Abstract
Innate immune signaling is an important feature in the pathology of alcohol use disorders. Alcohol abuse causes persistent innate immune activation in the brain. This is seen in postmortem human alcoholic brain specimens, as well as in primate and rodent models of alcohol consumption. Further, in vitro models of alcohol exposure in neurons and glia also demonstrate innate immune activation. The activation of the innate immune system seems to be important in the development of alcohol use pathology, as anti-immune therapies reduce pathology and ethanol self-administration in rodent models. Further, innate immune activation has been identified in each of the stages of addiction: binge/intoxication, withdrawal/negative affect, and preoccupation/craving. This suggests that innate immune activation may play a role both in the development and maintenance of alcoholic pathology. In this chapter, we discuss the known contributions of innate immune signaling in the pathology of alcohol use disorders, and present potential therapeutic interventions that may be beneficial for alcohol use disorders.
Keywords: Alcohol, Addiction, Neuroimmune, Treatment
1. Immune Signaling in the Brain
1.1 Immune Cells in the Brain
The brain has effective mechanisms for protection against infectious agents. Primarily, this is thought to occur via the maintenance of its “immune privileged” status by the physical protection of the blood-brain barrier. However, the brain also has resident immune defenses, which are primarily innate immune cells. In the brain the resident immune cells are primarily microglia and astrocytes. These cells are capable of recognizing and responding to viral, bacterial and fungal pathogens. Microglia, astrocytes and neurons contain innate immune signaling receptors and capabilities.
Microglia are often considered the resident macrophages of the brain. However, microglia are unique from peripheral macrophages. Microglia are the only myeloid cells that originate from yolk-sac progenitor cells[1, 2]. Microglia are formed early in embryonic brain development (E8 in mice) and maintain stable levels in adulthood through neuro-proliferation throughout the lifespan[3]. There remains debate about whether peripheral monocytes migrate into brain in normal physiological circumstances[4]. Both microglia and macrophages share multiple markers such as Cluster of Differentiation (CD)-45, CD11b, and Ionized calcium-binding adapter molecule 1 (Iba-1). However, microglia-retain unique functions from peripheral monocytes, such as an involvement in synaptic pruning, debris clearance, and the regulation of adult neurogenesis[5–7]. Microglia transition from physiological “resting” state to activated states in response to infections, stressors and drugs of abuse such as alcohol and cocaine[8–14]. Microglial activation state is traditionally classified as M1 (pro-inflammatory) or M2 (anti-inflammatory), though their function is likely more complicated. The M1 activation state is defined by the release of pro-inflammatory cytokines such as Tumor Necrosis Factor alpha (TNFα), Interleukin (IL)-1β and IL-6 with accompanying reactive oxygen species generation by Inducible Nitric Oxide Synthase (iNOS) and Nicotinamide Adenine Dinucleotide Phosphate-(NADPH) Oxidase expression. M2 state is defined by the release of “anti-inflammatory” cytokines such as IL-10 and IL-4. These activation states are a simplified framework to understand microglial activation. Alcohol and other drugs of abuse modulate microglial activation, contributing to disease pathology.
Astrocytes are also involved in neuroimmune responses in the brain[15]. Astrocytes express immune receptors and release cytokines when activated[16]. This activation, known as reactive gliosis, can limit tissue damage in several contexts[17]. Astrocytes might also have pro- and anti-inflammatory states, similar to microglia[18], but this has yet to be fully elucidated. Astrocytes can be activated by microglia to release neurotoxic factors that damage neurons[19–21]. We found that the expression of many immune genes and receptors are unchanged in brain after pharmacological depletion of microglia with the compound Plexicon[22]. Thus, astrocytes and neurons seem to express higher levels of immune mediators than previously believed. There continues to be debate regarding the exact immune function of astrocytes. In Sections 1.3 and 2.3 specific immune receptors on astrocytes are discussed. This is currently being investigated with in vitro culture models as well as chemogenetic in vivo models. In addition to immune function, astrocytes are also involved in fluid homeostasis, metabolic support of neurons and modulation of glutamate concentrations at the synapse[23]. Drugs of abuse such as alcohol and cocaine cause astrocyte activation[14, 24, 25]. It is important to note that since microglia and astrocytes regulate synaptic plasticity, activation of immune signaling in these cells might alter synaptic firing and neuroplasticity.
Though glia (i.e. microglia and astrocytes) are considered the primary neuroimmune cells, neurons also seem to play a role in innate immune responses[26, 27]. Neurons can regulate glial responses through factors such as fractalkine, and also express many cytokine receptors, such as those for TNFα, IL-1β, IL-6 and the interferons (IFNs)[28]. Immune molecules have normal physiological roles in neurons that regulate synaptic firing and plasticity. For instance IL-1β modulates γ-aminobutyric acid (GABA) transmission in the central nucleus of the amygdala[29, 30] and Monocyte Chemoattractant Protein (MCP-1) increases dopamine release in the rat substantia nigra[31]. The effects of cytokines and chemokines on ethanol-responses are discussed in Section 2. Thus, neurons contain and respond to immune signaling molecules. These cytokines and other immune signaling molecules not only regulate immune responses, but they modulate synapses and neurocircuits.
1.2 Innate immune signaling molecules as modulators of neurocircuitry
Increasing evidence from brain studies indicate the neuroimmune system is involved in the regulation of brain function, apart from its role in response to pathogens. Several immune signaling molecules have been found to regulate synaptic activity, learning and memory (see Table 1). TNFα is considered a classic pro-inflammatory cytokine. However, in the brain, TNFα also regulates long-term potentiation (LTP). LTP is a form of plasticity that involves increased synaptic excitability following a burst of firing that is thought to reflect components of memory formation. TNFα is required for proper LTP in visual cortical slices from rats and mice[32], but disrupts LTP at higher concentrations[33]. This results in behavioral dysfunction, with TNFα overexpressing mice having decreased performance on spatial learning and memory tasks[34]. TNFα also regulates synaptic strength in hippocampal neurons by increasing AMPA receptor surface expression[35]. The pro-inflammatory cytokine IL-1β also modulates LTP, promoting it at lower levels, and disrupting LTP at higher concentrations, similar to TNFα[36–38]. Pro-inflammatory chemokines Macrophage Inflammatory Protein Alpha (MIP-1α) and Fractalkine/Chemokine (C-X-C motif) ligand 1 (CX3CL1) also regulate synaptic plasticity and memory function[39, 40]. CX3CL1 is expressed in neurons and is an anti-inflammatory signal to microglia. CX3CL1 KO mice show impaired LTP, with exogenously added MIP-1α impairing LTP. These changes might be similar to those seen with IL-1β and TNFα where the dose response is critical for functions in LTP.
Table 1.
Innate Immune Molecules Involved in Neuroplasticity
| Neuroimmune Molecule | Synaptic Function |
|---|---|
| TNFα | LTP[32] |
| Synaptic strength[35] | |
| IL-1β | LTP[36–38] |
| GABA transmission in CeA[29, 30] | |
| IL-4 | Learning and memory[42, 43] |
| IL-13 | Learning and memory[42, 43] |
| CX3CL1 | LTP[40] |
| MIP-1 | LTP[39] |
| TGFβ | Promotes LTP[41] |
| CXCL16 | GABA transmission in Hippocampus[44] |
| HMGB1 | Excitatory Signaling[45, 46] |
Traditional anti-inflammatory molecules also regulate synaptic plasticity. The anti-inflammatory protein Transforming Growth Factor-β1 (TGF-β1) promotes LTP and object recognition memory[41]. Further, anti-inflammatory cytokines IL-4 and IL-13 regulate learning and memory. IL-4 and IL-13 knock-out (KO) mice show learning and memory impairments[42, 43]. Thus, since both cytokines and chemokines can regulate LTP and synaptic strength, neuroimmune signaling may actually be a form of neuroplasticity. In addition to LTP, cytokines can modulate inhibitory GABA and excitatory glutamatergic signaling. For instance, IL-1β modulates neuronal GABA transmission in the central amygdala (CeA), decreasing the amplitude of evoked inhibitory postsynaptic potentials (eIPSPs) and differentially modulating inhibitory postysynaptic currents (mIPSCs)[29, 30]. The chemokine CXCL16 also modulates GABA transmission in the hippocampal CA1 region by increasing the frequency of mIPSCs via increased presynaptic GABA release[44]. Thus, in the brain, immune signaling molecules act as neuromodulators. Many of these molecules are altered by drugs of abuse which could lead to altered synaptic activity and behavior.
1.3 Endogenous Toll-like Receptor (TLR) Agonists and Pattern Recognition Receptors
A key feature of the innate immune system is the recognition of foreign pathogens and endogenous damage-associated molecules. Specialized groups of receptors have been identified that recognize specific molecular signatures of pathogen-associated molecular patterns (PAMPs) from bacteria, fungi, viruses or endogenous damage-associated molecular patterns (DAMPs). These pattern recognition receptors (PRRs) are grouped into five different classes: Toll-like receptors (TLRs), C-type lectin receptors, nucleotide binding domain receptors (leucine-rich repeat containing or NOD-like receptors), RIG-I-like receptors (RLRs), and AIM 2-like receptors[47]. TLRs are the best characterized PRR and are involved in numerous disease states including alcohol and drug addiction[48–51]. Ten TLRs are known in humans and 12 in mice[47]. Ligands for TLRs include a variety of molecules from bacterial endotoxin to mammalian High-Mobility Group Box protein 1 (HMGB1) and heat shock proteins[52]. The ability of TLRs to recognize DAMPs creates the situation known as “sterile inflammation”. Sterile inflammation occurs when innate immune signaling is activated in the absence of an invading organism. Since immune signaling can modulate neurocircuitry, functional consequences of sterile inflammation are likely critical. Indeed, TLR signaling has been found to play a role in alcoholism and other neurological conditions. Table 2 illustrates some key TLRs, their foreign PAMP, endogenous DAMP, and associated neurological diseases.
Table 2.
Toll-like Receptors (TLRs) implicated in Alcoholism and Neurological Diseases
| TLR | Foreign Immunogen | Endogenous TLR Ligand |
Neuropsychiatric Disease |
|---|---|---|---|
| 2 | Bacterial di- and tri-aceylated polypeptides[68] Gram (+) lipoglyans[69] | α-synuclein[70] | Alcoholism Parkinson’s Disease [70] |
| 3 | dsRNA | Stathmin[71] | Alzheimer’s Disease [72] Multiple Sclerosis [71] |
| 4 | Bacterial endotoxin Peptidoglycans | HMGB1[73] HSPs 60, 70/72[52] | Alcoholism [74] Cocaine Abuse Stroke, Traumatic Brain Injury Chronic Pain |
| 7 | ssRNA[75] | miRNAs let-7[64] and miR-21[76] | Alcoholism [77] Alzheimer’s Disease [64] Chronic Pain [78] |
TLRs contain an N-terminal extracellular leucine-rich repeat sequence and an intracellular Toll/Interleukin-1 receptor/resistance motif (TIR)[53]. TLR signaling utilizes key adapter proteins to initiate a signaling cascade upon ligand recognition. Each of the TLRs, minus TLR3, utilizes the MyD88 adapter protein complex. TIRAP/MyD88 complex formation leads to activation of the IL-1 receptor-associated kinases (IRAKs) and the TNF receptor-associated factor 6 (TRAF6) leading to IκB Kinase (IKK) and Mitogen-Activated Protein Kinase (MAPK) activation, followed by activation of the Nuclear Factor kappa-light-chain-enhancer of Activated B cells (NF-κB) and Activated Protein-1 (AP-1) transcription factors respectively. Endosomal TLRs (i.e. TLR3, TLR7, and TLR9) cause activation of the Interferon Regulator Factors (IRFs), transcription factors that lead to interferon induction (see Figure 1). These key transcription factors subsequently induce the expression of several pro-inflammatory cytokines and mediators to perpetuate the immune response. The involvement of these transcription factors in addiction detailed is subsequent sections. Selected key TLRs, cytokines and chemokines are illustrated in Figure 1, as well as their expression on different brain cell types. The exact TLR signaling pathways in brain cells type is an ongoing work, as there are some differences between brain and peripheral immune cells, in which TLR signaling was initially described. Both microglia and astrocytes express TLRs, however, the effects of activation of specific TLRs may vary between cell types. For instance, there is question over whether astrocytes have TLR4 responses, which may depend on culture conditions[54, 55]. Astrocytes do, however, have strong TLR3 responses[56] and can release an assortment of immune mediators. Further, responses in neurons are less well understood. Neurons appear to be capable of activating NF-κB in some settings but not others[57]. Neuronal NF-κB might regulate plasticity, learning and memory[27, 58] in glutamatergic and neurons. Dorsal horn spinal neurons[59] have activated NF-κB and different neuronal cell lines exhibit NF-κB dependent regulation of μ-opioid receptors[60, 61]. Though the exact downstream signaling pathways in neurons needs to be studied in each specific context, TLRs undoubtedly play important roles in normal neuronal function. For instance TLR3 and TLR8 regulate axonal or neurite outgrowth, respectively[62, 63]. TLR7 activation can cause neurodegeneration[11, 64]. The presence of physiological, non-pathologic and non-damage associated TLR signaling in brain argues that endogenous ligands for these receptors play key roles in normal physiology.
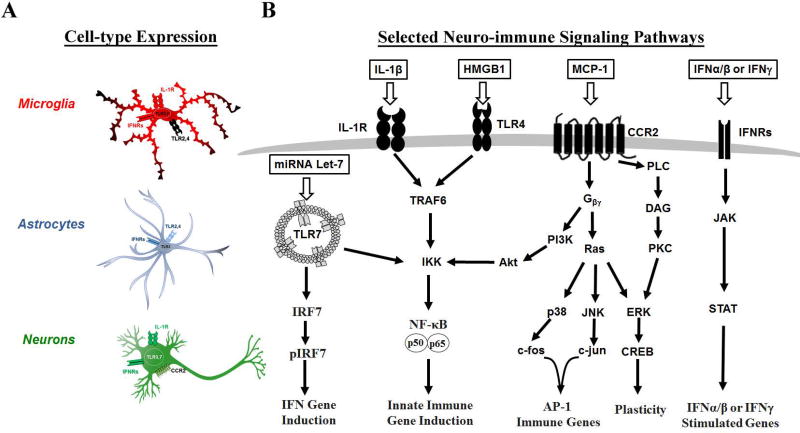
Figure 1. Selected Toll-like Receptors (TLRs), cytokine (IL-1β) and chemokine (MCP-1) signaling pathways in brain cells relevant to Alcoholism.
Selected immune signaling pathways that are involved in alcohol use pathologies are illustrated. (A) The three primary neuroimmune cell types-microglia, astrocytes, and neurons-are illustrated with selected immune receptors that are relevant to alcohol use disorders. Several TLRs are implicated in microglial and astrocyte activation including TLRs2–4 and 7. Microglia also contain the IL-1β receptor (IL-1R) and interferon (IFN) receptors. Astrocytes contain TLRs2–4 as well as IFN receptors. Neurons have TLR3 and 7 responses in the context of alcohol use disorders, as well as the MCP-1 receptor (CCR2) and IFN receptors. (B) Simplified versions of the signaling cascades for immune receptors that are relevant to alcoholism are shown. The TLR7 pathway, has been shown to be involved in alcoholic hippocampal neurodegeneration, and can lead to IFN gene induction via IRF7 as well as NFκB mediated immune gene induction. Both the IL-1R and surface TLRs such as TLR4 share the same downstream signaling pathway leading to NFκB mediated immune gene induction. The chemokine MCP-1 regulates ethanol self-administration and is a G-protein coupled receptor that can result in AP-1 mediated immune gene induction, NFκB activation, or neuronal plasticity via CREB signaling. The interferon receptors (IFN) are on all three cell types and are associated with depressive phenotypes. These activate the JAK/STAT signaling pathway to result in interferon response gene expression. See References: [64, 75, 77, 159, 163, 165, 214–216]
Since endogenous TLR agonists play key roles in normal brain physiology, a better understanding of these ligands in required. Traditionally called DAMPs (i.e. Damage Associated Molecular Patterns), in the brain these agonists may have normal biological functions. However, in the context of disease states, these DAMPs subsequently lead to further TLR activation by enhanced induction of their receptors. One DAMP that has been found to play a role in alcohol addiction in particular is the protein High-mobility group box 1 (HMGB1). HMGB1 is a nuclear chromatin binding protein that is released during bacterial infection, cellular stress, or damage. After its release HMGB1 can bind directly to TLR4 or Receptor for Advanced Glycation Endproducts (RAGE) receptors [65, 66]. However, HMGB1 also can modulate activity of several other TLRs such as TLR3, 7 and 9 [67]. HMGB1 might also regulate synaptic firing, as it is released just prior to hyperexicitable states, such as seizures, to modulate glutamatergic signaling[46]. The role of HMGB1 in the pathology of alcoholism is further described in Section 2.3. Neuroimmune activation and neuronal signaling might be interconnected by the release of DAMP such as HMGB1 in addition to cytokines. Thus, understanding DAMPs and cytokines play key roles in the neuroimmune system that may regulate neuron signaling and brain function.
2. Neuroimmune Activation in the Pathology of Alcoholism
2.1 The Natural History of Alcohol Use Disorders
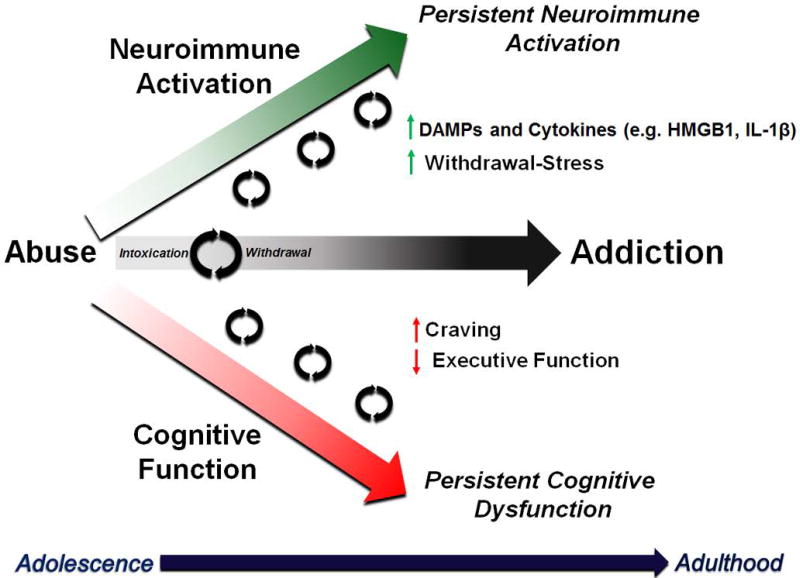
Neuroimmune activation in addiction has been elucidated by several laboratories, with several reviews on the topic[51, 79–92]. Alcoholism develops progressively over the course of an individual’s lifespan. This often begins during adolescence as drug exploration (Figure 4). The age of drinking onset is strongly associated with the risk of developing an alcohol use disorder in adulthood[93, 94]. Adolescence is a key developmental period during when maturation of frontal cortical structures regulating cognitive function and decision-making occurs (for review see Crews et al 2016[95]). A key cognitive feature in the progression to addiction is a loss in frontal-cortical mediated executive functions. This includes: motivation, planning, goal setting, and behavioral flexibility. Binge drinkers show impairments in executive functioning tasks[96–98]. Reversal learning (the ability to change previously learned behaviors) is impaired in both human alcoholics[99, 100] as well as cocaine addicts[101]. Mice and rats also have long-lasting impairment of reversal learning following binge ethanol [102–104] or cocaine[105, 106]. Prefrontal cortical regions regulate these behaviors in concert with striatum and amygdala[107] through reciprocal glutamatergic connections. Glutamatergic dysfunction is a well-known finding in alcoholism and drug abuse. Neuroimmune gene induction has been found to contribute to glutamatergic hyper-excitability in the frontal cortex and to impair executive function[90, 108]. Thus, neuroimmune activation might contribute to the development of cognitive dysfunction due to altering prefrontal cortical to limbic circuitry, though this needs to be elucidated. Recurrent immune activation across the lifespan, from adolescence into adulthood, may contribute to lasting cognitive dysfunction in alcoholism through these and other mechanisms.
Figure 4. Multiple Drug Exposures Amplify Neuroimmune Signals and Cognitive Decline.
The natural history of alcohol use disorders involves a progression from adolescence into adulthood of recurrent cycles of binge intoxication and withdrawal. Neuroimmune signaling is amplified with each cycle, as cognitive function progressively worsens.
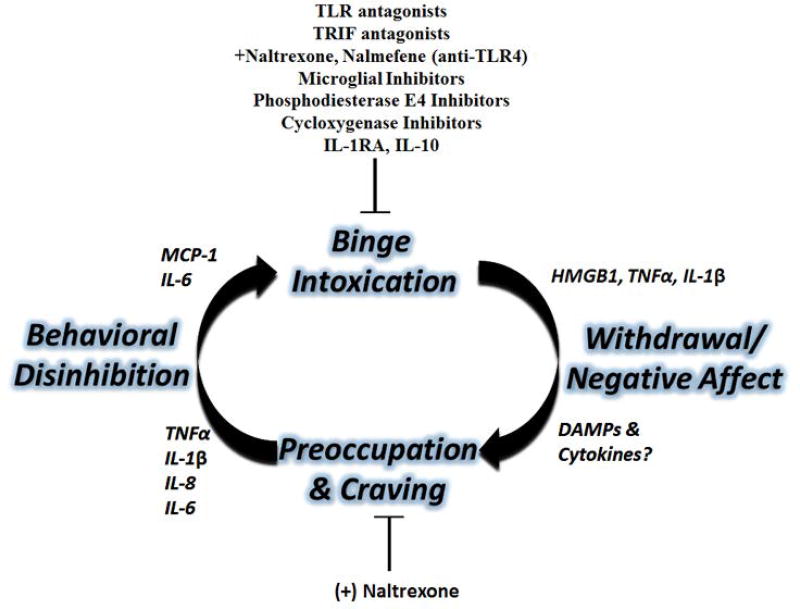
The pathology of addiction has been described as a cycle consisting of three recurring stages[109, 110]. This includes cycling through binge/intoxication, withdrawal and negative affect, craving and preoccupation, which leads to recurrent binge intoxication[111] (Figure 2). Specific neurocircuits are involved in the regulation of behavioral phenotypes associated with each stage in the cycle[109, 112]. For example, the binge/intoxication stage involves reward pathways that involve dopaminergic and opiod signaling originating in the basal ganglia. This includes the dorsal striatum (caudate nucleus and putamen), the ventral striatum (nucleus accumbens), globus pallidus, ventral pallidum, and substantia nigra. The withdrawal/negative affect stage involves circuits that regulate stress, fear and anxiety. This includes pathways such as the amydala-nucleus accumbens and ventral tegmental area (VTA). The craving/preoccupation stage is likely associated with prefrontal cortical dysfunction mentioned above. The complexity of the dysfunction in these circuits is significant, as reciprocal and overlapping connections cause interplay between the different circuits typically associated with each individual stage. Thus, each stage is connected, and the dysfunction of several circuits is likely linked. Interestingly, neuroimmune signaling has been implicated in each of the three stages of addiction, though there remains much to be further understood about the precise effects of neuroimmune activation on specific circuitry. The majority of the studies investigating neuroimmune contributions in vivo have been done on the binge/intoxication stage, leaving much to be examined in the other stages, especially the withdrawal/negative affect stage. However, several inflammatory mediators have been found to play important roles at different stages, and certain neuroimmune therapies are effective in reducing ethanol consumption in rodent models.
Figure 2. Neuroimmune Contributions to the Cycle of Addiction.
The three main stages of the cycle of addiction-binge/intoxication, withdrawal/negative affect, and preoccupation/craving-each have neuroimmune contributions. Multiple neuroimmune interventions reduce alcohol self-administration in rodent models. Binge intoxication causes the induction of several immune signaling molecules such as HMGB1, TNFα, and IL-1β. Neuroimmune molecules might also mediate some of the negative affect seen during withdrawal. The TLR4 antagonist (+)-Naltrexone reduces alcohol-induced conditioned-place preference (a feature of craving), and several immune molecules in plasma have been associated with craving in human alcoholics. See References: [119, 121, 126, 181, 189, 211, 212]
2.2 Neuroimmune Activation in the Stages of Addiction
2.2.1 Binge/Intoxication Stage
The binge/intoxication stage, is perhaps the most studied stage involving neuroimmune activation in alcoholism. Multiple immune regulating interventions have been found to alter ethanol consumption in rodents, suggesting neuroimmune activation can drive ethanol consumption. A genetic analysis found that high ethanol drinking rodents had increased expression of NF-κB and other pro-inflammatory genes[113]. A significant amount of work has been done surrounding TLR4. Sensitization of TLR4 responses by injection of the TLR4 agonist LPS increases ethanol self-administration in a two-bottle choice paradigm in mice[114]. Selective knockdown of TLR4 in the central amygdala decreases ethanol self-administration in alcohol-preferring strain of rats known as p-rats[115]. Knockdown of TLR4 in the ventral palladum, however, had no effect, suggesting brain regional specificity of TLR4 involvement in self-administration. Intracerebroventricular injection of the chemokine MCP-1 also increases alcohol self-administration in rats[116]. Inhibition of several traditional pro-inflammatory signaling has also been shown to reduce ethanol self-administration. Ablation of many key neuroimmune genes, including IL-6, Ccr2, MCP-1 and Ccl3 decrease ethanol consumption[117, 118]. The IL-1 receptor antagonist and the anti-inflammatory IL-10 both reduce alcohol self-administration when injected into the basolateral amygdala[119, 120]. Furthermore, IL-1β inhibition in the VTA also prevents cocaine-induced dopamine release in the nucleus accumbens[50], suggesting these signals are involved in other drugs of abuse. Broad acting inhibitors of microglial activation also reduce ethanol consumption in rodents. Minocycline, a tetracycline antibiotic and microglial inhibitor, reduces ethanol self-administration[121] as well as conditioned place preference after cocaine exposure[50]. These studies suggest that neuroimmune signaling plays a role in the rewarding properties of alcohol, or in the early stages of dependence. However, translating rodent drinking studies to humans can be challenging, as most rodent studies are not in dependent models. Further, in addicted humans, the rewarding properties of the drug seems to shift from the drug itself to cues associated with the drug[111]. Therefore, future work is needed in models that have more features of ethanol-dependence. Nonetheless, neuroimmune signaling is clearly involved in ethanol consumption and warrants further investigation.
2.2.2 Craving/Preoccupation Stage
Regarding the craving/preoccupation stage, innate immune activation both in the brain and periphery seem to be important. In human alcoholics, certain key inflammatory mediators are elevated in plasma that correlate with alcohol craving. This includes TNFα [122] (which also correlated with the severity of alcoholism), IL-1β, IL-6 and IL-8[122, 123]. Several of these cytokines such as TNFα, IL-6, and IL-1β can cross the blood brain barrier to exert effects on the brain[124, 125], and could drive craving in humans. However, they might also regulate craving via modulation of systemic stress responses. Recently, the systemic administration of the TLR4 antagonist (+)-naltrexone was found to reduce alcohol-induced conditioned place preference, which is likely a feature of craving[126]. Further, (+)-naltrexone reduces cue-induced heroine seeking[127]. These findings suggest that immune interventions may be effective at reducing alcohol and drug-associated craving or preoccupation. More work needs to be done to differentiate central from peripheral immune involvement in the craving/preoccupation stage. This can be challenging in animal models, as craving is a subjective measure in humans. However, assays such as conditioned place preference can identify features of craving and should be utilized to further dissect the neuroimmune contribution.
2.2.3 Withdrawal/Negative Affect and Stress
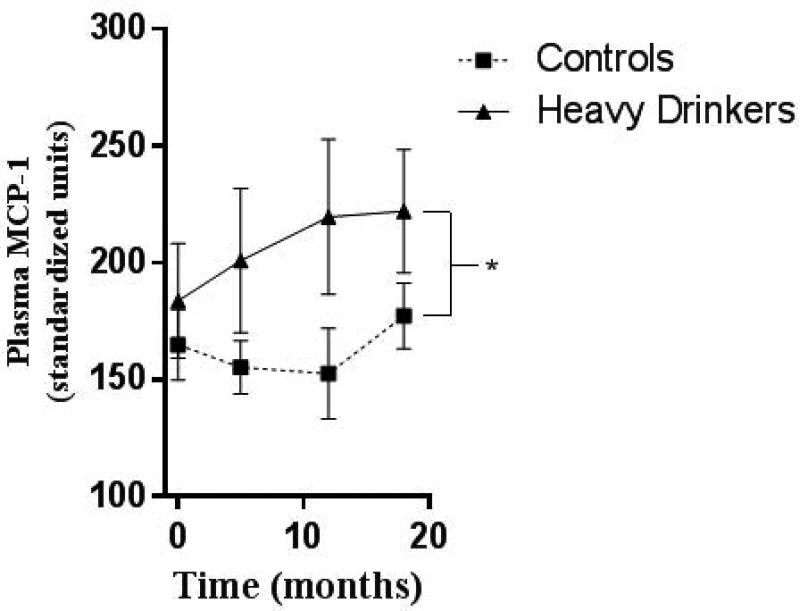
The withdrawal/negative affect stage of addiction is a key feature in the maintenance of addiction[128]. Negative affective states include stress, anxiety and dysphoria. Neuroimmune signaling seems to be involved in this stage as well. Several inflammatory cytokines including TNFα, MCP-1, as well as inflammatory transcription factors are increased in whole brain during withdrawal 24 hours after a 10-day chronic binge ethanol exposure in mice[12]. Transcription factors such as NFκB and CEBP are increased in the rodent hippocampus and amygdala respectively during withdrawal from ethanol[129, 130]. Further, intracerebroventricular (ICV) injection of TNFα, IL-1β and MCP-1 sensitize to anxiety-like behavior during alcohol withdrawal[131], suggesting that these cytokines contribute to withdrawal-associated negative affect. Interestingly, rhesus macaques that show heavy or binge drinking patterns during adolescence have elevated levels of MCP-1 in the plasma across the time of alcohol use (Figure 3). MCP-1 might cross the blood brain barrier and exert central effects, or it could alter stress responses. Stress pathways are critical in the negative affect stage. Neuroimmune activation is strongly associated with activation of stress pathways and may be a critical feature of modulating negative affect. Prior stress sensitizes microglia to inflammation in an HMGB1-dependent manner[132]. Ethanol sensitizes microglia, increasing microglial markers such as CD11b and Iba1[133, 134], priming the microglial response to subsequent systemic inflammatory responses[134]. Further, TLR4 activation alters serotonin transporter (SERT) function to increase depressive behavior[135]. Chronic restraint stress causes microglia activation throughout the brain[136] and leads to depression-like behavior. The pro-inflammatory cytokines TNFα, IL-6, and IL-1β contribute to the pathologies of mood disorders[137, 138]. Stress can also result in NF-κB activation. Psychosocial stress in humans drives NF-κB activation in blood monocytes[139]. Restraint stress in rodents causes NF-κB activation in rodents with production of TNFα and other pro-inflammatory prostaglandins[139, 140]. Ethanol itself activates NF-κB in rat and mouse brain [130, 141], and human astrocytes[142], and can interact with stress to enhance immune responses. On the other hand, agents that inhibit microglial activation can prevent the onset of depression-like behavior[136, 143–145]. Thus, it is clear that there is overlay between stress and alcohol effects on glia, via immune mechanisms. However, the exact role of neuroimmune activation during ethanol withdrawal/negative affect needs to be clearly delineated. Further, it remains unclear exactly which immune mediators are involved in the negative affect associated with withdrawal. Identification of these mediators could offer important therapeutic options to help individuals suffering from negative affect during withdrawal and abstinence, and hopefully improve remission rates.
Figure 3. Elevated Plasma MCP-1 in Heavy-Drinking Adolescent Rhesus Macaques.
Adolescent rhesus macaques had free access to either water or ethanol for 18 months. MCP-1 was measure by cytokine multiplex. Ethanol drinkers showed increased plasma MCP-1 across the exposure period: 2-way ANOVA F(1,38)=4.250, *p<0.05. Courtesy K. Grant et al, The Monkey Alcohol and Tissue Research Resource (MATRR)
2.3 Neuroimmune Signaling in Alcoholism: TLRs and Endogenous TLR agonists
The identification of neuroimmune activation in alcoholism has been supported by findings in postmortem human alcoholic tissue, rodent and cell culture experiments. Microglial and astrocytic markers are upregulated in postmortem human alcoholic brain[146, 147]. Also, other immune markers are increased such as TLR2, TLR3, TLR4, TLR7, MCP-1, and HMGB1[147, 148]. Studies in vivo also find increased expression of TLRs 2–4, 7 and HMGB1 in different brain regions with associated NF-κB activation and cytokine induction[74, 77, 149]. In vitro studies also find that ethanol activates brain cells. Microglial cell cultures find that ethanol causes activation, increasing expression of TNFα, IL-1β, iNOS and NADPH oxidase[133, 134, 150]. Ethanol causes NF-κB activation in neurons in brain slice culture [77, 151] and in vivo [141] as well as increased NF-κB–DNA binding[108, 151]. This leads to induction of proinflammatory cytokines (TNFα, IL-1β, and IL-6) [152], proinflammatory oxidases (inducible nitric oxide synthase [153, 154], COX [153, 155], and NOX [150]), and proteases (TNFα-converting enzyme [TACE] and tissue plasminogen activator [tPA]) [154]. In addition to NF-κB activation, ethanol also causes IRF3 activation in both neuronal and microglial cell cultures[27]. Astrocyte cell cultures similarly show activation with ethanol[153, 156]. Ex-vivo brain slice cultures agree, showing immune activation with ethanol exposure[154, 157, 158]. Thus, ethanol can directly cause neuroimmune activation, in the absence of peripheral immune involvement. Further, each of these studies show that ethanol neuroimmune induction involves TLR activation.
Activation of TLRs is a central feature in the neuroimmune responses to ethanol. This leads to pro-inflammatory transcription factor activation (e.g. NF-κB, AP-1, IRFs) and further amplification of immune responses. TLR4 has been the best studied TLR in alcohol-induced neuroimmune signaling[153]. TLR4 KO mice and TLR4 KO glia are protected from many features of the neuroimmune activation by ethanol. This includes protection from glial cell activation, NF-κB activation, caspase-3 cleavage, anxiety-like behavior, and memory impairment [24, 25, 133, 153, 159–162]. TLR4 siRNA in cultured astrocytes (or siRNA to critical TLR adaptor proteins MD-2 and CD14) prevents ethanol induction of NF-κB[163]. Ethanol-preferring p-rats have increased expression of TLR4 in the VTA[164], with TLR4 expression being regulated by GABA(A)α2 receptor[115] and the stress regulating corticotropin-releasing factor (CRF)[164]. TLR4 activation is also involved in cocaine and heroin abuse[48, 50, 127] suggesting a broader role in addictive pathologies. Though TLR4 is the best studied TLR in alcohol abuse, it is important to recognize that several TLRs are increased in the postmortem human alcoholic brain (TLRs 2, 3, 4, and 7) that show pathologic roles in vitro. Accordingly, TLR2 KO microglia are protected from ethanol-immune induction (iNOS and MAPK)[165]. Ethanol causes increased expression of TLR3 in neurons and TLR7, and TLR8 in both neuronal and microglial cell lines[27]. Further, TLR7 has recently been identified as important in ethanol-induced hippocampal neurodegeneration[77]. Thus, several TLRs are important in ethanol-induced neuroimmune induction. The exact contribution of each TLR, as well as the interplay between TLRs remains unclear. However, it does appear that the involvement of multiple TLRs, and the induction of multiple immune transcription factors leads to amplified and integrated responses.
It is clear that TLR signaling is key in neuroimmune responses for ethanol. However, the brain is sterile under normal operating conditions. This suggests that endogenous TLR-agonists (also known as DAMPs) mediate TLR responses to ethanol. Indeed, this has been found to be true in the cases of TLR4 and TLR7. The endogenous TLR4 ligand, HMGB1, has been found to be a critical immune mediator in alcoholism. Postmortem human alcoholic brain shows HMGB1 is increased in several brain regions, which correlates with lifetime alcohol consumption[11, 74, 166]. Rodent studies also find that ethanol administration increases HMGB1 in cortex and cerebellum [74, 149]. In response to ethanol, microglia release HMGB1[11, 27, 74, 157]. HMGB1 inhibition in vitro and in vivo protects against cytokine induction by ethanol [157, 167]. Methamphetamine also induces neuroimmune activation via HMGB1 induction both in vivo and in vitro[168], suggesting other drugs of abuse may also involve HMGB1 release. Ethanol also causes TLR7 activation through the release of its endogenous agonist. TLR7 is a single-strand RNA virus sensing TLR that has also been found to bind the endogenous miRNA let-7b when it is present in microvesicles[64]. Ethanol causes the secretion of the TLR7 agonist miRNA let-7b in microvesicles leading to TLR7-mediated neurodegeneration[77]. Let-7 isoforms have been shown previously to be increased in postmortem human alcoholic brain as well as in chronic ethanol models in rodents[169, 170]. Interestingly, HMGB1 served as a chaperone for let-7b, possibly mediating its vesicular secretion. Indeed, HMGB1 is critical for immune responses of each of the endosomal TLRs (i.e TLRs 3, 7 and 9)[67]. HMGB1 might represent a critical mediator for the induction of multiple TLRs in the context of alcohol addiction. These endogenous agonists, and perhaps others that have yet to be identified, may serve as novel targets against the neuroimmune activities of ethanol. More work is necessary to identify the precise functions of these agonists within specific brain circuits and stages of addiction.
2.4 Neuroimmune Contribution to the Progression to Addiction
The persistent activation of the neuroimmune system by alcohol abuse seems to contribute the development of addiction[51, 84, 91, 92]. Binge drinking during the adolescent developmental period has long lasting behavioral, functional, mood and cognitive effects[95, 103, 166]. A key feature of the neuroimmune activation in alcoholism that supports this hypothesis is the persistent upregulation of immune signals. The persistent upregulation of key immune molecules seen in postmortem human alcoholic brain tissue and rodents and their correlation with years of drinking links the degree of immune activation with the progression of disease. Neuroimmune markers in alcoholics not only correlate with lifetime alcohol consumption and age of drinking onset[74, 77, 92, 166, 171], but some remain elevated during prolonged abstinent periods [74, 77, 166]. Binge ethanol causes persistent upregulation of neuroimmune molecules including TLR3, TLR4, HMGB1, RAGE, etc. [166, 172]. Further, ethanol sensitizes TLRs to future activation by their agonists[12, 173]. The recurrent and persistent induction of pro-inflammatory transcription factors by repeated alcohol might result in a chronic inflammatory state in the brain (see Figure 5). This is supported by the increased innate immune markers in the postmortem brains of human alcoholics [74, 77], as well as the upregulation of NF-κB target genes in alcoholics[174]. Thus, chronic alcohol seems to shift the allosteric set point of immune activation in the brain. This is likely a result of multiple cycles of intoxication and withdrawal over time. It is well established that repeated cycles of binge and withdrawal amplify alcohol induced pathologies and behavioral dysfunction[175, 176]. Repeated neuroimmune induction may contribute to this amplification of pathology. The aforementioned effects of neuroimmune signals on plasticity suggest that changes in plasticity might be driven by repeated neuroimmune activation. Each acute inflammatory insult due to an alcohol binge may be additive, further increasing the “neuroimmune baseline”. Future work should investigate how the recurrent amplification of neuroimmune responses corresponds to known changes associated with the transition from alcohol abuse to addiction.
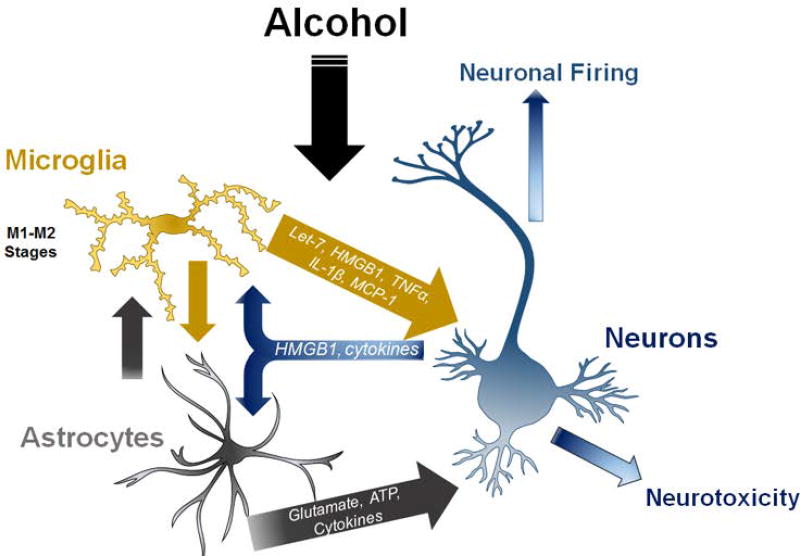
Figure 5. Neuron-glia cell-cell interactions in neuroimmune responses to alcohol.
Neuronalglial interactions underlie neuroimmune signaling in alcohol use disorders. Microglia release factors such as microRNA let-7, HMGB1 and cytokines that can cause either neurotoxicity or altered neuronal activity. Microglia and astrocytes likely release factors that alter each other’s activation status. Astrocytes modulate glutamate and ATP levels that affect neuronal signaling and vitality. Neurons release factors such as HMGB1, fractalkine and cytokines that can modulate microglial and astrocyte activation. See References: [19, 21, 27, 74, 77, 157]
2.5 Neuroimmune Basis of Addiction – An ongoing investigation
Much work has been presented in this chapter that supports a neuroimmune contribution to the development of alcoholism. However, much remains unknown. Particularly, the interactions between the immunocompetent brain cells– neurons, microglia, and astrocytes—need to be further investigated. These interactions could have very significant functional consequences. For example, a region-specific hyper-glutamatergic state has been demonstrated in both alcohol and drug addiction[177], with astrocytes being key regulators of synaptic glutamate levels. Ethanol exposure induces NF-κB activation in astrocytes leading to increased expression of pro-inflammatory genes[151, 154, 178] and impaired astrocyte glutamate transport [179]. Increased extracellular glutamate levels causes enhanced neuronal excitation, microglial activation, and excitotoxicity[151, 180]. TLR4 activation is involved in this interaction, as TLR4 KO mice are protected from the ethanol induced-hyperglutamatergic signaling and the associated neurotoxicity[153, 155]. The tripartite synapse is composed of three immunocompetent cells, thus the interactions between these cells must be elucidated. In Figure 5, we illustrate some of the known cell-cell interactions between neurons and glia regarding DAMPs (e.g. HMGB1 and miRNA let-7), cytokines (e.g. TNFα, IL-1β, and MCP-1), and glutamate. Several TLRs are involved in the alcohol-induced neuroimmune activation. There remains a paucity of understanding how these TLRs interact in the context of alcoholism, and at which stage of disease TLR antagonism might be effective. A recent report illustrates the difficulty in using TLR4 antagonism to reduce drinking behavior [181]. TLR4 antagonism dos, however, reduce conditioned place preference for ethanol [182]. Clearly, these interactions are complex, but they will likely produce novel therapeutic targets. Additionally, the interaction of stress and immune activation needs further investigation. Stress is a key feature in the cycle of addiction, however addiction-related stress is probably quite different from other non-addiction related stressors, and is difficult to model in rodents. Neuroimmune activation causes both cognitive and emotive effects, leading to dysfunction[183–186]. These mechanisms might contribute to the progressive and persistent nature of addiction. This work suggests that innate immune activation and TLR signaling are essential for ethanol-induced pathology. Though much of this work is convincing, important gaps remain regarding the precise mechanisms that cause immune induction and the precise impact of neuroimmune activation. Nonetheless, sufficient findings are present to warrant the investigation of neuroimmune therapies for the treatment or prevention of alcoholism.
3. Novel Immune Therapeutic Strategies for Addiction
3.1 Toward novel addiction treatments strategies based on immune pharmacology
Several potential and tested neuroimmune therapies are presented below in Table 3, some of which in clinical trials for alcohol use disorders. A challenge in interpreting rodent studies is the difficulty in translating animal drinking models to the human condition. Also, certain interventions may be more effective at different stages of addiction. Additionally, it will likely be important to consider at which point in the disease progression a therapeutic would be effective. For instance, immune therapies would likely not be of benefit in brain regions where permanent changes such as neurodegeneration have occurred. However, given the acute influences of cytokines on plasticity, neuroimmune therapies might also aid in the recovery of normal synaptic function in other regions. Below, we list several potential neuroimmune treatment strategies (Table 3). There are already several FDA approved drugs, which have immune modulating effects in the brain. Minocycline, for example, is a tetracycline antibiotic that also regulates microglial function[187] and reduces ethanol self-administration[121, 173] in vivo. Phosphodiesterase 4 (PDE4) inhibitors exert anti-inflammatory actions via NF-κB inhibition presumably through a cAMP-mediated mechanism[188], and have also been found reduce ethanol self-administration in vivo[189–191]. Previously, ibudilast was found to reduce some of the rewarding effects of methamphetamine in a placebo-controlled trial[192] and may have efficacy in alcoholism. Currently, a phase I clinical trial with the PDE4 inhibibitor ibudilast is underway for alcoholism. PPARγ agonists such as pioglitazone can act as microglial inhibitors and may be helpful in alcohol use disorders[193]. Pioglitazone, for example, is a PPARγ agonist that has been shown reduce neurotoxicity in models of fetal alcohol spectrum disorder[194, 195]. These drugs, and other, that have been shown to be effective in rodents should be considered for clinical therapeutic investigations. As the basic understanding of the neuroimmune contributions to the various stages of addiction increases, more targeted and strategic neuroimmune therapies will be developed.
Table 3.
Potential Neuroimmune Therapies for the Treatment of Addiction
| Drug | Mechanism Primary Immune |
CNS activity |
|---|---|---|
| Minocycline | Tetracycline antibiotic Microglial inhibitor | Reduces alcohol self-administration (free-choice voluntary drinking) [121] |
| Reduces ethanol microglia activation[173] | ||
| Prevents reinstatement of morphine and amphetamine seeking [196, 197] | ||
| Rapamycin | Macrolide antibiotic mTORC1 inhibitor | Reduces binge ethanol intake in male mice[198] |
| Neuroprotection via Autophagy Promotion [199, 200] | ||
| Azithromycin | Macrolide antibiotic Microglial inhibitor | Promotes anti-inflammatory M2 microglial activation state[201] |
| Rifampin | Bacterial RNA polymerase inhibitor TLR4 inhibition | Inhibits microglia activation to TLR4[202, 203] |
| Indomethacin | COX-2 inhibitor | Reduces ethanol self-administration in Sprague-Dawley rats[204] |
| Reduces ethanol neurotoxicity in cortex, hippocampus, and cerebellum[205] | ||
| Simvastatin | HMG-CoA Reductase Inhibitor NF-κB inhibition | Reduces inflammation and neurotoxicity to ischemia and traumatic brain injury in rodents[206, 207] |
| Glycyrrhizin | HMGB1 inhibition | Blocks ethanol-induced cytokine release in hippocampal slice culture[157] |
| Reduces neuroinflammation after traumatic brain injury in rodents[208] | ||
| Pioglitazone, DHA | PPARγ Agonists | Reduces toxicity and pro-inflammatory cytokines in a rodent fetal alcohol spectrum disorder model[194, 195] |
| Ibudilast, Mesopram, Rolipram, CDP 840 | Phosphodiesterase 4 inhibition | Reduces ethanol intake in two-bottle choice in C57BL/6J mice[189] |
| Reduces ethanol self-administration in p-rats and dependent mice[190] | ||
| Naltrexone/Naloxone, Nalmefene & GSK1521498 | μ-opioid antagonists TLR4 inhibition | Reduces alcohol self-administration in rodents[209] |
| Binds TLR4 adaptor protein MD2 [210, 211] | ||
| Prevent neuroimmune activation by ethanol [212] | ||
| Etanercept | TNFα antagonist | Prevents REM sleep disruption in alcoholics[213] |
| Amlexanox | IKK/TBK1 inhibitor | Reduces ethanol self-administration[181] |
3.2 Conclusions
In conclusion, the neuroimmune contributions to the pathology of alcoholism is a new and exciting field. This work suggests that innate immune activation and TLR signaling are essential for ethanol-induced pathology. Much of this work is convincing, however, critical gaps remain regarding the underlying mechanisms leading to immune induction and the precise impact of neuroimmune activation in the stages of addiction. Also, work is needed to identify particular circuits that may be more susceptible to deleterious effects of neuroimmune activation. Nonetheless, this field opens the possibility for new therapeutic interventions for alcoholism that could be efficacious at different stages of the disease. Nonetheless, sufficient findings are present to warrant the investigation of neuroimmune therapies for the treatment or prevention of alcoholism.
Acknowledgments
We thank the National Institute on Alcohol Abuse and Alcoholism for its support through the Neurobiology of Adolescent Drinking in Adulthood (NADIA) consortium (AA020024, AA020023), the Bowles Center for Alcohol Studies (AA011605), the U54 collaborative partnership between NCCU and UNC (AA019767), the K08 award program (AA024829), and the Monkey Alcohol and Tissue Research Resource (MATRR-R24 AA019431).
Footnotes
“Neuropharmacology of Alcohol” for the Handbook of Experimental Pharmacology series
K. Grant editor
References
- 1.Sheng J, Ruedl C, Karjalainen K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43(2):382–93. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Hoeffel G, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665–78. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginhoux F, et al. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 6.Kettenmann H, et al. Physiology of microglia. Physiological Reviews. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 7.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77(1):10–8. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Beynon SB, Walker FR. Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience. 2012;225:162–71. doi: 10.1016/j.neuroscience.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 10.Guo ML, et al. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy. 2015;11(7):995–1009. doi: 10.1080/15548627.2015.1052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman LG, Jr, Zou J, Crews FT. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation. 2017;14(1):22. doi: 10.1186/s12974-017-0799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin L, et al. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210(2):349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Periyasamy P, Guo ML, Buch S. Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy. 2016;12(8):1310–29. doi: 10.1080/15548627.2016.1183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in immunology. 2007;28(3):138–45. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Jensen CJ, Massie A, De Keyser J. Immune players in the CNS: the astrocyte. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8(4):824–39. doi: 10.1007/s11481-013-9480-6. [DOI] [PubMed] [Google Scholar]
- 17.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiological Reviews. 2014;94(4):1077–98. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 18.Jang E, et al. Phenotypic polarization of activated astrocytes: the critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. Journal of immunology. 2013;191(10):5204–19. doi: 10.4049/jimmunol.1301637. [DOI] [PubMed] [Google Scholar]
- 19.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashioka S, et al. Interferon-gamma-induced neurotoxicity of human astrocytes. CNS Neurol Disord Drug Targets. 2015;14(2):251–6. doi: 10.2174/1871527314666150217122305. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, McGeer E, McGeer PL. Neurotoxins released from interferon-gamma-stimulated human astrocytes. Neuroscience. 2013;229:164–75. doi: 10.1016/j.neuroscience.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Walter TJ, Crews FT. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. Journal of neuroinflammation. 2017;14(1):86. doi: 10.1186/s12974-017-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nature neuroscience. 2015;18(7):942–52. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valles SL, et al. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain pathology. 2004;14(4):365–71. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfonso-Loeches S, et al. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30(24):8285–95. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrimore C, Crews F. Neuron-like cell line SH-SY5Y displays different ethanol-induced immune signal molecule response and NFkB activation compared to microglia-like BV2; Research Society on Alcoholism Annual Meeting; 2016. Poster Abstract. [Google Scholar]
- 27.Lawrimore C, Crews F. Ethanol, TLR3, and TLR4 agonists have unique innate immune responses in neuron-like SH-SY5Y and microglia-like BV2. Alcoholism Clinical Experimental Research. 2017 doi: 10.1111/acer.13368. In Press(In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khairova RA, et al. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12(4):561–78. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajo M, et al. Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain Behav Immun. 2015;45:189–97. doi: 10.1016/j.bbi.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajo M, et al. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front Pharmacol. 2015;6:49. doi: 10.3389/fphar.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8(11):895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- 32.Sugimura T, Yoshimura Y, Komatsu Y. TNFalpha is required for the production of T-type Ca(2+) channel-dependent long-term potentiation in visual cortex. Neurosci Res. 2015;96:37–44. doi: 10.1016/j.neures.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Tancredi V, et al. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. 1992;146(2):176–8. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- 34.Aloe L, et al. Learning abilities, NGF and BDNF brain levels in two lines of TNF-alpha transgenic mice, one characterized by neurological disorders, the other phenotypically normal. Brain Res. 1999;840(1–2):125–37. doi: 10.1016/s0006-8993(99)01748-5. [DOI] [PubMed] [Google Scholar]
- 35.Beattie EC, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295(5563):2282–5. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 36.Prieto GA, Cotman CW. Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine Growth Factor Rev. 2017;34:27–33. doi: 10.1016/j.cytogfr.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prieto GA, et al. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1beta in the aged hippocampus. Proc Natl Acad Sci U S A. 2015;112(36):E5078–87. doi: 10.1073/pnas.1514486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goshen I, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8–10):1106–15. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Marciniak E, et al. The Chemokine MIP-1alpha/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep. 2015;5:15862. doi: 10.1038/srep15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bian C, et al. Involvement of CX3CL1/CX3CR1 signaling in spinal long term potentiation. PLoS One. 2015;10(3):e0118842. doi: 10.1371/journal.pone.0118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caraci F, et al. A key role for TGF-beta1 in hippocampal synaptic plasticity and memory. Sci Rep. 2015;5:11252. doi: 10.1038/srep11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brombacher TM, et al. IL-13-Mediated Regulation of Learning and Memory. J Immunol. 2017;198(7):2681–2688. doi: 10.4049/jimmunol.1601546. [DOI] [PubMed] [Google Scholar]
- 43.Derecki NC, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–80. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Castro MA, et al. The chemokine CXCL16 modulates neurotransmitter release in hippocampal CA1 area. Sci Rep. 2016;6:34633. doi: 10.1038/srep34633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang Y, et al. Toll-like receptor 4 is associated with seizures following ischemia with hyperglycemia. Brain Res. 2014;1590:75–84. doi: 10.1016/j.brainres.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Maroso M, et al. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J Intern Med. 2011;270(4):319–26. doi: 10.1111/j.1365-2796.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- 47.Brubaker SW, et al. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–90. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Periyasamy P, et al. Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bachtell R, et al. Targeting the Toll of Drug Abuse: The Translational Potential of Toll-Like Receptor 4. CNS Neurol Disord Drug Targets. 2015;14(6):692–9. doi: 10.2174/1871527314666150529132503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Northcutt AL, et al. DAT isn't all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry. 2015;20(12):1525–37. doi: 10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crews FT, et al. The role of neuroimmune signaling in alcoholism. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vabulas RM, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. The Journal of biological chemistry. 2002;277(23):20847–53. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Gorina R, et al. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59(2):242–55. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 55.Barbierato M, et al. Astrocyte-microglia cooperation in the expression of a pro-inflammatory phenotype. CNS Neurol Disord Drug Targets. 2013;12(5):608–18. doi: 10.2174/18715273113129990064. [DOI] [PubMed] [Google Scholar]
- 56.Serramia MJ, Munoz-Fernandez MA, Alvarez S. HIV-1 increases TLR responses in human primary astrocytes. Sci Rep. 2015;5:17887. doi: 10.1038/srep17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao XR, et al. Unique aspects of transcriptional regulation in neurons--nuances in NFkappaB and Sp1-related factors. Journal of neuroinflammation. 2009;6:16. doi: 10.1186/1742-2094-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaltschmidt B, Kaltschmidt C. NF-KappaB in Long-Term Memory and Structural Plasticity in the Adult Mammalian Brain. Front Mol Neurosci. 2015;8:69. doi: 10.3389/fnmol.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bai L, et al. Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull. 2014;30(6):936–48. doi: 10.1007/s12264-014-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borner C, Hollt V, Kraus J. Mechanisms of the inhibition of nuclear factor-kappaB by morphine in neuronal cells. Mol Pharmacol. 2012;81(4):587–97. doi: 10.1124/mol.111.076620. [DOI] [PubMed] [Google Scholar]
- 61.Wagley Y, et al. Inhibition of c-Jun NH2-terminal kinase stimulates mu opioid receptor expression via p38 MAPK-mediated nuclear NF-kappaB activation in neuronal and non-neuronal cells. Biochim Biophys Acta. 2013;1833(6):1476–88. doi: 10.1016/j.bbamcr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cameron JS, et al. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27(47):13033–41. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y, et al. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175(2):209–15. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehmann SM, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15(6):827–35. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 65.Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255(3):332–43. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 66.Janko C, et al. Redox modulation of HMGB1-related signaling. Antioxid Redox Signal. 2014;20(7):1075–85. doi: 10.1089/ars.2013.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanai H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462(7269):99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 68.Buwitt-Beckmann U, et al. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem. 2006;281(14):9049–57. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 69.Blanc L, et al. Gram-positive bacterial lipoglycans based on a glycosylated diacylglycerol lipid anchor are microbe-associated molecular patterns recognized by TLR2. PLoS One. 2013;8(11):e81593. doi: 10.1371/journal.pone.0081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim C, et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bsibsi M, et al. The microtubule regulator stathmin is an endogenous protein agonist for TLR3. J Immunol. 2010;184(12):6929–37. doi: 10.4049/jimmunol.0902419. [DOI] [PubMed] [Google Scholar]
- 72.Jackson AC, Rossiter JP, Lafon M. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol. 2006;12(3):229–34. doi: 10.1080/13550280600848399. [DOI] [PubMed] [Google Scholar]
- 73.Park JS, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279(9):7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 74.Crews FT, et al. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73(7):602–12. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lehmann SM, et al. Extracellularly delivered single-stranded viral RNA causes neurodegeneration dependent on TLR7. J Immunol. 2012;189(3):1448–58. doi: 10.4049/jimmunol.1201078. [DOI] [PubMed] [Google Scholar]
- 76.Yelamanchili SV, et al. MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PLoS Pathog. 2015;11(7):e1005032. doi: 10.1371/journal.ppat.1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coleman LG, Jr, Zou J, Crews F. Microglial-derived miRNA Let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation. 2017 doi: 10.1186/s12974-017-0799-4. In Press(In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park CK, et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82(1):47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neupane SP. Neuroimmune Interface in the Comorbidity between Alcohol Use Disorder and Major Depression. Front Immunol. 2016;7:655. doi: 10.3389/fimmu.2016.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305–314. doi: 10.1080/17512433.2017.1268916. [DOI] [PubMed] [Google Scholar]
- 81.Montesinos J, Alfonso-Loeches S, Guerri C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res. 2016;40(11):2260–2270. doi: 10.1111/acer.13208. [DOI] [PubMed] [Google Scholar]
- 82.Jacobsen JH, Hutchinson MR, Mustafa S. Drug addiction: targeting dynamic neuroimmune receptor interactions as a potential therapeutic strategy. Curr Opin Pharmacol. 2016;26:131–7. doi: 10.1016/j.coph.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 83.Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol. 2014;118:315–57. doi: 10.1016/B978-0-12-801284-0.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vetreno RP, Crews FT. Current hypotheses on the mechanisms of alcoholism. Handb Clin Neurol. 2014;125:477–97. doi: 10.1016/B978-0-444-62619-6.00027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Most D, Ferguson L, Harris RA. Molecular basis of alcoholism. Handb Clin Neurol. 2014;125:89–111. doi: 10.1016/B978-0-444-62619-6.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray LA, et al. Opportunities for the development of neuroimmune therapies in addiction. Int Rev Neurobiol. 2014;118:381–401. doi: 10.1016/B978-0-12-801284-0.00012-9. [DOI] [PubMed] [Google Scholar]
- 87.Loftis JM, Janowsky A. Neuroimmune basis of methamphetamine toxicity. Int Rev Neurobiol. 2014;118:165–97. doi: 10.1016/B978-0-12-801284-0.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui C, Shurtleff D, Harris RA. Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol. 2014;118:1–12. doi: 10.1016/B978-0-12-801284-0.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013;23(4):513–20. doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crews FT, et al. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res. 2015;37(2):331–41. 344–51. [PMC free article] [PubMed] [Google Scholar]
- 92.Crews FT, Vetreno RP. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 2016;233(9):1543–57. doi: 10.1007/s00213-015-3906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dawson DA, et al. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32(12):2149–60. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10(2):163–73. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- 95.Crews FT, et al. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev. 2016;68(4):1074–1109. doi: 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Townshend JM, Duka T. Mixed emotions: alcoholics' impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41(7):773–82. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- 97.Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology. 2003;165(3):306–12. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- 98.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, biochemistry, and behavior. 2009;93(3):237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fortier CB, et al. Delay discrimination and reversal eyeblink classical conditioning in abstinent chronic alcoholics. Neuropsychology. 2008;22(2):196–208. doi: 10.1037/0894-4105.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jokisch D, et al. Impairments in learning by monetary rewards and alcohol-associated rewards in detoxified alcoholic patients. Alcohol Clin Exp Res. 2014;38(7):1947–54. doi: 10.1111/acer.12460. [DOI] [PubMed] [Google Scholar]
- 101.Stalnaker TA, et al. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Obernier JA, et al. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, biochemistry, and behavior. 2002;72(3):521–32. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- 103.Coleman LG, Jr, et al. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism, clinical and experimental research. 2011;35(4):671–88. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci. 2011;125(6):879–91. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calu DJ, et al. Associative encoding in posterior piriform cortex during odor discrimination and reversal learning. Cerebral cortex. 2007;17(6):1342–9. doi: 10.1093/cercor/bhl045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schoenbaum G, et al. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. The European journal of neuroscience. 2004;19(7):1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 107.Izquierdo A, et al. The neural basis of reversal learning: An updated perspective. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.03.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crews F, et al. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcoholism, clinical and experimental research. 2006;30(11):1938–49. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 109.Cui C, et al. Brain pathways to recovery from alcohol dependence. Alcohol. 2015;49(5):435–52. doi: 10.1016/j.alcohol.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–71. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mulligan MK, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(16):6368–73. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blednov YA, et al. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain, behavior, and immunity. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu J, et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4465–70. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valenta JP, Gonzales RA. Chronic Intracerebroventricular Infusion of Monocyte Chemoattractant Protein-1 Leads to a Persistent Increase in Sweetened Ethanol Consumption During Operant Self-Administration But Does Not Influence Sucrose Consumption in Long-Evans Rats. Alcohol Clin Exp Res. 2016;40(1):187–95. doi: 10.1111/acer.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blednov YA, et al. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction biology. 2012;17(1):108–20. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blednov YA, et al. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural brain research. 2005;165(1):110–25. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marshall SA, et al. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun. 2016;51:258–67. doi: 10.1016/j.bbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marshall SA, et al. Modulation of Binge-like Ethanol Consumption by IL-10 Signaling in the Basolateral Amygdala. J Neuroimmune Pharmacol. 2016 doi: 10.1007/s11481-016-9709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agrawal RG, et al. Minocycline reduces ethanol drinking. Brain Behav Immun. 2011;25(Suppl 1):S165–9. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heberlein A, et al. TNF-alpha and IL-6 serum levels: neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol. 2014;48(7):671–6. doi: 10.1016/j.alcohol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Leclercq S, et al. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry. 2014;76(9):725–33. doi: 10.1016/j.biopsych.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 124.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2(4):241–8. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 125.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179(1–2):53–6. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 126.Jacobsen JHW, et al. The efficacy of (+)-Naltrexone on alcohol preference and seeking behaviour is dependent on light-cycle. Brain Behav Immun. 2018;67:181–193. doi: 10.1016/j.bbi.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Theberge FR, et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 2013;73(8):729–37. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nature neuroscience. 2005;8(11):1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 129.Freeman K, et al. Temporal changes in innate immune signals in a rat model of alcohol withdrawal in emotional and cardiorespiratory homeostatic nuclei. Journal of neuroinflammation. 2012;9:97. doi: 10.1186/1742-2094-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Breese GR, et al. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(4):867–76. doi: 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weber MD, et al. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(1):316–24. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. Journal of immunology. 2009;183(7):4733–44. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- 134.Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. Journal of neuroinflammation. 2012;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu CB, et al. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35(13):2510–20. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tynan RJ, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, behavior, and immunity. 2010;24(7):1058–68. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 137.Bhattacharya A, Drevets WC. Role of Neuro-Immunological Factors in the Pathophysiology of Mood Disorders: Implications for Novel Therapeutics for Treatment Resistant Depression. Curr Top Behav Neurosci. 2017;31:339–356. doi: 10.1007/7854_2016_43. [DOI] [PubMed] [Google Scholar]
- 138.Bhattacharya A, et al. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berl) 2016;233(9):1623–36. doi: 10.1007/s00213-016-4214-0. [DOI] [PubMed] [Google Scholar]
- 139.Bierhaus A, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100(4):1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Madrigal JL, et al. Stress-induced increase in extracellular sucrose space in rats is mediated by nitric oxide. Brain research. 2002;938(1–2):87–91. doi: 10.1016/s0006-8993(02)02467-8. [DOI] [PubMed] [Google Scholar]
- 141.Ward RJ, et al. Identification of the nuclear transcription factor NFkappaB in rat after in vivo ethanol administration. FEBS letters. 1996;389(2):119–22. doi: 10.1016/0014-5793(96)00545-5. [DOI] [PubMed] [Google Scholar]
- 142.Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neuroscience letters. 2004;371(2–3):128–32. doi: 10.1016/j.neulet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 143.Frank MG, et al. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21(1):47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 144.Wohleb ES, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(17):6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kreisel T, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Molecular Psychiatry. 2014;19(6):699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 146.Rubio-Araiz A, et al. Disruption of blood-brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model. Addiction biology. 2016 doi: 10.1111/adb.12376. [DOI] [PubMed] [Google Scholar]
- 147.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210(2):349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crews FT, et al. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological psychiatry. 2013;73(7):602–12. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lippai D, et al. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94(1):171–82. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qin L, et al. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zou J, Crews F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and molecular neurobiology. 2006;26(4–6):385–405. doi: 10.1007/s10571-006-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qin L, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alfonso-Loeches S, et al. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(24):8285–95. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-kappaB and proinflammatory cytokines. Alcoholism, clinical and experimental research. 2010;34(5):777–89. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 155.Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcoholism, clinical and experimental research. 1999;23(4):633–43. [PubMed] [Google Scholar]
- 156.Franke H. Influence of chronic alcohol treatment on the GFAP-immunoreactivity in astrocytes of the hippocampus in rats. Acta Histochem. 1995;97(3):263–71. doi: 10.1016/S0065-1281(11)80187-X. [DOI] [PubMed] [Google Scholar]
- 157.Zou JY, Crews FT. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 2014;9(2):e87915. doi: 10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zou J, Crews FT. Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal Neurogenesis. Front Neurosci. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183(7):4733–44. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- 160.Pascual M, et al. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain, behavior, and immunity. 2011;25(Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 161.Blanco AM, et al. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. Journal of immunology. 2005;175(10):6893–9. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 162.Pascual M, et al. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25(Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 163.Blanco AM, et al. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175(10):6893–9. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 164.June HL, et al. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 2015;40(6):1549–59. doi: 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandez-Lizarbe S, Montesinos J, Guerri C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J Neurochem. 2013;126(2):261–73. doi: 10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- 166.Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–88. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Whitman BA, et al. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin Exp Res. 2013;37(12):2086–97. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Frank MG, et al. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav Immun. 2016;51:99–108. doi: 10.1016/j.bbi.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lewohl JM, et al. Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res. 2011;35(11):1928–37. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nunez YO, et al. Positively correlated miRNA-mRNA regulatory networks in mouse frontal cortex during early stages of alcohol dependence. BMC Genomics. 2013;14:725. doi: 10.1186/1471-2164-14-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol Dis. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 2012;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Okvist A, et al. Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system. PLoS One. 2007;2(9):e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a "kindling"/stress hypothesis. Psychopharmacology (Berl) 2005;178(4):367–80. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marshall SA, Geil CR, Nixon K. Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sci. 2016;6(2) doi: 10.3390/brainsci6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behavioural pharmacology. 2010;21(5–6):514–22. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pascual M, et al. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. The European journal of neuroscience. 2007;25(2):541–50. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- 179.Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain research. 2005;1034(1–2):11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 180.Ward RJ, et al. Neuro-inflammation induced in the hippocampus of 'binge drinking' rats may be mediated by elevated extracellular glutamate content. Journal of neurochemistry. 2009;111(5):1119–28. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- 181.McCarthy GM, et al. Chronic ethanol consumption: role of TLR3/TRIF-dependent signaling. Addict Biol. 2017 doi: 10.1111/adb.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jacobsen JHW, et al. The efficacy of (+)-Naltrexone on alcohol preference and seeking behaviour is dependent on light-cycle. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dantzer R, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 185.Okun E, et al. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15625–30. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clinical science. 2011;121(9):367–87. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Plane JM, et al. Prospects for minocycline neuroprotection. Archives of neurology. 2010;67(12):1442–8. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jimenez JL, et al. Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J Pharmacol Exp Ther. 2001;299(2):753–9. [PubMed] [Google Scholar]
- 189.Blednov YA, et al. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci. 2014;8:129. doi: 10.3389/fnins.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bell RL, et al. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2015;20(1):38–42. doi: 10.1111/adb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hu W, et al. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 2011;218(2):331–9. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Worley MJ, et al. Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend. 2016;162:245–50. doi: 10.1016/j.drugalcdep.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Storer PD, et al. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005;161(1–2):113–22. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 194.Kane CJ, et al. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav Immun. 2011;25(Suppl 1):S137–45. doi: 10.1016/j.bbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Drew PD, et al. Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39(3):445–54. doi: 10.1111/acer.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Arezoomandan R, Haghparast A. Administration of the glial cell modulator, minocycline, in the nucleus accumbens attenuated the maintenance and reinstatement of morphine-seeking behavior. Can J Physiol Pharmacol. 2016;94(3):257–64. doi: 10.1139/cjpp-2015-0209. [DOI] [PubMed] [Google Scholar]
- 197.Attarzadeh-Yazdi G, Arezoomandan R, Haghparast A. Minocycline, an antibiotic with inhibitory effect on microglial activation, attenuates the maintenance and reinstatement of methamphetamine-seeking behavior in rat. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:142–8. doi: 10.1016/j.pnpbp.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 198.Cozzoli DK, et al. Functional regulation of PI3K-associated signaling in the accumbens by binge alcohol drinking in male but not female mice. Neuropharmacology. 2016;105:164–74. doi: 10.1016/j.neuropharm.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen G, et al. Autophagy is a protective response to ethanol neurotoxicity. Autophagy. 2012;8(11):1577–89. doi: 10.4161/auto.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pla A, Pascual M, Guerri C. Autophagy Constitutes a Protective Mechanism against Ethanol Toxicity in Mouse Astrocytes and Neurons. PLoS One. 2016;11(4):e0153097. doi: 10.1371/journal.pone.0153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang B, et al. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015;12:218. doi: 10.1186/s12974-015-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bi W, et al. Rifampicin inhibits microglial inflammation and improves neuron survival against inflammation. Brain Res. 2011;1395:12–20. doi: 10.1016/j.brainres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 203.Wang X, et al. Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J. 2013;27(7):2713–22. doi: 10.1096/fj.12-222992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.George FR. The role of arachidonic acid metabolites in mediating ethanol self-administration and intoxication. Ann N Y Acad Sci. 1989;559:382–91. doi: 10.1111/j.1749-6632.1989.tb22624.x. [DOI] [PubMed] [Google Scholar]
- 205.Pascual M, et al. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25(2):541–50. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- 206.Sironi L, et al. Activation of NF-kB and ERK1/2 after permanent focal ischemia is abolished by simvastatin treatment. Neurobiol Dis. 2006;22(2):445–51. doi: 10.1016/j.nbd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 207.Lim SW, et al. Simvastatin Therapy in the Acute Stage of Traumatic Brain Injury Attenuates Brain Trauma-Induced Depression-Like Behavior in Rats by Reducing Neuroinflammation in the Hippocampus. Neurocrit Care. 2016 doi: 10.1007/s12028-016-0290-6. [DOI] [PubMed] [Google Scholar]
- 208.Okuma Y, et al. Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1-RAGE interaction. Neuropharmacology. 2014;85:18–26. doi: 10.1016/j.neuropharm.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 209.Ripley TL, et al. The novel mu-opioid antagonist, GSK1521498, reduces ethanol consumption in C57BL/6J mice. Psychopharmacology (Berl) 2015;232(18):3431–41. doi: 10.1007/s00213-015-3995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hutchinson MR, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain, behavior, and immunity. 2010;24(1):83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang X, et al. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol. 2016;173(5):856–69. doi: 10.1111/bph.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Montesinos J, Gil A, Guerri C. Nalmefene Prevents Alcohol-Induced Neuroinflammation and Alcohol Drinking Preference in Adolescent Female Mice: Role of TLR4. Alcohol Clin Exp Res. 2017 doi: 10.1111/acer.13416. [DOI] [PubMed] [Google Scholar]
- 213.Irwin MR, et al. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66(2):191–5. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Montesinos J, et al. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav Immun. 2015;45:233–44. doi: 10.1016/j.bbi.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 215.Narayanan KB, Park HH. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis. 2015;20(2):196–209. doi: 10.1007/s10495-014-1073-1. [DOI] [PubMed] [Google Scholar]
- 216.Bose S, Cho J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharm Res. 2013;36(9):1039–50. doi: 10.1007/s12272-013-0161-z. [DOI] [PubMed] [Google Scholar]