Abstract
Background
Older, rural-dwelling Latinos face multiple health disparities.
Objective
We describe the protocol of a pilot study of a community health worker-occupational therapist-led lifestyle program, ¡Vivir Mi Vida! (¡VMV!), designed for delivery in primary care and adapted for late-midlife, Latino rural-living patients.
Methodology
Using mixed methods, we collected feasibility, acceptability, and preliminary efficacy data on ¡VMV!. Forty 50–64-year-old Latinos participated in a 16-week lifestyle intervention led by a community health worker-occupational therapist team. We conducted pre- and post-intervention assessments to evaluate the efficacy of ¡VMV! in improving psychosocial and clinical health outcomes. Focus groups and interviews were held post-intervention with participants and key stakeholders to assess feasibility and acceptability.
Findings
This is the first trial designed to evaluate a lifestyle intervention that includes collaboration between occupational therapists and community health workers within primary care.
Conclusion
The detailed description of methodology promotes research transparency and reproducibility of a community health worker-occupational therapist-led lifestyle intervention.
Keywords: community-based research, community health worker, Latino, lifestyle intervention, occupational therapy, rural
As the US population lives longer, efforts to help individuals lead healthier, disability-free lives during older adulthood have become an increasingly important public health focus. However, during the years leading up to old age, which we term late middle age (50–64 years old), individuals commonly experience incipient comorbidities such as arthritis or diabetes (Ornstein, Nietert, Jenkins, & Litvin, 2013). In addition, they often confront psychologically stressful challenges such as the death of parents or multiple caregiving responsibilities. This broad risk scenario is magnified for (a) individuals with socioeconomic vulnerabilities, including impoverished racial/ethnic minority group members (R. T. Brown et al., 2014) and (b) residents of rural communities, who commonly must negotiate access challenges to health care (Lutfiyya, Bianco, Quinlan, Hall, & Waring, 2012).
In response to the above concerns, our research team, in cooperation with local Los Angeles patients, healthcare providers, and community members, has been developing a culturally tailored, activity-focused lifestyle program, ¡Vivir Mi Vida! (¡VMV!; Schepens Niemiec et al., 2015). The goal of ¡VMV! is to improve the health and wellness of at-risk (i.e.., having social risk factors for poor health outcomes such as socioeconomic challenges and limited access to health care), late-midlife Latino adults. The intervention has several desirable features insofar as it: (a) is delivered by a community health worker (CHW)-occupational therapist (OT) team—a cutting-edge partnership—within a primary care setting that serves hard-to-reach, safety-net populations; (b) is underpinned with foundational principles of effective OT lifestyle intervention for ethnically diverse older adults (Clark et al., 2012); (c) attends to all facets of a healthy lifestyle; and (d) targets patient-prioritized outcomes. To address the dearth of health programs for non-urban Latinos, we made rural-targeted customizations to ¡VMV!. The purpose of this paper is to describe the ¡VMV! study design and protocol, as well as implementation challenges. This report provides information that may assist other OT researchers in developing similar community-based programs.
Methods
Using a single-arm design (i.e., all participants received the same treatment), we conducted a feasibility and pre-post pilot assessment of the adapted ¡VMV! intervention. The study, which also included brief qualitative exit interviews and focus groups, extended from January to August of 2016. The University of Southern California institutional review board (IRB) approved all study procedures.
Antelope Valley Community Clinic (AVCC)—our collaborating primary care clinic—conducted pre-screenings for eligibility using demographic information from a patient database. AVCC referred potentially eligible individuals to the study team by providing necessary patient contact information. Primary recruitment took place through randomized selection of patients from this list. A Spanish-speaking CHW telephoned and screened individuals to confirm eligibility. Inclusion criteria were as follows: contact with AVCC within the past year; documented as Latino; 50–64 years of age; fluency in Spanish; residence in Antelope Valley (AV) without plans to move within six months; availability by telephone; orientation to person, place, and time; and self-reported ability to participate in a 16-week intervention. Eligible persons interested in study participation were scheduled for an in-person visit to complete written informed consent and baseline assessment.
Sample size calculations indicated that the inclusion of 40 participants, given 10% attrition as found in a previous three-month lifestyle intervention for Latinos (Vincent, Pasvogel, & Barrera, 2007), would produce sufficient power (80%) to detect a change score with a medium effect size of .454 (two-sided alpha=.05, paired sample t-test) for a continuous outcome variable. We based our estimate of effect magnitude on the finding of medium effect sizes (d ranging from .46–.62) for significant variables in similar interventions (Koniak-Griffin et al., 2015; Parikh et al., 2010). Additionally, this sample size was adequate to generate useful information regarding the feasibility and acceptability of the planned intervention (Thabane et al., 2010).
Because our research headquarters was located in Los Angeles, 75 miles from AV where research took place, collaboration with a local community partner to fulfill personnel needs was crucial. We established a partnership with Antelope Valley Partners for Health (AVPH), a non-profit public health organization that provides wellness interventions to AV residents and patients of AVCC. AVPH supplied two Spanish-speaking CHWs and two assessors. Using recommended training processes for CHWs (O’Brien, Squires, Bixby, & Larson, 2009), we constructed a 40-hour instructional workshop for the intervening CHWs that covered: (1) research basics, (2) Lifestyle Redesign® principles, (3) ¡VMV! manual content, (4) ¡VMV! intervention tools and delivery techniques, (5) setting and revising participant goals, (6) motivational interviewing, and (7) documentation of treatment sessions.
General procedures
As individuals joined the study, they were allocated by convenience to one of four intervention groups (n=10 per group; 2 groups per CHW) based on individually specified times of availability. The intervention was initiated via an in-person, one-to-one session with a CHW combined with a video call from the treating OT. The CHW led subsequent, approximately weekly sessions over a 16-week period.
The assessors conducted baseline and post-intervention assessments in person at each participant’s location of choice. Participants had limited availability for group sessions due to reasons such as work schedules, childcare responsibilities, and lack of transportation. Consequently, it took an average of three weeks following the baseline assessment to initiate the intervention, which required coordinating 10 participants per group prior to intervention commencement. Following post-intervention assessment, each participant was briefly interviewed about the feasibility and acceptability of ¡VMV!. For the same purpose, two participant focus groups (n=8 and n=7)—size consistent with general practices for optimal group interactions (Masadeh, 2012)—and interviews with the community-based research personnel and administrators (n=6) were also conducted after all groups completed the intervention.
Assessments
The majority of health indicators were measured both at baseline and post-intervention. All tools were previously validated in Latino populations and available in Spanish. We gathered self-reported items via oral interviews. The primary study outcome—patient-identified symptom profile (PISP)—was measured using the Measure Yourself Medical Outcome Profile (MYMOP2), a questionnaire that requires respondents to identify personally bothersome symptoms. Patients rate well-being, symptom severity, as well as how much each symptom interferes with daily activities. This tool is sensitive to change and has construct and criterion validity (Paterson, 1996; Paterson et al., 2000; Polus, Kimpton, & Walsh, 2011).
Secondary outcomes included food frequency, measured with the Block 2005 Food Frequency Questionnaire Spanish Version (Block FFQ; Centers for Disease Control and Prevention, 2015); physical activity engagement, assessed using the short version of the International Physical Activity Questionnaires (IPAQ); social health satisfaction, measured with the Satisfaction with Social Roles–Short Form 7a and Satisfaction with Participation in Discretionary Social Activities–Short Form 7a (Cella et al., 2010); sleep quality, evaluated with the Pittsburgh Sleep Quality Index (PSQI; Backhaus et al., 2002, Cole et al., 2006); stress, assessed using Elo et al.’s (2003) Single Item Stress Index. Clinical measures hemoglobin A1c (HbA1c); disease risk, computed using the Framingham Risk Score LDL Points Total (Wilson et al., 1998) and European Prospective Investigation into Cancer and Nutrition (EPIC) Diabetes Risk Score (Schulze et al., 2007); patient activation, measured with the Patient Activation Measure 13-item short form (PAM 13; Hibbard et al., 2005). Patients’ electronic medical records (EMRs) were accessed to obtain diagnosis codes and prescribed medications covering the six-month period prior to baseline through two weeks past the end of intervention delivery.
¡VMV! intervention
A multi-year process of community-based participatory research was undertaken to develop ¡VMV!. Accordingly, a wide range of patient and stakeholder input influenced the intervention and study design. As a group, we identified late-midlife Latino safety-net patients as a population in need. To expand the reach and pragmatic sustainability of the program, healthcare administrators recommended that CHWs serve as front-line intervenors, with OT adopting a supervisory role. We conducted a formal needs assessment with the target population (Schepens Niemiec et al., 2015). Consistent with the community-engaged research approach that we adopted (C. H. Brown et al., 2012), we hired a Spanish-English bilingual and bicultural senior promotora—a specialized CHW with in-depth knowledge of the culture and life experiences of the Latino community—to co-lead development and implementation of the intervention.
Principles of the OT Lifestyle Redesign® approach served as the primary theoretical foundation for the intervention. These principles include: (a) a focus on wellness, (b) the importance of habits and routines, and (c) engagement in occupation—everyday activities that occupy one’s time—as a necessity of life and precursor to improved health (Clark et al., 2015). Lifestyle Redesign® has produced beneficial effects in underserved, ethnically diverse, older minority populations (Clark et al., 1997; Clark et al., 2012). The intervention was further molded to reflect behavior change strategies linked to social cognitive theory (Bandura, 1986, 2004; Michie et al., 2011) that aligned with CHWs’ traditional scope of practice. As such, health education, healthcare navigation support, patient advocacy, role modeling, and social support were embedded in the intervention (O’Brien et al., 2009).
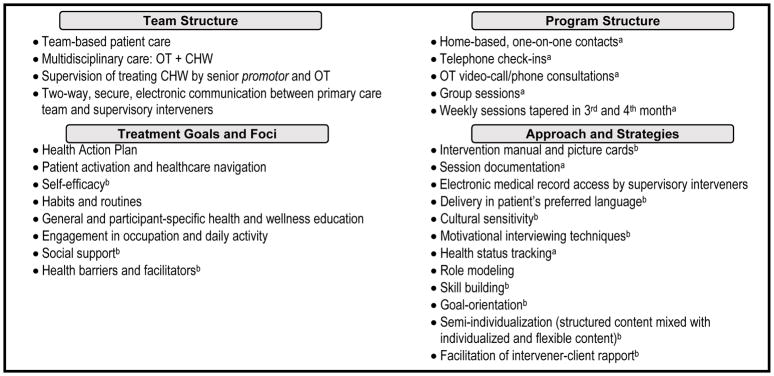
To articulate the scientific basis of the intervention, our research team identified its theoretically relevant active ingredients. We achieved consensus on the core content and processes that we postulated as critical to the intervention (Fig. 1). The ¡VMV! team structure included collaboration between OTs and CHWs, supplemented with a two-way line of communication between supervising interveners and the patient’s primary care team. Treatment goals and foci combined OT and CHW specialties such as attention to daily routines and provision of social support. Strategies utilized to achieve desired outcomes ranged from goal specification and motivational interviewing to building strong participant-intervener rapport.
Fig. 1.
Core elements of the ¡Vivir Mi Vida! intervention content and process.
aItems tracked for adherence and fidelity purposes using an electronic database shared by the interveners and research personnel
bItems assessed for fidelity as implemented by the CHWs
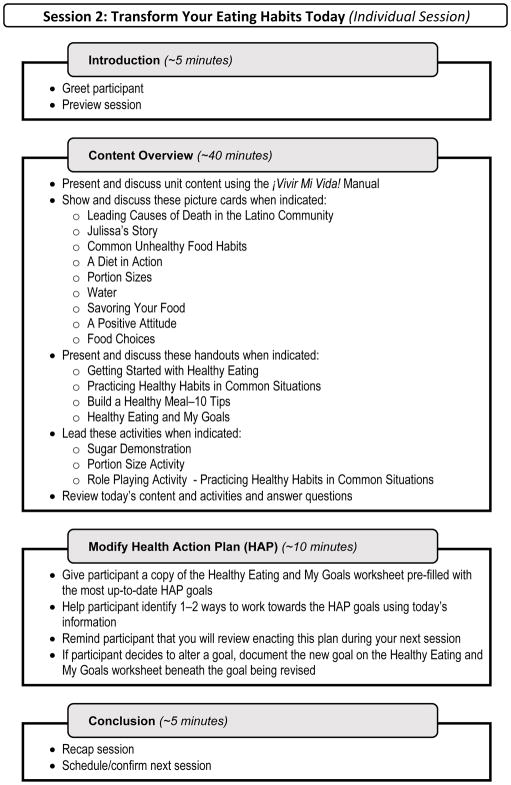
The ¡VMV! intervention included a Spanish-English scripted manual delivered by CHWs. Despite the structured nature of the manual, we incorporated open-ended discussion points and elective content to facilitate a semi-individualized experience. In contrast, OT interactions were fully individualized with consultations based on participants’ unique health portfolios. ¡VMV! included six overarching units: Welcome & Planning; Healthy Eating and Activity; Healthcare Navigation; Chronic Disease Management; Mental Well-being; and Wrap-Up (Table 1). Figure 2 presents a basic outline of a one-to-one session led by a CHW. The full intervention package included a one-hour introductory and planning session with a CHW (in-person) and an OT (video-call), seven one-hour individual in-home sessions and three one-hour group sessions led by a CHW, two 15-minute telephone check-ins from a CHW, and two 20-minute OT health consultations, all held over 16 weeks. Content included general information for all recipients (e.g., social eating), as well as discretionary topics selected by participants for customization purposes (e.g., diabetes). CHWs were equipped with a picture-based flip chart which complimented the scripted manual, as well as with a travelling intervener kit that included demonstration tools (e.g., MyPlate dishes, vascular atherosclerosis model), participant handouts, and miscellaneous supplies (e.g., markers, scale). The original program design called for the OT to send a final discharge report to the participant’s primary care physician that summarized the individual’s health status, progress towards goals, and the participant’s future health self-management. Due to budgetary and logistic constraints, the project manager, who is a licensed occupational therapist, completed this activity instead of the intervening OT.
Table 1.
¡Vivir Mi Vida! intervention units and associated session topics.
| Unit | Week | Session Type | Topic |
|---|---|---|---|
| Welcome & Planning | 1 | Individual + Video-OT | Introduction and Health Action Plan development |
| Healthy Eating & Physical Activity | 2 | Individual | Transforming eating habits |
| 3 | Individual Outing/Activity | Selecting healthy food in real-life situations | |
| 4 | Group | Social eating | |
| Telephone-OT | Individualized OT consultation | ||
| 5 | Individual | Physical activity | |
| Healthcare Navigation | 6 | Individual | Maximizing one’s healthcare |
| Chronic Disease Management | 7 | Individual | Condition-specific management (flexible session options): Diabetes; Weight management; Heart health; Arthritis; Cancer |
| Mental Well-Being | 8 | Group | Gratitude and positivity |
| Telephone-OT | Individualized OT consultation | ||
| 9 | Individual | Mental well-being | |
| 10 | -- | Off week | |
| 11 | Individual | Stressors (flexible session options): Finances and health; Sleep; Effective communication; Coping with loss; Safety | |
| 12 | Telephone | Community health worker follow-up | |
| Wrap-Up | 13 | -- | Off week |
| 14 | Group | Graduation | |
| 15 | -- | Off week | |
| 16 | Telephone | Community health worker follow-up |
Notes: OT= occupational therapist; Video-OT= Video-call consultation with an occupational therapist. All sessions were led by the intervening community health worker except where OT is indicated.
Fig. 2.
Sample session outline of a community health worker-led individualized visit.
Implementation in a rural setting
Multiple tailoring strategies foster successful implementation of health interventions in rural communities (Calancie et al., 2015; Lutfiyya et al., 2012). The original design of ¡VMV!, without adaptation, adhered to several of these recommendations. First, employing resident CHWs—trusted members of the local community—to perform recruitment and intervention delivery allowed us to gain “insider” access to hard-to-reach individuals. Second, linking ¡VMV! to participants’ primary care services promoted the goal of coordinated care. Third, building in telemedicine consultations, telephone check-ins, and home-based visits, as opposed to multiple group sessions, mitigated the necessity for participants to travel long-distances. Fourth, we supplemented instructional content with picture cards to address issues of literacy—a problem identified in minority, low socioeconomic status, and rural-dwelling communities (Zahnd, Scaife, & Francis, 2009).
In addition to the above features already included in ¡VMV!, we enacted specific adaptations to enhance its suitability for the targeted community. First, though we provided all interveners with wireless hotspot devices and laptops, the lack of reliable cellular network and Wi-Fi signals in remote locations was a concern. Therefore, activities requiring the internet were minimized and alternative options made available (e.g., permitting use of the basic telephone to conduct originally planned video-based calls). Second, we altered content related to healthcare navigation to address unique issues that rural-living patients encounter. For example, we added strategies for accessing alternative health resources to overcome barriers related to limited service availability and accessibility. Third, due to higher suicide rates documented for rural residents (Eberhardt & Pamuk, 2004), we added content on mental health and well-being, along with an in-depth suicide prevention protocol. Finally, the lack of an available OT from our community partners required hiring a research-affiliated OT to conduct consultations via video conference or telephone rather than in person, as originally designed.
Process evaluation
To determine the feasibility and acceptability of ¡VMV!, our research team conducted a mixed-methods process evaluation of study implementation. Following the post-intervention assessment, assessors interviewed participants using both a survey and semi-structured interview guide. The first part of the participant interview included closed-ended and Likert-style survey questions regarding acceptability of and experiences during the program. The second section included open-ended questions on the following three topics: facilitators to electing to participate, overall experience in the study, and suggestions for improvements. Participants were also invited to one of two 90-minute focus groups. A semi-structured guide with open-ended questions was used by an experienced qualitative researcher to lead the group discussion. The sessions were audio-recorded and a student research volunteer wrote detailed field notes. We held separate semi-structured interviews, approximately one hour in length, with stakeholders including the supervising senior promotora and OT, assessors, CHW interveners, and AVPH administrator.
Interveners tracked participant adherence via electronic attendance logs. Intervention fidelity was formally assessed in two ways. First, beginning at week three, the OT was instructed to schedule random, in-person fidelity visits for two individual and two group sessions per CHW. While attending these visits, the OT used a study-specific fidelity index that included a list and description of selected core elements of ¡VMV!. Using “completed,” “not completed,” “unsure,” or “not applicable” response options, the OT documented whether the CHW incorporated various components into the sessions. Second, we instructed each CHW to audio-record two individual sessions, which the OT later rated for fidelity using the aforementioned index.
Data management and analyses plans
During assessment sessions, the assessors entered participant responses into a Research Electronic Data Capture (REDCap) database—a web-based application designed to support data collection for research (Harris et al., 2009). Focus group and interview responses were audio-recorded, transcribed, and, when necessary, translated into English. Presently, we are in the process of coding and analyzing the transcripts using Dedoose (Version 7.0.23) qualitative data analysis software. Given the brevity of our qualitative methods employed, surface-level content analysis (Berg, 2001) is being conducted to identify themes related to feasibility and acceptability. To ensure rigor, two research team members are coding qualitative data independently, with checking by a third member for analyst triangulation. Analysts are discussing themes and deliberating disagreements or concerns until they reach consensus. An audit trail of analytic memos is being kept to document decision pathways.
¡VMV! is being assessed for feasibility and acceptability by examining a variety of factors related to scientific merit and study processes, resources, and management domains. Table 2 summarizes each relevant factor by domain, the type of information to be gathered, and data sources that we are analyzing. For each domain, success is being gauged by comparison to previously published standards (e.g., fidelity [Perepletchikova & Kazdin, 2005], enrollment and retention of minority participants [Las Nueces, Hacker, DiGirolamo, & Hicks, 2012]), with overall program success achieved by satisfying the criteria across the majority of program outcome domains.
Table 2.
Feasibility, acceptability, and efficacy data to be collected.
| Factor/Outcome | Information Gathered | Data Source/Measure | |
|---|---|---|---|
| Feasibility & Acceptability | |||
| Process | Recruitment | % (of # approached) recruited over 1 mo; % recruited from various sources | Recruitment logs; Screening forms |
| Reach | Representativeness of individuals willing and eligible to participate | Recruitment logs; Screening forms; Antelope Valley census data; AVCC patient statistics | |
| Retention | % completing post-intervention assessment | Testing logs | |
| Adherence | 16-wk session-by-session attendance | Intervener attendance logs | |
| Fidelity | Proportion of applicable core intervention elements incorporated; Perceived use of core intervention elements | Fidelity index; Stakeholder interviews; Intervener session notes | |
| Implementation barriers/facilitators | Perceived implementation barriers/facilitators | Participant focus groups; Participant interviews; Stakeholder interviews | |
| Implementation alterations | Changes made to the intervention during implementation | Intervener session notes; Stakeholder interviews; Study team daily operations communications | |
| Satisfaction | Participant and stakeholder experiences | Participant interview and survey; Participant focus groups; Stakeholder interviews | |
| Adoption | Willingness by staff and primary care provider to initiate the program | Stakeholder interviews | |
| Resources | Equipment reliability | Equipment availability and functionality | Stakeholder interviews; Study team daily operations communications; Staff time logs; Expenditures log |
| Personnel | Sufficiency of personnel to carry out all aspects of the study | ||
| Space | Sufficiency of space to carry out study | ||
| Time | Sufficiency of time to carry out study | ||
| Budget | Study expenditures | ||
| Management | Data | Database set-up; Data accessibility, accuracy and maintenance; EMR availability | REDCap; Stakeholder interviews; Study team daily operations communications; EMRs |
| Administration | Research team’s administrative capacity to manage the study; Compliance with human subjects protection protocol | Stakeholder interviews; Study team daily operations communications; Intervener notes; Adverse event logs | |
| Preliminary Efficacy | |||
| Scientific Assessment | Safety | # and type of adverse events documented | Adverse event logs |
| Patient-identified symptom profile (PISP) | Change in symptom impact/severity | MYMOP2 | |
| Food frequency | Change in dietary intake | Block FFQ | |
| Physical activity | Change in physical activity level | IPAQ | |
| Social functioning satisfaction | Change in satisfaction with social participation | PROMIS Short Form 7a: Satisfaction with Social Roles; Satisfaction with Participation in Discretionary Social Activities | |
| Sleep quality | Change in quality of sleep | PSQI | |
| Stress | Change in perceived stress level | Single Item Stress Index | |
| Hemoglobin A1c | Change in hemoglobin A1c | Non-fasting hemoglobin A1c test via finger prick | |
| Blood pressure | Change in systolic/diastolic blood pressure | Digital blood pressure reading | |
| BMI (kg/m) | Change in BMI | Digital scale and stadiometer reading | |
| Waist/hip circumference | Change in waist and hip circumference | Tape measure | |
| Coronary heart disease risk | Change in coronary heart disease risk | Framingham Risk Score LDL Points Total | |
| Diabetes risk | Change in risk for diabetes | EPIC Diabetes Risk Score | |
| Patient activation | Activation level (baseline not available) | PAM 13 | |
AVCC= Antelope Valley Community Clinic; Block FFQ= Block 2005 Food Frequency Questionnaire Spanish Version; BMI= body mass index; EPIC= European Prospective Investigation into Cancer and Nutrition; EMR= electronic medical record; IPAQ = International Physical Activity Questionnaire; MYMOP2= Measure Yourself Medical Outcome Profile; PAM 13= Patient Activation Measure 13-item short form; PROMIS= Patient Reported Outcomes Measurement Information System; PSQI= Pittsburgh Sleep Quality Index; SES= socioeconomic status; SRT= signed rank test.
A preliminary analysis of scientific feasibility of the intervention (i.e., efficacy) is underway. We are calculating descriptive statistics for demographics, as well as baseline and post-intervention outcome variable values. To assess scientific merit of the intervention, we are performing paired sample t-tests or Wilcoxon signed rank tests to compare pre-post change scores on outcomes. Results are presented in a forthcoming paper featured in Primary Health Care Research & Development (Schepens Niemiec et al., in press).
Discussion
This paper provides a detailed account of the methodology used to implement the adapted ¡VMV! program for the purpose of research transparency, a practice consistent with recent guidelines issued by the U.S. National Institutes of Health (National Institutes of Health, 2016) for fundable trials as a means of improving the rigor and utility of research. One important component of our documented methodology is the proposed core elements thought to underpin ¡VMV!. Determining the critical characteristics of a complex intervention is prerequisite to establishing fidelity procedures, which in turn can be used to isolate intervention characteristics that may serve as active ingredients and maintain implementation quality and consistency over time (Kazdin, 1997). We intend to further investigate potential key ingredients in a future trial that allows for analyses of effect mediators.
To further promote research transparency and reproducibility, our protocol delineates adaptations made to the original ¡VMV! intervention in an effort to make the program pragmatically viable for a rural-dwelling Latino population. Documenting these alterations is important, as even seemingly small changes in an intervention within a new setting can fundamentally disrupt program effectiveness (Stanton et al., 2005). Other research teams may choose to replicate relevant adaptations when transferring an intervention to a new context.
Novel aspects of the ¡VMV! lifestyle program are also worth noting. The intervention features a pioneering collaboration between OTs and CHWs within primary care. Though both fields of practice are separately emerging as promising players in this arena (Brownstein, Hirsch, Rosenthal, & Rush, 2011; Donnelly, Brenchley, Crawford, & Letts, 2013), their potential for addressing community health disparities as a partnered team is virtually untapped. Second, we approached healthy lifestyle through a holistic lens, moving beyond the traditional limited focus on diet and exercise to encompass less commonly addressed aspects of wellness such as mental well-being and life satisfaction. Indeed, holistic lifestyle interventions have produced encouraging results (Clark et al., 2012; Johansson & Bjorklund, 2016). Third, the age group for which ¡VMV! is intended (i.e., late-midlife) was purposefully narrow. Three factors influenced our decision to target this group: (a) recent theory stresses that healthy aging should happen continuously throughout the lifespan, not just in older adulthood (Hansen-Kyle, 2005); (b) healthy habits could potentially be instilled prior to major health declines often experienced in older age (Sudano & Baker, 2006); and (c) at a late middle-aged life stage, Latinos are amenable to adopting health-promoting lifestyle changes (Osuna et al., 2011).
The study protocol featured contemporary research practices. In connection with a community-based participatory research approach, patients and other stakeholders were involved in all stages of the research process, ranging from conceptualization of the intervention and study design to provision of feedback on program feasibility post-implementation. Presently, science is shifting towards this degree of patient and stakeholder engagement to produce evidence that is meaningful to end-users and readily adoptable in real-life contexts (Frank, Basch, Selby, & Patient-Centered Outcomes Research, 2014). Correspondingly, patients and all ranks of stakeholders have provided their input into the design of ¡VMV! (Schepens Niemiec et al., 2015), identified meaningful health outcomes, and assisted in the successful implementation of the pilot program. Conducting research in this manner is advantageous, as it approximates realistic implementation of the program in a community-based primary care setting.
Highlighting the patient-centeredness of our design, the primary outcome selected for this study, PISP, focuses on the self-perceived severity and daily life impact of personally bothersome health-related symptoms. We also opted to include more traditional objective clinical health indicators (e.g., blood pressure) to track outcomes of interest to our primary care stakeholders. This design acknowledges the centrality of patients’ perspectives about the intervention’s health benefits, while still allowing for collection of important data that primary care institutions may be required to report.
Despite its strengths and unique features, our study protocol has potential limitations. First, because the MYMOP2 centers on self-reported, currently bothersome symptoms, it is susceptible to regression to the mean. Further, because we used a single group pre-post design, void of a follow-up measurement period, we will not be able to establish causality or determine the period over which intervention effects persist. Moreover, the small sample size may provide low precision effect size estimates. At this stage of research, however, a formal assessment of statistically significant changes was not the end goal, but rather our primary aim was to evaluate the intervention’s feasibility. Though lack of a control group will remain a limitation, we recently received funding to add a 12-month follow-up assessment. This additional measurement period will strengthen the protocol and allow us to preliminarily evaluate long-term effects. Finally, resource limitations (as in rural settings) mandated that the research team identify creative accommodations. The brevity of the intervention (16 weeks) in comparison to other lifestyle programs (Clark et al., 2012; Koniak-Griffin et al., 2015) exemplified this issue.
Conclusion
The ¡VMV! program is an occupation-based lifestyle intervention adapted for rural-living, late-midlife Latinos and delivered by a CHW-OT team as a part of primary care services for safety-net patients. By providing a detailed report of the pilot study methodology for assessing ¡VMV!, we are supporting research transparency, scientific rigor, and reproducibility. As such, our study protocol is replicable and may thereby prove useful to other occupational therapy scientists developing lifestyle interventions for similar populations. Findings from our trial will uncover feasibility challenges that can be avoided for future large-scale studies, provide the necessary preliminary evidence supporting intervention efficacy, and elucidate the scalability of ¡VMV! in a broader primary care context.
Acknowledgments
The authors would like to thank the participants and also Antelope Valley Partners for Health, Antelope Valley Community Clinic, and Southern California Clinical and Translational Science Institute for their support of this project. This work was supported by the National Institutes of Health – National Center for Medical and Rehabilitation Research and National Institute for Neurological Disorders & Stroke (Schepens Niemiec, grant # K12 HD055929); and an internal award through the University of Southern California OS/OT Initiatives Program (Schepens Niemiec).
Footnotes
Conflict of Interest
The authors declare that there is no conflicts of interest.
Research Ethics
The institutional review board for this study was the University of Southern California Health Sciences Review Board (HSIRB). The approval number for the study was HS-14-00725.
References
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- Bandura A. Health promotion by social cognitive means. Health Education & Behavior. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- Berg BL. Qualitative Research Methods for the Social Sciences. 4. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- Brown CH, Kellam SG, Kaupert S, Muthen BO, Wang W, Muthen LK, … McManus JW. Partnerships for the design, conduct, and analysis of effectiveness, and implementation research: experiences of the prevention science and methodology group. Administration and Policy in Mental Health and Mental Health Services Research. 2012;39(4):301–316. doi: 10.1007/s10488-011-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RT, Pierluissi E, Guzman D, Kessell ER, Goldman LE, Sarkar U, … Kushel MB. Functional disability in late-middle-aged and older adults admitted to a safety-net hospital. J Am Geriatr Soc. 2014;62(11):2056–2063. doi: 10.1111/jgs.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein JN, Hirsch GR, Rosenthal EL, Rush CH. Community health workers “101” for primary care providers and other stakeholders in health care systems. The Journal of ambulatory care management. 2011;34(3):210–220. doi: 10.1097/JAC.0b013e31821c645d. [DOI] [PubMed] [Google Scholar]
- Calancie L, Leeman J, Jilcott Pitts SB, Khan LK, Fleischhacker S, Evenson KR, … Ammerman A. Nutrition-related policy and environmental strategies to prevent obesity in rural communities: A systematic review of the literature, 2002–2013. Preventing Chronic Disease. 2015;12:E57. doi: 10.5888/pcd12.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, … Choi S. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2015 Retrieved from http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- Clark F, Azen SP, Zemke R, Jackson J, Carlson M, Mandel D, … Lipson L. Occupational therapy for independent-living older adults: A randomized controlled trial. Journal of the American Medical Association. 1997;278(16):1321–1326. doi: 10.1001/jama.1997.03550160041036. [DOI] [PubMed] [Google Scholar]
- Clark F, Blanchard J, Sleight A, Cogan A, Floríndez L, Gleason S, … Vigen C. Lifestyle Redesign: The Intervention Tested in the USC Well Elderly Studies. 2. Bethesda, MD: AOTA Press; 2015. [Google Scholar]
- Clark F, Jackson J, Carlson M, Chou CP, Cherry BJ, Jordan-Marsh M, … Azen SP. Effectiveness of a lifestyle intervention in promoting the well-being of independently living older people: Results of the Well Elderly 2 Randomised Controlled Trial. Journal of Epidemiology and Community Health. 2012;66(9):782–790. doi: 10.1136/jech.2009.099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- Donnelly C, Brenchley C, Crawford C, Letts L. The integration of occupational therapy into primary care: a multiple case study design. BMC Family Practice. 2013;14:60. doi: 10.1186/1471-2296-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt MS, Pamuk ER. The importance of place of residence: examining health in rural and nonrural areas. American Journal of Public Health. 2004;94(10):1682–1686. doi: 10.2105/ajph.94.10.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo AL, Leppänen A, Jahkola A. Validity of a single-item measure of stress symptoms. Scandinavian Journal of Work, Environment & Health. 2003;29(6):444–451. doi: 10.5271/sjweh.752. [DOI] [PubMed] [Google Scholar]
- Frank L, Basch E, Selby JV Patient-Centered Outcomes Research I. The PCORI perspective on patient-centered outcomes research. Journal of the American Medical Association. 2014;312(15):1513–1514. doi: 10.1001/jama.2014.11100. [DOI] [PubMed] [Google Scholar]
- Hansen-Kyle L. A concept analysis of healthy aging. Nursing Forum. 2005;40(2):45–57. doi: 10.1111/j.1744-6198.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Services Research. 2005;40(6 Pt 1):1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Bjorklund A. The impact of occupational therapy and lifestyle interventions on older persons’ health, well-being, and occupational adaptation. Scandinavian Journal of Occupational Therapy. 2016;23(3):207–219. doi: 10.3109/11038128.2015.1093544. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. A model for developing effective treatments: progression and interplay of theory, research, and practice. Journal of Clinical Child Psychology. 1997;26(2):114–129. doi: 10.1207/s15374424jccp2602_1. [DOI] [PubMed] [Google Scholar]
- Koniak-Griffin D, Brecht ML, Takayanagi S, Villegas J, Melendrez M, Balcazar H. A community health worker-led lifestyle behavior intervention for Latina (Hispanic) women: Feasibility and outcomes of a randomized controlled trial. International Journal of Nursing Studies. 2015;52(1):75–87. doi: 10.1016/j.ijnurstu.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Las Nueces D, Hacker K, DiGirolamo A, Hicks LS. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Services Research. 2012;47(3pt2):1363–1386. doi: 10.1111/j.1475-6773.2012.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfiyya MN, Bianco JA, Quinlan SK, Hall C, Waring SC. Mental health and mental health care in rural America: The hope of redesigned primary care. Disease-a-Month. 2012;58(11):629–638. doi: 10.1016/j.disamonth.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Masadeh M. Focus group: Reviews and practices. The Journal of Applied Science and Technology. 2012;2(10) [Google Scholar]
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychology & Health. 2011;26(11):1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Rigor and reproducibility. 2016 8/18/16. Retrieved from https://grants.nih.gov/reproducibility/index.htm.
- O’Brien MJ, Squires AP, Bixby RA, Larson SC. Role development of community health workers: an examination of selection and training processes in the intervention literature. American Journal of Preventive Medicine. 2009;37(6 Suppl 1):S262–269. doi: 10.1016/j.amepre.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein SM, Nietert PJ, Jenkins RG, Litvin CB. The prevalence of chronic diseases and multimorbidity in primary care practice: a PPRNet report. Journal of the American Board of Family Medicine. 2013;26(5):518–524. doi: 10.3122/jabfm.2013.05.130012. [DOI] [PubMed] [Google Scholar]
- Osuna D, Barrera M, Jr, Strycker LA, Toobert DJ, Glasgow RE, Geno CR, … Doty AT. Methods for the cultural adaptation of a diabetes lifestyle intervention for Latinas: An illustrative project. Health Promotion Practice. 2011;12(3):341–348. doi: 10.1177/1524839909343279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. American Journal of Public Health. 2010;100(S1):S232–239. doi: 10.2105/AJPH.2009.170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson C. Measuring outcomes in primary care: A patient generated measure, MYMOP, compared with the SF-36 health survey. BMJ. 1996;312(7037):1016–1020. doi: 10.1136/bmj.312.7037.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson C, Langan C, McKaig G, Anderson P, Maclaine G, Rose L, … Campbell M. Assessing patient outcomes in acute exacerbations of chronic bronchitis: The measure your medical outcome profile (MYMOP), medical outcomes study 6-item general health survey (MOS-6A) and EuroQol (EQ-5D) Quality of Life Research. 2000;9(5):521–527. doi: 10.1023/a:1008930521566. [DOI] [PubMed] [Google Scholar]
- Perepletchikova F, Kazdin AE. Treatment integrity and therapeutic change: Issues and research recommendations. Clinical Psychology: Science and Practice. 2005;12(4):365–383. [Google Scholar]
- Polus BI, Kimpton AJ, Walsh MJ. Use of the measure your medical outcome profile (MYMOP2) and W-BQ12 (Well-Being) outcomes measures to evaluate chiropractic treatment: An observational study. Chiropractic & Manual Therapies. 2011;19:7. doi: 10.1186/2045-709X-19-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens Niemiec SL, Blanchard J, Vigen CL, Martínez J, Guzmán L, Concha A, … Carlson M. Evaluation of ¡Vivir Mi Vida! to improve health and wellness of rural-dwelling, late middle-aged Latino adults: Results of a feasibility and pilot study of a lifestyle intervention. Primary Health Care Research & Development. doi: 10.1017/S1463423617000901. Accepted for publication, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens Niemiec SL, Carlson M, Martínez J, Guzmán L, Mahajan A, Clark F. Developing occupation-based preventive programs for late-middle-aged Latino patients in safety-net health systems. American Journal of Occupational Therapy. 2015;69:1–11. doi: 10.5014/ajot.2015.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Möhlig M, … Häring H-U. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care. 2007;30(3):510–515. doi: 10.2337/dc06-2089. [DOI] [PubMed] [Google Scholar]
- Stanton B, Guo J, Cottrell L, Galbraith J, Li X, Gibson C, … Harris C. The complex business of adapting effective interventions to new populations: an urban to rural transfer. Journal of Adolescent Health. 2005;37(2):163. doi: 10.1016/j.jadohealth.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Sudano JJ, Baker DW. Explaining US racial/ethnic disparities in health declines and mortality in late middle age: The roles of socioeconomic status, health behaviors, and health insurance. Social Science & Medicine. 2006;62(4):909–922. doi: 10.1016/j.socscimed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, … Goldsmith CH. A tutorial on pilot studies: The what, why and how. BMC Medical Research Methodology. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent D, Pasvogel A, Barrera L. A feasibility study of a culturally tailored diabetes intervention for Mexican Americans. Biological Research for Nursing. 2007;9(2):130–141. doi: 10.1177/1099800407304980. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Zahnd WE, Scaife SL, Francis ML. Health literacy skills in rural and urban populations. American Journal of Health Behavior. 2009;33(5):550–557. doi: 10.5993/ajhb.33.5.8. [DOI] [PubMed] [Google Scholar]