Abstract
Paralysis of the upper extremities following cervical spinal cord injury (SCI) significantly impairs one's ability to live independently. While regaining hand function or grasping ability is considered one of the most desired functions in tetraplegics, limited therapeutic development in this direction has been demonstrated to date in humans with a high severe cervical injury. The underlying hypothesis is that after severe cervical SCI, nonfunctional sensory-motor networks within the cervical spinal cord can be transcutaneously neuromodulated to physiological states that enable and amplify voluntary control of the hand. Improved voluntary hand function occurred within a single session in every subject tested. After eight sessions of non-invasive transcutaneous stimulation, combined with training over 4 weeks, maximum voluntary hand grip forces increased by ∼325% (in the presence of stimulation) and ∼225% (when grip strength was tested without simultaneous stimulation) in chronic cervical SCI subjects (American Spinal Injury Association Impairment Scale [AIS] B, n = 3; AIS C, n = 5) 1-21 years post-injury). Maximum grip strength improved in both the left and right hands and the magnitude of increase was independent of hand dominance. We refer to the neuromodulatory method used as transcutaneous enabling motor control to emphasize that the stimulation parameters used are designed to avoid directly inducing muscular contractions, but to enable task performance according to the subject's voluntary intent. In some subjects, there were improvements in autonomic function, lower extremity motor function, and sensation below the level of the lesion. Although a neuromodulation-training effect was observed in every subject tested, further controlled and blinded studies are needed to determine the responsiveness of a larger and broader population of subjects varying in the type, severity, and years post-injury. It appears rather convincing, however, that a “central pattern generation” phenomenon as generally perceived in the lumbosacral networks in controlling stepping neuromodulator is not a critical element of spinal neuromodulation to regain function among spinal networks.
Keywords: : cervical spinal cord injury, non-invasive spinal cord stimulation, tetraplegia, upper extremity rehabilitation
Introduction
More than 1 million people in the United States cannot walk and have minimal function in the upper limbs, and hundreds of thousands have lost control of vital bodily functions due to spinal cord injury (SCI). While there has been considerable progress in rehabilitation efforts focusing on adapting the individual to the disability, improvements beyond the immediate spontaneous lost function has been limited. One approach has been to use brain–muscle interfaces to trigger muscle stimulation directly, thus bypassing the control that is intrinsic among the spinal networks.1,2 We have reported that the cervical spinal networks can be modulated with epidurally implanted electrodes to achieve significant functional recovery of the upper limb in SCI patients3 as previously shown for regaining function of the lower limbs with lumbosacral epidural stimulation.4
More recently, we have developed a prototype stimulation device that can non-invasively neuromodulate the lumbosacral spinal networks (transcutaneous enabling motor control [tEmc]). When tEmc is combined with training, a rapid recovery of the ability to voluntarily generate bilateral rhythmic stepping-like movements in individuals who had been completely paralyzed for more than a year.4–6 The present study was designed to determine whether the same non-invasive tEmc approach would be effective in neuromodulating the cervical spinal networks to improve upper limb motor function. The objective of this study was to test the efficacy of using tEmc in combination with upper extremity exercises over the course of 4 weeks in recovering sensory-motor function. We hypothesized that the cervical spinal segments can be neuromodulated to physiological states that can enhance voluntary motor control when used in conjunction with motor training.
Methods
Experimental design
This study was registered with ClinicalTrials.gov, number NCT01906424. Subjects were invited to participate in the study based on the following inclusion and exclusion criteria:
Inclusion criteria: 1) age >18 years; 2) SCI 1 or more years prior; 3) American Spinal Injury Association Impairment Scale (AIS) A, B and C; 4) non-progressive traumatic SCI at C7 (vertebral level) or higher; 5) ability to commit to 12 week participation; 6) stable medical condition without cardiopulmonary disease or dysautonomia that would contraindicate participation in upper extremity rehabilitation or testing activities; 7) not dependent on ventilation support; 8) no painful musculoskeletal dysfunction, unhealed fracture, pressure sore, or urinary tract infection that might interfere with upper extremity rehabilitation or testing activities; 9) No clinically significant depression or ongoing drug abuse; 10) adequate social support network to be able to participate in weekly training and assessment sessions for the duration of the 12 week study period; 11) no current anti-spasticity regimen; 12) must not have received Botox injections in the prior 6 months; and 13) limited use upper extremity for functional tasks.
Exclusion criteria: 1) pregnancy, and 2) no functional segmental reflexes below the lesion.
From those that qualified, a select group were asked to complete a pre-screening questionnaire by phone interview; from those who qualified in the interview, 11 were selected to undergo a neurological and electrophysiological evaluation, of which eight subjects (SCI AIS-B, n = 3 and AIS-C, n = 5) qualified for the study. Neurological evaluations by the team physician included testing sensory (soft touch via Q-tip and pin prick) and motor scores. All subjects signed an informed consent form that was approved by the Institutional Review Board at the University of California, Los Angeles. Six of the eight subjects completed the trial. Subject 452495 discontinued due to a urinary tract infection and subject 259463 discontinued due to educational obligations after four sessions.
For each subject, three baseline sessions were conducted over a period of 10 days including:
-
1.
Neurophysiological tests (spinally evoked potentials, 1 Hz with a 1 msec pulse width and monophasic waveform) to determine recruitment profiles of proximal and distal motor pools with increasing stimulation intensity ranging from 10 mA to 200 mA (or if noted to be uncomfortable)
-
2.
Hand grip with maximum voluntary contraction (MVC) without stimulation
-
3.
Identifying appropriate stimulation parameters for each site of stimulation (30 Hz, 1 msec pulse width, biphasic waveform [AIS C] or monophasic waveform [AIS B])
-
4.
Efficacy of one site versus a second site versus combined two-site stimulation in generating a MVC based on stimulation parameters identified in step # 3
At the end of the baseline sessions, the formal 4-week intervention program (two sessions/week) using a proprietary multi-channel transcutaneous stimulator to neuromodulate the spinal cord began. Each session consisted of 1-2 h/day, including a series of voluntary hand grip tasks, beginning without tEmc, followed by series of 18 attempts to generate an MVC (for ∼5 secs) for each hand with tEmc. At the end of the session, MVCs were repeated without tEmc. At the end of the intervention, six subjects who completed the study underwent final neurological, functional, and electrophysiological evaluation. Functional changes associated with quality of life were self-reported by all subjects before every session. Of special note, during and between all sessions, no adverse events were reported. The stimulation intensity used caused no discomfort of concern to the patients, did not negatively affect their breathing patterns, heart rate, or blood pressure, cause any adverse skin reaction at the stimulation site, nor result in any adverse effects on severity of self-reported spasticity.
tEmc stimulation protocol
The experiments were carried out with use of a proprietary non-invasive Transcutaneous Electrical Spinal Cord Stimulator (NeuroRecovery Technologies Inc.). During baseline and final evaluation, each subject was evaluated for functional responses to single versus two-site stimulation. In each case, it was noted that applying stimulation simultaneously at two sites was consistently more effective. This finding was consistent with previous studies of lower extremity function showing that multi-site spinal cord stimulation using tEmc was more effective than single site stimulation for inducing involuntary stepping movements.7,8 With this determination, we delivered transcutaneous stimulation simultaneously at two sites along the midline between spinous processes C3-C4 and C6-C7 during every interventional session. The intensity of stimulation at each spinal level was adjusted sufficiently to enable maximal grip strength when applied in isolation, without causing discomfort (range, 10–250 mA). Tonic electromyography (EMG) responses from proximal and distal muscles along with MVC forces were observed to optimize the stimulation intensity. Further, the stimulation parameters also were adjusted based on patient feedback. Stimulation was continuously delivered using 2.0 cm diameter hydrogel adhesive electrodes (Axelgaard, ValuTrode® Cloth) as cathodes and two 5.0 × 10.0 cm2 rectangular electrodes (Axelgaard, ValuTrode® Cloth) placed symmetrically on the skin over the iliac crests as anodes. tEmc was delivered using biphasic or monophasic rectangular 1.0-msec pulses at a frequency of 30 Hz, with each pulse filled with a carrier frequency of 10 kHz (Table 1). It should be noted that since this was a proof of concept study, the patients were made aware when the stimulation was turned on to check for any adverse events.
Table 1.
Stimulation Parameters Used for the Six Individuals Who Completed the Study
| Sub ID | Mode | Frequency | PW | Site | Amp |
|---|---|---|---|---|---|
| 265582 | Biphasic | 30 Hz | 1 msec | C3-4/C6-7 | 110/110 mA |
| 491863 | Biphasic | 30 Hz | 1 msec | C3-4/C6-7 | 90/100 mA |
| 058613 | Biphasic | 30 Hz | 1 msec | C3-4/C6-7 | 120/120 mA |
| 773762 | Monophasic | 30 Hz | 1 msec | C3-4/C6-7 | 90/140 mA |
| 739144 | Monophasic | 30 Hz | 1 msec | C3-4/C6-7 | 180/210 mA |
| 511282 | Biphasic | 30 Hz | 1 msec | C3-4/C6-7 | 70/70 mA |
PW, pulse width.
EMG recording
Muscle activity was recorded from select proximal (bicep brachia) and distal (flexor digitorium and extensor digitorium) muscles via surface EMG electrodes (LabChart and PowerLab; ADInstruments). Data were recorded and sampled at a rate of 10 kHz and were analyzed using LabChart software. EMG data were filtered using a 60-Hz notch filter and a Butterworth bandpass filter of 10 to 1000 Hz. Peak-to-peak analyses were conducted using the LabChart software to analyze spinally evoked EMG responses. The EMG data were filtered, rectified to analyze area under the curve during MVC tasks to calculate the integrated EMG.
Hand grip function
Over the course of the 1-2 h training session, the subjects performed two tasks: 1) maximum voluntary contraction (isometric) to assess and train for grip strength, and 2) voluntary rhythmic efforts of submaximal contraction (isometric) to evaluate and train for opening and closing one's hand, equivalent to squeezing/grasping, and releasing objects (voluntary movement). Each voluntary rhythmic effort maneuver was performed for 10-30 sec (one every 1 to 2 sec) and was repeated 18 times with each hand (left and right) over a 1-2 h period. The size and shape of the transducer easily accommodated the variety of hand sizes. Due to the isometric nature of the contraction, the transducer allowed subjects to train for gripping, grasping, and squeezing using primarily their forearm muscles. We found that the isometric device made it was more feasible to minimize forces that could be contributed by the subject's shoulder and upper arm muscles, compared with when pulling a spring-loaded grip device.3,9 In addition, the subjects were instructed and closely monitored to assure that a neutral wrist position was maintained while performing the maximum voluntary efforts and voluntary rhythmic efforts to avoid any compensatory mechanisms such as tenodesis. The possibility of the transfer of forces emanating from movements of the more proximal arm, shoulder and trunk muscles also were monitored for each effort, followed by examining video recordings. (Supplementary Figs.1 and 2; see online supplementary material at www.liebertpub.com).
Statistical analysis
All data are reported as mean ± standard error of the mean. Two-way repeated measures analysis of variance was used to determine overall differences across time. Wilcoxon matched-paired signed rank test was used as a post hoc test to identify difference between “tEmc Off” and “tEmc On” on a day-to-day basis. Mann Whitney U test was used to determine individual differences between “tEmc On” versus “tEmc Off” at pre-intervention and post-intervention and between “tEmc Off” and “tEmc On” at pre-intervention versus post-intervention. Coefficient of variation (cv) was calculated to determine consistency of responses at baseline. The criterion for statistical difference was set at p < 0.05 for all comparisons.
Results
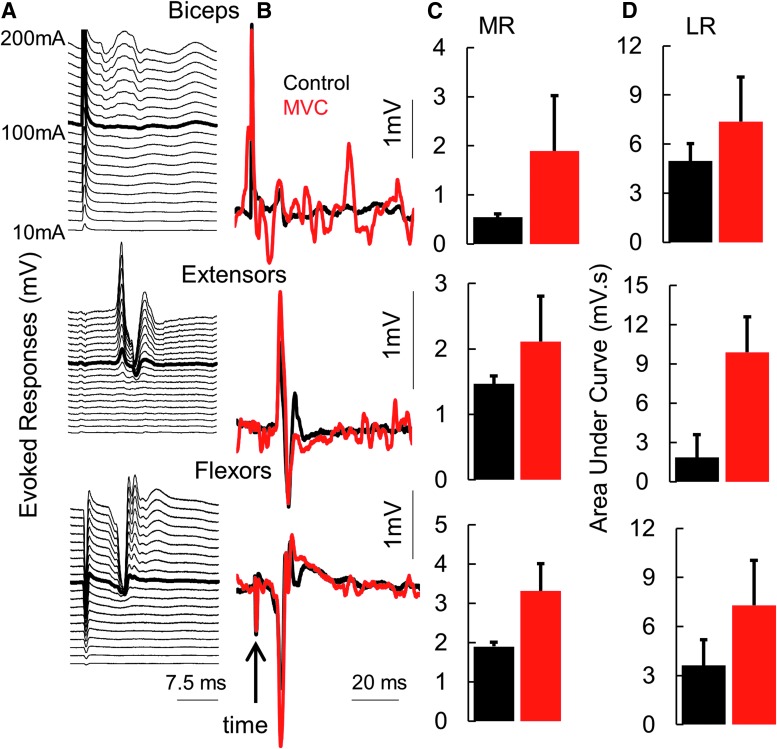
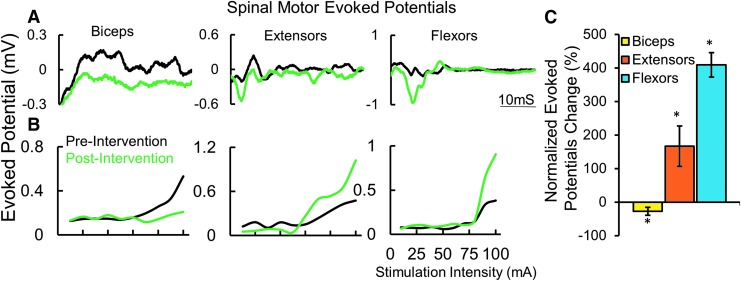
All subjects demonstrated some detectable level of grip strength, ranging from ∼0.1 N to ∼5 N (mean cv = 8.78%; n = 6 subjects), without stimulation during the three baseline tests over 10 days (Supplementary Fig. 1; see online supplementary material at www.liebertpub.com). We also observed that spinal networks controlling multiple primary upper extremity muscles can be activated via non-invasive spinal stimulation (single pulses) over a range of intensities (stimulation at C6-C7, 1 msec pulse width (PW), 10 mA-200 mA; Fig. 1A). At submaximal levels of stimulation, the responses were further amplified when combined with voluntary effort to make a fist (similar to an MVC). Evoked responses (middle [MR; ∼17-20 msec] and long [LR; 20-100 msec] latency responses) at 1 Hz spinal stimulation were amplified during MVC, suggesting a highly integrated and synergistic interface between the spinal and voluntarily controlled supraspinal networks (Fig. 1B). The increase in MR amplitude from control (no voluntary effort) to MVC was highest in the biceps while the increase in the LRs were higher in the forearm flexors and extensors, suggesting the activation of a larger interneuronal network projecting to the more distal motor pools (Fig. 1C, 1D).
Fig. 1.
(A) A series of spinally evoked responses (mean of five responses at each intensity) from proximal and distal muscles from one subject 491863 at rest with increasing intensities of stimulation at pre-intervention. In (B), the thick black line represents the average control response when stimulated at 110 mA, while the red trace was generated during a maximal voluntary effort to generate a grip force. (C) Mean ± standard deviation (n = 5 response) spinally evoked middle responses (latency ∼15 msec) in (B). (D) Area under the curve of the rectified long latency electromyography (latency 30 msec-100 msec) in B.
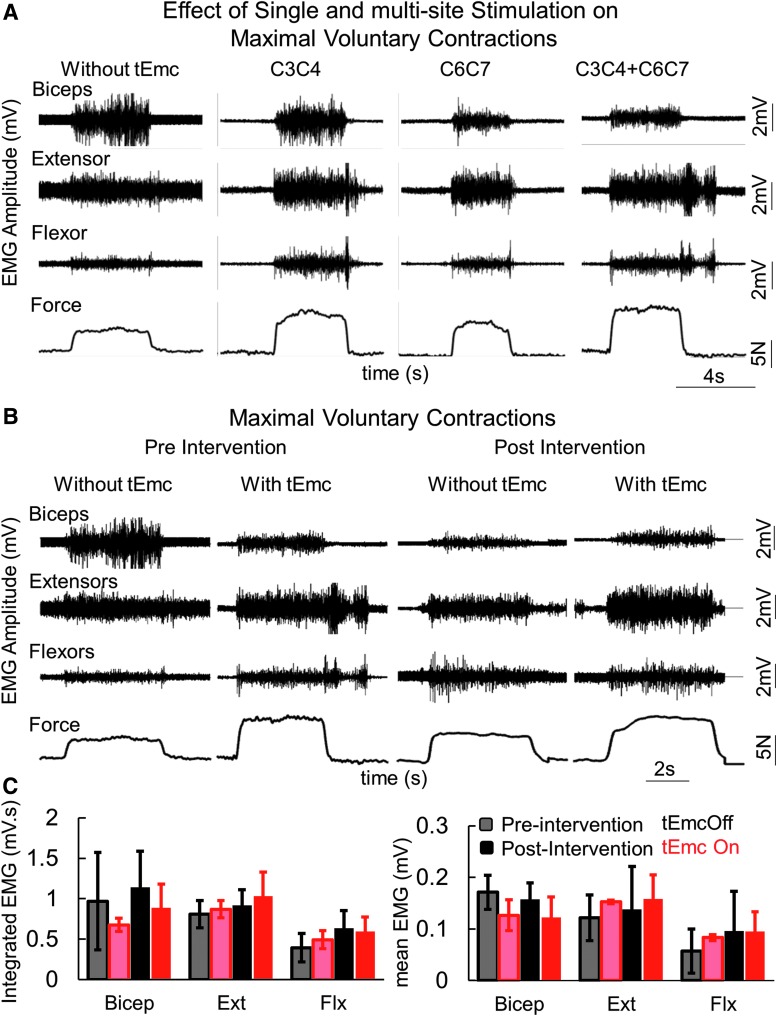
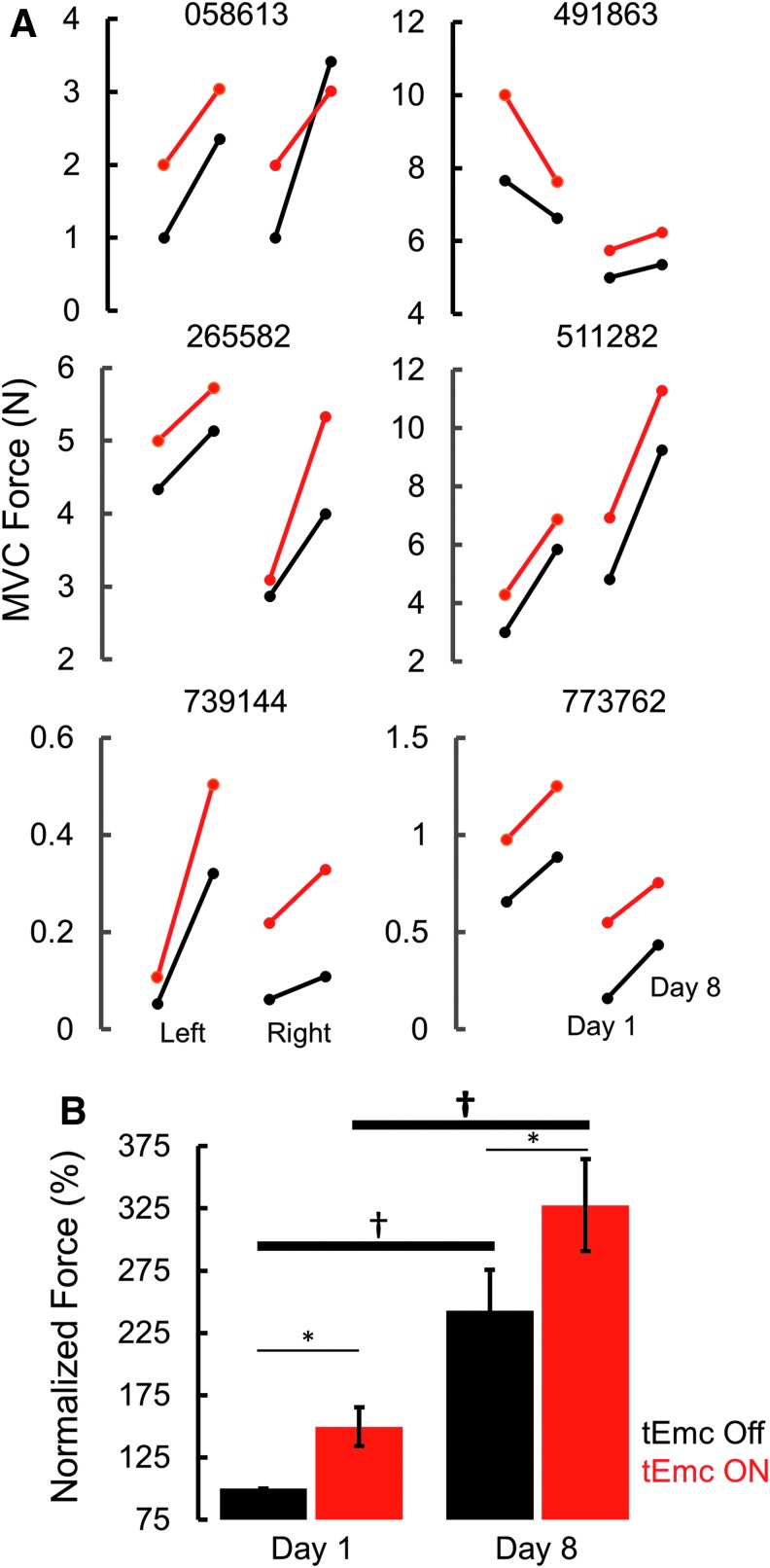
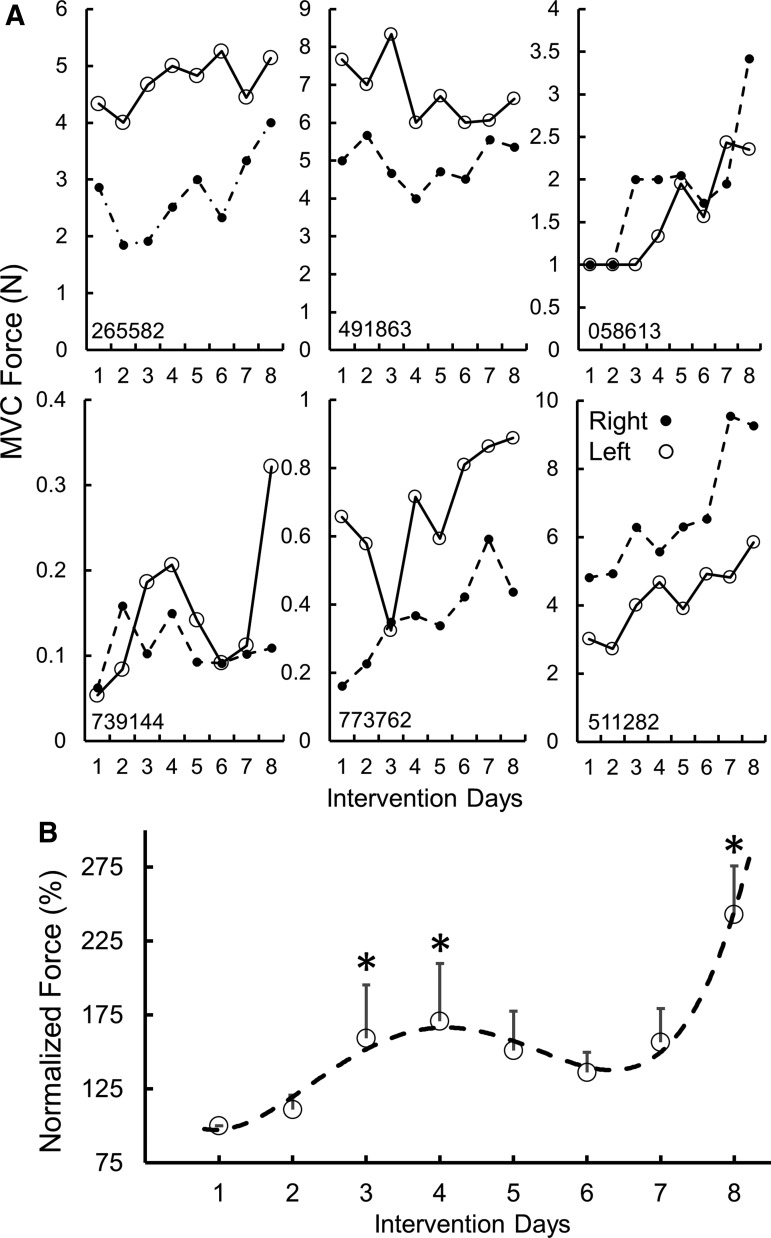
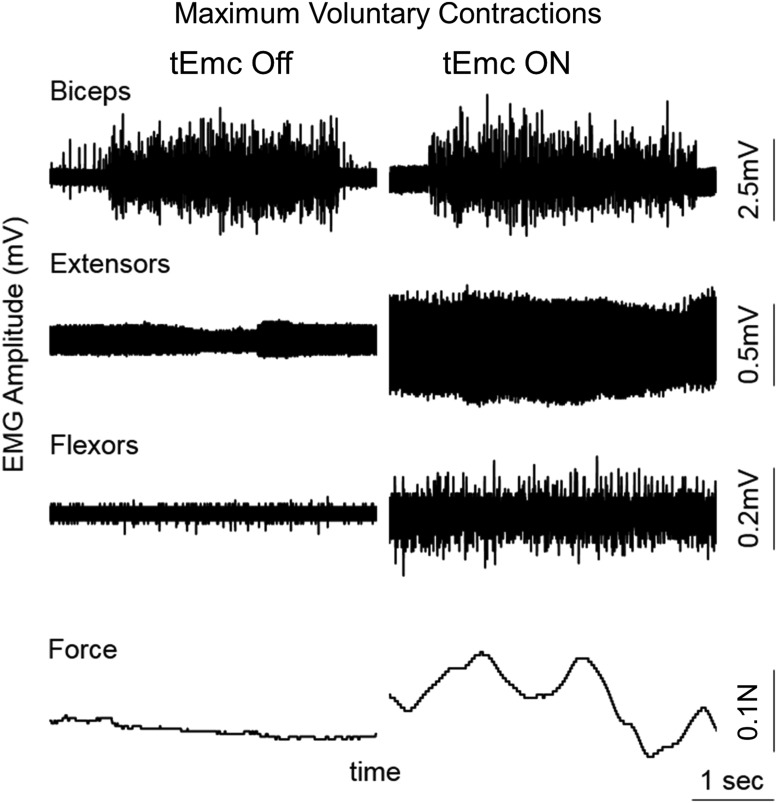
During the first treatment session, all subjects were capable of generating greater grip force with tEmc, compared with without tEmc. Further, the levels of activation of distal (forearm) muscles increased while the activation in proximal muscles decreased when exposed to multi-site stimulation (Fig. 2A). This is consistent with previous findings when stimulating the lumbosacral spinal cord in our studies of the lower extremity showed a more favorable effect of multi-site stimulation, compared with single site stimulation, to control locomotor activity10 and other motor functions.11,12 Based on the increased force voluntarily generated by the subjects, we elected to use multi-site stimulation over the course of the 4 weeks of intervention. The subjects (n = 5 both hands; n = 1 [491863] one hand) consistently generated higher forces both with and without stimulation at the end of an intervention session, compared with pre-intervention (Fig. 3 and Fig. 4). Along with an increase in force, all subjects demonstrated a reduced reliance on proximal upper arm muscles and an increased activation of distal forearm muscles consistent with the need to stabilize the wrist during MVC,13 suggesting some functional reorganization of brain-to-spinal network connectivity projecting to different motor pools (Fig. 2C). Consistent with this functional reorganization, we observed that the spinal cord evoked responses were larger for distal muscles at the end of the intervention, compared with before intervention, while the amplitudes of evoked responses were lower for the biceps at post-intervention, compared with pre-intervention (Fig. 4). Significant intersubject variability was observed both during baseline (Supplementary Fig. 1), as well as during the day-to-day performances (Fig. 4A). Further, over the course of multiple treatments, all subjects demonstrated a progressive increase in grip strength in both hands without any stimulation at the start of every session compared with before the intervention, demonstrating a residual and therefore a cumulative impact. That is, a significant level of the elevated performance, compared with baseline, persisted (2–5 days) until the next training session occurred (Fig. 4B).
Fig. 2.
(A) Representative electromyography (EMG) and force during a maximum voluntary contraction (MVC) during the first treatment session in an American Spinal Injury Association Impairment Scale C subject without stimulation; then with one site stimulation at different locations; and then with two-site simultaneous stimulation. (B) Representative EMG and force during a MVC in the same subject as in (A) before (pre-intervention) and after (post-intervention) and with and without stimulation. (C) mean ± standard error (SE) integrated EMG (mV.s) and mean ± SE EMG amplitude (n = 6 subjects) during the MVC at pre- and post-intervention with and without two-site stimulation shown in (A). biceps, biceps brachia; extensors, extensor digitorium; flexors, flexor digitorium.
Fig. 3.
(A) Six individual subjects' maximum voluntary contraction (MVC) force for left and right hands at the start and end of intervention with (red) and without (black) stimulation. (B) Normalized mean ± standard error (n = 6 subjects) at Day 1 (pre-intervention) and Day 8 (post-intervention) during transcutaneous enabling motor control (tEmc) ON and tEmc Off. *tEmc On significantly different from tEmc off. †Day 8 significantly different from Day 1.
Fig. 4.
(A) A Six individual subjects' maximum voluntary contraction (MVC) force for left and right hands during the eight treatment sessions without stimulation. (B) Normalized mean ± standard error (n = 8 subjects, Day 1–4, n = 6, Day 5–8) forces generated without transcutaneous enabling motor control for the stronger hand. *Significantly different from Day 1. The dotted line represents a 4th order curve fitted to the data.
The electrophysiological evidence from spinal cord evoked potentials suggests that an increased level of spinal network excitability occurred with tonic tEmc and training. This higher excitability, in turn, enabled greater voluntarily generated forces of networks projecting to the distal forearm motor pools, reflecting more motor units exceeding their motor threshold and/or activated at a higher frequency (Fig. 5).
Fig. 5.
(A) An example of mean (n = 5 responses) evoked potential at pre-intervention (black) and post-intervention (green). (B) An example of mean evoked potential recruitment curve for proximal and distal muscles recorded at pre-intervention (black) and post-intervention (green). (C) Normalized change in maximum evoked responses (n = 6 subjects, both hands) shown in (C). *Significantly different from 0 at p < 0.05.
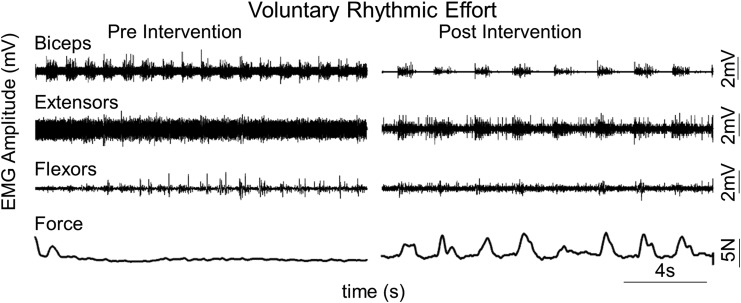
One subject (739144) classified as motor complete (AIS B) generated a barely detectable force and evidence of some minimal level of flexor and extensor EMG during the first day of testing without any tEmc (Fig. 6). When tEmc accompanied a voluntary effort on the first day of testing, the subject generated a larger voluntary force along with increased tonic EMG activity in distal muscles. The oscillating force of <0.1 N in the presence of tEmc as shown in Figure 6 occurred although the effort was intended to be tonic. Along with an increase in maximal grip strength, all subjects demonstrated an increased capability to generate submaximal rhythmic voluntary contractions, as well (Fig. 7). This suggests an improvement in not just the ability to squeeze the force transducer but also demonstrated better rhythmic control in opening and closing their hands.
Fig. 6.
Representative electromyography and force during maximum voluntary contraction with and without transcutaneous enabling motor control in an American Spinal Injury Association Impairment Scale B subject (739144) on the first treatment session.
Fig. 7.
Representative electromyography and force during rhythmic submaximal voluntary efforts in an American Spinal Injury Association Impairment Scale B subject (739144) before (pre-intervention) and after (post-intervention) without transcutaneous enabling motor control.
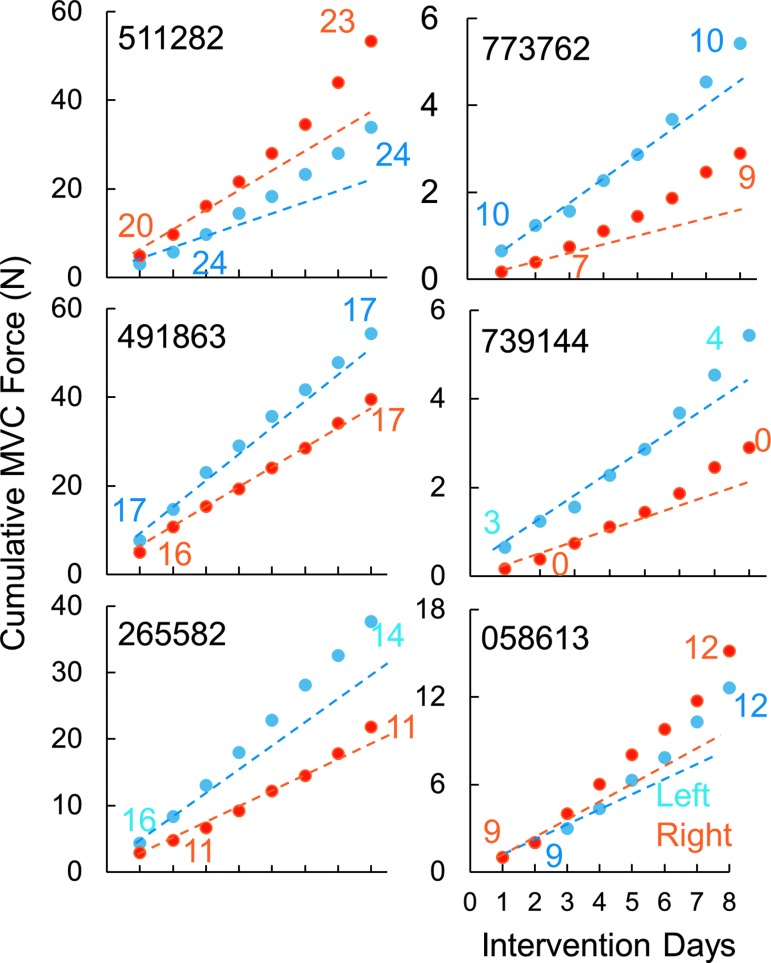
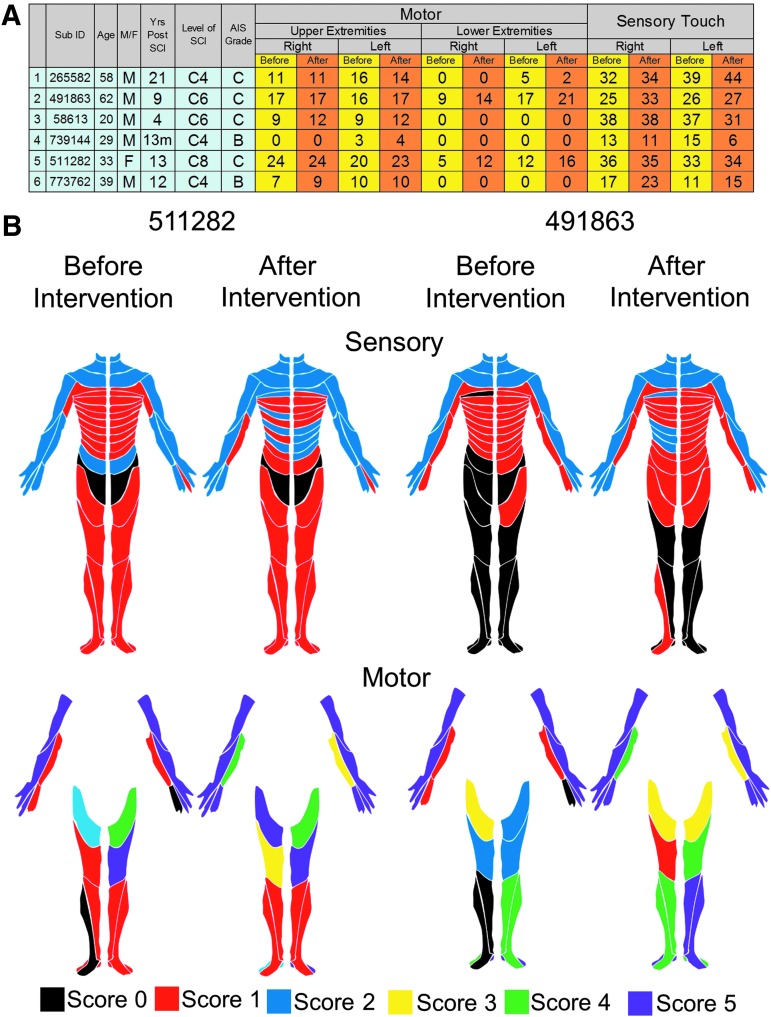
Given that the stimulation electrodes were placed at the midline of the spinal column and both of the upperlimbs were trained similarly, we sought to ascertain the improved function was dependent on hand dominance before the injury or to the upperlimb with the higher level of function remaining post-injury. The dominant hand (before SCI) did not determine the stronger hand (after SCI). In this study, all eight participants were right hand dominant (before SCI); however, three of the eight were stronger on the right side and five were stronger on the left side (at pre- and post-intervention). The strength of each hand and rate of recovery of grip strength varied based on the severity of the injury to that hand (Fig. 8). Smaller increases in grip forces were reported in subjects with the lower initial motor scores and lower initial grip forces, while at higher initial motor scores and higher initial grip forces, the increased grip strength was exponentially higher (Fig. 9). However, one subject (491863) was markedly different, wherein the subject's left arm did not improve even though the subject was one of the strongest at pre-intervention. Clinically, most of the subjects also showed an increase in their sensory and/or motor scores in International Standards for Neurological Classification of Spinal Cord Injury examination, with an overall mean increase of 4.4 ± 3.3 points (n = 6 subjects) in the motor score (p > 0.05) and a mean increase of 8.4 ± 2.9 points (n = 6 subjects) in the sensory scores (significant at p < 0.05; Fig. 10).
Fig. 8.
Cumulative maximal voluntary contraction (MVC) force and numerical motor score for the left and right hands (all right dominant at pre-injury) for six individuals over the course of the eight treatment sessions. Note the blue and orange lines are plotted based on the first and second days of intervention; thus, those data points that fall above the line represent greater response compared with the responses seen in the first to the second intervention.
Fig. 9.
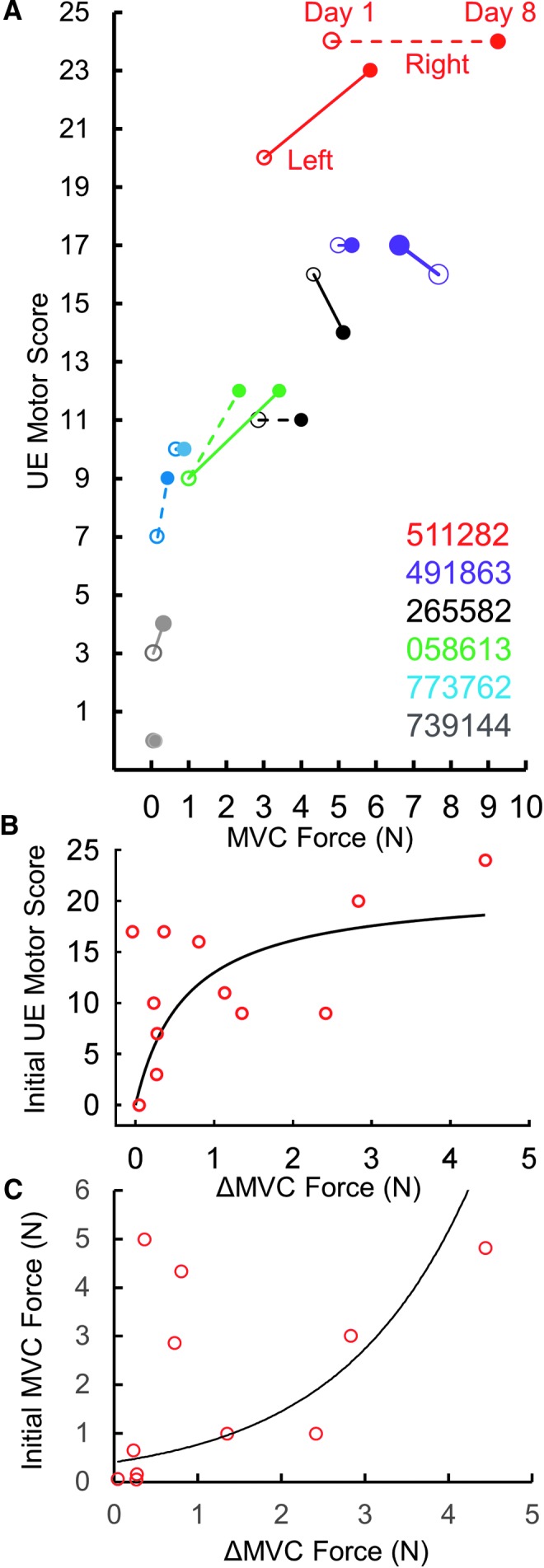
(A) Individual maximum voluntary contraction (MVC) forces at the Day 1 (hollow) and Day 8 (solid) of intervention relative to the start and end upper extremity (UE) motor scores for left and right hands. Note no change in motor score of subject 511282 but with one of greater improvement in grip force. Right hand of subject 739144 did not change in UE motor score and had minimal increase in grip force. Also note subject 491863's left hand did not improve even though the subject had a strong intial MVC and UE motor score. (B) Increased MVC force relative to initial UE motor scores for the subjects shown in (A). The 12 dots represent the left and right hands of six individuals listed in (A), r2 (linear) = 0.259, r2 (exponential) = 0.452. (C) Increased MVC force relative to initial grip force for the subjects shown in (A). 491863's left data point (non-responder) is not included in (C). Black line represents an exponential curve fitted to the data points. Note the marginal increase in grip force at lower initial motor scores and lower initial grip forces, compared with higher increased grip strength at higher initial motor scores and higher initial grip forces.
Fig. 10.
(A) Subject characteristics (n = 6) including motor and sensory scores before (yellow) and after the intervention (orange). Level of spinal cord injury neurological level based on International Standards for Neurological Classification of Spinal Cord Injury examination. (B) Examples of dermatomes for motor and sensory scores before and after the intervention for two subjects. Note: Subject 511282 had suffered an injury to the C7 vertebrae qualifying the subject for the study; however, based on the American Spinal Injury Association Impairment Scale (AIS) examination (motor and sensory scores) the subject level of injury was classified as a C8 AIS C.
Along with improved hand strength, some patients subjectively self-reported an improvement in performing tasks typically associated with activities of daily living. For example, improved trunk control made it more feasible to sit upright on the edge of their bed without back support and the ability to maintain an upright posture while standing in a standing frame. All subjects reported improvements in finger and hand dexterity. For example, the ability to successfully pinch and withdraw a debit card from the ATM machine; to pinch a clothes hanger clip to release the garment from the hanger; and to open their hand to hold a cup single handed, use a cell phone, rotate a door knob, turn a key lock, and open a water bottle with a twist-off cap were reported by at least one subject (Table 2; Supplementary Fig. 3; see online supplementary material at www.liebertpub.com). Along with changes in upper extremity, some of the patients reported an improvement in lower extremity function, including the ability to march in place between parallel bars and the ability to voluntarily flex specific joints of the lower limbs (without stimulation). These motor function changes were accompanied by improvement in one or more autonomic functions, including improved bowel function, increased perspiration below the level of injury, increased duration of reflex erections in males, and increased sensation of bladder fullness (Table 2). Patients classified as AIS B with barely detectable voluntary movement of their fingers at the start of the intervention were capable of voluntarily moving their fingers both in a gross fashion to make a fist, as well as selective activation of specific fingers on command (Supplementary Video 1; see online supplementary material at www.liebertpub.com). Anecdotally, all subjects routinely reported the maintenance of the improved function in subsequent treatment session (2–5 days between treatments on Tuesday and Thursday). Further, two subjects were capable of maintaining their grip strength when tested after 60 days of no stimulation (data not shown). It seems likely that the persistence of function without continuing tEmc interventions results from the persistence of the newly enabled and/or enlarged networks that can now be used in a plethora of voluntary movements to complete a range of motor task as desired throughout the normal day's activities. It appears that this enabling phenomenon is an important feature that can improve the quality of daily life of these individuals. It also seems likely that this persistence can lead to further recovery, enabling them to regain more fine motor skills in carrying out a wider range of activities of daily living such as grasping a cup of water to drink or holding a utensil to eat (details of improvements in specific subjects are summarized in Table 2).
Table 2.
Quality-of-Life Changes Self-Reported by the Eight Subjects Involved in the Study
| Subject ID | Years post-SCI | AIS | Upper extremity | Trunk | Lower extremity | Other |
|---|---|---|---|---|---|---|
| 265582 | 21 | C | Regained ability to pinch-withdraw debit card from ATM machine | Improved sensation in arms and trunk | ||
| 452495 | 11 | C | More fluid hand and finger movement | Significantly lower spasms in trunk and legs | ||
| 491863 | 9 | C | Improved ability to type on a computer | Improved trunk stability while sitting | Regained ability to flex hips and ankles and step in place | Improved sensation; regained ability to perspire below level of injury |
| 058613 | 4 | C | Regained ability to hold a spoon and fork and handle a cell phone | Able to sit upright | Regained ability to flex ankles | |
| 739144 | 13 months | B | Regained ability to move fingers on command | Improved control of posture during sitting | ||
| 773762 | 13 | B | Regained ability to move fingers on command | Improved ability to sit upright | Regained ability to perspire; regained sexual function | |
| 511282 | 13 | C | Improved ability to grasp, ping and hold a utensil, handle a door knob, hold a cup of water and open a sealed water bottle | Improved trunk control while sitting and while standing in a stand chair | Ability to march in place | Improved bowel control |
| 259463 | 18 months | B | Regained voluntary control of hand and fingers | Improved trunk control and awareness of core | Improved sensation in lower extremities |
SCI, spinal cord injury, American Spinal Injury Association Impairment Scale.
Discussion
The present study was designed as a proof of concept and feasibility to use tEmc to neuromodulate the cervical spinal segments to improve upperlimb function. Using a completely non-invasive intervention, we have modulated the functional potential of cervical spinal networks of severely injured human subjects to physiological states that enabled greater voluntary controlled sensory-motor function of the upper limbs in individuals that had been paralyzed up to 20+ years. We show the following effects of non-invasive neuromodulation of cervical spinal networks:
-
1.
Recovery of increased hand grip function within one session and a mean increase of ∼325% (with tEmc), from baseline measurements after eight treatment sessions over 4 weeks.
-
2.
Direct physiological evidence of reorganization of supraspinal-spinal networks (e.g., changes in amplitude of spinally evoked motor responses after training with tEmc and a significant increase of hand grip strength without tEmc after only eight treatment sessions). The present data demonstrate that the levels of connectivity of descending-ascending networks between the brain and spinal cord that initially were not detectable in the absence of tEmc can be transformed with tEmc and training to a significantly greater level of function, including strength and control of grip forces.
-
3.
Improvement in the functional state of autonomic control systems (in some individuals) emerged in response to neuromodulatory-training interventions. These improvements parallel the changes reported using epidural stimulation and locomotor training.14 We are not aware of any studies reporting improvement in autonomic function in individuals with chronic SCI.4 All subjects' self-reported improvement in one or more measures of quality of life’ parameters including increased sensation below the level of lesion, improved trunk control, improved bladder and bowel control (Table 2).
Limitations and immediate questions to address
The present data suggest that the novel transcutaneous modulation intervention used facilitated the recovery of sensory-motor function in individuals with a severe cervical spinal injury. A number of issues need to be addressed, however, before it can and should be available for general clinical use. This study was designed to carefully and systematically explore the relative responsiveness of individuals with severe chronic paralysis after a cervical spinal injury to an array of experimental neuromodulatory parameters. The present results call for further critical tests of the effectiveness of the neuromodulatory intervention, one being a fully blinded control design and including other clinically relevant tests such as Graded Redefined Assessment of Strength, Sensation, and Prehension (GRASSP) and Spinal Cord Independence Measure (SCIM). A broader, even more heterogeneous cohort with larger number of subjects (e.g., severity, spinal level of injury, and years post-injury) will also provide a clearer perspective on the potential impact of this intervention on upperlimb function. Additionally, the correlation of the frequency and duration of the intervention to the improvement in sensorimotor function is still unknown, considering that patients implanted with epidural spinal electrodes several years ago4,15 are still improving their lower extremity function.16
How do the spinal neuromodulatory procedures noted above compare with other methodologies? For example, surgical tendon transfers,17 implantable functional electrical stimulation systems18 and bionic gloves19 have been reported to improve grip strength in tetraplegics. The improved grip strength of 225% observed in the present study after eight sessions (without tEmc) was significantly higher and occurred more rapidly than has been observed with surgical interventions,17 bionic gloves19 or implantable muscle stimulators. We are unaware of an interventional device that has been applied to subjects with injuries as severe and as chronic as those studied here that has recovered similar magnitudes of sensory, motor, and autonomic functions as rapidly as observed in the present study. Further, these data do not provide a direct comparison of efficacy with many other strategies that have shown some facilitation of motor function. Obviously, head-to-head comparisons of the potential of tEmc intervention with other interventions, will be in order so that comparisons of the cost-to-benefit ratios with respect to magnitude of effects, technical costs, time commitment for the subject, for example, can be weighed. It also is important to recognize, however, that we have no evidence that the intervention as used here has been developed to its optimal potential.
Comparisons of the present non-invasive neuromodulatory strategy with other interventions designed to recover functions post paralysis
A common question of high relevance in the area of neuromodulation of the cervical, as well as the other spinal segments, is: What are the advantages of epidural spinal stimulation over non-invasive transcutaneous spinal stimulation? Though some answers are becoming clear, much uncertainty remains and several observations reflect important advantages of both non-invasive and invasive modulation.20 Multiple functions can be treated with the transcutaneous approach in the same individual simply by moving the electrodes to stimulate at different spinal levels at the most efficacious site for a given function along the entire length of the spine. Some patients with less severe injuries may need the stimulation only a brief time. For growing children or for older patients, there will be advantages to avoid the surgical procedure. The economic consequences are likely to be >10-fold, compared with an epidural implant. A disadvantage of the tEmc approach is the inconvenience of the subject being able to readily don and doff the electrodes over the spine. In some cases there may be significant advantages of selecting a more focused network that could be achieved with an implant, but in other cases a broader network neuromodulation can improve motor functions involving large groups of motor pools as needed for standing, stepping and upper body functions.
For example, the success in executing most hand movements depends on positioning the more proximal muscles of the arm, the shoulder, and even the trunk. In our opinion, however, at this early stage in realizing the potential of improving multiple upper extremity functions with neuromodulatory strategies and the fact that we still have only a modest understanding of the systemic, network, cellular, and synaptic mechanisms involved, multiple neuromodulatory technologies should continue to be developed. Thus, we predict that it will be an advantage to have the option to use spinal epidural or transcutaneous approaches to neuromodulate the cervical, thoracic, and/or lumbosacral spinal cord to facilitate sensory-motor function, as well as autonomic functions such as for cardiovascular,21,22 bladder,23,24 bowel and sexual function.14
A key advantage of spinal cord stimulation, either via spinal epidural or transcutaneous stimulation, is to engage rather than bypass this exquisite control system by directly stimulating the skeletal muscles. In addition to capitalizing on the intrinsic control of a wide range of movements, as the supraspinal networks reorganize in concert with the spinal networks, spinal cord stimulation results in recruitment of motor units in a more normal order.15,25–27 than occurs with direct muscle stimulation. This recruitment order of motor unit phenotypes provides a greater resistance to neuromuscular fatigue. In addition, the modulation of the physiological state of spinal interneurons plays a key role in enabling a continuum of the coordinated activation of more motor pools to enable and/or facilitate successful execution of a wide range of intended motor events.
Improvement in hand function facilitates the integration of multiple physiological systems
Complex muscle synergies can be engaged by activating the relevant spinal networks.28 For example, neuromodulation of spinal networks at segments C3-C4 facilitated the control of upper and lower extremity movements. These observations are consistent with the idea that propriospinal neurons from the cervical pre-enlargement project to more caudal, as well as rostral spinal networks, thus engaging a broad spectrum of motor pools, approximately as they might occur normally to accommodate the presumed support system needed to execute upperlimb functions.29,30–32
Other evidence of the integration of cervical and the more caudal spinal segments in spinal injury models have been demonstrated. For example, quadrupedal stepping of rats on a treadmill with an incomplete thoracic spinal cord injury activates cervical spinal networks that enable locomotor activity of the hind limbs.31 Similarly, passive movement of the forelimbs in a rhythmic stepping pattern in a decerebrated cat can induce stepping in the hindlimbs even after hindlimb stepping has been blocked with a serotonergic antagonist.33 It appears that the extensive remodeling capacity of spinal networks34 and its robust capability to respond to therapeutic interventions32,35 after spinal cord injury can result in a persistent synergistic presence of cervical, thoracic, and lumbosacral spinal networks. Upper extremity rehabilitation, which integrates hand, arm, and trunk function is also important from other perspectives. For example, small improvements in upper extremity function can amplify the quality of life of subjects with paralysis.36 The major point here is that theoretically, the networks along the sensory motor axis will be most effective if the reorganization among networks at all levels of this axis is functionally highly synergistic. The evidence to date suggests that this synergism among networks can occur and probably be facilitated via activity dependent mechanisms following a spinal injury.
Need for neuromodulation during training
Although there could be some question of how much of the improvements in function observed in the present study could be attributed to training alone (i.e., without the motivation of receiving tEmc during training), this seems remote for the following reasons. During the first three baseline sessions of the present study, all subjects showed very stable responses without stimulation (∼0.1 N – 5 N, cv = 8.78%; Supplementary Fig. 1). Further, in a study of 17 subjects performing a similar MVC task once per week for 20 weeks an insignificant increase in MVC (3.78 N to 6.14 N) of subjects with AIS scores ranging from A-D (A [12], B [1], C [2], and D [2]) were reported.9 Neither were there any changes in the SCIM scores. The very severely injured subjects (AIS A, B and some C) cannot train if they have no or only minimal voluntary movement. In another previous study,26 we used tEmc to modulate rhythmic stepping movements in AIS A and B subjects, when initially, no movement and therefore no training could occur without the neuromodulation. In subsequent treatment sessions of tEmc combined with voluntary effort, however, all of the subjects eventually recovered rhythmic stepping without tEmc. Some subjects recovered to the point that their voluntary performance was better without than with simultaneous stimulation. It is important to recognize, however, that it was the neuromodulation-training paradigm that enabled this transformation, from no voluntary movement, to a bilateral coordinated stepping-like movement in less than 18 treatment sessions. By that stage in the treatment, significant function had emerged even when no stimulation was provided.26
The criticalness of combining the neuromodulation with an activity-dependent mechanism in the recovery of multiple sensory-motor and autonomic functions already was clearly evident in our initial paralyzed subjects that we implanted for epidural stimulation of the lumbosacral spinal networks, when no improved motor functions emerged with 80 sessions of training without stimulation. Only when they were combined did the recovered functions emerge.4 We have reported a similar interdependence of enabling and activity-dependent mechanisms phenomenon in adult rats with a complete mid-thoracic spinal cord transection37 in which spontaneous cage activity increased 5-fold when a tonic subthreshold stimulus strength (80% of motor threshold) was applied, compared with when all conditions were identical but no stimulation was provided. These examples of modulation by either implanted or transcutaneous electrodes show that the stimulation can enable one to move, which in turn enables training. Thus, there seems to be a clear interdependence of these two variables as they are used in improving multiple physiological systems.4 The interactive effects of the two interventions are highly dynamic and for all practical purposes, spinal neuromodulation and training are functionally inseparable. What remains unclear and important to define, however are some basic principles that can provide a strategy for optimizing the dose of tEmc and of training at any given functional deficit state in a given subject at any given point post-injury and in a given state of ‘wellness.” For very practical reasons (how often and how much training or stimulation), these questions are and will remain a challenge as we gain an increasingly clearer understanding of the mechanisms involved.
Conclusion
Our working hypothesis is that there are spinal neuronal networks above, within, and below a spinal lesion in a significant number of individuals with chronic, severe paralysis that can be neuromodulated into an elevated functional state. This can occur with specific modes of repetitive spinal stimulation that facilitates an emergence of supraspinal-spinal connectivity when simultaneously receiving highly coordinated and predictable proprioceptive and cutaneous input.38 A consistent concept that is evolving is that the improved functions observed in previous and the present study is that it is possible to engage surviving, but non-functional spinal networks to ones with greater intrinsic automaticity in generating coordinated motor tasks. This result reflects the presence of functional supraspinal-spinal connectivity that can mediate a conscious effort to generate a maximum grip force.
This is a feature of these spinal and supraspinal networks that, to date, has been largely overlooked in efforts to regain upper and lower sensorimotor as well as autonomic function after paralysis. We propose, therefore, that the present data provide compelling reasons to further define the critical variables and parameters to optimize functional recovery. The increasing number of examples of regained/improved supraspinal control after “complete” paralysis suggest that the basic biology of a spinal lesion that is presently clinically defined to be motor complete must, at least, be challenged.
Supplementary Material
Acknowledgments
This research was funded by the Christopher & Dana Reeve Foundation, Broccoli Foundation, the Walkabout Foundation, NIH U01EB007615, NIH R43EB017641 and Y.P.G. was supported by the Russian Foundation for Fundamental Research (Grant No. 16-29-08173-ofi-m). We would like to thank Mr. Anirudh Bushan for his help with patient training and Ms. Nelly Kokikian for her help with data analysis.
Author Disclosure Statement
V.R.E, Y.P.G, N.T., and PG, researchers on the study team, hold shareholder interest in NeuroRecovery Technologies and hold certain inventorship rights on intellectual property licensed by the Regents of the University of California to NeuroRecovery Technologies and its subsidiaries.
References
- 1.Ethier C. and Miller L.E. (2015). Brain-controlled muscle stimulation for the restoration of motor function. Neurobiol. Dis. 83, 180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensmaia S.J. and Miller L.E. (2014). Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nature Rev. Neurosci. 15, 313–325 [DOI] [PubMed] [Google Scholar]
- 3.Lu D.C., Edgerton V.R., Modaber M., AuYong N., Morikawa E., Zdunowski S., Sarino M.E., Sarrafzadeh M., Nuwer M.R., Roy R.R. and Gerasimenko Y. (2016). Engaging Cervical Spinal Cord Networks to Reenable Volitional Control of Hand Function in Tetraplegic Patients. Neurorehabil. Neural Repair 30, 951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.G., and Edgerton V.R. (2011). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rejc E., Angeli C., and Harkema S. (2015). Effects of Lumbosacral Spinal Cord Epidural Stimulation for Standing after Chronic Complete Paralysis in Humans. PloS One 10, e0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grahn P.J., Lavrov I.A., Sayenko D.G., Van Straaten M.G., Gill M.L., Strommen J.A., Calvert J.S., Drubach D.I., Beck L.A., Linde M.B., Thoreson A.R., Lopez C., Mendez A.A., Gad P.N., Gerasimenko Y.P., Edgerton V.R., Zhao K.D., and Lee K.H. (2017). Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin. Proc. 92, 544–554 [DOI] [PubMed] [Google Scholar]
- 7.Gerasimenko Y.P., Lu D.C., Modaber M., Zdunowski S., Gad P., Sayenko D.G., Morikawa E., Haakana P., Ferguson A.R., Roy R.R., and Edgerton V.R. (2015). Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 32, 1968–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerasimenko Y., Gad P., Sayenko D., McKinney Z., Gorodnichev R., Puhov A., Moshonkina T., Savochin A., Selionov V., Shigueva T., Tomilovskaya E., Kozlovskaya I., and Edgerton V.R. (2016). Integration of sensory, spinal, and volitional descending inputs in regulation of human locomotion. J. Neurophysiol. 116, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman H., Sierro T., Niu T., Sarino M.E., Sarrafzadeh M., McArthur D., Edgerton V.R., and Lu D.C. (2017). Rehabilitation of hand function after spinal cord injury using a novel handgrip device: a pilot study. J. Neuroeng. Rehabil. 14, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerasimenko Y., Gorodnichev R., Puhov A., Moshonkina T., Savochin A., Selionov V., Roy R.R., Lu D.C., and Edgerton V.R. (2015). Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J. Neurophysiol. 113, 834–842 [DOI] [PubMed] [Google Scholar]
- 11.Shah P.K., Sureddi S., Alam M., Zhong H., Roy R.R., Edgerton V.R., and Gerasimenko Y. (2016). Unique spatiotemporal neuromodulation of the lumbosacral circuitry shapes locomotor success after spinal cord injury. J. Neurotrauma 33, 1709–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah P.K. and Gerasimenko Y. (2016). Multi-site spinal stimulation strategies to enhance locomotion after paralysis. Neural Regen. Res. 11, 1926–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charissou C., Amarantini D., Baures R., Berton E., and Vigouroux L. (2017). Effects of hand configuration on muscle force coordination, co-contraction and concomitant intermuscular coupling during maximal isometric flexion of the fingers. Eur. J. Applied Physiol. 117, 2309–2320 [DOI] [PubMed] [Google Scholar]
- 14.Hubscher C.H., Herrity A.N., Williams C.S., Montgomery L.R., Willhite A.M., Angeli C.A., and Harkema S.J. (2018). Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PloS One 13, e0190998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angeli C.A., Edgerton V.R., Gerasimenko Y.P., and Harkema S.J. (2014). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rejc E., Angeli C.A., Atkinson D., and Harkema S.J. (2017). Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci. Rep. 7, 13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.House J.H., Gwathmey F.W., and Lundsgaard D.K. (1976). Restoration of strong grasp and lateral pinch in tetraplegia due to cervical spinal cord injury. J. Hand Surg. 1, 152–159 [DOI] [PubMed] [Google Scholar]
- 18.Carroll S., Cooper C., Brown D., Sormann G., Flood S., and Denison M. (2000). Australian experience with the Freehand System for restoring grasp in quadriplegia. Aust. N. Z. J. Surg. 70, 563–568 [DOI] [PubMed] [Google Scholar]
- 19.Prochazka A., Gauthier M., Wieler M., and Kenwell Z. (1997). The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch. phys.med. rehabil. 78, 608–614 [DOI] [PubMed] [Google Scholar]
- 20.Ievins A. and Moritz C.T. (2017). Therapeutic stimulation for restoration of function after spinal cord injury. Physiology 32, 391–398 [DOI] [PubMed] [Google Scholar]
- 21.Phillips A.A., Squair J.W., Sayenko D.G., Edgerton V.R., Gerasimenko Y., and Krassioukov A.V. (2017). An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J. Neurotrauma 35, 446-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West C.R., Phillips A.A., Squair J.W., Williams A.M., Walter M., Lam T., and Krassioukov A.V. (2018). Association of epidural stimulation with cardiovascular function in an individual with spinal cord injury. JAMA Neurol. 2018. February 19; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gad P.N., Roy R.R., Zhong H., Lu D.C., Gerasimenko Y.P., and Edgerton V.R. (2014). Initiation of bladder voiding with epidural stimulation in paralyzed, step trained rats. PloS One 9, e108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gad P.N., Roy R.R., Zhong H., Gerasimenko Y.P., Taccola G., and Edgerton V.R. (2016). Neuromodulation of the neural circuits controlling the lower urinary tract. Exp. Neurol. 285, 182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman R., He J., D'Luzansky S., Willis W., and Dilli S. (2002). Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 40, 65–68 [DOI] [PubMed] [Google Scholar]
- 26.Gerasimenko Y.P., Lu D.C., Modaber M., Zdunowski S., Gad P., Sayenko D.G., Morikawa E., Haakana P., Ferguson A.R., Roy R.R., and Edgerton V.R. (2015). Noninvasive reactivation of motor descending control after paralysis. J. Neurotrauma 32, 1968–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gad P., Gerasimenko Y., Zdunowski S., Turner A., Sayenko D., Lu D.C., and Edgerton V.R. (2017). Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front. Neurosci. 11, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunshine M.D., Cho F.S., Lockwood D.R., Fechko A.S., Kasten M.R., and Moritz C.T. (2013). Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J. Neural Eng. 10, 036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwahara T., Atsuta Y., Garcia-Rill E., and Skinner R.D. (1992). Spinal cord stimulation-induced locomotion in the adult cat. Brain Res. Bull. 28, 99–105 [DOI] [PubMed] [Google Scholar]
- 30.Beaumont E., Onifer S.M., Reed W.R., and Magnuson D.S. (2006). Magnetically evoked inter-enlargement response: an assessment of ascending propriospinal fibers following spinal cord injury. Exp. Neurol. 201, 428–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah P.K., Garcia-Alias G., Choe J., Gad P., Gerasimenko Y., Tillakaratne N., Zhong H., Roy R.R., and Edgerton V.R. (2013). Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain 136, 3362–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtine G., Song B., Roy R.R., Zhong H., Herrmann J.E., Ao Y., Qi J., Edgerton V.R., and Sofroniew M.V. (2008). Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 14, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerasimenko Y., Musienko P., Bogacheva I., Moshonkina T., Savochin A., Lavrov I., Roy R.R., and Edgerton V.R. (2009). Propriospinal bypass of the serotonergic system that can facilitate stepping. J. Neurosci. 29, 5681–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bareyre F.M., Kerschensteiner M., Raineteau O., Mettenleiter T.C., Weinmann O., and Schwab M.E. (2004). The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 [DOI] [PubMed] [Google Scholar]
- 35.Houle J.D., Tom V.J., Mayes D., Wagoner G., Phillips N., and Silver J. (2006). Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J. Neurosci. 26, 7405–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peckham P.H., Keith M.W., Kilgore K.L., Grill J.H., Wuolle K.S., Thrope G.B., Gorman P., Hobby J., Mulcahey M.J., Carroll S., Hentz V.R., and Wiegner A; Implantable Neuroprosthesis Research Group. (2001). Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch. Phys. Med. Rehabil. 82, 1380–1388 [DOI] [PubMed] [Google Scholar]
- 37.Gad P., Choe J., Shah P., Garcia-Alias G., Rath M., Gerasimenko Y., Zhong H., Roy R.R., and Edgerton V.R. (2013). Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. J. Neuroeng. Rehabil. 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerasimenko Y., Sayenko D., Gad P., Liu C.T., Tillakaratne N.J., Roy R.R., Kozlovskaya I., and Edgerton V.R. (2016). Feed-forwardness of spinal networks in posture and locomotion. Neuroscientist 2016. December 1; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.