Abstract
Integrity of the default mode network (DMN) is believed to be essential for human consciousness. However, the effects of acute severe traumatic brain injury (TBI) on DMN functional connectivity are poorly understood. Furthermore, the temporal dynamics of DMN reemergence during recovery of consciousness have not been studied longitudinally in patients with acute severe TBI. We performed resting-state functional magnetic resonance imaging (rs-fMRI) to measure DMN connectivity in 17 patients admitted to the intensive care unit (ICU) with acute severe TBI and in 16 healthy control subjects. Eight patients returned for follow-up rs-fMRI and behavioral assessment six months post-injury. At each time point, we analyzed DMN connectivity by measuring intra-network correlations (i.e. positive correlations between DMN nodes) and inter-network anticorrelations (i.e. negative correlations between the DMN and other resting-state networks). All patients were comatose upon arrival to the ICU and had a disorder of consciousness (DoC) at the time of acute rs-fMRI (9.2 +/- 4.6 days post-injury): 2 coma, 4 unresponsive wakefulness syndrome, 7 minimally conscious state, and 4 post-traumatic confusional state. We found that, while DMN anticorrelations were absent in patients with acute DoC, patients who recovered from coma to a minimally conscious or confusional state while in the ICU showed partially preserved DMN correlations. Patients who remained in coma or unresponsive wakefulness syndrome in the ICU showed no DMN correlations. All eight patients assessed longitudinally recovered beyond the confusional state by 6 months post-injury and showed normal DMN correlations and anticorrelations, indistinguishable from those of healthy subjects. Collectively, these findings suggest that recovery of consciousness after acute severe TBI is associated with partial preservation of DMN correlations in the ICU, followed by long-term normalization of DMN correlations and anticorrelations. Both intra-network DMN correlations and inter-network DMN anticorrelations may be necessary for full recovery of consciousness after acute severe TBI.
Keywords: Coma, Consciousness, Traumatic brain injury, Default mode network, Resting-state functional MRI
1. Introduction
The default mode network (DMN) comprises brain regions that share a correlated pattern of spontaneous neuronal activity in the resting, conscious brain (Raichle et al., 2001; Shulman et al., 1997). Although its component circuits are still being mapped (Braga & Buckner, 2017), the DMN's role in sustaining human consciousness is well established and supported by multiple lines of evidence. Functional magnetic resonance imaging (fMRI) studies of healthy subjects show that resting self-awareness, introspection, and spontaneous cognition are mediated by the DMN (Andrews-Hanna, Reidler, Huang, & Buckner, 2010; Buckner, Andrews-Hanna, & Schacter, 2008; Mason et al., 2007; Whitfield-Gabrieli et al., 2011). In patients with chronic disorders of consciousness (DoC) caused by severe brain injuries, resting-state fMRI (rs-fMRI) studies show that DMN functional connectivity differs between conscious patients (i.e. minimally conscious state [MCS] and post-traumatic confusional state [PTCS]) and unconscious patients (i.e. unresponsive wakefulness syndrome [UWS], also known as the vegetative state) (Demertzi et al., 2015; Di Perri et al., 2016; Rosazza et al., 2016; Vanhaudenhuyse et al., 2010). Moreover, DMN connectivity is altered in the sleeping, anesthetized, and comatose brain (Boveroux et al., 2010; Greicius et al., 2008; Horovitz et al., 2009; Kirsch et al., 2017; Norton et al., 2012; Stamatakis, Adapa, Absalom, & Menon, 2010), suggesting a relationship between DMN connectivity and consciousness under a broad range of physiological and pathological conditions.
Yet DMN connectivity alone is not sufficient for recovery of consciousness after severe brain injury. Other resting-state functional networks such as the executive control network (ECN) and salience network (SN) contribute to human consciousness (Seeley et al., 2007), and there is emerging evidence that dynamic interactions between these networks are important for maintaining or recovering consciousness (Qin et al., 2015). The DMN is an “intrinsic” network most active during rest and introspection, while the ECN and SN are “extrinsic” networks most active during tasks and externally directed cognition (Buckner et al., 2008; Fox et al., 2005; Golland et al., 2007; Vanhaudenhuyse et al., 2011). DMN connectivity in the resting, conscious brain is thus characterized not only by DMN intra-network correlations (i.e. positive correlations between DMN nodes), but also by DMN inter-network anticorrelations (i.e. negative correlations between the DMN, ECN, and SN) (Amico et al., 2017; Boly et al., 2009; Di Perri et al., 2016; Fox et al., 2005; He et al., 2014). It is hypothesized that the resting, conscious brain undergoes spontaneous, dynamic toggling between anticorrelated brain networks engaged in awareness of self (DMN) and awareness of the environment (ECN and SN) (Crone et al., 2011; Di, Gohel, Kim, & Biswal, 2013; Fox et al., 2005; Fransson, 2005; Keller et al., 2013; Spadone et al., 2015).
Given that DMN intra-network correlations and inter-network anticorrelations both appear to be features of human consciousness, it is likely that both are necessary for recovery of consciousness after severe brain injury (Demertzi et al., 2015; Di Perri et al., 2016; Kondziella et al., 2017; Norton et al., 2012; Vanhaudenhuyse et al., 2010). DMN connectivity in acute DoC has been studied in patients with cardiac arrest and in heterogeneous groups of patients with cardiac arrest, trauma, and other causes of DoC (Norton et al., 2012; Sair et al., 2017; Silva et al., 2015). However, with the exception of a recent case series (Kondziella et al., 2017), DMN studies of patients with severe traumatic brain injury (TBI) have focused almost exclusively on patients with chronic DoC and have been cross-sectional (Bodien, Chatelle, & Edlow, 2017), precluding investigation of changes in DMN connectivity over time. Given the technical challenges of performing rs-fMRI acutely in the intensive care unit (ICU) and the difficulty of acquiring follow-up imaging data after discharge, little is known about DMN correlations or anticorrelations and how they reemerge in patients with acute traumatic DoC. Understanding the role of DMN correlations and anticorrelations in early recovery of consciousness has implications for identifying novel therapeutic targets and formulating prognoses that families rely upon to make time-sensitive decisions about continuation of life-sustaining therapy.
In this prospective study, we cross-sectionally and longitudinally analyze DMN correlations and anticorrelations in patients with acute DoC caused by severe TBI. We test three hypotheses: 1) DMN correlations and anticorrelations are decreased in patients with acute traumatic DoC compared to healthy subjects; 2) DMN correlations and anticorrelations differ between unconscious patients (i.e. coma and UWS) and patients with early recovery to MCS or PTCS; and 3) DMN correlations and anticorrelations normalize in patients who recover from DoC.
2. Materials and Methods
2.1. Experimental design
We prospectively enrolled 17 patients with acute traumatic coma admitted to the ICU at an academic institution. We enrolled patients who met the following inclusion criteria: 1) 18 to 65 years of age, 2) head trauma; 3) at least one neurological examination prior to ICU admission consistent with coma, defined by a Glasgow Coma Scale (GCS) score of ≤ 6 with no eye opening; and 4) no eye opening for at least 24 hours after injury. We excluded patients with a life expectancy of less than six months, prior severe brain injury or neurodegenerative disease, metal precluding MRI, or lack of English fluency prior to injury (as we provided instructions for the resting-state scan in English and collected data alongside previously described stimulus-based testing conducted in English) (Bodien, Giacino, & Edlow, 2017; Edlow et al., 2017). We acquired rs-fMRI data once the patient was deemed stable for transport by treating clinicians. Sedatives, anxiolytics, and analgesics were allowed for patient safety or comfort. We enrolled sixteen healthy age- and sex-matched controls. We obtained written, informed consent from patients' surrogates and from healthy volunteers. Investigators attempted to contact all surviving patients or surrogates after hospital discharge to schedule a 6-month follow-up rs-fMRI. The Institutional Review Board approved the research protocol.
2.2. Behavioral assessments
Immediately prior to MRI, each patient's level of consciousness was categorized as coma, UWS, MCS, or PTCS using one Coma Recovery Scale-Revised (CRS-R) assessment and components of the Confusion Assessment Protocol (CAP) (Giacino, Kalmar, & Whyte, 2004; Sherer, Nakase-Thompson, Yablon, & Gontkovsky, 2005; Stuss et al., 1999). Although patients with PTCS demonstrate no deficit in arousal, they exhibit abnormal awareness characterized by disorientation and marked behavioral dysregulation (Giacino, Fins, Laureys, & Schiff, 2014; Russell, 1932; Symonds, 1928). We therefore consider PTCS a DoC, and resolution of PTCS a marker of full recovery of consciousness.
2.3. MRI data acquisition
We performed all scans on a 3 Tesla Skyra MRI scanner (Siemens Medical Solutions) using a 32-channel head coil. For registration, we acquired T1-weighted multi-echo magnetization prepared gradient echo (MEMPRAGE) images in the sagittal plane [176 slices, echo time=1.69, 3.55, 5.41, 7.27 ms, repetition time=2530 ms, 1.0 mm3 isotropic resolution, matrix size=256×256, flip angle=7°, inversion time=1200-1300 ms; (van der Kouwe, Benner, Salat, & Fischl, 2008)]. We performed rs-fMRI using a blood-oxygen-level-dependent sequence with 432 seconds of data acquisition, 49 axial slices, echo time=30 ms, repetition time=2400 ms, voxel size=3.4×3.4×3.5 mm, and matrix size=64×64. Prior to rs-fMRI, we instructed each subject to “close your eyes and relax.” We chose the “eyes closed” condition because the comatose patients could not open their eyes for the scan.
2.4. Functional connectivity analysis
We analyzed rs-fMRI data using the CONN functional connectivity toolbox (www.nitrc.org/projects/conn) (Whitfield-Gabrieli & Nieto-Castanon, 2012). Preprocessing included slice-timing correction, realignment, structural segmentation, normalization into Montreal Neurological Institute (MNI-152) space, and smoothing with a 6 mm full-width at half-maximum Gaussian kernel. Scans were inspected to ensure appropriate normalization (Supplementary Fig. 1), and no lesion extraction or masking was performed. We included motion parameters and first temporal derivatives as first-level covariates. The artifact rejection tool (ART) was used to reject outliers satisfying at least one of the following thresholds: normalized global BOLD signal Z ≥ 3.0, absolute subject motion ≥ 0.5 mm, absolute subject rotation ≥ 0.05 radians, scan-to-scan motion ≥ 1.0 mm, and scan-to-scan rotation ≥ 0.02 radians. Number of volumes rejected per subject was compared between groups and included as a regressor in second-level analysis. For denoising, we used CSF and white matter principal components as nuisance covariates in accordance with the anatomical component-based noise correction method (aCompCor) (Behzadi, Restom, Liau, & Liu, 2007; Muschelli et al., 2014). After denoising, we isolated low-frequency fluctuations with a low-pass temporal filter (0.0080.09Hz).
We investigated functional connectivity using a seed-based approach, in which the mean time series in a seed region is compared with the time series of all other voxels in the brain. Consistent with established DMN nodes (Raichle, 2011), we used four 10-mm diameter spherical seeds: one in the medial prefrontal cortex (MPFC), one in the posterior cingulate cortex (PCC), and one in each of the left and right lateral parietal cortices (LP). To develop functional correlation maps, we generated a mean time series by averaging the time series among the four DMN seeds (Demertzi et al., 2015). We calculated Pearson's correlation coefficients between the mean time series and that of each voxel in the brain, followed by Fisher z-transformation for inter-subject comparison. Sedation was ordinally stratified: (1) no sedation; (2) intermittent administration of nonanesthetic agents; (3) low-dose continuous infusion of anesthetic agents; and (4) high-dose continuous infusion of anesthetic agents. The MRI and behavioral examinations were performed independently. The investigator who performed the functional connectivity analysis (Z.D.T.) was blinded to the results of the behavioral evaluations.
2.5. Statistical analysis
We adjusted correlation maps for significance with P<0.001 height-level and false discovery rate (FDR)-corrected P<0.05 cluster-level thresholds. We generated average DMN correlation maps for patients stratified by level of consciousness. We investigated the following group differences using the general linear model with motion as a covariate, accounting for repeated measures as appropriate in longitudinal comparisons: 1) healthy subjects versus all patients with acute DoC; 2) conscious patients (MCS, PTCS) versus unconscious patients (coma, UWS); 3) patients at 6-month follow-up versus healthy subjects; and 4) patients at 6-month follow-up versus the same patients with acute traumatic DoC. Mean connectivity z-scores were extracted from the largest clusters and compared between groups to assess the directionality of observed differences. Connectivity was compared with CRS-R scores.
To calculate global mean DMN correlation and anticorrelation values, we computed an average connectivity map for all subjects using the four seeds described above, with motion as a regressor. Regions with positive correlations delineated a correlation mask, and regions with negative correlations delineated an anticorrelation mask (Supplementary Fig. 2). We extracted the mean z-score within each mask to generate a global mean correlation value and anticorrelation value at the single-subject level. We tested for group differences between coma/UWS, MCS/PTCS, and healthy subjects using analysis of variance followed by post-hoc Tukey's test. Group differences between acute and follow-up scans of the same patients were analyzed with a paired t-test.
Both FDR and family-wise error (FWE) correction are means of controlling for multiple comparisons in connectivity studies. Although Type 1 error in cluster-level significance is mitigated by the use of a conservative height-level threshold of P<0.001 in the present study, FWE correction may be a more conservative method and may reduce the false positive rate (Woo, Krishnan, & Wager, 2014). In a post-hoc investigation, we repeated the analysis using FWE-corrected cluster-level thresholds. All statistical analyses were performed in GraphPad Prism v7.0 (GraphPad; LaJolla, CA).
3. Results
We enrolled 17 patients (12 male, mean+/-standard deviation age=29+/-9 years; Supplementary Table 1) and 16 healthy subjects (12 male, 29+/-8 years). At the time of acute rs-fMRI (9.2+/-4.6 days after injury), 2 patients were in coma, 4 UWS, 7 MCS, and 4 PTCS. DMN inter-network anticorrelations were absent acutely in both the conscious and unconscious patient groups. DMN intra-network correlations were absent in unconscious patients (coma/UWS) but partially intact in conscious patients (MCS/PTCS) (Fig. 1). Compared with unconscious patients, conscious patients had significantly stronger DMN connectivity in the PCC and MPFC (Fig. 2 and 3), and connectivity in these clusters was associated with the CRS-R total score (Supplementary Fig. 3; ρ=0.88).
Fig. 1. Default mode network functional connectivity.
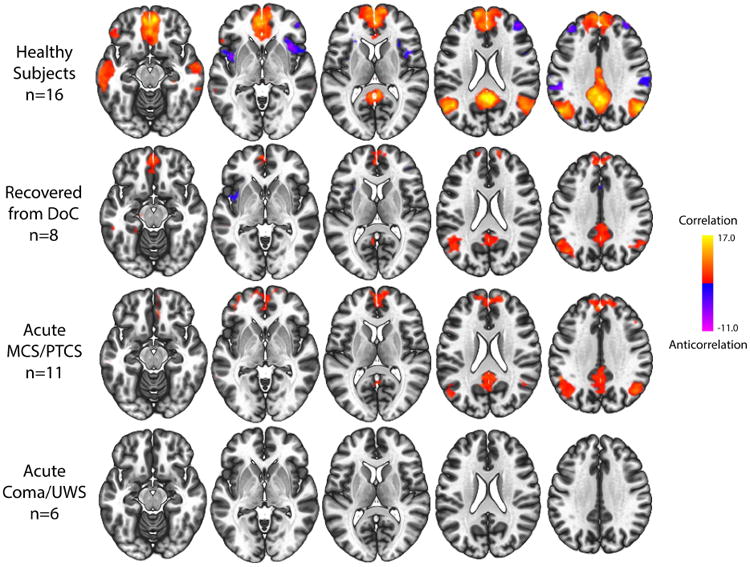
Fisher's z-transformed correlation maps of resting-state functional connectivity using four default mode network (DMN) seeds (thresholded height-level P<0.001; cluster-level false discovery rate-corrected P<0.05) in healthy controls, patients who fully recovered consciousness at 6-month follow-up, patients who acutely recovered to MCS/PTCS, and patients with coma or UWS.
Fig. 2. Differences in default mode network functional connectivity between groups.
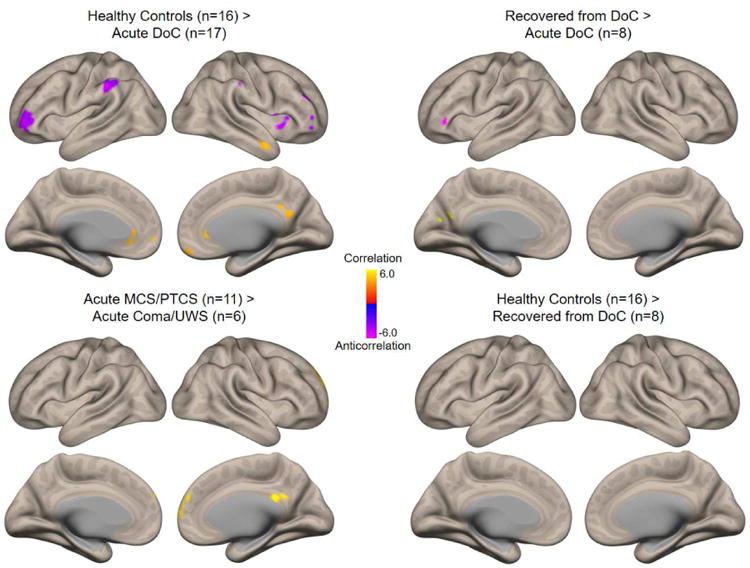
Comparisons of resting-state functional connectivity among groups of interest (thresholded height-level P<0.001; cluster-level false discovery rate-corrected P<0.05). Clockwise from upper left: regions with stronger default mode network (DMN) connectivity in healthy subjects than in patients with acute traumatic disorders of consciousness (DoC); regions with stronger connectivity in patients who fully recovered consciousness at 6 months than in the same patients acutely after TBI; connectivity in healthy subjects compared with patients who fully recovered consciousness by 6-month follow-up; and regions with stronger connectivity in patients with acute MCS/PTCS than in patients with acute coma/UWS. Comparisons used the general linear model with motion as a regressor, accounting for paired measures in the longitudinal comparison of recovered versus acute patients.
Fig. 3. Quantitative connectivity within significant clusters.
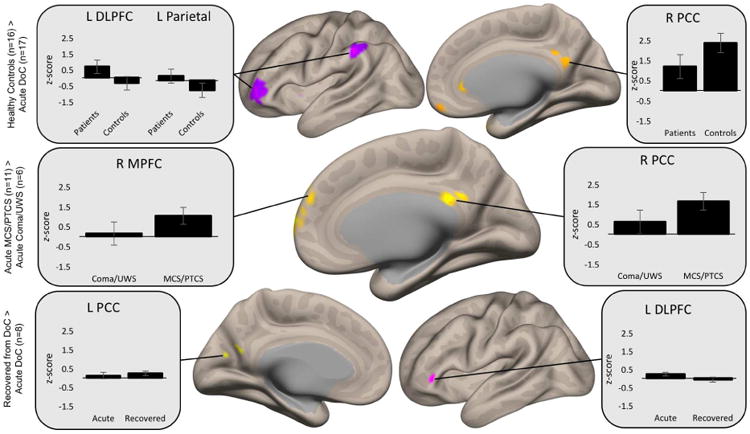
Mean connectivity z-scores were extracted from the largest clusters generated within each between-group comparison to assess the directionality of observed differences. Compared to healthy subjects, patients demonstrate diminished correlation in DMN nodes (right posterior cingulate cortex [R PCC] shown), as well as pathologic correlation in regions typically anticorrelated with the DMN (left dorsolateral prefrontal cortex [L DLPFC] and left parietal cortex). Unconscious patients have reduced correlation in the right medial prefrontal cortex (R MPFC) and R PCC compared to conscious patients. Among patients followed longitudinally, correlations strengthen in the left PCC (L PCC), and pathological correlations in the L DLPFC appropriately become anticorrelated.
Motion differed between patients and healthy controls, with a mean of 38 volumes rejected per patient versus 3 volumes per control subject (P<0.01). However, there was no difference in the number of volumes rejected in conscious versus unconscious patients (P=0.40). Among longitudinally followed patients, there was no difference in the number of volumes rejected in the acute and follow-up scans (P=0.09). The number of rejected volumes per subject was included as a regressor in all subsequent analyses.
Among coma/UWS patients, MCS/PTCS patients, and healthy subjects, the magnitudes of global DMN correlations (F[2,30]=9.09, P<0.001) and anticorrelations (F[2,30]=11.8, P<0.001) differed (Fig. 4). Five DoC patients (1 coma, 1 UWS, 2 MCS, 1 PTCS) had positive mean global DMN anticorrelations, suggesting pathological DMN correlations within typically anticorrelated regions. Control subjects showed no pathological correlations. Although some patients received anxiolytic, sedative, or analgesic agents (Supplementary Table 2), analysis of variance showed no difference in global DMN connectivity among sedation groups for either correlations (F[3,13]=0.13, P=0.94) or anticorrelations (F[3,13]=1.42, P=0.28).
Fig. 4. Global default mode network correlation and anticorrelation.
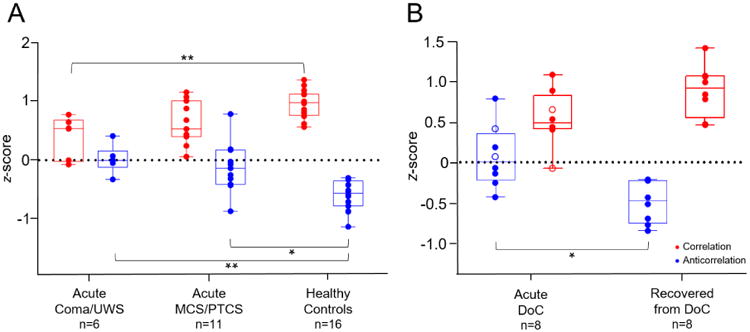
A) Mean global default mode network (DMN) correlation and DMN anticorrelation Fisher's transformed z-scores, by group. Boxes show interquartile range; whiskers extend to minimum and maximum values. Comparisons between coma/UWS, MCS/PTCS, and healthy subjects were conducted with analysis of variance followed by Tukey's test. * = P<0.002; ** = P<0.001. B) Mean global DMN correlation and DMN anticorrelation z-scores in eight patients followed longitudinally. Comparison was conducted with a paired t-test. Open circles represent unconscious patients (coma/UWS), while closed circles represent conscious patients (MCS/PTCS). * = P<0.01.
Eight patients (1 coma, 1 UWS, 3 MCS, 3 PTCS) underwent repeat rs-fMRI and standardized behavioral assessment (median [IQR]=199 [189-269] days post-injury). At the time of follow-up, all patients had recovered from DoC, as indicated by CAP scores, and all had DMN correlations and anticorrelations indistinguishable from those in healthy subjects (Fig. 2). Seven patients demonstrated longitudinal increases in magnitude of correlations and anticorrelations. Paired t-tests showed that changes in anticorrelations were significant (P=0.01), while changes in correlations trended towards significance (P=0.054; Supplementary Fig. 4). Of the nine patients who did not return for follow-up, two died in the ICU and seven were unable to return to the hospital.
Post-hoc re-analysis with FWE cluster-level correction instead of FDR yielded the same results except for loss of significance of the right PCC cluster in the Controls > Acute contrast (Fig. 3). This cluster remained significant in all other comparisons tested (Figs. 2 and 3). Global DMN correlation and anticorrelation values did not change significantly (Supplementary Table 3). Post-hoc analysis confirmed no difference in age (ANOVA F[2,30]=0.22; P=0.80) or sex (Fisher's exact test; P=0.05) among the subgroups analyzed.
4. Discussion
This prospective, longitudinal study provides initial evidence that both DMN correlations and anticorrelations are disrupted in patients with acute severe TBI, then normalize sequentially as level of consciousness improves. In our sample, anticorrelations were absent in all patients with acute traumatic DoC. Correlations were absent in unconscious patients. However, correlations were partially intact in patients who regained consciousness acutely. This finding suggests that inter-network DMN anticorrelations are more severely altered than intra-network DMN correlations in the acute stage of recovery from severe TBI. At follow-up, all patients fully recovered consciousness and DMN correlations and anticorrelations normalized, suggesting that anticorrelations reemerge after correlations. Moreover, among patients, anticorrelations significantly improved over time and correlations trended toward improvement. Collectively, these observations suggest that both intra- and inter-network DMN connectivity contribute to full recovery of consciousness after acute severe TBI.
This study, while limited in size, is the first demonstration of longitudinal reemergence of DMN correlations and anticorrelations in patients recovering from traumatic coma. Our observation that correlations reemerge before anticorrelations is consistent with prior findings of stepwise reemergence of DMN connectivity in healthy subjects regaining consciousness after anesthetic-induced coma (Boveroux et al., 2010; Greicius et al., 2008). The physiologic mechanisms by which DMN anticorrelations contribute to consciousness remain unclear, but evidence suggests that anticorrelations reflect toggling between introspective self-awareness and externally directed awareness of the environment (Crone et al., 2011; Di Perri et al., 2016; Di et al., 2013; Fox et al., 2005; Fransson, 2005; Keller et al., 2013; Spadone et al., 2015). This interpretation is supported by our observation that DMN anticorrelations were present within the dorsolateral prefrontal cortex, frontoinsular cortex, and lateral parietal cortices, nodes of the ECN and SN that mediate externally directed cognition (Seeley et al., 2007).
We also observed preservation of DMN correlations within the PCC and MPFC in conscious (MCS/PTCS) but not unconscious (coma/UWS) patients acutely, concordant with prior studies in chronic DoC patients (Demertzi et al., 2015; Vanhaudenhuyse et al., 2010). Our findings thus extend the time window during which the PCC and MPFC appear to act as central “hub” nodes of the DMN from the chronic setting to the acute setting of the ICU. Interestingly, we did not detect partial DMN correlations in patients with acute traumatic coma and UWS. Partial preservation of DMN connectivity has been observed in patients with chronic UWS caused by multiple types of brain injury (Demertzi et al., 2015; Vanhaudenhuyse et al., 2010). Our findings suggest that, even in the unconscious state, reemergence of DMN correlations may be time-dependent. It is also possible that sample inhomogeneity or administration of anesthetic agents in 1 of 4 UWS patients in this study contributed to lack of DMN intra-network correlations in coma and UWS patients.
Our results should be interpreted in the context of several limitations. First, given the inherent challenges of performing rs-fMRI in critically ill patients with acute DoC, our sample size is relatively small compared with those of recently published studies of patients with chronic DoC (Demertzi et al., 2015; Di Perri et al., 2016; Qin et al., 2015; Rosazza et al., 2016; Wu et al., 2015). Nevertheless, we found significant group differences both cross-sectionally and longitudinally, suggesting the possibility of a large effect size of DMN connectivity on behavioral measures of consciousness.
Additionally, though we did not find a significant effect of sedation on DMN connectivity in our patient sample, it remains possible that sedation confounded our results. The relationship between sedation and functional connectivity is complex and difficult to measure because multiple patient-specific factors, including body mass, tolerance, metabolism, and clearance may alter the effect of a sedative on cortical connectivity. Recent fMRI studies suggest that severe brain injury alters cortical function more than does sedation (Bodien, Giacino, et al., 2017; Davis et al., 2007; Edlow et al., 2017; Greicius et al., 2008; Kirsch et al., 2017), but more work is needed to elucidate how sedation affects functional connectivity in brain-injured patients.
We evaluated level of consciousness using a single CRS-R assessment conducted immediately prior to the MRI. A recent study suggests that, in patients with chronic DoC, at least five CRS-R assessments are required to reliably detect MCS (Wannez et al., 2017). In patients with acute DoC being treated in the ICU, it remains unknown how many assessments are required to achieve a reliable diagnosis because the natural rate of recovery is faster than in the chronic state and there may be frequent fluctuations in level of arousal. Furthermore, critically ill patients with acute DoC are often sedated or medically unstable. Therefore, obtaining even one full CRS-R examination in this patient population is challenging. Future studies will need to address the need for repeated CRS-R examinations in the ICU setting.
Patients with DoC moved in the scanner more than did healthy control subjects. However, we found that the number of rejected volumes did not differ between unconscious (coma/UWS) and conscious (MCS/PTCS) patients acutely or between baseline and follow-up scans in the eight subjects analyzed longitudinally. Nevertheless, we controlled for the potential contribution of motion by rejecting motion-corrupted volumes and including the number of rejected volumes as a covariate in the linear regression model. Although non-linear interactions could not be accounted for, our approach minimized the potential confounding effects of motion on the results. It is also notable that the duration of rs-fMRI data acquisition in this study was limited to 7 minutes and 12 seconds due to concerns for the safety of critically ill patients, though longer scan times may increase intra- and inter-session reliability (Birn et al., 2013). Additionally, subjects may fall asleep during scanning, which potentially influences functional connectivity results (Tagliazucchi & Laufs, 2014). All subjects undergoing fMRI were asked if they were awake at the end of the scan. All healthy subjects and patients who returned for the follow-up assessment remained awake, but the state of arousal could not be assessed in patients with acute DoC. Finally, although this is the first longitudinal study of the temporal dynamics of DMN connectivity in acute DoC, only eight patients returned for follow-up imaging. Because all eight recovered from DoC, the prognostic value of early DMN connectivity could not be assessed. Future rs-fMRI investigations with larger samples and additional time points are needed to determine the prognostic relevance of early DMN connectivity and to more precisely map the temporal dynamics of DMN connectivity during recovery from severe TBI. A key unanswered question is whether patients with early recovery from coma to MCS experience a reemergence of partial DMN correlations or if these correlations are present even during coma, and thus may have predicted the early behavioral recovery.
In summary, we show that recovery of consciousness after severe TBI is associated with sequential normalization of DMN correlations and anticorrelations. Our findings support the growing body of evidence suggesting that both DMN correlations and anticorrelations are essential for full recovery of consciousness after severe brain injury. In addition to elucidating the physiologic mechanisms underlying recovery of consciousness, early analysis of DMN connectivity has the potential to improve the accuracy of behavioral diagnosis and prognostication for critically ill patients with acute severe TBI. If our findings are validated in larger studies, DMN connectivity may also be used as a pharmacodynamic biomarker to test the efficacy of therapies aimed at promoting recovery of consciousness.
Supplementary Material
Acknowledgments
The authors thank the nursing staff of the Massachusetts General Hospital Neurosciences ICU, Multidisciplinary ICU, and Surgical ICU. We are grateful to the patients and families in this study for their participation and support. We acknowledge Dylan Tisdall and Andre van der Kouwe (Athinoula A. Martinos Center for Biomedical Imaging) and Himanshu Bhat (Siemens Medical Center) for the provision of WIP711D (vNav Motion-Corrected Multiecho MPRAGE) used to acquire MEMPRAGE data. This work was supported by grants from the NIH National Institute of Neurological Disorders and Stroke (K23NS094538), the Center for Integration of Medicine & Innovative Technology (Boston, MA, USA), the American Academy of Neurology/American Brain Foundation, the James S. McDonnell Foundation, the Massachusetts General Hospital Department of Neurology and Division of Neurocritical Care and Emergency Neurology, and the National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR), Administration for Community Living (90DP0039, Spaulding-Harvard TBI Model System). The contents of this manuscript do not necessarily represent the policy of the U.S. Department of Health and Human Services, and endorsement by the federal government should not be assumed.
Footnotes
Conflict of Interest: There is no conflict of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amico E, Marinazzo D, Di Perri C, Heine L, Annen J, Martial C, et al. Goni J. Mapping the functional connectome traits of levels of consciousness. Neuroimage. 2017;148:201–211. doi: 10.1016/j.neuroimage.2017.01.020. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104(1):322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, et al. Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodien YG, Chatelle C, Edlow BL. Functional Networks in Disorders of Consciousness. Semin Neurol. 2017;37(5):485–502. doi: 10.1055/s-0037-1607310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodien YG, Giacino JT, Edlow BL. Functional MRI Motor Imagery Tasks to Detect Command Following in Traumatic Disorders of Consciousness. Front Neurol. 2017;8:688. doi: 10.3389/fneur.2017.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Tshibanda L, Vanhaudenhuyse A, Noirhomme Q, Schnakers C, Ledoux D, et al. Laureys S. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. [Research Support, Non-U.S. Gov't] Hum Brain Mapp. 2009;30(8):2393–2400. doi: 10.1002/hbm.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, et al. Boly M. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. [Comparative Study Research Support, Non-U.S. Gov't] Anesthesiology. 2010;113(5):1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- Braga RM, Buckner RL. Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron. 2017;95(2):457–471 e455. doi: 10.1016/j.neuron.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Crone JS, Ladurner G, Holler Y, Golaszewski S, Trinka E, Kronbichler M. Deactivation of the default mode network as a marker of impaired consciousness: an fMRI study. PLoS One. 2011;6(10):e26373. doi: 10.1371/journal.pone.0026373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, et al. Menon DK. Dissociating speech perception and comprehension at reduced levels of awareness. [Research Support, Non-U.S. Gov't] Proc Natl Acad Sci U S A. 2007;104(41):16032–16037. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demertzi A, Antonopoulos G, Heine L, Voss HU, Crone JS, de Los Angeles C, et al. Laureys S. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain. 2015;138(Pt 9):2619–2631. doi: 10.1093/brain/awv169. [DOI] [PubMed] [Google Scholar]
- Di Perri C, Bahri MA, Amico E, Thibaut A, Heine L, Antonopoulos G, et al. Laureys S. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: a cross-sectional multimodal imaging study. Lancet Neurol. 2016;15(8):830–842. doi: 10.1016/S1474-4422(16)00111-3. [DOI] [PubMed] [Google Scholar]
- Di X, Gohel S, Kim EH, Biswal BB. Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Front Hum Neurosci. 2013;7:493. doi: 10.3389/fnhum.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Chatelle C, Spencer CA, Chu CJ, Bodien YG, O'Connor KL, et al. Wu O. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399–2414. doi: 10.1093/brain/awx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. [Comparative Study Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S.] Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10(2):99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020–2029. doi: 10.1016/j.apmr.2004.02.033. doi:S0003999304004770 [pii] [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, et al. Malach R. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17(4):766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Hum Brain Mapp. 2008;29(7):839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JH, Yang Y, Zhang Y, Qiu SY, Zhou ZY, Dang YY, et al. Xu RX. Hyperactive external awareness against hypoactive internal awareness in disorders of consciousness using resting-state functional MRI: highlighting the involvement of visuo-motor modulation. NMR Biomed. 2014;27(8):880–886. doi: 10.1002/nbm.3130. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009;106(27):11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, et al. Mehta AD. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci. 2013;33(15):6333–6342. doi: 10.1523/JNEUROSCI.4837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch M, Guldenmund P, Ali Bahri M, Demertzi A, Baquero K, Heine L, et al. Laureys S. Sedation of Patients With Disorders of Consciousness During Neuroimaging: Effects on Resting State Functional Brain Connectivity. Anesth Analg. 2017;124(2):588–598. doi: 10.1213/ANE.0000000000001721. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Fisher PM, Larsen VA, Hauerberg J, Fabricius M, Moller K, Knudsen GM. Functional MRI for Assessment of the Default Mode Network in Acute Brain Injury. Neurocrit Care. 2017 doi: 10.1007/s12028-017-0407-6. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage. 2014;96:22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Disruptions of functional connectivity in the default mode network of comatose patients. [Research Support, Non-U.S. Gov't] Neurology. 2012;78(3):175–181. doi: 10.1212/WNL.0b013e31823fcd61. [DOI] [PubMed] [Google Scholar]
- Qin P, Wu X, Huang Z, Duncan NW, Tang W, Wolff A, et al. Northoff G. How are different neural networks related to consciousness? Ann Neurol. 2015;78(4):594–605. doi: 10.1002/ana.24479. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain Connect. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C, Andronache A, Sattin D, Bruzzone MG, Marotta G, Nigri A, et al. Coma Research Centre - Besta, I. Multimodal study of default-mode network integrity in disorders of consciousness. Ann Neurol. 2016 doi: 10.1002/ana.24634. [DOI] [PubMed] [Google Scholar]
- Russell WR. Cerebral involvement in head injury: a study based on the examination of two hundred cases. Brain. 1932;55(4):549–603. [Google Scholar]
- Sair HI, Hannawi Y, Li S, Kornbluth J, Demertzi A, Di Perri C, et al. Recovery, C. Early Functional Connectome Integrity and 1-Year Recovery in Comatose Survivors of Cardiac Arrest. Radiology. 2017;162161 doi: 10.1148/radiol.2017162161. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer M, Nakase-Thompson R, Yablon SA, Gontkovsky ST. Multidimensional assessment of acute confusion after traumatic brain injury. Arch Phys Med Rehabil. 2005;86(5):896–904. doi: 10.1016/j.apmr.2004.09.029. doi:S0003999305000080[pii]10.1016/j.apmr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Silva S, de Pasquale F, Vuillaume C, Riu B, Loubinoux I, Geeraerts T, et al. Peran P. Disruption of posteromedial large-scale neural communication predicts recovery from coma. Neurology. 2015;85(23):2036–2044. doi: 10.1212/WNL.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadone S, Della Penna S, Sestieri C, Betti V, Tosoni A, Perrucci MG, et al. Corbetta M. Dynamic reorganization of human resting-state networks during visuospatial attention. Proc Natl Acad Sci U S A. 2015;112(26):8112–8117. doi: 10.1073/pnas.1415439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis EA, Adapa RM, Absalom AR, Menon DK. Changes in resting neural connectivity during propofol sedation. PLoS One. 2010;5(12):e14224. doi: 10.1371/journal.pone.0014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Binns MA, Carruth FG, Levine B, Brandys CE, Moulton RJ, et al. Schwartz ML. The acute period of recovery from traumatic brain injury: posttraumatic amnesia or posttraumatic confusional state? J Neurosurg. 1999;90(4):635–643. doi: 10.3171/jns.1999.90.4.0635. [DOI] [PubMed] [Google Scholar]
- Symonds CP. Observations on the differential diagnosis and treatment of cerebral states consequent upon head injuries. Br Med J. 1928;2(3540):829–832. doi: 10.1136/bmj.2.3540.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. 2014;82(3):695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, et al. Laureys S. Two distinct neuronal networks mediate the awareness of environment and of self. [Research Support, Non-U.S. Gov't] J Cogn Neurosci. 2011;23(3):570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. Boly M. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Brain. 2010;133(Pt 1):161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannez S, Heine L, Thonnard M, Gosseries O, Laureys S Coma Science Group, c. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol. 2017;81(6):883–889. doi: 10.1002/ana.24962. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55(1):225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zou Q, Hu J, Tang W, Mao Y, Gao L, et al. Yang Y. Intrinsic Functional Connectivity Patterns Predict Consciousness Level and Recovery Outcome in Acquired Brain Injury. J Neurosci. 2015;35(37):12932–12946. doi: 10.1523/JNEUROSCI.0415-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


