Abstract
Loss of ovarian function has important effects on neurotransmitter production and release with corresponding effects on cognitive performance. To date, there has been little direct comparison of the effects of surgical and transitional menopause on neurotransmitter pathways in the brain. In this study, effects on monoamines, monoamine metabolites, and the amino acids tryptophan (TRP) and tyrosine (TYR) were evaluated in adult ovariectomized (OVX) rats and in rats that underwent selective and gradual ovarian follicle depletion by daily injection of 4-vinylcyclohexene-diepoxide (VCD). Tissues from the hippocampus (HPC), frontal cortex (FCX), and striatum (STR) were dissected and analyzed at 1- and 6-weeks following OVX or VCD treatments. Tissues from gonadally intact rats were collected at proestrus and diestrus to represent neurochemical levels during natural states of high and low estrogens. In gonadally intact rats, higher levels of serotonin (5-HT) were detected at proestrus than at diestrus in the FCX. In addition, the ratio of 5-hydroxyindoleacetic acid (5-HIAA)/5HT in the FCX and HPC was lower at proestrus than at diestrus, suggesting an effect on 5-HT turnover in these regions. No other significant differences between proestrus and diestrus were observed. In OVX- and VCD-treated rats, changes were observed which were both brain region- and time point-dependent. In the HPC levels of norepinephrine, 5-HIAA, TRP and TYR were significantly reduced at 1 week, but not 6 weeks, in both OVX and VCD-treated rats relative to proestrus and diestrus. In the FCX, dopamine levels were elevated at 6 weeks after OVX relative to diestrus. A similar trend was observed at 1 week (but not 6 weeks) following VCD treatment. In the STR, norepinephrine levels were elevated at 1 week following OVX, and HVA levels were elevated at 1 week, but not 6 weeks, following VCD treatment, relative to proestrus and diestrus. Collectively, these data provide the first comprehensive analysis comparing the effects of two models of menopause on multiple neuroendocrine endpoints in the brain. These effects likely contribute to effects of surgical and transitional menopause on brain function and cognitive performance that have been reported.
Keywords: Loss of ovarian function, Ovariectomy, VCD, Hippocampus, Striatum, Frontal Cortex
1. Introduction
Estrogens have widespread effects in the brain, both during development and in adulthood. These include effects on neuronal organization and function, synaptic plasticity, and neuronal survival, with corresponding effects on cognitive function, neurodegenerative disease, and age-related cognitive decline. Studies also show that, in women, loss of estrogens following oophorectomy or menopause can contribute significantly to risk for neurodegenerative diseases like Alzheimer’s disease and Parkinson’s disease, as well as late-life mood disorders and age-related cognitive decline [1–3]. A few studies have documented effects on neurotransmitter systems that are known to play a role in mood and cognitive changes. These include effects on monoaminergic pathways [4, 5], as well as cholinergic [6], glutamatergic [7–9], and GABAergic [10, 11] transmission. Most studies have used ovariectomy models to study the effects of rapid hormone depletion followed by estrogen replacement to study effects on one or two neurotransmitter systems in a specific region of the brain. Few studies have focused specifically on the effects of menopause, and in particular on comparing effects associated with surgical vs. transitional menopause.
In primates, natural or transitional menopause is characterized by an accelerated loss of ovarian follicles, resulting in irregular fluctuations in ovarian hormones during the transition period, and culminating in a substantial decline in ovarian production of estrogens and progesterone [12]. As a consequence, the release of LH and FSH from the pituitary rises significantly due to the loss of negative feedback regulation. In addition, ovarian stromal cells continue to produce androgens post-menopause, resulting in a substantial increase in the ratio of androgens:estrogens in the systemic circulation. In contrast, ovariectomy produces a surgical menopause characterized by a rapid and complete loss of all ovarian hormones. In the United States, most women (>80%) experience a transitional menopause. Far fewer women (~13%) experience a surgical menopause, and many of these surgeries are conducted in younger women (<40 years of age) to prevent ovarian and breast cancer associated with BRCA1 and BRCA2 mutations [13].
Unlike primates, rodents do not experience follicular atresia resulting in a natural menopause. Rodents enter a condition of reproductive senescence, characterized by long periods of elevated levels of circulating estradiol during middle-age, and followed by persistent diestrus in old age [14]. This change is related to a dysfunction of the hypothalamic-pituitary-gonadal (HPG) axis that occurs as a result of normal aging [15]. Thus, until recently, rodents have not served as a good model of natural menopause in humans.
Notably, a rodent model of progressive ovarian failure has recently been characterized [16–19]. This model involves daily injection of 4-vinylcyclohexene diepoxide (VCD), a chemical that selectively and over time destroys the majority of primordial and primary ovarian follicles [20]. Rodents treated with appropriate daily doses of VCD experience a progressive loss of ovarian follicles. Stromal cells remain intact and continue to produce androgens, thus mirroring conditions that occur in natural menopause. Using this model, studies have shown that transitional and surgical menopause produce differing effects on measures of brain function and cognitive performance [21]. The most recent evidence suggests that some of these differences may be due to elevated levels of androstenedione following transitional vs. surgical menopause, and the local conversion of androstenedione to estrone in the brain [22, 23].
To date, there has been little direct comparison of the effects of surgical vs. transitional menopause on neurotransmitter pathways in the brain. We hypothesize that surgical and transitional menopause produce different effects on cognitive functioning due to differential effects on multiple interacting neurotransmitter pathways. In this study, we utilized a high-pressure liquid chromatography-coulometric multi-electrode array system (HPLC-CMEAS) method to characterize neurochemical changes associated with surgical vs. VCD-induced transitional menopause in three regions of the rat brain. Results show that while many of the effects of surgical vs. transitional menopause are the same, there also are some important differences that vary by brain region and by time following the menopausal transition.
2. Methods
2.1. Animals
Female Sprague–Dawley rats (~11 weeks of age) were purchased from Hilltop Laboratories, Inc. Young adults were selected as opposed to middle-aged rats based on several considerations. Rodents do not experience a natural menopause. Instead, they experience what is known as reproductive senescence associated with dysregulation of the HPG axis. Characteristics of reproductive senescence include irregular cycles, a period of constant estrous where levels of circulating estradiol are chronically elevated, followed eventually by constant diestrus. The timing of these events varies by strain and typically begin in middle-age [24]. Humans do not experience reproductive senescence, but instead experience accelerated follicular atresia resulting in a natural menopause [25]. We therefore chose to use young adults as opposed to middle-aged rats in order to study the effects of chemically-induced follicular atresia in the absence of confounding factors associated with the process of reproductive senescence. Another reason to use young adult rats is that, with respect to surgical menopause, the majority of women who experience surgical menopause are young adults (e.g., early 30s) whose ovaries are removed to prevent ovarian and breast cancers associated with BRCA1 and BRCA2 mutations [13]. Hence, we concluded that young adult rats would be more appropriate for comparing models of surgical vs. natural menopause with respect to neurochemical endpoints.
All rats were individually-housed and maintained on a 12/12 h light–dark cycle (lights on at 7:00 AM) with free access to food and water and were allowed to acclimate to the housing environment for two weeks prior to use. All experiments were conducted in accordance with the NIH Guide for Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
2.2. Gonadally Intact Controls
Tissues were collected from rats at proestrus, and at diestrus, to represent neurochemical levels during natural states of high and low estrogen over the course of the estrous cycle. Daily vaginal smears were collected from these rats to determine stage of the estrous cycle. Cycle stage was characterized by vaginal cytology and the presence/appearance of three cell types, leukocytes, nucleated epithelial cells, and cornified epithelial cells [26]. After collecting at least 2 weeks of cycle data, rats were killed either on proestrus or diestrus and serum as well as brain tissues were collected as described below.
2.3. Menopausal Models
Rats were randomly assigned to either the VCD or OVX group. Rats in the VCD group received 4-vinylcyclohexene diepoxide (VCD; Sigma Chemicals; St Louis, MO) at a dose of 80 mg/kg I.P. daily for a period of 30 days as previously described [27]. During this time, rats in the OVX group received daily injections of sesame oil (1ml/kg i.p.). At the end of 30 days, rats that had received sesame oil underwent bilateral ovariectomy, and rats that received VCD received sham surgery. Rats were anesthetized with ketamine (100 mg/kg) and xylazine (7 mg/kg) (0.1 cc/100 g.b.w.). Ovariectomy was performed using a lateral approach. The apical tips of the uterus were ligated and the ovaries removed. The peritoneal muscle was closed with 6.0 suture silk, and the overlying skin was closed with metal wound clips. Sham surgery, consisted of skin and muscle incisions and wound closure only. Antibiotic cream was applied to the wound to reduce the chance of infection. Rats received Ketofen (3 mg/kg, i.p.) every day for 3 days to reduce post-surgical pain.
2.4. Tissue Collection
At 1 week or 6 weeks following surgery, rats were anesthetized with an overdose of ketamine (3 mg) and xylazine (0.6 mg) and euthanized by decapitation. These time points were chosen to evaluate early and late effects of the two menopausal models and to evaluate changes in effects as a function of time. Trunk blood was collected for determination of serum levels of estradiol, testosterone, and androstenedione. Dissections were performed according to plate designations in Paxinos & Watson (1998) [28] and were as follows: hippocampus (plates 21–41), frontal cortex (plates 11–21), and striatum (plates 11–21). These brain regions were selected based on known monoaminergic innovation, their roles in learning, memory and attentional functions, and evidence that neuronal organization and functioning in these regions can be affected by gonadal hormones. Tissues were stored at −80 °C until processed.
2.5. Brain sample Preparation
500 μL 10 mM sodium acetate buffer (pH 6.5) were added to 50 mg tissue in a 1.5 mL microfuge tube. Tissue was sonicated until completely dissolved. After sonication, tissues were spun at 14,000 g for 10 min at 4°C. Supernatant was collected and placed at 4 °C. This step was repeated three times and the three supernatants were combined. The cell free supernatants were spun at 15,000g for an additional 40 minutes. A 200 μL aliquot was taken for the determination of protein concentration using the Bradford method [29]. The remaining volume of each supernatant were filtered through a disposable membrane (0.22μm pore size) micropartition device (Millipore Ultrafree-MC) under centrifugation at 14,000g for 30 min at 4 °C to remove any compounds above 10,000 nominal molecular weight limit.
2.6. Hormone assays
2.6.1. E2 assay
Serum levels of E2 were quantified as recently described [30]. Briefly, samples were spiked with internal standard 25μl 2,4,16,16,17-d5–17 beta-estradiol (1 ng/ml in methanol). 3–4 ml n-Butyl chloride was added and samples were vortexed for 1 min. Samples were then centrifuged at 4,770 × g at room temperature for 10 min, and the organic layer was transferred to salinized culture tubes and dried under a steam of nitrogen at 37°C for 20 min. Residues were derivatized in 0.1 ml buffered dansyl chloride solution (a 1:1 mix of acetonitrile: water, pH 10.5) and heated to 60°C for 3 min. The reconstituted sample was then transferred into glass vials for LC-MS/MS analysis. E2 was eluted using a Waters Acquity UPLC BEH C18, 1.7 μm, 2.1 × 150 mm reversed-phase column, with an acetonitrile: water (0.1% formic acid) gradient. MS detection and quantification were achieved in the positive mode with a Thermo Fisher TSQ Quantum Ultra mass spectrometer interfaced via an electrospray ionization (ESI) probe with the Waters UPLC Acquity solvent delivery system. Transitions used for analysis were 506 → 171 for E2, and 511 →171 for the deuterated internal standard. Area under the peak was quantified and used to determine absolute levels of E2/mL of sample by comparison with a series of standards. The limit of detectability for this assay is 2.5 pg/mL. Intra-assay statistics show relative standard errors below 8.1% and relative standard deviations below 10.4%. Inter-assay statistics show relative standard errors below 5.0% with relative standard deviations below 7.4%. The E2 serum concentration was calculated as pg/mL.
2.6.2. Testosterone and Androstenedione assay
Testosterone (T) and Androstenedione (AD) levels in serum were quantified by a modification of the method described by Cawood [31] and using methods similar to the E2 detection method described above. Briefly, samples were spiked with 0.25 ng/ml D3-testosterone or D7-androstenedione as the internal standard and then extracted with 3 ml n-butyl chloride. After centrifugation and evaporation, the residue was reconstituted in methanol and water (80 μl: 20 μl), and was transferred into glass vials for UPLC-MS/MS analysis. T and AD was eluted from the same column as E2, with a methanol: water (0.1% formic acid and 2 mM ammonium acetate) gradient from 50 to 85% methanol. Transitions used for T analysis were 289 →97 for T and 292 →97 for the deuterated T; transitions used for AD analysis were 287→100 for AD and 294→ 100 for the deuterated AD. The limit of detectability for this assay is 25 pg/mL for both T and AD.
2.7. Monoamine analysis by HPLC-CMEAS
Monoamines and metabolites were measured with a modified version of a HPLC-ECD method described by Yao et al [32]. High-performance liquid chromatography (HPLC) with electrochemical detection was used to detect and quantify levels of amino acids, monoamines and metabolites, including tryptophan (TRP) and tyrosine (TYR); dopamine (DA) and its metabolites, 3–4-dihydroxyphenylalanine (DOPAC) and homovanillic acid (HVA); norepinephrine (NE) and epinephrine (EPI) and serotonin (5-HT) and its metabolite 5-hydroxyindole acetic acid (5-HIAA). These neurochemical endpoints were selected not only because they are known to be involved in a variety of cognitive functions but also are reported to be regulated by estrogens. Within each sample, 100 μl was injected into an ESA CoulArray Model 5600 HPLC system, consisting of two Model 582 pumps, one dynamic gradient mixer, two PEEK pulse dampers, a Model 542 refrigerated autosampler injector, a CoulArray organizer module, and a serial array of 16 coulometric electrodes. The system was controlled and chromatograms were analyzed using the ESA CoulArray for Windows-32 software program. Each sample was run on a single column (ESA Meta-250, 5 μm ODS, 250 × 4.6 mm ID) under a 68-minute gradient elution that ranged from 0% to 100% Mobile Phase B. Mobile Phase A consisted of 1.1% (w/v) of 1-pentane-sulfonic acid (Specrum, Inc.) pH was adjusted to 3.0 using acetic acid (Sigma-Aldrich, Inc.). Mobile Phase B consisted of 0.1 M lithium acetate (Sigma-Aldrich, Inc.) in a solvent mixture of methanol (Avantor Performance Materials, Inc.), acetonitrile (Honeywell, Inc.) and isopropanol (Mallinckrodt Baker, Inc.) at the ratio of 80/10/10 (v/v/v). pH was adjusted to 6.5 using acetic acid (Sigma-Aldrich, Inc.). The mobile phase was filtered through a 0.2 μm nylon filter (Sartorius Stedim Biotech, Inc.), and delivered at a fixed flow rate of 0.6 mL/min. The temperature of both cells and the column was set at 25 °C. The retention time and area of the peaks in tissue homogenates were measured and compared to an external calibrating standard solution containing TYR, TRP, DA, DOPAC, HVA, NE, EPI, 5-HT, and 5-HIAA (Sigma, St. Louis, MO, USA). Concentrations of these substances in the samples were calculated and expressed as ng/mg protein. Turnover ratios (metabolite/monoamine) were calculated as a measure of activity.
2.8. Statistical Analysis
Results are presented as mean ± standard error of the mean (SEM). A p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed by three steps. First, comparisons of proestrus with diestrus were performed using Student’s t-test. Second, all 6 groups were compared by one-way analysis of variance (ANOVA) followed by Tukey test if overall p < 0.05. Third, a two-way analysis of variance (ANOVA) was performed with ovarian status (OVX or VCD) as one factor and Time (1 week or 6 weeks) as a second factor to investigate to investigate interactions between menopausal model and time.
2.9. Presentation of the Data
For each of the brain region, data for DA, 5-HT and NE are shown. Data for additional monoamines, metabolites, and metabolite/monoamine ratios also are included for those endpoints that were statistically significant. A supplementary table summarizing all of the data for monoamines and metabolites also is provided.
3. Results
3.1. Serum levels of hormones
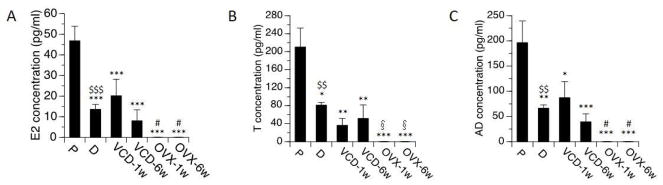
Levels of E2, T, and AD in each of the treatment groups are summarized in Figure 1. Significantly higher levels of E2, T, and AD were detected on proestrus than on diestrus, (t(14)=3.0, p<0.01 for AD; t(12)=3.1, p<0.01 for T; t(14)=4.5, p<0.001 for E2). In proestrus rats, mean serum levels of circulating E2, AD and T were 46.8 ± 7.0 pg/ml, 196.5 ± 42.9 pg/ml and 210.5 ± 41.8 pg/ml. In diestrus rats, levels of E2, AD and T were 13.6 ± 2.4 pg/ml, 66.5 ± 6.3 pg/mL and 81.4 ± 6.0 pg/ml. None of these hormones were detectable in OVX rats. In rats treated with VCD, levels of E2, T, and AD were significantly lower than levels detected in proestrus rats (p<0.05 in all cases), and did not differ significantly from levels detected in diestrus rats. This was the case at both 1 week and at 6 weeks following the completion of VCD treatments.
Fig. 1.
Serum (A) E2 (17β-estradiol), (B) T (testosterone) and (C) AD (androstenedione) levels by treatment groups. Rats at P (proestrous) and D (diestrous) are used as controls. Bars indicate Mean±SEM. One-way ANOVA: *p<0.05, **p<0.01, ***p<0.001, relative to P; # p<0.05 relative to VCD-1w; §p<0.05 relative to VCD-6w. T-test: $$p<0.01, $$$p<0.001, relative to P. None of these hormones were detectable in OVX rats.
3.2. Monoamines, Monoamine metabolites, and Amino Acids Levels
3.2.1. Comparison of Proestrus vs. Diestrus
Few differences were detected between brain tissues collected from gonadally intact cycling rats. In the frontal cortex, higher levels of 5-HT were detected at proestrus than at diestrus (t (14) = 2.2, p<0.05), and the ratio of 5-HIAA/5-HT was significantly lower at proestrus than at diestrus (t (14) = 2.4, p<0.05). Likewise, in the hippocampus, the ratio of 5-HIAA/5-HT was significantly lower at proestrus than at diestrus (t (11) = 2.7, p<0.05). In the striatum, higher levels of TYR were detected at proestrus than at diestrus (t (13) = 2.2, p<0.05). No other significant differences were detected. There was, however, a trend for reduction in the ratio of 5-HIAA at proestrus vs. diestrus (t (11) = 1.8, p=0.1) in the HPC, and a strong trend for an increase in HVA (t (14) = 2.1, p=0.06) levels at proestrous vs. diestrous in STR.
3.3.2. Effects of OVX and VCD Treatments
Significant effects of OVX and VCD treatments on monoamines, monoamine metabolites, and amino acid levels were detected.
HPC
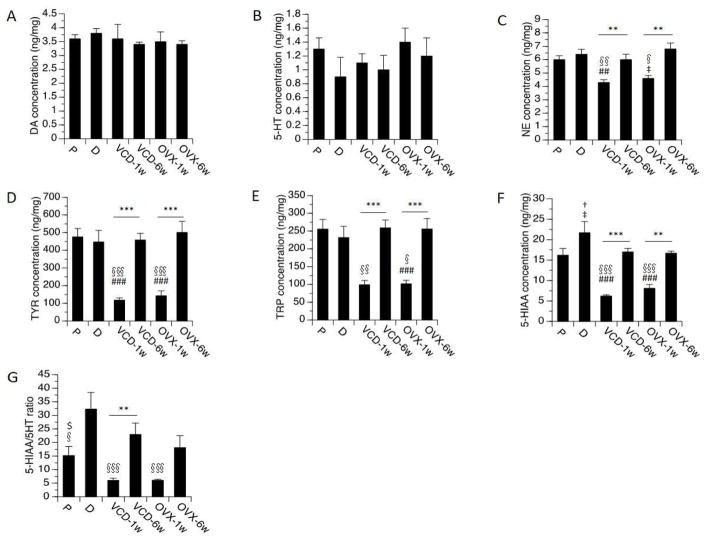
One-way ANOVAs consistently revealed significant effects of OVX and VCD treatments on levels of NE (F[5,35]=8.6, p<0.0001), 5-HIAA (F[5,35]=20.6, p<0.0001), TYR and TRP (p<0.0001 in each case). Effects were observed primarily at 1 week following OVX and VCD treatment (Fig 2). Specifically, 1 week following OVX, levels of 5-HIAA were reduced relative to both diestrus (p<0.0001) and proestrus (p<0.001). There was a trend for reduction in 5-HIAA levels at proestrus vs. diestrus (p=0.07). Levels of NE (p<0.05) likewise were reduced relative to diestrus, with a trend towards reduced levels relative to proestrus (p=0.08). Similar effects were observed following VCD treatments. At 1 week following VCD treatments levels of both 5-HIAA and NE were reduced relative to both diestrus and proestrus (p<0.0001 for 5-HIAA; p<0.01 for NE in each case). These effects were not observed 6 weeks after OVX or 6 weeks following the completion of VCD treatments. Levels of the amino acids (TYR and TRP) also were significantly reduced (up to 80%) at 1 week following OVX and VCD treatments relative to both proestrus and diestrus in gonadally intact controls (Fig 2; p<0.0001 in each case). By 6 weeks the levels of the amino acids had returned to levels comparable to normal cycling rats. Notably, the levels of DA and 5-HT were not affected. Levels of DOPAC, HVA and EPI were undetectable.
Fig. 2.
Monoamines, metabolites, amino acids levels and metabolite/monoamine ratio by treatment groups in HPC. Rats at P (proestrous) and D (diestrous) are used as controls. Bars indicate Mean ± SEM. One-way ANOVA: **p<0.01, ***p<0.001 relative to VCD/OVX-1w; ## p<0.01, ### p<0.001 relative to P; § p<0.05, §§ p<0.01, §§§ p<0.001 relative to D; ‡ 0.05<p<0.1, relative to P. T-test: $p<0.05, relative to D; †0.05<p<0.1, relative to P.
Significant effects on the ratios of DA/TYR (F[5,35]=10.6, p<0.0001) and 5-HIAA/5-HT (F[5,35]=7.4, p<0.0001) also were detected (Fig 2). The ratios of DA/TYR at 1 week following OVX or VCD treatments were significantly higher than the ratios detected at proestrus and diestrus (p<0.05 following OVX; P<0.01 following VCD). The ratios of 5-HT/TRP at 1 week following OVX or VCD treatments were significantly higher than the ratios detected at proestrus and diestrus (p<0.001 in each case). The ratio of 5-HIAA/5-HT was significantly lower at 1 week after OVX or VCD treatment relative to diestrus (p<0.05 in each case), but did not differ significantly from proestrus.
In no case did one-way or two-way ANOVAs reveal significant differences between the two menopausal models on any of the endpoints measured in the hippocampus. Results of the two-way ANOVAs on each of the measures revealed a significant overall effect (F[3,24]>8.0, p<0.001), a significant effect of time-point (p<0.0001 in each case), no main effect of model, and no interaction between model × time-point.
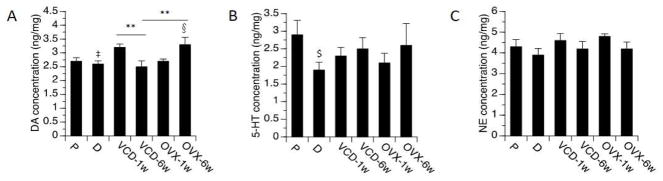
FCX
One-way ANOVA revealed significant effects on the levels of DA (F[5,40]=3.8, p<0.01). Elevated levels of DA were detected at 6 weeks following OVX relative to diestrus (p<0.05), as well as relative to VCD-treated rats at the 6-week time-point (p<0.05). Levels of DA 1 week following VCD treatments also were significantly higher than levels detected 6 weeks following VCD treatments (p<0.05), with a trend towards being higher relative to diestrus (p=0.07) (Figure 3). One-way ANOVA also revealed a significant effect on the ratio of NE/DA (F(5,40)=3.14, p<0.05). This ratio was significantly lower 6 weeks following OVX than at 1 week following OVX. A trend for reduction in the ratio of NE/DA was detected at 6 weeks following OVX vs. VCD treatments (p=0.07). In no case did the ratio of NE/DA in OVX or VCD-treated rats differ significantly from the ratio detected in normal cycling rats.
Fig. 3.
Monoamines levels by treatment groups in FCX. Rats at P (proestrous) and D (diestrous) are used as controls. Bars indicate Mean ± SEM. One-way ANOVA: **p<0.01 relative to VCD-6w; § p<0.05 relative to D; ‡ 0.05<p<0.1, relative to VCD-1w. T-test: $p<0.05, relative to P.
Two-way ANOVA of DA levels revealed a significant overall effect (F[3,26]=3.9, p<0.05), no main effects of model or time-point, but a significant interaction between model × time-point (p<0.01) with the levels of DA higher at 6 weeks following OVX than at 6 weeks following VCD treatments (p<0.05). Likewise, two-way ANOVA of NE/DA ratio revealed a significant overall effect (F[3,26]=4.6, p<0.01), no main effects of model or time-point, but a significant interaction between model × time-point (p<0.01) with the ratio of NE/DA lower at 6 weeks following OVX than at 6 weeks following VCD treatments (p<0.05).
STR
One-way ANOVA revealed significant effects on the levels of NE (F[5,40]=3.9, p<0.01) and HVA (F[5,40]=5.4, p<0.001) in the STR. Elevated levels of NE were detected at 1 week following OVX relative to proesturs (p<0.05), diestrus (p<0.01), and at 1 week following VCD treatment (p<0.01). Elevated levels of HVA were detected 1 week following OVX and VCD treatment relative to diestrus, and 1 week following VCD treatment relative to proestrus (p<0.05 in each case) (Figure 4). No significant effects on the levels of TYR or TRP were detected in the STR.
Fig. 4.
Monoamines and metabolite levels by treatment groups in STR. Rats at P (proestrous) and D (diestrous) are used as controls. Bars indicate Mean ± SEM. One-way ANOVA: **p<0.01 relative to VCD-1w; # p<0.05 relative to P; § p<0.05, §§ p<0.01, §§§ p<0.001 relative to D. T-test: † 0.05<p<0.1, relative to P.
Two-way ANOVA of NE levels revealed a significant overall effect (F[3,26]=3.6, p<0.05), a main effect of model (p<0.05), no main effect of time-point, and a significant interaction between model × time-point (p<0.05) with the levels of NE higher 1 week following OVX than 1 week following VCD treatments (p<0.05). Two-way ANOVA of HVA levels revealed no significant effects of model or time-point.
4. Discussion
4.1. Serum hormone measurements
Our goal was to characterize neurochemical changes in the brain associated with two models of menopause, surgical vs. transitional, and to compare effects with changes observed at two stages of the estrous cycle, proestrus and diestrus. Circulating levels of E2, T and AD confirm that representation of each of these conditions was successfully accomplished. Consistent with previous study using GC-MS/MS for hormone measurement [33], levels of E2, T and AD levels were significantly lower at diestrous than at proestrous. In addition, E2, T and AD were not detected 1 week and 6 weeks after OVX, as expected with complete removal of the ovaries. Unlike OVX, VCD treatments eliminate only the ovarian follicles, while the androgen-producing interstitial cells remain intact [34]. Consequently, in VCD-treated rats E2 levels were reduced, whereas androgens continued to be detected at levels comparable to diestrus. These findings are consistent with previous characterizations of this model [21]. In addition to AD levels, our study provides new information regarding T levels in two distinct estrous stages in comparison to VCD and OVX treatment.
4.2. Fluctuations in neurochemical endpoints detected in gonadally intact rats
Results show that significant effects are detected in the HPC and FCX associated with cycle stage and are limited primarily to the serotonin pathway. Specifically, levels of 5-HT in the FCX were significantly higher at proestrus than at diestrus, and the ratio of 5-HIAA/5-HT was significantly lower in the HPC and FCX in rats euthanized at proestrus vs. diestrus. Similar results were reported by Shimizu, where the ratio of 5-HIAA/5-HT was significantly reduced during proestrous in nucleus accumbens [35]. The increase in 5-HT coupled with a decrease in the ratio of 5-HIAA/5-HT in the FCX suggests a decrease in 5-HT release, resulting in elevated 5-HT and less 5-HIAA production. A similar reduction in 5-HT turnover may be occurring in the HPC, although levels of 5-HT were not elevated at proestrus in this region. There also was a trend for a reduction in the ratio of 5-HT/TRP in the HPC at proestrus vs. diestrus, though this did not reach statistical significance. This may reflect greater susceptibility of the HPC to effects on amino acid levels with corresponding effects on neurotransmitter endpoints. These effects appear minimal and difficult to detect in normal cycling rats, but were exacerbated in the two models of menopause (see below).
Some of our results differ from published studies. For example, we detected no significant differences in neurochemical endpoints as a function of cycle in the striatum. In contrast, Xiao et al. [36] reported that in female rats extracellular DA concentrations in the STR were significantly higher at proestrus than at diestrus. In contrast to the elevation in 5HT we detected in the FCX, Desan et al.[37] reported no significant differences in 5-HT levels in anterior cerebral cortex, hippocampus or cerebellum across the estrous cycle. Differences may be due in part to methodologies and to the absence of verification of hormone levels. Advantages of the current study include the measurement of hormone levels, as well as multiple neurochemical endpoints, thus giving a more comprehensive characterization of the neurochemical signatures.
4.3. Effects of OVX and VCD treatments on neurochemical endpoints
A strength of the metabolomics approach is the ability to provide a comprehensive analysis of multiple endpoints for a variety of brain structures, all from the same set of tissues. Multiple effects were detected and most effects were both region-specific and time-dependent. One of the key findings of our analysis is the relatively few differences in the effects of the two models on neurochemical endpoints, despite the fact that the hormonal profiles, particularly androgen levels, were quite different. This suggests that surgical and transitional menopause produce similar effects on these endpoints, at least during the early weeks following menopause. This, in turn, suggests that any differences in cognitive performance between OVX and VCD-treated rats are not due to differential effects on monoaminergic pathways.
As we dissect the regional effects, we find that the greatest number of effects were detected in the HPC, suggesting that this region of the brain is particularly sensitive to loss of ovarian function. The hippocampus plays a critical role in memory consolidation as well as age-related cognitive decline and many studies have demonstrated significant effects of estrogens on synaptic plasticity and neuronal function in this region [38]. In the current study, effects of both surgical and VCD-induced loss of ovarian function in the HPC were detected at 1 week, but not at 6 weeks, suggesting that these are temporary effects. The results are in agreement with previous reports showing decreased levels of NE [39, 40] and 5-HIAA [39] following ovariectomy in mice. Furthermore, our finding of reduced 5-HIAA/5-HT ratio observed at 1 week and not 6 weeks following OVX or VCD treatments agrees with previous results in rats where the ratio was reduced at 2 weeks, but not 4 weeks, after OVX [41]. The substantial reduction in TRP as well as elevated ratios of 5-HT/TRP and 5-HIAA/TRP suggest that at an early time-point following either OVX or VCD treatments serotonin turnover is significantly reduced, possibly due to reductions in the amino acid TRP. The fact that levels of DA and 5-HT in HPC remained unchanged following estrogen deprivation also concurs with earlier findings reported in mice [39].
The fact that 5-HIAA was reduced without a reduction in 5-HT suggests that less 5-HT was released, perhaps in response to a reduction in 5-HT, resulting from a reduction in TRP. A similar effect may be occurring in the DA and NE pathways. Levels of NE were reduced, possibly in response to a reduction in TYR. Since levels of DOPAC and HVA were not detectable in this region we were unable to evaluate potential effects on DA turnover; however, it may be that temporary reductions in TYR resulted in reduced DA release (thereby preserving DA levels) along with reduced metabolism of DA to NE.
Some of our findings were surprising and not entirely consistent with previous reports. For example, in contrast with the HPC, relatively few effects of OVX and VCD treatments were detected in the FCX. The FCX plays a critical role in attention as well as executive functions and significant effects of estrogen treatment on monoaminergic innervation of the FCX have been reported [42]. Nevertheless, in the present study none of the sizable decreases in 5-HIAA, NE and amino acids that were detected in the HPC 1 week following OVX or VCD treatments were detected in the FCX. This was unexpected given the significantly higher levels of 5HT and the lower ratio of 5-HIAA/5-HT detected in the FCX at proestrus relative to diestrus. Hence, we anticipated lower levels of 5-HT in the FCX following OVX and VCD treatments compared with proestrus in association with the lower levels of gonadal hormones. The unexpected result suggests that levels of 5-HT cease to fluctuate and are restored in non-cycling animals, even in a state of chronically low estrogen levels.
The significant increase in DA in the FCX of OVX-6W rats also was surprising, and contrasts with previous results showing a decrease in DA at 20 days following OVX treatment in mice [40]. This could reflect a species difference, or possibly differences associated with the time points when neurotransmitter levels were evaluated. In contrast to OVX rats, higher DA levels were detected in the FCX at 1 week relative to 6 weeks following VCD treatments. This was one of the few instances where effects of the two models differed. These increases in DA in OVX and VCD-treated rats may reflect compensatory changes in the expression or activity of metabolic enzymes involved in the production and degradation of the monoamines (see below).
In contrast with effects in the HPC and FCX, OVX and VCD treatments significantly affected the noradrenergic pathway in the STR. The striatum is part of the extrapyramidal motor system. Not only is it critically involved in extrapyramidal motor control but also plays a significant role in motor learning [43]. Notably, elevated estrogens have been shown to favor the use of hippocampal learning strategies whereas low estrogens have been shown to favor the use of striatal learning strategies [44].
Our data show an increase in NE in the STR at 1 week following OVX, but not following VCD-treatment. This again is one of the few model differences that were detected and may contribute to short-term differences in striatal function and motor learning following loss of ovarian function. We also detected increases in HVA at 1 week following OVX or VCD treatment. This agrees with results reported by Bitar et al. which showed markedly elevated concentrations of HVA in male and female rats following gonadectomy, whereas the level of 5-HT and 5-HIAA remained unaltered in STR [45]. However, they also reported detected elevated DA and DOPAC levels after OVX whereas we did not. Using a microdialysis approach, a separate study found that female rats had significantly higher extracellular striatal dopamine concentrations at proestrus than after ovariectomy [36]. The reasons for these inconsistencies are unknown. Differences in analytical methodologies and in the length of time between ovariectomy and day of sacrifice could potentially account for differences.
In the present study, increases in NE and HVA following OVX suggests an increase in NE production and DA release at the 1-week time point in the surgical model. The increases in HVA detected in the OVX-1W and VCD-1W groups may indicate an increase in DA release at the early time-point in response to the loss of ovarian function. It was unexpected that elevated HVA levels were accompanied by unaltered DOPAC level in this brain structure. One possible explanation is that DA metabolism was shifted toward the DA-3MT (3-methoxytyramine)-HVA pathway (15% of DA turnover in normal condition), however additional study is required to elucidate the underlying mechanisms.
4.4 Potential Mechanisms – Anabolic and Catabolic Enzymes
Possible mechanism for some of the effects observed on the neurochemical endpoints could be alterations in synthetic and catabolic enzymes involved in monoamine regulation. Tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) are the rate limiting enzymes responsible for the conversion of tyrosine and tryptophan to dopamine and serotonin. Both of these enzymes are potently regulated by phosphorylation and are negatively regulated by monoamines [46, 47]. Numerous reports show that these enzymes also can be regulated by estrogens. Some reports are contradictory. For example, Beattie et al. [48] reported that ovariectomy caused a 2–3 fold increase in hypothalamic TH activity in the rat, whereas Krieger et al. [49] reported no significant increase in TH activity in the hypothalamus or medial preoptic area following ovariectomy. Ivanova and Beyer [50] reported that local estrogen production increases TH mRNA and protein expression in the ventral midbrain of mice during late prenatal and early postnatal development. Sabban et al. [51] reported differing effects of estradiol on TH expression in the nucleus of the solitary tract and in the locus coeruleus that differed depending on mode of delivery. Kritzer et al.[52] reported that the density of TH immunoreactive fibers decreased in the dorsolateral prefrontal cortex of rhesus monkeys following ovariectomy, which was reversed by combined treatment with estrogen + progesterone. These findings demonstrate the potential for TH expression to be regulated by estradiol in a region-specific way.
TPH expression also has been shown to be regulated by estrogen signaling. Smith et al. [53] reported that 1 month of estradiol treatment increased TPH mRNA in the dorsal raphe of rhesus monkeys. These same treatments also decreased expression of the serotonin transporter and decreased expression of monoamine oxidase (MAO)-A mRNAs. Hiroi et al. [54] showed similar increases in TPH-2 mRNA in the rat raphe in response to estradiol. More recently, Hiroi et al. [55] reported that treating with estradiol or with conjugated equine estrogens increased TPH-2 mRNA in the raphe with some differences in subregion-specific effects depending on which estrogens were administered. Collectively, these findings are consistent with estrogen enhancement of serotonin signaling.
Other enzymes that may contribute to the effects of menopause on neurochemical endpoints are dopamine beta hydroxylase (DBH), a key enzyme in NE synthesis, as well as MAO and catechol-O-methyl transferase (COMT), key enzymes responsible for the inactivation of monoamines. Zhang et al. [56] reported a reduction in DBH in the locus coeruleus following OVX which was associated with a reduction NE levels, and Sabban et al. [51] reported that estradiol potently increased activation of the DBH promoter in PC12 cells. Studies also have reported significant increases in MAO activity in the hypothalamus [57] and COMT activity in the prefrontal cortex [58] following ovariectomy in rats. Hence, estrogen regulation of these enzymes is brain region specific and may differ as a function of menopause and length of time in a hypoestrogenic state. Further clarification of how these enzymes contribute to the effects of surgical and transitional menopause on neurochemical endpoints will require a detailed analysis of the anabolic and catabolic enzymes associated with the regulation of monoamines in each of the affected brain structures.
4.5 Effects on TRP and TYR levels
This study is also the first to analyze levels of TRP and TYR following loss of ovarian function in the same tissues in which multiple monoaminergic endpoints were measured. Most striking was the reduction in the levels of TRP and TYR detected in the HPC, but not in the FCX or STR, at 1 week following OVX or VCD treatments, which suggests loss of ovarian function has substantial acute effects on amino acid homeostasis in this region of the brain. This effect was only observed in the HPC, was not observed in normal cycling rats, and levels returned to normal within 6 weeks. To our knowledge this is the first report of a substantial reduction in amino acid levels detected in the HPC following the loss of ovarian function.
At present, the mechanisms responsible for the reductions in TRP and TYR at the 1-week time-point are unknown. TYR and TRP are large, neutral, aromatic amino acids. Their brain levels are controlled in part by their plasma concentrations, but are even more dependent on plasma concentrations of other large neutral amino acid (LNAA) and Branched Chain Amino Acids (BCAA). Previous research has demonstrated that certain LNAA (e.g. Phenlyalanine, PHE) as well as BCAAS (leucine, LEU; isoleucine, ILE and valine, VAL) [59] compete with TYR and TRP for shared transportation through the BBB by System L (e.g. LAT1), which is located on both luminal and abluminal sides of the BBB. In addition, it is known that LAT1 in brain capillary endothelial cells is typically saturated by amino acids in plasma under normal conditions because its Km value is smaller than the plasma concentrations of substrate amino acids. Therefore, an elevation in the plasma concentration of either LNAA or BCAA will influence transport activity of LAT1 and could reduce brain uptake of TRP and TYR. One study showed that OVX caused an array of metabolic changes in amino acid metabolism. Plasma levels of PHE, LEU, ILE and VAL, for example, all significantly increased in OVX treated rats compared with controls [60]. Another study reported similar effects (i.e. increases in PHE and decreases in ALA, GLN, TRP) in rat serum following OVX [61]. It is possible, therefore, that in our study OVX and VCD treatments resulted in increased levels of competing amino acids in plasma, which contributed to a reduction in TYR, TRP transportation through the BBB. This, however, would not account for the selective reduction of TRP and TYR in the HPC and not in other brain regions.
Other possibilities include effects on amino acid metabolism, and the rate of protein synthesis, all of which have been reported to be affected by estrogen deficiency. Estrogen plays an important role of sustaining glucose as the primary fuel source in the brain. One study previously showed OVX caused substantial reductions in plasma glucose levels compared with controls [60]. This appears at first to be contrary to clinical observations that the prevalence of the metabolic syndrome such as hyperglycemia increases with menopause [62]. However, it should be noted that in rats hyperglycemia was observed at 13 weeks after OVX [63] while hypoglycemia was observed at 10 days after OVX [60], which is similar to the current study. Therefore, it is likely that estrogen deficiency leads to hypoglycemia at early time-points. Glucose transporter (GLUT-1) mediates glucose transport across the blood brain barrier (BBB) and is highly sensitive to changes in plasma glucose levels. Under normal conditions, GLUT-1 expression is closely regulated by glucose availability and is upregulated in the BBB during hypoglycemia [64]. However, it is found that OVX may induce a significant decline in expression of GLUT-1 in the hippocampus. Furthermore, the protein expression and activity of hexokinase, which irreversibly phosphorylates glucose to glucose-6-phosphate in the glycolysis process, also was significantly reduced following OVX [65]. Therefore, it is possible that by compromising these two rate-limiting steps, ovarian hormone loss further exacerbates decreased glucose availability and utilization in this brain structure. Previous research has reported that increased amino acid metabolism took place in rat neuron-enriched aggregate cultures when exposed to hypoglycaemic conditions [66]. Although not used primarily for cerebral energy production due to their low ATP production efficiency, glycogenic and ketogenic TYR and TRP might be degraded as alternative energy sources to form ketone bodies and TCA cycle intermediates (e.g. acetoacetate, fumarate and pyruvate) in response to short glucose supply.
5. CONCLUSIONS
Collectively, the results of our analysis describe significant changes in local levels of serotoninergic, dopaminergic, and noradrenergic endpoints in association with validated models of surgical and transitional menopause. Most notably, effects were brain-region specific and time-dependent. These changes are likely relevant to changes in neural function and cognitive performance associated with early and late time periods following menopause. However, interpretation of these findings need to be carried out with caution, since the levels of these neurochemical endpoints do not necessarily represent a dynamic measure of neuronal activities. Similar studies that focus on the anabolic and catabolic enzymes involved in monoamine regulation will further add to our understanding of how monoaminergic regulation is affected by loss of ovarian function. Studies evaluating the effects of treating OVX and VCD-treated rats with estrogen receptor agonists are currently in progress.
Supplementary Material
Highlights.
Comparison of surgical vs. transitional menopause on neurochemical markers in the brain.
Significant effects on serotoninergic, dopaminergic, and noradrenergic endpoints, as well as on levels of tryptophan and tyrosine, were detected.
Effects were brain-region specific and time-dependent.
Few differences between the two models on neurochemical endpoints were detected.
Acknowledgments
The study was supported by the National Institutes of Health (NIH) grant R21 AG043817.
Footnotes
Conflict of Interest Statement
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–98. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol. 2014;389(1–2):7–12. doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol. 2014;142:90–8. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez MG, et al. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16(3):e43–71. doi: 10.1111/j.1755-5949.2010.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839–50. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31(2):224–53. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(48):16137–48. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170(4):1045–55. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. Journal of chemical neuroanatomy. 2011;42(4):236–41. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moura PJ, Petersen SL. Estradiol acts through nuclear- and membrane-initiated mechanisms to maintain a balance between GABAergic and glutamatergic signaling in the brain: implications for hormone replacement therapy. Rev Neurosci. 2010;21(5):363–80. doi: 10.1515/revneuro.2010.21.5.363. [DOI] [PubMed] [Google Scholar]
- 11.Noriega NC, et al. Influence of 17beta-estradiol and progesterone on GABAergic gene expression in the arcuate nucleus, amygdala and hippocampus of the rhesus macaque. Brain Res. 2010;1307:28–42. doi: 10.1016/j.brainres.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman BL, Schorge JO, Schaffer JI. Reroductive endocrinology. In: Hoffman BL, Schorge JO, Schaffer JI, et al., editors. Williams Gynecology. Vol. 2. New York: McGraw-Hill; 2012. pp. 400–39. [Google Scholar]
- 13.Metcalfe K, et al. Effect of Oophorectomy on Survival After Breast Cancer in BRCA1 and BRCA2 Mutation Carriers. JAMA Oncol. 2015;1(3):306–13. doi: 10.1001/jamaoncol.2015.0658. [DOI] [PubMed] [Google Scholar]
- 14.LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 1988;38(4):780–9. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- 15.Wilkes MM, et al. Hypothalamic-pituitary-ovarian interactions during reproductive senescence in the rat. Adv Exp Med Biol. 1978;113:127–47. doi: 10.1007/978-1-4684-8893-7_8. [DOI] [PubMed] [Google Scholar]
- 16.Hoyer PB, et al. Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol. 2001;29(1):91–9. doi: 10.1080/019262301301418892. [DOI] [PubMed] [Google Scholar]
- 17.Lohff JC, et al. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp Med. 2005;55(6):523–7. [PubMed] [Google Scholar]
- 18.Lohff JC, et al. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause. 2006;13(3):482–8. doi: 10.1097/01.gme.0000191883.59799.2e. [DOI] [PubMed] [Google Scholar]
- 19.Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–87. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer LN, et al. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996;139(2):394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- 21.Acosta JI, et al. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150(9):4248–59. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camp BW, et al. High serum androstenedione levels correlate with impaired memory in the surgically menopausal rat: a replication and new findings. Eur J Neurosci. 2012;36(8):3086–95. doi: 10.1111/j.1460-9568.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mennenga SE, et al. Pharmacological blockade of the aromatase enzyme, but not the androgen receptor, reverses androstenedione-induced cognitive impairments in young surgically menopausal rats. Steroids. 2015;99(Pt A):16–25. doi: 10.1016/j.steroids.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengupta P. The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med. 2013;4(6):624–30. [PMC free article] [PubMed] [Google Scholar]
- 25.te Velde ER, et al. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;145(1–2):67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 26.Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. Mem Univ Calif. 1922;6:1–148. [Google Scholar]
- 27.Muhammad FS, et al. Effects of 4-vinylcyclohexene diepoxide on peripubertal and adult Sprague-Dawley rats: ovarian, clinical, and pathologic outcomes. Comp Med. 2009;59(1):46–59. [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Li J, et al. A microsomal based method to detect aromatase activity in different brain regions of the rat using ultra performance liquid chromatography-mass spectrometry. J Steroid Biochem Mol Biol. 2016;163:113–20. doi: 10.1016/j.jsbmb.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Cawood ML, et al. Testosterone measurement by isotope-dilution liquid chromatography-tandem mass spectrometry: validation of a method for routine clinical practice. Clin Chem. 2005;51(8):1472–9. doi: 10.1373/clinchem.2004.044503. [DOI] [PubMed] [Google Scholar]
- 32.Yao JK, Cheng P. Determination of multiple redox-active compounds by high-performance liquid chromatography with coulometric multi-electrode array system. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810(1):93–100. doi: 10.1016/j.jchromb.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson ME, et al. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology. 2015;156(7):2492–502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 34.Mayer LP, et al. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71(1):130–8. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu H, Bray GA. Effects of castration, estrogen replacement and estrus cycle on monoamine metabolism in the nucleus accumbens, measured by microdialysis. Brain Res. 1993;621(2):200–6. doi: 10.1016/0006-8993(93)90107-x. [DOI] [PubMed] [Google Scholar]
- 36.Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180(2):155–8. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 37.Desan PH, et al. Monoamine neurotransmitters and metabolites during the estrous cycle, pregnancy, and the postpartum period. Pharmacol Biochem Behav. 1988;30(3):563–8. doi: 10.1016/0091-3057(88)90066-4. [DOI] [PubMed] [Google Scholar]
- 38.Baudry M, Bi X, Aguirre C. Progesterone-estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–94. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heikkinen T, et al. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav. 2002;41(1):22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- 40.Toriizuka K, et al. Acupuncture inhibits the decrease in brain catecholamine contents and the impairment of passive avoidance task in ovariectomized mice. Acupunct Electrother Res. 1999;24(1):45–57. doi: 10.3727/036012999816356408. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, et al. Neurochemical characteristics and behavioral responses to psychological stress in ovariectomized rats. Pharmacol Res. 1999;39(6):455–61. doi: 10.1006/phrs.1999.0468. [DOI] [PubMed] [Google Scholar]
- 42.Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847–65. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pisani A, et al. Striatal synaptic plasticity: implications for motor learning and Parkinson’s disease. Mov Disord. 2005;20(4):395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- 44.Korol DL, Pisani SL. Estrogens and cognition: Friends or foes?: An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–15. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bitar MS, et al. Modification of gonadectomy-induced increases in brain monoamine metabolism by steroid hormones in male and female rats. Psychoneuroendocrinology. 1991;16(6):547–57. doi: 10.1016/0306-4530(91)90038-u. [DOI] [PubMed] [Google Scholar]
- 46.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508(1):1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts KM, Fitzpatrick PF. Mechanisms of tryptophan and tyrosine hydroxylase. IUBMB Life. 2013;65(4):350–7. doi: 10.1002/iub.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beattie CW, Rodgers CH, Soyka LF. Influence of ovariectomy and ovarian steroids on hypothalamic tyrosine hydroxylase activity in the rat. Endocrinology. 1972;91(1):276–9. doi: 10.1210/endo-91-1-276. [DOI] [PubMed] [Google Scholar]
- 49.Krieger A, Wuttke W. Effects of ovariectomy and hyperprolactinemia on tyrosine hydroxylase and dopamine-beta-hydroxylase activity in various limbic and hypothalamic structures. Brain Res. 1980;193(1):173–80. doi: 10.1016/0006-8993(80)90954-3. [DOI] [PubMed] [Google Scholar]
- 50.Ivanova T, Beyer C. Estrogen regulates tyrosine hydroxylase expression in the neonate mouse midbrain. J Neurobiol. 2003;54(4):638–47. doi: 10.1002/neu.10193. [DOI] [PubMed] [Google Scholar]
- 51.Sabban EL, et al. Divergent effects of estradiol on gene expression of catecholamine biosynthetic enzymes. Physiol Behav. 2010;99(2):163–8. doi: 10.1016/j.physbeh.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395(1):1–17. [PubMed] [Google Scholar]
- 53.Smith LJ, et al. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29(11):2035–45. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- 54.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60(3):288–95. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 55.Hiroi R, et al. Benefits of Hormone Therapy Estrogens Depend on Estrogen Type: 17beta-Estradiol and Conjugated Equine Estrogens Have Differential Effects on Cognitive, Anxiety-Like, and Depressive-Like Behaviors and Increase Tryptophan Hydroxylase-2 mRNA Levels in Dorsal Raphe Nucleus Subregions. Front Neurosci. 2016;10:517. doi: 10.3389/fnins.2016.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, et al. Mechanisms underlying alterations in norepinephrine levels in the locus coeruleus of ovariectomized rats: Modulation by estradiol valerate and black cohosh. Neuroscience. 2017;354:110–121. doi: 10.1016/j.neuroscience.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi T, et al. Fluctuations in Monoamine Oxidase Activity in the Hypothalamus of Rat during the Estrous Cycle and after Castration. Endocrinol Jpn. 1964;11:283–90. doi: 10.1507/endocrj1954.11.283. [DOI] [PubMed] [Google Scholar]
- 58.Schendzielorz N, et al. Complex estrogenic regulation of catechol-O-methyltransferase (COMT) in rats. J Physiol Pharmacol. 2011;62(4):483–90. [PubMed] [Google Scholar]
- 59.Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135(6 Suppl):1539S–46S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, et al. Metabonomic analysis reveals efficient ameliorating effects of acupoint stimulations on the menopause-caused alterations in mammalian metabolism. Sci Rep. 2014;4:3641. doi: 10.1038/srep03641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Assadi-Porter F, Selen E, Shen C. NMR-based metabolomics analysis in muscle and serum of middle-aged ovariectomized rats supplemented with 6-month green tea polyphenols. The FASEB Journal. 2015;29(1 Supplement):745.2. [Google Scholar]
- 62.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–11. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 63.Liu ML, et al. Influence of ovariectomy and 17beta-estradiol treatment on insulin sensitivity, lipid metabolism and post-ischemic cardiac function. Int J Cardiol. 2004;97(3):485–93. doi: 10.1016/j.ijcard.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 64.Carruthers A, et al. Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab. 2009;297(4):E836–48. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding F, et al. Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer’s. PLoS One. 2013;8(3):e59825. doi: 10.1371/journal.pone.0059825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honegger P, et al. Alteration of amino acid metabolism in neuronal aggregate cultures exposed to hypoglycaemic conditions. J Neurochem. 2002;81(6):1141–51. doi: 10.1046/j.1471-4159.2002.00888.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.