Abstract
Background
Arboviral disease transmitted by Aedes albopictus such as dengue fever is an important threat to human health. Pyrethroid resistance raises a great challenge for mosquito control. A systematic assessment of Ae. albopictus resistance status in China is urgently needed, and the study of correlation between pyrethroid resistance and knockdown resistance (kdr) mutations would provide information to guide the control of the Ae. albopictus vector.
Methods
Five field populations of Ae. albopictus were collected from Jinan (JN), Hangzhou (HZ), Baoshan (BS), Yangpu (YP) and Haikou (HK), China in 2017. Insecticide-impregnated papers were prepared with four pyrethroid chemicals, deltamethrin, permethrin, beta-cypermethrin and lambda-cyhalothrin. The susceptibility of Ae. albopictus to pyrethroids was tested by the WHO tube assay. Kdr mutations were identified by PCR and sequencing. Moreover, the correlation analysis between kdr alleles and pyrethroid resistance was performed.
Results
All five populations of Ae. albopictus showed resistance to four pyrethroid insecticides. One kdr mutant allele at codon 1532 and three at 1534 were detected with frequency of 5.33% (I1532T), 44.20% (F1534S), 1.83% (F1534 L) and 0.87% (F1534C), respectively. Both 1532 and 1534 mutation mosquitoes were found in the BS and YP populations. Allele I1532T was negatively correlated with deltamethrin resistance phenotype (OR < 1), while F1534S mutation was positively correlated with deltamethrin and permethrin resistance (OR > 1).
Conclusions
The five field populations of Ae. albopictus adults were all resistant to deltamethrin, permethrin, beta-cypermethrin and lambda-cyhalothrin. Mutant F1534S was clearly associated with pyrethroid resistance phenotype in Ae. albopictus and this could be developed as a molecular marker to monitor the pyrethroid resistance problem in China.
Electronic supplementary material
The online version of this article (10.1186/s40249-018-0471-y) contains supplementary material, which is available to authorized users.
Keywords: Aedes albopictus, Pyrethroid, Insecticide resistance, kdr mutation, WHO tube bioassay
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the five official working languages of the United Nations.
Background
Aedes albopictus Skuse, also called the Asian tiger mosquito, is widely distributed in China [1], and is the primary vector of dengue fever and chikungunya fever in China [2, 3]. The spraying of chemical insecticides to control the vector is one of the most important methods to prevent the arboviral diseases due to absence of effective vaccines and treatments [4, 5]. Pyrethroids have been widely used as indoor/outdoor residual or space sprays for mosquito control in China since the 1980s because of their effectiveness and low toxicity. Especially during the dengue fever outbreaks, pyrethroids were heavily used to control mosquito adults [6, 7]. However, pyrethroid resistance was reported in more and more populations [4, 8], raising a great challenge for mosquito control. The evaluation of chemical susceptibility could provide important information to determine mosquito management strategies. Moreover, understanding the mechanism of mosquito resistance to pyrethroids would be beneficial to development of novel insecticides or application methods.
The mechanisms that mosquitoes have developed for the resistance to pyrethroids include behavioral resistance, target insensitivity and metabolic detoxification [9]. Target insensitivity, also known as knockdown resistance (kdr) caused by mutations in the voltage-gated sodium channel (VGSC) gene, which is the target site of pyrethroids [10]. Several surveys indicated that pyrethroid resistance in Culex pipiens pallens Coquillett, Anopheles sinensis Wiedemann and Ae. aegypti Linnaeus were associated with kdr mutations [11–15]. However, there are few studies about kdr mutations in Ae. albopictus. In 2011, Kasai et al. [10] firstly reported that the F1534C mutant allele was detected in Ae. albopictus from Singapore, with a frequency of 73.1%. Then, in 2015, our research team detected new mutant alleles F1534S and F1534 L in Ae. albopictus larvae from Haikou, Hainan, China, and identified that the F1534S mutant allele was associated with pyrethroid resistance [16, 17]. The F1534S/L mutations were also found in Ae. albopictus from Guangzhou, Guangdong, China [4, 18].
Aedes albopictus has a wide distribution in China, north to Liaoning Province and west to Tibet Autonomous Region [19]. A lot of investigations about the susceptible status of Ae. albopictus field populations have been reported in China [16, 20–22], most of which focused on the larvae stage [23]. However, pyrethroid insecticides space spraying was mainly targeted to adult mosquitoes. The susceptible status of the adult stage of Ae. albopictus tested by the World Health Organization (WHO) tube assay was limited [7, 18, 24–26]. The diagnostic doses of deltamethrin, permethrin, beta-cypermethrin and lambda-cyhalothrin of Ae. albopictus in China were established [27], which could be used as reference for surveillance of physiological resistance. Furthermore, as outbreaks of dengue fever have expanded to new regions in recent years [28–30], a systematic evaluation of Ae. albopictus adult resistance status in China is urgently needed.
In this study, we investigated insecticide resistance of Ae. albopictus adults collected from five populations in Central, East and South China to four kinds of pyrethroids using the WHO tube assay. The corresponding kdr mutations in tested Ae. albopictus adults were detected using PCR and sequencing. Moreover, the associations between pyrethroid resistance and kdr mutations were analyzed.
Methods
Mosquito samples
The field populations of Ae. albopictus were collected from five sites in 2017, as Jinan Shandong (JN), Hangzhou Zhejiang (HZ), Baoshan Shanghai (BS), Yangpu Shanghai (YP) and Haikou Hainan (HK), China (Table 1). Larvae and pupae were scooped from breeding sites, such as used tire dumps, flower pot trays, metal containers, terraria, stone holes, ceramic vessels, plastic containers, and other water containers. They were brought back and reared to adults under standard conditions at 26 ± 1 °C and 65 ± 5% relative humidity with a photoperiod of 12-h light: 12-h dark at the insectary of the Second Military Medical University, Shanghai, China. The F0 generation adults were used for the susceptibility test.
Table 1.
The summary information of the Ae. albopictus collection sites in China.
| Population | Date | Coordinates | Sampling environment | Mosquito-borne diseasesa |
|---|---|---|---|---|
| JN | September 2017 | 117°03′E, 36°39′N | Park, Urban | Malaria [52], Filariasis [53], Japanese B encephalitis [54] |
| HZ | September 2017 | 120°08′E, 30°15′N | Park, Suburban | Malaria [52], Dengue fever [8], Filariasis [53], Japanese B encephalitis [54] |
| BS | September 2017 | 121°31′E, 31°24′N | Residential area, Urban | Malaria [52], Dengue fever [30], Filariasis [53], Japanese B encephalitis [54] |
| YP | September 2017 | 121°33′E, 31°19′N | Park, Suburban | Malaria [52], Filariasis [53], Japanese B encephalitis [54] |
| HK | August 2017 | 110°21′E, 20°05′N | Residential area, Urban | Malaria [52], Dengue fever [8], Filariasis [53], Japanese B encephalitis [54] |
aLocal mosquito-borne diseases cases reported in the last 50 years
Insecticide susceptibility bioassay
Ae. albopictus species were identified by morphology [19] and the molecular marker of ITS2 [31]. Female mosquitoes of 3 to 5 days old after emergence and not blood fed were tested for susceptibility to four pyrethroid insecticides by the tube bioassay following the WHO protocol [32]. The insecticide-impregnated test papers of deltamethrin (0.1036%), permethrin (1.0634%), beta-cypermethrin (0.2400%) and lambda-cyhalothrin (0.2372%) were made in our laboratory [27]. The above four technical-grade pyrethroid chemicals were provided by Jiangsu Yangnong Chemical Group Co., Ltd. (deltamethrin, 98.37% purity; lambda-cyhalothrin, 95.85% purity), and Jiangsu Gongcheng Bio-Tech Co., Ltd. (permethrin, 96.20% purity; beta-cypermethrin, 95.00% purity). Silicone oil-treated papers without insecticide were used as a control. Tests with each insecticide paper and untreated paper were repeated for at least 4 times, including approximately 100 female mosquitoes. The number of mosquitoes knocked down was recorded at 1 h. If the mosquitoes lay with ventral side up and could not fly up, they were considered to be knocked down. After 1 h exposure, the mosquitoes were transferred to a recovery tube and maintained on 10% of sucrose solution for 24 h when the number of dead mosquitoes was recorded to calculate mortality rate, which was used to evaluate the insecticide susceptibility status. After 24 h, if the mosquito can fly, it was considered to be alive, regardless of the number of legs remaining; if it was knocked down, whether or not it had lost legs or wings, was considered moribund and was counted as dead. The 24 h mortality was used to calculate the insecticide sensitivity. Dead and surviving mosquitoes were collected and preserved in 95% ethanol for subsequent DNA analysis.
DNA extraction and kdr alleles detection
Genomic DNA was extracted from a single mosquito using DNAzol Reagent (Invitrogen, USA). To identify kdr alleles, partial sequences of domains II, III and IV of the VGSC gene were amplified using the primers aegSCF3 and aegSCR22, aegSCF7 and albSCR9 (designed in this study, 5’-CTG ATC CTC CGT CAT GAA CA-3′), albSCF6 and albSCR8, developed by Kasai [10]. The PCR kit was purchased from Aidlab, China. PCR reaction was carried out in Veriti 96 well Thermal Cycler (Applied Biosystems, USA). The cycling parameters used were those of Kasai [10]. After electrophoresis, PCR products were purified and directly sequenced in both directions with the same primers.
Statistical analysis
The mortality data were adjusted according to Abbott’s formula if any mosquitoes in the control tube were dead [33]. The susceptibility status of Ae. albopictus was identified by WHO’s criteria, using 90% mortality rate as threshold for resistance [32]. Sequences were aligned and analyzed by DNASTAR Lasergene 12.0 software [34]. The codons were examined and genotypes were determined.
Because none of the populations showed mortality of > 90%, the dead mosquito individuals after the test were considered to have a susceptible phenotype while surviving mosquitoes were categorized as resistant. The kdr allele frequencies in resistant and susceptible samples were calculated in each population. Chi-squared tests were used to examine the association between kdr alleles and the resistant phenotype. The dependent variables were the mosquito phenotypes (resistant or susceptible) at 24 h after bioassay. Then, the odds ratio (OR) values and 95% confidential intervals (CI) of kdr alleles were calculated using SPSS 20.0 (IBM Corp., Armonk, NY, USA) [35]. The relationship between the kdr allele and resistant phenotype was considered as positive when OR > 1, while as negative when OR < 1. If 95% CI of OR value was a range across 1 or P-value > 0.05, it was considered as statistically insignificant.
Results
Pyrethroid susceptibility status of Ae. albopictus field populations
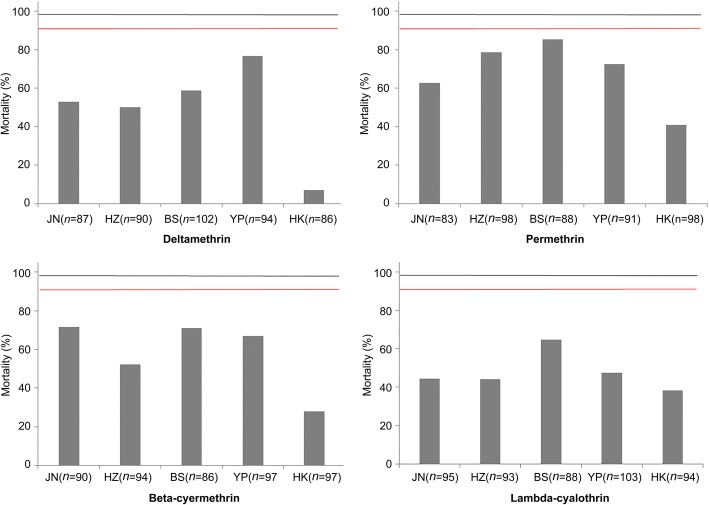
All five populations of Ae. albopictus showed resistance to four pyrethroid insecticides (Fig. 1). The range of mortality was 6.98% (HK) − 76.60% (YP) after exposure to deltamethrin, 40.82% (HK) − 85.23% (BS) to permethrin, 27.84% (HK) − 71.58% (JN) to beta-cypermethrin and 38.30% (HK) − 64.77% (BS) to lambda-cyhalothrin (Additional file 2: Table S1). Of the four pyrethroid insecticides tested, the mortality was the lowest after exposure to deltamethrin across all populations. The resistance level of Ae. albopictus HK population was all the highest to four pyrethroids tested, while BS or YP samples was relatively low in the five populations.
Fig. 1.
The mortality of Aedes albopictus field populations after exposure to four pyrethroids using the WHO tube bioassay. Red lines represent mortality at 90%, black lines as 98%, error bars represent 95% confidential interval (CI)
kdr alleles frequency in Ae. albopictus field populations
Sequences of domains II, III and IV of the VGSC gene were obtained from 303 mosquitoes exposed to deltamethrin and 326 individuals exposed to permethrin. Non-synonymous kdr mutations were detected at codon 1532 and 1534 in domain III of the VGSC gene. Synonymous mutations were not recorded in this study, but were found in all three domains.
Two alleles were detected at codon 1532, one mutant allele ACC/T and the wildtype allele ATC/I (GenBank accession No.: MH384955 − MH384958). The allele I1532T was only detected in BS and YP populations with a frequency of 8.03 and 18.00%, respectively. There were three genotypes including the wildtype genotype I/I (75.57%), wildtype/mutant heterozygote I/T (22.90%) and mutant genotype T/T(1.53%) in two populations (Additional file 2: Table S2).
At codon 1534, the wildtype TTC/F frequency was 53.10%, and three kdr mutant alleles TCC/S, TTA/L and TGC/C were detected with frequency of 44.20, 1.83 and 0.87%, respectively (GenBank accession No.: MH384950 − MH384961). There were a total of seven genotypes, including wildtype genotype F/F (37.52%), wildtype/mutant heterozygote F/S (28.30%), F/L (2.38%) and F/C (0.48%), and mutant genotype S/S (29.41%), L/L (0.64%) and S/C (1.27%). The mutant allele F1534S was found in all Ae. albopictus populations, namely JN, HZ, BS, YP and HK with frequency of 1.39, 90.83, 22.63, 37.20 and 64.75%, respectively, while F1534 L was only found in the JN population with a frequency of 10.65%, and F1534C was only found in the HK population with a frequency of 3.96% (Additional file 2: Table S3).
In the BS and YP populations, we found some individual mosquitoes that showed a kdr mutation in both the 1532 and 1534 codons. The genotypes included wildtype+mutant type (I/I + F/S, I/I + S/S, I/T + F/F, T/T + F/F) and both mutant type (I/T + F/S) (Table 2). However, there were no double mutant homozygotes (T/T + S/S) found, which may be due to the low numbers sampled or due to some other reason to do with the genotype itself which causes it to be rare.
Table 2.
Frequency of simultaneous mutations at codon I1532T and F1534S in Ae. albopictus of BS and YP populations from China
| Population | n | (Wild+mutant) type | (Mutant+mutant) type | |||
|---|---|---|---|---|---|---|
| I/I + F/S | I/I + S/S | I/T + F/F | T/T + F/F | I/T + F/S | ||
| BS | 137 | 39(28.06) | 9(6.47) | 18(12.95) | 1(0.72) | 5(3.60) |
| YP | 125 | 38(30.40) | 18(14.40) | 20(16.00) | 3(2.40) | 19(15.20) |
Note: n indicates the sample number. Data outside brackets is the number of individuals; data inside brackets means its frequency (%)
Correlations between pyrethroid resistance and kdr mutations
The OR values and 95% CI of kdr mutant alleles at codon 1532 and 1534 in Ae. albopictus field populations after exposure to deltamethrin and permethrin were calculated. At codon 1532, OR value was 0.49 (P < 0.05), and 95% CI ranging from 0.23 to 1.00 indicating that I1532T mutant allele was negatively correlated with the deltamethrin resistance phenotype in total samples, and there was no significant correlation in YP and BS populations (Table 3). However, there is no significant correlation between I1532T mutation and permethrin resistant across the total sample, nor in each of the YP and BS populations individually.
Table 3.
kdr mutant allele frequency at codon 1532 of Ae. albopictus and association with pyrethroid resistance in BS and YP populations from China
| Insecticide | Population | Phenotype | n | kdr allele | ||
|---|---|---|---|---|---|---|
| I1532T | I1532 | OR (95% CI) | ||||
| Deltamethrin | BS | R | 32 | 6(9.38) | 58(90.63) | 2.24 (0.54, 9.37) |
| S | 34 | 3(4.41) | 65(95.59) | |||
| YP | R | 28 | 6(10.71) | 50(89.29) | 0.39 (0.14, 1.05) | |
| S | 38 | 18(23.68) | 58(76.32) | |||
| Total | R | 161 | 12(3.73) | 310(96.27) | 0.49* (0.23, 1.00) | |
| S | 142 | 21(7.39) | 263(92.61) | |||
| Permethrin | BS | R | 25 | 4(8.00) | 46(92.00) | 0.80 (0.23, 2.75) |
| S | 46 | 9(9.78) | 83(90.22) | |||
| YP | R | 23 | 7(15.22) | 39(84.78) | 0.74 (0.28, 2.01) | |
| S | 36 | 14(19.44) | 58(80.56) | |||
| Total | R | 138 | 11(3.99) | 265(96.01) | 0.64 (0.31, 1.33) | |
| S | 188 | 23(6.12) | 353(93.88) | |||
Note: n indicates the sample number. S was the susceptible phenotype; R indicates the resistant phenotype. Data outside brackets indicate the number of individuals; data inside brackets indicate frequency (%).
*P < 0.05
At codon 1534, F1534S mutant allele was positively correlated with deltamethrin and permethrin resistance in total samples, both OR values was above 1 (Table 4). Also, the OR values of F1534S mutant allele were all above 1 in three populations (BS, YP and HK) to deltamethrin and two (YP and HK) to permethrin, showed that this allele was positively correlated with the resistance phenotype. Furthermore, there were no statistical significances between F1534 L/F1534C and resistance to two pyrethroids.
Table 4.
kdr mutant allele frequency at codon 1534 of Ae.albopictus populations and association with pyrethroid resistance in China
| Insecticide | Population | Phenotype | n | kdr allele | OR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1534S | F1534 L | F1534C | F1534 | F1534S | F1534 L | F1534C | ||||
| Deltamethrin | JN | R | 26 | 1 (1.92) | 6 (11.54) | 0 (0.00) | 45 (86.54) | 2.35 (0.21, 26.73) | 1.01 (0.32, 3.22) | – |
| S | 30 | 0 (0.00) | 7 (11.67) | 0 (0.00) | 53 (88.33) | |||||
| HZ | R | 25 | 43 (86.00) | 0 (0.00) | 0 (0.00) | 7 (14.00) | 0.28 (0.06, 1.42) | – | – | |
| S | 23 | 44 (95.65) | 0 (0.00) | 0 (0.00) | 2 (4.35) | |||||
| BS | R | 32 | 18 (28.13) | 0 (0.00) | 0 (0.00) | 46 (71.88) | 2.94* (1.17, 7.34) | – | – | |
| S | 34 | 8 (11.76) | 0 (0.00) | 0 (0.00) | 60 (88.24) | |||||
| YP | R | 28 | 30 (53.57) | 0 (0.00) | 0 (0.00) | 26 (46.43) | 2.35* (1.16, 4.79) | – | – | |
| S | 38 | 25 (32.89) | 0 (0.00) | 0 (0.00) | 51 (67.11) | |||||
| HK | R | 50 | 77 (77.00) | 0 (0.00) | 6 (6.00) | 17 (17.00) | 7.55* (3.11, 18.34) | – | 3.53 (0.63, 19.83) | |
| S | 17 | 12 (35.29) | 0 (0.00) | 2 (5.88) | 20 (58.82) | |||||
| Total | R | 161 | 169 (52.48) | 6 (1.86) | 6 (1.86) | 141 (43.79) | 2.50* (1.79, 3.51) | 1.13 (0.37, 3.44) | 3.96 (0.79, 19.90) | |
| S | 142 | 89 (31.34) | 7 (2.46) | 2 (0.70) | 186 (65.49) | |||||
| Permethrin | JN | R | 20 | 2 (5.00) | 4 (10.00) | 0 (0.00) | 34 (85.00) | 5.06 (0.51, 50.52) | 1.14 (0.30, 4.32) | – |
| S | 32 | 0 (0.00) | 6 (9.38) | 0 (0.00) | 58 (90.63) | |||||
| HZ | R | 34 | 60 (88.24) | 0 (0.00) | 0 (0.00) | 8 (11.76) | 0.53 (0.16, 1.70) | – | – | |
| S | 38 | 71 (93.42) | 0 (0.00) | 0 (0.00) | 5 (6.580 | |||||
| BS | R | 25 | 12 (24.00) | 0 (0.00) | 0 (0.00) | 38 (76.00) | 0.90 (0.40, 1.99) | – | – | |
| S | 46 | 24 (26.09) | 0 (0.00) | 0 (0.00) | 68 (73.91) | |||||
| YP | R | 23 | 20 (43.48) | 0 (0.00) | 0 (0.00) | 26 (56.52) | 2.31* (1.05, 5.09) | – | – | |
| S | 36 | 18 (25.00) | 0 (0.00) | 0 (0.00) | 54 (75.00) | |||||
| HK | R | 36 | 60 (83.33) | 0 (0.00) | 0 (0.00) | 12 (16.67) | 6.13* (2.81, 13.38) | – | 0.43 (0.05, 3.82) | |
| S | 36 | 31 (43.06) | 0 (0.00) | 3 (4.17) | 38 (52.78) | |||||
| Total | R | 138 | 154 (55.80) | 4 (1.45) | 0 (0.00) | 118 (42.75) | 2.02* (1.47, 2.78) | 1.26 (0.35, 4.55) | 0.47 (0.05, 4.26) | |
| S | 188 | 144 (38.30) | 6 (1.60) | 3 (0.80) | 223 (59.31) | |||||
Note: n indicates the sample number. S was the susceptible phenotype; R indicates the resistant phenotype. Data outside brackets indicate the number of individuals; data inside brackets indicate frequency (%)
*P < 0.05, −, no data
The kdr mutant allele F1534S showed a positive correlation with resistance to two pyrethroids, while I1532T as a negative correlation with deltamethrin. It is interesting that the two adjacent mutations have opposite effects on pyrethroid resistance, so we chose the samples with F1534S + I1532T and analyzed the possible interactions using the Chi-squared method. The results showed no statistical significance (Table 5), indicating that there were no associations between I1532T with F1534S with respect to pyrethroid resistance. However, a relatively small sample size may be the cause of insignificant results.
Table 5.
Association between I1532T and pyrethroid resistance caused by F1534S in Ae. albopictus BS and YP populations from China
| Insecticide | Population | Phenotype | n | I1532T | I1532 | P-value |
|---|---|---|---|---|---|---|
| Deltamethrin | BS | R | 16 | 2 (6.25) | 30 (93.75) | 1.000 |
| S | 8 | 1 (6.25) | 15 (93.75) | |||
| YP | R | 24 | 5 (10.42) | 43 (89.58) | 0.181 | |
| S | 22 | 9 (20.45) | 35 (79.55) | |||
| Total | R | 40 | 7 (8.75) | 73 (91.25) | 0.156 | |
| S | 30 | 10 (16.67) | 50 (83.33) | |||
| Permethrin | BS | R | 9 | 1 (5.56) | 17 (94.44) | 0.555 |
| S | 20 | 1 (2.50) | 39 (97.50) | |||
| YP | R | 16 | 2 (6.25) | 30 (93.75) | 0.588 | |
| S | 15 | 3 (10.00) | 27 (90.00) | |||
| Total | R | 25 | 3 (6.00) | 47 (94.00) | 0.948 | |
| S | 35 | 4 (5.71) | 66 (94.29) |
Note: All samples have kdr mutate allele F1534S. n indicates the sample number. Data outside brackets indicate the number of individuals; data inside brackets indicate frequency (%)
Discussion
In this study, five field populations of Ae. albopictus were collected ranging from Central (Jinan Shandong), East (Baoshan Shanghai, Yangpu Shanghai and Hangzhou Zhejiang) and South (Haikou Hainan) China. WHO tube bioassay results showed that all populations were resistant to the four tested pyrethroids. The corresponding kdr mutations were detected in the five field populations of Ae. albopictus. I1532T (5.33%) at codon 1532 and F1534S (44.20%), F1534 L (1.83%) and F1534C (0.87%) at codon 1534 were found in the tested mosquitoes. Correlative analysis showed that I1532T mutation was negatively correlated with deltamethrin resistance and F1534S mutation was positively correlated with deltamethrin and permethrin resistance phenotype in Ae. albopictus.
To explain the resistance of Ae. albopictus to pyrethroid insecticides, a survey about the insecticide sales and usage was conducted with the staff of Jiangsu Gongcheng Bio-Tech Co., Ltd., members of the community and pest control organizations in China, and by searching the relevant reports. There were 23 active ingredients of pyrethroids allowed as public health insecticides by the government of the People’s Republic of China [6]. Over the past decade, beta-cypermethrin, lambda-cyhalothrin and permethrin were the most commonly used and marketed pyrethroid insecticides. Deltamethrin was less common because its resistance was reported in many places in China [22, 36–38]. Moreover, according to Wang’s report [6] and “general situation of pesticide production and usage in China in 2015” [39], these four pyrethroids in this testing were heavily used in agriculture, gardens and public health in China. The space spraying frequency was usually once a week in the season of mosquito activity, and even once a day during dengue outbreaks.
In recent years, there have been several outbreaks of dengue fever in China, which have had a tendency to spread to the north [8, 28–30]. As one of the most important control measures, pyrethroid insecticides spraying was widely used in epidemic outbreaks. Few investigations of pyrethroid insecticides resistance of Ae. albopictus adult in China have been reported, and those that have been published used a different diagnostic dose from that tested in the current study [18, 26, 40, 41]. Compared to previous reports, we tested the Ae. albopictus adult samples from more areas including Central, East and South China. The environment of collecting sites were residential areas or parks, where spraying was applied outside on a regular basis to reduce mosquito adult density, and garden pest control insecticides also aggravated the selection pressure of local mosquitoes. Moreover, the five collecting sites were all located in the mosquito-borne diseases (malaria, filariasis, Japanese B encephalitis) epidemic region in the last 50 years (Table 1), where the usage quantity of chemical insecticides has been high throughout history. Many investigations indicated that Ae. albopictus larvae from these areas had developed resistance to pyrethroids [16, 20–22]. Aedes albopictus adults from Hangzhou, Zhejiang were all susceptible [26], which was tested by insecticide-impregnated papers with 0.1% deltamethrin, 3% permethrin and 3% beta-cypermethrin. The differences should be further analyzed because there were many factors affecting bioassay results, such as testing samples, breeding sites, and assessment of mortality standard. These results enriched the information on the pyrethroid resistance of Ae. albopictus populations from a wider area in China. In addition, pyrethroid resistance was detected in adult mosquitoes, which was more direct evidence to guide the use of insecticides.
Among the five populations, Ae. albopictus from Haikou Hainan showed higher resistance level to four pyrethroids. The reason may be that Haikou is in the south of China, located at marginal zone of the tropics where the mosquito population density was high almost all the year. In the past, dengue fever outbreaks have occurred twice in 1979–1982 and 1985–1988 in Hainan Island and surrounding areas; the mortality rate was 0.0785% [42–45]. All the factors led to the continuous use of insecticides and contributed to the development of resistance [16, 17, 37].
The correlation between the kdr mutations and pyrethroid resistance was reported in An. gambiae [46], An. sinensis [15] and Ae. aegypti [14]. However, there are few reports about the correlation between the kdr mutations and the pyrethroid resistance in despite kdr mutant alleles being detected in Ae. albopictus field populations [4, 16–18]. In order to test for a correlation between kdr mutations and pyrethroid resistance of Ae. albopictus, we ensured that the mosquitoes tested by the bioassay and the kdr gene detection were the same in this study, which was important for determining the association between mutations and pyrethroid resistance. Our results further confirmed that the significant positive correlation between F1534S and pyrethroid resistance phenotype in Ae. albopictus (OR value was 2.50 and 2.02 for deltamethrin and permethrin), suggesting that F1534S mutant allele is a potential biomarker for surveillance of physiological resistance in China. Similarly, OR value of F1534S for deltamethrin was from 9.3 to 33.6 in Ae. albopictus Guangzhou populations reported by Li et al. [18]. We are the first to report that the I1532T mutation is negatively correlated with pyrethroid resistance. A similar pattern has been found in which the F1534C mutation in Ae. aegypti is negatively correlated with pyrethroid resistance in a study from southern China [47]. A different pattern was found from the F1534S mutation in these Ae. albopictus samples, I1532T mutation only showed negative correlation with deltamethrin resistance, but no correlation with permethrin. The reason may be that deltamethrin and permethrin are two different types of pyrethroids. Permethrin is a type I pyrethroid, which is without an α-cyano group, while deltamethrin is type II pyrethroid which does contain α-cyano. In this study, F1534 L showed no correlation with pyrethroid resistance (no statistical significance in OR value) (Table 4), which was inconsistent with the results in Ae. albopictus Guangdong population, China (range of OR value from 15.7 to 19.8) [18]. The correlation between different kdr mutant alleles and pyrethroid resistance requires further confirmation in more samples.
The interactions between mutation positions may lead to phenotypic changes in Ae. aegypti [48], which is also an interesting issue in mosquito resistance. We found some individuals with both I1532T + F1534S and I1532T + F1534 L mutations in Ae. albopictus Shanghai (BS and YP) and Yunnan (JH) population [49]. The occurrence of multiple mutations may be a result of the long-term insecticide pressure on mosquitoes. The analyzed results showed there was no significant correlation between I1532T and pyrethroid resistance caused by F1534S in Ae. albopictus BS and YP populations from China. However, a relatively small sample size of the individuals with simultaneous mutations may have led to insignificant results. The interaction between the I1532 with F1534 mutations needs further clarification.
The mechanisms of mosquito insecticide resistance are often multiple [9, 18, 35]. For example, in An. sinensis, no kdr mutant alleles were found in Yunnan, but metabolic detoxification enzymes (monooxygenases, glutathion S-transferase and carboxylesterases) play major roles in pyrethroids and DDT resistance while kdr alleles play a minor role [35]. The kdr mutations were reported largely from An. sinensis populations in central China, where the resistance to DDT and pyrethroids were conferred primarily by the metabolic detoxification mechanisms as well as the kdr mutation [50]. To date, there are few reports of metabolic detoxification mechanisms in Ae. albopictus insecticides resistance, and no regular pattern and potential correlations were found [18, 51]. Although we focused on the correlations between kdr mutations with the pyrethroid resistance in this research, we believe metabolic detoxification enzymes may also play important roles in pyrethroid resistance in Ae. albopictus and will be a topic of future research.
Conclusions
The field populations of Ae. albopictus adults collected from Jinan, Hangzhou, Baoshan, Yangpuand Haikou in China were resistant to deltamethrin, permethrin, beta-cypermethrin and lambda-cyhalothrin tested by WHO tube bioassay. The kdr mutation F1534S was positively correlated with deltamethrin and permethrin resistance phenotype, while the I1532T mutation was negatively correlated only with deltamethrin resistance in Ae. albopictus. The I1532T + F1534S mutations in the same individual were also detected; the interaction between them needs further study.
Additional files
Multilingual abstracts in the five official working languages of the United Nations. (PDF 1038 kb)
Table S1. The knockdown rate and mortality of Ae. albopictus field populations exposed to four pyrethroids. Table S2. Kdr alleles and genotypes at codon 1532 of Aedes albopictus field populations. Table S3. Kdr alleles and genotypes at codon 1534 of Aedes albopictus field populations. (DOCX 31 kb)
Acknowledgments
We are grateful to Pei-En Leng and Xiao-Qing Lin (Shanghai), Li-Nong Yao (Zhejiang), Hai-Tao Huang, Jian-Bin Wu and Fang Cai (Hainan), who provided field assistance in the study.
Funding
This work was supported by National Natural Sciences Foundation of China (No. 81371848) and the Infective Diseases Prevention and Cure Project of China (No. 2017ZX10303404–002).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request, and sequences are available in GenBank.
Abbreviations
- BS
Baoshan
- CI
Confidence intervals
- HK
Haikou
- HZ
Hangzhou
- JN
Jinan
- kdr
Knockdown resistance
- OR
Odds ratio
- VGSC
Voltage-gated sodium channels
- WHO
World Health Organization
- YP
Yangpu
Authors’ contributions
All authors contributed to the collection of mosquitoes. YM and HP designed the study. YM and HS identified specimens by morphological characters. JG and HC performed tube bioassay and rearing mosquitoes. JG performed PCR assay. JG, HP and YM did data analysis and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was carried out in strict accordance with the National Natural Science Foundation of China ethical guidelines for biomedical research involving living animals and human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Jing-Peng Gao, Email: hello_gaojingpeng@163.com.
Han-Ming Chen, Email: 13482329184@163.com.
Hua Shi, Email: placdc@qq.com.
Heng Peng, Email: pengheng0923@126.com.
Ya-Jun Ma, Email: yajun_ma@163.com.
References
- 1.Yang SR, Liu QY. Trend in global distribtuion and spread of Aedes albopictus. Chin J Vector Biol Control. 2013;24(3):1–4. [Google Scholar]
- 2.Lu X, Li X, Mo Z, Jin F, Wang B, Huang J, et al. Chikungunya emergency in China: microevolution and genetic analysis for a local outbreak. Virus Genes. 2014;48(1):15–22. doi: 10.1007/s11262-013-0991-2. [DOI] [PubMed] [Google Scholar]
- 3.Li MT, Sun GQ, Yakob L, Zhu HP, Jin Z, Zhang WY. The driving force for 2014 dengue outbreak in Guangdong, China. PLoS One. 2016;11(11):e0166211. doi: 10.1371/journal.pone.0166211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu JB, Bonizzoni M, Zhong DB, Zhou GF, Cai SW, Li YJ, et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) gene in Aedes albopictus. PLoS Negl Trop Dis. 2016;10(5):e0004696. doi: 10.1371/journal.pntd.0004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11(7):e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YY, Jiang ZK. Development and application of public health pesticides in China, 2013-2016. Chin J Vector Biol Control. 2016;27(5):421–425. [Google Scholar]
- 7.Wang YG, Liu X, Li CL, Su TY, Jin JC, Guo YH, et al. A survey of insecticide resistance in Aedes albopictus (Diptera: Culicidae) during a 2014 dengue fever outbreak in Guangzhou, China. J Econ Entomol. 2017;110(1):239–244. doi: 10.1093/jee/tow254. [DOI] [PubMed] [Google Scholar]
- 8.Meng FX, Wang YG, Feng L, Liu QY. Review on dengue prevention and control and integrated mosquito management in China. Chin J Vector Biol Control. 2015;26(1):4–10. [Google Scholar]
- 9.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45(3):71–91. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 10.Kasai S, Ng LC, Lam-Phua SG, Tang CS, Itokawa K, Komagata O, et al. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus. Jpn J Infect Dis. 2011;64(3):217–221. [PubMed] [Google Scholar]
- 11.Smith LB, Kasai S, Scott JG. Voltage-sensitive sodium channel mutations S989P + V1016G in Aedes aegypti confer variable resistance to pyrethroids, DDT and oxadiazines. Pest Manag Sci. 2018;74(3):737–745. doi: 10.1002/ps.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu HM, Cheng P, Huang X, Dai YH, Wang HF, Liu LJ, et al. Identification of TCT, a novel knockdown resistance allele mutation and analysis of resistance detection methods in the voltage-gated Na+ channel of Culex pipiens pallens from Shandong Province, China. Mol Med Rep. 2013;7(2):525–530. doi: 10.3892/mmr.2012.1184. [DOI] [PubMed] [Google Scholar]
- 13.Wuliandari JR, Lee SF, White VL, Tantowijoyo W, Hoffmann AA, Endersby-Harshman NM. Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in Aedes aegypti from Yogyakarta, Indonesia. Insects. 2015;6(3):658–685. doi: 10.3390/insects6030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushwah RB, Dykes CL, Kapoor N, Adak T, Singh OP. Pyrethroid-resistance and presence of two knockdown resistance (kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLoS Negl Trop Dis. 2015;9(1):e3332. doi: 10.1371/journal.pntd.0003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong D, Chang X, Zhou G, He Z, Fu F, Yan Z, et al. Relationship between knockdown resistance, metabolic detoxification and organismal resistance to pyrethroids in Anopheles sinensis. PLoS One. 2013;8(2):e55475. doi: 10.1371/journal.pone.0055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XH, Chen HY, Yang XY, Lin Y, Cai F, Zhong WB, et al. Resistance to pyrethroid insecticides and analysis of knockdown resistance (kdr) gene mutations in Aedes albopictus form Haikou City. Acad J Second Mil Univ. 2015;36(8):832–838. doi: 10.3724/SP.J.1008.2015.00832. [DOI] [Google Scholar]
- 17.Chen H, Li K, Wang X, Yang X, Lin Y, Cai F, et al. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou city, Hainan island, China. Infect Dis Poverty. 2016;5(1):31. doi: 10.1186/s40249-016-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Xu J, Zhong D, Zhang H, Yang W, Zhou G, et al. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China. Parasit Vectors. 2018;11(1):4. doi: 10.1186/s13071-017-2581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu BL. Insecta: Diptera. In: Zhu HF, editor. History of animals in China. Beijing: Science Press; 1997. [Google Scholar]
- 20.Gong ZY, Hou J, Ren ZY, Ling F, Guo S. Resistance investigation of Culex pallens and Aedes albopictus to eight pesticides and resistance control strategy in Zhejiang Province. Chin J Vector Biol Control. 2012;23(5):458–60.
- 21.Zhang X, Wang D, Wang YM, Xin Z. Resistance monitoring of Culex pipiens and Aedes albopictus to nine insecticides in Ji'nan City. Chin J Hyg Insect Equip. 2016;22(2):121–123. [Google Scholar]
- 22.Liu HX, Zhu J, Liu Y, Xu JQ, Leng PE. Study on the seasonal dynamics and insecticides resistance of Aedes albopictus larvae in shanghai, 2015-2016. Chin J Vector Biol Control. 2017;28(4):305–307. [Google Scholar]
- 23.WHO . Guidelines for laboratory and field testing of mosquito larvicides. Geneva: World Health Organization; 2005. [Google Scholar]
- 24.Thanispong K, Sathantriphop S, Malaithong N, Bangs MJ, Chareonviriyaphap T. Establishment of diagnostic doses of five pyrethroids for monitoring physiological resistance in Aedes albopictus in Thailand. J Am Mosq Control Assoc. 2015;31(4):346–352. doi: 10.2987/moco-31-04-346-352.1. [DOI] [PubMed] [Google Scholar]
- 25.Rahim J, Ahmad AH, Ahmad H, Ishak IH, Rus AC, Maimusa HA. Adulticidal susceptibility evaluation of Aedes albopictus using new diagnostic doses in Penang Island, Malaysia. J Am Mosq Control Assoc. 2017;33(3):200–208. doi: 10.2987/16-6607R.1. [DOI] [PubMed] [Google Scholar]
- 26.Hou J, Meng FX, Wu YY, Wang JN, Guo S, Gong ZY. Resistance of adult Aedes albopictus to commonly used insecticides in Zhejiang province. Chin J Vector Biol Control. 2017;28(3):230–2.
- 27.Gao JP, Chen HM, Ma YJ. Establishment of diagnostic doses of three pyrethroid insecticides for detecting resistance in Aedes albopictus (Diptera: Culicidae) in China. Acta Entomol Sin. 2018;61(1):18–24. [Google Scholar]
- 28.Health and Family Planning Commission of Zhejiang Province. Epidemic information of Dengue fever in Zhejiang Province. http://www.zjwjw.gov.cn/art/2017/10/10/art_1202112_12301762.html. Accessed 29 Apr 2018.
- 29.General Office of the People’s Government of Jiaxiang County. Notification of emergency response of dengue fever outbreak in General Office of the People’s Government of Jiaxiang County. http://www.jiaxiang.gov.cn/content.jsp?id=bc5372855f484da0015f57270e0b01cc&classid=2c17a4be401f4817aecce1ab7fb2d1be. Accessed 29 Apr 2018.
- 30.Shanghai Municipal Commission of Health and Family Planning. First case of local dengue fever has been confirmed in Shanghai. http://www.wsjsw.gov.cn/wsj/n422/n424/u1ai142084.html?from=groupmessage&isappinstalled=0. Accessed 29 Apr 2018.
- 31.Manni M, Gomulski LM, Aketarawong N, Tait G, Scolari F, Somboon P, et al. Molecular markers for analyses of intraspecific genetic diversity in the asian tiger mosquito, Aedes albopictus. Parasit Vectors. 2015;8(1):188. doi: 10.1186/s13071-015-0794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO . Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: World Health Organization; 2013. [Google Scholar]
- 33.Abbott WS. A method of computing the effectiveness of an insecticide. 1925. J Am Mosq Control Assoc. 1987;3(2):302–303. [PubMed] [Google Scholar]
- 34.Burland TG. DNASTAR’s Lasergene sequence analysis software. Methods Mol Biol. 2000;132:71–91. doi: 10.1385/1-59259-192-2:71. [DOI] [PubMed] [Google Scholar]
- 35.Chang X, Zhong D, Fang Q, Hartsel J, Zhou G, Shi L, et al. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: a major obstacle to mosquito-borne diseases control and elimination in China. PLoS Negl Trop Dis. 2014;8(5):e2889. doi: 10.1371/journal.pntd.0002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kou JX, Liu HM, Gong MQ, Wang HW. Preliminary investigation of Aedes albopictus resistant to commonly used insecticides in Shangdong province. Parasit Infect Dis. 2015;13(3):115–117. [Google Scholar]
- 37.Gao JP, Li KK, Chen HY, Yang XY, Cai F, Lin Y, et al. Bioassay effects of pyrethroid insecticide commodities on Aedes albopictus populations from Haikou. Chin Trop Med. 2017;17(9):867–870. [Google Scholar]
- 38.Xiong JF, Yang R, Tan LF, Yao X. Analysis of population density and insecticide resistance of Aedes albopictus in Hubei province, China, in 2016. Chin J Vector Biol Control. 2017;28(4):383–385. [Google Scholar]
- 39.Shu F. Production and application of pesticides in China. 2015. [Google Scholar]
- 40.Wang YG, Shi CN, Lin GS, Liu QY, Liu SQ, Du GW, et al. Resistance of Aedes albopictus in Chaozhou city, China to commonly used insecticides. Chin J Vector Biol Control. 2016;27(4):228–231. [Google Scholar]
- 41.Qu BW, Zhang JM, Zhu YP, Huang MC, Huang XJ, Huang YZ. Resistance of Aedes albopictus to commonly used insecticides in Jiangmen city, Guangdong province, 2016. Chin J Vector Biol Control. 2017;28(4):386–389. [Google Scholar]
- 42.Li FS, Yang FR, Song JC, Gao H, Tang JQ, Zou CH, et al. Etiologic and serologic investigations of the 1980 epidemic of dengue fever on Hainan Island, China. Am J Trop Med Hyq. 1986;35(5):1051–1054. doi: 10.4269/ajtmh.1986.35.1051. [DOI] [PubMed] [Google Scholar]
- 43.Qiu FX, Chen QQ, Ho QY, Chen WZ, Zhao ZG, Zhao BW. The first epidemic of dengue hemorrhagic fever in the People’s Republic of China. Am J Trop Med Hyq. 1991;44(4):364–370. doi: 10.4269/ajtmh.1991.44.364. [DOI] [PubMed] [Google Scholar]
- 44.Qiu FX, Gubler DJ, Liu JC, Chen QQ. Dengue in China: a clinical review. Bull World Health Organ. 1993;71(3–4):349–359. [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu FX, Zhao ZG. A pandemic of dengue fever on the Hainan Island. Epidemiologic investigations. Chin Med J. 1988;101(7):463–467. [PubMed] [Google Scholar]
- 46.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7(2):179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 47.Li CX, Kaufman PE, Xue RD, Zhao MH, Wang G, Yan T, et al. Relationship between insecticide resistance and kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasit Vectors. 2015;8:325. doi: 10.1186/s13071-015-0933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black Iv WC. Coevolution of the IIe1,016 and Cys1,534 mutations in the voltage gated sodium channel gene of Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2015;9(12):e0004263. doi: 10.1371/journal.pntd.0004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen HM, Gao JP, Jiang JY, Peng H, Ma YJ. Detection of the I1532 and F1534 kdr mutations and a novel mutant allele I1532T in VGSC gene in the field populations of Aedes albopictus from China. Chin J Vector Biol Control. 2018;29(2):120–125. [Google Scholar]
- 50.Wang Y, Yu W, Shi H, Yang Z, Xu J, Ma Y. Historical survey of the kdr mutations in the populations of Anopheles sinensis in China in 1996-2014. Malar J. 2015;14(1):120. doi: 10.1186/s12936-015-0644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu NJ, Chen WW, Wu SL, Luo NH. Resistance to permethrin and activity of carboxylesterase in Aedes albopictus in Pingshan New District, Shenzhen City, Pract Prev Med. 2016;23(9):1123–5.
- 52.Yin JH, Zhou SS, Xia ZG, Wang RB, Qian YJ, Yang WZ, et al. Historical patterns of malaria transmission in China. Adv Parasitol. 2014;86:1–19. doi: 10.1016/B978-0-12-800869-0.00001-9. [DOI] [PubMed] [Google Scholar]
- 53.Sun DJ, Deng XL, Duan JH. The history of the elimination of lymphatic filariasis in China. Infect Dis Poverty. 2013;2(1):30. doi: 10.1186/2049-9957-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang G, Li X, Gao X, Fu S, Wang H, Li M, et al. Arboviruses and their related infections in China: A comprehensive field and laboratory investigation over the last 3 decades. Rev Med Virol. 2017;e1959. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multilingual abstracts in the five official working languages of the United Nations. (PDF 1038 kb)
Table S1. The knockdown rate and mortality of Ae. albopictus field populations exposed to four pyrethroids. Table S2. Kdr alleles and genotypes at codon 1532 of Aedes albopictus field populations. Table S3. Kdr alleles and genotypes at codon 1534 of Aedes albopictus field populations. (DOCX 31 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request, and sequences are available in GenBank.