Abstract
As a result of great diversity in life histories and a large number of described species, taxonomic and phylogenetic uncertainty permeates the entire crustacean order of Isopoda. Large molecular datasets capable of providing sufficiently high phylogenetic resolution, such as mitochondrial genomes (mitogenomes), are needed to infer their evolutionary history with confidence, but isopod mitogenomes remain remarkably poorly represented in public databases. We sequenced the complete mitogenome of Cymothoa indica, a species belonging to a family from which no mitochondrial genome was sequenced yet, Cymothoidae. The mitogenome (circular, 14484 bp, A+T = 63.8%) is highly compact, appears to be missing two tRNA genes (trnI and trnE), and exhibits a unique gene order with a large number of rearrangements. High compactness and the existence of palindromes indicate that the mechanism behind these rearrangements might be associated with linearization events in its evolutionary history, similar to those proposed for isopods from the Armadillidium genus (Oniscidea). Isopods might present an important model system to study the proposed discontinuity in the dynamics of mitochondrial genomic architecture evolution. Phylogenetic analyses (Bayesian Inference and Maximum Likelihood) conducted using nucleotide sequences of all mitochondrial genes resolved Oniscidea and Cymothoida suborders as paraphyletic. Cymothoa indica was resolved as a sister group (basal) to all remaining isopods, which challenges the accepted isopod phylogeny, where Cymothoida are the most derived, and Phreatoicidea the most basal isopod group. There is growing evidence that Cymothoida suborder might be split into two evolutionary distant clades, with parasitic species being the most basal split in the Isopoda clade, but a much larger amount of molecular resources carrying a high phylogenetic resolution will be needed to infer the remarkably complex evolutionary history of this group of animals with confidence.
Introduction
Isopoda is an exceptionally speciose (>10,000) order of crustaceans that mostly but not exclusively inhabit aquatic habitats [1,2]. As a result of great diversity in life histories and a large number of described species, taxonomic and phylogenetic uncertainty permeate the entire order [1,3–7]. As studies relying on morphology and small molecular datasets (such as single gene datasets) did not manage to resolve their phylogeny, this is an indication that phylogenetic resolution provided by the commonly used markers (such as cox1 or 18S) is too low to unequivocally resolve the evolutionary history of Isopoda. Therefore, availability of molecular resources carrying a high phylogenetic resolution is indispensable for identification and evolutionary history studies of Isopoda.
Mitochondrial genomes (mitogenomes) carry a large amount of data, which makes them capable of providing much higher resolution than traditionally used morphological and (single-gene) molecular markers, so mitochondrial phylogenomics is increasingly used to address phylogenetic and taxonomic controversies [8–10]. Furthermore, isopod mitogenomes generally exhibit a large number of gene order rearrangements [4,11], and some groups of isopods even possess a non-standard, linearised, mitogenome organisation and unique tRNA-encoding mechanisms [12–16]. As the evolution of mitogenomic architecture appears to be highly discontinuous [17,18], with some major animal taxa exhibiting a highly conserved mitochondrial architecture (most vertebrates being a good example), and other taxa exhibiting a rapidly-evolving architecture [17–21], we hypothesise that isopods might present an important model system to study the complex dynamics of the evolution of mitochondrial genomic architecture. However, isopod mitogenomes remain remarkably poorly represented in the GenBank, with only five complete and 19 partial mitogenomes currently (Apr, 2018) available for the entire order (20 species in total), which presents a major obstacle to their application.
Among the non-represented taxa (taxa from which no mitochondrial genome was sequenced yet) is the entire large (≈366 species and ≈42 genera [22]) family Cymothoidae Leach 1818 (suborder Cymothoida, superfamily Cymothooidea). Species belonging to this family are largely obligate parasites of fishes that feed on host tissues and fluids at least at some stage of their life [1,22,23]. Cymothoid isopods are mostly protandrous hermaphrodites that have a biphasic life cycle: after the free-swimming micropredatory stage, they attach permanently to fishes (or other crustaceans), upon which they change sex and morphology [24]. They exhibit a range of parasitic feeding strategies: on the external body surfaces, in the buccal and opercular cavities, or burrowing into the muscle of their fish hosts [1,22]. The buccal mode sometimes results in the intriguing phenomenon of parasitic castration [25].
Identification and taxonomy of cymothoid isopods are complicated by a number of factors, including morphological similarity, sequential hermaphroditism, sexual dimorphism (females up to three times larger than males), flexible host preference almost completely unrelated to phylogeny, habitat flexibility (sea, brackish and fresh water), global distribution of many species, etc. Along with limited molecular resources currently available, this causes frequent incorrect identifications and misuse of species names, so synonymies and paraphyly are widespread, which is reflected in widely varying estimates of the number of valid species and genera within the family Cymothoidae [1,3,22–24,26–28]. Additionally, although the monophyly of Cymothoida is believed to be supported by morphological data and rejected by molecular data [7], a relatively recent morphological study also failed to find evidence for the monophyly of Cymothoida [29], so it is increasingly likely that the suborder is indeed paraphyletic [5,11,29]. This phylogenetic and taxonomic uncertainty permeates the deep-level phylogeny of Isopoda as well: there are some indications that the position of Cymothoida, which was traditionally regarded as the most derived isopod taxon [4,7,26,30], may be relatively basal within the Isopoda [6,7,27] (throughout the manuscript we use these two terms, basal and derived, to refer to common ancestors, not extant species). The few attempts to apply mitochondrial phylogenomics to study the evolutionary history of Isopoda resolved this suborder relatively ‘centrally’ within the Isopod clade, clustering with Sphaeromatidea and Valvifera [4–6,11]. As only two cymothoid mitogenomes are currently available, both belonging to free-living Cirolanidae species, interpretation and reliability of these findings are severely hampered by the low number of mitogenomes available.
To address this problem, we sequenced and characterised the first complete mitochondrial genome of a parasitic cymothoid isopod, Cymothoa indica Schiödte & Meinert 1884 (Cymothoidae), and conducted comparative mitogenomic and phylogenomic analyses. Our results indicate that the availability of this mitogenomic sequence has the potential to advance our understanding of the evolution of mitogenomic architecture and phylogeny of Isopoda, but the amount of available data remains too limited to draw conclusions with confidence.
Results and discussion
Genome architecture and characteristics
The complete mitochondrial genome of C. indica is a circular, 14,484 bp-long molecule, somewhat smaller than most of the remaining complete isopod mitogenomes (Worksheet A in S1 File). Although there is evidence for a linear mitogenomic organisation in Armadillidium vulgare [12], an isopod species belonging to a different suborder (Oniscidea), we did not find any indications of such organisation in C. indica (i.e., all fragments overlapped during the assembly). The mitogenome possesses the standard 13 protein-coding genes and two rRNA genes (12S and 16S), but only 20 tRNA genes, as trnI and trnE could not be detected (Table 1). A 390 bp-long putative control region (CR) was found between trnS and nad1 genes. The A+T content of the complete mitogenome (63.8%) is average for isopods (54 to 72%; Worksheet A in S1 File). It should be emphasised here again that only five complete mitogenomes were available for the entire order Isopoda when we conducted these analyses, so all comparative analyses in this study were hampered by the fact that the remaining 18 mitogenomes were partial, and should be interpreted with that limitation in mind.
Table 1. Organisation of the mitochondrial genome of Cymothoa indica.
| Gene | From | To | Length | IGR | Start | Stop | Anticodon | Strand |
|---|---|---|---|---|---|---|---|---|
| trnQ | 1 | 62 | 62 | TTG | - | |||
| trnM | 67 | 129 | 63 | 4 | CAT | + | ||
| nad2 | 130 | 1131 | 1002 | ATT | TAG | + | ||
| trnC | 1129 | 1182 | 54 | -3 | GCA | - | ||
| trnY | 1186 | 1246 | 61 | 3 | GTA | - | ||
| cox1 | 1245 | 2786 | 1542 | -2 | ATG | TAA | + | |
| cox2 | 2830 | 3508 | 679 | 43 | ATA | T | + | |
| trnK | 3509 | 3569 | 61 | TTT | + | |||
| trnD | 3567 | 3619 | 53 | -3 | GTC | + | ||
| atp8 | 3626 | 3781 | 156 | 6 | ATA | TAA | + | |
| atp6 | 3775 | 4450 | 676 | -7 | ATG | T–– | + | |
| cox3 | 4451 | 5236 | 786 | ATG | TAA | + | ||
| trnR | 5243 | 5295 | 53 | 6 | TCG | + | ||
| trnG | 5296 | 5353 | 58 | TCC | + | |||
| nad3 | 5351 | 5701 | 351 | -3 | ATC | TAA | + | |
| trnA | 5702 | 5756 | 55 | TGC | + | |||
| trnV | 5758 | 5819 | 62 | 1 | TAC | + | ||
| trnN | 5816 | 5879 | 64 | -4 | GTT | + | ||
| rrnS | 5880 | 6604 | 725 | + | ||||
| CR | 6605 | 6994 | 390 | |||||
| nad1 | 6995 | 7927 | 933 | ATT | TAA | - | ||
| trnL1 | 7937 | 7996 | 60 | 9 | TAG | - | ||
| trnL2 | 8043 | 8102 | 60 | 46 | TAA | - | ||
| trnS | 8100 | 8158 | 59 | -3 | TCT | - | ||
| trnW | 8155 | 8215 | 61 | -4 | TCA | - | ||
| cytb | 8220 | 9344 | 1125 | 4 | TTG | TAG | - | |
| trnT | 9345 | 9398 | 54 | TGT | - | |||
| nad5 | 9398 | 11095 | 1698 | -1 | ATA | TAA | + | |
| trnF | 11097 | 11154 | 58 | 1 | GAA | + | ||
| trnH | 11147 | 11206 | 60 | -8 | GTG | - | ||
| nad4 | 11181 | 12515 | 1335 | -26 | ATG | TAA | - | |
| nad4L | 12514 | 12781 | 268 | -2 | TGG | T | - | |
| trnP | 12797 | 12860 | 64 | 15 | TGG | - | ||
| nad6 | 12863 | 13342 | 480 | 2 | ATA | TAA | + | |
| trnS | 13342 | 13401 | 60 | -1 | TGA | + | ||
| rrnL | 13402 | 14475 | 1074 | - |
CR is control region. IGR is intergenic region, where a negative value indicates an overlap.
Gene order
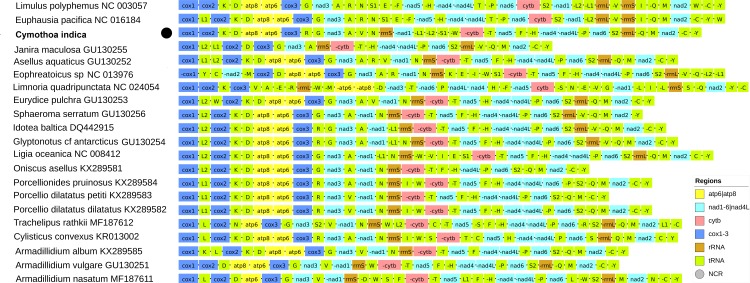
The ancestral arthropod architecture, almost identical to the one exhibited by Limulus polyphemus (Fig 1), has remained almost unchanged for over 400 million years in many crustacean lineages [31], but not among the isopods [32,33]. Gene order rearrangements in this group of animals were discussed in detail in previous studies [4,11,32], so here we only briefly discuss the idiosyncrasies of the new mitogenome. Cymothoa indica also exhibits a completely unique order with a large number of rearrangements (Fig 1). The only available isopod mitogenome exhibiting somewhat similar architecture is that of the only other available Cymothoida (family Cirolanidae) species, Bathynomus sp. [11]. In terms of PCG and rRNA arrangement, the two mitogenomes are almost identical, with the exception of the position of nad1: in C. indica it is on the minus strand, which corresponds to the putative pancrustacean ground pattern [4,34], but in Bathynomus sp. it is on the plus strand (Worksheet B in S1 File). However, the two mitogenomes also differ in the arrangement of a number of tRNAs (L2, A, V, E, W, H, L1, S1), and the location of the putative control region (CR). In terms of uniquely derived gene positions of single species in relation to the putative pancrustacean ancestral gene order discussed by Shen et al. [11], C. indica shares the unique position of trnS-nad1 with Bathynomus sp. In comparison to the putative ancestral isopod architecture [4], C. indica differs in the arrangement of nad1 and 12S rRNA genes, which appear to have switched places, and a number of tRNAs: L2, R, V, S1, W, E and I (the last two are missing). Intriguingly, it shares large, completely conserved (in terms of gene order) segments of the mitogenome of 16/11 genes (cytb through trnY/rrnL) with Asellus aquaticus/Eophreatoicus sp. respectively, which may bear relevance for resolving the conflicting topologies produced by different datasets for Isopoda (see discussion in the ‘Phylogeny’ section for further details).
Fig 1. Gene order in isopod mitogenomes.
The newly sequenced species, Cymothoa indica, is bolded and marked with a black dot. GenBank accession numbers are shown next to the species names, and gene legend is incorporated in the figure. Two outgroups, Limulus polyphemus and Euphausia pacifica, are also shown.
Mitogenomic gene order rearrangements are usually attributed to the TDRL (tandem duplication and subsequent evolutionary loss of duplicated genes) mechanism [19,35], but high compactness of isopod mitogenomes indicates that this is not the most parsimonious explanation in this group of animals, as TDRL events result in pseudogenes which are then usually [17,36] ‘erased’ from the mitogenome in the course of evolution [37]. In terrestrial isopods (Oniscidea: Armadillidium), a different mechanism was proposed: the atypical organisation of their mitogenomes, which are composed of linear monomers and circular dimers, might facilitate architecture conversions via creation of telomeric hairpins [14,15]. Doublet et al. [14] have characterised the (highly conserved) CR structure in isopod species that undergo (occasional) mitogenome linearization, but the putative CR of C. indica does not appear to possess any of those features. However, regardless of the different organisation, its CR sequence does contain a large number of palindromes (24 ≥ 6bp), which are required for the formation of hairpins. Therefore, we hypothesise that occasional linearization events in their evolutionary history might be the most parsimonious explanation for the large number of gene rearrangements observed in this species and Isopoda in general.
tRNAs
Whereas the majority of sequenced metazoan mitogenomes contain the full set of 22 tRNAs, the number of identified tRNA genes often varies in arthropod mitogenomes [16]. Most sequenced isopod mitogenomes appear to possess an incomplete set of tRNA genes [5,32]. As trnI and trnE appear to be missing from nine and 16 (respectively) of the available mitogenomes, C. indica is not an outlier in this aspect. Another tRNA gene commonly missing from isopod mitogenomes is trnW (13 species), but we annotated this gene upstream of cytb, as observed in some other isopods (Fig 1), and successfully folded it into a relatively standard cloverleaf structure. The loss of mitochondrial tRNA genes is usually compensated by the import of nuclear tRNAs [16,38], but some unique features described in oniscidean isopod mitogenomes raise suspicion that these missing tRNAs might be actually encoded in their mitogenomes: heteroplasmic mitochondrial DNA, which may allow for the presence of two tRNA genes with different anticodons at the same locus [15,39,40], and tRNA genes partially or fully overlapping with protein coding genes, that have been reported in the oniscidean Armadillidium genus [16]. Although we did find an unusually large overlap (26 bp between nad4 and trnH), we did not find any evidence for the existence of fully overlapping genes, nor for heteroplasmy (by examining electropherograms), so these features appear to remain limited to oniscidean isopods. A 46 bp-long non-coding fragment between trnL1 and L2 genes bears high similarity to trnE orthologues in other isopods (including the conserved anticodon), but it is missing the 3’ end. We attempted to create a 19-bp overlap with the downstream trnL2 gene, but the ARWEN tool [41] still did not manage to fold the gene into a cloverleaf structure. Therefore, although the conserved 5’ end and anticodon make us suspect that this tRNA gene might be functional after undergoing post-translational editing [42], as we have no evidence for this, it remains merely a speculation.
NCRs and overlaps
Mitogenomes of isopods are believed to be very compact, with many overlaps between genes and short non-coding sequences [32]. In agreement with this, we found 12 intergenic regions ranging from 1 to 46 bp in size (excluding the putative control region). The largest was located between cox1 and cox2 genes, followed by 15 bp between nad4L and trnP genes, whereas the remaining nine were smaller than 10 bp (Table 1). Further evidence for this high compactness is a relatively large number (13) of gene overlaps observed, ranging from 1 bp to 26 bp. Eleven of these overlaps, including the largest, between nad4 and trnH, involved at least one tRNA gene. This is expected and believed to be a consequence of lesser evolutionary constraints on tRNA genes [16]. The only overlaps involving two PCGs were those between atp8/atp6 (7 bp) and nad4/nad4L (2 bp). However, there are several indications that atp8 and nad4L are generally under relaxed evolutionary constraints: both are exceptionally small (in C. indica: atp8 = 156bp, and nad4l = 268bp); nad4 and nad4L often overlap in mitogenomes of many different groups of animals including isopods [32]; atp8 is often even completely absent from mitogenomes [19]; and atp6/atp8 overlaps of 4–12 bp were also reported in other isopods [5,11,32]. Therefore, we do not suspect an annotation artefact in either of the two overlaps. In conclusion, this mitogenome can also be characterised as highly compact, which bears relevance for inferring the evolutionary history of its architectural rearrangements.
Gene features
Most PCGs of C. indica exhibit sizes and start/stop codons standard for isopods (Worksheet C in S1 File). An exception is cytb, which (putatively) uses a non-standard TTG start codon. Alternatively, it might use a standard ATT start codon, but that would create a 12-bp overlap with the neighbouring trnT gene. The atp6 gene exhibited a unique 6 bp-long insertion from positions 56 to 61, but this fragment of the sequence is generally poorly conserved so we don’t deem this as suspicious. Whereas other isopod atp6 genes end with a TAA stop codon, in the studied mitogenome this codon appears to have mutated into TAT (presuming this is not a sequencing artefact). This is not likely to affect its transcription, as it can still use T—as the stop codon [43]. In cox1, a frameshift mutation (or insertion) near the end of the gene appears to have caused a minor extension of the gene: 1542 bp. vs. 1531–1539 bp. in orthologs (Limnoria quadripunctata is an outlier with 1596 bp, but as all other genes are highly conserved in size, we suspect an annotation artefact here; Worksheet C in S1 File). Alternatively, it might use a non-standard stop codon, or span only 1524 bp. The 5’ end of nad5 gene is very divergent, so the start codon is questionable: we selected the standard ATA, creating a 1 bp overlap with the neighbouring tRNA, but if it uses one of alternative start codons, it might start 9, 15, 21, or 24 bp downstream with an ATC.
Several genes exhibit unusually broad size variability in isopods (Worksheet C in S1 File), but in most cases we suspect annotation artefacts, especially as most mitogenomes are incomplete. For example, cytb gene exhibits a huge variation in size in the available isopods, from 303 to 1206 bp, but outliers are mostly incomplete, unpublished or unverified mitogenomes, or from a study wherein a large number of mitogenomes were sequenced with the aim to focus on gene rearrangements [4]. An intriguing outlier is Bathynomus sp. [11], which exhibited a very divergent 3’ end of the sequence, much longer that the rest of the orthologs. As the authors did not discuss this issue, we also suspect an annotation or a sequencing artefact here. Finally, nad1 gene also exhibits a suspiciously wide size range (876 to 972 bp). Here again, Bathynomus sp. is among the outliers, but in this case its large size (969 bp) is very likely to be an artefact, as it can easily be shortened by 15 bp (to 954 bp) to use the same start codon as most other orthologs, ATA (S2 File). Other three outliers, Oniscus asellus (876 bp), Porcellionides pruinosus (882 bp), and Armadillidium album (972 bp) are unverified and come from the same unpublished study, so their unusual sizes would have to be independently confirmed.
Phylogeny
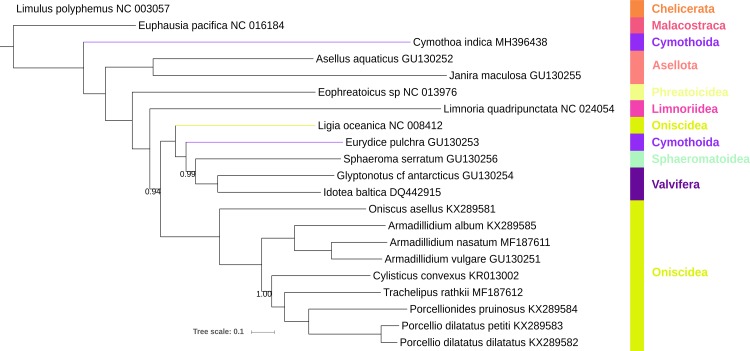
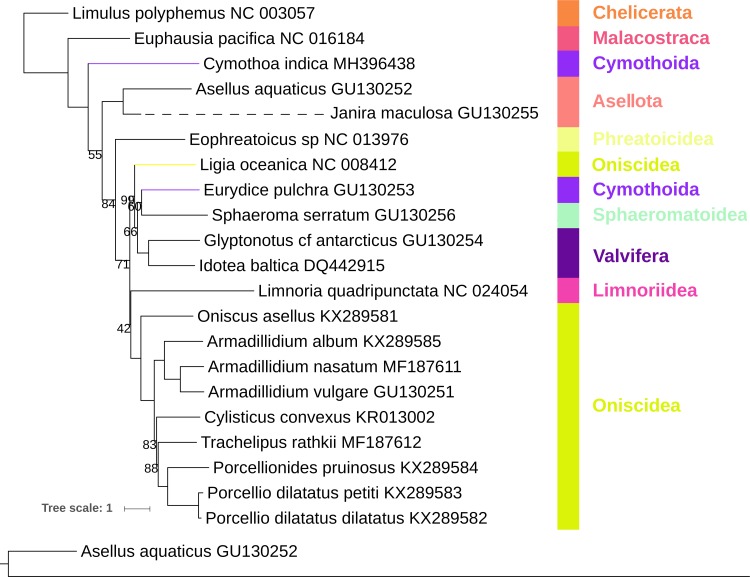
The two phylogenetic analyses, Bayesian Inference (BI) and Maximum Likelihood (ML), produced topologies (Figs 2 and 3) differing in three details: the position of Limnoria quadripunctata (sister-clade to Oniscidea in ML, a relatively basal position in BI), the relationship of Eurydice pulchra and Sphaeroma serratum (sister-clades in ML), and the length of the Janira maculosa branch (extremely long in ML). In other aspects, the results of the two analyses were congruent, with the BI topology exhibiting very high statistical support, and the ML topology a mix of mostly high and several lower values. The suborder Oniscidea was resolved as paraphyletic by Ligia oceanica [32] exhibiting a sister-clade relationship with a cluster of other taxa (Eurydice pulchra, Sphaeromatoidea, and Valvifera; see Worksheet A in S1 File for detailed taxonomy). Paraphyly of Oniscidea caused by this genus is a relatively well-established fact [6], and all recent mito-phylogenomic analyses resolved this species approximately in the same position [5,6,11]. Although it is tempting to interpret this as a sign that its position is resolved, we advise that additional mitogenomes belonging to this genus should be sequenced, to exclude the possibility of misidentification or unusual evolutionary rates, before any taxonomic changes are officially proposed. The Limnoria genus was described as ‘rogue’ a long time ago [7], and its position also remains unresolved to this day, including by our study. In recent mitochondrial phylogenomic studies it was mostly resolved as a sister-clade to Oniscidea (which corresponds to our ML topology) using different datasets (nucleotides and amino acids) and algorithms (BI and ML) [5,11]. However, Lins et al. [6] resolved it as a sister-clade to all other isopoda (BI, amino acid dataset), and a combined mitonuclear dataset (18S, 28S, and cox1) in the same study resolved it as a sister-clade to a derived Asellota clade (Asellota was paraphyletic in that analysis). Therefore, although most mitogenomic analyses resolve it as a sister-clade to Oniscidea, extreme variations in its position indicate that the evolution of this species is very peculiar, and that more molecular data of closely related species should be sequenced to identify the reasons underlying this instability. As the J. maculosa mitogenome is incomplete (<10,000 bp), we suspected that the long branch produced by the ML analysis may have been an artefact. To test this hypothesis, we removed the four genes (atp6 and 8, nad1 and 5) missing from J. maculosa from the entire dataset and re-conducted the ML analysis. This analysis produced a congruent topology (S1 Fig.) and resolved the issue, with the length of the branch comparable to the one produced by the BI analysis.
Fig 2. Mitochondrial phylogenomics of Isopoda: Bayesian inference analysis.
The analysis was conducted using nucleotide sequences of all genes. Limulus polyphemus (branch cropped) and Euphausia pacifica are outgroups. Scale bar corresponds to the estimated number of substitutions per site. Bayesian posterior probability values (lower than 1.0) are shown next to corresponding nodes. GenBank accession numbers are shown next to species names. Taxonomic rank (suborder/superfamily) is shown to the right. Coloured branches highlight paraphyly.
Fig 3. Mitochondrial phylogenomics of Isopoda: Maximum likelihood analysis.
The analysis was conducted using nucleotide sequences of all genes. Limulus polyphemus and Euphausia pacifica are outgroups. Scale bar corresponds to the estimated number of substitutions per site. Bootstrap support values (lower than 100) are shown next to corresponding nodes. GenBank accession numbers are shown next to species names. Taxonomic rank (suborder/superfamily) is shown to the right. Coloured branches highlight paraphyly. Janira maculosa branch (dashed line) is shortened, with its original size shown below the phylogram.
Most importantly, the newly-sequenced C. indica species was resolved as a sister group (basal) to all remaining isopods, which challenges the accepted isopod phylogeny, where Phreatoicidea (represented by Eophreatoicus sp.) is usually regarded as the most basal split within the Isopoda [4,7,30], and Cymothoida as the most derived group [4,7,26,30]. The most basal position of Phreatoicidea was also challenged by the position of Asellota clade (A. aquaticus and J. maculosa), which was derived in relation to Cymothoidae, but basal to Phreatoicidea and all other Isopoda. This topology (Asellota basal to Phreatoicidea) was produced by all other recent mitochondrial phylogenomic analyses as well [5,6,11] (with the exception of an amino acid dataset in [11]). Although all of these topologies support the close relationship of these two clades, and their relatively basal position within the Isopoda clade, none of them show support for the proposed monophyly of a combined asellotan and phreatoicidean clade [4,44]. The suborder Cymothoida was paraphyletic: the other cymothoid species included in the analysis, Eurydice pulchra (Cymothoida: Cirolanidae), clustered with Sphaeromatoidea and Valvifera (low support in ML). This position of E. pulchra is congruent with the topologies produced by most other mito-phylogenomic studies [4–6,11]. A topology highly congruent to ours, including the deep evolutionary split between free-living (Cymothoidae) and parasitic (Cirolanidae) cymothoid species, was produced before using a combined 18S-morphology dataset [7]. Similar topologies were also produced by two Bayesian analyses of combined mitonuclear gene datasets [6,27], but this was not further discussed by the authors. Intriguingly, that same mitonuclear dataset resolved E. pulchra within the basal cymothoid clade [6]. The basal position of C. indica within the isopod clade is further indirectly supported by the similarity in gene order with A. aquaticus and Eophreatoicus sp. (J. maculosa is incomplete, so it is difficult to assess the level of similarity). Therefore, although the issue cannot be declared resolved yet, it appears that there is increasing evidence from different types of data (gene order, mitochondrial phylogenomics, mitochondrial and nuclear single-marker, and morphological data) for deep paraphyly of the suborder Cymothoida, and for the parasitic Cymothoidae being sister group to all other Isopoda.
Conclusions
As the absence of a sufficient number of sequenced mitogenomes is currently the foremost limiting factor to their broader application, we sequenced the first mitogenome of a species belonging to the large family Cymothoidae, Cymothoa indica. The results of our phylogenetic analyses, which resolved C. indica as the most basal split in the isopod clade, present a major challenge to the accepted deep phylogeny of Isopoda. There is growing evidence that Cymothoida might be split into two evolutionary distant clades, with parasitic species being the most basal split in the isopod clade. However, the small number of isopod mitogenomes currently available (as well as the minor topological instability observed) prevents us from making any conclusions with confidence. Therefore, a much larger amount of molecular resources carrying a high phylogenetic resolution will be needed to infer the remarkably complex evolutionary history of this group of animals with confidence. Aside from the importance of mitochondrial genomes for taxonomy and phylogenetics, the highly rearranged and unique gene order found in Cymothoa indica is a further indication that isopods might present an important model system to study the discontinuous dynamics of the evolution of mitochondrial genomic architecture. Therefore, sequencing of further isopod mitogenomes is strongly urged.
Materials and methods
Samples, identification, and DNA extraction
Two adult specimens were collected on 10/07/2017 in Dayawan Town, Guangdong Province, China (22°42’58”– 22°42’56” N; 114°32’16”– 114°32’25” E) from the mouth of a euryhaline fish species, Mugil cephalus Linnaeus 1758. Live parasites were kept alive in 0.6% saline as long as possible (one day) to ensure that they were starved, and then stored in 75% ethanol at 4°C. Specimens were morphologically identified under a dissecting microscope as described before [1,3,45,46]. After washing in distilled water, DNA was isolated from one specimen using Aidlab DNA extraction kit (Aidlab Biotechnologies, Beijing, China). This study has been reviewed and approved by the ethics committee of the Institute of Hydrobiology, Chinese Academy of Sciences. As the study involved an unregulated parasitic invertebrate, and as we obtained the samples from already dead fish bought on a local fish market, no permits were required to retrieve and process the samples.
Genome sequencing and assembly
Ten primer pairs used to amplify and sequence the entire mitogenome were designed to match conserved regions of mitochondrial genes and to overlap by approximately 100 bp (Table 2). Amplification reaction mixture and conditions used were described before [17]; briefly: 50μL with 5 U/μL of TaKaRa LA Taq polymerase (TaKaRa, Japan), 10×LATaq Buffer II, 2.5μM dNTP mixture, 0.2–1.0μM each primer, 60ng DNA template. Conditions: denaturation 98°C/2min, and 40 cycles of 98°C/10s, 50°C/15s, 68°C/1min per kb. When the product was not specific enough, PCR conditions were optimized by increasing the annealing temperature and decreasing the number of cycles. PCR products were sequenced using Sanger method and the same set of primers. All obtained fragments were quality-proofed by visually inspecting the electropherograms and BLASTed [47] to confirm that the amplicon is the actual target sequence. Mitogenome was assembled, annotated, and comparative analyses conducted, roughly as described previously [17,48]. Briefly: assembly was conducted manually using DNAstar v7.1 [49]. In each step we checked whether overlaps were identical, thereby making sure that the mitogenome is circular, and avoiding incorporation of numts [50] into the sequence. The same software was also used to locate the putative ORFs for protein-coding genes. BLAST and BLASTx were used to compare the inferred ORFs with nucleotide and amino acid sequences of available orthologs, and manually determine the exact initiation and termination codon positions accordingly. tRNAs were annotated using tRNAscan [51] and ARWEN [41] tools, and the results checked manually. The annotation was recorded in a Word (Microsoft Office) document, and then parsed and extracted using an in-house MitoTool software [52]. The same software was also used for file conversions (including the creation of the GenBank file) and to generate tables with comparative mitogenomic statistics (including the file used to visualise gene orders in iTOL). Palindromes in the CR were predicted using Palindrome analyser [53]. The mitogenome is available from the GenBank repository under the accession number MH396438.
Table 2. Primers used for amplification and sequencing of the mitochondrial genome of Cymothoa indica.
| Gene/region | Name | Sequence (5’-3’) | Length |
|---|---|---|---|
| COX1 | LYF1 | GCTGGGATATTAGGTCTTAG | 1436 |
| LYR1 | GAGTGTTCGGAGGGAGGGAA | ||
| COX1-COX2 | LYF2 | GACGTTATTCAGATTACCCTG | 663 |
| LYR2 | GGATAACAAGTTTGTTATCTG | ||
| COX2 | LYF3 | CTGATGAAACTTTTTCATCAC | 356 |
| LYR3 | GAAACTATGATTTGCACCAC | ||
| COX2-COX3 | LYF4 | GGACAATCCCATCACTTGGG | 1462 |
| LYR4 | TTAGGAGACAATCTTCTATG | ||
| COX3 | LYF5 | GATGTCTCACGAGAAGCAAG | 442 |
| LYR5 | GAAAGCCATGAAAACCAGTAG | ||
| COX3-16S | LYF6 | CAATTATTCTTGGGATTAC | 2402 |
| LYR6 | GACCCTAAGAATTTGAAGATC | ||
| 16S | LYF7 | TACGCTGTTATCCCTAGAG | 828 |
| LYR7 | CGTACCTTTAGCATTAGGG | ||
| 16S-CYTB | LYF8 | GAAAAGAATTTCACATCTAAAG | 5995 |
| LYR8 | CCAAAAGGGTTTCTTGATCC | ||
| CYTB | LYF9 | GCAATCCCATATATCGGTTC | 370 |
| LYR9 | GAAAGTACCATTCAGGTTG | ||
| CYTB- COX1 | LYF10 | CGATCATTTACCCTTATAGAC | 2247 |
| LYR10 | CGCCAATTATGATAGGTATAAC |
Phylogenetic analyses
Three complete and 15 partial isopod sequences were retrieved from the GenBank for the phylogenetic analyses. A basal arthropod, Limulus polyphemus [31], and a Malacostraca species basal to Isopoda [32], Euphausia pacifica [54], were used as outgroups. To maximize the amount of phylogenetic signal, we conducted the analyses on a dataset containing all genes (PCGs and RNAs). Genes were extracted from GenBank files using MitoTool. Nucleotide sequences of protein-coding genes were aligned in batches (using codon-alignment mode) with MAFFT [55] integrated into another in-house software package—BioSuite [56]. As described before [57], RNAs were aligned by an algorithm that takes secondary structure information into account, Q-INS-i, incorporated into MAFFT-with-extensions software [58]. BioSuite was then used to concatenate the alignments, and another plug-in program in BioSuite, Gblocks [59,60], was used to remove ambiguously aligned regions [59,60]. As a result, although a majority of mitogenomes used for the analysis were incomplete, the final alignment (S3 File) had a relatively low proportion of gaps and undetermined characters (7.68%) and a high number of distinct alignment patterns (9,641). Best partitioning scheme and evolutionary models for partitions (GTR+I+G, GTR+G; S4 File) were selected using PartitionFinder [61], also implemented in BioSuite. Phylogenetic analyses were conducted using Bayesian Inference method implemented in MrBayes 3.2.6 [62] (default settings, two parallel runs, five million MCMC generations) and Maximum Likelihood method implemented in RAxML 8.1.21 [63] (1000 rapid bootstrap replicates). Phylograms and gene orders were visualized in iTOL [64]. WoRMS database [65] was used as the authority for the taxonomic nomenclature.
Supporting information
Worksheet A: taxonomy and basic statistics for all available isopod mitogenomes. Worksheet B: ancestral gene orders. Worksheet C: gene statistics for all available isopod mitogenomes (+ outgroups): gene sizes, start and terminal codons. Species are represented by acronyms of their binomial scientific names (see footnote). The new sequence (C. indica) and outgroups are shaded grey.
(XLSX)
(FAS)
(FAS)
(TXT)
Four genes missing from Janira maculosa were removed from the entire dataset: atp6, atp8, nad1 and nad5. See caption for Fig 3 for other details.
(TIF)
Acknowledgments
The authors (KAA and SM) would like to express their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-1435-012. We would also like thank the anonymous reviewer for investing time and expertise into reviewing our manuscript.
Data Availability
The newly sequenced mitochondrial genome is available from the GenBank database (accession number MH396438). All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Earmarked Fund for China Agriculture Research System (CARS-45-15); the National Natural Science Foundation of China (grant number 31572658); the Major Scientific and Technological Innovation Project of Hubei Province (grant number 2015ABA045); and the Deanship of Scientific Research at King Saud University (Research Group Project No. 1435-012). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Poore GCB, Bruce NL. Global Diversity of Marine Isopods (Except Asellota and Crustacean Symbionts) MacKenzie BR, editor. PLoS One. Public Library of Science; 2012;7: e43529 10.1371/journal.pone.0043529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson GDF. Global diversity of Isopod crustaceans (Crustacea; Isopoda) in freshwater. Hydrobiologia. 2008. pp. 231–240. 10.1007/s10750-007-9019-z [DOI] [Google Scholar]
- 3.Martin MB, Bruce NL, Nowak BF. Review of the fish-parasitic genus Cymothoa Fabricius, 1793 (Crustacea: Isopoda: Cymothoidae) from Australia. Zootaxa. 2016;4119: 1–72. doi: 10.11646/zootaxa.4119.1.1 [DOI] [PubMed] [Google Scholar]
- 4.Kilpert F, Held C, Podsiadlowski L. Multiple rearrangements in mitochondrial genomes of Isopoda and phylogenetic implications. Mol Phylogenet Evol. Elsevier Inc.; 2012;64: 106–117. 10.1016/j.ympev.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 5.Yu J, An J, Li Y, Boyko CB. The first complete mitochondrial genome of a parasitic isopod supports Epicaridea Latreille, 1825 as a suborder and reveals the less conservative genome of isopods. Syst Parasitol. Springer Netherlands; 2018; 10.1007/s11230-018-9792-2 [DOI] [PubMed] [Google Scholar]
- 6.Lins LSF, Ho SYW, Lo N. An evolutionary timescale for terrestrial isopods and a lack of molecular support for the monophyly of Oniscidea (Crustacea: Isopoda). Org Divers Evol. Organisms Diversity & Evolution; 2017;17: 813–820. 10.1007/s13127-017-0346-2 [DOI] [Google Scholar]
- 7.Wilson GDF. The phylogenetic position of the Isopoda in the Peracarida (Crustacea: Malacostraca). Arthropod Syst Phylogeny. 2009;67: 159–198. [Google Scholar]
- 8.Cameron SL. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu Rev Entomol. 2014;59: 95–117. 10.1146/annurev-ento-011613-162007 [DOI] [PubMed] [Google Scholar]
- 9.Lan T, Gill S, Bellemain E, Bischof R, Nawaz MA, Lindqvist C. Evolutionary history of enigmatic bears in the Tibetan Plateau-Himalaya region and the identity of the yeti. Proc R Soc B Biol Sci. The Royal Society; 2017;284: 20171804 10.1098/rspb.2017.1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Der Sarkissian C, Vilstrup JT, Schubert M, Seguin-Orlando A, Eme D, Weinstock J, et al. Mitochondrial genomes reveal the extinct Hippidion as an outgroup to all living equids. Biol Lett. 2015;11: 20141058 10.1098/rsbl.2014.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Y, Kou Q, Zhong Z, Li X, He L, He S, et al. The first complete mitogenome of the South China deep-sea giant isopod Bathynomus sp. (Crustacea: Isopoda: Cirolanidae) allows insights into the early mitogenomic evolution of isopods. Ecol Evol. 2017;7: 1869–1881. 10.1002/ece3.2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raimond R, Marcadé I, Bouchon D, Rigaud T, Bossy JP, Souty-Grosset C. Organization of the large mitochondrial genome in the isopod Armadillidium vulgare. Genetics. 1999;151: 203–210. Available: http://www.genetics.org/content/151/1/203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peccoud J, Chebbi MA, Cormier A, Moumen B, Gilbert C, Marcadé I, et al. Untangling heteroplasmy, structure, and evolution of an atypical mitochondrial genome by pacbio sequencing. Genetics. 2017;207: 269–280. 10.1534/genetics.117.203380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doublet V, Helleu Q, Raimond R, Souty-Grosset C, Marcadé I. Inverted repeats and genome architecture conversions of terrestrial isopods mitochondrial DNA. J Mol Evol. 2013;77: 107–118. 10.1007/s00239-013-9587-7 [DOI] [PubMed] [Google Scholar]
- 15.Doublet V, Raimond R, Grandjean F, Lafitte A, Souty-Grosset C, Marcadé I, et al. Widespread atypical mitochondrial DNA structure in isopods (Crustacea, Peracarida) related to a constitutive heteroplasmy in terrestrial species. Genome. 2012;55: 234–244. 10.1139/g2012-008 [DOI] [PubMed] [Google Scholar]
- 16.Doublet V, Ubrig E, Alioua A, Bouchon D, Marcade I, Marechal-Drouard L. Large gene overlaps and tRNA processing in the compact mitochondrial genome of the crustacean Armadillidium vulgare. RNA Biol. 2015;12: 1159–1168. 10.1080/15476286.2015.1090078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou H, Jakovlić I, Chen R, Zhang D, Zhang J, Li W-X, et al. The complete mitochondrial genome of parasitic nematode Camallanus cotti: extreme discontinuity in the rate of mitogenomic architecture evolution within the Chromadorea class. BMC Genomics. 2017;18: 840 10.1186/s12864-017-4237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DR, Keeling PJ. Mitochondrial and plastid genome architecture: Reoccurring themes, but significant differences at the extremes. Proc Natl Acad Sci. 2015;112: 10177–10184. 10.1073/pnas.1422049112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gissi C, Iannelli F, Pesole G. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity (Edinb). 2008;101: 301–320. 10.1038/hdy.2008.62 [DOI] [PubMed] [Google Scholar]
- 20.Smith DR. The past, present and future of mitochondrial genomics: Have we sequenced enough mtDNAs? Brief Funct Genomics. 2016;15: 47–54. 10.1093/bfgp/elv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballard JWO, Whitlock MC. The incomplete natural history of mitochondria. Mol Ecol. 2004;13: 729–744. 10.1046/j.1365-294X.2003.02063.x [DOI] [PubMed] [Google Scholar]
- 22.Hata H, Sogabe A, Tada S, Nishimoto R, Nakano R, Kohya N, et al. Molecular phylogeny of obligate fish parasites of the family Cymothoidae (Isopoda, Crustacea): evolution of the attachment mode to host fish and the habitat shift from saline water to freshwater. Mar Biol. Springer Berlin Heidelberg; 2017;164 10.1007/s00227-017-3138-5 [DOI] [Google Scholar]
- 23.Smit NJ, Bruce NL, Hadfield KA. Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int J Parasitol Parasites Wildl. Australian Society for Parasitology; 2014;3: 188–197. 10.1016/j.ijppaw.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones CM, Miller TL, Grutter AS, Cribb TH. Natatory-stage cymothoid isopods: Description, molecular identification and evolution of attachment. Int J Parasitol. 2008;38: 477–491. 10.1016/j.ijpara.2007.07.013 [DOI] [PubMed] [Google Scholar]
- 25.Fogelman RM, Kuris AM, Grutter AS. Parasitic castration of a vertebrate: Effect of the cymothoid isopod, Anilocra apogonae, on the five-lined cardinalfish, Cheilodipterus quinquelineatus. Int J Parasitol. Australian Society for Parasitology Inc.; 2009;39: 577–583. 10.1016/j.ijpara.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 26.Brusca RC. A monograph on the Isopoda Cymothoidae (Crustacea) of the eastern Pacific. Zool J Linn Soc. 1981;73: 117–199. 10.1111/j.1096-3642.1981.tb01592.x [DOI] [Google Scholar]
- 27.Lins LSF, Ho SYW, Wilson GDF, Lo N. Evidence for Permo-Triassic colonization of the deep sea by isopods. Biol Lett. 2012;8: 979–982. 10.1098/rsbl.2012.0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joca LK, Leray VL, Zigler KS, Brusca RC. A new host and reproduction at a small size for the “snapper-choking isopod” Cymothoa excisa (Isopoda: Cymothoidae). J Crustac Biol. Oxford University Press; 2015;35: 292–294. 10.1163/1937240X-00002312 [DOI] [Google Scholar]
- 29.Wilson GDF, Sims C a., Grutter AS. Toward a Taxonomy of the Gnathiidae (Isopoda) Using Juveniles: The External Anatomy of Gnathia aureamaculosa Zuphea Stages Using Scanning Electron Microscopy. J Crustac Biol. 2011;31: 509–522. 10.1651/10-3432.1 [DOI] [Google Scholar]
- 30.Wetzer R. Mitochondrial genes and isopod phylogeny (Peracarida: Isopoda). J Crustac Biol. 2002;22: 1–14. 10.2307/1549602 [DOI] [Google Scholar]
- 31.Lavrov DV, Boore JL, Brown WM. The complete mitochondrial DNA sequence of the horseshoe crab Limulus polyphemus. Mol Biol Evol. 2000;17: 813–824. Available: http://www.ncbi.nlm.nih.gov/pubmed/10779542 10.1093/oxfordjournals.molbev.a026360 [DOI] [PubMed] [Google Scholar]
- 32.Kilpert F, Podsiadlowski L. The complete mitochondrial genome of the common sea slater, Ligia oceanica (Crustacea, Isopoda) bears a novel gene order and unusual control region features. BMC Genomics. 2006;7: 241 10.1186/1471-2164-7-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podsiadlowski L, Bartolomaeus T. Major rearrangements characterize the mitochondrial genome of the isopod Idotea baltica (Crustacea: Peracarida). Mol Phylogenet Evol. 2006;40: 893–899. 10.1016/j.ympev.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 34.Boore JL, Lavrov DV., Brown WM. Gene translocation links insects and crustaceans. Nature. Nature Publishing Group; 1998;392: 667–668. 10.1038/33577 [DOI] [PubMed] [Google Scholar]
- 35.Boore JL. The Duplication/Random Loss Model for Gene Rearrangement Exemplified by Mitochondrial Genomes of Deuterostome Animals In: Sankoff D, Nadeau JH, editors. Comparative Genomics. Springer; Netherlands; 2000. pp. 133–147. 10.1007/978-94-011-4309-7_13 [DOI] [Google Scholar]
- 36.Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151: 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch M, Koskella B, Schaack S. Mutation pressure and the evolution of organelle genomic architecture. Science. 2006. pp. 1727–1730. 10.1126/science.1118884 [DOI] [PubMed] [Google Scholar]
- 38.Duchêne AM, Pujol C, Maréchal-Drouard L. Import of tRNAs and aminoacyl-tRNA synthetases into mitochondria. Curr Genet. 2009;55: 1–18. 10.1007/s00294-008-0223-9 [DOI] [PubMed] [Google Scholar]
- 39.Chandler CH, Badawi M, Moumen B, Grève P, Cordaux R. Multiple Conserved Heteroplasmic Sites in tRNA Genes in the Mitochondrial Genomes of Terrestrial Isopods (Oniscidea). G3 Genes|Genomes|Genetics. 2015;5: 1317–1322. 10.1534/g3.115.018283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doublet V, Souty-Grosset C, Bouchon D, Cordaux R, Marcadé I. A thirty million year-old inherited heteroplasmy. PLoS One. 2008;3 10.1371/journal.pone.0002938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laslett D, Canbäck B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 2008;24: 172–175. 10.1093/bioinformatics/btm573 [DOI] [PubMed] [Google Scholar]
- 42.Segovia R, Pett W, Trewick S, Lavrov DV. Extensive and Evolutionarily Persistent Mitochondrial tRNA Editing in Velvet Worms (Phylum Onychophora). Mol Biol Evol. Oxford University Press; 2011;28: 2873–2881. 10.1093/molbev/msr113 [DOI] [PubMed] [Google Scholar]
- 43.Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290: 470–474. 10.1038/290470a0 [DOI] [PubMed] [Google Scholar]
- 44.Wilson GDF. Some of the deep-sea fauna is ancient. Crustaceana. 1999;72: 1019–1030. 10.1163/156854099503915 [DOI] [Google Scholar]
- 45.Yu H, Li X. Study on the Cymothoidae from Chinese waters. Stud Mar Sin. 2003; 223–238 (In Chinese, with English Abstract). [Google Scholar]
- 46.Nagasawa K, Doi H. The spotfin burrfish (chilomycterus reticulatus), a new host record for cymothoa pulchra (isopoda, cymothoidae). Crustaceana. 2012;85: 893–896. 10.1163/156854012X649900 [DOI] [Google Scholar]
- 47.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25: 3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D, Zou H, Wu SG, Li M, Jakovlić I, Zhang J, et al. Sequencing of the complete mitochondrial genome of a fish-parasitic flatworm Paratetraonchoides inermis (Platyhelminthes: Monogenea): tRNA gene arrangement reshuffling and implications for phylogeny. Parasit Vectors. 2017;10: 462 10.1186/s13071-017-2404-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burland TG. DNASTAR’s Lasergene sequence analysis software In: Misener S, Krawetz SA, editors. Methods in Molecular BiologyTM. Totowa, NJ: Humana Press; 2000. pp. 71–91. 10.1385/1-59259-192-2:71 [DOI] [PubMed] [Google Scholar]
- 50.Hazkani-Covo E, Zeller RM, Martin W. Molecular poltergeists: Mitochondrial DNA copies (numts) in sequenced nuclear genomes. PLoS Genet. 2010;6: e1000834 10.1371/journal.pgen.1000834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33: W686–9. 10.1093/nar/gki366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D. MitoTool [Internet]. [cited 29 May 2018]. Available: https://github.com/dongzhang0725/BioSuite
- 53.Brázda V, Kolomazník J, Lýsek J, Hároníková L, Coufal J, Št’astný J. Palindrome analyser—A new web-based server for predicting and evaluating inverted repeats in nucleotide sequences. Biochem Biophys Res Commun. 2016;478: 1739–1745. 10.1016/j.bbrc.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 54.Shen X, Wang H, Wang M, Liu B. The complete mitochondrial genome sequence of Euphausia pacifica (Malacostraca: Euphausiacea) reveals a novel gene order and unusual tandem repeats. Genome. 2011;54: 911–22. 10.1139/g11-053 [DOI] [PubMed] [Google Scholar]
- 55.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D. BioSuite [Internet]. [cited 28 May 2018]. Available: https://github.com/dongzhang0725/BioSuite
- 57.Liu F-F, Li Y-P, Jakovlić I, Yuan X-Q. Tandem duplication of two tRNA genes in the mitochondrial genome of Tagiades vajuna (Lepidoptera: Hesperiidae). Eur J Entomol. 2017;114: 407–415. doi: 10.14411/eje.2017.052 [Google Scholar]
- 58.Katoh K, Toh H. Improved accuracy of multiple ncRNA alignment by incorporating structural information into a MAFFT-based framework. BMC Bioinformatics. 2008;9: 212 10.1186/1471-2105-9-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castresana J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol Biol Evol. 2000;17: 540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 60.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56: 564–577. 10.1080/10635150701472164 [DOI] [PubMed] [Google Scholar]
- 61.Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol Biol Evol. Oxford University Press; 2012;29: 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- 62.Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, et al. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Letunic I, Bork P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23: 127–128. 10.1093/bioinformatics/btl529 [DOI] [PubMed] [Google Scholar]
- 65.Horton T, Kroh A, Ahyong S, Bailly N, Boury-Esnault N, Brandão SN, et al. World Register of Marine Species (WoRMS). In: Available from http://www.marinespecies.org at VLIZ. [Internet]. WoRMS Editorial Board; 2018 [cited 2 May 2018]. doi:10.14284/170
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Worksheet A: taxonomy and basic statistics for all available isopod mitogenomes. Worksheet B: ancestral gene orders. Worksheet C: gene statistics for all available isopod mitogenomes (+ outgroups): gene sizes, start and terminal codons. Species are represented by acronyms of their binomial scientific names (see footnote). The new sequence (C. indica) and outgroups are shaded grey.
(XLSX)
(FAS)
(FAS)
(TXT)
Four genes missing from Janira maculosa were removed from the entire dataset: atp6, atp8, nad1 and nad5. See caption for Fig 3 for other details.
(TIF)
Data Availability Statement
The newly sequenced mitochondrial genome is available from the GenBank database (accession number MH396438). All other relevant data are within the paper and its Supporting Information files.