Abstract
Background:
There is a growing body of evidence in animal and cell based models of Parkinson's disease (PD) to suggest that overexpression and / or abnormal accumulation and aggregation of α-synuclein can trigger neuronal death. This important role of α-synuclein in PD pathogenesis is supported by the fact that duplication, triplication and mutations of α-synuclein gene cause familial forms of PD.
Methods:
A review of literature was performed by searching PubMed and Google Scholar for relevant articles highlighting the pathogenic role of α-synuclein and the potential therapeutic implications of targeting various pathways related to this protein.
Results:
The overexpression and accumulation of α-synuclein within neurons may involve both transcriptional and post-transcriptional mechanisms including a decreased degradation of the protein through proteasomal or autophagic processes. The mechanisms of monomeric α-synuclein aggregating to oligomers and fibrils have been investigated intensively, but it is still not certain which form of this natively unfolded protein is responsible for toxicity. Likewise the proteotoxic pathways induced by α-synuclein leading to neuronal death are not elucidated completely but mitochondrial dysfunction, endoplasmic reticulum (ER) stress and altered ER-golgi transport may play crucial roles in this process. At the molecular level, the ability of α-synuclein to form pores in biomembranes or to interact with specific proteins of the cell organelles and the cytosol could be determining factors in the toxicity of this protein.
Conclusion:
Despite many limitations in our present knowledge of physiological and pathological functions of α-synuclein, it appears that this protein may be a target for the development of neuroprotective drugs against PD. This review has discussed many such potential drugs which prevent the expression, accumulation and aggregation of α-synuclein or its interactions with mitochondria or ER and thereby effectively abolish α-synuclein mediated toxicity in different experimental models.
Keywords: α-Synuclein, autophagy, proteasomal degradation, mitochondrial dysfunction, chaperone, endoplasmic reticulum stress, Parkinson's disease, microRNA, neuroprotective therapy
1. INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder affecting the elderly population. A recent meta-analysis study on the world wide data indicates a rise in PD prevalence with age (from 41 per 100,000 at 40-49 years to 1,903 per 100,000 at over 80 years) [1]. The classic tetrad of Parkinson’s symptoms comprises- resting tremor, bradykinesia, rigidity and loss of postural reflexes [2]. Bradykinesia is the most characteristic clinical feature of PD and is also responsible for the other salient Parkinsonian features namely hypophonia, hypomimia resulting in a mask-like facies, sialorrhoea due to impaired swallowing and reduced arm swing while walking. Apart from these typical motor symptoms and signs, a host of non-motor features complicate the course of PD.
The typical pathological signatures of the disease are the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the presence of proteinaceous inclusions, primarily composed of α-synuclein, within the surviving neurons [3]. The round inclusions in the neuronal soma are called Lewy bodies (LBs) while the thread-like ones, Lewy neurites (LNs), are seen in the neuronal processes [3, 4]. Although neurodegeneration in the SNpc is pronounced, the neuronal loss is spread over many other regions in the central nervous system (CNS) including non-dopaminergic neurons [5]. There is good post-mortem evidence to suggest that the neurodegeneration begins in the olfactory bulb and dorsal nucleus of vagus and progressively extends to affect the midbrain including substantia nigra (SN), thalamus and mesocortical areas and finally neocortex although contrasting views also exist [5, 6].
The majority of PD cases (>95%) are sporadic in nature, but some patients (<5%) suffer from the familial variety of the disease. Genetic studies have identified about 18 PD related gene loci (PARK loci) and 11 genes associated with these loci in connection with familial PD [7]. Several of these genes like α-synuclein (SNCA), parkin, PTEN-induced putative kinase 1 (PINK1), Daisuke-Junko1 (DJ1) and leucine-rich repeat kinase 2 (LRRK2) have been extensively studied, and mutations in these genes give rise to familial forms of the disease with autosomal dominant or recessive inheritance. Although genetic causes of PD have helped us to understand the pathogenesis of the disease to some extent, the vast majority of PD cases are sporadic which present with a complex multi-factorial and uncertain etiopathogenesis.
2. PATHOGENESIS OF SPORADIC PD: Α SYNUCLEIN AND PROTEOTOXIC MECHANISMS
2.1. α-Synuclein
α-Synuclein, coded by SNCA gene, is a 140 amino acid protein abundantly expressed in the neurons of brain and localizing predominantly at presynaptic regions [3]. The physiological function of α-synuclein is still not clearly established, but some studies have indicated its role in synaptic plasticity and vesicular transport of neurotrans-mitters [13]. The protein remains in a natively unfolded structure and has three distinct regions - the N-terminal region contains several repeats of a consensus sequence KTKEGV having homology with apolipoprotein lipid-binding domain, a central hydrophobic region known as the non-amyloid β-component domain (NAC) and the C-terminal region with multiple negatively charged amino acids [3, 14, 15]. The natively unfolded structure of α-synuclein attains some degrees of secondary structures at low pH or higher temperature, and further upon binding to synthetic lipid vesicles or lipid-mimetics, the protein undergoes significant changes in the conformational state from unfolded to partially α-helical structures [14, 16]. The different structural forms of α-synuclein within cells under physiological conditions have not been established with certainty as yet. Some studies have indicated that α-synuclein under physiological conditions in neural and non-neural tissues exists as partially α-helical tetramers with minor amounts of higher oligomeric forms in equilibrium with monomeric forms; while others have shown that it exists primarily in unfolded monomeric forms [17-19].
2.2. Oligomerization of α-Synuclein
Although the different conformational states of soluble α-synuclein under physiological conditions have been the subject of controversies, the protein is known to form oligomers with predominantly β-strand conformations which subsequently aggregate to give rise to insoluble fibrillar forms [3, 20, 21]. The oligomerization progresses with intermediates of various sizes and shapes that can be visualized by electron microscopy or detected by native or denaturing polyacryl-amide gel electrophoresis (PAGE) or size-exclusion chromato-graphy [3, 22]. The fibrils, rich in anti-parallel β-sheets, are typical amyloid type and can bind Thioflavin T and Congo red, and the process of fibrillization is nucleation-dependent and follows first-order kinetics [21, 23]. The process of oligomerization has been studied with a variety of biophysical techniques like far ultra-violet (UV) circular dichroism (CD), light scattering, small angle X-ray scattering, nuclear magnetic resonance (NMR) spectroscopy, single molecule Fӧrster Resonance Energy Transfer (FRET) etc., and a number of models are proposed [14, 24-26]. It is known that the hydrophobic NAC segments of α-synuclein molecules are important for aggregation which is also facilitated by the binding of α-synuclein to lipid membranes [27]. Some mutations of α-synuclein present in familial PD like A30P and A53T can enhance the oligomerization, but different mutations may affect the subsequent fibrillization process in different ways [21, 28]. Some of the post-trans-lational modifications of α-synuclein like phosphorylation, proteolysis and oxidation affect the aggregation process in different ways [3, 15]. Moreover, several studies have indicated that dopamine (DA) oxidation products like quinones can form adducts with α-synuclein leading to the formation of non-amyloidogenic oligomers which are resistant to sodium dodecyl sulphate (SDS) and have structures very different from native oligomers or fibrils of α-synuclein [29]. The pathophysiological significance of such DA-α-synuclein adducts is not known. There is conflicting evidence on the toxic effects of different forms of endogenous α-synuclein and their role in PD pathogenesis, but a significant number of studies hold the oligomers as the toxic species [3, 15, 22]. It is, however, not clear if the different forms of α-synuclein could produce different toxic consequences. A recent study using proximity ligation assay (PLA) has demonstrated diffused α-synuclein oligomers in mildly affected neurons in post-mortem brain samples of PD, while in more severely affected degenerating neurons fibrillar α-synuclein is detected by immunohistochemistry in pale bodies and Lewy bodies (LBs) [30].
2.3. Toxicity of α-Synuclein
The hypothesis that the toxic action of α-synuclein is an important contributor to PD pathogenesis is based on both direct and indirect evidence. It is established that mutations, duplication and triplication of SNCA gene are responsible for autosomal dominant types of familial PD implying clearly the toxicity of α-synuclein as the possible mediator of neurodegeneration in PD [7]. The dominant presence of α-synuclein in LBs and LNs in post-mortem PD brains again emphasizes the pathogenic role of this protein in PD. The α-synuclein transgenic mouse models expressing wild-type or mutant human α-synuclein or even wild-type murine α-synuclein resembling A53T mutant human α-synuclein have been widely studied, and these models develop neuro-degeneration in different parts of the brain and spinal cord with motor deficits and accumulation of α-synuclein inclusions [31-34]. Although these models are not true transgenic PD models, they are good tools to understand the cytotoxic actions of α-synuclein. On the other hand, in a large number of studies, viral vectors carrying α-synuclein gene, wild type or mutant, injected into substantia nigra of rodents have led to progressive loss of nigral dopaminergic neurons with other features of PD pathogenesis [35, 36]. Recombinant adeno-associated virus (AAV) mediated overexpression of human wild-type or mutant α-synuclein gene in nigral dopaminergic neurons of primates has produced slow, progressive neuropathology with intraneuronal inclusions immunoreactive for α-synuclein along with motor impairment [37]. In another primate model, synthetic α-synuclein has been injected into the striatum of marmoset resulting in α-synucleinopathy and loss of nigral dopaminergic neurons which indicates not only the toxicity but also internalization and retrograde transport of the protein [38]. Furthermore, α-synuclein derived from LBs of post-mortem PD brain and injected into striatum of monkeys or mice leads to progressive degeneration of nigrostriatal dopaminergic neurons after internalization of the protein, and this process depends on the presence of endogenous α-synuclein [36]. Similar studies where intracerebral injections of misfolded wild-type or mutant α-synuclein protein have led to progressive neurodegeneration and α-synucleinopathy, have been reported [39, 40]. Such studies lend credence to the hypothesis that α-synuclein toxicity may be propagated in a prion-like manner.
The toxicity of accumulated wild-type or mutant α-synuclein has also been shown in cultured cell lines of neural origin (PC 12, SH-SY5Y, BE (2) - M17 etc.) or non-neural cells like HEK or primary culture of mesencephalic neurons in a variety of experimental conditions [41-45]. Thus, there is a growing body of evidence from animal and cell based studies suggesting the direct cytotoxic potential of both wild-type and mutant α-synuclein when present in excess amount, which strengthens the view that α-synuclein is a key mediator of PD neurodegeneration.
2.4. Accumulation and Aggregation of α-Synuclein in PD
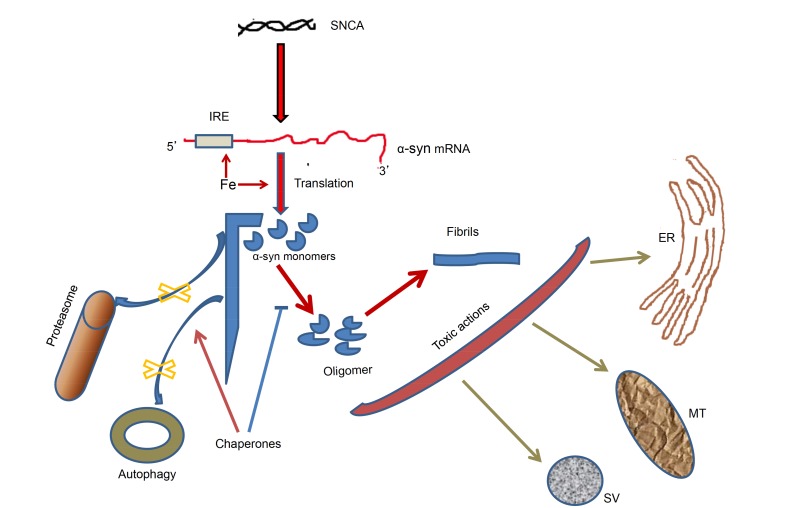
The accumulation of α-synuclein and subsequent aggregation within the neurons in the affected brain regions of PD may result from impaired removal of this protein by proteasomal or lysosomal systems, translational upregulation by iron and impaired chaperone function leading to increased misfolding and aggregation of the protein as well as defective removal of the misfolded protein through chaperone-mediated autophagy (CMA) (Fig. 1).
Fig. (1).
Altered homeostasis and toxic actions of α-synuclein in PD brain. Increased accumulation of α-synuclein takes place because of translational upregulation by iron / IRE (arrows); and impaired degradation of the monomeric and oligomeric forms via proteasomal pathway and autophagy (cross marks). Chaperones can inhibit oligomerization of α-synuclein (bar headed line) and activate autophagic removal of the protein (arrow). The oligomers and fibrils of α-synuclein have potential toxic actions on mitochondria, endoplasmic reticulum, synaptic vesicles etc. SNCA: α-synuclein gene; α-syn: α-synuclein; IRE: iron-responsive element; MT: mitochondria; ER: endoplasmic reticulum; SV: synaptic vesicle.
2.4.1. Degradation of α-Synuclein: Role in PD Pathogenesis
α-synuclein can be degraded both by the ubiquitin-proteasome system (UPS) and the autophagy-lysosome pathway (ALP) involving both macroautophagy and chaperone-mediated autophagy [46-48]. In proteasomal degradation pathway, the target protein is first conjugated to multiple ubiquitin (an evolutionarily conserved protein of 76 residues) units through the complex actions of E1, E2, E3 enzymes [49, 50]. In the first place ubiquitin is activated by E1 in ATP-dependent manner, and the activated ubiquitin is finally ligated to the Ɛ-amino group of a lysine residue of the target protein via the formation of several high-energy thioester intermediates with the help of E2 and E3 enzymes [49, 50]. Usually after poly-ubiquitination, the target protein is delivered to the multimeric 26S proteasomal complex composed of 20S catalytic complex and 19S regulatory particle [49, 50]. The target protein undergoes de-ubiquitination followed by proteolysis by trypsin-like, chymotrypsin-like and caspase-like activities in a complex process requiring ATP hydrolysis [49, 50]. The degradation kinetics of wild and mutant α-synuclein has been studied in SH-SY5Y cells where the degradation can be halted by proteasomal inhibitors and the mutant protein is degraded much slower than the wild one [51]. Another study has reported ubiquitin-independent proteasomal degradation of α-synuclein when it is overexpressed in SH-SY5Y cells [52]. The mechanism of α-synuclein degradation by 20S proteasome complex has been elaborately studied in vitro as well as in a cell line which reveals that the N-terminal segment of the protein is necessary for proteasomal degradation and oxidation of two methionine residues at this segment hinders the proteolysis [53]. Using α-synuclein transgenic mice and a technique to follow in vivo protein degradation in the brain, it has been shown that UPS is the main pathway of α-synuclein degradation under normal conditions in vivo and ALP is recruited when the protein burden is increased within the cell [54]. Thus, proteasomal inhibition as observed in the substantia nigra (SN) of post-mortem brains of PD subjects may be an important causative factor for the accumulation of α-synuclein within dopaminergic neurons in this condition [55, 56]. The accumulated α-synuclein can impair proteasomal activity by inhibiting the 26S subunit and thus may lead to further build up of the protein [57]. The importance of the proteasomal system in PD pathogenesis has been tested by developing an animal model of PD by using proteasomal inhibitors. Several reports have claimed to induce nigral dopaminergic neuronal loss and the appearance of typical motor deficits of PD after systemic administration of proteasomal inhibitors, but several other studies have failed to reproduce it [58, 59].
The other pathway of α-synuclein clearance involves lysosomal autophagy which occurs in three forms, and out of which macroautophagy and chaperone-mediated autophagy are useful in the present context. In macroautophagy, cytosolic components such as aggregated proteins, dysfunctional mitochondria, ribosomes etc. are engulfed in a double-membrane vesicle called autophagosome which matures and fuses with lysosomes to produce an autophagic vesicle in which proteins undergo degradation by lysosomal enzymes [60, 61]. This process thoroughly studied in yeast is regulated by a large number of autophagy related proteins (Atg), and in mammalian system the process of autophagosome formation and maturation involves many different proteins of which some are homologous to yeast Atg proteins [60, 61]. Beclin 1 (homologue of yeast Atg6) and LC3 II, derived from microtubule-associated protein light chain 3 (LC3, a homologue of yeast Atg8), are useful markers to study mammalian autophagy [60-62]. In chaperone-mediated autophagy, the target protein containing KFERQ domain in complex with a chaperone like heat-shock cognate protein of 70 kDa molecular weight (HSc70) is delivered to lysosomal lumen by a receptor-mediated translocation after binding to lysosome-associated membrane protein 2A (LAMP-2A) [47, 62, 63]. Chaperone mediated lysosomal degradation of α-synuclein has been shown in midbrain of mice treated with paraquat or of transgenic mice overexpressing α-synuclein [48]. Likewise in PC12 and SH-SY5Y cells and primary neuronal cultures from cortex and midbrain, the involvement of both macroautophagy and chaperone mediated autophagy have been demonstrated for α-synuclein degradation [64]. The autophagic removal of wild and mutant α-synuclein under different experimental conditions has been well-reviewed in a recent article [63]. Further, in different experimental systems, α-synuclein overexpression inhibits macroautophagy via Rab1a inhibition or other mechanisms involving Beclin 1, high-mobility group box 1 (HMGB 1) protein etc., and these processes would eventually result in further accumulation of α-synuclein [65-67]. Though the relative contributions of UPS and ALP in clearing the load of monomeric and aggregated α-synuclein within neurons under different contexts are yet to be established with certainty, it is plausible that autophagic failure contributes partially to accumulation of α-synuclein in PD brain [67, 68].
2.4.2. Translational Upregulation of α-Synuclein: Role of Iron
There are complex transcriptional and translational regulation of α-synuclein expression, but the role of intracellular iron is particularly important with respect to identification of new drug targets. The 5' untranslated region (5'-UTR) of α-synuclein mRNA contains a predicted iron-responsive element (IRE), which implies a translational control of the protein by intracellular iron much in the same fashion as with amyloid precursor protein (APP) or ferritin [69, 70]. Thus, it is interesting to note an iron dependent translational increase of α-synuclein in HEK293 exposed to ferric ammonium citrate presumably mediated through the predicted iron responsive element (IRE) in 5'-UTR of α-synuclein mRNA [71]. The importance of possible iron-dependent increase in α-synuclein protein expression in PD could be easily appreciated because of the fact that several transition metals including iron accumulate in PD brain in substantia nigra [72].
2.4.3. Role of Chaperones in α-Synuclein Accumulation and Aggregation
We have already indicated how chaperones may help in α-synuclein degradation through autophagy. However, chaperones may also prevent misfolding and aggregation of α-synuclein mitigating its cellular toxicity. The key observations on chaperones affecting α-synuclein aggregation and toxicity may be summarized here. In Drosophila, the directed expression of the wild-type or the mutant α-synuclein transgene causes dopaminergic neuronal loss which can be prevented by overexpressing Hsp70, while interference with endogenous fly Hsp70 aggravates the neuronal loss [73]. In a rat model of PD with lentivirus mediated overexpression of A30P α-synuclein, the dopaminergic neuronal loss and formation of α-synuclein inclusions could be ameliorated by simultaneous over-expression of yeast-chaperone Hsp104 [74]. In a mouse model of PD, striatal injections of recombinant adeno-associated virus (AAV) carrying human wild type SNCA has led to dopaminergic neuronal loss along with overexpression of several chaperones like Hsp27, Hsp40 and Hsp70 [75]. In multiple in vitro studies, chaperones like Hsp70, Hsp90 and Hsc70 have been shown to inhibit oligomerization and fibrillization of α-synuclein [76, 77]. Further, mutant α-synucleins A30P and A53T have been shown to inhibit chaperone-mediated autophagy (CMA) preventing their own degradation by this pathway, and in the substantia nigra (SN) and amygdala of post-mortem PD brain, the expression levels of Hsc70 and lysosomal-associated membrane protein 2A (LAMP2A), which are both involved in chaperone-mediated autophagy (CMA), are diminished [78]. Likewise, the overexpression of LAMP2A increases chaperone-mediated autophagy (CMA) and prevents neurotoxicity caused by adeno-viral mediated overexpression of wild-type α-synuclein in cultured cells or in the substantia nigra of experimental animals [79]. In other studies, the oligomers of α-synuclein have been shown to inhibit Hsp70-Hsp40 system which may lead to altered proteostasis within neurons, and additionally α-synuclein may co-aggregate with Hsp70 in an ADP-dependent process leading to the depletion of the chaperone inside the cells [80, 81]. Thus, chaperones may affect intraneuronal accumulation and aggregation of α-synuclein in multiple ways thereby regulating the toxicity of the misfolded protein.
2.5. Mechanisms of α-Synuclein Toxicity
Although there is overwhelming evidence supporting the toxicity of accumulated wild-type or mutant α-synuclein within neurons, the mechanism of toxicity is still uncertain to an extent, and the uncertainty lies primarily with the physiological versus pathological and neuroprotective versus neurotoxic functions of the protein. Multiple studies indeed have indicated that accumulated α-synuclein in monomeric or oligomeric form can cause endoplasmic reticulum (ER) stress, altered ER-golgi transport, pre-synaptic functional alterations with impaired vesicular exocytosis and release of neurotransmitters, altered cytoskeletal dynamics, calcium dysregulation, mitochondrial dysfunction, oxidative stress etc. which have been widely reported and reviewed [3, 36, 82-89]. In this context, it may be stated that α-synuclein effects on mitochondria should be more enthusiastically pursued with regard to possible alterations in mitochondrial dynamics, mitophagy and mitochondria-mediated cell death mechanisms [82, 83, 90]. The Parkin / PINK1 dependent mitophagy, a special type of macroautophagy to remove dysfunctional or depolarized mitochondria, has been worked out in considerable details from different experimental studies, and it is held that altered mitophagy has a probable role in PD pathogenesis which agrees with the fact that mutations of Parkin, or PINK1 gene give rise to several forms of familial PD [36, 91, 92]. However, the relationship of α-synuclein with Parkin / PINK 1 mediated mitophagy is yet to be elucidated clearly. The varied mechanisms of α-synuclein toxicity mentioned here may presumably lead to autophagic or apoptotic or caspase-dependent non-apoptotic neuronal death observed under different conditions [41-45]. On the other hand, α-synuclein plays a role in trafficking and exocytosis of synaptic vesicles through interaction with SNARE proteins [3, 85, 93, 94]. In agreement with such studies, it has been shown that transgenic expression of α-synuclein ameliorates the neurodegeneration seen in cysteine-string protein-α (CSP-α) knock-out mice and ablation of endogenous α-synuclein aggravates CSP-α knock-out phenotype [95]. This suggests a neuroprotective function of α-synuclein as opposed to a neurotoxic role in PD, and an interesting paper suggests that different domains of α-synuclein may be involved in physiological and pathological functions of this protein [96]. The figure (Fig. 1) summarizes the various aspects of α-synuclein accumulation, aggregation and toxicity in PD affected brain regions.
It may be envisaged that all the toxic actions of α-synuclein are related fundamentally to interactions of the latter with lipid bilayer membranes and proteins of different organelles, and these interactions may be analyzed further. The interaction of α-synuclein with lipid membranes not only triggers the conformational alterations and aggregation of the protein, but also leads to altered membrane dynamics, increased permeabilization and formation of pores and channels through the membranes [97-100]. Both monomeric and oligomeric forms of the protein may lead to channel formation in synthetic lipid vesicles, cultured cell lines or primary culture or dissociated neurons of brain as identified by measurements of conductance changes at these channels by patch-clamp technique and models involving activation states of such channels have been proposed [99, 101]. The role of increased intracellular Ca2+ in α-synuclein mediated toxicity has been shown by linking entry of Ca2+ through these membrane channels [84, 101, 102].
Apart from its interactions with lipid membranes, α-synuclein interacts directly with a large number of mitochondrial, synaptic and other vesicular membrane bound proteins that may play pivotal roles in neurotoxicity of this protein in PD. In particular, it interacts with mitochondrial voltage-dependent anion selective channel (VDAC), TOM 20, cytochrome c oxidase (COX) etc., several Rab proteins involved in vesicular transport, tyrosine hydroxylase (TH), dopamine transporter (DAT), tubulin, synphilin, 14-3-3 protein and a variety of other intracellular proteins [3, 103, 104-106]. In a recent proteomics based study on brain synaptosomes, multiple interacting proteins have been identified with 10 proteins showing preferential binding to monomeric and 76 exhibiting preference to oligomeric forms of α-synuclein [107]. The significance of such protein-protein interactions in the pathological functions of α-synuclein is yet to be established.
3. NEUROPROTECTIVE THERAPY FOR PD: TARGETING Α-SYNUCLEIN
Before proceeding to analyze the newer opportunities of drug development for PD, it would be pertinent to make a brief summary of the existing therapeutic options and their limitations.
3.1. Present Therapeutic Options
PD therapy is centred on boosting the dopaminergic system in the basal ganglia either by supplementation of levodopa - a prodrug of DA or by means of dopaminergic receptor agonists and inhibitors of DA catabolism. Because DA by itself does not cross the blood-brain barrier, levodopa which is metabolized to DA in the body is used as the bedrock of Parkinson’s therapy. The existing therapeutic options for PD including surgical intervention and cell replacement procedures are summarized in Table 1 [108-115]. In contradistinction to other age related neuro-degenerative disorders, PD is atypical in the sense that treatment response to traditional agents is nearly uniformly good. The chief concerns regarding traditional PD therapy are, however, the inevitability of disease progression, the prolongation of off-periods or wearing off of the drug effects and the development of drug induced dyskinesias and other adverse effects. Furthermore, the non-motor symptoms of PD are less amenable to therapy [108]. This implies that newer avenues of research in Parkinson’s therapy are warranted.
Table 1.
Conventional therapeutic options for Parkinson’s Disease.
| Pharmacological Options | |||
|---|---|---|---|
| Drug Class | Drug | Mechanism of Action | Remarks |
| Dopamine prodrug | Levodopa | Metabolized to DA and replaces depleted DA in brain | Large part metabolized to dopamine in periphery by dopa decarboxylase and lost; as dopamine cannot cross the BBB |
| Enhancer of brain dopamine availability 1. Peripheral DOPA-decarboxylase inhibitor 2. Catechol-O-methyl transferase (COMT) inhibitor 3. Monoamine oxidase (MAO-B) inhibitor |
Carbidopa Entacapone Selegiline Rasagiline |
Inhibits the action of peripheral dopa decarboxylase so that levodopa is available in adequate quantity to cross the BBB and exert its action after metabolism to DA Inhibits the peripheral metabolism of levodopa to 3-methyl dopa and the brain metabolism of dopamine to homovanillic acid Inhibits the brain metabolism of dopamine to homovanillic acid |
Always used with levodopa Add-on therapy with levodopa Add-on therapy with levodopa |
| Dopamine agonists 1. Ergot derived 2. Non-ergot derived |
Bromocriptine Ropinirole Pramipexole Apomorphine |
Agonist at dopamine D2, D3 receptors Dopamine receptor agonist D1/D2/D3/D4/D5 receptor agonist |
Alternative to levodopa, especially in younger population Alternative to levodopa, especially in younger population Rescue therapy for freezing episodes |
| N-methyl-D-aspartate (NMDA) receptor antagonist | Amantadine | Increases dopamine release and stimulatory action on post-synaptic dopamine receptors in brain (exact mechanism unclear) | Add-on therapy with levodopa |
| Centrally acting anti-cholinergics | Trihexiphenidyl Benztropine | Inhibits relative cholinergic overactivity in brain | Add-on therapy for control of tremors in PD |
| Non-Pharmacological Options | |||
| Technique | Mechanism of Action | Remarks | |
| Deep Brain Stimulation -Subthalamic nucleus -Globus pallidus interna |
Stimulation of nerve fibres in the target areas while inhibiting the neuronal cell bodies | FDA approved and actively used in many centers. Solves the problem of dyskinesias and drug refractoriness. Used late in disease. | |
| Stem cell therapies -fetal ventral mesencephalic allografts - human embryonic stem cell-derived |
Dopamine replacement | Experimental therapy | |
3.2. Potential Therapeutic Avenues Targeting α-synuclein
As in the case of other neurodegenerative diseases, neuroprotective therapy to halt or substantially diminish the disease progression is not currently available for PD. Whatever has been discussed about the role of α-synuclein in PD neurodegeneration, it is likely that new approaches of neuroprotective therapy could be developed targeting α-synuclein expression, degradation, oligomerization and interactions with cellular organelles like mitochondria and ER. Thus, iron-chelators could diminish the protein expression level of α-synuclein and its oligomerization as also the interaction of this protein with iron to generate ROS. In many toxin-based experimental models of PD, iron-chelators have been shown to prevent neurodegeneration, and it is presumable that the protective action of such chelators is mediated partly by prevention of accumulation and aggregation of α-synuclein in the brain and partly by abolishing the iron-catalyzed oxidative stress [116-120]. However, a few small clinical trials in PD patients have not yet confirmed the efficacy of the metal-chelators [121]. The multiple and inter-related effects of chaperones on α-synuclein accumulation, aggregation and degradation make them useful targets of drug development for PD through inhibition of α-synuclein toxicity. Three compounds of the ansamycin group of antibiotics which are small molecule inhibitors of Hsp90, namely geldanamycin, tanespimycin and alvespimycin have in preclinical studies been shown to attenuate α-synuclein toxicity and facilitate its clearance but success in clinical practice could be difficult owing to pharmacokinetic and adverse effect profiles [122]. It may seem a bit surprising why Hsp90 inhibitors should reduce α-synuclein toxicity especially when it has been demonstrated that Hsp90 inhibits α-synuclein aggregation in vitro [77]. It is plausible that the drugs inhibit Hsp90, which leads to the release of HSF1 from its complex with Hsp90 and thus to an increased expression of Hsp70 and other stress-induced chaperones [123]. Another group of synthetic small molecule inhibitors of Hsp90 is designed by performing a compound library screen for scaffolds that bind the ATP binding pocket of Hsp90. More than one leads in this group of molecules have been identified, and in cell culture models of PD they cause a reduction of high molecular weight and monomeric α-synuclein as well as a significant reduction of α-synuclein toxicity [124]. Direct enhancers of Hsp70 expression such as arimoclomol, celastrol and valproate may show benefits in PD. Valproate is shown to induce Hsp70 through its action as a histone deacetylase (HDAC) inhibitor, and it is protective against rotenone-induced toxicity in treated cell culture and rats [125-127]. Several techniques are being tested in order to get the chaperones to reach beyond the blood brain barrier, including the use of viral mediated delivery and cell penetrating peptide technology [128]. The mucolytic drug ambroxol which also functions as a small molecule chaperone, increases glucocerebrosidase activity and decreases α-synuclein in mice with over-expression of this Parkinsonian protein. A lot of recent literature suggests a therapeutic role for ambroxol in PD [129]. Similar effects have been observed in cell lines derived from fibroblasts [130]. In transgenic mouse model with A53T α-synuclein mutation, overexpression of sirtuin-1 (SIRT1) decreases the accumulation of α-synuclein in the brain and increases the life span of the animal presumably by increasing Hsp70 level, and thus drugs enhancing SIRT1 levels should be explored further in pre-clinical studies with PD models. Resveratrol which activates SIRT1 has been shown to protect against α-synuclein toxicity in a cellular model [131].
The removal of α-synuclein from the neuron depends on both autophagy and proteasomal degradation as indicated earlier. The small molecule enhancer of autophagy trehalose has been shown to accelerate the clearance of mutant α-synuclein in experimental models [132]. Oleuropein obtained from the leaf extract of olive enhances proteasomal activity and retards senescence of fibroblasts in culture, and this drug should be tested in experimental PD models [133]. The tyrosine kinase inhibitor nilotinib, used for adult leukemia, has been shown to increase autophagic removal of α-synuclein in transgenic mice, and the drug also prevents α-synuclein induced neurodegeneration in mice and increases α-synuclein clearance by autophagy in a lentiviral gene transfer model [134]. The neuroleptic drug trifluperazine enhances macroautophagy and prevents the death of human post-mitotic dopaminergic cells induced by overexpressing α-synuclein [135]. A group of synthetic compounds called molecular tweezers have been designed which specifically bind to the aggregation-prone proteins through lysine/arginine residues and prevent pathological protein aggregation or even de-aggregate the protein oligomers to non-toxic forms [136, 137]. One such molecular tweezer CLR01 can bind through Lys side-chains of α-synuclein and inhibit its oligomerization in vitro and in vivo in a zebra fish model. CLR01 prevents the accumulation and toxicity of α-synuclein within neurons [137, 138]. It appears that molecular tweezers would be effective drugs for PD in future.
The varied interactions of α-synuclein with mitochondria may contribute to mitochondrial dysfunctions characteristic of clinical PD, and such mitochondrial functional impairment plays a crucial role in α-synuclein- induced toxicity models of PD also. These studies do indicate that several drugs may thwart the effects of α-synuclein on mitochondria and thereby protect the cells from the toxicity of this protein. For example, α-synuclein induced mitochondrial toxicity and cell death in SH-SY5Y cells could be prevented by cyclosporine A which blocks the activation of mitochondrial permeability transition pore (mPTP) [82]. Likewise, in neuronally differentiated SH-SY5Y cells, cell death and mitochondrial dysfunction induced by overexpressing α-synuclein could be prevented by olesoxime [139]. In yeast cells, α-synuclein mediated toxicity is dependent upon sirtuin-2 (SIRT2) dependent mitophagy, and interestingly SIRT2 inhibitors have been shown earlier to prevent dopaminergic cell death in vitro and in drosophila model of PD [140, 141]. In a yeast model, 1,15,000 compounds have been screened to identify many small molecular weight compounds which can ameliorate the α-synuclein toxicity including mitochondrial dysfunction and altered ER-Golgi transport in this system as well as in nematode neurons or rat cortical neurons in culture [142]. All these drugs are to be explored further for their potential benefit in PD. ER stress or the impairment of vesicular transport or ER-Golgi trafficking may underlie the toxicity of overexpressed and accumulated α-synuclein in experimental models of PD, as already described in this review. It has been shown that alterations in ER-Golgi transport induced by α-synuclein in yeast and mammalian cell based models of PD can be prevented by overexpression of Rab1 belonging to Rab guanine nucleotide triphosphatase family of proteins, and this may provide new drug targets for PD [143]. We have earlier discussed the study in a yeast model of α-synuclein toxicity identifying many compounds that could rectify the ER-Golgi transport defect caused by α-synuclein [142]. Several other compounds such as 4-phenylbutyrate, tauroursodeoxycholic acid, dibenzoyl methane derivative etc. have been shown to prevent ER stress in α-synuclein transgenic mice or different toxin-based models of PD [144]. Similarly, a phenyl-sulphonamide compound has been isolated which reverses the disruption of vesicular transport and dopaminergic neuronal death in an α-synuclein-induced toxicity model of PD [145].
In conclusion it may be stated that the role of α-synuclein in PD neurodegeneration could hardly be overemphasized and this has opened new avenues of neuroprotective therapy which should thwart the disease progression instead of bringing symptomatic relief only. Although there are important leads for the development of such drugs, most of these are in pre-clinical testing phase. It is uncertain at this moment how the actions of these drugs would be targeted to degenerating dopaminergic neurons only and whether disruption of normal physiological functions of α-synuclein in other neurons would produce adverse effects. Moreover, drugs like ansamycin group of antibiotics, the tyrosine kinase inhibitor nilotinib, the neuroleptic trifluperazine or the immunosuppressive cyclosporine A would have intrinsic toxic effects which could be true for other putative drugs as well. Thus, a more concerted effort is needed to prove the efficacy of such α-synuclein-targeting drugs in clinical cases of PD and to ascertain their toxicity and bioavailability in the human body.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Sasanka Chakrabarti thanks the Department of Science and Technology, Govt. of India for their continued support for research in the area neurodegenerative diseases. UG acknowledges the Council for Scientific and Industrial Research, Govt. of India, for providing research fellowship to her. SSC and UK thank the National Programme for Healthcare of the Elderly, Ministry of Health and Family Welfare, Govt. of India for support in their research work.
Footnotes
Several excellent reviews have elaborately described the possible pathogenic mechanisms of sporadic PD that include oxidative stress, mitochondrial dysfunction, inflammation and proteotoxicity [8, 9]. There is a growing body of evidence however which amply suggests that proteotoxicity caused by the accumulation and subsequent oligomerization of α-synuclein is the major driving force in PD pathogenesis [10-12]. This review will primarily deal with the molecular pathogenesis of sporadic PD with α-synuclein in the centre stage and suggest new therapeutic avenues targeting this protein.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hirsch L., Jette N., Frolkis A., Steeves T., Pringsheim T. The Incidence of Parkinson’s disease: A systematic review and meta-analysis. 2016 doi: 10.1159/000445751. http://dx.doi.org/10 [DOI] [PubMed]
- 2.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 3.Stefanis L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2(2):a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forno L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996;55(3):259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Braak H., Del Tredici K., Rüb U., de Vos R.A., Jansen S.E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 6.Braak H., Ghebremedhin E., Rüb U., Bratzke H., Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 7.Klein C., Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2(1):a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subhramaniam S.R., Chesselet M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. 2013. [DOI] [PMC free article] [PubMed]
- 9.Xie W., Wan O.W., Chung K.K. New insights into the role of mitochondrial dysfunction and protein aggregation in Parkinson’s disease. Biochim. Biophys. Acta. 2010;1802:935–941. doi: 10.1016/j.bbadis.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Cookson M.R. 2009 http://doi.org/10
- 11.Shulman J.M., De Jager P.L., Feany M.B. Parkinson’s disease: genetics and pathogenesis. Annu. Rev. Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 12.Houlden H., Singleton A.B. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124(3):325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng F., Vivacqua G., Yu S. The role of alpha-synuclein in neurotransmission and synaptic plasticity. J. Chem. Neuroanat. 2011;42(4):242–248. doi: 10.1016/j.jchemneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Uversky V.N., Eliezer D. Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr. Protein Pept. Sci. 2009;10(5):483–499. doi: 10.2174/138920309789351921. http://www. eurekaselect.com/85064/article# [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villar-Pique A., Lopes da Fonseca T., Outerio T.F. Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J. Neurochem. 2016;1:240–255. doi: 10.1111/jnc.13249. [DOI] [PubMed] [Google Scholar]
- 16.Ferreon A.C., Gambin Y., Lemke E.A., Deniz A.A. Interplay of alpha-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc. Natl. Acad. Sci. USA. 2009;106(14):5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartels T., Choi J.G., Selkoe D.J. 2011 https://doi.org/10
- 18.Fauvet B., Mbefo M.K., Fares M.B., Desobry C., Michael S., Ardah M.T., Tsika E., Coune P., Prudent M., Lion N., Eliezer D., Moore D.J., Schneider B., Aebischer P., El-Agnaf O.M., Masliah E., Lashuel H.A. α-synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 2012;287(19):15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dettmer U., Newman A.J., Luth E.S., Bartels T., Selkoe D. In-vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells. J. Biol. Chem. 2013;288(9):6371–6385. doi: 10.1074/jbc.M112.403311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narhi L., Wood S.J., Steavenson S., Jiang Y., Wu G.M., Anafi D., Kaufman S.A., Martin F., Sitney K., Denis P., Louis J.C., Wypych J., Biere A.L., Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J. Biol. Chem. 1999;274(14):9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 21.Conway K.A., Lee S.J., Rochet J.C., Ding T.T., Harper J.D., Williamson R.E., Lansbury P.T., Jr Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. 2000 doi: 10.1111/j.1749-6632.2000.tb06903.x. http://doi.org/10 [DOI] [PubMed]
- 22.Pieri L., Madiona K., Melki R. Structural and functional properties of prefibrillar α-synuclein oligomers. Sci. Rep. 2016;6:24526. doi: 10.1038/srep24526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood S.J., Wypych J., Steavenson S., Louis J.C., Citron M., Biere A.L. alpha-synuclein fibrillogenesis is nucleation dependent. Implications for the pathogenesis of Parkinson’s disease. J. Biol. Chem. 1999;274(28):19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad B., Chen Y., Lapidus L.J. Aggregation of α-synuclein is kinetically controlled by intramolecular diffusion. Proc. Natl. Acad. Sci. USA. 2012;109(7):2336–2341. doi: 10.1073/pnas.1109526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lashuel H.A., Overk C.R., Oueslati A., Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013;14(1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iljina M., Garcia G.A., Horrocks M.H., Tosatto L., Choi M.L., Ganzinger K.A., Abramov A.Y., Gandhi S., Wood N.W., Cremades N., Dobson C.M., Knowles T.P., Klenerman D. Kinetic model of the aggregation of alpha-synuclein provides insights into prion-like spreading. 2016. [DOI] [PMC free article] [PubMed]
- 27.Pfefferkorn C.M., Jiang Z., Lee J.C. Biophysics of alpha-synuclein membrane interactions. 2012. [DOI] [PMC free article] [PubMed]
- 28.Dikiy I., Eliezer D. Folding and misfolding of alpha synuclein on membranes. Biochim. Biophys. Acta. 2012;1818(14):1013–1018. doi: 10.1016/j.bbamem.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rekas A., Knott R.B., Sokolova A., Barnham K.J., Perez K.A., Masters C.L., Drew S.C., Cappai R., Curtain C.C., Pham C.L. The structure of dopamine-induced alpha-synuclein oligomers. Eur. Biophys. J. 2010;39(10):1407–1419. doi: 10.1007/s00249-010-0595-x. [DOI] [PubMed] [Google Scholar]
- 30.Roberts R.F., Wade-Martins R., Alegre-Abarrategui J. Direct visualization of alpha synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015;138(6):1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernagut P.O., Chesselet M.F. Alpha-synuclein and transgenic mouse models. Neurobiol. Dis. 2004;17(2):123–130. doi: 10.1016/j.nbd.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Martin L.J., Pan Y., Price A.C., Sterling W., Copeland N.G., Jenkins N.A., Price D.L., Lee M.K. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006;26(1):41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amschl D., Neddens J., Havas D., Flunkert S., Rabl R., Rӧmer H., Rockenstein E., Masliah E., Windisch M., Hutter-Paier B. Time course and progression of wild-type α-synuclein accumulation in a transgenic mouse model. BMC Neurosci. 2013;14:6. doi: 10.1186/1471-2202-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieker C., Dev K.K., Lehnhoff K., Barbieri S., Ksiazek L., Kauffman S., Danner S., Schell H., Boden C., Ruegg M.A., Kahle P.J., van der Putten H., Shimshek D.R. Neuropathology in mice expressing mouse alpha-synuclein. 2011. [DOI] [PMC free article] [PubMed]
- 35.Mochizuki H., Yamada M., Mizuno Y. Alpha-synuclein overexpression model. J. Neural Transm. Suppl. 2006;70:281–284. [PubMed] [Google Scholar]
- 36.Ganguly G., Chakrabarti S., Chatterjee U., Saso L. Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer’s disease and Parkinson’s disease. 2017. [DOI] [PMC free article] [PubMed]
- 37.Kirik D., Annett L.E., Burger C., Muzyczka N., Mandel R.J., Bjӧrklund A. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2003;100(5):2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimozawa A., Ono M., Takahara D., Tarutani A., Imura S., Masuda-Suzukake M., Higuchi M., Yanai K., Hisanaga S.I., Hasegawa M. Propagation of pathological α-synuclein in marmoset brain. Acta Neuropathol. Commun. 2017;5(1):12. doi: 10.1186/s40478-017-0413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Recasens A., Dehay B., Bové J., Carballo-Carbajal I., Dovero S., Pérez-Villalba A., Fernagut P.O., Blesa J., Parent A., Perier C., Farińas I., Obeso J.A., Bezard E., Vila M. Lewy body extracts from Parkinson disease brain trigger α-synuclein pathology and neurodegeneration in mice and monkeys. 2014. [DOI] [PubMed]
- 40.Luk K.C., Kehm V.M., Zhang B. OʹBrien, P.; Trojanowski, J.Q.; Lee, V.M. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefanis L., Larsen K.E., Rideout H.J., Sulzer D., Greene L.A. Expression of A53T mutant but not wild type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release and autophagic cell death. J. Neurosci. 2001;21(24):9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bisaglia M., Greggio E., Maric D., Miller D.W., Cookson M.R., Bubacco L. Alpha-synuclein overexpression increases dopamine toxicity in BE2-M17 cells. BMC Neurosci. 2010;11:41. doi: 10.1186/1471-2202-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabrizi S.J., Orth M., Wilkinson J.M., Taanman J.W., Warner T.T., Cooper J.M., Schapira A.H. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum. Mol. Genet. 2000;9(18):2683–2689. doi: 10.1093/hmg/9.18.2683. [DOI] [PubMed] [Google Scholar]
- 44.Vekrellis K., Xilouri M., Emmanouilidou E., Stefanis L. Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J. Neurochem. 2009;109(5):1348–1362. doi: 10.1111/j.1471-4159.2009.06054.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W., Hurlbert M.S., Schaack J., Prasad K.N., Freed C.R. Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 2000;866(1-2):33–43. doi: 10.1016/S0006-8993(00)02215-0. [DOI] [PubMed] [Google Scholar]
- 46.Webb J.L., Ravikumar B., Atkins J., Skepper J.N., Rubinsztein D.C. Alpha-Synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 2003;278(27):25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 47.Wong E., Cuervo A.M. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb. Perspect. Biol. 2010;2(12):a006734. doi: 10.1101/cshperspect.a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mak S.K., McCormack A.L., Manning-Bog A.B., Cuervo A.M., Di Monte D.A. Lysosomal degradation of alpha-synuclein in vivo. J. Biol. Chem. 2010;285(18):13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glickman M.H., Ciechanover A. The ubiquitin proteasome proteolytic pathway: destruction for the sake of construction. 2002 doi: 10.1152/physrev.00027.2001. https://doi.org/10 [DOI] [PubMed]
- 50.Bentea E., Verbruggen L., Massie A. The proteasome inhibition model of Parkinson’s disease. J. Parkinsons Dis. 2017;7(1):31–63. doi: 10.3233/JPD-160921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett M.C., Bishop J.F., Leng Y., Chock P.B., Chase T.N., Mouradian M.M. Degradation of alpha-synuclein by proteasome. J. Biol. Chem. 1999;274(48):33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 52.Tofaris G.K., Layfield R., Spillantini M.G. alpha-synuclein metabolism and aggregation is linked to ubiquitin-dependent degradation by the proteasome. FEBS Lett. 2001;509(1):22–26. doi: 10.1016/S0014-5793(01)03115-5. [DOI] [PubMed] [Google Scholar]
- 53.Alvarez-Castelao B., Goethals M., Vandekerckhove J., Castaño J.G. 2014.
- 54.Ebrahimi-Fakhari D., Cantuti-Castelvetri I., Fan Z., Rockenstein E., Masliah E., Hyman B.T., McLean P.J., Unni V.K. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J. Neurosci. 2011;31(41):14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McNaught K.S., Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci. Lett. 2001;297(3):191–194. doi: 10.1016/S0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- 56.McNaught K.S., Belizaire R., Isacson O., Jenner P., Olanow C.W. Altered proteasomal function in sporadic Parkinson’s disease. Exp. Neurol. 2003;179(1):38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 57.Zhang N.Y., Tang Z., Liu C.W. alpha-Synuclein protofibrils inhibit 26 S proteasome-mediated protein degradation: Understanding the cytotoxicity of protein protofibrils in neurodegenerative disease pathogenesis. J. Biol. Chem. 2008;283(29):20288–20298. doi: 10.1074/jbc.M710560200. [DOI] [PubMed] [Google Scholar]
- 58.McNaught K.S., Perl D.P., Brownell A.L., Olanow C.W. Systemic exposure to proteasome inhibitors cause a progressive model of Parkinson’s disease. Ann. Neurol. 2004;56(1):149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- 59.Cook C., Petrucelli L. A critical evaluation of the ubiquitin-proteasome system in Parkinson’s disease. Biochim. Biophys. Acta. 2009;1792(7):664–675. doi: 10.1016/j.bbadis.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang R., Zeh H.J., Lotze M.T., Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nedelsky N.B., Todd P.K., Taylor J.P. Autophagy and the ubiquitin-proteasome system: collaborators in neuroprotection. Biochim. Biophys. Acta. 2008;1782(12):691–699. doi: 10.1016/j.bbadis.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopes da Fonseca T., Villar-Pique A., Outerio T.F. The interplay between alpha-synuclein clearance and spreading. Biomolecules. 2015;5(2):435–471. doi: 10.3390/biom5020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogiatzi T., Xilouri M., Vekrellis K., Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J. Biol. Chem. 2008;283(35):23542–23556. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winslow A.R., Chen C.W., Corrochano S., Acevedo-Arozena A., Gordon D.E., Peden A.A., Lichtenberg M., Menzies F.M., Ravikumar B., Imarisio S., Brown S. OʹKane, C.J.; Rubinsztein, D.C. α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J. Cell Biol. 2010;190(6):1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang C., Zhao C., Li D., Tian Z., Lai Y., Diao J., Liu C. Versatile structures of α-Synuclein. Front. Mol. Neurosci. 2016;9:48. doi: 10.3389/fnmol.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song J.X., Lu J.H., Chen L.L., Durairajan S.S., Yue Z., Zhang H.Q., Li M. HMGB1 is involved in autophagy inhibition caused by SNCA/α-synuclein overexpression: a process modulated by the natural autophagy inducer corynoxine B. Autophagy. 2014;10(1):144–154. doi: 10.4161/auto.26751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xilouri M., Brekk O.R., Stefanis L. Autophagy and Alpha-Synuclein: relevance to Parkinson’s disease and related synucleiopathies. Mov. Disord. 2016;31(2):178–192. doi: 10.1002/mds.26477. [DOI] [PubMed] [Google Scholar]
- 69.Friedlich A.L., Tanzi R.E., Rogers J.T. 2007.
- 70.Cahill C.M., Lahiri D.K., Huang X., Rogers J.T. Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. 2009 doi: 10.1016/j.bbagen.2008.12.001. https://doi.org/10 [DOI] [PMC free article] [PubMed]
- 71.Febbaro F., Giorgio M., Caldarola S., Loreni F., Romero-Ramos M. α-Synuclein expression is modulated at the translational level by iron. Neuroreport. 2012;23(9):576–580. doi: 10.1097/WNR.0b013e328354a1f0. [DOI] [PubMed] [Google Scholar]
- 72.Dexter D.T., Wells F.R., Lees A.J., Agid F., Agid Y., Jenner P., Marsden C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. 1989 doi: 10.1111/j.1471-4159.1989.tb07264.x. https://doi.org/10 [DOI] [PubMed]
- 73.Auluck P.K., Chan H.Y., Trojanowski J.Q., Lee V.M., Bonini N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295(5556):865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 74.Lo Bianco C., Shorter J., Réquiler E., Lashuel H., Iwatsubo T., Lindquist S., Aebischer P. Hsp 104 antagonizes alpha-synuclein aggregation and reduces dopaminergic degeneration in a rat model of Parkinson’s disease. J. Clin. Invest. 2008;118(9):3087–3097. doi: 10.1172/JCI35781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.St Martin J.L., Klucken J., Outerio T.F., Nguyen P., Keller-McGandy C., Cantuti-Castelvetri I., Grammatopoulos T.N., Standaert D.G., Hyman B.T., McLean P.J. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 2007;100(6):1449–1457. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- 76.Pemberton S., Melki R. The interaction of Hsc 70 protein with fibrillar α-Synuclein and its therapeutic potential in Parkinson’s disease. Commun. Integr. Biol. 2012;5(1):94–95. doi: 10.4161/cib.18483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daturpalli S., Waudby C.A., Meehan S., Jackson S.E. Hsp90 inhibits α-synuclein aggregation by interacting with soluble oligomers. J. Mol. Biol. 2013;425(22):4614–4628. doi: 10.1016/j.jmb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez-Erviti L., Rodriguez-Oroz M.C., Cooper J.M., Caballero C., Ferrer I., Obeso J.A., Schapira A.H. Chaperone-mediated autophagy markers in Parkinson disease brains. 2010 doi: 10.1001/archneurol.2010.198. https://doi.org/10 [DOI] [PubMed]
- 79.Xilouri M., Brekk O.R., Landeck N., Pitychoutis P.M., Papasilekas T., Papadopoulou-Daifoti Z., Kirik D., Stefanis L. Boosting chaperone-mediated autophagy in vivo mitigates α-synuclein induced neurodegeneration. Brain. 2013;136(7):2130–2146. doi: 10.1093/brain/awt131. [DOI] [PubMed] [Google Scholar]
- 80.Hinault M.P., Cuendet A.F., Mattoo R.U., Mensi M., Dietler G., Lashuel H.A., Goloubinoff P. Stable alpha-synuclein oligomers strongly inhibit chaperone activity of the Hsp70 system by weak interactions with J-domain co-chaperones. J. Biol. Chem. 2010;285(49):38173–38182. doi: 10.1074/jbc.M110.127753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roodveldt C., Bertoncini C.W., Andersson A., van der Good A.T., Hsu S.T., Fernández-Montesinos R., de Jong J., van Ham T.J., Nollen E.A., Pozo D., Christodoulou J., Dobson C.M. 2009. [DOI] [PMC free article] [PubMed]
- 82.Bir A., Sen O., Anand S., Khemka V.K., Banerjee P., Cappai R., Sahoo A., Chakrabarti S. α-Synuclein-induced mitochondrial dysfunction in isolated preparation and intact cells: implications in the pathogenesis of Parkinson’s disease. J. Neurochem. 2014;131(6):868–877. doi: 10.1111/jnc.12966. [DOI] [PubMed] [Google Scholar]
- 83.Xie W., Chung K.K. Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J. Neurochem. 2012;122(2):404–414. doi: 10.1111/j.1471-4159.2012.07769.x. [DOI] [PubMed] [Google Scholar]
- 84.Angelova P.R., Ludtmann M.H.R., Horrocks M.H., Negoda A., Cremades N., Klenerman D., Dobson C.M., Wood N.W., Pavlov E.V., Gandhi S., Abramov A.Y. Calcium is a key factor in α-synuclein induced neurotoxicity. J. Cell Sci. 2016;129:1792–1801. doi: 10.1242/jcs.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang T., Hay J.C. Alpha-synuclein Ttoxicity in the early secretory pathway: How it drives nneurodegeneration in Parkinson’s Disease. Front. Neurosci. 2015;9:433. doi: 10.3389/fnins.2015.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waxman E.A., Giasson B.I. Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim. Biophys. Acta. 2009;1792(7):616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bendor J.T., Logan T.P., Edwards R.H. The function of α-synuclein. Neuron. 2013;79(6):1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mercado G., Castillo V., Vidal R., Hetz C. ER proteostasis disturbances in Parkinson’s disease: novel insights. 2015. [DOI] [PMC free article] [PubMed]
- 89.Mullin S., Schapira A. α-Synuclein and mitochondrial dysfunction in Parkinson’s disease. Mol. Neurobiol. 2013;47(2):587–597. doi: 10.1007/s12035-013-8394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Norris K.L., Hao R., Chen L.F., Lai C.H., Kapur M., Shaughnessy P.J., Chou D., Yan J., Taylor J.P., Engelender S., West A.E., Lim K.L., Yao T.P. Convergence of Parkin, PINK1, and α-Synuclein on stress-induced mitochondrial morphology remodeling. J. Biol. Chem. 2015;290(22):13862–13874. doi: 10.1074/jbc.M114.634063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deas E., Wood N.W., Plun-Favreau H. Mitophagy and Parkinson’s disease: the PINK1-parkin link. Biochim. Biophys. Acta. 2011;1813(4):622–633. doi: 10.1016/j.bbamcr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang B., Abraham N., Gao G., Yang Q. Dysregulation of autophagy and mitochondrial function in Parkinson’s disease. Transl. Neurodegener. 2016;5:19. doi: 10.1186/s40035-016-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M.R., Südhof T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burré J., Sharma M., Südhof T.C. α-Synuclein assembles into higher order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA. 2014;111(40):4274–4283. doi: 10.1073/pnas.1416598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chandra S., Gallardo G., Fernández-Chacón R., Schülter O.M., Südhof T.C. alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 96.Burré J., Sharma M., Südhof T.C. Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. 2012. [DOI] [PMC free article] [PubMed]
- 97.Zhu M., Li J., Fink A.L. The association of alpha-synuclein with membranes affects bilayer structure, stability and fibril formation. J. Biol. Chem. 2003;278(41):40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 98.Tsigelny I.F., Sharikov Y., Wrasidlo W., Gonzalez T., Desplats P.A., Crews L., Spencer B., Masliah E. Role of α-Synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J. 2012;279(6):1000–1013. doi: 10.1111/j.1742-4658.2012.08489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tosatto L., Andrighetti A.O., Plotegher N., Antonini V., Tessari I., Ricci L., Bubacco L., Dalla S.M. Alpha-synuclein pore forming activity upon membrane association. Biochim. Biophys. Acta. 2012;1818(11):2876–2883. doi: 10.1016/j.bbamem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Ouberai M.M., Wang J., Swann M.J., Galvagnion C., Guilliams T., Dobson C.M., Welland M.E. α-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodeling. J. Biol. Chem. 2013;288(29):20883–20895. doi: 10.1074/jbc.M113.478297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pacheco C.R., Morales C.N., Ramírez A.E., Muñoz F.J., Gallegos S.S., Caviedes P.A., Aguayo L.G., Opazo C.M. Extracellular α-synuclein alters synaptic transmission in brain neurons by perforating the neuronal plasma membrane. J. Neurochem. 2015;132(6):731–741. doi: 10.1111/jnc.13060. [DOI] [PubMed] [Google Scholar]
- 102.Rcom-H’cheo-Gauthier A., Goodwin J., Pountney D.L. Interactions between calcium and alpha-synuclein in neurodegeneration. Biomolecules. 2014;4(3):795–811. doi: 10.3390/biom4030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Snead D., Eliezer D. Alpha-synuclein function and dysfunction on cellular membranes. Exp. Neurobiol. 2014;23(4):292–313. doi: 10.5607/en.2014.23.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elkon H., Don J., Melamed E., Ziv I., Shirvan A., Offen D. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J. Mol. Neurosci. 2002;18(3):229–238. doi: 10.1385/JMN:18:3:229. [DOI] [PubMed] [Google Scholar]
- 105.Rostovtseva T.K., Gurnev P.A., Protchenko O., Hoogerheide D.P., Yap T.L., Philpott C.C., Lee J.C., Bezrukov S.M. 2015. [DOI] [PMC free article] [PubMed]
- 106.Di Maio R., Barrett P.J., Hoffman E.K., Barrett C.W., Zharikov A., Borah A., Hu X., McCoy J., Chu C.T., Burton E.A., Hastings T.G., Greenamyre J.T. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 2016;8(342):342–378. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Betzer C., Movius A.J., Shi M., Gai W.P., Zhang J., Jensen P.H. Identification of synaptosomal proteins binding to monomeric and oligomeric α-synuclein. PLoS One. 2015;10(2):e0116473. doi: 10.1371/journal.pone.0116473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jankovic J., Aguilar L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008;4(4):743–757. doi: 10.2147/NDT.S2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ribarič S. The pharmacological properties and therapeutic use of apomorphine. Molecules. 2012;17(5):5289–5309. doi: 10.3390/molecules17055289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mehanna R., Lai E.C. Deep brain stimulation in Parkinson’s disease. Transl. Neurodegener. 2013;2(1):22. doi: 10.1186/2047-9158-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tekriwal A., Baltuch G. Deep brain stimulation: expanding applications. Neurol. Med. Chir. (Tokyo) 2015;55(12):861–877. doi: 10.2176/nmc.ra.2015-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McIntyre C.C., Hahn P.J. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol. Dis. 2010;38(3):329–337. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vitek J.L. Mechanisms of deep brain stimulation: excitation or inhibition. Mov. Disord. 2002;17(3):69–72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 114.Barker R.A., Drouin-Ouellet J., Parmar M. Cell-based therapies for Parkinson’s disease-past insights and future potential. Nat. Rev. Neurol. 2015;11(9):492–503. doi: 10.1038/nrneurol.2015.123. [DOI] [PubMed] [Google Scholar]
- 115.Tabar V.S. The Development of human embryonic stem cell-derived dopamine neurons for clinical use in Parkinson’s Disease. Neurosurgery. 2016;63(Suppl. 1):154–155. doi: 10.1227/01.neu.0000489703.68466.c4. [DOI] [Google Scholar]
- 116.Youdim M.B., Stephenson G., Ben S.D. Ironing out iron in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann. N. Y. Acad. Sci. 2004;1012:306–325. doi: 10.1196/annals.1306.025. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X., Xie W., Qu S., Pan T., Wang X., Le W. Neuroprotection by iron chelator against proteasome inhibitor-induced nigral degeneration. 2005. [DOI] [PubMed]
- 118.Jiang H., Luan Z., Wang J., Xie J. Neuroprotective effects of iron chelator Desferal on dopaminergic neurons in the substantia nigra of rats with iron overload. Neurochem. Int. 2006;49(6):605–609. doi: 10.1016/j.neuint.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 119.Xu K., Kanthasamy A.G., Reddy M.B. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology. 2008;245(1-2):101–108. doi: 10.1016/j.tox.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 120.Aguirre P., Mena N.P., Carrasco C.M., Muñoz Y., Pérez-Henriquez P., Morales R.A., Cassels B.K., Méndez-Galvez C., Garcia-Beltran O., Gonzalez-Billault C., Nüñez M.T. 2015 doi: 10.1371/journal.pone.0144848. https://doi.org/10 [DOI] [PMC free article] [PubMed]
- 121.Martin-Bastida A., Ward R.J., Newbould R., Piccini P., Sharp D., Kabba C., Patel M.C., Spino M., Connelly J., Tricta F., Crichton R.R., Dexter D.T. Brain iron chelation by Deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 2017;7(1):1398. doi: 10.1038/s41598-017-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim Y.S., Alarcon S.V., Lee S., Lee M.J., Giaccone G., Neckers L., Trepel J.B. Update on Hsp90 inhibitors in clinical trial. Curr. Top. Med. Chem. 2009;9(15):1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zou J., Guo Y., Guettouche T., Smith D.F., Voellmy R. 1998 doi: 10.1016/s0092-8674(00)81588-3. http://dx.doi.org/10 [DOI] [PubMed]
- 124.Putcha P., Danzer K.M., Kranich L.R., Scott A., Silinski M., Mabbett S., Hicks C.D., Veal J.M., Steed P.M., Hyman B.T., McLean P.J. Brain-permeable small-molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein induced toxicity. J. Pharmacol. Exp. Ther. 2010;332(3):849–857. doi: 10.1124/jpet.109.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cleren C., Calingasan N.Y., Chen J., Beal M.F. Celastrol protects against MPTP and 3-nitropropionic acid-induced neurotoxicity. J. Neurochem. 2005;94(4):995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- 126.Monti B., Gatta V., Piretti F., Raffaelli S.S., Virgili M., Contestabile A. Valproic acid is neuroprotective in the rotenone rat model of Parkinson’s disease: involvement of alpha-synuclein. Neurotox. Res. 2010;17(2):130–141. doi: 10.1007/s12640-009-9090-5. [DOI] [PubMed] [Google Scholar]
- 127.Pan T., Li X., Xie W., Jankovic L., Le W. Valproic acid-mediated Hsp70 induction and anti-apoptotic neuroprotection in SHSY5Y cells. FEBS Lett. 2005;579(30):6716–6720. doi: 10.1016/j.febslet.2005.10.067. [126]. [DOI] [PubMed] [Google Scholar]
- 128.Dimant H., Ebrahimi-Fakhari D., McLean P.J. Molecular chaperones and co-chaperones in Parkinson’s disease. Neuroscientist. 2012;18(16):589–601. doi: 10.1177/1073858412441372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Migdalska-Richards A., Daly L., Bezard E., Schapira A.H. Ambroxol effects in glucocerebrosidase and α-synuclein transgenic mice. Ann. Neurol. 2016;80(5):766–775. doi: 10.1002/ana.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McNeill A., Magalhaes J., Shen C., Chau K.Y., Hughes D., Mehta A., Foltynie T., Cooper J.M., Abramov A.Y., Gegg M., Schapira A.H. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. 2014 doi: 10.1093/brain/awu020. https://doi.org/10 [DOI] [PMC free article] [PubMed]
- 131.Albani D., Polito L., Batelli S., De Mauro S., Fracasso C., Martelli G., Colombo L., Manzoni C., Salmona M., Caccia S., Negro A., Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. 2009;110(5):1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 132.Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem. 2007;282(8):5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 133.Katsiki M., Chondrogianni N., Chinou I., Rivett A.J., Gonos E.S. The olive constituent oleuropein exhibits proteasome-stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007;10(2):157–172. doi: 10.1089/rej.2006.0513. [DOI] [PubMed] [Google Scholar]
- 134.Hebron M.L., Lonskaya I., Moussa C.E. Nilotinib reverses loss of dopamine neurons and improves motor behaviour via autophagic degradation of α-synuclein in Parkinson’s disease models. Hum. Mol. Genet. 2013;22(16):3315–3328. doi: 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hӧllerhage M., Goebel J.N., de Andrade A., Hildebrandt T., Dolga A., Culmsee C., Oertel W.H., Hengerer B., Hӧglinger G.U. Trifluoperazine rescues human dopaminergic cells from wild-type α-synuclein-induced toxicity. Neurobiol. Aging. 2014;35(7):1700–1711. doi: 10.1016/j.neurobiolaging.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 136.Schrader T., Bitan G., Klärner F.G. 2016. [DOI] [PMC free article] [PubMed]
- 137.Prabhudesai S., Sinha S., Attar A., Kotagiri A., Fitzmaurice A.G., Lakshmanan R., Ivanova M.I., Loo J.A., Klärner F.G., Schrader T., Stahl M., Bitan G., Bronstein J.M. A novel “molecular tweezer” inhibitor of α-synuclein neurotoxicity in vitro and in vivo. Neurotherapeutics. 2012;9(2):464–476. doi: 10.1007/s13311-012-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Acharya S., Safaie B.M., Wongkongkathep P., Ivanova M.I., Attar A., Klärner F.G., Schrader T., Loo J.A., Bitan G., Lapidus L.J. Molecular basis for preventing α-synuclein aggregation by a molecular tweezer. J. Biol. Chem. 2014;289(15):10727–10737. doi: 10.1074/jbc.M113.524520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gouarné C., Tracz J., Paoli M.G., Deluca V., Seimandi M. [DOI] [PMC free article] [PubMed]; Tardif G., Xilouri M., Stefanis L., Bordet T., Pruss R.M. Protective role of olesoxime against wild-type α-synuclein-induced toxicity in human neuronally differentiated SHSY-5Y cells. Br. J. Pharmacol. 2015;172(1):235–245. doi: 10.1111/bph.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sampaio-Marques B., Felgueiras C., Silva A., Rodrigues M., Tenreiro S., Franssens V., Reichert A.S., Outerio T.F., Winderickx J., Ludovico P. SNCA (α-synuclein)-induced toxicity in yeast cells is dependent on sirtuin 2 (Sir2)-mediated mitophagy. Autophagy. 2012;8(10):1494–1509. doi: 10.4161/auto.21275. [DOI] [PubMed] [Google Scholar]
- 141.Outeiro T.F., Kontopoulos E., Altmann S.M., Kufareva I., Strathearn K.E., Amore A.M., Volk C.B., Maxwell M.M., Rochet J.C., McLean P.J., Young A.B., Abagyan R., Feany M.B., Hyman B.T., Kazantsev A.G. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317(5837):516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 142.Su L.J., Auluck P.K., Outeiro T.F., Yeger-Lotem E., Kritzer J.A., Tardiff D.F., Strathearn K.E., Liu F., Cao S., Hamamichi S., Hill K.J., Caldwell K.A., Bell G.W., Fraenkel E., Cooper A.A., Caldwell G.A., McCaffery J.M., Rochet J.C., Lindquist S. Compounds from an unbiased chemical screen reverse both ER-to-Golgi trafficking defects and mitochondrial dysfunction in Parkinson’s disease models. Dis. Model. Mech. 2010;3(3-4):194–208. doi: 10.1242/dmm.004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cooper A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F., Cao S., Caldwell K.A., Caldwell G.A., Marsischky G., Kolodner R.D., Labaer J., Rochet J.C., Bonini N.M., Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s Models. Science. 2006;313(5785):324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mercado G., Valdés P., Hetz C. An ERcentric view of Parkinson’s disease. 2013. [DOI] [PubMed]
- 145.Tóth G., Gardai S.J., Zago W., Bertoncini C.W., Cremades N., Roy S.L., Tambe M.A., Rochet J.C., Galvagnion C., Skibinski G., Finkbeiner S., Bova M., Regnstrom K., Chiou S.S., Johnston J., Callaway K., Anderson J.P., Jobling M.F., Buell A.K., Yednock T.A., Knowles T.P., Vendruscolo M., Christodoulou J., Dobson C.M., Schenk D., McConlogue L. Targeting the intrinsically disordered structural ensemble of α-synuclein by small molecules as a potential therapeutic strategy for Parkinson’s disease. PLoS One. 2014;9(2):e87133. doi: 10.1371/journal.pone.0087133. [DOI] [PMC free article] [PubMed] [Google Scholar]