Abstract
The Lin−c-Kit+ Sca-1+ cell population in the bone marrow (BM) serves as the direct precursor for differentiation of myeloid cells. In this study, we report that deficiency in Fpr2, a G protein–coupled chemoattractant receptor in mice, is associated with reduced BM nucleated cells, including CD31+Ly6C+ (granulocytes and monocytes), CD31−/Ly6Cint (granuloid cells), and CD31−/Ly6Chigh (predominantly monocytes) cells. In particular, the number of Lin−c-Kit+Sca-1+ (LKS) cells was reduced in Fpr2−/− mouse BM. This was supported by observations of the reduced incorporation of intraperitoneally injected bromodeoxyuridine by cells in the c-Kit+ population from Fpr2−/− mouse BM. Purified c-Kit+ cells from Fpr2−/− mice showed reduced expansion when cultured in vitro with stem cell factor (SCF). SCF/c-Kit-mediated phosphorylation of P38, STAT1, Akt (Thr-308), and Akt (Ser-473) was also significantly reduced in c-Kit+ cells from Fpr2−/− mice. Furthermore, Fpr2 agonists enhanced SCF-induced proliferation of c-Kit+ cells. Colony-forming unit assays revealed that CFU–granulocyte–macrophage formation of BM cells from Fpr2−/− mice was significantly reduced. After heat-inactivated bacterial stimulation in the airway, the expansion of c-kit+ Sca-1+ cells in BM and recruitment of Ly6G+ cells to the lungs and CD11b+Ly6C+TNFα+ cells to the spleen of Fpr2−/− mice was significantly reduced. These results demonstrate an important role for Fpr2 in the development of myeloid lineage precursors in mouse BM.
Keywords: mouse, bone marrow, myeloid cell, proliferation, cytokine, c-KIT, Fpr2, Lin−c-Kit+Sca1+cells, SCF
Introduction
Hematopoietic stem cells (HSCs)2 are able to self-renew and generate all lineages of the hematopoietic system (1). In the mouse model, they are enriched in a population that does not express markers of mature hematopoietic cells (i.e. lineage-negative (Lin−) cells) and expresses Sca1 and c-kit (Lin−c-Kit+Sca-1+ (LKS) cells) (2, 3). The interaction of the receptor tyrosine kinase c-kit with its ligand stem cell factor (SCF) sustains HSC survival, proliferation, adhesion, homing, activation, and multipotent differentiation (4–6). Thus, c-kit expression is also the phenotypic hallmark of HSCs and early hematopoietic progenitors (3, 7, 8).
Accumulating evidence has shown that hematopoietic activity in BM is altered during bacterial infection toward production of granulocytes, which is critical for enhancing host defense (9–12). In response to bacterial infection, BM generation of granulocytes or polymorphonuclear leukocytes (PMNs) from their precursors is accelerated to constitute the first line of phagocytic defense in the systemic circulation (13). By investigating the hematopoietic precursor cell response to bacterial infection, the Lin−c-Kit+Sca-1+ cell population in BM was found to constitute a key component of the host defense response to bacteremia (13). The BM Lin−c-Kit+Sca-1+ cell population is rapidly expanded following Escherichia coli bacteremia, and these cells in the expanded marrow Lin−c-Kit+Sca-1+ cell pool are functionally activated for CFU-GM formation. Moreover, mobilization of Lin−c-kit+Sca-1+ cells into the circulation was significantly enhanced from 12 to 48 h after initiation of bacteremia (13).
Formylpeptide receptors (FPRs) belong to the G protein–coupled receptor (GPCR) family and are increasingly recognized as important mediators in inflammatory and immune responses (14, 15). The human FPRs consists of three members: FPR1, FPR2, and FPR3 (14, 15). FPR1 is a high-affinity receptor for the bacterial and mitochondrial formylpeptides and mediates phagocyte chemotaxis and activation. FPR1 also mediates the leukocyte chemotactic activity of a host-derived neutrophil granule protein, cathepsin G (16). In vivo, FPR1 plays a role in host defense against infection by Listeria monocytogenes, as shown by evidence obtained with mice deficient in the FPR1 homolog Fpr1 (17). FPR2 and its mouse counterpart Fpr2 are low-affinity receptors for bacterial formylmethionine-leucyl-phenylalanine (fMLF), but they interact with a greater number of endogenous chemotactic agonist peptides released during inflammatory and immune responses (15). Similar to Fpr1, Fpr2 plays an essential role in mouse resistance against Listeria infection (17, 18). FPR2 and Fpr2 have been reported to also recognize the N-terminal peptides of Annexin I (Anx-A1) that trigger anti-inflammatory responses (19, 20). FPR3 in humans recognizes a chemotactic peptide fragment derived from heme-binding protein that chemoattracts dendritic cells (DCs) (21). In mice, Fpr2 is likely a receptor that functions as human FPR2 and FPR3 (17, 22).
Recently, FPR2 (Fpr2) has been implicated in a number of pathophysiological processes, including severely reduced allergic airway inflammation in Fpr2−/− mice (23). This is associated with a significant reduction in the recruitment of Ly6C+/CD11c+ inflammatory DCs into the bronchiolar regions, which is mediated by an endogenous Fpr2 ligand, CRAMP (24). We also found that the autocrine interaction between Fpr2 and CRAMP promotes normal DC maturation in response to Toll-like receptor (TLR) agonists (25). In addition, FPR2 in mice (Fpr2) promotes M1 macrophage polarization and antitumor host responses (26). These results suggest that Fpr2, in addition to mediating leukocyte chemotaxis, may also participate in the fundamental process of myeloid cell development. We therefore investigated the capacity of Fpr2 and its ligands to affect the c-kit/SCF–mediated generation and expansion of Lin−c-Kit+Sca-1+ cells. We also investigated the expansion of Lin−c-Kit+Sca-1+ cells and accumulation of Ly6G+ cells in the lungs and CD11b+Ly6C+TNFα+ cells in the spleen after the airway was stimulated with killed L. monocytogenes. Here we report defective generation of Lin−c-Kit+Sca-1+ cells and CFU-GM formation in the BM of Fpr2−/− mice and an additive effect of Fpr2 agonists and SCF in inducing the proliferation of c-Kit+ cells. The expansion of Lin−c-Kit+Sca-1+ cells in the BM and accumulation of Ly6G+ cells in the lungs and CD11b+Ly6C+TNFα+ cells in the spleen of Fpr2−/− mice were significantly reduced after acute inflammatory stimulation in the airway.
Results
Reduction of BM nucleated cells and myeloid progenitors in Fpr2−/− mice
We first examined the number of BM nucleated cells in mice and found that the total number of both populations from Fpr2−/− mice at the age of 3 and 6 weeks was significantly reduced compared with WT littermates (Fig. 1, A and B). As shown in Fig. 1, C and D, the BM contains six morphologically and phenotypically distinct cell subsets based on ER-MP12/CD31 and ER-MP20/Ly-6C expression (23). Fpr2 deficiency was associated with reduced myeloid progenitor cells in the BM, including CD31+/Ly6C+ (granulocytes and monocytes), CD31−/Ly6Chigh (predominantly monocytes), and CD31−/Ly6Cint (granuloid cells) populations (Fig. 1D and Fig. S1, A and B). These results suggest a potentially important role of Fpr2 in normal myelopoiesis.
Figure 1.
Reduction of BM nucleated cells and progenitor subpopulations in Fpr2−/−mice. A and B, reduction of the total number of BM nucleated cells in Fpr2−/− mice. BMCs from the femora of male mice at the age of 3 or 6 weeks (WK) were obtained and counted. A, the mean number of BMCs from two femora of each male mouse at the age of 3 weeks. n = 11–13 mice/group. *, p < 0.05. B, the number of BMCs from two femora of each male mouse at the age of 6 weeks. n = 9–10 mice/group. *, p < 0.05. C and D, reduction of BM progenitor cells in Fpr2−/− mice. BMCs from male mice at the age of 3 weeks were preincubated in FACS buffer with anti-mouse CD16/32 mAb for 20 min at 4 °C to eliminate nonspecific Ab binding to FcγII/IIIR. The cells were then stained with anti-mouse Ly6C-PE and CD31-FITC Abs and analyzed by FACS. C, two-color FACS analysis of CD31 and Ly6C expression by total BM cells. D, six phenotypically distinct subpopulations in the BM of WT and Fpr2−/− mice. *, p < 0.05, significantly reduced cell number in the BM of Fpr2−/− mice. n = 4 mice/group with three independent experiments.
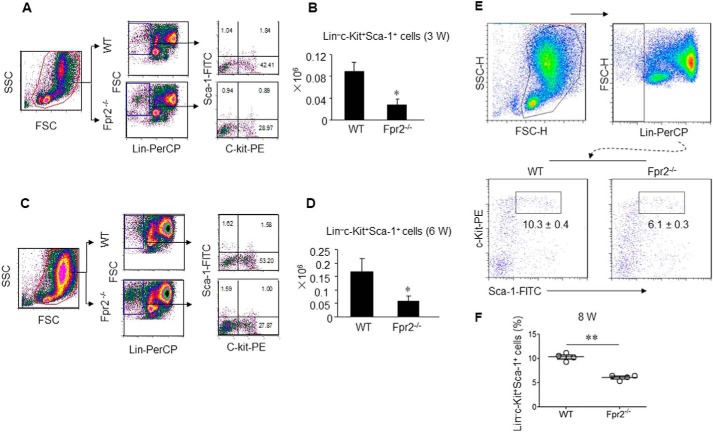
Reduced KLS HSC in Fpr2−/− mouse BM
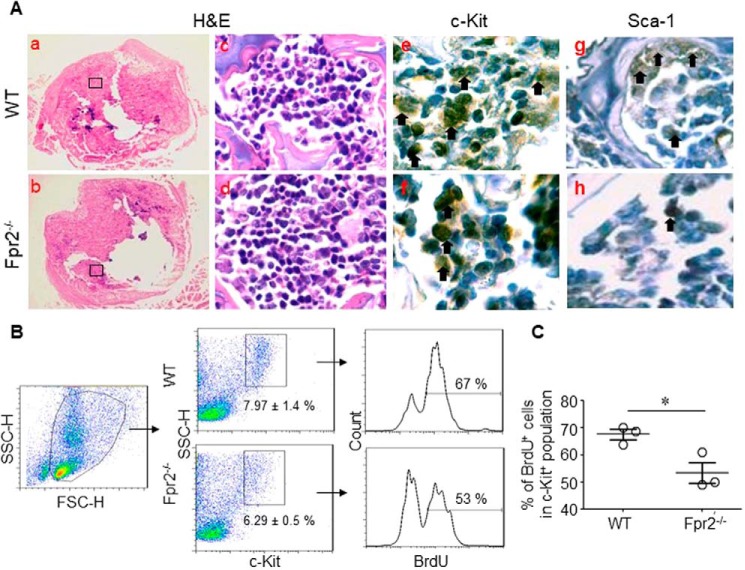
HSCs expressing the SCF receptor c-Kit can self-renew and generate all lineages of the hematopoietic system (1). Because mutations in genes for SCF or c-Kit result in impaired HSC self-renewal and differentiation (24, 25), we examined the state of the KLS HSC-enriched population in the BM. The proportion of the KLS population in 3-week-old (Fig. 2, A and B), 6-week-old (Fig. 2, C and D), and 8-week-old (Fig. 2, E and F) Fpr2−/− mouse BM was significantly reduced, as measured by flow cytometry. The reduction was confirmed by immunohistology results showing that c-Kit+ and Sca-1+ cells in Fpr2−/− mouse BM were diminished (Fig. 3A).
Figure 2.
Reduced number of Lin−c-Kit+Sca-1+ cells in BM of Fpr2−/− mice. BMCs from male mice at the age of 3, 6, or 8 weeks were stained with anti-mouse Sca-1–PE, c-Kit–FITC, and Lin-PerCP Abs and analyzed by FACS. A and B, cumulative results for Lin−c-Kit+Sca-1+ cells in the BM of mice at the age of 3 weeks. C and D, cumulative results for Lin−c-Kit+Sca-1+ cells in the BM of mice at the age of 6 weeks. E and F, cumulative results for Lin−c-Kit+Sca-1+ cells in the BM of mice at the age of 8 weeks. Results are expressed as the mean ± S.E. *, p < 0.05; **, p < 0.01. n = 4–6 mice/group with three independent experiments. FSC, forward-scattered light; SSC, side-scattered light; FSC-H, FSC–height; SSC-H, SSC–height.
Figure 3.
Reduced proliferation of c-Kit+ or Sca-1+ cells in BM of Fpr2−/− mice. A, immunohistochemical staining for c-Kit+ or Sca-1+ cells in BM. Femur sections from mice at the age of 3 weeks were stained for c-Kit or Sca-1. Original magnification, ×400. Parts of c-Kit+ or Sca-1+ cells are denoted by arrows. H&E, hematoxylin and eosin. B and C, c-Kit+ cell proliferation in BM. Mice were i.p. injected with 1 ml of BrdU (1 mg/ml). BM was harvested after 24 h, and BrdU+ cells were detected by incubation with biotin-conjugated anti-BrdU mAb, followed by streptavidin-FITC and anti-mouse c-Kit–PE Ab. B, representative frequency of BrdU+ cells in the c-Kit+ cell population. C, cumulative results for BrdU+ cells in the c-Kit+ cell population. Results are expressed as the mean ± S.E. n = 3 mice/group with three independent experiments. *, p < 0.05. FSC-H, forward-scattered light–height; SSC-H, side-scattered light–height.
Reduced proliferation of Fpr2−/− mouse c-Kit+ cells
To verify that the reduced number of c-Kit+ cells in Fpr2−/− mouse BM was due to decreased proliferation of the population, we i.p. injected BrdU into mice and analyzed BrdU-labeled c-Kit+ cells from mouse BM 24 h later. We found that there was reduced expansion of the c-Kit+ population from BM of Fpr2−/− mice compared with WT littermates (Fig. 3, B and C).
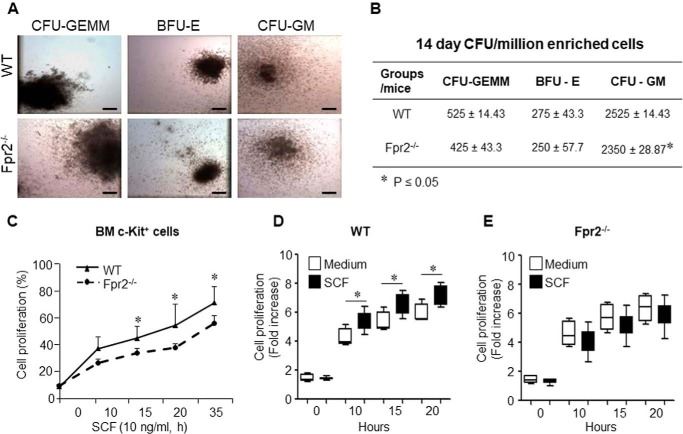
Lin−c-kit+Sca-1+ cells isolated from mice exhibited a significant increase in CFU activity when cultured on Methocult GF M3434 after stimulation with E. coli. This increase in CFU formation resulted essentially from an increase in CFU-GM (including CFU-G and CFU-M) activity of these cells (13). We assayed CFU formation with BM cells isolated from Fpr2−/− and WT mice and found that CFU-GM formation was significantly reduced in BM cells from Fpr2−/− mice compared with WT mouse cells (Fig. 4, A and B). The change of BFU-E and CFU-GEMM was not statistically significant (Fig. 4, A and B). These results suggest that Fpr2 is required for the proliferation of Lin−c-kit+Sca-1+ cells.
Figure 4.
Reduced CFU-GM formation and proliferation of c-Kit+ cells induced by SCF in Fpr2−/− mice. A and B, CFU assays. BM cells from WT and Fpr2−/− mice were treated with the EasySep® mouse hematopoietic progenitor cell enrichment mixture. The enriched cells were cultured in MethoCult® M3434 medium. BFU-E, CFU-GM, and CFU-GEMM were scored, and representative photographs were taken on day 14. A, representative photographs for CFU-GEMM, BFU-E, and CFU-GM from WT and Fpr2−/− mouse BM. Scale bars = 30 μm. B, cumulative results for CFU-GEMM, BFU-E, and CFU-GM from WT and Fpr2−/− mice. C–E, BM cells were harvested, and c-Kit+ cells were isolated by incubation with anti-mouse c-Kit-FITC Ab, followed by anti-FITC MicroBeads (Miltenyi Biotec). c-Kit+ cells were then triple-seeded in 96-well plates in 100 μl of RPMI 1640 (5 × 104/ml) in the presence or absence of SCF (10 ng/ml). alamarBlue was added (10 μl/well) for measurement at 570 nm and 600 nm at the indicated time points. C, c-Kit+ cell proliferation stimulated by SCF. *, p < 0.05. D, proliferation of c-Kit+ cells from WT mice in the presence or absence of SCF (10 ng/ml). *, p < 0.05. E, the proliferation of c-Kit+ cells from Fpr2−/− mice in the presence or absence of SCF (10 ng/ml). *, p < 0.05. c-Kit+ cells isolated from 10 mice/group were pooled with three independent experiments.
In vitro, SCF-induced proliferation of c-Kit+ cells isolated from Fpr2−/− mouse BM was significantly reduced compared with the cells from WT littermates (Fig. 4C). In addition, SCF potently stimulated the proliferation of c-Kit+ cells from WT mouse BM (Fig. 4D) at different time points tested. In contrast, SCF failed to stimulate the proliferation of c-Kit+ cells isolated from Fpr2−/− mouse BM (Fig. 4E). These results suggest that Fpr2 is required for SCF signaling in the proliferation of Lin−c-kit+Sca-1+ cells via stimulation of c-Kit.
Fpr2 agonists enhance the activity of SCF on c-Kit+ cell proliferation
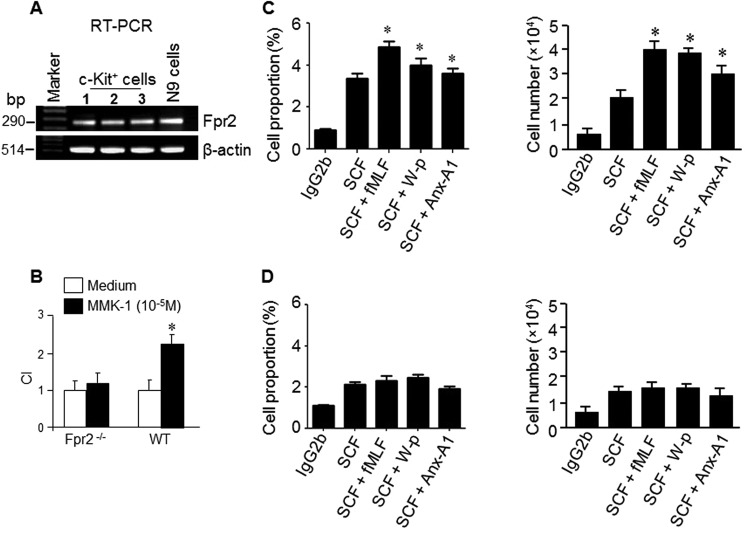
The unexpected consequence of Fpr2 deficiency on c-Kit+ myeloid progenitor cell expansion motivated us to examine the function of Fpr2 on c-Kit+ myeloid progenitor cells. c-Kit+ myeloid progenitor cells from WT mice expressed Fpr2 mRNA (Fig. 5A). More than 90% of Lin−c-Kit+ cells expressed Fpr2 (Fig. S2A) on the cell surface (Fig. S2B), and these cells migrated in response to the Fpr2 ligand MMK-1 (22) (Fig. 5B). We then examined the capacity of Fpr2 ligands to promote SCF-induced proliferation of c-Kit+ cells. Many Fpr2 ligands, including fMLF, W-p, and Anx-A1, enhanced the capacity of SCF to stimulate the proliferation of c-Kit+ cells from WT mice (Fig. 5C) despite their inability to directly stimulate c-Kit+ cell proliferation (data not shown). Fpr2 agonists failed to enhance the stimulatory activity of SCF on c-Kit+ cells from Fpr2−/− mice (Fig. 5D). Another Fpr2 ligand, MMK-1, also enhanced SCF-mediated proliferation of c-Kit+ cells from WT mouse BM. The effect of MMK-1 was attenuated by the Fpr2 antagonist WRW4 (Fig. S3A). In contrast, MMK-1 failed to enhance SCF-mediated proliferation of c-Kit+ cells from BM of Fpr2−/− mice. (Fig. S3B). We also found that combination of Fpr2 ligands (W-peptide and fMLF) with SCF improved the survival of BM nucleated cells (data not shown). It is interesting to note that the expression of the prototype Fpr, Fpr1, was reduced in Lin−c-Kit+ cells from Fpr2−/− mouse BM compared with WT mouse cells, suggesting that Fpr2 deficiency impairs the expression of Fpr1 (Fig. S4, A–C). The reason for this impairment is under further investigation. SCF improved the responses of c-Kit+ cells and neutrophils to the Fpr2 ligand MMK-1 (Fig. S5, A and B) and fMLF (Fig. S6, A and B). These results indicate that the cooperation of Fpr2 and SCF plays an important role in maintaining the normal function of c-Kit+ myeloid progenitor cells.
Figure 5.
Fpr2 expression by c-Kit+ cells and enhancement of SCF-induced proliferation of c-Kit+ cells. A, Fpr2 mRNA expression in c-Kit+ cells. n = 3 mice/group. B, the migration of c-Kit+ cells in response to the Fpr2 ligand MMK-1. c-Kit+ cells from 10 mice/group were used, and the experiments were performed three times. *, p < 0.05. C and D, synergism between Fpr2 agonists and SCF in stimulating c-Kit+ cell proliferation. BM nucleated cells from WT or Fpr2−/− mice were labeled with CFSE (10 μm) and then divided into five groups: control (IgG), SCF (100 ng/ml), SCF (100 ng/ml) + fMLF (10−5 m), SCF (100 ng/ml) + W-peptide (W-p, 10−6 m), and SCF (100 ng/ml) + Anx-A1 (20 μg/ml). The cells were cultured at 37 °C for 72 h and then analyzed by FACS. C, the proliferation of c-Kit+ cells from WT mice. Left panel, the percentage of c-Kit+CFSE+ cells from WT mice. Right panel, the number of c-Kit+CFSE+ cells from WT mice. *, p < 0.05; significantly increased cell number in the presence of Fpr2 agonists compared with SCF alone. D, the proliferation of c-Kit+ cells from Fpr2−/− mice. Left panel, the percentage of c-Kit+CFSE+ cells from Fpr2−/− mice. Right panel, the number of c-Kit+CFSE+ cells from Fpr2−/− mice. c-Kit+ cells isolated from 10 mice/group were pooled for experiments, which were performed three times.
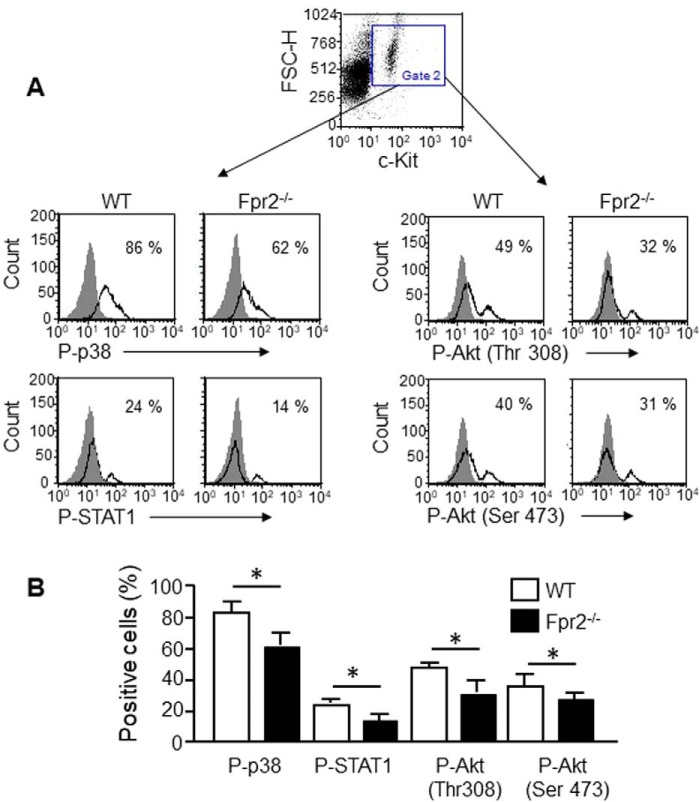
Reduction of P38, STAT1, Akt (Thr-308), and Akt (Ser-473) phosphorylation induced by SCF in Fpr2−/− mouse c-Kit+ cells
SCF/c-Kit signals through phosphatidylinositol 3-kinase (PI3K)/Akt, which regulates gene transcription, proliferation, survival, and metabolic homeostasis of c-Kit+ cells (26). Activation of PI3K results in the phosphorylation of the serine–threonine kinase Akt, an important mediator of cell growth, survival, and migration. We thus measured the phosphorylation of P38, STAT1, Akt (Thr-308), and Akt (Ser-473) in c-Kit+ cells from Fpr2−/− mice in response to SCF. Compared with c-Kit+ cells from WT mice, the percentage of cells with P38, STAT1, and Akt phosphorylation was reduced in Fpr2−/− mice after stimulation with SCF (Fig. 6, A and B), although mean fluorescence intensity (MFI) shown by cells from Fpr2−/− mice was not significantly different compared with cells from WT mice (data not shown). The Fpr2 antagonist WRW4 reduced SCF-induced phosphorylation of p38 in c-Kit+ cells and neutrophils (Fig. S7A). The JAK3 inhibitor CP-690550 and the JAK2 inhibitor AG-490 attenuated SCF-induced cell proliferation, indicating that JAK–STAT signaling pathway mediates SCF-induced c-Kit+ cell proliferation (Fig. 7B). The Fpr2 antagonist WRW4 also attenuated the phosphorylation of STAT1 in Lin−c-Kit+ cells induced by SCF (Fig. S7C). These results, combined with our previous observation that both c-Kit+ cell proliferation (Fig. 4C) and STAT1 phosphorylation (Fig. 6) were reduced in Fpr2−/− mice, indicate the requirement of Fpr2 for normal signaling of SCF in c-Kit+ HSCs in mouse BM.
Figure 6.
The phosphorylation of P38, STAT1, Akt (Thr-308) and Akt (Ser-473) induced by SCF in c-Kit+ cells. A, SCF-induced phosphorylation. BM nucleated cells were stimulated with SCF (10 ng/ml) for 1 h and then stained with anti-mouse c-Kit-FITC Ab and anti-mouse phosphorylated p-p38, p-Akt (Thr-308), p-Akt (Ser-473), and p-STAT1 Abs as well as matched IgG as a control. The cells were analyzed by FACS. B, cumulative results for the phosphorylation of p38, STAT1, Akt (Thr-308), and Akt (Ser-473) induced by SCF. Results are expressed as the mean ± S.E. n = 3 mice/group, and the experiments were performed three times. *, p < 0.05. FSC-H, forward-scattered light–height.
Reduced accumulation of Gr-1+ cells in the lung and CD11b+Ly6C+TNFα+cells in the spleen of Fpr2−/− mice after stimulation with heat-inactivated bacteria
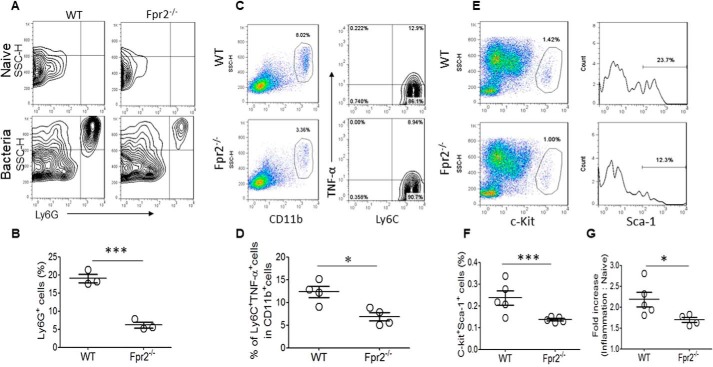
During bacterial infection, granulopoiesis becomes predominant, with inhibition of other lineage (lymphoid and erythroid) development in the BM (9, 10, 27), and the granulocytes (neutrophils or PMNs) egress from the BM to the blood and then to inflamed tissue to constitute the first line of defense. To examine the role of Fpr2 in the expansion of granulocyte–macrophage progenitors, we investigated the accumulation of Ly6G+ cells (PMNs) in the lung and CD11b+Ly6C+TNFα+ cells (inflammatory monocytes or TipDCs) in the spleen of Fpr2−/− mice after stimulation with heat-inactivated bacteria. We found that Ly6G+ neutrophils recruited into the inflamed airway were significantly reduced in Fpr2−/− mice compared with WT mice (Fig. 7, A and B). The infiltration of CD11b+Ly6C+TNFα+ inflammatory monocytes to the spleen was also significantly diminished in Fpr2−/− mice compared with WT mice (Fig. 7, C and D). These results indicate that the capacity of migration of inflammatory cells from the blood to damaged tissue is impaired in Fpr2−/− mice.
Figure 7.
Reduced recruitment of myeloid cells and diminished expansion of Lin−c-Kit+ Sca-1+ cells in Fpr2−/− mice after stimulation with heat-inactivated bacteria. Mice were intranasally administered 100 μl of heat-inactivated Listeria (5 × 108 CFU/ml of PBS) and then euthanized after 24 h to harvest the lungs, spleens, and BM cells. A and B, cumulative results for the frequency of Ly6G+ neutrophils isolated from lung tissue. ***, p < 0.001; significantly reduced frequency of the cells in Fpr2−/− mouse lungs. Results are expressed as the mean ± S.E. n = 3 mice/group. C and D, cumulative results for the frequency of CD11b+Ly6C+TNFα+ monocytes in the spleen. *, p < 0.05; significantly reduced frequency of CD11b+Ly6C+TNFα+ monocytes in Fpr2−/− mouse spleens. E and F, cumulative results for the frequency of c-Kit+Sca-1+ cells in BM. ***, p < 0.001; significantly reduced frequency of the cells in BM of Fpr2−/− mice. *, p < 0.05; significantly reduced expansion of c-Kit+Sca-1+ cells in BM of Fpr2−/− mice. Results are expressed as the mean ± S.E. n = 4 mice/group with three experiments performed. SSC-H, side-scattered light–height.
Reduced proliferation of Lin−c-Kit+Sca-1+ cells in the BM of Fpr2−/− mice after stimulation with heat-inactivated bacteria
Hematopoiesis in normal hosts is tightly regulated to maintain daily blood cell turnover among different lineages (1, 28). After bacterial infection, cells in the Lin−c-Kit+Sca-1+ pool are rapidly expanded in the BM and are activated for granulopoiesis (27). Our previous studies showed that Fpr2 plays an important role in mediating neutrophil migration from the blood to inflamed tissues in bacterial infection and skin wounds (18, 29). To examine the relationship between Lin−c-Kit+Sca-1+ cells and inflammatory cells, we investigated the responses of Lin−c-Kit+Sca-1+ cells in the BM of Fpr2−/− mice after stimulation with heat-inactivated bacteria. We found that the percentage of c-Kit+Sca-1+ cells in the BM of WT mice was significantly higher compared with Fpr2−/− mice (Fig. 7, E and F). In contrast, the -fold increase of c-Kit+Sca-1+ cells in the BM of Fpr2−/− mice was significantly lower compared with WT mice after stimulation with heat-inactivated bacteria (Fig. 7G). We thus conclude that the reduced accumulation of neutrophils in the airway and inflammatory monocytes in the spleen after stimulation with heat-inactivated bacteria is a result of reduced expansion of Lin−c-Kit+Sca-1+ cells in the BM and their reduced capacity to accumulate at inflamed tissues.
Reduced maturation of neutrophils in Fpr2−/− mice
Multiple factors may affect the recruitment of neutrophils to inflamed tissues, including granulopoiesis and differentiation. Because granulocytes are derived from Lin−c-kit+Sca-1+ BM cells, we hypothesized that Fpr2 may also affect the maturation of neutrophils in addition to the number of progenitor cells. We found that the CD11b+Ly6G+ cell population was significantly reduced in the BM of Fpr2−/− mice compared with WT mice (Fig. S8A), and the number of neutrophils recruited into the peritoneal cavity was significantly reduced in Fpr2−/− mice compared with WT mice after i.p. injection with casein (Fig. S8B) (30). Furthermore, the expression of the chemokine receptor CXCR2 was reduced in casein-induced neutrophils from Fpr2−/− mice, and these cells also showed reduced migration in response to the CXCR2 ligand CXCL2 (Fig. S8, C and D). These results indicate that Fpr2 deficiency not only diminishes the number of Lin−c-kit+Sca-1+ progenitor cells but also granulocyte maturation.
Discussion
Fpr2 was originally identified as a chemoattractant receptor that interacts with a variety of pathogen- and host-derived chemotactic ligands to mediate myeloid cell chemotaxis (15, 25, 26, 31, 32, 34, 35). Recently, Fpr2 has been implicated in more fundamental processes, including DC differentiation (25, 36) and homeostasis of the colon epithelial layer (34). Fpr2 and its prototype variant Fpr1 promote osteoblast differentiation (37). Also, Fpr1 and Fpr2 expressed by mesenchymal stem cells promote cell adhesion, migration, and homing to injured tissue for repair (38). In this study, the CD31+/Ly6C+ (granulocytes and monocytes), CD31−/Ly6Chigh (predominantly monocytes), and CD31−/Ly6Cint (granuloid cells) populations were reduced in Fpr2−/− mouse BM but with an increase in the CD31−/Ly6− cell (erythroid cell) population. It has been reported that significant expansion of granulocytes and monocytes inhibits other lineage (such as erythroid) development (9, 11, 12). It is therefore not surprising that the BM erythroid population was increased with a reduction in granulocyte and monocyte populations in Fpr2−/− mice. We also demonstrated that Fpr2 is required for SCF/c-Kit–mediated Lin−c-Kit+Sca-1+ cell proliferation, as shown by the capacity of Fpr2 ligands to enhance SCF/c-Kit signaling in WT mouse cells with significantly reduced expansion of Lin−c-Kit+Sca-1+ cells in the BM of Fpr2 deficient mice.
SCF is a potent hematopoietic growth factor in humans and mice (39–41). In combination with some cytokines, SCF synergistically enhances the proliferation, differentiation, and survival of hematopoietic cells, a process critical for adequate lineage expansion and development (42–52). For example, the chemokine SDF-1 (CXCL12) is constitutively produced by BM stromal cells. Its receptor, CXCR4, is expressed by CD34+ hematopoietic cells, including primitive progenitor cells. However, SDF-1 (CXCL12) alone was not able to stimulate quiescent cells without co-stimulation by SCF and other cytokines (52). SCF/c-Kit and Epo/Epo-R function as key switches for erythropoiesis. SCF/c-Kit are essential for the proliferation and differentiation of BFU-Es to CFU-Es, and Epo/Epo-R promote the survival, proliferation, and terminal differentiation of CFU-Es. SCF synergizes with Epo in regulating the proliferation, differentiation, and apoptosis of erythroid progenitors (53). We found that c-Kit+ HSC cells express Fpr2, but ligands alone did not stimulate c-Kit+ cell proliferation. Instead, Fpr2 ligands significantly enhanced SCF/c-Kit signaling in HSC cells. Therefore, Fpr2 is of considerable importance as a regulator of hematopoiesis in synergy with SCF for BM cells.
Cytokine synergy with SCF in enhancing HSC cell proliferation involves diverse mechanisms. For example, SDF-1 (CXCL12) may transiently desensitize HSCs and protect the cells from damage by the chemotherapeutic agent fluorouracil (5-FU). Alternatively, SDF-1 (CXCL12) may trigger quiescent HSCs to express other cytokine receptors, such as IL-6R, IL-12R, or c-Kit. In fact, SCF was reported to increase the chemotaxis response of HSCs to SDF-1 (CXCL12) (52). SCF and G-CSF also show synergism on HSC cell cycle distribution with a marked shortening of the duration of G0/G1 by substantially decreasing the expression of cdki p27kip-1. Consequently, the loss of p27kip-1 facilitates cell proliferation (54). In this study, we found that the phosphorylation of P38, STAT1, Akt (Thr-308), and Akt (Ser-473) of c-Kit+ cells from Fpr2−/− mouse BM was significantly reduced after stimulation with SCF compared with cells from WT mice. PI3K mediates SCF-induced proliferation of BM-derived mast cells by activating the pathways of the small GTP-binding proteins Rac1 and JNK (55). Therefore, SCF-induced JNK phosphorylation and cell proliferation were down-regulated in PI3K−/− BM-derived mast cells (56). PI3K was also required for the survival and proliferation of early myeloid cells in the BM (57). In this regard, Fpr2 may play a role in promoting SCF-induced signaling and HSC proliferation through PI3K, an issue currently under further investigation.
Similar to Fpr2 ligands fMLF, W-peptide, Anx-A1, and MMK-1, cathelin-related antimicrobial peptide (CRAMP), which is an endogenous ligand for Fpr2, did not directly stimulate c-Kit+ cell proliferation but, rather, enhanced the capacity of SCF to stimulate the proliferation of c-Kit+ cells from WT mouse BM. However, our previous studies showed that the Fpr2 ligands fMLF, MMK-1, and CRAMP directly stimulated the proliferation of normal colon epithelial cells and cancer cell lines (34, 58). Therefore, the response of hematopoietic cells appears different compared with epithelial cells. This was demonstrated by the failure of CRAMP to directly stimulate DC maturation, but it increased the sensibility of immature DCs to lipopolysaccharide-stimulated maturation (25). Therefore, Fpr2 function may depend on cell type. Further study of the involvement of Fpr2 and its endogenous ligands in hematopoiesis by synergy with cytokines should be useful for the potential development of novel therapeutic agents for hematopoietic defects.
Experimental procedures
Animals
The generation of Fpr2−/− mice was described previously (31). Mice used in the experiments were 3–8 weeks old and both male and female. They were allowed free access to standard laboratory chow and tap water. All animals were housed in an air-conditioned room with controlled temperature (22 °C), humidity (65–70%), and day/night cycle (12:12 h light:dark). Animal care was provided in accordance with the procedures outlined in the Guide for Care and Use of Laboratory Animals (National Research Council, 1996). Animal studies were approved by the Animal Care and Use Committee of the NCI-Frederick, National Institutes of Health.
Reagents
FITC-, phycoerythrin (PE)-, and PerCP-Cy5.5–conjugated, affinity-purified rat IgG anti-mouse mAbs against CD16/32, Ly6C, CD31, Sca-1, and c-Kit and PerCP-conjugated antibodies (Abs) indicating the lineage-positive phenotype (CD4, NK, CD8, B220, CD11b, and Gr-1) were from eBiosciences (San Diego, CA). Alexa Fluor 488–conjugated anti-mouse phospho-P38, -JNK, -ERK1/2, -STAT1, -AKT (Thr-308), and -AKT (Ser-473) antibodies were from Cell Signaling Technology (Beverly, MA). SCF was from PeproTech (Rocky Hill, NJ). The Fpr2 agonist peptides MMK-1 and W-peptide (WKYMVm, W-pep) were synthesized at the Department of Biochemistry, Colorado State University (Fort Collins, CO) (33). Anti-FITC MicroBeads were from Miltenyi Biotec Inc. (Auburn CA). alamarBlue® was from Invitrogen. Bromodeoxyuridine (BrdU) was from Pharmingen, and the carboxyfluorescein succinimidyl ester (CFSE) Cell Division Tracker Kit was from Biolegend (San Diego, CA).
Isolation and purification of c-Kit+ mouse BMCs
BMCs from 3- and 6-week-old male mice were obtained by flushing femora with PBS as described previously (25). Red cells were lysed with ACK lysing buffer (Cambrex Bio Science). The number of nucleated cells from two femora per mouse was calculated. For purification of c-Kit+ cells, nucleated cells isolated from BM were incubated with anti-mouse c-Kit–FITC antibody, followed by anti-FITC MicroBeads (Miltenyi Biotec) according to the instruction manual of the manufacturer.
Flow cytometry
The phenotype of murine cells was examined by immunostaining, followed by flow cytometry analysis on a FACSCalibur (BD Biosciences) with CellQuest software. Single-cell suspension was prepared in FACS buffer. Rat CD16/32 was used to block murine Fc receptors. Staining was performed at 4 °C for 30 min. Murine BM cells were triple-stained with PerCP-conjugated antibodies, indicating a lineage-positive phenotype (CD4, NK, CD8, B220, CD11b, or Gr-1), together with anti Sca-1–FITC and anti c-Kit–PE antibodies to determine the levels of c-Kit+/Lin−/Sca-1+ stem cell–enriched populations. The number of c-Kit+/Lin−/Sca-1+ cells in one femur was counted using flow cytometry. In some experiments, progenitor cells were double-stained with FITC-conjugated anti-mouse Ly6C and PE-conjugated anti-mouse CD31 antibodies. For detection of c-Kit+ cell proliferation in vivo, the nucleated cells were stained with anti-mouse c-Kit–FITC and anti-PE–conjugated BrdU (BioLegend) antibodies. To measure c-Kit+ cell proliferation in vitro, the cells were stained with anti-mouse c-Kit–PE. Cell surface c-Kit expression and labeled CFSE were analyzed with flow cytometry.
Chemotaxis assays
Chemotaxis of c-Kit+ cells was measured with 48-well microchambers and polycarbonate filters (5-μm pore size) (NeuroProbe, Cabin John, MD) as described previously (32). Results are expressed as the mean ± S.E. of the chemotaxis index, representing the -fold increase in the number of migrated cells in response to chemoattractants over spontaneous cell migration (to control medium).
RT-PCR
The expression of Fpr2 mRNA in c-Kit+ cells was examined by RT-PCR with primers as follows: 5′-GTGTCCCCTGAATCTGGAAA-3′ (sense) and 5′-TAATTCAGGTGCTGTGGGTG-3′ (antisense), which yield a 290-bp product. Mouse β-actin primers were 5′-TGTGATGGTGGGAATGGGTGAG-3′ (sense) and 5′-TTTGATGTCACGCACGATTTCC-3′ (antisense), which yield a 514-bp product. The RT-PCR was performed as described previously (19). All PCR products were resolved by 1.5% agarose gel electrophoresis and visualized with ethidium bromide staining.
Immunohistology
Femora from mice at the age of 3 weeks were fixed in formalin for 24 h. After decalcification, the femora, embedded in paraffin, were sectioned (5 μm) and stained with hematoxylin and eosin. For immunohistological examination, the sections were stained with anti-mouse c-Kit or Sca-1 antibodies, followed by a biotinylated anti-Ig secondary Ab (BD Biosciences) and streptavidin–horseradish peroxidase/diaminobenzidine (DAB) with hematoxylin counterstaining (Surgipath, Richmond, IL).
Detection of c-Kit+ cell proliferation in vivo
Mice were i.p. injected with 1 ml BrdU (1 mg). After 24 h, bone marrow was harvested, and the incorporation of BrdU was detected by incubation with biotin anti-BrdU mAb, followed by streptavidin-FITC and anti-mouse c-Kit–PE Ab for analysis by FACS.
Assay for c-Kit+ cell proliferation in vitro
c-Kit+ cells were isolated from BM by incubation with anti-mouse c-Kit–FITC antibody, followed by anti-FITC MicroBeads (Miltenyi Biotec). c-Kit+ cells were seeded in 96-well plates with 100 μl of RPMI 1640, 10% fetal calf serum (5 × 104/ml) in the presence or absence of SCF (10 ng/ml). alamarBlue (Invitrogen) was added to the wells (10 μl/well). Absorbance was recorded at 570 nm and 600 nm at the indicated time points. The following equation was used to calculate cell percentage:% = [(ϵOX)λ2Aλ1 − (ϵOX)λ1Aλ2/(ϵRED)λ1A'λ2 − (ϵRED)λ2A'λ1] × 100, where λ1 = 570, λ2 = 600; (ϵOX)λ2 = 117,216; (ϵOX)λ1 = 80,586; (ϵRED)λ1 = 155,677; (ϵRED)λ2 = 14,652; Aλ1, absorbance reading from the test well; Aλ2, absorbance reading from the test well; A'λ2, absorbance reading from the negative control well; A'λ1, absorbance reading from the negative control well.
Measurement of the effect of Fpr2 agonists on SCF-induced proliferation of cKit+ cells in vitro
BM nucleated cells labeled with CFSE were divided into five groups: a, IgG2b group; b, SCF group; c, SCF plus fMLF group; d, SCF plus W-peptide group; and e, SCF plus Anx-A1 group. The cells were cultured at 37 °C for 72 h and analyzed by FACS.
CFU assay
CFU assays of freshly isolated bone marrow were performed using MethoCultTM (Stemcell Technologies, Inc., Seattle, WA). Briefly, nucleated cells were treated with biotinylated antibodies (CD5, CD11b, CD19, CD45R, 7–4, Ly-6G/C (Gr-1), and TER119) (Stemcell Technologies, Seattle, WA). EasySep® mouse progenitor magnetic microparticles were added to segregate unwanted cells. The cell suspension was mixed gently with M3434 medium and dispensed into 35-mm culture dishes that were placed in a 100-mm Petri dish and incubated at 37 °C, 5% CO2, and 95% humidity for 14 days. Burst-forming unit erythroid (early erythroid precursors (BFU-E)), granulocyte–macrophage (CFU-GM), and multipotential progenitor (granulocyte, erythrocyte, monocyte, and megakaryocyte (CFU-GEMM)) were scored, and representative photographs were taken on day 14.
Stimulation with heat-inactivated listeria
Listeria strain EGD was cultured in brain–heart infusion broth and stored in 30% glycerol at −80 °C at a concentration of 5 × 108 CFU/ml. Heat-inactivated Listeria were prepared by incubating the bacteria at 60 °C for 1 h. For airway stimulation, male mice were intranasally given with 100 μl of Listeria suspension (5 × 108 CFU/ml). 24 h later, mice were euthanized by CO2 inhalation. The mouse lung, spleen, and bone marrow were harvested. Lung was digested with triple enzyme mixture solution (10× stock solution: 1 g of collagenase, 100 mg of hyaluronidase, and 20,000 units of DNase in 100 ml of Hanks' balanced salt solution) and treated with ammonium–chloride–potassium (ACK) lysing buffer (Cambrex) to remove erythrocytes. The cell suspension from spleen and bone marrow was treated with ACK to remove erythrocytes. Lung cells and red cell–free blood leukocytes were preincubated in buffer (PBS with 1% fetal calf serum, 5 mm EDTA, and 0.1% NaN3) containing anti-CD16/32 mAb for 20 min at 4 °C to eliminate nonspecific binding of the mAb to the FcγII/IIIR, followed by anti-mouse Ly6G (lung cells), anti-mouse CD11b, Ly6C (spleen cells), and anti-mouse C-kit, Sca-1 (BM cells) antibodies for 30 min at 4 °C. For intracellular staining, the spleen cells were then fixed and permeabilized with BD Cytofix/Cytoperm, resuspended in BD Perm/Wash buffer, and stained with fluorochrome-conjugated anti-TNFα Abs or appropriate negative control Abs. Cell suspension was analyzed with flow cytometry.
Statistical analysis
All experiments were performed at least three times. Representative and reproducible results are shown. Statistical analysis was performed with the Prism software (GraphPad Software, La Jolla, CA). Values are expressed as the means ± S.E. The significance of the differences between testing and control groups was assessed by Student's t test or one-way analysis of variance where appropriate. p < 0.05 was considered statistically significant.
Author contributions
K. C., V. K. S., P. T., Z. B., T. H., Y. X., W. G., T. Y., and X. C. investigation; K. C. writing-original draft; J. M. W. conceptualization; J. M. W. supervision; J. M. W. project administration; J. M. W. writing-review and editing; Y. L. gene knockout; L. T. gene knockout.
Supplementary Material
Acknowledgment
We thank Laraine A. Main for secretarial assistance.
This project was funded in part by federal funds from the NCI, National Institutes of Health, under Contract HHSN261200800001E and supported in part by the Intramural Research Program of the NCI, National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S8.
- HSC
- hematopoietic stem cell
- SCF
- stem cell factor
- M
- bone marrow
- PMN
- polymorphonuclear leukocyte
- CFU
- colony-forming unit
- GM
- granulocyte–macrophage
- fMLF
- formyl-methionyl-leucyl-phenylalanine
- FPR
- formylpeptide receptor
- DC
- dendritic cell
- KLS
- c-Kit+Lin-Sca-1+ cells
- CRAMP
- cathelin-related antimicrobial peptide
- i.p.
- intraperitoneal(ly)
- BrdU
- bromodeoxyuridine
- BFU-E
- burst-forming unit erythroid
- GEMM
- granulocyte, erythrocyte, monocyte, and megakaryocyte
- JAK
- Janus kinase
- TNF
- tumor necrosis factor
- PI3K
- phosphatidylinositol 3-kinase
- JNK
- c-Jun N-terminal kinase
- PE
- phosphatidylethanolamine
- Ab
- antibody
- CFSE
- carboxyfluorescein succinimidyl ester
- PE
- phycoerythrin
- BMC
- bone marrow cell
- ACK
- ammonium–chloride–potassium.
References
- 1. Kondo M., Wagers A. J., Manz M. G., Prohaska S. S., Scherer D. C., Beilhack G. F., Shizuru J. A., and Weissman I. L. (2003) Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21, 759–806 10.1146/annurev.immunol.21.120601.141007 [DOI] [PubMed] [Google Scholar]
- 2. Spangrude G. J., Heimfeld S., and Weissman I. L. (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 10.1126/science.2898810 [DOI] [PubMed] [Google Scholar]
- 3. Okada S., Nakauchi H., Nagayoshi K., Nishikawa S., Nishikawa S., Miura Y., and Suda T. (1991) Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood 78, 1706–1712 [PubMed] [Google Scholar]
- 4. Broudy V. C. (1997) Stem cell factor and hematopoiesis. Blood 90, 1345–1364 [PubMed] [Google Scholar]
- 5. Rönnstrand L. (2004) Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol. Life Sci. 61, 2535–2548 10.1007/s00018-004-4189-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma S., Gurudutta G. U., Satija N. K., Pati S., Afrin F., Gupta P., Verma Y. K., Singh V. K., and Tripathi R. P. (2006) Stem cell c-KIT and HOXB4 genes: critical roles and mechanisms in self-renewal, proliferation, and differentiation. Stem Cells Dev. 15, 755–778 10.1089/scd.2006.15.755 [DOI] [PubMed] [Google Scholar]
- 7. Ikuta K., and Weissman I. L. (1992) Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Natl. Acad. Sci. U.S.A. 89, 1502–1506 10.1073/pnas.89.4.1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li C. L., and Johnson G. R. (1995) Murine hematopoietic stem and progenitor cells: I: enrichment and biologic characterization. Blood 85, 1472–1479 [PubMed] [Google Scholar]
- 9. Ueda Y., Kondo M., and Kelsoe G. (2005) Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 201, 1771–1780 10.1084/jem.20041419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartmann D. W., Entringer M. A., Robinson W. A., Vasil M. L., Drebing C. J., Morton N. J., and True L. (1981) Regulation of granulopoiesis and distribution of granulocytes in early phase of bacterial infection. J. Cell. Physiol. 109, 17–24 10.1002/jcp.1041090103 [DOI] [PubMed] [Google Scholar]
- 11. Barthlen W., Zantl N., Pfeffer K., Heidecke C. D., Holzmann B., and Stadler J. (1999) Impact of experimental peritonitis on bone marrow cell function. Surgery 126, 41–47 10.1067/msy.1999.99060 [DOI] [PubMed] [Google Scholar]
- 12. Santangelo S., Gamelli R. L., and Shankar R. (2001) Myeloid commitment shifts toward monocytopoiesis after thermal injury and sepsis. Ann. Surg. 233, 97–106 10.1097/00000658-200101000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang P., Nelson S., Bagby G. J., Siggins R. 2nd, Shellito J. E., and Welsh D. A. (2008) The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in BALB/c mice. Stem Cells 26, 1778–1786 10.1634/stemcells.2007-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., and Murphy P. M. (2009) International Union of Basic and Clinical Pharmacology: LXXIII: nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 10.1124/pr.109.001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Y., Murphy P. M., and Wang J. M. (2002) Formyl-peptide receptors revisited. Trends Immunol. 23, 541–548 10.1016/S1471-4906(02)02316-5 [DOI] [PubMed] [Google Scholar]
- 16. Liu X., Ma B., Malik A. B., Tang H., Yang T., Sun B., Wang G., Minshall R. D., Li Y., Zhao Y., Ye R. D., and Xu J. (2012) Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat. Immunol. 13, 457–464 10.1038/ni.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao J. L., Lee E. J., and Murphy P. M. (1999) Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 189, 657–662 10.1084/jem.189.4.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Wang A., Gao J. L., Murphy P. M., and Wang J. M. (2012) Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2, 786 10.1038/srep00786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiang N., Fierro I. M., Gronert K., and Serhan C. N. (2000) Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 191, 1197–1208 10.1084/jem.191.7.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy B. D., De Sanctis G. T., Devchand P. R., Kim E., Ackerman K., Schmidt B. A., Szczeklik W., Drazen J. M., and Serhan C. N. (2002) Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat. Med. 8, 1018–1023 10.1038/nm748 [DOI] [PubMed] [Google Scholar]
- 21. Migeotte I., Riboldi E., Franssen J. D., Grégoire F., Loison C., Wittamer V., Detheux M., Robberecht P., Costagliola S., Vassart G., Sozzani S., Parmentier M., and Communi D. (2005) Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J. Exp. Med. 201, 83–93 10.1084/jem.20041277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devosse T., Guillabert A., D'Haene N., Berton A., De Nadai P., Noel S., Brait M., Franssen J. D., Sozzani S., Salmon I., and Parmentier M. (2009) Formyl peptide receptor-like 2 is expressed and functional in plasmacytoid dendritic cells, tissue-specific macrophage subpopulations, and eosinophils. J. Immunol. 182, 4974–4984 10.4049/jimmunol.0803128 [DOI] [PubMed] [Google Scholar]
- 23. Jung S. K., Lee M. H., Lim D. Y., Kim J. E., Singh P., Lee S. Y., Jeong C. H., Lim T. G., Chen H., Chi Y. I., Kundu J. K., Lee N. H., Lee C. C., Cho Y. Y., Bode A. M., et al. (2014) Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J. Biol. Chem. 289, 35839–35848 10.1074/jbc.M114.585513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kular J., Tickner J. C., Pavlos N. J., Viola H. M., Abel T., Lim B. S., Yang X., Chen H., Cook R., Hool L. C., Zheng M. H., and Xu J. (2015) Choline kinase β mutant mice exhibit reduced phosphocholine, elevated osteoclast activity, and low bone mass. J. Biol. Chem. 290, 1729–1742 10.1074/jbc.M114.567966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen K., Xiang Y., Huang J., Gong W., Yoshimura T., Jiang Q., Tessarollo L., Le Y., and Wang J. M. (2014) The formylpeptide receptor 2 (Fpr2) and its endogenous ligand cathelin-related antimicrobial peptide (CRAMP) promote dendritic cell maturation. J. Biol. Chem. 289, 17553–17563 10.1074/jbc.M113.535674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y., Chen K., Wang C., Gong W., Yoshimura T., Liu M., and Wang J. M. (2013) Cell surface receptor FPR2 promotes antitumor host defense by limiting M2 polarization of macrophages. Cancer Res. 73, 550–560 10.1158/0008-5472.CAN-12-2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang P., Welsh D. A., Siggins R. W. 2nd, Bagby G. J., Raasch C. E., Happel K. I., and Nelson S. (2009) Acute alcohol intoxication inhibits the lineage- c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. J. Immunol. 182, 1568–1576 10.4049/jimmunol.182.3.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akala O. O., and Clarke M. F. (2006) Hematopoietic stem cell self-renewal. Curr. Opin. Genet. Dev. 16, 496–501 10.1016/j.gde.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 29. Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Le Y., Gao J. L., Zhao J., Wang J. M., and Wang A. (2014) Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. PLoS ONE 9, e90613 10.1371/journal.pone.0090613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swamydas M., Luo Y., Dorf M. E., and Lionakis M. S. (2015) Isolation of mouse neutrophils. Curr. Protoc. Immunol. 110, 3.20.1–3.20.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen K., Le Y., Liu Y., Gong W., Ying G., Huang J., Yoshimura T., Tessarollo L., and Wang J. M. (2010) A critical role for the G protein-coupled receptor mFPR2 in airway inflammation and immune responses. J. Immunol. 184, 3331–3335 10.4049/jimmunol.0903022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen K., Liu M., Liu Y., Wang C., Yoshimura T., Gong W., Le Y., Tessarollo L., and Wang J. M. (2013) Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J. Biol. Chem. 288, 16262–16273 10.1074/jbc.M113.450635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu J. Y., Le Y., Gong W., Dunlop N. M., Gao J. L., Murphy P. M., and Wang J. M. (2001) Synthetic peptide MMK-1 is a highly specific chemotactic agonist for leukocyte FPRL1. J. Leukocyte Biol. 70, 155–161 [PubMed] [Google Scholar]
- 34. Chen K., Liu M., Liu Y., Yoshimura T., Shen W., Le Y., Durum S., Gong W., Wang C., Gao J. L., Murphy P. M., and Wang J. M. (2013) Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J. Clin. Invest. 123, 1694–1704 10.1172/JCI65569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iribarren P., Zhou Y., Hu J., Le Y., and Wang J. M. (2005) Role of formyl peptide receptor-like 1 (FPRL1/FPR2) in mononuclear phagocyteresponses in Alzheimer disease. Immunol. Res. 31, 165–176 10.1385/IR:31:3:165 [DOI] [PubMed] [Google Scholar]
- 36. Yang D., Chen Q., Stoll S., Chen X., Howard O. M., and Oppenheim J. J. (2000) Differential regulation of responsiveness to fMLP and C5a upon dendritic cell maturation: correlation with receptor expression. J. Immunol. 165, 2694–2702 10.4049/jimmunol.165.5.2694 [DOI] [PubMed] [Google Scholar]
- 37. Shin M. K., Jang Y. H., Yoo H. J., Kang D. W., Park M. H., Kim M. K., Song J. H., Kim S. D., Min G., You H. K., Choi K. Y., Bae Y. S., and Min do S. (2011) N-formyl-methionyl-leucyl-phenylalanine (fMLP) promotes osteoblast differentiation via the N-formyl peptide receptor 1-mediated signaling pathway in human mesenchymal stem cells from bone marrow. J. Biol. Chem. 286, 17133–17143 10.1074/jbc.M110.197772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viswanathan A., Painter R. G., Lanson N. A. Jr, and Wang G. (2007) Functional expression of N-formyl peptide receptors in human bone marrow-derived mesenchymal stem cells. Stem Cells 25, 1263–1269 10.1634/stemcells.2006-0522 [DOI] [PubMed] [Google Scholar]
- 39. Broxmeyer H. E., Maze R., Miyazawa K., Carow C., Hendrie P. C., Cooper S., Hangoc G., Vadhan-Raj S., and Lu L. (1991) The kit receptor and its ligand, steel factor, as regulators of hemopoiesis. Cancer Cells 3, 480–487 [PubMed] [Google Scholar]
- 40. Morstyn G., Brown S., Gordon M., Crawford J., Demetri G., Rich W., McGuire B., Foote M., and McNiece I. (1994) Stem cell factor is a potent synergistic factor in hematopoiesis. Oncology 51, 205–214 10.1159/000227335 [DOI] [PubMed] [Google Scholar]
- 41. Hoffman R., Tong J., Brandt J., Traycoff C., Bruno E., McGuire B. W., Gordon M. S., McNiece I., and Srour E. F. (1993) The in vitro and in vivo effects of stem cell factor on human hematopoiesis. Stem Cells 11, 76–82 [DOI] [PubMed] [Google Scholar]
- 42. Broxmeyer H. E., Lu L., Cooper S., Ruggieri L., Li Z. H., and Lyman S. D. (1995) Flt3 ligand stimulates/costimulates the growth of myeloid stem/progenitor cells. Exp. Hematol. 23, 1121–1129 [PubMed] [Google Scholar]
- 43. McNiece I. K., Langley K. E., and Zsebo K. M. (1991) Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp. Hematol. 19, 226–231 [PubMed] [Google Scholar]
- 44. Sui X., Krantz S. B., You M., and Zhao Z. (1998) Synergistic activation of MAP kinase (ERK1/2) by erythropoietin and stem cell factor is essential for expanded erythropoiesis. Blood 92, 1142–1149 [PubMed] [Google Scholar]
- 45. Horie M., and Broxmeyer H. E. (1995) The combination of Steel factor and GM-CSF blocks apoptosis induced by retinoic acid and upregulates AP-1 in a human growth factor-dependent cell line. Exp. Hematol 23, 168–173 [PubMed] [Google Scholar]
- 46. Hendrie P. C., Miyazawa K., Yang Y. C., Langefeld C. D., and Broxmeyer H. E. (1991) Mast cell growth factor (c-kit ligand) enhances cytokine stimulation of proliferation of the human factor-dependent cell line, M07e. Exp. Hematol. 19, 1031–1037 [PubMed] [Google Scholar]
- 47. Gehling U. M., Ryder J. W., Hogan C. J., Hami L., McNiece I., Franklin W., Williams S., Helm K., King J., and Shpall E. J. (1997) Ex vivo expansion of megakaryocyte progenitors: effect of various growth factor combinations on CD34+ progenitor cells from bone marrow and G-CSF-mobilized peripheral blood. Exp. Hematol. 25, 1125–1139 [PubMed] [Google Scholar]
- 48. Keller J. R., Ortiz M., and Ruscetti F. W. (1995) Steel factor (c-kit ligand) promotes the survival of hematopoietic stem/progenitor cells in the absence of cell division. Blood 86, 1757–1764 [PubMed] [Google Scholar]
- 49. Fehniger T. A., Carson W. E., Mrózek E., and Caligiuri M. A. (1997) Stem cell factor enhances interleukin-2-mediated expansion of murine natural killer cells in vivo. Blood 90, 3647–3653 [PubMed] [Google Scholar]
- 50. Avraham H., Vannier E., Cowley S., Jiang S. X., Chi S., Dinarello C. A., Zsebo K. M., and Groopman J. E. (1992) Effects of the stem cell factor, c-kit ligand, on human megakaryocytic cells. Blood 79, 365–371 [PubMed] [Google Scholar]
- 51. Uittenbogaart C. H., Schmidc I., Kiertscher S., Shau H., Anisman D. J., Zsebo K. M., and Clement L. T. (1996) Human thymocyte responsiveness to stem cell factor: synergy with interleukin-2 for the generation of NK/LAK cytotoxicity. Immunol. Lett. 52, 45–52 10.1016/0165-2478(96)02580-1 [DOI] [PubMed] [Google Scholar]
- 52. Grafte-Faure S., Leveque C., Ketata E., Jean P., Vasse M., Soria C., and Vannier J. P. (2000) Recruitment of primitive peripheral blood cells:synergism of interleukin 12 with interleukin 6 and stromal cell-derived FACTOR-1. Cytokine 12, 1–7 10.1006/cyto.1999.0520 [DOI] [PubMed] [Google Scholar]
- 53. Wang J., Tang Z. Y., Ka W., Sun D., Yao W., Wen Z., and Chien S. (2007) Synergistic effect of cytokines EPO, IL-3 and SCF on the proliferation, differentiation and apoptosis of erythroid progenitor cells. Clin. Hemorheol. Microcirc. 37, 291–299 [PubMed] [Google Scholar]
- 54. Duarte R. F., and Frank D. A. (2000) SCF and G-CSF lead to the synergistic induction of proliferation and gene expression through complementary signaling pathways. Blood 96, 3422–3430 [PubMed] [Google Scholar]
- 55. Timokhina I., Kissel H., Stella G., and Besmer P. (1998) Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 17, 6250–6262 10.1093/emboj/17.21.6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fukao T., Yamada T., Tanabe M., Terauchi Y., Ota T., Takayama T., Asano T., Takeuchi T., Kadowaki T., Hata Ji J., and Koyasu S. (2002) Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat. Immunol. 3, 295–304 10.1038/ni768 [DOI] [PubMed] [Google Scholar]
- 57. Young S. M., Cambareri A. C., and Ashman L. K. (2006) Role of c-KIT expression level and phosphatidylinositol 3-kinase activation in survival and proliferative responses of early myeloid cells. Cell Signal. 18, 608–620 10.1016/j.cellsig.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 58. Xiang Y., Yao X., Chen K., Wang X., Zhou J., Gong W., Yoshimura T., Huang J., Wang R., Wu Y., Shi G., Bian X., and Wang J. (2016) The G-protein coupled chemoattractant receptor FPR2 promotes malignant phenotype of human colon cancer cells. Am. J. Cancer Res. 6, 2599–2610 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.