Abstract
Artisanal and small-scale gold mining (ASGM) in Madre de Dios, Peru, continues to expand rapidly, raising concerns about increases in loading of mercury (Hg) to the environment. We measured physicochemical parameters in water and sampled and analyzed sediments and fish from multiple sites along one ASGM-impacted river and two unimpacted rivers in the region to examine whether Hg concentrations were elevated and possibly related to ASGM activity. We also analyzed the 308 fish samples, representing 36 species, for stable isotopes (δ15N and δ13C) to estimate their trophic position. Trophic position was positively correlated with the log-transformed Hg concentrations in fish among all sites. There was a lack of relationship between Hg concentrations in fish and either Hg concentrations in sediments or ASGM activity among sites, suggesting that fish Hg concentrations alone is not an ideal bioindicator of site-specific Hg contamination in the region. Fish Hg concentrations were not elevated in the ASGM-impacted river relative to the other two rivers; however, sediment Hg concentrations were highest in the ASGM-impacted river. Degraded habitat conditions and commensurate shifts in fish species and ecological processes may influence Hg bioaccumulation in the ASGM-impacted river. More research is needed on food web dynamics in the region to elucidate any effects caused by ASGM, especially through feeding relationships and food sources.
Keywords: mercury, fish, sediment, artisanal and small-scale gold mining, Madre de Dios, Peruvian Amazon
1. Introduction
Mercury (Hg) is a global pollutant that can affect both human and ecosystem health. It is a naturally-occurring element, but concentrations have been enriched due to mobilization by humans for thousands of years into the atmosphere, as well as aquatic and terrestrial ecosystems [1,2]. Over the past century, Hg emissions have increased due to increased industrialization associated with fossil fuel combustion, mining, and industrial products and processes [2,3].
Artisanal and small-scale gold mining (ASGM) currently accounts for an estimated 37% of global Hg emissions into the atmosphere [4,5]. Mercury-laden waste is also directly discharged into water bodies after gold refining processes. Mercury exposure can threaten wildlife and human health through the consumption of Hg-contaminated fish [6,7,8]. Thus, remote regions and communities near gold mining activities, such as the Amazonian region of Madre de Dios (MDD) in Peru, are highly susceptible to Hg contamination [9,10].
Peru is Latin America’s top gold producer, and currently the sixth largest gold producer globally, processing approximately 150 Mg in 2017 [11,12,13]. ASGM has been occurring in MDD for over 40 years [14]. From 1999 to 2012, land conversion for gold mining in MDD increased 400%, with approximately 50,000 ha of forest removed by 2012 [15]. From 2012 to 2016, another 20,000 ha was deforested due to gold mining in MDD [16]. Asner et al. [15,16] indicate that coinciding with the rapid expansion of mining zones there is also a marked increase in the number of miners. It is estimated that there are over 60,000 miners working in this region [17,18].
Peru signed the United Nations’ Minamata Convention in October 2013 and ratified it in January 2016. The Minamata Convention is a global treaty to limit Hg emissions and releases to the environment and protect human and environmental health from adverse effects of Hg. Despite this international management effort, extrajudicial gold mining continues to expand in Peru. ASG miners mix liquid elemental Hg with river sediments or soils to capture and amalgamate fine alluvial gold particles. The gold-mercury amalgam is heated in an open flame to volatilize the Hg and extract the gold [19,20]. A similar practice of adding liquid Hg to mined sediment slurries was practiced in California during the ‘Gold Rush’ (latter half of 1800s), leaving a legacy of Hg contamination that continues to plague the environment [21].
The toxicity and exposure of Hg is closely linked to the formation of methylmercury (MeHg). Ionic Hg can be converted by bacteria and archaea in anaerobic waters and sediments into the bioaccumulative and highly toxic form MeHg. Fish concentrate both ionic Hg and MeHg through consumption of water and aquatic organisms. However, the half-life of MeHg in fish is approximately two years, compared to almost three months for ionic Hg [22]. This slow loss, combined with continuous exposure, results in substantial MeHg accumulation along the food chain. Dietary consumption of fish is the primary pathway of human and wildlife exposure to MeHg, a potent neurotoxin that can significantly impair human health [2]. More than 95% of the total Hg present in fish muscle tissue is MeHg [23]. Consequently, larger predatory fish generally have the highest MeHg concentrations [24]. Approximately 75% of total fish captures in the Peruvian Amazon are from subsistence fisheries; therefore, MeHg exposure in regions such as MDD is an important concern for riverine and indigenous populations that subsist on local fish [25].
Mercury concentrations are difficult to predict and track in aquatic ecosystems because of their complex interactions with multiple physical and biogeochemical factors that drive its transport, transformations and fate [26]. Diringer et al. [27] found higher Hg concentrations in fish (Hgfish) and sediment (Hgsed) at sites downstream from ASGM activities in comparison to upstream sites along the Madre de Dios River. Previous research in the study area have reported elevated Hg concentrations in human hair and in commercial fish species in locations with ASGM activities as well as in locations without ASGM activities [19,27,28,29,30,31,32]. Fish sampling in these rivers is difficult because waters tend to be uninhabitable by fish due to high turbidity from very high suspended solids loads [33].
In this study, we evaluated Hgsed and Hgfish in a river where mining activities were occurring upstream and compared these values to those from two proximate rivers without mining to characterize the level and extent of Hg contamination associated with ASGM. The objective of our study was to evaluate if proximity to ASGM is enriching Hgsed and Hgfish in the rivers. We also wanted to address two subsidiary questions: (1) How do Hgfish vary by species, trophic position, and size? and (2) Do Hgfish vary seasonally?
2. Materials and Methods
2.1. Site Description
MDD is located in the Amazon rainforest in southeast Peru bordering Brazil and Bolivia. Puerto Maldonado (12.5909° S, 69.1963° W) is the capital city of MDD. Madre de Dios, which directly translates as “Mother of God,” is known as the “biodiversity capital of Peru”. It is considered one of the most biodiverse ecosystems in the world and has been prioritized for conservation [34,35]. The construction of the Interoceanic Highway, a roadway which spans approximately 2600 km connecting Brazil and Peru (Figure 1, dashed line), has led to increased urbanization, industrialization, and natural resource extraction, all of which have degraded the environment [36]. In particular, 70,000 ha of virgin forest have been cleared for gold mining and an estimated 40–45 Mg of elemental Hg are used annually by miners to amalgamate gold flakes in excavated soil [14,16].
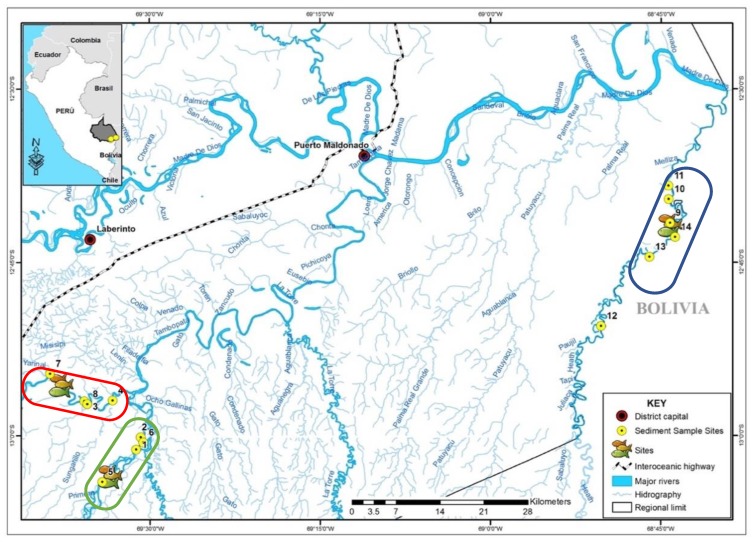
Figure 1.
Map of the study region. Fish sampling for each season was conducted in the circled reaches for each river Tambopata River (green), Malinowski River (red), and Heath River (blue). Circles indicate sediment sample sites. The Interoceanic Highway is denoted with a dashed line.
This study was conducted on three rivers in MDD. The Tambopata and Malinowski rivers are located southwest of the district capital, in the Tambopata National Reserve. Tambopata River receives inputs from the mining-impacted Malinowski River (Figure 1, circled in red). We sampled Tambopata River upstream from its confluence with Malinowski River (Figure 1, circled in green). Studies show that forest loss from ASGM on the upper Malinowski River has largely increased in the last decade [16]. The Heath River (circled in blue, Figure 1) is located southeast of the district capital in an undisturbed watershed within the Bahuaja Sonene National Park. This river forms the border between Peru and Bolivia. There have been no known mining activities in the national park up to the time of this study.
2.2. Sampling and Sample Processing Procedures
Wet season sampling was conducted in January–February 2017. Dry season sampling was conducted in August 2017. Wet season sampling was challenging because fish dispersed into floodplains and debris hindered boat travel. Nevertheless, fish and sediment samples were collected in both seasons. Sediment samples were collected using an Ekman dredge in quiescent pools with predominantly fine sediments. To complement river sediments, shoreline samples were also collected from recently submerged areas (following storm peaks) using a 2-cm deep, 10-cm wide stainless-steel scoop. All sediment collection equipment was rinsed thoroughly with deionized water between sample collections.
Fish samples were collected using drag nets, gill nets, and hook and line based on conditions at each site. Lengths (L, mm) and wet weight (WW, kg) were measured for each individual fish in the field. During wet season sampling, strong currents proved difficult for certain fishing techniques, particularly drag nets. During dry season sampling, with low-flow and low-water levels, all fishing techniques were viable. The majority of fish species caught were commercially important species, easily identified in the field. For less common fish species, identification was based on feeding habits and sampling location according to taxonomy keys [37,38]. For fish longer than 10 cm, a scalpel was used to remove the skin and scales from just below the dorsal fin of the left side of each fish. An 8-mm Acuderm biopsy punch (Acuderm Inc, Ft. Lauderdale, FL, USA) was then used to extract a small, subcutaneous “plug” of fish tissue for analysis. Several studies have shown that there is little variation between traditional, fish filet samples and fish plug samples [39,40,41,42]. For fish less than 10-cm long, a scalpel was used to remove the skin and a standard fillet sample was taken from the left side of the fish. All research protocols involving fish subjects were approved by Servicio Nacional de Áreas Naturales Protegidas por el Estado (Resolución Directoral N° 010-2017-SERNANP-DGANP).
We visited a total of 25 fish sampling sites: nine on Tambopata River, six on Malinowski River, and ten on Heath River (Supplementary Materials, Table S1). All fish sampling sites for Tambopata and Malinowski rivers were located upstream from their confluence. A total of 308 fish samples were collected during both seasons. During each cruise, fish and sediment samples were preserved on wet ice. Upon arrival in Puerto Maldonado, all samples were immediately stored at −20 °C. Samples were then transported with ice in a Crēdo ProMed® cooler (Pelican BioThermal, Plymouth, MN, USA) to Syracuse University in Syracuse, NY for analyses.
Physicochemical water quality parameters (pH, temperature, dissolved oxygen, and nitrate) were measured in-situ at each site using a YSI Sonde (YSI Inc., Yellow Springs, OH, USA), Hanna pH Tester (Hanna Instruments, Woonsocket, RI, USA), and a LaMotte Dissolved Oxygen Test Kit (LaMotte Company, Chestertown, MD, USA). Turbidity was measured using a Hach 2100Q Portable Turbidimeter (Hach Company, Loveland, CO, USA).
2.3. Chemical Analyses
Upon arrival at Syracuse University, all samples were freeze-dried dried at −80 °C and 0.080 mBar using FreeZone: Type 6 plus freeze drier by Labconco (Labconco Corp., Kansas City, MO, USA). After freeze-drying, fish samples were homogenized and analyzed for total Hg using a Milestone Direct Mercury Analyzer (DMA-80, Milestone Inc., Shelton CT, USA) following US EPA Method 7473 [43]. Sediment samples were sieved and the fraction of particle sizes <63 µm were analyzed for total Hg (EPA Method 7473) [43] as well as organic carbon and total nitrogen using an elemental analyzer (EPA Method 440.0) (Costech, Valencia, CA, USA) [43,44]. Samples were weighed before and after freeze-drying in order to calculate percent moisture.
Duplicate measurements were taken for each fish and sediment sample analyzed for Hg, and the two results were averaged for the reported result. The average relative percent difference in total Hg measurements of duplicate samples was 4.5%. Quality control measures included testing of standard reference materials. Continuing calibration verification and continuing calibration blank measurements were determined on every tenth sample analyzed. The Method Detection Limit for total Hg analysis is 2 ng/g. No fish or sediment samples were rejected based on quality control results or duplicate relative percent differences.
Stable isotope analysis for fish tissue samples was completed at the University of California Davis Stable Isotope Facility using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK). Stable isotope composition is expressed in parts per thousand (‰ or ‘per mil’) as a deviation from a standard material. Nitrogen isotopic values were standardized against N2 gas in air as follows:
| (1) |
where R = 15N/14N [45]. Similarly, carbon isotope samples (δ13C) were standardized against Vienna Pee Dee Belemnite, where R = 13C/12C. For every 11 field samples, one sample was randomly chosen for analysis as a lab duplicate.
Because there is considerable variation in δ15N at the base of the food web among ecosystems [46,47], we corrected δ15N values for each river using the average δ15N of Prochilodus nigricans, a primary consumer (e.g., a fish species that primarily feed on autochthonous algae and detritus). We calculated δ15N baseline-corrected trophic positions according to the following equation:
| (2) |
2.4. Statistical Analyses
Three-way ANOVA without interaction terms was conducted to investigate the differences in physicochemical water quality parameters in the three rivers, between mainstem and tributary sites, and for two seasons. If there was a significant difference among rivers, then Tukey’s Honest Significant Difference (HSD) tests were used to perform pairwise comparisons among the three rivers.
Quantile–quantile plots were used to examine the normality of Hgfish (Figure S1). Results suggested that log transformation would improve the normality of the dataset. Therefore, all statistical analyses were performed on log-transformed values. All Hgsed reported were normalized to the average carbon content for comparison among sites.
Two-way ANOVA was conducted to investigate the differences in Hgsed in the three rivers and two seasons. Two-way ANOVA was used to evaluate the differences in isotopic values (δ15N and δ13C) between the three rivers and two seasons. One-way ANOVA was used to examine any differences between mean log Hgfish among the three rivers. Tukey’s HSD tests were used to perform pairwise comparisons among the three rivers. Due to the diversity of fish species being collected, fish size (length or mass) was not available as a covariate because of the wide ranges in fish species’ morphologies and growth rates did not provide a suitable correlation between size and Hgfish. Results from statistical analyses were considered significant when p < 0.05. p-values were adjusted for the multiple comparisons [50]. Analyses were performed in R 3.4.3 [51].
3. Results
3.1. Field Measurements and Physical Observations
Physicochemical water quality parameters were measured at each sampling site during both seasons. Values from sites in each river were averaged for mainstem and tributary sites separately (Table 1). Tributaries to Malinowski River were not measured for water quality during the dry season due to lack of flow.
Table 1.
Average physicochemical water quality parameters gathered from mainstem (M) and tributary (T) sites from each river for both wet and dry seasons. Values presented are means (± standard deviation).
| River | Season | Site (M/T) | pH | Temperature (°C) | Dissolved Oxygen (mg/L) | NO3− (mg N/L) | Turbidity (NTU) |
|---|---|---|---|---|---|---|---|
| Tambopata | Wet | M | 7.5 | 26.3 (0.41) | 7.4 (0.04) | 0.29 (0.06) | 581 (362) |
| Wet | T | 7.6 | 27.5 | 7.0 | 0.26 | 215 | |
| Dry | M | 7.4 (0.23) | 25.7 (0.28) | 7.6 (0.17) | 0.05 (0.03) | 42 (31) | |
| Dry | T | 7.3 | 24.6 | 7.4 | 0.03 | 12 | |
| Malinowski | Wet | M | 7.1 (0.42) | 28.1 (0.55) | 6.9 (0.17) | 0.25 (0.14) | 359 (169) |
| Wet | T | 6.3 | 24.8 | 6.7 | 0.12 | 9 | |
| Dry | M | 6.9 (0.02) | 28.9 (1.6) | 6.3 (0.36) | 0.09 | 401 (56) | |
| Dry | T | NS 1 | NS | NS | NS | NS | |
| Heath | Wet | M | 6.5 (0.40) | 26.1 (1.1) | 5.0 (0.33) | 0.28 (0.12) | 305 (188) |
| Wet | T | 6.4 (0.02) | 25.1 (1.3) | 4.9 | 0.34 (0.03) | 79 (18) | |
| Dry | M | 7.2 (0.04) | 27.5 (1.9) | 6.2 (0.84) | 0.23 (0.02) | 644 (221) | |
| Dry | T | 6.6 | 25.9 | 6.2 | 0.06 | 33 |
1 NS (no sample) was collected or measurement taken due to tributaries being dry.
Across all sites pH values were circum-neutral (6.3–7.6). Tambopata River had the highest mean (± standard deviation) pH values (7.4 ± 0.2) followed by Malinowski River (6.7 ± 0.4) and Heath River (6.7 ± 0.3). Tukey’s HSD showed Tambopata River had significantly higher pH values than both Malinowski and Heath rivers (p < 0.02). On average all temperature values were in the same range (26.4 ± 1.3 °C), but mainstem sites had significantly higher temperatures (27.1 ± 1.3 °C) than tributary sites (25.6 ± 1.1 °C, p = 0.041). Tambopata River had the highest mean dissolved oxygen (DO) concentrations (7.3 ± 0.2 mg/L), followed by Malinowski River (6.6 ± 0.3 mg/L) and Heath River (5.6 ± 1.6 mg/L). Tukey’s HSD identified a significant difference in DO concentrations among all rivers (Tambopata-Malinowski, p = 0.039; Malinowski-Heath, p = 0.003; Tambopata-Heath, p < 0.00001). Analysis showed significantly higher nitrate (NO3−) concentrations in the wet season (0.27 ± 0.09 mg N/L) compared to the dry season (0.11 ± 0.08 mg N/L, p = 0.0002), but not among rivers or tributaries vs. mainstem sites.
We noted that turbidity was significantly higher in the mainstem rivers (388 ± 272 NTU) than their tributaries (71 ± 77 NTU, p = 0.014) (Table 1). The single highest turbidity (890 NTU) was recorded at a mainstem site on Tambopata River during the wet season. The lowest turbidity (9 NTU) was recorded in a tributary to Malinowski River during the wet season. During the low-flow, dry season, the difference between the clear Tambopata (42 ± 31 NTU) and turbid Malinowski (401 ± 56 NTU) rivers was evident at their confluence (Figure 2). Despite the large difference in mean turbidity, only two sites measured in Malinowski River led to an adjusted p-value from Tukey’s HSD of 0.069. For Heath River, turbidity in the mainstem was higher during the dry season (644 ± 220 NTU) due to uncharacteristically large, daily precipitation events. However, in the wet season, Heath River had the lowest mean turbidity (305 ± 187 NTU) in mainstem sites in comparison to both Tambopata (581 ± 362 NTU) and Malinowski (359 ± 168 NTU) rivers.
Figure 2.
Photo looking upstream at the confluence of the relatively clear Tambopata River (left) and the more turbid Malinowski River (right), during the dry season sampling event in August 2017.
3.2. Total Hg and % C in Sediment
Hgsed were positively, linearly correlated with % C (R2 = 0.85) for all samples combined. Thus, Hgsed were normalized to the average % C (0.39) using the linear relationship. % C values ranged from 0.03 to 0.98% (0.39 ± 0.29%). % C-normalized Hgsed ranged from 13.9 to 367.2 μg/kg (21.3 ± 3.9 μg/kg). The highest Hgsed was found immediately downstream from an ongoing ASGM site which was releasing mining tailings directly into Malinowski River. This value is considered an outlier, and therefore was excluded from the normalizing and statistical analyses.
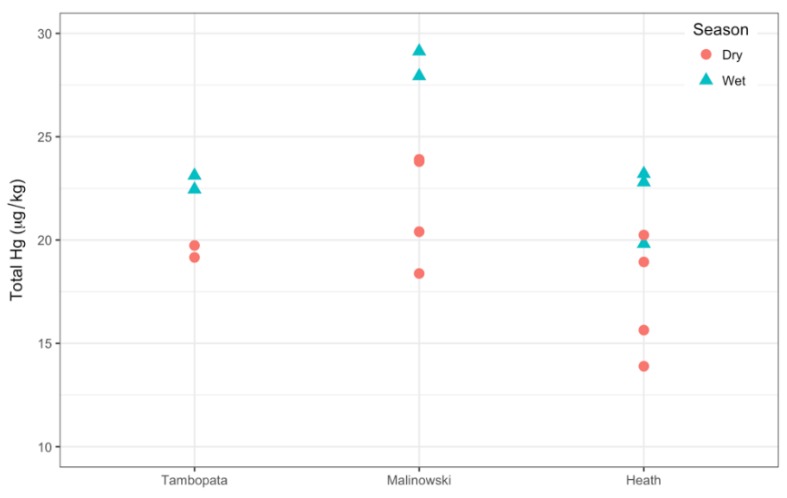
The two highest Hgsed values were measured in Malinowski River during the wet season (29.1 and 27.9 μg/kg) (Figure 3). The two lowest Hgsed values were measured in samples from Heath River during the dry season (13.9 and 15.6 μg/kg). On average, Hgsed were significantly higher in the wet season (24.1 ± 3.3 μg/kg) than in the dry season (19.4 ± 3.1 μg/kg, p < 0.001). The ASGM-impacted Malinowski River had the highest mean Hgsed (23.9 ± 4.1 μg/kg), followed by Tambopata River (21.1 ± 1.9 μg/kg) and Heath River (19.2 ± 3.4 μg/kg). There was no evidence of interaction between river and season (p = 0.49), but there was a significant difference in Hgsed among the three rivers (p = 0.01). There was a significant difference in Hgsed between Malinowski and Heath rivers (p = 0.008), but no difference between other pairwise comparisons.
Figure 3.
The % C-normalized Hgsed (μg/kg) for each river. Dry season Hgsed are shown with circles and wet season Hgsed are shown with triangles. All wet season Hgsed are higher than dry season Hgsed for each river, except one value in Heath River. An outlier collected directly from an ASGM-site was not included (Malinowski River = 367.2 μg/kg).
3.3. Hg in Fish
Hgfish data indicated significant differences among the three rivers. Tukey’s HSD showed that Hgfish in Malinowski River were significantly different than Tambopata (p = 0.02) and Heath (p = 0.0001) rivers. Various factors were explored (length, season, and baseline-corrected trophic position) to further investigate differences in Hgfish after controlling for these factors.
3.3.1. Fish Length
More than 35 species of fish were collected among all sites (Table S2), ranging in length from 66 mm to 980 mm (median 205 mm). Fish length is a common scaling factor for Hgfish; therefore, we attempted to normalize Hgfish to median lengths for species for which >5 individuals were collected. However, Hgfish were not strongly correlated with length. Therefore, we present unadjusted Hgfish. Hgfish ranged from 0.01 mg/kg to 1.50 mg/kg (median 0.14 mg/kg). The highest Hgfish was found in a piscivorous paña Serrasalmus spp. (1.50 mg/kg) sampled in the ASGM-impacted Malinowski River. The fish was only 79 mm long. The longest fish sampled was 980 mm, a piscivorous puma zungaro Pseudoplatystoma tigrinum (0.82 mg/kg) caught in Heath River.
3.3.2. Wet vs. Dry Season
The mean Hgfish in Malinowski River was significantly higher in the dry season (0.28 ± 0.24 mg/kg) than in the wet season (0.18 ± 0.15 mg/kg, p = 0.028) (Table 2). However, for Tambopata and Heath rivers mean Hgfish were similar in the wet (Tambopata: 0.23 ± 0.23 mg/kg; Heath; 0.20 ± 0.22 mg/kg) and dry seasons (Tambopata: 0.22 ± 0.27 mg/kg, p = 0.508; Heath: 0.19 ± 0.27 mg/kg, p = 0.072). ANOVA among rivers and seasons showed there was no significant difference between seasons (p = 0.33). Therefore, subsequent comparisons are based on data combined for both seasons.
Table 2.
Mean fish Hg concentrations (±standard deviation) for each season.
| River | Season | Mean Hgfish (mg/kg) |
|---|---|---|
| Tambopata | Wet | 0.23 ± 0.23 |
| Dry | 0.22 ± 0.27 | |
| Malinowski | Wet | 0.18 ± 0.15 |
| Dry | 0.28 ± 0.24 | |
| Heath | Wet | 0.20 ± 0.22 |
| Dry | 0.19 ± 0.27 |
3.3.3. Fish Isotopic Values (δ15N and δ13C)
The δ15N values ranged from 3.9 to 12.4‰ (9.4 ± 1.5‰) for the entire dataset. Mean δ15N values were lowest in Tambopata River (8.6 ± 1.4‰), followed by Heath River (9.6 ± 1.4‰) and Malinowski River (9.8 ± 1.4‰). Two-way ANOVA showed there was no significant difference between seasons (p = 0.82), but there was a significant difference among the three rivers (p < 0.000001). Tukey’s HSD indicated a significant difference in δ15N values between Tambopata and both Heath and Malinowski rivers (p < 0.00001).
The δ13C values ranged from −39.4 to −14.9‰ (−31.3 ± 3.9‰) for the entire dataset. Mean δ13C values were most depleted in Heath River (−33.2 ± 3.6‰), followed by Malinowski River (−29.8 ± 2.3‰) and Tambopata River (−28.4 ± 3.6‰). Two-way ANOVA showed there was no significant difference between seasons (p = 0.10), but there was a significant difference among the three rivers (p < 0.000001). Tukey’s HSD showed there was a significant difference in δ13C values among all rivers (Tambopata-Malinowski, p = 0.037; Malinowski-Heath, p < 0.000001; Tambopata-Heath, p < 0.000001).
Log-transformed Hgfish were positively, linearly correlated with δ15N baseline corrected trophic position for each river: Tambopata River, R2 = 0.52; Malinowski River, R2 = 0.51; Heath River, R2 = 0.45. Thus, log Hgfish were normalized to median δ15N baseline-corrected trophic position (2.3) for all fish samples, using the linear model constructed for each river. Once normalized, the highest mean Hgfish were in Tambopata River (0.22 ± 0.14 mg/kg), followed by Malinowski River (0.17 ± 0.10 mg/kg), and lastly Heath River (0.16 ± 0.13 mg/kg). ANOVA showed significant differences in mean log Hgfish among the three rivers (p = 0.03). Tukey’s HSD indicated there was a significant difference in mean log Hgfish only between Tambopata and Heath rivers (p = 0.037).
Tambopata River had the highest fraction of fish (27%) above the United States Environmental Protection Agency fish tissue-based water quality criterion of 0.3 mg Hg/kg [52]. Heath River had the intermediate fraction of fish caught above the health consumption concentration criterion (13%), while Malinowski River had the lowest fraction of fish above the criterion (9%). Although the criterion is for MeHg, the USEPA suggests that 90 to 95 (or greater) percent of total Hg in fish tissue is MeHg. Therefore, we assumed the Hgfish occurred as MeHg, and compare results directly to this criterion. Heath River had the most fish, 20 of (154), with Hgfish above the health consumption criteria. Tambopata River and Malinowski River had 16 (of 61) fish and 7 (of 80) fish, respectively, above the criterion.
4. Discussion
Although the major waterways studied in MDD are generally similar to each other in terms of their physicochemical water quality conditions, environmental degradation was apparent in Malinowski River’s very turbid water indicative of upstream land disturbance. Hgsed measured for this study are consistent with prior studies that evaluated Hgsed from the ASGM-impacted Malinowski River [27,30,33]. Riverine sites closer to ASGM activities tended to have higher Hgsed (i.e., Malinowski and Tambopata rivers), while rivers more remote from mining activities tended to have lower Hgsed (i.e., Heath River). The highest Hgsed measured directly downstream from an ASGM site on the Malinowski River was an order of magnitude higher than all other Hgsed in this study, similar to concentrations measured in other studies near mining activity [10,53,54]. Hgsed varied significantly between seasons, with higher Hgsed during the wet season. These higher Hgsed could be due to transport of Hg contaminated soil from denuded landscapes where mining occurs or the remobilization of river sediments from turbulence associated with runoff events [9,10]. This observation raises concern of the impacts of ASGM on Hg mobilization by sediment transport due to tailings from mining activities that are either directly released or washed into waterways.
In spite of the higher turbidity and Hgsed, we do not find that Hg contamination is strongly expressed as higher Hgfish downstream of ASGM. The lack of a relationship between Hgsed and Hgfish is consistent with other regional Hg studies [55,56,57,58]. Gold mining along the Malinowski River, and elsewhere in MDD, remains localized relative to their large watersheds. Thus, local activities appear to have local impacts, but are not so large contaminate to the entire river downstream from mining sites. It is not clear how much gold mining is contributing to the somewhat elevated concentrations. Apparently, Hg supply is only one factor influencing Hgfish. Likely, complex interactions among multiple factors that influence the transport, bioavailability, methylation and trophic transfer of Hg contribute to Hgfish in MDD.
Larger fish generally have higher Hgfish than smaller fish of the same species [59,60]. However, Hgfish in MDD rivers were not directly correlated with fish size, similar to results reported from other studies in the Amazon [9,27,61]. The tremendous diversity in habitat, feeding patterns and trophic structure in the Peruvian Amazon likely over-shadowed typical bioaccumulation processes for any particular specie [62]. Moreover, high species diversity among sampling sites precluded direct comparisons of any one specie. However, δ15N baseline-corrected trophic position were positively, linearly correlated with Hgfish for each river. Suggesting that, similar to previous studies in the region, feeding behavior and trophic position are better bioindicators of Hgfish than fish size in the region [27,33]. We found only one detritivorous fish, yulilla, with Hgfish higher than the USEPA criterion. All other species above the criterion were omnivores or piscivores, i.e., chambira, corvina, dentón, doncella, paña, pejeperro, pirillo, pico de pato, puma zungaro, raya, and toa.
Even though the highest individual Hgfish was observed in the Malinowski River, mean normalized Hgfish from the Malinowski River were similar to Hgfish in the other rivers. Unexpectedly, Malinowski (0.17 ± 0.10 mg/kg) and Heath (0.16 ± 0.13 mg/kg) rivers had similar mean normalized Hgfish. Tambopata River had the highest mean normalized Hgfish (0.22 ± 0.14 mg/kg). There was not a seasonal difference in Hgfish, in contrast to the seasonal difference in Hgsed. Therefore, future studies should consider sampling in both wet and dry seasons to more fully capture any seasonal variation. Not portrayed in these Hgfish results, however, are differences in aquatic habitats within a given river.
Mean δ15N in fish were lower in Tambopata River (8.6 ± 1.4‰), in comparison to both Heath (9.6 ± 1.4‰), and Malinowski rivers (9.8 ± 1.4‰). δ15N ratio is a proxy for the relative trophic position. δ15N becomes more enriched with each subsequent trophic position. Higher Hgfish in Tambopata River were unexpected given their lower δ15N because lower trophic levels fish tend to have lower Hg concentrations [27,28,33,63]. Mean δ13C was most depleted in Heath River throughout both seasons (−33.2 ± 3.6‰), followed by Malinowski (−29.8 ± 2.3‰) and Tambopata (−28.4 ± 3.6‰) rivers. Although there were no statistical differences in isotopic values between seasons, differences were evident among rivers. δ13C ratio is a signature of the source of energy at the base of the food chain because it fractionates very little in biota. More depleted δ13C values are usually associated with photosynthetic C fixation [64]. The lighter δ13C in Heath River indicate a phytoplankton-driven ecosystem and fish eating on detritus material [65,66]. The more isotopically fractionated (more positive) δ13C ratios in fish from Tambopata and Malinowski rivers are most probably a result of fish feeding on carbon-limited benthic phytoplankton. The differences in the energy source to these ecosystems is probably driven by the different biogeochemistry. This linkage and potential influence on the fish Hg accumulation warrens a further study.
While fish in the ASGM-impacted Malinowski River did not exhibit particularly high Hgfish, mining activities still appear to negatively impact their aquatic ecosystem. Studies suggest that elevated turbidity, resulting from an increased load of suspended sediment such as observed in Malinowski River, leads to a reduction of fish diversity and shifts community structure [67]. The turbidity reduces light transmission, which in turn affects the aquatic food web by both reducing photosynthesis and limiting visual foraging efficiency of many fishes [68,69].
Similar to previous studies conducted in ASGM-impacted river reaches, we found it difficult to collect fish where ASGM was most intense [33,70]. Nonetheless, after spending 20+ hours in Malinowski River, we were able to collect 80 fish samples over both seasons. Sight-feeding detritivores made up only 7% of fish samples for Malinowski River compared to more than 50% of the fish samples in both Tambopata and Heath rivers. Lower trophic position fish, which consume detritus and plants, seem less successful in the turbid waters of Malinowski River with no submerged aquatic vegetation. Economically important fishes present in MDD fish markets (e.g., bocachico, yahuarachi, doncella) are apparently absent from Malinowski River [70].
ASGM-enhanced soil erosion plays an important role in altering the physical structure of habitats by limiting or eliminating food sources for certain fish species [69]. Future research of Hgfish in this region should use carbon and nitrogen isotopes of both fish and primary food sources to investigate Hgfish among fish species with similar feeding habits, as well as the effects of ASGM activities on aquatic food webs. Our findings suggest that further monitoring of Hgfish from MDD could be important to evaluate exposure to Hg in these sensitive environments.
Fish in MDD can be a good indicator of Hg risk and exposure for nearby riverine communities as studies have shown that persons who consume more fish tend to have higher Hg in hair [19,32]. Mercury in human hair may not be the best indicator of Hg contamination at a given riverine site due to many extrinsic and intrinsic drivers which influence pathways of Hg bioaccumulation in food webs [57]. For example, Wyatt et al., (2017) found that the highest hair Hg in communities strongly connected to Madre de Dios River occurred upstream—but downwind—from active mining. For most organisms, exposure to MeHg mainly occurs through diet, whereas for humans associated with ASGM inorganic Hg exposure can also occur through inhalation [7,71].
In order to obtain a better understanding of Hg risk to wildlife and to avoid confounding factors such as migration and variation in trophic position, future studies might utilize juvenile fish, which typically provide a more site-specific measure of relative Hg exposure and uptake in comparison to larger, predatory fish [59,71,72].
5. Conclusions
Hgfish results from this study are similar to results reported by others in the Amazon. Accounting for fish size, species and trophic position, Hgfish were not found to be elevated in the ASGM-impacted Malinowski River in comparison to the other two rivers sampled. Higher Hgfish were correlated with higher trophic position fish, predominantly piscivores, thus indicating that regardless of the proximity to ASGM activity, feeding behavior and food availability play an important role in Hg bioaccumulation. Future investigations in the region should focus on food web dynamics to elucidate effects caused by ASGM.
Most water quality parameters did not portray effects of ASGM; however, the increased turbidity indicated environmental degradation in the ASGM-impacted Malinowski River. Although, Hg loadings from ASGM activities did not translate to higher Hgfish, it did account for higher Hgsed. Sediment samples proved to be better indicators of Hg contamination for local river conditions. Our findings highlight the importance of annual sediment sampling for monitoring Hg contamination and transport along riverine sites.
Due to the high complexity of the Amazonian aquatic ecosystem studied, a direct comparison of Hgfish among sites may not fully explain the variations in Hg contamination. Hgfish concentrations are a good indicator of Hg exposure to riverine communities that rely heavily on fish as a source of food and protein; however, these values alone are not a full representation of ecosystem health. Many confounding variables need to be considered when comparing Hgfish among different sites, such as: species, trophic position, feeding behavior, habitat degradation, and energy sources. Follow-up Hg studies in biota should include isotopic signatures from distinct seasons in order to examine the variation in food availability and energy sources.
Acknowledgments
We thank Miguel Macedo, Shamir Delgado, David Salas, Mael Apaza, Edgar Corazao, Edi Chirinos, Aldo Rivas, Randi Villacorta, and Martin Pillaca for their assistance with field data collections. We would like to thank Reserva Nacional Tambopata (RNTMB), Parque Nacional Bahuaja-Sonene (PNBS), Dirección Regional de la Producción (DIREPRO) and Organismo Nacional de Sanidad Pesquera (SANIPES, Puerto Maldonado) for logistical support. We would also like to thank Mario Montesdeoca and Mariah Taylor for assistance with sample analyses.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-4601/15/8/1584/s1, Table S1: Sample site list with Global Positioning System coordinates. Sites 1-9 correspond to Tambopata River, sites 10-15 correspond to Malinowski River, and sites 16–25 correspond to Heath River. Sites with * were also sampled for sediment, Figure S1: Quantile–quantile plots examining normality of Hgfish data: (a) raw Hgfish data and (b) log-transformed Hgfish data, Table S2: Raw mercury concentrations for fish samples collected during both wet and dry season sampling events for all rivers combined. Sampling Location identifies where each specie was collected—Tambopata River (T), Malinowski River (M), and Heath River (H).
Author Contributions
Conceptualization, G.M. and S.T.; Formal analysis, G.M. and S.W.; Funding acquisition, S.T.; Investigation, G.M.; Methodology, S.A.M.; Resources, C.T.D.; Supervision, L.E.F.; Visualization, G.M.; Writing–original draft, G.M.; Writing—review and editing, J.F.A. and C.M.V.
Funding
Funding for this research was provided in part by EPA STAR Fellowship FP91782701 and UC Davis Civil & Environmental Engineering Fellowship.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.World Health Organization . Exposure to Mercury: A Major Public Health Concern. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- 2.Driscoll C.T., Mason R.P., Chan H.M., Jacob D.J., Pirrone N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013;47:4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundseth K., Pacyna J.M., Pacyna E.G., Pirrone N., Thorne R.J. Global Sources and Pathways of Mercury in the Context of Human Health. Int. J. Environ. Res. Public Health. 2017;14:105. doi: 10.3390/ijerph14010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telmer K.H., Veiga M.M. World emissions of mercury from artisanal and small scale gold mining. In: Mason R., Pirrone N., editors. Mercury Fate and Transport in the Global Atmosphere. Springer; Boston, MA, USA: 2009. pp. 131–172. [Google Scholar]
- 5.United Nations Environment Programme (UNEP) Global Mercury Assessment 2013: Sources, Emissions, Releases, and Environmental Transport. UNEP; Geneva, Switzerland: 2013. [Google Scholar]
- 6.Driscoll C.T., Han Y.-J., Chen C.Y., Evers D.C., Lambert K.F., Holsen T.M., Kamman N.C., Munson R.K. Mercury Contamination in Forest and Freshwater Ecosystems in the Northeastern United States. BioScience. 2007;57:17–28. doi: 10.1641/B570106. [DOI] [Google Scholar]
- 7.Mergler D., Anderson H.A., Chan L.H.M., Mahaffey K.R., Murray M., Sakamoto M., Stern A.H. Methylmercury Exposure and Health Effects in Humans: A Worldwide Concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:MEAHEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Eagles-Smith C.A., Silbergeld E.K., Basu N., Bustamante P., Diaz-Barriga F., Hopkins W.A., Kidd K.A., Nyland J.F. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio. 2018;47:170–197. doi: 10.1007/s13280-017-1011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malm O., Branches F.J.P., Akagi H., Castro M.B., Pfeiffer W.C., Harada M., Bastos W.R., Kato H. Mercury and methylmercury in fish and human hair from the Tapajós river basin, Brazil. Sci. Total Environ. 1995;175:141–150. doi: 10.1016/0048-9697(95)04910-X. [DOI] [PubMed] [Google Scholar]
- 10.Bastos W.R., Gomes J.P., Oliveira R.C., Almeida R., Nascimento E.L., Bernardi J.V., de Lacerda L.D., da Silveira E.G., Pfeiffer W.C. Mercury in the environment and riverside population in the Madeira River Basin, Amazon, Brazil. Sci. Total Environ. 2006;368:344–351. doi: 10.1016/j.scitotenv.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 11.United States Geological Survey (USGS) Mineral Commodity Summaries 2016. USGS; Reston, VA, USA: 2016. p. 202. [Google Scholar]
- 12.World Atlas. [(accessed on 28 February 2018)]; Available online: https://www.worldatlas.com/articles/top-14-gold-producers-in-the-world.html.
- 13.Investing News Network. [(accessed on 28 February 2018)]; Available online: https://investingnews.com/daily/resource-investing/precious-metals-investing/gold-investing/top-gold-producing-countries/
- 14.Brack A., Ipenza C., Alvarez J., Sotero V. In: Minería Aurífera en Madre de Dios y Contaminación con Mercurio—Una Bomba de Tiempo. 1st ed. Editorial Súper Gráfica E.I.R.L., editor. Ministerio del Ambiente; Lima, Peru: 2011. [Google Scholar]
- 15.Asner G.P., Llactayo W., Tupayachi R., Luna E.R. Elevated rates of gold mining in the Amazon revealed through high-resolution monitoring. Proc. Natl. Acad. Sci. USA. 2013;110:18454–18459. doi: 10.1073/pnas.1318271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asner G.P., Tupayachi R. Accelerated losses of protected forests from gold mining in the Peruvian Amazon. Environ. Res. Lett. 2017;12:094004. doi: 10.1088/1748-9326/aa7dab. [DOI] [Google Scholar]
- 17.Pachas V.H. Historia de una Incertidumbre: Hábitat, Conflicto y Poder en la Minería Artesanal de oro en el Perú. Earth First SAC; Lima, Perú: 2013. p. 292. Conservación y Desarrollo. [Google Scholar]
- 18.Valencia L. Políticas de Pequeña Minería y Deforestación: El Caso de Madre de Dios. SPDA; Lima, Peru: 2016. [Google Scholar]
- 19.Ashe K. Elevated mercury concentrations in humans of Madre de Dios, Peru. PLoS ONE. 2012;7:e33305. doi: 10.1371/journal.pone.0033305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerson J.R., Driscoll C.T., Hsu-Kim H., Berhhardt E.S. Senegalese artisanal gold mining leads to elevated total mercury and methylmercury concentrations in soils, sediments, and rivers. Elementa Sci. Anthr. 2018;6 doi: 10.1525/elementa.274. [DOI] [Google Scholar]
- 21.Alpers C.N., Hunerlach M.P., May J.T., Hothem R.L., Taylor H.E., Antweiler R.C., De Wild J.F., Lawler D.A. U.S. Geological Survey Scientific Investigations Report 2004-5251. U.S. Department of Interior and the U.S. Geological Survey; Reston, VA, USA: 2005. Geochemical characterization of water, sediment, and biota affected by mercury contamination and acidic drainage from historical gold mining, Greenhorn Creek, Nevada County, California, 1999–2001; p. 278. [Google Scholar]
- 22.Tollefson L., Cordle F. Methylmercury in Fish: A Review of Residue Levels, Fish Consumption and Regulatory Action in the United States. Environ. Health Perspect. 1986;68:203–208. doi: 10.1289/ehp.8668203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloom N.S. On the Chemical Form of Mercury in Edible Fish and Marine Invertabrate Tissue. Can. J. Fish. Aquat. Sci. 1992;49:1010–1017. doi: 10.1139/f92-113. [DOI] [Google Scholar]
- 24.United States Geological Survey (USGS) Mercury Contamination of Aquatic Ecosystems. USGS; Reston, VA, USA: 1995. [(accessed on 25 July 2018)]. Available online: https://pubs.usgs.gov/fs/1995/fs216-95/pdf/fs21695.pdf. [Google Scholar]
- 25.Tello S., Bayley P.B. La pesquería comercial de Loreto con énfasis en el análisis de la relación entre captura y esfuerzo pesquero de la flota comercial de Iquitos, cuenca del Amazonas (Perú) Folia Amaz. 2001;12:123–139. doi: 10.24841/fa.v12i1-2.128. [DOI] [Google Scholar]
- 26.United States Environmental Protection Agency (USEPA) Mercury Study Report to Congress, Volume III: Fate and Transport of Mercury Fate in the Environment. USEPA; Washington, DC, USA: 1997. [Google Scholar]
- 27.Diringer S.E., Feingold B.J., Ortiz E.J., Gallis J.A., Araujo-Flores J.M., Berky A., Pan W.K., Hsu-Kim H. River transport of mercury from artisanal and small-scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru. Environ. Sci. Process Impacts. 2015;17:478–487. doi: 10.1039/C4EM00567H. [DOI] [PubMed] [Google Scholar]
- 28.Centro de Atención Médica Especializada y Preventiva (CAMEP) Mercury in Madre de Dios—Mercury Concentrations in Fish and Humans in Puerto Maldonado. CAMEP; Vista Alegre, Panama: 2013. [(accessed on 26 April 2018)]. Available online: https://dge.carnegiescience.edu/research/CAMEP/CAMEP%20Research%20Brief%20-%20Puerto%20Maldonado%20English%20-%20FINAL.pdf. [Google Scholar]
- 29.Luis Fernández V.G. Niveles del Mercurio en Peces de Madre de Dios. [(accessed on 26 April 2018)]; Available online: http://www.minam.gob.pe/mineriailegal/wp-content/uploads/sites/43/2013/10/Carnegie-mercurio-Madre-de-Dios.pdf.
- 30.Roach K.A., Jacobsen N.F., Fiorello C.V., Stronza A., Winemiller K.O. Gold Mining and Mercury Bioaccumulation in a Floodplain Lake and Main Channel of the Tambopata River, Perú. J. Environ. Prot. 2013;4:51–60. doi: 10.4236/jep.2013.41005. [DOI] [Google Scholar]
- 31.Langeland A.L., Hardin R.D., Neitzel R.L. Mercury Levels in Human Hair and Farmed Fish near Artisanal and Small-Scale Gold Mining Communities in the Madre de Dios River Basin, Peru. Int. J. Environ. Res. Public Health. 2017;14:302. doi: 10.3390/ijerph14030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt L., Ortiz E.J., Feingold B., Berky A., Diringer S., Morales A.M., Jurado E.R., Hsu-Kim H., Pan W. Spatial, Temporal, and Dietary Variables Associated with Elevated Mercury Exposure in Peruvian Riverine Communities Upstream and Downstream of Artisanal and Small-Scale Gold Mining. Int. J. Environ. Res. Public Health. 2017;14:1582. doi: 10.3390/ijerph14121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Brush M., Rydberg J., Gamboa N., Storch I., Biester H. Is mercury from small-scale gold mining prevalent in the southeastern Peruvian Amazon? Environ. Pollut. 2016;218:150–159. doi: 10.1016/j.envpol.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 34.Myers N. Threatened Biotas: “Hot Spots” in Tropical Forests. Environmentalist. 1988;8:187–208. doi: 10.1007/BF02240252. [DOI] [PubMed] [Google Scholar]
- 35.Olson D.M., Dinerstein E. The Global 200: A Representation Approach to Conserving the Earth’s Most Biologically Valuable Ecoregions. Conserv. Biol. 1998;12:502–515. doi: 10.1046/j.1523-1739.1998.012003502.x. [DOI] [Google Scholar]
- 36.Jensen K.E., Naik N.N., O’Neal C., Salmon-Mulanovich G., Riley-Powell A.R., Lee G.O., Hartinger S.M., Bausch D.G., Paz-Soldan V.A. Small scale migration along the interoceanic highway in Madre de Dios, Peru: An exploration of community perceptions and dynamics due to migration. BMC Int. Health Hum. Rights. 2018;18:12. doi: 10.1186/s12914-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis R.E., Kullander S.O., Ferraris C.J.J. Check List of the Freshwater Fishes of South and Central America. EDIPUCRS; Porto Alegre, Brasil: 2003. p. 742. [Google Scholar]
- 38.Van der Sleen P. Albert, J.S. Field Guide to the Fishes of the Amazon, Orinoco, & Guianas. Princeton University Press; Princeton, NJ, USA: 2018. [Google Scholar]
- 39.Baker R.F., Blanchfield P.J., Paterson M.J., Flett R.J., Wesson L. Evaluation of Nonlethal Methods for the Analysis of Mercury in Fish Tissue. Am. Fish. Soc. 2004;133:568–576. doi: 10.1577/T03-012.1. [DOI] [Google Scholar]
- 40.Peterson S.A., Van Sickle J., Hughes R.M., Schacher J.A., Echols S.F. A biopsy procedure for determining filet and predicting whole-fish mercury concentration. Arch. Environ. Contam. Toxicol. 2005;48:99–107. doi: 10.1007/s00244-004-0260-4. [DOI] [PubMed] [Google Scholar]
- 41.Ackerson J.R., McKee M.J., Schmitt C.J., Brumbaugh W.G. Implementation of a non-lethal biopsy punch monitoring program for mercury in smallmouth bass, Micropterus dolomieu Lacepede, from the Eleven Point River, Missouri. Bull. Environ. Contam. Toxicol. 2014;92:125–131. doi: 10.1007/s00128-013-1145-x. [DOI] [PubMed] [Google Scholar]
- 42.Henderson C.J., Stevens T.F., Lee S.Y. Assessing the suitability of a non-lethal biopsy punch for sampling fish muscle tissue. Fish Physiol. Biochem. 2016;42:1521–1526. doi: 10.1007/s10695-016-0237-z. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Environmental Protection Agency (USEPA) Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. USEPA; Washington, DC, USA: 1998. [Google Scholar]
- 44.Zimmerman C.F., Keefe C.W., Bashe J. Method 440.0 Determination of Carbon and Nitrogen in Sediments and Particulatesof Estuarine/Coastal Waters Using Elemental Analysis. U.S. Environmental Protection Agency; Washington, DC, USA: 1997. [Google Scholar]
- 45.Stewart A.R., Saiki M.K., Kuwabara J.S., Alpers C.N., Marvin-DiPasquale M., Krabbenhoft D.P. Influence of plankton mercury dynamics and trophic pathways on mercury concentrations of top predator fish of a mining-impacted reservoir. Can. J. Fish. Aquat. Sci. 2008;65:2351–2366. doi: 10.1139/F08-140. [DOI] [Google Scholar]
- 46.Post D.M. Using Stable Isotopes to Estimate Trophic Position: Models, Methods, and Assumptions. Ecology. 2002;83:703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2. [DOI] [Google Scholar]
- 47.Carscallen W.M.A., Vandenberg K., Lawson J.M., Martinez N.D., Romanuk T.N. Estimating trophic position in marine and estuarine food webs. Ecosphere. 2012;3 doi: 10.1890/ES11-00224.1. [DOI] [Google Scholar]
- 48.Vander Zanden J., Rasmussen J. Variation in d15N and d13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 2001;46:2061–2066. doi: 10.4319/lo.2001.46.8.2061. [DOI] [Google Scholar]
- 49.Minagawa M., Wada E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between d15N and animal age. Geochim. Cosmochim. Acta. 1984;48:1135–1140. doi: 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- 50.Sokal R.R., Rohlf F.J. Biometry: The Principles and Practice of Statistics in Biological Research. 4th ed. W. H. Freeman and Company; New York, NY, USA: 2011. [Google Scholar]
- 51.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [Google Scholar]
- 52.United States Environmental Protection Agency . Water Quality Criterion for the Protection of Human Health: Methylmercury Final. EPA; Washington, DC, USA: 2001. [Google Scholar]
- 53.Malm O., Pfeiffer W.C., Souza C.M.M., Reuther R. Mercury pollution due to gold mining in the Madeira River Basin, Brazil. Ambio. 1990;19:11–15. [Google Scholar]
- 54.Lacerda L.D., Marins R.V., Souza C.M.M., Rodrigues S., Pfeiffer W.C., Bastos W.R. Mercury dispersal in water, sediments and aquatic biota of a gold mining tailings drainage in Pocone’, Brazil. Water Air Soil Pollut. 1991;55:283–294. doi: 10.1007/BF00211194. [DOI] [Google Scholar]
- 55.Malm O., Castro M.B., Bastos W.R., Branches F.J.P., Guimarães J.R.D., Zuffo C.E., Pfeiffer W.C. An assessment of Hg pollution in different goldmining areas, Amazon Brazil. Sci. Total Environ. 1995;175:127–140. doi: 10.1016/0048-9697(95)04909-6. [DOI] [Google Scholar]
- 56.Lechler P.J., Miller J.R., Lacerda L.D., Vinson D., Bonzongo J.-C., Lyons W.B., Warwick J.J. Elevated mercury concentrations in soils, sediments, water, and fish of the Madeira River basin, Brazilian Amazon: A function of natural enrichments? Sci. Total Environ. 2000;260:87–96. doi: 10.1016/S0048-9697(00)00543-X. [DOI] [PubMed] [Google Scholar]
- 57.Eagles-Smith C.A., Ackerman J.T., Willacker J.J., Tate M.T., Lutz M.A., Fleck J.A., Stewart A.R., Wiener J.G., Evers D.C., Lepak J.M., et al. Spatial and temporal patterns of mercury concentrations in freshwater fish across the Western United States and Canada. Sci. Total Environ. 2016;568:1171–1184. doi: 10.1016/j.scitotenv.2016.03.229. [DOI] [PubMed] [Google Scholar]
- 58.Eagles-Smith C.A., Wiener J.G., Eckley C.S., Willacker J.J., Evers D.C., Marvin-DiPasquale M., Obrist D., Fleck J.A., Aiken G.R., Lepak J.M., et al. Mercury in western North America: A synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci. Total Environ. 2016;568:1213–1226. doi: 10.1016/j.scitotenv.2016.05.094. [DOI] [PubMed] [Google Scholar]
- 59.Wiener J.G., Krabbenhoft D.P., Heinz G.H., Scheuhammer A.M. Ecotoxicology of Mercury. In: Hoffman D.J., Rattner B.A., Burton G.A. Jr., Cairns J. Jr., editors. Handbook of Ecotoxicology. 2nd ed. CRC Press; Boca Raton, FL, USA: 2002. pp. 409–463. [Google Scholar]
- 60.Davis J.A., Ross J.R., Bezalel S., Sim L., Bonnema A., Ichikawa G., Heim W.A., Schiff K., Eagles-Smith C.A., Ackerman J.T. Hg concentrations in fish from coastal waters of California and Western North America. Sci. Total Environ. 2016;568:1146–1156. doi: 10.1016/j.scitotenv.2016.03.093. [DOI] [PubMed] [Google Scholar]
- 61.Barbosa A.C., De Souza J., Dorea J.G., Jardim W.F., Fadini P.S. Mercury Biomagnification in a Tropical Black Water, Rio Negro, Brazil. Arch. Environ. Contam. Toxicol. 2003;45:235–246. doi: 10.1007/s00244-003-0207-1. [DOI] [PubMed] [Google Scholar]
- 62.Ortega H., Hidalgo M., Trevejo G., Correa E., Cortijo A.M., Meza V., Espino J. Lista Anotada de los Peces de Aguas Continentales del Perú: Estado Actual del Conocimiento, Distribución, Usos y Aspectos de Conservación. Ministerio del Ambiente; Lima, Peru: 2012. p. 56. [Google Scholar]
- 63.Bastos W.R., Dorea J.G., Bernardi J.V., Lauthartte L.C., Mussy M.H., Lacerda L.D., Malm O. Mercury in fish of the Madeira river (temporal and spatial assessment), Brazilian Amazon. Environ. Res. 2015;140:191–197. doi: 10.1016/j.envres.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 64.Campbell L.M., Hecky R.E., Wandera S.B. Stable Isotope Analyses of Food Web Structure and Fish Diet in Napoleon and Winam Gulfs, Lake Victoria, East Africa. J. Great Lakes Res. 2003;29:243–257. doi: 10.1016/S0380-1330(03)70552-8. [DOI] [Google Scholar]
- 65.Araujo-Lima C.A., Forsberg B.R., Victoria R., Martinelli L. Energy Sources for Detritivorous Fishes in the Amazon. Science. 1986;234:1256–1258. doi: 10.1126/science.234.4781.1256. [DOI] [PubMed] [Google Scholar]
- 66.Wantzen K.M., Machado F.D.A., Voss M., Boriss H., Junk W.J. Seasonal isotopic shifts in fish of the Pantanal wetland, Brazil. Aquat. Sci. 2002;64:239–251. doi: 10.1007/PL00013196. [DOI] [Google Scholar]
- 67.Mol J., Ouboter P.E. Downstream Effects of Erosion from Small-Scale Gold Mining on the Instream Habitat and Fish Community of a Small Neotropical Rainforest Stream. Conserv. Biol. 2004;18:201–214. doi: 10.1111/j.1523-1739.2004.00080.x. [DOI] [Google Scholar]
- 68.Aksnes D.L., Nejstgaard J., Sædberg E., Sørnes T. Optical control of fish and zooplankton populations. Limnol. Oceanogr. 2004;49:233–238. doi: 10.4319/lo.2004.49.1.0233. [DOI] [Google Scholar]
- 69.Wantzen K., Mol J. Soil Erosion from Agriculture and Mining: A Threat to Tropical Stream Ecosystems. Agriculture. 2013;3:660–683. doi: 10.3390/agriculture3040660. [DOI] [Google Scholar]
- 70.Lujan N.K., Roach K.A., Jacobsen D., Winemiller K.O., Vargas V.M., Ching V.R., Maestre J.A., Ladle R. Aquatic community structure across an Andes-to-Amazon fluvial gradient. J. Biogeogr. 2013;40:1715–1728. doi: 10.1111/jbi.12131. [DOI] [Google Scholar]
- 71.Slotton D.G., Ayers S.M., Suchanek T.H., Weyand R.D., Liston A.M., MacDonald C., Nelson D.C., Johnson B. The Effects of Wetland Restoration on the Production and Bioaccumulation of Methylmercury in the Sacramento-San Joaquin Delta, California: Draft Final Report. CALFED; Davis, CA, USA: 2002. [(accessed on 25 July 2018)]. p. 48. Available online: http://loer.tamug.edu/calfed/Report/DraftFinal/UCD_Delta_Report.pdf. [Google Scholar]
- 72.Slotton D.G., Ayers S.M., Suchanek T.H., Weyand R.D., Liston A.M. Mercury Bioaccumulation and Trophic Transfer in the Cache Creek Watershed of California, in Relation to Diverse Aqueous Mercury Exposure Conditions. [(accessed on 25 July 2018)];2004 :74. Assessment of Ecological and Human Health Impacts of Mercury in the San Francisco Bay-Delta Watershed. A CALFED Bay-Delta Program Project. Available online: http://loer.tamug.edu/calfed/Report/Final/UCDavis_Cache_Bio_Final.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.