Abstract
It has been shown in both human and mouse placentas that follicle stimulating hormone receptor (FSHR) is expressed in fetal vascular endothelium. There are conflicting reports, however, on the role of FSH to stimulate angiogenesis in vitro in cultured endothelial cells from umbilical veins. Therefore, in this study we undertook an in vivo approach utilizing Fshr null mice to definitively address this question. In the context where all pregnant dams have identical Fshr genotypes, we generated fetuses and associated fetal portions of placenta that were Fshr wt or Fshr null and analyzed angiogenesis within the placental labyrinths. Quantitative morphometric analyses of placentas obtained at mid-gestation revealed that the percentage of the placenta composed of labyrinth is significantly decreased in Fshr null placentas relative to wt placentas. Furthermore, data presented demonstrate that within the Fshr null labyrinths, fetal vessel angiogenesis was significantly reduced relative to wt labyrinths. The results obtained with this combination of in vivo and genetic approaches conclusively demonstrate that signaling through endothelial FSHR does indeed stimulate angiogenesis and that placental Fshr is essential for normal angiogenesis of the fetal placental vasculature.
Keywords: Follicle stimulating hormone, FSH receptor, angiogenesis, placenta
1. Introduction
Female fertility is critically dependent upon the actions of follicle stimulating hormone (FSH) on the ovary, where FSH promotes follicular growth and estrogen synthesis. Indeed, these actions of FSH serve as the foundation for its widespread use in assisted reproductive technologies. Historically, it was thought that FSH receptors (FSHR) were expressed exclusively in the gonads and therefore the actions of FSH in females were not required once ovulation had occurred. In recent years, however, there have been several studies reporting the extra-gonadal expression of FSHR, suggesting that FSH may exert additional functions that contribute to a successful pregnancy and delivery (Celik et al., 2008; Hascalik et al., 2010; Kumar, 2018; Mancinelli et al., 2009; Mizrachi and Shemesh, 1999; Onori et al., 2013; Ponikwicka-Tyszko et al., 2016; Popovici et al., 2000; Radu et al., 2010; Robin et al., 2016; Stilley et al., 2014a; Stilley et al., 2014b; Stilley et al., 2016; Sun et al., 2006; Zhu et al., 2012a).
Indeed, some studies have documented physiological actions of FSH on extra-gonadal tissues. These include reports showing effects of FSH on bone resorption (Sun et al., 2006; Zhu et al., 2012b) and myometrial contractile activity (Stilley et al., 2016). Of particular relevance to the present study, it has also been reported that endothelial cells, including those in the chorionic villi of human placenta and the labyrinth of mouse placenta, express FSHR (Radu et al., 2010; Stilley et al., 2014a; Stilley et al., 2014b). These anatomical regions of the human and mouse placentas, respectively, mediate the maternal-fetal exchange of nutrients and gasses. A physiological role for endothelial FSHR within the placental vasculature was demonstrated by decreased mid-gestational placental weights when Fshr was deleted from the fetal portion of the mouse placenta (Stilley et al., 2014a). That the decreased placental weights may have resulted as a consequence of impaired placental angiogenesis was suggested by a study reporting that FSH addition to primary cultures of human umbilical vein endothelial cells (HUVECs) stimulated several processes associated with angiogenesis (Stilley et al., 2014b). A subsequent report from a different laboratory, however, described the absence of FSH effects on HUVECs (Stelmaszewska et al., 2016). Since these apparent discrepancies could be attributable to methodological differences that would influence expression of FSHR in vitro in cultured HUVECs, they prompted us to examine the potential role of the FSHR in promoting placental angiogenesis using an in vivo gene knockout approach in mice. Towards this end, the present study demonstrates that deletion of Fshr from fetal portions of the placenta does indeed significantly inhibit angiogenesis of fetal vessels within the placental labyrinth.
2. Materials and Methods
2.1 Forko mice
FORKO mice were originally generated and characterized by Dr. R. Sairam and colleagues (Danilovich et al., 2000; Danilovich and Sairam, 2002; Dierich et al., 1998). The mice were created using homologous recombination to replace exon 1 of the Fshr with a PGK-Neo cassette, thus yielding a global knockout of the Fshr. The FORKO mice in our colony were housed under standard conditions, on a 12h light cycle, with ad libitum access to water and food. Animal care procedures were approved by the Institutional Animal Care and Use Committee and performed in accordance with the standards set by the National Institutes of Health.
Frozen ear punches or fetal tissue (approximately 20 mg) were used to genotype the FORKO mice. DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) as per the manufacturer's instructions. PCR for Fshr genotyping was performed as described (Stilley et al., 2014a).
The placentas used in this study were those previously collected for another study (Stilley et al., 2014a). Briefly, Fshr +/- females were mated with Fshr +/- males, and pregnant dams were sacrificed 15 days post conception (dpc) to obtain fetoplacental tissues of the desired genotypes. Each fetus was snap frozen and used for genotyping, and its placenta was formalin fixed and paraffin embedded. Placentas of three and six Fshr wt fetuses and Fshr null fetuses, respectively, were analyzed in the current study.
2.2 Analyses of placental tissue
Paraffin-embedded placental tissue was sectioned and stained for histologic analyses using Harris hematoxylin (Leica Biosystems, Buffalo Grove, IL) and eosin Y (Sigma-Aldrich, St. Louis, MO) protocols. Images spanning the entirety of the placental section were captured and tiled using an Olympus BX61 Light Microscope (Center Valley, PA). Computer-assisted quantitative morphometric analysis was performed using Image J (National Institutes of Health, Bethesda, MD) by an investigator blinded to genotypes of the samples. The perimeters of the labyrinth layer and the placenta were traced by hand using the software's tracing tool, and the area of each was determined. Morphometric tracing of the labyrinth layer was specifically selected by the presence of nucleated fetal blood cells within blood vessels. The junctional layer adjacent to the labyrinth was excluded based on the presence of giant cells (Natale et al., 2006). Within the labyrinth layer, all individual fetal sinuses and maternal sinuses were traced, using nucleated and non-nucleated blood cells as markers for fetal and maternal blood sinuses, respectively, and pseudo-colored (Li et al., 2013). Within a given labyrinth, the numbers of fetal sinuses versus maternal sinuses were calculated. To correct for potential differences in numbers of sinuses due to differences in placental size and/or labyrinth area, these data were normalized to labyrinth size. All fetal and maternal sinuses within a given labyrinth were used to calculate the mean size of sinuses of each type, based on the total number of pixels within a traced sinus.
2.3 Statistical analyses
Quantified data are expressed as the mean ± SEM. For the tracing study, the statistical significance of differences between groups was determined using the Student's t test. All analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Significance was defined as P < 0.05.
3. Results
The goal of our studies was to resolve the overriding question as to whether or not endothelial FSHR mediates angiogenesis (Stelmaszewska et al., 2016; Stilley et al., 2014b) by using an in vivo approach comparing mid-gestational placentas from three wt and six Fshr null fetuses (Stilley et al., 2014a). Because the genotype of the fetal portion of the placenta, which includes the labyrinth, is the same as that of the fetus, angiogenesis within the placental labyrinth could therefore be analyzed as a function of the specific knockout of Fshr from the fetus/fetal placenta. It should be noted that this was the only experimental variable because the pregnant dams were all of the same Fshr genotype (Fshr +/-).
Previous studies have demonstrated that the vascular endothelium within the placental labyrinth from a Fshr wt fetus expresses FSHR protein, but that from an Fshr null fetus does not (Stilley et al., 2014a). To examine the impact of the deletion of Fshr, and thus FSHR protein, from the fetal portion of the placenta on the size of the labyrinth layer, we morphometrically quantified the area of the labyrinth layer in each placenta. To account for potentially different sizes of the overall placentas and for potential different placental areas in slices from a given placenta, the area of each placental labyrinth layer was normalized to the area of the entire placenta on the placental slice being analyzed. Our analyses revealed that the labyrinth layer in the Fshr null samples comprised only 56% of the placenta as compared to 75% of the placenta in the wt samples (Figure 1).
Figure 1. Quantification of labyrinth size in placentas of wild-type (Fshr+/+) and null (Fshr-/-) fetuses, expressed as a percentage of the area of the entire placenta.
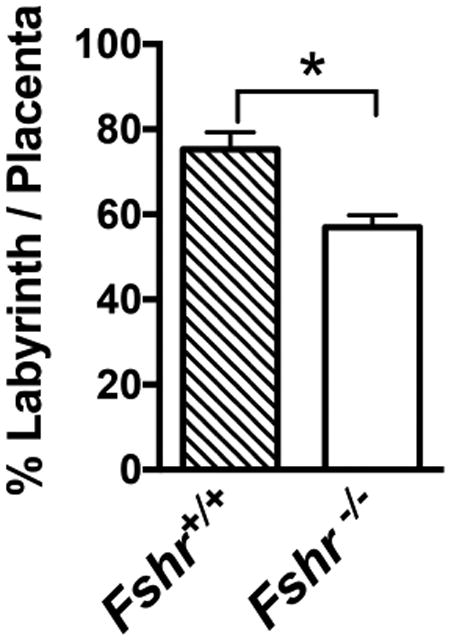
Data shown are mean ± SEM of quantifications of 3 Fshr+/+ and 6 Fshr-/- placentas. Asterisk denotes statistically significant difference, P<0.05.
We then sought to determine if FSHR in the vascular endothelium of the placenta mediates the stimulation of angiogenesis. There are two different experimental approaches typically used to quantify placental angiogenesis. One utilizes vascular corrosion casts of placental fetal vessels that are scanned by x-ray microcomputed tomography and quantified (Rennie et al., 2015). The other method utilizes a quantitative morphometric approach in which maternal vs. fetal sinuses in the placental labyrinth are manually traced (identified by the presence vs. the absence of red blood cells, respectively), pseudo-colored, and quantified with Image J (Harper et al., 2015; Li et al., 2013; Schulz et al., 2012; Van Gronigen Caesar et al., 2016). We chose the latter method for this study because it afforded us the ability to quantitatively analyze potential changes in both the fetal and the maternal sinuses of the labyrinth. Due to the labor-intensive nature of this approach, other studies have typically quantified only one or more selected regions within a placental labyrinth. However, to obviate any potential differences between different regions of a given labyrinth, we analyzed the entire labyrinth area of each slice of placenta. As such, within the labyrinth layer of all examined placentas, we traced the fetal sinuses (representing the fetal vasculature) and maternal sinuses (representing maternal pools of blood). Fetal sinuses were identified based on the presence of nucleated red blood cells, whereas maternal sinuses were based on the presence of non-nucleated red blood cells. Representative images of placentas from Fshr null and Fshr wt fetuses are shown in Figure 2, where the maternal and fetal sinuses have been pseudo-colored as red and blue, respectively. A qualitative visual inspection of enlarged labyrinth images from each Fshr genotype suggests that fetal vessels in the Fshr wt placenta are generally small, in contrast to apparently larger fetal vessels in a Fshr null placenta (lower portion of Figure 2). Quantitative morphometric analyses of the sinuses throughout the entire labyrinth layer of three Fshr wt and six Fshr null placentas were performed to calculate the sizes and numbers of fetal and maternal sinuses. To correct for differences between the sizes of labyrinths of the Fshr wt and Fshr null genotypes, we normalized the number of sinuses in each placenta to the total labyrinth area. The data revealed that deletion of fetoplacental Fshr led to a ∼4-fold increase in the size of fetal vessels (Figure 3A), concomitant with a ∼60% decrease in the number of fetal vessels (Figure 3B), demonstrating significantly reduced fetal vessel angiogenesis (Li et al., 2013). When maternal sinuses of each genotype were similarly analyzed, no statistical differences were observed with respect to either their size or quantity (Figures 3C and D, respectively). Taken together, the quantitative morphometric data demonstrate that the deletion of Fshr from the fetal vessels of the placental labyrinth is associated with a significant impairment of fetal vessel angiogenesis.
Figure 2. Fetal and maternal sinuses in the labyrinth of placentas of wild-type (Fshr+/+) and null (Fshr-/-) fetuses.
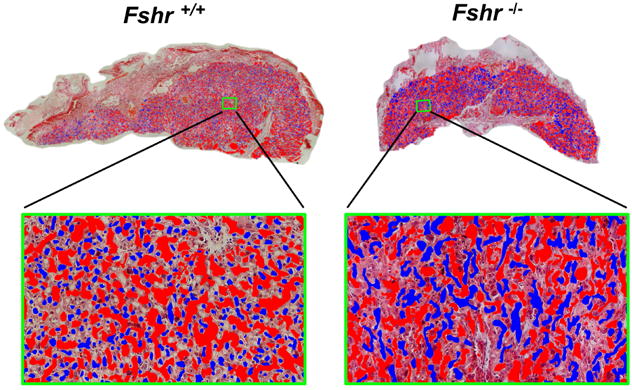
Tracings of sinuses in the placental labyrinth are shown, with fetal and maternal sinuses identified based on the presence of nucleated and non-nucleated blood cells, respectively. Blue pseudo-coloring indicates fetal sinuses, and red indicates maternal sinuses. Top: Images shown are placentas of fetuses 3 and 5 of Fig 1 and they are representative of 3 Fshr+/+ and 6 Fshr-/- 15 dpc placentas. Each image was tiled at 200× magnification. Bottom: Enlargement of the portion of each labyrinth that is highlighted by a green box in the image above.
Figure 3. Quantification of angiogenesis in the placental labyrinth of wild-type (Fshr+/+) and null (Fshr-/-) fetuses.
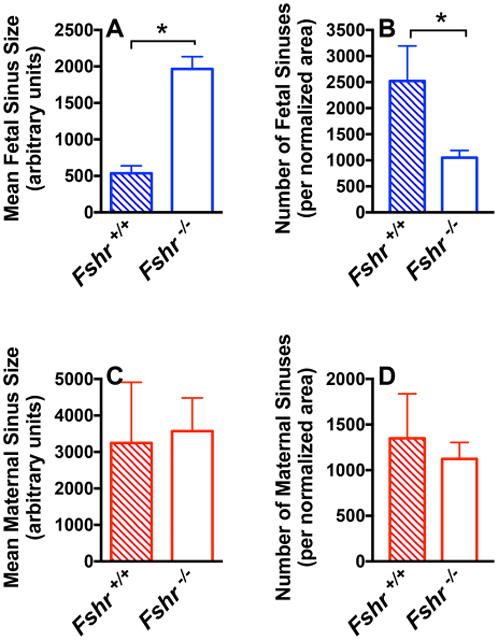
The A number and B size of fetal sinuses, and the C number and D size of maternal sinuses in Fshr+/+ and Fshr-/- labyrinths of 15 dpc placentas, with number of sinuses normalized to overall labyrinth size. Data shown are the mean ± SEM of sinus quantifications of 3 Fshr+/+ and 6 Fshr-/- placentas. Asterisks denote statistically significant differences, P<0.05.
4. Discussion
A healthy pregnancy is predicated upon the normal development and function of the placenta. Impairments in placental development can result in placental insufficiency, fetal growth retardation, and fetal death. The ability of the placenta to mediate bi-directional maternal-fetal exchange of nutrients and respiratory gases requires extensive angiogenesis throughout its component tissues. Indeed, abnormalities in the placental vasculature account for the most common placental pathologies identified in various pregnancy complications (Chen and Zheng, 2014; Macara et al., 1996; Mayhew et al., 2004; Redman and Sargent, 2005; Reynolds et al., 2006). The appropriate timing and growth of the placental vasculature is finely regulated by a number of pro-angiogenic and anti-angiogenic factors, many of which are synthesized within the placenta. The studies presented herein expand upon the repertoire of known hormones and growth factors regulating placental angiogenesis by demonstrating that signaling through fetal placental FSHR is essential for appropriate angiogenesis of placental fetal vessels.
The expression of FSHR in endothelial cells was first reported by Radu et. al (Radu et al., 2010). In addition to demonstrating FSHR expression on endothelial cells of blood vessels on the outer surface of human tumors, they also demonstrated the expression of the FSHR on fetal vessels within the chorionic villi of the human placenta. Subsequent studies from our laboratory more fully examined FSHR expression in human placentas from eight-weeks of gestation (the earliest time point examined) through term pregnancy, revealing FSHR expression on endothelial cells and arterial smooth muscle of fetal blood vessels in the chorionic villi as well as the umbilical vein and arteries (Stilley et al., 2014a). Notably, the FSHR expressed in human endothelial cells is not the full-length receptor, but a splice variant lacking exon 9 (Stilley et al., 2014b), which is the same splice variant expressed in osteoclasts (Robinson et al., 2010; Zhu et al., 2012b). Using primary cultures of HUVECs, we reported the FSH-provoked stimulation of several angiogenic processes including tube formation (Stilley et al., 2014a; Stilley et al., 2014b). More recently, however, Stelmaszewska et al. reported the absence of FSH effects on HUVECs (Stelmaszewska et al., 2016). There are several methodological differences that could readily account for the apparent discrepant results. Most notably, the source of HUVECs and culture conditions differed significantly between the studies. It is possible that the conditions used in the latter studies, while generally appropriate for maintaining HUVECs in culture, may not have been suitable for maintaining FSHR expression. The present study resolves this apparent discordance by using an in vivo approach that compares angiogenesis within the Fshr wt vs. Fshr null placental labyrinths of mice. Our results show that fetal vessel angiogenesis is significantly reduced in the Fshr null placental labyrinth, thus conclusively demonstrating that signaling through endothelial FSHR stimulates angiogenesis.
Previous studies from our laboratory demonstrated that the deletion of fetoplacental Fshr resulted in decreased mid-gestational weights of placentas and fetuses as compared to wt littermates as well as an increase in mid-gestational intra-uterine demise of Fshr null fetuses (Stilley et al., 2014a). In light of the data presented herein, we conclude that these impairments are due, at least in part, to decreased angiogenesis of the fetal placental vasculature. Our previous studies also revealed quantitatively similar decreases in mid-gestational fetal and placental weights in Fshr+/- samples. While the quantification of angiogenesis in the Fshr+/- placental labyrinths was beyond the scope of the present study, we speculate that they would most likely similarly exhibit decreased fetal vessel angiogenesis. Female mice that are haploinsufficient for the global deletion of Fshr exhibit decreased fertility and decreased estrogen and progesterone levels, indicating that decreased ovarian function is at least partially accountable for the subfertility (Danilovich et al., 2000; Danilovich and Sairam, 2002). That the impact of Fshr deletion on the ovary appears to be milder than that of deletion of Fshr from the placental vascular endothelium may be due to the much higher density of ovarian FSHR and thus spare receptors on granulosa cells.
In addition to placental FSHR exerting a role in pregnancy by promoting angiogenesis of the fetal placental vasculature, uterine myometrial FSHR has also been shown to play a role in pregnancy (Stilley et al., 2016). Thus, whereas lower levels of FSHR expression in non-pregnant and early pregnancy myometrium suppress contractile activity in response to FSH, the higher densities of FSHR in myometrium from term pregnancy respond to FSH with increased contractile activity.
As one considers the role of the placental endothelial FSHR and myometrial FSHR during pregnancy, it is important to consider that the secretion of pituitary FSH is suppressed during pregnancy (Jaffe et al., 1969). It is also important to consider that the levels of FSHR in endothelial cells and extra-gonadal tissues of the female reproductive tract are considerably lower than those in the ovary (Stilley et al., 2014a; Stilley et al., 2014b). However, human placenta, decidua and myometrium, but not endothelial cells, were shown to express mRNAs for both subunits of FSH (Stilley et al., 2014a). Furthermore, microarray and sequence data available from NCBI GEO reveal expression of CGA mRNA and FSHB mRNA in placental tissue from each trimester, the uterine endometrium, and the uterine myometrium (Dezso et al., 2008; Eyster et al., 2007; Hawkins et al., 2011; Huuskonen et al., 2008; Mikheev et al., 2008; Mutter et al., 2004; Su et al., 2004; Talbi et al., 2006; Votavova et al., 2011; Winn et al., 2007). Because NCBI GEO sets further reveal FSHB expression in human trophoblast cells (ID: 59683968; ID: 28793668), these cells are most likely the source of FSH within the placenta. Based on these observations, we hypothesize that locally synthesized FSH, where concentrations may be quite high, may be the source of FSH for the stimulation of the relatively low densities of FSHR in placenta and the extra-gonadal reproductive tract. Ultimately, signaling through extra-gonadal FSHR appears to exert several actions to establish and maintain pregnancy, including, but not necessarily limited to, the regulation of placental angiogenesis and uterine contractile activity.
It is noteworthy to point out that several studies have associated the underlying maternal causes of infertility, rather than the assisted reproductive technologies used to address the infertility, with increased risks of failed implantation, spontaneous miscarriage, and adverse perinatal outcomes (Basso and Baird, 2003; Henriksen et al., 1997; Jaques et al., 2010; Joffe and Li, 1994; Raatikainen et al., 2012; Romundstad et al., 2008; Thomson et al., 2005; Wisborg et al., 2010; Zhu et al., 2007). It should be considered that a decrease in FSHR expression or responsiveness that is sufficient to attenuate the ovarian response to FSH may also attenuate extra-ovarian responses to FSH, thereby potentially contributing to adverse pregnancy outcomes. In particular, as demonstrated herein, insufficient signaling through FSHR in the fetal vascular endothelium of the placenta would severely impair placental angiogenesis.
In conclusion, the data presented show that FSH signaling through endothelial FSHR does indeed stimulate angiogenesis by demonstrating that targeted deletion of Fshr from the fetal portion of the placenta results in decreased fetal vessel angiogenesis.
Highlights.
Deletion of Fshr from the mouse placenta inhibits labyrinth development
Deletion of Fshr from the mouse placenta inhibits fetal vessel angiogenesis
Endothelial FSHR mediates angiogenesis
Acknowledgments
The authors thank Dr. Rongbin Guan for technical assistance and Dr. Mario Ascoli for critical review of the manuscript.
Funding: J.A.W.S. was supported in part by grant T32DK007690 from the National Institutes of Health. The University of Iowa Central Microscopy Research Facility is supported by the Office of the Vice President for Research and Economic Development, the Holden Comprehensive Cancer Center and the Carver College of Medicine.
Footnotes
The authors have no competing interests to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basso O, Baird DD. Infertility and preterm delivery, birthweight, and Caesarean section: a study within the Danish National Birth Cohort. Hum Reprod. 2003;18:2478–2784. doi: 10.1093/humrep/deg444. [DOI] [PubMed] [Google Scholar]
- Celik O, Tagluk ME, Hascalik S, Elter K, Celik N, Aydin NE. Spectrotemporal changes in electrical activity of myometrium due to recombinant follicle stimulating hormone preparations follitropin alfa and beta. Fert Steril. 2008;90:1348–1356. doi: 10.1016/j.fertnstert.2007.07.1391. [DOI] [PubMed] [Google Scholar]
- Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21:15–25. doi: 10.1111/micc.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141:4295–4308. doi: 10.1210/endo.141.11.7765. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Sairam MR. Haploinsufficiency of the follicle-stimulating hormone receptor accelerates oocyte loss inducing early reproductive senescence and biological aging in mice. Biol Reprod. 2002;67:361–369. doi: 10.1095/biolreprod67.2.361. [DOI] [PubMed] [Google Scholar]
- Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, et al. A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol. 2008;6:49. doi: 10.1186/1741-7007-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fert Steri. 2007;88:1505–1533. doi: 10.1016/j.fertnstert.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Harper JL, Caesar GA, Pennington KA, Davis JW, Schulz LC. Placental changes caused by food restriction during early pregnancy in mice are reversible. Reproduction. 2015;150:165–172. doi: 10.1530/REP-15-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascalik S, Celik O, Tagluk ME, Yildirim A, Aydin NE. Effects of highly purified urinary FSH and human menopausal FSH on uterine myoelectrical dynamics. Mol Hum Reprod. 2010;16:200–206. doi: 10.1093/molehr/gap076. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen TB, Baird DD, Olsen J, Hedegaard M, Secher NJ, Wilcox AJ. Time to pregnancy and preterm delivery. Obstet Gynecol. 1997;89:594–599. doi: 10.1016/s0029-7844(97)00045-8. [DOI] [PubMed] [Google Scholar]
- Huuskonen P, Storvik M, Reinisalo M, Honkakoski P, Rysa J, Hakkola J, Pasanen M. Microarray analysis of the global alterations in the gene expression in the placentas from cigarette-smoking mothers. Clin Pharmacol Ther. 2008;83:542–550. doi: 10.1038/sj.clpt.6100376. [DOI] [PubMed] [Google Scholar]
- Jaffe RB, Lee PA, Midgley AR., Jr Serum gonadotropins before, at the inception of, and following human pregnancy. J Clin Endocrinol Metab. 1969;29:1281–1283. doi: 10.1210/jcem-29-9-1281. [DOI] [PubMed] [Google Scholar]
- Jaques AM, Amor DJ, Baker HW, Healy DL, Ukoumunne OC, Breheny S, Garrett C, Halliday JL. Adverse obstetric and perinatal outcomes in subfertile women conceiving without assisted reproductive technologies. Fert Steril. 2010;94:2674–2679. doi: 10.1016/j.fertnstert.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Joffe M, Li Z. Association of time to pregnancy and the outcome of pregnancy. Fert Steril. 1994;62:71–75. doi: 10.1016/s0015-0282(16)56818-6. [DOI] [PubMed] [Google Scholar]
- Kumar TR. Extragonadal Actions of FSH: A Critical Need for Novel Genetic Models. Endocrinology. 2018;159:2–8. doi: 10.1210/en.2017-03118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Schwerbrock NM, Lenhart PM, Fritz-Six KL, Kadmiel M, Christine KS, Kraus DM, Espenschied ST, Willcockson HH, Mack CP, et al. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest. 2013;123:2408–2420. doi: 10.1172/JCI67039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, Lyall F, Greer IA. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996;17:37–48. doi: 10.1016/s0143-4004(05)80642-3. [DOI] [PubMed] [Google Scholar]
- Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastroint Liver Physiol. 2009;297:G11–G26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, Unadkat JD. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci. 2008;15:866–877. doi: 10.1177/1933719108322425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrachi D, Shemesh M. Follicle-stimulating hormone receptor and its messenger ribonucleic acid are present in the bovine cervix and can regulate cervical prostanoid synthesis. Biol Reprod. 1999;61:776–784. doi: 10.1095/biolreprod61.3.776. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Zahrieh D, Liu C, Neuberg D, Finkelstein D, Baker HE, Warrington JA. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics. 2004;5:88. doi: 10.1186/1471-2164-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale DR, Starovic M, Cross JC. Phenotypic analysis of the mouse placenta. Methods Mol Med. 2006;121:275–293. doi: 10.1385/1-59259-983-4:273. [DOI] [PubMed] [Google Scholar]
- Onori P, Mancinelli R, Franchitto A, Carpino G, Renzi A, Brozzetti S, Venter J, Francis H, Glaser S, Jefferson DM, et al. Role of follicle-stimulating hormone on biliary cyst growth in autosomal dominant polycystic kidney disease. Liver Int. 2013;33:914–925. doi: 10.1111/liv.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikwicka-Tyszko D, Chrusciel M, Stelmaszewska J, Bernaczyk P, Sztachelska M, Sidorkiewicz I, Doroszko M, Tomaszewski J, Tapanainen JS, Huhtaniemi I, et al. Functional Expression of FSH Receptor in Endometriotic Lesions. J Clin Endocrinol Metab. 2016;101:2905–2914. doi: 10.1210/jc.2016-1014. [DOI] [PubMed] [Google Scholar]
- Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]
- Raatikainen K, Kuivasaari-Pirinen P, Hippelainen M, Heinonen S. Comparison of the pregnancy outcomes of subfertile women after infertility treatment and in naturally conceived pregnancies. Hum Reprod. 2012;27:1162–1169. doi: 10.1093/humrep/des015. [DOI] [PubMed] [Google Scholar]
- Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, Fromont G, Hai MT, Ghinea N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621–1630. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Rennie MY, Rahman A, Whiteley KJ, Sled JG, Adamson SL. Site-specific increases in utero- and fetoplacental arterial vascular resistance in eNOS-deficient mice due to impaired arterial enlargement. Biol Reprod. 2015;92:48. doi: 10.1095/biolreprod.114.123968. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin B, Planeix F, Sastre-Garau X, Pichon C, Olesen TK, Gogusev J, Ghinea N. Follicle-Stimulating Hormone Receptor Expression in Endometriotic Lesions and the Associated Vasculature: An Immunohistochemical Study. Reprod Sci. 2016;23:885–891. doi: 10.1177/1933719115623647. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Tourkova I, Wang Y, Sharrow AC, Landau MS, Yaroslavskiy BB, Sun L, Zaidi M, Blair HC. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem Biophys Res Commun. 2010;394:12–17. doi: 10.1016/j.bbrc.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Gunnell D, Vatten LJ. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet. 2008;372:737–743. doi: 10.1016/S0140-6736(08)61041-7. [DOI] [PubMed] [Google Scholar]
- Schulz LC, Schlitt JM, Caesar G, Pennington KA. Leptin and the placental response to maternal food restriction during early pregnancy in mice. Biol Reprod. 2012;87:1–9. doi: 10.1095/biolreprod.112.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmaszewska J, Chrusciel M, Doroszko M, Akerfelt M, Ponikwicka-Tyszko D, Nees M, Frentsch M, Li X, Kero J, Huhtaniemi I, et al. Revisiting the expression and function of follicle-stimulation hormone receptor in human umbilical vein endothelial cells. Sci Rep. 2016;6:37095. doi: 10.1038/srep37095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilley JA, Christensen DE, Dahlem KB, Guan R, Santillan DA, England SK, Al-Hendy A, Kirby PA, Segaloff DL. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod. 2014a;91:74. doi: 10.1095/biolreprod.114.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilley JA, Guan R, Duffy DM, Segaloff DL. Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis. J Clin Endocrinol Metab. 2014b;99:E813–820. doi: 10.1210/jc.2013-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilley JA, Guan R, Santillan DA, Mitchell BF, Lamping KG, Segaloff DL. Differential Regulation of Human and Mouse Myometrial Contractile Activity by FSH as a Function of FSH Receptor Density. Biol Reprod. 2016;95:1–10. doi: 10.1095/biolreprod.116.141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Thomson F, Shanbhag S, Templeton A, Bhattacharya S. Obstetric outcome in women with subfertility. BJOG. 2005;112:632–637. doi: 10.1111/j.1471-0528.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- Van Gronigen Caesar G, Dale JM, Osman EY, Garcia ML, Lorson CL, Schulz LC. Placental development in a mouse model of spinal muscular atrophy. Biochem Biophys Res Commun. 2016;470:82–87. doi: 10.1016/j.bbrc.2015.12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votavova H, Dostalova Merkerova M, Fejglova K, Vasikova A, Krejcik Z, Pastorkova A, Tabashidze N, Topinka J, Veleminsky M, Jr, Sram RJ, et al. Transcriptome alterations in maternal and fetal cells induced by tobacco smoke. Placenta. 2011;32:763–770. doi: 10.1016/j.placenta.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, Feng KT, Bernlohr DA, McDonagh S, Pereira L, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148:1059–1079. doi: 10.1210/en.2006-0683. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Ingerslev HJ, Henriksen TB. IVF and stillbirth: a prospective follow-up study. Hum Reprod. 2010;25:1312–1316. doi: 10.1093/humrep/deq023. [DOI] [PubMed] [Google Scholar]
- Zhu JL, Obel C, Hammer Bech B, Olsen J, Basso O. Infertility, infertility treatment, and fetal growth restriction. Obstet Gynecol. 2007;110:1326–1334. doi: 10.1097/01.AOG.0000290330.80256.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF, Sun L, et al. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A. 2012a;109:14574–14579. doi: 10.1073/pnas.1212806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Tourkova I, Yuen T, Robinson LJ, Bian Z, Zaidi M, Blair HC. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun. 2012b;422:54–58. doi: 10.1016/j.bbrc.2012.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


