erythroid progenitors for the higher -567GγZF-DBD concentrations compared to no change for the NC-ZF-DBD (Figure 2C). Quantitation of 3 independent experiments revealed an average 2-fold increase in HbF protein after delivery of the -567GγZF-DBD (Figure 2C, right panel).
Figure 2.
Increased number of F-cells and fetal hemoglobin (HbF) production in sickle erythroid progenitors treated with the -567GγZF-DBD. Peripheral blood mononuclear cells were induced to differentiate toward erythroid progenitors and exposed to hydroxyurea (HU), -567GγZF-DBD (ZF-DBD), or NC-ZF-DBD (Neg) as described in the legend to Figure 1. (A) Cells were stained with FITC labeled anti-γ-globin antibody and analyzed by flow cytometry. Shown is the number of HbF positive cells (F-cells) after treatment with the various agents. Graphical quantification of F-cell counts from 3 independent experiments analyzed in triplicate (n=9), with error bars reflecting the Standard Deviation (SD). (B) Graphical representation of the mean fluorescence intensity of cells described in (A) from 3 independent experiments. (C) Analysis of protein expression in erythroid progenitors treated under the different conditions shown. Western blot analysis was performed with antibodies specific for human HbF and b-actin. (Left) Representative western blot. (Right) Densitometry data from 3 independent western blot experiments performed in triplicate (n=9); error bars represent SD.
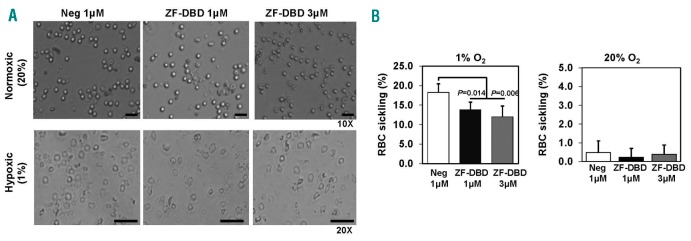
Although the pathophysiology of SCD is complex, the process of hemoglobin S polymerization and erythrocyte sickling remains central to the vaso-occlusion process, painful episodes, and tissue ischemia.1 Therefore, we investigated whether HbF induction by the -567GγZF-DBD could reach levels to produce a functional anti-sickling effect. Under hypoxic conditions (1% O2) sickle erythroid progenitors exhibit distinct sickling, visualized by light microscopy (Figure 3A). Delivery of the -567GγZF-DBD reduced the number of sickle cells from 18% to 14% at 1 µM or to 12% at 3 µM, while the delivery of the NC-ZF-DBD had no effect (Figure 3B).
Figure 3.
Decreased sickling in erythroid progenitors exposed to the -567GγZF-DBD. Peripheral blood mononuclear cells isolated from sickle cell patients were differentiated toward erythroid progenitors and exposed to ZF-DBDs at the indicated concentrations. After ZF-DBD treatments, cells were exposed to different oxygen levels for 24 hours (h) and then analyzed. See Online Supplementary Methods for details of the hypoxia protocol. (A) Images of erythroid progenitors (20× and 10× as indicated) exposed to the -567GγZF-DBD (ZF-DBD) or the NC-ZF-DBD (Neg) and incubated under normoxic (20% O2) or hypoxic (1% O2) conditions. (B) Graphical representation of erythroid progenitor sickling after exposure to the -567GγZF-DBD (ZF-DBD) or the NC-ZF-DBD (Neg) at the indicated concentrations. The number of sickle erythroid progenitors was quantified under light microscopy in 3 independent experiments (analyzed in triplicate, n=9) and the error bars reflect the Standard Deviation (SD).
Numerous strategies including pharmacological drug treatment,6,7 gene therapy,8 gene editing,9,10 and, more recently, expression of non-coding RNA11–13 are under development for reactivating β-globin gene transcription. For example, Miller et al. demonstrated that overexpression of Lin28B inhibits let7 miRNA expression as a mechanism of HbF induction in erythroid progenitors.12 Furthermore, our laboratory demonstrated the ability of miR-34a to mediate HbF induction by silencing Stat3 expression, a repressor of γ-globin transcription.13
Our findings herein provide another innovative molecular approach for HbF induction. We demonstrate that direct delivery of a ZF-DBD targeting a GATA-1 repressor-binding site in the Gγ-globin promoter ex vivo to primary erythroid progenitors derived from patients with SCD elevated expression of HbF and produced an anti-sickling effect. Protein therapy offers a promising alternative to genome editing or gene therapy strategies, which are expensive and associated with stem cell transplantation. Despite recent advances, gene-editing procedures are often associated with undesired changes to the genome due to off-site targeting of nucleases. Furthermore, perturbations of gene expression patterns due to the insertion of viruses with strong regulatory potential is a concern in gene therapy, as recent evidence points to the structuring of nuclear chromatin into topologically-associated domains.14
By contrast, ZF-DBDs without effector domains offer several advantages. The lack of effector domains minimizes non-specific activation or repression of genes at off-site binding events. Furthermore, ZF-DBDs can be directly delivered to primary erythroid cells due to small size and high positive surface charges, thus eliminating the need for stem cell transplantation. Moreover, direct protein delivery allows controlled dosing of the therapeutic ZF-DBDs. Finally, simultaneous delivery of different ZF-DBDs targeting repressor binding sites in the γ-globin promoters or activator binding sites in genes expressing γ-globin repressors could lead to synergistic activation of HbF production. However, successful protein therapy depends on efficient strategies for the stabilization and in vivo delivery of potentially therapeutic ZF-DBDs. We are currently pursuing experiments aimed at tracking and targeting ZF-DBDs in sickle cell mice in vivo.
Supplementary Material
Acknowledgments
We thank Blanca Ostmark for technical support. This work was funded through NIH grants R01DK052356 (to JB) and R01HL069234 (to BSP) as well as a training grant from the National Heart Lung and Blood Institute (HL117684 to XZ) and a pre-doctoral fellowship from the American Heart Association (AHA 16PRE31290001, to MAH).
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127(3):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paikari A, Sheehan VA. Fetal haemoglobin induction in sickle cell disease. Br J Haematol. 2018;180(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, Luo HY, Basran RK, et al. A T-to-G transversion at nucleotide -567 upstream of HBG2 in a GATA-1 binding motif is associated with elevated hemoglobin F. Mol Cell Biol. 2008;28(13):4386–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossain MA, Shen Y, Knudson I, et al. Activation of Fetal γ-globin Gene Expression via Direct Protein Delivery of Synthetic Zinc-finger DNA-Binding Domains. Mol Ther Nucleic Acids. 2016;5(10):e378. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamoorthy S, Pace B, Gupta D, et al. Dimethyl fumarate increases fetal hemoglobin, provides heme detoxification, and corrects anemia in sickle cell disease. JCI Insight. 2017;2(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang WC. Minireview: Prognostic factors and the response to hydroxurea treatment in sickle cell disease. Exp Biol Med (Maywood). 2016;241(7):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molokie R, Lavelle D, Gowhari M, et al. Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study. PLoS Med. 2017;14(9):e1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. Gene Therapy in a Patient with Sickle Cell Disease. N Engl J Med. 2017;376(9):848–855. [DOI] [PubMed] [Google Scholar]
- 9.Bauer DE, Brendel C, Fitzhugh CD. Curative approaches for sickle cell disease: A review of allogeneic and autologous strategies. Blood Cells Mol Dis. 2017;67(155–168. [DOI] [PubMed] [Google Scholar]
- 10.Hossain MA, Bungert J. Genome Editing for Sickle Cell Disease: A Little BCL11A Goes a Long Way. Mol Ther. 2017;25(3):561–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankaran VG, Menne TF, Šćepanović D, et al. MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc Natl Acad Sci USA. 2011;108(4):1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YT, de Vasconcellos JF, Byrnes C, et al. Erythroid-Specific Expression of LIN28A Is Sufficient for Robust Gamma-Globin Gene and Protein Expression in Adult Erythroblasts. PLoS One. 2015;10(12):e0144977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward CM, Li B, Pace BS. Original Research: Stable expression of miR-34a mediates fetal hemoglobin induction in K562 cells. Exp Biol Med (Maywood). 2016;241(7):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krijger PH, de Laat W. Regulation of disease-associated gene expression in the 3D genome. Nat Rev Mol Cell Biol. 2016;17(12):771–782. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Li B, Pace BS. NRF2 mediates γ-globin gene regulation and fetal hemoglobin induction in human erythroid progenitors. Haematologica. 2017;102(8):e285–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.