Abstract
Cognitive-behavioral therapy (CBT) improves coping and daily functioning in adolescents with juvenile fibromyalgia (JFM), but is less effective in reducing pain. This pilot trial evaluated the efficacy of a novel intervention (Fibromyalgia Integrative Training for Teens; FIT Teens) which integrates CBT with specialized neuromuscular exercise training to enhance the impact of treatment on reducing pain and disability. Forty adolescents with JFM (12–18 years) were randomized to CBT-only or FIT Teens. Treatment was conducted in group-based sessions over 8 weeks with assessments at baseline, post-treatment, and 3-month follow-up (primary endpoint). Primary outcomes were pain intensity and functional disability. Secondary outcomes were depressive symptoms, fear of movement and pain catastrophizing. Thirty-six participants (Mage=15.33 years; 90% female) completed the program. Intent-to-treat analysis was conducted to evaluate differences between the FIT Teens and CBT groups from baseline to 3-month follow-up, controlling for baseline group differences. Participants in the FIT Teens group demonstrated significantly greater decreases in pain than the CBT group. FIT Teens participants also showed significant improvements in disability, but did not differ from CBT-only at the 3-month endpoint. Results provide preliminary evidence that the FIT Teens intervention provides added benefits beyond CBT in the treatment of JFM, particularly in pain reduction.
Keywords: Pediatric pain, fibromyalgia, neuromuscular exercise, Cognitive-Behavioral Therapy
Introduction
Juvenile fibromyalgia (JFM) is a chronic and disabling condition characterized by widespread musculoskeletal pain, fatigue, sleep disturbance and other associated symptoms.19–21, 31, 44 Cognitive-behavioral therapy (CBT), a well-studied approach for the management of pediatric chronic pain,9, 11 is effective in reducing functional disability in adolescents with JFM.24 However, CBT treatments have a relatively small impact on pain reduction in JFM and physical activity levels may remain unchanged. 17 Although research strongly indicates that physical exercise is beneficial in reducing pain1, 10; adherence with traditional exercise regimens remains challenging for patients with fibromyalgia. Therefore, a new approach that can enhance CBT with more well-tolerated forms of exercise may be needed to produce stronger treatment gains and impact both pain and disability outcomes.
The fibromyalgia integrative training program for teens (FIT Teens) is a novel intervention developed to enhance the positive effects of CBT via coping skills practice integrated with increasing engagement in physical exercise. FIT Teens is a group-based treatment which combines CBT skills training with neuromuscular exercise, an approach derived from injury prevention research that focuses on improving core strength, balance and movement biomechanics to enhance functional movements through a specialized progression of resistance training and movement based exercises.29, 30 The FIT Teens program was iteratively developed - first with qualitative testing of feasibility, tolerability and engagement of teens with JFM25, followed by initial efficacy testing using a pre-post study design.41 Qualitative feedback during program development and preliminary results during feasibility testing indicated high levels of engagement and acceptability among adolescents with JFM. Refinements were made to the treatment protocol based on patient and interventionist feedback. The final FIT Teens intervention was an 8-week (16 session) protocol conducted in small groups of 4–5 patients with JFM. Results of initial efficacy testing of the FIT Teens intervention (N=22 adolescents with JFM across two study sites) showed significant reductions in measures of pain, disability, catastrophizing, depressive symptoms, and fear of movement at the end of treatment.25, 41
Based on these promising early findings, the current study was designed to test whether the combined FIT Teens intervention provided stronger benefits than CBT alone. Specifically, the primary objective of the current study was to conduct a pilot controlled trial to test whether FIT Teens was more effective than CBT alone in reducing pain intensity and functional disability (co-primary outcomes) in adolescents with JFM. Assessments were conducted at baseline, post-treatment (8 weeks) and 3-month follow-up, with the 3-month follow-up selected as the primary endpoint to evaluate the durability of treatment gains. It was hypothesized that 1) participants assigned to FIT Teens would show significantly greater reduction in pain intensity and functional disability than those who received CBT alone and 2) FIT Teens would also lead to greater improvements in the secondary outcomes of depressive symptoms, fear of movement, and pain catastrophizing. The selection of secondary outcome measures was based on prior studies of JFM in which CBT was associated with reduction in depressive symptoms and catastrophizing22, 24 and the specific expectation that the neuromuscular training component of the FIT Teens intervention would result in decreased fear of movement.41
Methods
Participants
Adolescents (between the ages of 12 and 18 years) were recruited from pediatric rheumatology and pain clinics at a large children’s hospital in the US Midwest. Participants were eligible if they 1) met criteria for JFM based on the 2010 American College of Rheumatology (ACR) criteria modified for pediatric use,39 2) had at least moderate functional disability, represented by Functional Disability Score ≥1316 and 3) average pain intensity in the past week ≥ 4 on a 0–10 cm Visual Analog Scale. Additionally, participants were required to be on stable medications that were part of their usual medical care for 4 weeks prior to enrollment. Medications used for JFM as part of standard clinical practice may include tricyclic or other antidepressant medications, analgesics or non-steroidal anti-inflammatory medications and/or anticonvulsants. While it was not possible to control for medications in this preliminary trial, all patients were required to be on 4 weeks of stable medication prior to their baseline assessment to minimize the possibility of confounding effects of medication changes during the trial. Participants were not eligible if 1) they presented with comorbid rheumatic disease (e.g., juvenile arthritis, systemic lupus erythematous), 2) documented developmental delay, 3) any medical condition determined by their physician to be a contraindication for exercise participation (e.g., acute injury) or 4) untreated major psychiatric diagnoses (e.g., major depression, bipolar disorder, psychoses). Participants with co-morbid psychiatric diagnoses (such as depression, bipolar disorder) were further assessed by a doctoral-level psychologist on the study team and were deemed eligible for inclusion if they reported 1) being under the ongoing care of a medical or mental health professional, 2) were on stable medication and 3) did not report any severe symptoms that required immediate attention (such as suicidal ideation) or would interfere with their participation in the group-based treatments. Finally, those who were currently enrolled in pain-focused CBT or a structured physical therapy program were excluded to prevent overlapping treatments that may confound outcomes. Prior engagement in standard outpatient pain care that included CBT or physical therapy was not exclusionary and participants were free to keep appointments with their physicians throughout the study.
Potential participants were screened by trained research assistants. Physicians confirmed medical eligibility and introduced the study to participants and caregivers. If interested, research assistants provided an overview of the study, answered questions, obtained written informed consent and verbal assent and scheduled a baseline assessment. This study was approved by the hospital Institutional Review Board.
Study Design
A two-arm randomized controlled trial design with equal group allocation was used in which enrolled participants were randomly assigned to receive either the FIT Teens intervention or CBT alone, both delivered in a group-based format. The CBT alone condition was selected as the comparison arm because it is now commonly offered as part of usual multidisciplinary care in most pediatric pain treatment settings and has already been demonstrated to be superior to attention control in prior studies.9, 24 Despite the fact that several participants may have had exposure to CBT as part of usual care prior to enrolling in the study, none had received structured group-based CBT.
Randomization
A group-randomization procedure was chosen based on our extensive experience of enrolling JFM participants24, 41 in which the timeline for recruiting a group of 4–5 eligible JFM participants is about 2 months. This methodology was chosen to minimize the wait time and potential drop out while sufficient participants were enrolled. Therefore, after 4–5 consecutive participants were found to meet eligibility criteria, they were enrolled into the study. Randomization sequencing was obtained by using a random group generation algorithm in R.37 The biostatistician maintained the concealed block randomization schedule and subsequently revealed the treatment assignment (FIT or CBT) for the group to the research coordinator and interventionists after baseline assessments were completed.
Blinding
At baseline, assessment staff were blinded to the upcoming group assignment. For post-treatment and 3-month follow-up evaluations, standardized protocols were used by assessment staff but they were not blinded due to limited resources available in this small-scale pilot study. Interventionists did not participate in the assessment to avoid therapist bias in influencing outcomes and participants’ attending physicians were blinded to treatment group assignment throughout. To maintain blinding, study participants were asked not to discuss their treatment allocation during any physician appointments during the trial.
Intervention
Adolescents in each of the treatment arms participated in group-based (4–5 participants per group; ~1.5 hours per session) treatment sessions twice a week over 8 weeks (16 sessions total). Sessions were held in the Sports Medicine and Biodynamics laboratory which includes equipment and adjacent conference room space for the exercise and CBT components of treatment respectively. Parents were also were included in treatment and attended 6 of the 16 sessions, during which they were introduced to the coping skills and exercises their children were learning to help foster continued practice at home and after the treatment period. Additionally, during several of the treatment sessions, parents were taught operant behavioral strategies to encourage independent pain coping and functioning in their teens (e.g., not asking about pain, promoting normal function, etc.). Treatment sessions were conducted according to manualized protocols and led jointly by a psychology post-doctoral fellow/pain psychologist and an exercise physiologist. For the CBT-only sessions and the CBT component of FIT Teens, the psychologist provided coping skills training and the exercise physiologist supported the psychologist as a co-leader. During the neuromuscular exercise component of FIT Teens, the exercise physiologist led participants through the exercise program and the psychologist supported participants in the use of coping skills while engaged in physical exercise. If participants missed a session, a brief (~15–20 min) make-up session was conducted prior to the start of the next session to cover any missed content.
FIT Teens
The FIT Teens intervention has been previously described in detail in prior publications including the full neuromuscular exercise protocol.25, 38, 41 Briefly, FIT Teens consists of an integration of both CBT skills training and neuromuscular exercise training (NMT) components with approximately 45 minutes dedicated to each component (NMT and CBT) per session. CBT included psychoeducation about the gate control theory of pain, use of behavioral strategies (e.g., muscle relaxation, activity pacing), and cognitive strategies (e.g., distraction, problem solving, self-calming statements). The NMT exercise component used a resistive and movement training protocol focused on improving core strength, balance, and posture. Exercises followed a phasic progression, systematically progressing in difficulty level every two weeks, beginning with Level 1: Holding Movement Exercises (isometric focused exercises), and proceeding to Level 2: Creating Movement Exercises (concentric focused exercises), Level 3: Resisting Movement Exercises (eccentric focused exercises) and Level 4: Functional Movement Exercises (combining all previous levels of movement; see Thomas et al., 2013 for full description of NMT exercise protocol).38 Exercises were individualized to the participants’ ability and modified as needed. Participants received education about proper technical form and technique, as well as the benefits (e.g., activities of daily living supported by the environment) of each of the exercises in facilitating functional daily activities. In addition, group trainers incorporated instruction in how to use behavioral coping skills while engaged in exercise. Home practice of NMT exercises consisted of exercises similar to those learned in session, that were modified to be performed at home with limited or no equipment needed. Participants were assigned home practice for both coping skills and NMT exercises. Additionally, beginning with session 5, participants were instructed to gradually increase engagement in more vigorous forms of physical activity of their choice (brisk walking, dancing, swimming etc.) as part of home practice.
CBT-only
The content of the CBT-only intervention was based on an established individual CBT protocol24 modified for a group-based 16-session format which matched the FIT Teens protocol described above in terms of number of sessions and therapist contact time. Content of the CBT-only training was similar to the coping skills portion in the FIT Teens intervention with greater time spent on practicing each of the skills in session. Of note, while adolescents were encouraged to increase their participation in physical, social and recreational activities as part of the CBT intervention, they were not specifically instructed to engage in physical exercise.
Incentives
Participants were provided a modest incentive for completing assessments. At the end of the 8 weeks of treatment, those who completed CBT treatment received a gift basket with relaxation CDs, stress balls, and distraction activities to encourage continued practice of adaptive skills at the end of treatment. Those participating in FIT Teens were provided with a BOSU ® Balance Trainer4 ball at the conclusion of treatment to encourage continued exercise practice at home.
Study Measures
Demographic Information: Parents completed a form providing information about the participants’ age, gender, race, ethnicity, and family income.
Primary Outcomes
Pain Intensity
The Visual Analog Scale (VAS), has been recommended for the assessment of pain intensity by the PedIMMPACT guidelines,28 and has been validated for use with children over the age of 5 years.33, 36 Participants marked their average pain intensity over the past two weeks on a 0–10 cm VAS scale, anchored by “no pain” and “pain as bad as it can be.” The VAS has been extensively used in pediatric chronic pain research including in adolescents with JFM.15, 35, 41
Functional Disability
The Functional Disability Inventory (FDI- Child Report) is a 15-item scale developed to assess perceived difficulty in daily activities in home, school, recreational, and social domains due to pain. Participants selected one of 5 response choices ranging from 0 (i.e., No Trouble) to 4 (i.e., Impossible), with a total score being the sum of responses on the 15 items. The FDI has been used in numerous studies of pediatric chronic pain,23, 31, 42 was recommended by the Pediatric Initiative of Methods, Measurement and Pain Assessment in Clinical Trials (PedIMMPACT) guidelines28 and has published clinical cut-off scores16. Disability scores can be classified as None/Minimal (0–12), Mild (13–20), Moderate (21–29), and Severe (≥30). It has shown to have excellent internal reliability (Cronbach α = .86 – .91), and good concurrent and predictive validity.6 Reliability of the FDI for the present sample was adequate (α = .79).
Secondary Outcomes
Depressive symptoms
The Children’s Depression Inventory (CDI) assesses self-reported symptoms of depression in children and adolescents27. Participants selected 1 of 3 statements for each item (scored from 0 to 2), with higher scores indicating greater frequency and/or severity of symptoms. Total scores on the CDI range from 0 to 54, and raw scores can be converted to T-scores based on a normative sample. It is well-validated and is the most frequently used scale to measure depressive symptoms in children and adolescents with chronic pain.7, 18, 26 Internal consistency reliability for the CDI in this study was good (Cronbach α = .83).
Fear of Movement
The Tampa Scale for Kinesiophobia (TSK-11)40 measures fear of movement due to pain and consists of 11 statements rated on a scale of 1 (strongly disagree) to 4 (strongly agree). Total scores range from 11 to 44 with higher scores indicating increased fear of movement, activity avoidance, and somatic focus. Although the TSK has not been formally validated in children, the scale has been validated across various pain conditions in adults32 and has been previously used in pediatric pain studies including JFM,41, 43 and demonstrated good reliability (α = .76-.84) and sensitivity to change.
Pain Catastrophizing
The Pain Catastrophizing Scale for Children (PCS-C; 40) is a validated and reliable measure consisting of 13 statements describing negative and catastrophic thoughts and feelings about pain. Participants rate how intensely they experience each thought or feeling on a scale of 0 (not at all) to 4 (extremely) when they have pain. Total scores range from 0 to 52 with higher scores indicating greater levels of pain catastrophizing (α = .87).8
Monitoring of Adverse Events
Adverse events (AEs) were monitored throughout the study. Adverse events were defined as a participant having any new or worsening symptoms, hospitalizations, or emergency department visits - whether or not they were thought to be related to the study. The study coordinator recorded each adverse event, contacted the participant and/or their parent to gather further details if necessary and consulted with the principal investigator, interventionists and study physician to evaluate the seriousness and relatedness of the adverse event and take appropriate action as needed. Based on the nature of the event, the study physician determined if it was safe for the participant to remain in the study and/or be referred for additional care. Adverse events were reported to the IRB per institutional guidelines.
Analytic Plan
Power calculation
Modeled from previous intervention studies in fibromyalgia, a-priori power analyses were conducted based upon anticipated 3-month pain reductions with effect sizes of Cohen’s d = 1.31 for the FIT Teens and d = 0.60 for the CBT group.5, 14, 24 Power analyses were conducted with the following assumptions: (a) no differences between the FIT and CBT groups at baseline due to random assignment, (b) up to 25% participant attrition, resulting in post-attrition sample size ranging between N = 30 to 48 and (c) that pain scores at baseline, along with possible additional control covariates (e.g., age), could reduce the variance in pain scores at 3-month follow-up between 3% and 12%. Given these assumptions, results from multiple Monte Carlo simulation power analyses showed that power was at least .80 to detect an incremental treatment effect for pain reduction of d = .70 for FIT Teens versus CBT at the 3-month follow-up. Thus, the post attrition size of the present sample (n = 36) provided adequate power to detect moderate treatment effect size differences.
Preliminary Analyses
Data were entered into SPSS Version 24, where they were initially evaluated for missing data, skew, and baseline differences that would inform statistical analyses. This study demonstrated high retention, with all the study drop-out (n = 4) occurring prior to the post-treatment assessment, and no-drop out between post-treatment and 3-month follow-up. Drop out did not significantly differ between the FIT Teens and CBT groups, Pearson’s χ2(1) = 1.11, p = .29. In addition, distributional properties of primary and secondary variables outcomes were examined to ensure there were no marked deviations from assumptions of normality.
Further analyses were conducted to evaluate 1) whether treatment groups significantly differed from each other at baseline, and 2) whether outcome variables were significantly correlated at baseline. No differences were observed between treatment groups at baseline for age (p = .48) or gender (p = .61). Also, treatment groups did not differ at baseline on either of the co-primary outcomes or depressive symptoms (see Table 1 for descriptive statistics). However, FIT Teens and CBT groups differed marginally at baseline for pain catastrophizing [t(38) = −1.99, p = .053] with higher scores in the FIT Teens group. As expected, several of the baseline outcome variables were significantly correlated with both 3-month and post-assessment time-points. Notably, baseline values for the co-primary outcomes were both significantly related to values at the 3-month primary endpoint (rFDI = .467, p = .004; rVAS = .332, p = .048).
Table 1.
Descriptive statistics for treatment groups across time-points
| CBT-Only | FIT Teens | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 20) | Post Treatment (n = 19) | 3-Month Follow-Up (n = 19) | Baseline (n = 20) | Post Treatment (n = 17) | 3-Month Follow-Up (n = 17) | Cohen’s dɸ | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Base vs Post | Base vs 3 Month | |
| Pain VAS | 6.41 | 1.59 | 6.38 | 2.31 | 6.26 | 2.06 | 6.55 | 1.22 | 4.69ᵻ | 2.13 | 4.62* | 1.90 | 0.762 | 0.770 |
| Functional Disability Inventory | 24.45 | 8.29 | 23.95 | 11.04 | 22.68 | 9.01 | 26.70 | 7.27 | 18.71ᵻ | 4.61 | 19.76 | 5.55 | 0.859 | 0.632 |
| Childhood Depression Inventory | 14.05 | 6.18 | 13.79 | 7.56 | 12.95 | 7.55 | 15.80 | 6.85 | 11.71 | 5.70 | 11.35 | 6.52 | 0.666 | 0.491 |
| Pain Catastrophizing Scale | 24.25 | 8.46 | 20.85 | 10.69 | 22.0 | 9.45 | 30.20 | 10.56 | 19.53 | 7.23 | 19.65 | 8.57 | 0.638 | 0.733 |
| Tampa Scale of Kinesiophobia | 25.5 | 4.86 | 23.53 | 4.39 | 24.16 | 5.74 | 28.35 | 5.44 | 23.29 | 3.89 | 22.71 | 5.25 | 0.555 | 0.652 |
Mean scores represent observed values.
Statistically significant difference between FIT Teens and CBT for 3-month follow-up vs. baseline change scores
Statistically significant difference between FIT Teens and CBT for post-treatment vs. baseline change scores
Effect sizes for treatment differences between FIT Teens and CBT
Primary Analysis
Primary analysis consisted of multiple-group structural equation modeling in MPlus, carried out on the full intent-to-treat sample at the 3-month follow-up. In general, a small N trial of this type raises multiple potential statistical issues including 1) missing data due to attrition, 2) differences between treatment groups on baseline characteristics, and 3) covariability between baseline and follow-up measures. The use of a structural equation modeling approach was selected over traditional repeated measures multivariate analyses because it simultaneously provides 1) missing data estimations, 2) baseline covariation, and 3) multiple group analyses. Maximum likelihood estimation with 500 bootstrapped samples was used to account for missing data and to obtain observed standard errors. Baseline variables (i.e., age, Functional Disability Inventory, Pain, Tampa Scale for Kinesiophobia, Pain Catastrophizing Scale, and Child Depression Inventory) were included as covariates. This provides expected mean estimates of the co-primary and secondary outcomes controlling for age and the respective baseline value of that outcome (i.e., FDI at 3-months controlling for baseline FDI).
After missing data handling (drop-out n = 4) and control of baseline variables, expected values of the co-primary outcomes and secondary outcomes were obtained by evaluating intercept values of the regressed observed values of outcome variables at 3-month follow-up onto their respective baseline values separately. The intercepts of these regression lines represent the expected means at 3-month follow-up, covarying for age and baseline value, and were calculated for FIT Teens and CBT-only groups separately. Age was specifically chosen as a covariate due to possible developmental differences in treatment response across the wide age range (12–18 years). The CBT and FIT teens groups did not differ in JFM symptom severity at baseline, so we did not use symptom severity as a co-variate. Post-hoc comparisons in structural equation modeling were used to compare the magnitude of change in the FIT Teens group vs. the CBT group.
Analysis of post-treatment outcomes
Analyses of differences between groups at post-treatment were similar to primary analyses through the use of structural equation modeling with MPlus, and multiple group analysis option. Identical procedures were used to account for missing data and controlling for baseline variables. Expected means were calculated for the co-primary and secondary outcome variables at post-treatment, controlling for baseline values.
Evaluation of within group changes
Within-group changes for CBT-only and FIT Teens on primary outcomes at both post-treatment and 3-month follow-up were evaluated by plotting age-adjusted means with 95% confidence interval error bars for each group across time points. Age-adjusted means were calculated by first regressing baseline age onto each response variable and obtaining unstandardized residual variability. These residual scores were then added to the grand mean to yield the adjusted means. Non-overlapping error bars indicate significant within-group change between time-points. Significance testing for results with non-overlapping error bars was conducted via within-subjects MANOVA in Mplus. All possible pairwise comparisons were tested using false discovery rate to control family-wise Type 1 error.
Results
Enrollment and Retention
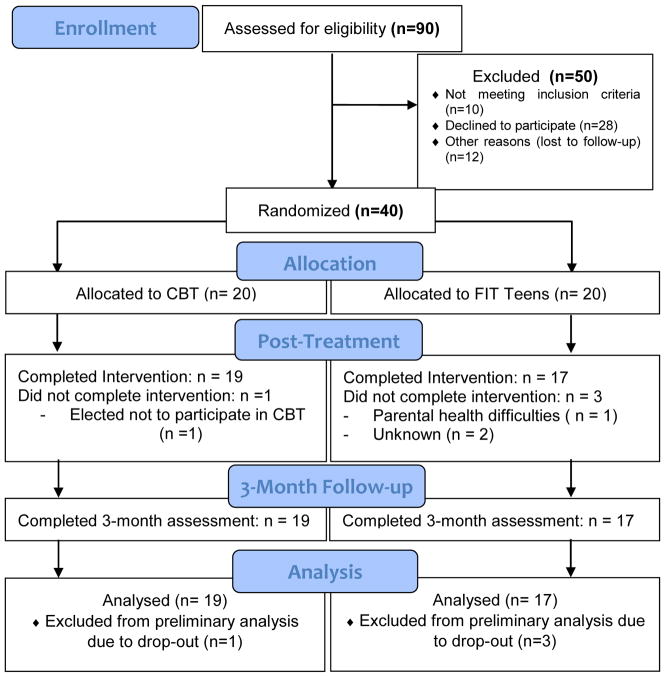
A total of 90 adolescents met initial criteria for participation and were referred to the study, and 40 (Mage =15.33, SD = 1.526) agreed to participate in the study. All 40 of the participants who agreed to participate in the study met criterial for enrollment and were subsequently randomized to one of the two treatment conditions. Those not enrolled either did not meet inclusion criteria, declined to participate, or were lost to follow-up between initial recruitment and enrollment (see CONSORT flow chart, Figure 1). Demographic characteristics of those who were referred but not enrolled in the study were not collected. Enrollment occurred from 12/16/2013 to 4/01/2016 and follow-up occurred concurrently, from 7/28/2014 to 8/31/2016. Once enrolled, retention at post-treatment and 3-month follow-up was 90% (n=36; CBT = 19, FIT = 17). Reasons for withdrawal from the study include needing further treatment for complications due to a preexisting medical condition (n=1), dissatisfaction of group assignment (n=1), and gradual discontinuation of group attendance (n=2). Recruitment and enrollment ended after sufficient sample size was obtained.
Figure 1.
Participant characteristics
Participants were 90% female (n=36) and 93% Caucasian (n=37) (see Table 2 for demographic information) and had been seen at the Rheumatology or Pain Clinics for their care. Most (70%) were currently taking one or more medications for JFM and associated symptoms. With regard to other treatments prior to enrollment in trial, 47.5% of participants had seen a psychologist for at least one session of pain management and 72.5% had received physical therapy as part of their usual care. At their baseline assessment, adolescents reported levels of disability in the moderate range (MFDI = 25.58, SD = 7.78) and pain levels in the moderate-severe range (MVAS = 6.48, SD = 1.40). Depressive symptoms were in the mildly elevated range (MCDI = 14.93, SD = 6.50). Pain catastrophizing (M = 27.18, SD = 9.93) and fear of movement (M = 26.93, SD = 5.29) scores were comparable to those in our prior JFM studies.34, 41 Baseline values and unadjusted post-treatment and 3-month values per treatment group are presented in Table 1. Of the 36 participants that completed the study, 34 participants completed at least 12 of the 16 sessions, and the average number of sessions attended was 14.06 (87.8%).
Table 2.
Demographic Information
| n | % | M | SD | |
|---|---|---|---|---|
| Age | 40 | 15.38 | 1.531 | |
|
| ||||
| Sex | ||||
| Female | 36 | 90 | ||
| Male | 4 | 10 | ||
|
| ||||
| Race | ||||
| Caucasian | 37 | 92.5 | ||
| American Indian/Alaskan Native | 2 | 5.0 | ||
| More than one race | 1 | 2.5 | ||
|
| ||||
| Ethnicity | ||||
| Non-Hispanic | 40 | |||
|
| ||||
| Household Income | ||||
| <$25,000 | 3 | 7.7 | ||
| $25,000 – $50,000 | 6 | 15.4 | ||
| $50,000 –$75,000 | 11 | 28.2 | ||
| $75,000 – $100,000 | 7 | 17.9 | ||
| $100,000 – $125,000 | 4 | 10.3 | ||
| $125,000 – $150,000 | 4 | 10.3 | ||
| >$150,000 | 4 | 10.3 | ||
|
| ||||
| Family History of Chronic Pain | ||||
| None | 15 | 37.5 | ||
| Mother | 22 | 55.0 | ||
| Father | 5 | 12.5 | ||
| Sibling | 7 | 17.5 | ||
| Extended Family | 3 | 7.5 | ||
|
| ||||
| Grade Level | ||||
| 7th | 3 | 7.5 | ||
| 8th | 3 | 7.5 | ||
| 9th | 9 | 22.5 | ||
| 10th | 5 | 12.5 | ||
| 11th | 9 | 22.5 | ||
| 12th | 9 | 22.5 | ||
|
| ||||
| School Attendance | ||||
| Full Time | 32 | 80.0 | ||
| Part Time | 3 | 7.5 | ||
| Not Attending | 1 | 2.5 | ||
| Homebound w. Tutor | 2 | 5.0 | ||
Changes in Pain and Functional Disability (Primary Outcomes) at 3-month endpoint
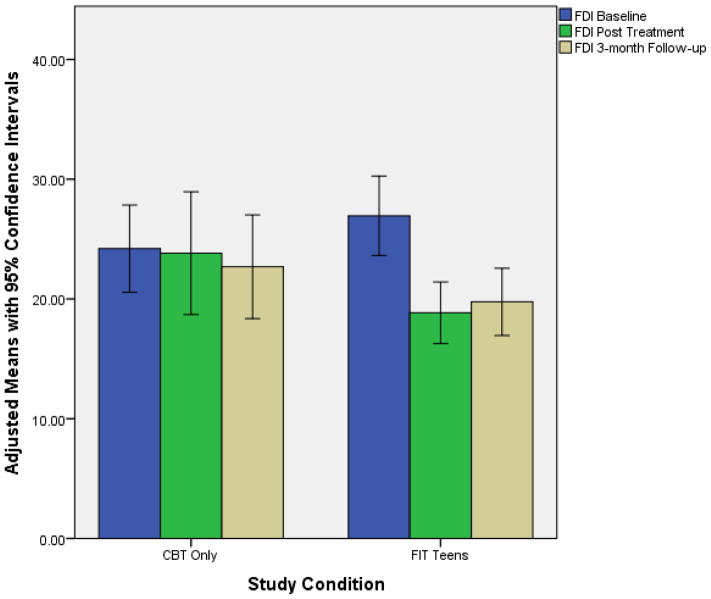
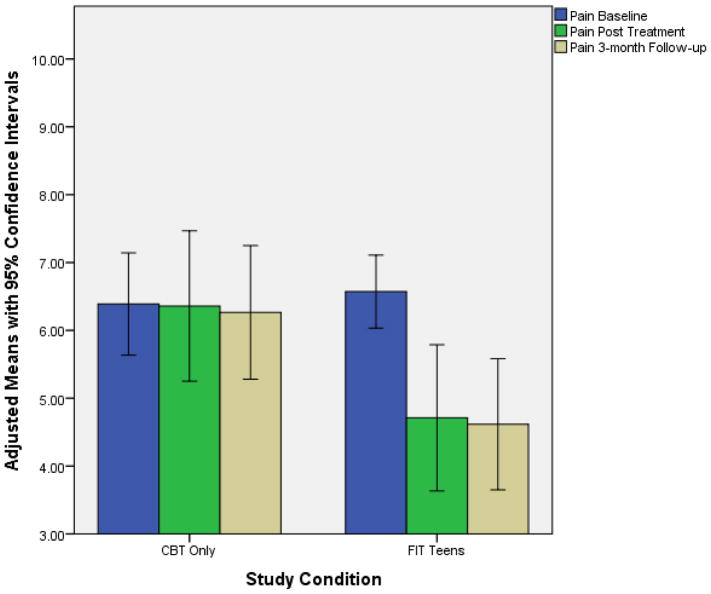
While controlling for baseline values, at 3-month follow-up those that participated in the FIT Teens group exhibited significantly greater decrease in pain than participants who received CBT-only (MDiff. = −1.62, p = .011, Cohen’s d effect size difference = 0.77). Group differences in functional disability at 3-month follow-up tended to be stronger for FIT Teens than CBT but did not achieve statistical significance at the .05 level (MDiff. = −4.58, p = .055, Cohen’s d effect size difference = .63). Those who received the FIT Teens intervention did demonstrate a significant within-group decrease in functional disability from baseline to 3-months (p < .05, d = 1.21). Figures 2 & 3 demonstrate the co-primary treatment outcomes for both CBT-only and FIT Teens groups with 95% confidence ranges.
Figure 2.
Age-adjusted means of the Functional Disability Inventory across time points
Figure 3.
Age-adjusted means of the Pain VAS across time points
Changes in Pain Catastrophizing, Fear of Movement, and Depression (Secondary Outcomes) at 3-month endpoint
For the secondary outcomes, although participants who received FIT Teens demonstrated stronger improvements than CBT-only for depressive symptoms (MDiff. = −4.58, d = .49), pain catastrophizing (MDiff. = −3.812, d = .73), and fear of movement (MDiff. = −2.679, d = .65), the differences between groups were not statistically significant (see Table 3). When evaluating 95% confidence intervals for age-adjusted means of within group differences (Table 4), non-overlapping confidence intervals between baseline scores and 3-month follow-up demonstrate that those in FIT Teens groups reported improvement in both pain catastrophizing and fear of movement scales, while those in the CBT-only group did not change from baseline to 3-month follow-up. Thus, while the treatment differences between groups did not significantly differ for pain catastrophizing or fear of movement, within-group analyses show significant (p < .05) improvement for the FIT Teens group but not the CBT-only group. Pairwise comparisons for the FIT Teens group remained statistically significant after false discovery rate correction.
Table 3.
Post-hoc pairwise comparisons of treatment effects
| CBT | FIT Teens | ||||||
|---|---|---|---|---|---|---|---|
| Expected Means* | Diff. Estimate | S.E. of Diff. | Wald Z | pᵻ | |||
| Functional Disability Inventory | Baseline v. Post-Treatment | 14.623 | 7.949 | −6.674 | 2.499 | −2.671 | 0.008 |
| Baseline vs. 3-months | 16.216 | 11.635 | −4.581 | 2.389 | −1.917 | 0.055 | |
| Pain VAS | Baseline v. Post-Treatment | 5.058 | 3.38 | −1.678 | .702 | −2.391 | 0.017 |
| Baseline vs. 3-months | 4.861 | 3.241 | −1.62 | 0.641 | −2.530 | 0.011 | |
| Childhood Depression Inventory | Baseline v. Post-Treatment | 8.793 | 4.967 | −3.826 | 2.282 | −1.667 | 0.094 |
| Baseline vs. 3-months | 10.971 | 7.86 | −3.111 | 2.451 | −1.269 | 0.204 | |
| Pain Catastrophizing Scale | Baseline v. Post-Treatment | 23.381 | 19.579 | −3.802 | 3.166 | −1.201 | 0.23 |
| Baseline vs. 3-months | 8.127 | 4.315 | −3.812 | 3.193 | −1.194 | 0.233 | |
| Tampa Scale of Kinesiophobia | Baseline v. Post-Treatment | 11.674 | 9.812 | −1.862 | 1.671 | −1.114 | 0.265 |
| Baseline vs. 3-months | 20.459 | 17.78 | −2.679 | 1.800 | −1.488 | 0.137 | |
Expected means at post-treatment and 3-month follow-up controlling for age and baseline scores;
Bolded p-values represent statistically significant group differences.
Table 4.
Age-adjusted mean scores with 95% confidence intervals
| CBT Only | FIT Teensa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95%Confidence Interval | 95%Confidence Interval | |||||||||
| Adj. Mean | Lower Limit | Upper Limit | Std. Error Mean | Adj. Mean | Lower Limit | Upper Limit | Std. Error Mean | pb | ||
| Functional Disability Inventory | Baseline | 24.20 | 20.56 | 27.85 | 1.74 | 26.95 | 23.63 | 30.26 | 1.58 | |
| Post-Treatment | 23.82 | 18.68 | 28.97 | 2.45 | 18.85 | 16.24 | 21.45 | 1.23 | .006 | |
| 3-Month Follow-Up | 22.69 | 18.34 | 27.03 | 2.07 | 19.76 | 16.92 | 22.60 | 1.34 | .002 | |
| Pain VAS | Baseline | 6.39 | 5.63 | 7.14 | 0.36 | 6.57 | 6.03 | 7.11 | 0.26 | |
| Post-Treatment | 6.36 | 5.25 | 7.47 | 0.53 | 4.71 | 3.62 | 5.80 | 0.52 | .014 | |
| 3-Month Follow-Up | 6.26 | 5.28 | 7.25 | 0.47 | 4.62 | 3.64 | 5.60 | 0.46 | .005 | |
| Childhood Depression Inventory | Baseline | 13.77 | 10.96 | 16.57 | 1.34 | 16.08 | 13.30 | 18.87 | 1.33 | |
| Post-Treatment | 13.67 | 10.03 | 17.30 | 1.73 | 11.84 | 9.13 | 14.55 | 1.28 | .033 | |
| 3-Month Follow-Up | 12.92 | 9.31 | 16.53 | 1.72 | 11.39 | 8.01 | 14.77 | 1.59 | .024 | |
| Pain Catastrophizing Scale | Baseline | 23.85 | 19.92 | 27.79 | 1.88 | 30.50 | 25.94 | 35.05 | 2.18 | |
| Post-Treatment | 20.74 | 15.62 | 25.87 | 2.44 | 19.64 | 16.00 | 23.28 | 1.72 | <.001 | |
| 3-Month Follow-Up | 21.92 | 17.49 | 26.35 | 2.11 | 19.73 | 15.23 | 24.24 | 2.12 | <.001 | |
| Tampa Scale of Kinesiophobia | Baseline | 25.33 | 23.05 | 27.61 | 1.09 | 28.52 | 26.24 | 30.81 | 1.09 | |
| Post-Treatment | 23.46 | 21.28 | 25.63 | 1.04 | 23.37 | 21.58 | 25.16 | 0.84 | <.001 | |
| 3-Month Follow-Up | 24.19 | 21.43 | 26.95 | 1.31 | 22.67 | 19.99 | 25.36 | 1.27 | .001 | |
Within groups MANOVA only conducted for the FIT Teens groups due to non-overlapping confidence intervals in post-hoc analyses
All comparisons are to baseline levels (i.e., Post-Treatment vs. Baseline, 3-Month Follow-up vs. Baseline); all p-values reported are significant at the .05 level after False Discovery Rate Type-1 error control.
Post-treatment Outcomes
Supplemental analyses were conducted to evaluate treatment gains at the immediate conclusion of active treatment. Participants in FIT Teens demonstrated significantly greater decreases in both pain ratings (MDiff. = −1.678, p = .017, d = .76) and in functional disability (MDiff. = −6.674, p = .008, d = .86) than adolescents in the CBT only group. In comparing treatment gains at post-treatment to 3-month follow-up, FIT Teens demonstrated superiority over CBT in the maintained reduction of pain. With respect to functional disability, the CBT group continued to show slight improvement over time whereas the FIT Teens group showed a small loss of gains in functional disability, potentially explaining the lack of significant between group differences at 3-month follow-up.
All secondary measures of depressive symptoms, catastrophizing and fear of movement improved in the anticipated direction favoring FIT Teens over CBT; however, the differences between groups were not statistically significant. In examining within group changes, 95% confidence of age-adjusted means demonstrated that participants in the FIT Teens group demonstrated significant improvement in both pain catastrophizing and fear of movement (Table 4), with no significant improvement in the CBT only group. Pairwise comparisons for the FIT Teens group remained statistically significant after false discovery rate correction.
Adverse Events
Adverse events reported during the study included accidental injury (n = 2), joint pain (n = 5), somatic symptoms (n = 2) and other illness (n =1). None of these events were associated with participation in the study. As expected (given that most participants were mostly sedentary), several participants in the FIT Teens intervention reported mild muscle soreness associated with learning new exercises. The soreness was temporary and resolved within 1–2 days without need for intervention. Participants reported that they were able to distinguish muscle soreness related to exercise from fibromyalgia pain “flares.”
Discussion
Results of this preliminary randomized trial of the FIT Teens intervention are promising and indicate that combining CBT with neuromuscular exercise may be synergistic and offer a more powerful approach to the treatment of pain and disability in adolescents with JFM compared to CBT alone. Prior studies indicate that standard forms of multidisciplinary care and CBT are effective in improving psychological coping and daily functioning.9, 11, 23, 24 However the small reductions in pain and lack of impact on physical activity engagement after treatment pointed to the need to enhance coping skills training with more engaging forms of exercise training. Prior studies of physical exercise in fibromyalgia indicate beneficial effects on pain reduction but difficulties with exercise tolerance and adherence.13 Therefore, it is important for exercise programming to take a more tailored approach that is geared towards the needs of adolescents with JFM. Neuromuscular training is a specialized form of exercise derived from injury prevention research and focused on functional movement and dynamic strength stabilization, including improvements in, posture, strength and balance. This type of training is primarily based on body-weight resistance and movement training, requires relatively little equipment and can be easily practiced at home. As such, it offers a convenient method than can be easily blended with CBT-based skills training. Additionally, neuromuscular training was found to be very well tolerated by adolescents in our pilot work. Treatment delivery in the form of group sessions is efficient and was well-received by adolescents who greatly enjoyed the group support and interaction based on their consistent qualitative feedback during feasibility testing as well as in this trial.
Based on our prior studies of CBT24 and step-by-step development of the new FIT Teens intervention,25 both group-based treatments used in this study were designed to be developmentally appropriate, well tolerated, and engaging for adolescents with JFM. Both intervention arms had high retention (90%) of participants at post-treatment and 3-month follow-up, with no notable difference in drop-out between the two groups. Additionally, consistent with previous studies,25, 41 FIT Teens was safe with no adverse responses to treatment other than expected initial and temporary muscle soreness associated with beginning new exercises.
Pain reductions for the FIT Teens intervention were significantly greater than CBT-only and these reductions were maintained even 3-months after the end of active treatment. The effect size for the FIT Teens intervention on pain reduction (Baseline to 3-month follow-up Cohen’s d = 1.24) also was superior to effect sizes reported in prior studies of CBT in fibromyalgia (ES ranging from .052 .457.2, 3, 12, 24 Although some studies of exercise for fibromyalgia have demonstrated strong treatment effects (up to effect sizes of 1.3) post treatment, these effects were not maintained at follow-up.14 In this study, pain reduction for FIT Teens was nearly as great as previous exercise studies, and maintained even at the 3-month post-treatment follow-up, demonstrating the potential durability of the intervention. These findings are encouraging, and suggest that two unique aspects of this intervention, namely the combined application of coping skills training and the specialized neuromuscular training, may have facilitated maintenance of treatment effects.
With regard to the co-primary outcome of functional disability, the hypothesis of significantly greater improvement in the FIT Teens group compared to CBT-only at 3-month follow up was not supported. Although significantly greater improvements in the FIT Teens group compared to CBT-only were evident immediately at the end of treatment, these differences were not statistically significant at the primary 3-month endpoint due to slight loss in treatment gains for the FIT Teens group and slight treatment gains in the CBT group over the follow-up period (Figure 2). Nevertheless, effect sizes for improvement in the FIT Teens group at both post-treatment and 3-month follow-up (dpost = 1.35; d3mo =1.08) were superior to CBT-only (dpost = .05; d3mo = .23). Clearly, there is great potential for the FIT Teens intervention in being more effective than CBT in improving disability but greater efforts may be required to sustain these gains over time.
It is worth mentioning that in this study the effects of group-based CBT delivered as a stand-alone treatment did not appear to be as strong as we predicted based our prior results of CBT delivered in individual format.24 However, effect sizes for FIT Teens in this study were still stronger when compared to outcomes of prior studies of CBT-only interventions.2, 24 Given that this study included a smaller sample size, a shorter time frame (3 months versus 6 months) than our previous trial of CBT, and did not include any booster sessions as in the prior trial, the results of this trial may under-represent the treatment effectiveness of group-based CBT as an independent treatment. Additional investigation with larger sample sizes and longer-term follow-up may be needed to more definitively understand the effectiveness of FIT Teens and CBT only for the treatment of pain and functional disability in JFM.
A number of secondary outcomes were also assessed in this study, including depressive symptoms, pain catastrophizing, and fear of movement. FIT Teens and CBT only groups did not differ in any of these domains at either post-treatment or 3-month follow-up. However, when within-group changes were examined, FIT Teens did exhibit significant improvement in pain catastrophizing and fear of movement. Though not significantly different than CBT only, this might indicate that the combination of pain coping skills and neuromuscular training (FIT Teens) may offer slightly greater benefits in reducing pain catastrophizing and fear of movement.
Although this trial was a relatively small-scale trial, strengths of the study include - a rigorous approach using clear a priori selection of primary outcomes and primary study endpoints, use of a randomized design, an intent-to-treat analyses that included careful attention to missing data, consideration of baseline values on primary and secondary outcome variables and adjustments for age demographics. A-priori power calculations were based on previously conducted trials, providing greater confidence in statistical results given the sample size of the study.
There are also several limitations that restrict generalizability and conclusiveness of this study. First, while this study was adequately powered for evaluation of the primary outcomes, apriori power analyses were not conducted to determine the necessary sample size to evaluate secondary outcomes. While group differences on the secondary outcomes trended in the direction of superiority for the FIT Teens group, a larger sample size is needed to provide adequate power to further provide further confirmation of the efficacy of FIT Teens on depressive symptoms, catastrophizing and fear of movement. In addition, the impact of FIT Teens on other potential outcomes recommended for use in clinical trials (PedIMMPACT),28 such as sleep and anxiety, might be useful to assess in future studies. Second, this study utilized group randomization instead of individual randomization. Though this method of randomization was chosen for feasibility of recruitment, it does limit the assumption of independence in treatment effects for individual participants. This potential issue could be addressed by investigating group effects with multi-level modeling through larger sample trials. Third, this study only utilized partial blinding due to limited staff and resources with a small trial. However, standardized collection procedures were used at each time point to minimize the possibility of bias. Fourth, while this study can draw conclusions of FIT Teens compared to a CBT only intervention, this study did not utilize an exercise-only control group. Therefore, it is unknown whether improved treatment effects were due to participating in NMT specifically or whether similar effects would be seen from engaging in more traditional forms of exercise (e.g., graded aerobic training). Fifth, due to the preliminary nature of this study, it was not possible to examine covariates including pain duration, prior treatments, co-morbid psychiatric diagnoses and medications, and how they affected treatment outcomes beyond the impact of the FIT Teens or CBT interventions. Sixth, although it has been used in previous studies, the Tampa Scale for Kinesiophobia has not been formally validated in children. Last, while this study evaluated a primary endpoint of 3-months post treatment, it is unknown to what extent treatment gains are maintained over a longer period.
Based on the findings of this study, there are several implications for future work in enhancing the FIT Teens treatment protocol and further testing its efficacy. Participant feedback requesting periodic “booster sessions” after the end of the 8-week treatment should be considered to maintain treatment gains. These sessions would provide opportunities for group members to reconnect, review and practice their skills, problem solve around maintenance of practice and treatment gains, and receive additional support from fellow group members, which was consistently noted by participants as a key benefit of the program.25 Other methods of improving maintenance could also be considered and evaluated, such as text-message reminders for skill utilization and establishing group “check-ins” through video platforms, such as Skype. In addition to booster sessions, additional follow-up assessments over the course of 6 months - 1 year would be helpful evaluate the long-term maintenance of treatment effects. Also, comparing FIT Teens with an exercise-only treatment arm would help distinguish whether this specific form of intervention should be the preferred approach. Finally, further research is needed into the specific mechanisms of how each treatment exerts its particular effects. Many relevant factors such as – number of sessions attended, adherence to practice outside treatment sessions, perception of group support, pain coping efficacy, exercise self-efficacy, movement competence and objective changes in movement biomechanics need more detailed investigation. A greater understanding of mechanisms of treatment effects would help to optimize treatments and better tailor them for the needs of adolescents with JFM.
Conclusions
Results of this pilot trial investigating the treatment efficacy of FIT Teens in comparison to CBT-only are promising. The FIT Teens program appeared to offer stronger benefits, particularly in pain reduction. If these results are supported in future more rigorous randomized controlled studies, the implications for clinical care of patients with JFM will be significant and improve the current standard of care for this poorly understood pain condition.
Perspective.
Results from this pilot randomized controlled trial of a new combined cognitive behavioral therapy (CBT) and specialized neuromuscular exercise intervention (FIT Teens), compared to CBT alone suggested that FIT Teens offers stronger treatment benefits than CBT alone at initial treatment follow-up, especially with respect to the outcome of pain reduction.
Highlights.
Results of this pilot trial of the FIT Teens program were promising
FIT Teens was more effective than CBT-only in the reduction of disability and pain
Mood symptoms improved, and fear of movement was reduced after FIT Teens
Acknowledgments
The authors would like to acknowledge the contributions of postdoctoral fellows Soumitri Sil, PhD and Susan Tran, PhD and research coordinator Daniel Strotman, BS who assisted in the conduct of this study, as well as referring physicians from the Divisions of Rheumatology and Pain Management – Drs. Brunner, Henrickson, Lovell, Morgan, Huggins, Grom, Goldschneider, Szabova and Rose. The study team would also like to acknowledge the time, effort and feedback provided by the participating teens with JFM and their parents. This study was funded by the NIH/National Institutes of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grants R21AR063412 and K24AR056687 (PI: Kashikar-Zuck).
Footnotes
Disclosures: The authors declare no conflicts of interest with this work.
Trial registration number: NCT # 01981096.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract Res Clin Rheumatol. 2015;29:120–130. doi: 10.1016/j.berh.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- 3.Bernardy K, Klose P, Busch AJ, Choy E, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;9 doi: 10.1002/14651858.CD009796.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BOSU. BOSU Balance Trainer. 2017 [Google Scholar]
- 5.Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007:CD003786. doi: 10.1002/14651858.CD003786.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte P, Walco G, Kimura Y. Temperament and stress response in children with juvenile primary fibromyalgia syndrome. Arthritis and Rheumatism. 2003;48:2923–2930. doi: 10.1002/art.11244. [DOI] [PubMed] [Google Scholar]
- 8.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 9.Eccleston C, Palermo TM, Williams AC, Lewandowski Holley A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2014;5:CD003968. doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ericsson A, Palstam A, Larsson A, Lofgren M, Bileviciute-Ljungar I, Bjersing J, Gerdle B, Kosek E, Mannerkorpi K. Resistance exercise improves physical fatigue in women with fibromyalgia: a randomized controlled trial. Arthritis Res Ther. 2016;18:176. doi: 10.1186/s13075-016-1073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher E, Heathcote L, Palermo TM, de CWAC, Lau J, Eccleston C. Systematic review and meta-analysis of psychological therapies for children with chronic pain. J Pediatr Psychol. 2014;39:763–782. doi: 10.1093/jpepsy/jsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280–295. doi: 10.1016/j.pain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Gowans SE, de Hueck A. Effectiveness of exercise in management of fibromyalgia. Curr Opin Rheumatol. 2004;16:138–142. doi: 10.1097/00002281-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Hooten WM, Qu W, Townsend CO, Judd JW. Effects of strength vs aerobic exercise on pain severity in adults with fibromyalgia: a randomized equivalence trial. Pain. 2012;153:915–923. doi: 10.1016/j.pain.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, Peugh J, Noll J, Ting TV, Powers SW. Long-term outcomes of adolescents with juvenile- onset fibromyalgia in early adulthood. Pediatrics. 2014;133:e592–e600. doi: 10.1542/peds.2013-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory (FDI) among a multicenter sample of youth with chronic pain. Pain. 2011;152:1600–1607. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashikar-Zuck S, Flowers SR, Strotman D, Sil S, Ting TV, Schikler KN. Physical activity monitoring in adolescents with juvenile fibromyalgia: findings from a clinical trial of cognitive-behavioral therapy. Arthritis Care Res (Hoboken) 2013;65:398–405. doi: 10.1002/acr.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clinical Journal of Pain. 2001;17:341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis and Rheumatism. 2007;57:474–480. doi: 10.1002/art.22615. [DOI] [PubMed] [Google Scholar]
- 20.Kashikar-Zuck S, Lynch AM, Slater S, Graham TB, Swain NF, Noll RB. Family factors, emotional functioning, and functional impairment in juvenile fibromyalgia syndrome. Arthritis and Rheumatism. 2008;59:1392–1398. doi: 10.1002/art.24099. [DOI] [PubMed] [Google Scholar]
- 21.Kashikar-Zuck S, Parkins IS, Graham TB, Lynch AM, Passo M, Johnston M, Schikler KN, Hashkes PJ, Banez G, Richards MM. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clin J Pain. 2008;24:620–626. doi: 10.1097/AJP.0b013e31816d7d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashikar-Zuck S, Sil S, Lynch-Jordan AM, Ting TV, Peugh J, Schikler KN, Hashkes PJ, Arnold LM, Passo M, Richards-Mauze MM, Powers SW, Lovell DJ. Changes in Pain Coping, Catastrophizing, and Coping Efficacy After Cognitive-Behavioral Therapy in Children and Adolescents With Juvenile Fibromyalgia. The journal of pain : official journal of the American Pain Society. 2013;14:492–501. doi: 10.1016/j.jpain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashikar-Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. Journal of Rheumatology. 2005;32:1594–1602. [PubMed] [Google Scholar]
- 24.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MH, Schikler KN, Hashkes PJ, Spalding S, Lynch-Jordan AM, Banez G, Richards MM, Lovell DJ. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: A multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012;64:297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashikar-Zuck S, Tran ST, Barnett K, Bromberg MH, Strotman D, Sil S, Thomas SM, Joffe N, Ting TV, Williams SE, Myer GD. A Qualitative Examination of a New Combined Cognitive-behavioral and Neuromuscular Training Intervention for Juvenile Fibromyalgia. Clin J Pain. 2015;32:70–81. doi: 10.1097/AJP.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping and functional disability in juvenile primary fibromyalgia syndrome. Journal of Pain. 2002;3:412–419. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs M. Children’s Depression Inventory. Multi-Health systems, Inc; 908 Niagara Falls Blvd., North Tonawanda, N.Y: 1992. [Google Scholar]
- 28.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Myer GD, Faigenbaum AD, Chu DA, Falkel J, Ford KR, Best TM, Hewett TE. Integrative training for children and adolescents: techniques and practices for reducing sports-related injuries and enhancing athletic performance. Phys Sportsmed. 2011;39:74–84. doi: 10.3810/psm.2011.02.1854. [DOI] [PubMed] [Google Scholar]
- 30.Myer GD, Faigenbaum AD, Ford KR, Best TM, Bergeron MF, Hewett TE. When to initiate integrative neuromuscular training to reduce sports-related injuries and enhance health in youth? Curr Sports Med Rep. 2011;10:155–166. doi: 10.1249/JSR.0b013e31821b1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid GJ, Lang BA, McGrath PJ. Primary juvenile fibromyalgia: psychological adjustment, family functioning, coping, and functional disability. Arthritis and Rheumatism. 1997;40:752–760. doi: 10.1002/art.1780400423. [DOI] [PubMed] [Google Scholar]
- 32.Roelofs J, Sluiter JK, Frings-Dresen MH, Goossens M, Thibault P, Boersma K, Vlaeyen JW. Fear of movement and (re)injury in chronic musculoskeletal pain: Evidence for an invariant two-factor model of the Tampa Scale for Kinesiophobia across pain diagnoses and Dutch, Swedish, and Canadian samples. Pain. 2007;131:181–190. doi: 10.1016/j.pain.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Scott PJ, Ansell BM, Huskisson EC. Measurement of pain in juvenile chronic polyarthritis. Ann Rheum Dis. 1977;36:186–187. doi: 10.1136/ard.36.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sil S, Arnold LM, Lynch-Jordan A, Ting TV, Peugh J, Cunningham N, Powers SW, Lovell DJ, Hashkes PJ, Passo M. Identifying treatment responders and predictors of improvement after cognitive-behavioral therapy for juvenile fibromyalgia. PAIN®. 2014 doi: 10.1016/j.pain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sil S, Thomas S, DiCesare C, Strotman D, Ting TV, Myer G, Kashikar-Zuck S. Preliminary evidence of altered biomechanics in adolescents with juvenile fibromyalgia. Arthritis Care Res (Hoboken) 2015;67:102–111. doi: 10.1002/acr.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125:143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2013. [Google Scholar]
- 38.Thomas SM, Sil S, Kashikar-Zuck S, Myer GD. Can Modified Neuromuscular Training Support the Treatment of Chronic Pain in Adolescents? Strength & Conditioning Journal. 2013;35:12–26. [Google Scholar]
- 39.Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology adult fibromyalgia criteria for use in an adolescent female population with juvenile fibromyalgia. The Journal of pediatrics. 2016;169:181–187.e181. doi: 10.1016/j.jpeds.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tkachuk GA, Harris CA. Psychometric Properties of the Tampa Scale for Kinesiophobia-11 (TSK-11) J Pain. 2012;13:970–977. doi: 10.1016/j.jpain.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Tran ST, Guite JW, Pantaleao A, Pfeiffer M, Myer GD, Sil S, Thomas SM, Ting TV, Williams SE, Edelheit B. Preliminary outcomes of a cross-site cognitive-behavioral and neuromuscular integrative training intervention for juvenile fibromyalgia. Arthritis Care & Research. 2017;69:413–424. doi: 10.1002/acr.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of Pediatric Psychology. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 43.Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain--a randomized controlled trial. Pain. 2009;141:248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty- three patients and matched normal controls. Arthritis & Rheumatism. 1985;28:138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]