Abstract
Aims/Introduction
Emerging evidence has suggested that the genetic background of gestational diabetes mellitus (GDM) was analogous to type 2 diabetes mellitus. In contrast to type 2 diabetes mellitus, the genetic studies for GDM were limited. Accordingly, the aim of the present study was to extensively explore the influence of micro‐ribonucleic acid‐binding single‐nucleotide polymorphisms (SNPs) in type 2 diabetes mellitus candidate loci on GDM susceptibility in Chinese.
Materials and Methods
A total of 839 GDM patients and 900 controls were enrolled. Six micro‐ribonucleic acid‐binding SNPs were selected from 30 type 2 diabetes mellitus susceptibility loci and genotyped using TaqMan allelic discrimination assays.
Results
The minor allele of three SNPs, PAX4 rs712699 (OR 1.366, 95% confidence interval 1.021–1.828, P = 0.036), KCNB1 rs1051295 (OR 1.579, 95% confidence interval 1.172–2.128, P = 0.003) and MFN2 rs1042842 (OR 1.398, 95% confidence interval 1.050–1.862, P = 0.022) were identified to significantly confer higher a risk of GDM in the additive model. The association between rs1051295 and increased fasting plasma glucose (b = 0.006, P = 0.008), 3‐h oral glucose tolerance test plasma glucose (b = 0.058, P = 0.025) and homeostatic model assessment of insulin resistance (b = 0.065, P = 0.017) was also shown. Rs1042842 was correlated with higher 3‐h oral glucose tolerance test plasma glucose (b = 0.056, P = 0.028). However, no significant correlation between the other included SNPs (LPIN1 rs1050800, VPS26A rs1802295 and NLRP3 rs10802502) and GDM susceptibility were observed.
Conclusions
The present findings showed that micro‐ribonucleic acid‐binding SNPs in type 2 diabetes mellitus candidate loci were also associated with GDM susceptibility, which further highlighted the similar genetic basis underlying GDM and type 2 diabetes mellitus.
Keywords: Gestational diabetes mellitus, Type 2 diabetes, Variants
Introduction
Gestational diabetes mellitus (GDM) has become a major public health problem owing to its increased prevalence, and its associated short‐ and long‐term complications for the mother and her offspring1, 2. Similar to type 2 diabetes mellitus, GDM is a multifactorial disease induced by genetics interacting with environmental factors, and genetic factors are known to be a major determinant3. Accumulating evidence shows that the genetic architecture underlying GDM and type 2 diabetes mellitus overlap considerably4. First, GDM and type 2 diabetes mellitus share similar pathophysiologies characterized by increased insulin resistance and impaired islet β‐cell function5, 6. Second, GDM is a strong risk factor for developing type 2 diabetes mellitus in later life7, and women with a family history of type 2 diabetes mellitus are more likely to develop GDM8. Additionally, some known type 2 diabetes mellitus risk variants have been identified to contribute to GDM susceptibility. In contrast to type 2 diabetes mellitus, the genetic basis for GDM remains poorly defined, and the limited GDM genetic studies only center on exploring the association with common variants of type 2 diabetes mellitus candidate loci.
Micro‐ribonucleic acids (miRNAs) are small, non‐coding RNAs of approximately 22 nucleotides that regulate >60% of protein‐coding genes at the post‐transcriptional level, indicating that miRNAs hold critical roles in a multitude of biological pathways9. miRNAs bind to the 3′ untranslated region (3′UTR) of target messenger RNAs (mRNAs) and act by translation repression or mRNA degradation10. Emerging evidence has shown that single‐nucleotide polymorphisms (SNPs) in the miRNA binding site (miR‐binding SNPs) conferred a higher risk of a variety of diseases by disturbing the interaction between miRNAs and targeted mRNAs11. Furthermore, our previous study had first shown that several miR‐binding SNPs of reported GDM candidate loci were associated with GDM susceptibility in Chinese Han pregnant women12, 13.
Considering GDM shares a similar genetic predisposition with type 2 diabetes mellitus and combined with our previous findings, the present study broadened the scope of screening and focused on type 2 diabetes mellitus candidate loci that had been confirmed in the Chinese Han population and had not been studied in our previous research. We aimed to intensively investigate the association between miR‐binding SNPs of type 2 diabetes mellitus candidate loci and the risk of GDM, which would further improve our knowledge about the role of miR‐binding SNPs in GDM pathogenesis, and provide additional insights into the similar genetic basis underlying GDM and type 2 diabetes mellitus.
Methods
Ethics statement
The ethics committee of Peking Union Medical College Hospital approved the study protocol. The study was carried out in accordance with the principles of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). Written informed consent was obtained from each participant.
Study participants
The recruitment flow of study participants has been previously reported13. In brief, the study participants consisted of 900 controls and 839 GDM patients who were recruited between January 2006 and June 2011. All pregnant women who had not been previously diagnosed with diabetes underwent a 50‐g glucose challenge test at 24–28 weeks‐of‐gestation. Individuals with a positive result (1‐h plasma glucose levels ≥7.8 mmol/L) were then followed by a 3‐h, 100‐g oral glucose tolerance test (OGTT). The diagnosis of GDM was in accordance with the American Diabetes Association criteria14. We classified participants with negative glucose challenge test results or normal glucose tolerance tests as the control group. The exclusion criteria were severe pregnancy complications, taking medicines affecting glucose metabolism and diabetes diagnosed before pregnancy.
Clinical and laboratory analysis
Clinical data including weight before pregnancy, height and blood pressure were collected at 24–28 weeks‐of‐gestation by a trained physician. The measurement methods and instruments for plasma glucose and serum insulin levels have been reported previously13. Homeostatic model assessments of islet β‐cell function (HOMA‐β) and basal insulin resistance (HOMA‐IR) were evaluated. The calculation formulas were as follows: HOMA‐β = fasting serum insulin (mU/L) × 20 / (fasting plasma glucose in mmol/L − 3.5), HOMA‐IR = fasting serum insulin (mU/L) × fasting plasma glucose (mmol/L) / 22.5.
SNP selection and genotyping
The flow diagram of SNP selection is shown in Figure 1. We selected 28 type 2 diabetes mellitus susceptibility genes that were identified by a genome‐wide association study and or confirmed in the Chinese Han population as candidate genes. These genes were as follows: KCNB1, GIPR, MADD, CRY2, NLRP3, PAX4, GRK5, RASGRP1, BDNF, EXT2, MFN2, ADRB3, JAZF1, EXT2, VPS26A, DUSP9, ARHGEF11, POU2F1, FGF21, LPIN1, C5L2, HSPA5, TP53INP1, CAMK1D, SPRY2, G6PC2, HNF4A and GLIS3 (Table S1 shows the reference of these genes). The other detailed information about the process of SNP selection has been described in our previous study13. Finally, six potential miRNA‐binding site SNPs that were predicted to be located in the same miRNA‐binding site by at least two different types of software were included in the present study. TaqMan allelic discrimination assays were applied for SNP genotyping. Each 384‐well plate included four blank samples to ensure the genotyping quality. In addition, we carried out replicate assays and Sanger sequencing to further confirm the genotyping accuracy. The results of replicate assays and Sanger sequencing were completely consistent with the original analysis. The genotyping success rate of six SNPs was 97.87%, 95.63%, 97.35%, 99.37%, 98.50% and 99.31% for rs712699, rs1051295, rs1042842, rs1050800, rs1802295 and rs10802502, respectively. Sequence validation of SNPs is supplied in Figure S1.
Figure 1.
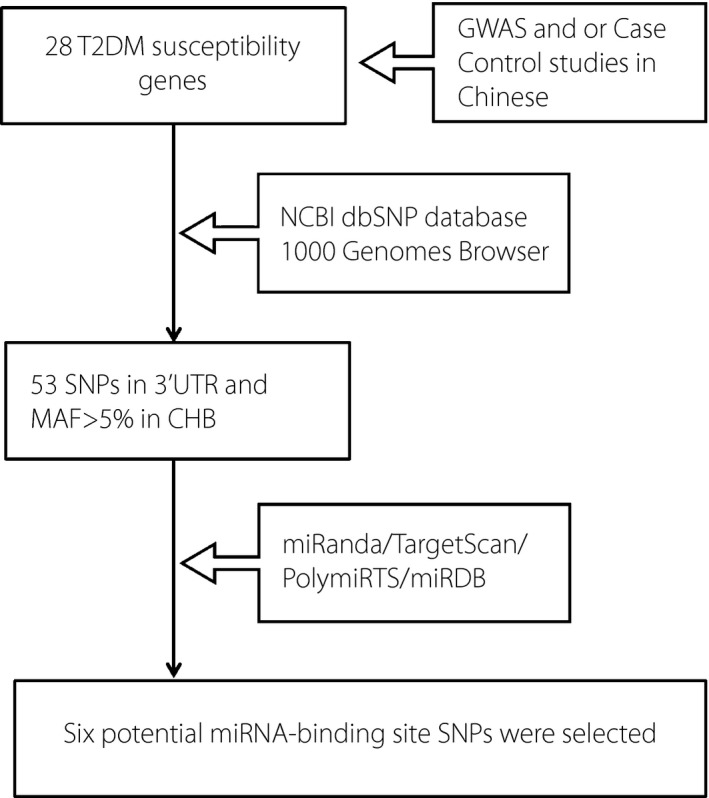
Flow diagram of single‐nucleotide polymorphism (SNP) selection. 3′UTR, 3′ untranslated region; CHB, Chinese Han in Beijing; GWAS, genome‐wide association study; MAF, minor allele frequency; miRNA, micro‐ribonucleic acid; NCBI dbSNP, National Center for Biotechnology Information SNP database; T2DM, type 2 diabetes mellitus.
Statistical analysis
Statistical analysis was carried out using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The χ2‐test was used to assess the Hardy–Weinberg equilibrium of genotype distribution for each SNP in control groups. Skewed distribution variables (age, pre‐pregnancy body mass index, parity, fasting plasma glucose and insulin, HOMA‐β and HOMA‐IR) are expressed as the median and interquartile range (IQR), and comparisons between GDM and controls were carried out using non‐parametric tests. The association between SNPs and the risk of GDM was evaluated by multivariate logistic regression analysis adjusting for age in three genetic models. Multiple linear regression with adjustment for age was applied to determine the effect of the minor alleles of each SNP on glucose metabolism traits (including plasma glucose, HOMA‐β and HOMA‐IR), assuming an additive model of inheritance. The regression coefficients (b) and 95% confidence interval (CI) were calculated. Before linear regression analysis, HOMA‐IR and HOMA‐β were logarithmically transformed to nearly normal distribution.
Results
The median age of GDM patients was 32.0 years (IQR 30.0–35.0 years), which was older than the control group (median 31.0, IQR 28.0–4.0; P < 0.05). GDM patients presented with higher fasting glucose, 1‐h OGTT glucose, 2‐h OGTT glucose and 3‐h OGTT glucose than controls. Furthermore, GDM patients showed higher insulin resistance and lower islet function. However, pre‐pregnancy body mass index was not significantly different between the GDM and control groups, as well as parity (median 1 [IQR 0–3] vs 1 [IQR 0–3]). Detailed information on the clinical characteristics is supplied in Table S2.
The six selected SNPs, PAX4 rs712699, KCNB1 rs1051295, MFN2 rs1042842, LPIN1 rs1050800, VPS26A rs1802295 and NLRP3 rs10802502, were predicted to be located in the binding site of hsa‐miR‐223, hsa‐miR‐216a, hsa‐miR‐183, hsa‐miR‐96, hsa‐miR‐510 and hsa‐miR‐548, respectively. The minor allele frequency of each SNP in the present study was similar to the data supplied by the 1,000 Genome Browser. The genotype distribution of these SNPs was in accordance with the Hardy–Weinberg equilibrium in the control groups (P > 0.05). Table 1 shows detailed information on the six SNPs.
Table 1.
Six micro‐ribonucleic acid‐binding single‐nucleotide polymorphisms and the Hardy–Weinberg equilibrium test
| SNP | Gene | Main function | Min/Maj | Chr. position | MAF (CHB)† | Sample MAF | Predicted binding miRNA | Algorithm | P |
|---|---|---|---|---|---|---|---|---|---|
| rs712699 | PAX4 | Pancreatic development | A/G | Chr.7:127250597 | 0.356 | 0.403 | hsa‐miR‐223 | TargetScan;MiRDB | 0.122 |
| rs1051295 | KCNB1 | Insulin secretion | C/T | Chr.20:47988905 | 0.444 | 0.413 | hsa‐miR‐216a | PolymiRTS;TargetScan | 0.485 |
| rs1042842 | MFN2 | Mitochondrial fusion | A/G | Chr.1: 12071680 | 0.376 | 0.397 | hsa‐miR‐183 | PolymiRTS;TargetScan | 0.116 |
| rs1050800 | LPIN1 | Adipocyte differentiation | T/C | Chr.2: 11965814 | 0.211 | 0.256 | hsa‐miR‐96 | PolymiRTS;TargetScan | 0.727 |
| rs1802295 | VPS26A | Transport proteins | T/C | Chr.10:70931474 | 0.108 | 0.116 | hsa‐miR‐510 | PolymiRTS;TargetScan;MiRDB | 0.861 |
| rs10802502 | NLRP3 | Regulate inflammation | C/T | Chr.1: 247612295 | 0.423 | 0.484 | hsa‐miR‐548 | PolymiRTS;TargetScan;microRNA.org | 0.074 |
†From 1,000‐genome database. CHB, Chinese Han in Beijing; Chr., chromosome; MAF, frequency of minor allele; Maj, major allele; Min, minor allele; miRNA, micro‐ribonucleic acid.
The allele and genotype frequencies of the six SNPs in GDM patients and controls are presented in Table 2 and Table S3. SNP rs712699 of PAX4 significantly increased the risk of GDM with an odds ratio (OR) of 1.366 (95% CI 1.021–1.828, P = 0.036) in an additive model adjusting for age at pregnancy, and the statistically significant effect remained in a recessive model (adjusted OR 1.433, 95% CI 1.101–1.866, P = 0.007). In addition, the CC genotype of KCNB1 rs1051295 was found to have a 1.579‐fold, 1.378‐fold and 1.331‐fold increased risk of GDM in the additive model (95% CI 1.172–2.128, P = 0.003), dominant model (95% CI 1.055–1.799, P = 0.008) and recessive model (95% CI 1.055–1.799, P = 0.019), respectively. The frequency of rs1051295 C allele was also significantly higher in GDM patients, with an OR of 1.230 (95% CI 1.071–1.413) and a P‐value of 0.003. Furthermore, the AA genotype of MFN2 rs1042842 conferred elevated GDM susceptibility both in the additive model (adjusted OR 1.398, 95% CI 1.050–1.862, P = 0.022) and the dominant model (adjusted OR 1.269, 95% CI 1.035–1.555, P = 0.022). The A allele of rs1042842 was also significantly correlated with an increased risk of GDM, with an OR of 1.224 (95% CI 1.066–1.405, P = 0.004). In addition, the pregnant women carrying the T allele of LPIN1 rs1050800 had a nominally significant 1.169‐fold risk of GDM (95% CI 1.000–1.354, P = 0.049) after adjustment for age compared with the women harboring the C allele.
Table 2.
Genotype distribution and gestational diabetes mellitus susceptibility in additive model
| SNP ID | Gene | Model | Genotypes/minor allele | GDM | Controls | OR (95% CI) | P‐value | ||
|---|---|---|---|---|---|---|---|---|---|
| rs712699 | PAX4 | Additive | A/A vs G/G | 154 | 293 | 126 | 317 | 1.366 (1.021–1.828) | 0.036* |
| rs1051295 | KCNB1 | Additive | C/C vs T/T | 149 | 247 | 125 | 316 | 1.579 (1.172–2.128) | 0.003* |
| rs1042842 | MFN2 | Additive | A/A vs G/G | 153 | 278 | 135 | 358 | 1.398 (1.050–1.862) | 0.022* |
| rs1050800 | LPIN1 | Additive | T/T vs C/C | 46 | 461 | 51 | 513 | 1.027 (0.665–1.587) | 0.903 |
| rs1802295 | VPS26A | Additive | T/T vs C/C | 12 | 642 | 12 | 699 | 0.932 (0.411–2.113) | 0.866 |
| rs10802502 | NLRP3 | Additive | C/C vs T/T | 195 | 228 | 201 | 221 | 0.945 (0.716–1.247) | 0.691 |
*Significant P‐values (P < 0.05). CI, confidence interval; GDM, gestational diabetes mellitus; OR, odds ratio; SNP, single‐nucleotide polyporphism.
The results of multiple linear regression analysis adjusted for age (Table 3, Table S4) showed that the minor C allele of rs1051295 was associated with increased fasting plasma glucose (FPG; b = 0.006, P = 0.008), 2‐h OGTT plasma glucose (b = 0.056, P = 0.023), 3‐h OGTT plasma glucose (b = 0.058, P = 0.025) and HOMA‐IR (b = 0.065, P = 0.017). In addition, the A allele of rs1042842 was correlated with higher 3‐h OGTT plasma glucose with a per‐allele effect of 0.056 mmol/L (P = 0.028). The A allele of rs712699 also had an effect on 2‐h OGTT plasma glucose (b = 0.051, P = 0.036). We also observed the minor T allele of rs1050800 was associated with elevated 3‐h OGTT plasma glucose (b = 0.061, P = 0.016) and decreased HOMA‐β (b = –0.059, P = 0.029). The T allele of rs1802295 had a mild effect on FPG with an increase of 0.05 mmol/L per risk allele (P = 0.040), but the association with mildly decreased HOMA‐IR was also found (b = –0.056, P = 0.042).
Table 3.
Association between minor allele and glucose metabolism‐related quantitative traits
| SNP ID | Gene | Minor allele | Fasting plasma glucose (n = 1,739) | 3‐h OGTT glucose (n = 1,471) | HOMA‐β (n = 1,413) | HOMA‐IR (n = 1,413) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| b (95% CI) | P | b (95% CI) | P | b (95% CI) | P | b (95% CI) | P | |||
| rs712699 | PAX4 | A | 0.016 (−0.023, 0.046) | 0.504 | 0.013 (−0.079, 0.136) | 0.601 | −0.036 (−0.075, 0.015) | 0.186 | −0.019 (−0.068, 0.032) | 0.484 |
| rs1051295 | KCNB1 | C | 0.006 (0.013, 0.083) | 0.008* | 0.058 (0.016, 0.234) | 0.025* | 0.045 (−0.007, 0.085) | 0.099 | 0.065 (0.011, 0.114) | 0.017* |
| rs1042842 | MFN2 | A | 0.029 (−0.013, 0.054) | 0.236 | 0.056 (0.013, 0.222) | 0.028* | −0.003 (−0.047, 0.042) | 0.902 | 0.011 (−0.039, 0.060) | 0.688 |
| rs1050800 | LPIN1 | T | 0.024 (−0.019, 0.060) | 0.318 | 0.061 (0.029, 0.274) | 0.016* | −0.059 (−0.109, −0.006) | 0.029* | −0.027 (−0.087, 0.028) | 0.310 |
| rs1802295 | VPS26A | T | 0.050 (−0,108, −0.002) | 0.040* | 0.019 (−0.100, 0.224) | 0.453 | 0.008 (−0.058, 0.080) | 0.759 | −0.056 (−0.155, −0.003) | 0.042* |
| rs10802502 | NLRP3 | C | 0.020 (−0.020, 0.048) | 0.414 | −0.011 (−0.129, 0.083) | 0.673 | −0.027 (−0.068, 0.022) | 0.315 | −0.019 (−0.068, 0.031) | 0.468 |
P‐value was adjusted for age in the linear regression model. *Significant P‐values (P < 0.05). CI, confidence interval; HOMA‐β, homeostatic model assessments of islet β‐cell function; HOMA‐IR, homeostatic model assessments of insulin resistance; OGTT, oral glucose tolerance test; OR, odds ratio; SNP, single‐nucleotide polymorphism.
Discussion
A growing body of evidence suggests that GDM and type 2 diabetes mellitus share a similar genetic background. In particular, our previous research discovered that miR‐binding SNPs played a critical role in the genetic pathogenesis of GDM. Accordingly, in the present study, we extensively investigated the effect of miR‐binding SNPs in type 2 diabetes mellitus candidate loci on the risk of GDM in the Chinese Han population. The present findings showed that three SNPs of type 2 diabetes mellitus risk genes were also associated with GDM.
The PAX4 gene functions as a transcription repressor and plays essential roles in islet β‐cell development during embryogenesis, and regulating β‐cell survival and proliferation during adulthood15. PAX4‐knockout mice presented with the absence of mature insulin‐producing β‐cells and hyperplasia of glucagon‐producing α‐cells16. Variants in the PAX4 gene have been found to be associated with several forms of diabetes mellitus. The PAX4 mutation causes maturity‐onset diabetes of the young, type 917. Genetic association studies showed that SNPs of PAX4 were linked to both type 1 diabetes mellitus and type 2 diabetes mellitus susceptibility across various ethnic groups18, 19. Subsequently, rs6467136 near the PAX4 gene and rs10229583 locating downstream of the PAX4 were identified as risk variants for type 2 diabetes mellitus by a genome‐wide association study in a Chinese population20 and a large meta‐analysis in an East Asian population21, respectively. It is worth mentioning that quite recently an exome‐chip association analysis revealed an Asian‐specific coding variant rs2233580 of PAX4 associated with type 2 diabetes mellitus in Chinese individuals22. Such observations suggested PAX4 was a crucial locus for diabetes mellitus, especially in Asian people. The present study found for the first time that the A allele of PAX4 rs712699 increased the risk of GDM in Chinese Han pregnant women. The A allele was predicted to create a novel hsa‐miR‐223 binding site, which might impair PAX4 gene expression. It was speculated that the defect had an influence on β‐cells differentiation or mature β‐cells survival contributing to GDM susceptibility. The possible molecular mechanisms need to be clarified by functional studies in the future.
The KCNB1 gene encoded the voltage‐dependent K+ (Kv) channel isoform Kv2.1, and was highly enriched in islet β‐cells. In the process of insulin secretion, Kv2.1 mediated the repolarization of pancreatic β‐cells action potential to control the duration of depolarization23. Kv2.1‐null mice had improved glucose tolerance with reduced fasting glucose24. Pancreatic β‐cells from Kv2.1‐null mice showed greater glucose‐stimulated insulin secretion, and pharmacological inhibition of Kv2.1 enhanced glucose‐stimulated insulin secretion25. The present study showed that the carriers of the rs1051295‐CC genotype in KCNB1 had a higher risk of developing GDM, and the minor C allele was associated with elevated FPG, 3‐h OGTT plasma glucose and HOMA‐IR. Given the T allele of rs1051295 was predicted to be located in the hsa‐miR‐216a binding site, we supposed that the presence of the minor C allele might block the interaction and increase KCNB1 expression, thereby contributing to the glucose metabolism disorder. Certainly, the biological mechanisms of miR‐216a and rs1051295 in the GDM pathogenesis need to be elucidated in the future. Interestingly, the significant association between rs1051295 and type 2 diabetes mellitus susceptibility and decreased insulin sensitivity were also discovered in the Chinese population, but the risk allele (T) was inconsistent with the present study26. The small sample size of the previous study might be the principal explanation for the differences. Larger well‐designed studies across multiple ethnic groups should further validate the association.
The MFN‐2 gene encoded mitofusin 2 (Mfn2), which explained outer mitochondrial membrane fusion, regulated oxidative metabolism and autophagy, and maintained mitochondrial deoxyribonucleic acid stability27, 28, 29. In addition, Mfn2 was required for metabolic homeostasis28, 30. Glucose intolerance was observed in liver‐specific Mfn2 knockout mice. Mfn2 deficiency also caused endoplasmic reticulum stress and mitochondrial dysfunction triggering reactive oxygen species, by which c‐Jun N‐terminal kinase was activated and led to insulin resistance29. Importantly, type 2 diabetes mellitus was related to reduced expression of Mfn231, and SNPs of the MFN‐2 gene were reported to be associated with type 2 diabetes mellitus32. The present study first identified that the A allele of rs1042842 in the MFN‐2 gene was significantly associated with GDM and increased 3‐h OGTT plasma glucose. The significant association might be explained by the repression of the MFN‐2 gene due to the binding between the hsa‐miR‐183 and rs1042842 A allele. Nevertheless, functional studies are warrant to confirm the speculation.
The LPIN1 gene, which was primarily expressed in skeletal muscle and adipose tissue, was discovered as the causative gene in the fatty liver dystrophy mouse33. Except for its important roles in adipocyte differentiation and lipid metabolism, recent studies showed that the LPIN1 gene was also expressed in pancreatic β‐cells and implicated in glucose metabolism34. Furthermore, SNPs and a rare haplotype of the LPIN1 gene were reported to be significantly associated with type 2 diabetes mellitus35, 36. So far, the relationship between variants of the LPIN1 gene and GDM has not been investigated. In the present study, we found that the T allele of rs1050800‐LPIN1 was not associated with GDM susceptibility, but was significantly correlated with increased fasting glucose and decreased HOMA‐β. It was possible that the disturbed interaction between rs1050800 and predicted binding miRNA (hsa‐miR‐96) decreased LPIN1 expression, contributing to the observed association with glucose metabolism traits. In parallel, a strong negative correlation was shown between lipin mRNA levels in human adipose tissue and fasting glucose37. In contrast, miR‐96 was shown to play a key role in insulin exocytosis modulating the plasma glucose homeostasis38. However, the real influence of rs1050800 on LPIN1 expression has not been verified.
The VPS26A gene is located at 10q22, and is expressed in pancreatic and adipose tissues, which encode a subunit of the retromer complex involved in the transport of proteins from endosomes to the trans‐Golgi complex39. The VPS26A gene was identified as type 2 diabetes mellitus susceptibility loci by a genome‐wide association study suggesting its role in glucose metabolism40. A case–control study in Chinese participants confirmed the association between rs1802295‐VPS26A and the risk of type 2 diabetes mellitus41. However, the present study observed that the T allele of rs1802295 was not associated with GDM susceptibility, only a significant association with higher FPG and decreased HOMA‐IR were indicated. Consistent with the present findings, rs1802295 was recently reported to have no effect on GDM in an Asian Indian population as well. Therefore, the role of the VPS26A gene in the development of diabetes mellitus requires further clarification.
There were some limitations to the present study. First, functional studies were not carried out to further confirm the binding between predicted miRNA and targeted mRNA. Second, only common variants in 3′UTR of type 2 diabetes mellitus candidate loci were examined, thus some rare SNPs with large effects were probably omitted. Third, Bonferroni adjustments were not carried out so as to avoid missing possible SNPs predisposed to GDM. In addition, because no replication was carried out, it is possible that some of the findings might be false positives.
In conclusion, the present study extensively investigated the influence of miR‐binding SNP of type 2 diabetes mellitus candidate loci on GDM susceptibility. The minor alleles of three genes (PAX4 rs712699, KCNB1 rs1051295, MFN2 rs1042842) were identified to increase the risk of GDM. In addition, four miR‐binding SNPs (rs1051295, rs1042842, LPIN1 rs1050800, VPS26A rs1802295) were significantly associated with glucose metabolism traits. The present findings facilitated a better understanding of the genetic contribution to the risk of GDM, and further underscored the similar genetic architecture underlying GDM and type 2 diabetes mellitus. In future, functional studies are required to elucidate the biological mechanisms underlying these significant associations.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Sanger sequencing trace of six micro‐ribonucleic acid‐binding single‐nucleotide polymorphisms.
Table S1 | Reference of 28 selected genes.
Table S2 | Clinical characteristics of the participants.
Table S3 | Genotype distribution and gestational diabetes mellitus susceptibility in the recessive and dominant model.
Table S4 | Association between minor allele and 1‐h oral glucose tolerance test and 2‐h oral glucose tolerance test plasma glucose.
Acknowledgments
We thank all the participants in this study. The study was funded by research grants from the National Natural Science Foundation of China (Grant No. 81270879) and National Key Program of Clinical Science (WBYZ2011873).
J Diabetes Investig 2018; 9: 1196–1202
References
- 1. Group HSCR, Metzger BE, Lowe LP, et al Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 2. Hillier TA, Pedula KL, Schmidt MM, et al Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 2007; 30: 2287–2292. [DOI] [PubMed] [Google Scholar]
- 3. Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009; 373: 1789–1797. [DOI] [PubMed] [Google Scholar]
- 4. Kleinberger JW, Maloney KA, Pollin TI. The genetic architecture of diabetes in pregnancy: implications for clinical practice. Am J Perinatol 2016; 33: 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Homko C, Sivan E, Chen X, et al Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab 2001; 86: 568–573. [DOI] [PubMed] [Google Scholar]
- 6. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 2004; 88: 787–835, ix. [DOI] [PubMed] [Google Scholar]
- 7. Clausen TD, Mathiesen ER, Hansen T, et al High prevalence of type 2 diabetes and pre‐diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008; 31: 340–346. [DOI] [PubMed] [Google Scholar]
- 8. Williams MA, Qiu C, Dempsey JC, et al Familial aggregation of type 2 diabetes and chronic hypertension in women with gestational diabetes mellitus. J Reprod Med 2003; 48: 955–962. [PubMed] [Google Scholar]
- 9. Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol 2011; 3: 83–92. [PMC free article] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moszynska A, Gebert M, Collawn JF, et al SNPs in microRNA target sites and their potential role in human disease. Open Biol 2017; 7: 170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Li W, Ma L, et al Association study of the miRNA‐binding site polymorphisms of CDKN2A/B genes with gestational diabetes mellitus susceptibility. Acta Diabetol 2015; 52: 951–958. [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Li W, Ma L, et al Investigation of miRNA‐binding site variants and risk of gestational diabetes mellitus in Chinese pregnant women. Acta Diabetol 2017; 54: 309–316. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33 (Suppl 1): S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenzo PI, Juarez‐Vicente F, Cobo‐Vuilleumier N, et al The diabetes‐linked transcription factor PAX4: from gene to functional consequences. Genes (Basel) 2017; 8: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sosa‐Pineda B, Chowdhury K, Torres M, et al The Pax4 gene is essential for differentiation of insulin‐producing beta cells in the mammalian pancreas. Nature 1997; 386: 399–402. [DOI] [PubMed] [Google Scholar]
- 17. Sujjitjoon J, Kooptiwut S, Chongjaroen N, et al Aberrant mRNA splicing of paired box 4 (PAX4) IVS7‐1G>A mutation causing maturity‐onset diabetes of the young, type 9. Acta Diabetol 2016; 53: 205–216. [DOI] [PubMed] [Google Scholar]
- 18. Biason‐Lauber A, Boehm B, Lang‐Muritano M, et al Association of childhood type 1 diabetes mellitus with a variant of PAX4: possible link to beta cell regenerative capacity. Diabetologia 2005; 48: 900–905. [DOI] [PubMed] [Google Scholar]
- 19. Sujjitjoon J, Kooptiwut S, Chongjaroen N, et al PAX4 R192H and P321H polymorphisms in type 2 diabetes and their functional defects. J Hum Genet 2016; 61: 943–949. [DOI] [PubMed] [Google Scholar]
- 20. Ma RC, Hu C, Tam CH, et al Genome‐wide association study in a Chinese population identifies a susceptibility locus for type 2 diabetes at 7q32 near PAX4. Diabetologia 2013; 56: 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cho YS, Chen CH, Hu C, et al Meta‐analysis of genome‐wide association studies identifies eight new loci for type 2 diabetes in East Asians. Nat Genet 2011; 44: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheung CY, Tang CS, Xu A, et al Exome‐chip association analysis reveals an Asian‐specific missense variant in PAX4 associated with type 2 diabetes in Chinese individuals. Diabetologia 2017; 60: 107–115. [DOI] [PubMed] [Google Scholar]
- 23. MacDonald PE, Wheeler MB. Voltage‐dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia 2003; 46: 1046–1062. [DOI] [PubMed] [Google Scholar]
- 24. Jacobson DA, Kuznetsov A, Lopez JP, et al Kv2.1 ablation alters glucose‐induced islet electrical activity, enhancing insulin secretion. Cell Metab 2007; 6: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li XN, Herrington J, Petrov A, et al The role of voltage‐gated potassium channels Kv2.1 and Kv2.2 in the regulation of insulin and somatostatin release from pancreatic islets. J Pharmacol Exp Ther 2013; 344: 407–416. [DOI] [PubMed] [Google Scholar]
- 26. Zhang YX, Liu Y, Dong J, et al An exploratory study of the association between KCNB1 rs1051295 and type 2 diabetes and its related traits in Chinese Han population. PLoS ONE 2013; 8: e56365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rovira‐Llopis S, Banuls C, Diaz‐Morales N, et al Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol 2017; 11: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zorzano A, Hernandez‐Alvarez MI, Sebastian D, et al Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal 2015; 22: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 29. Sebastian D, Hernandez‐Alvarez MI, Segales J, et al Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA 2012; 109: 5523–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bach D, Pich S, Soriano FX, et al Mitofusin‐2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 2003; 278: 17190–17197. [DOI] [PubMed] [Google Scholar]
- 31. Hernandez‐Alvarez MI, Thabit H, Burns N, et al Subjects with early‐onset type 2 diabetes show defective activation of the skeletal muscle PGC‐1{alpha}/Mitofusin‐2 regulatory pathway in response to physical activity. Diabetes Care 2010; 33: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li P, Zhu S, Wu X, et al Association of polymorphisms in mitofusin‐2 gene with type 2 diabetes in Han Chinese. J Biomed Biotechnol 2012; 2012: 205752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peterfy M, Phan J, Xu P, et al Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet 2001; 27: 121–124. [DOI] [PubMed] [Google Scholar]
- 34. Lan H, Rabaglia ME, Stoehr JP, et al Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 2003; 52: 688–700. [DOI] [PubMed] [Google Scholar]
- 35. Zhang R, Jiang F, Hu C, et al Genetic variants of LPIN1 indicate an association with Type 2 diabetes mellitus in a Chinese population. Diabet Med 2013; 30: 118–122. [DOI] [PubMed] [Google Scholar]
- 36. Chang YC, Chang LY, Chang TJ, et al The associations of LPIN1 gene expression in adipose tissue with metabolic phenotypes in the Chinese population. Obesity (Silver Spring) 2010; 18: 7–12. [DOI] [PubMed] [Google Scholar]
- 37. Suviolahti E, Reue K, Cantor RM, et al Cross‐species analyses implicate Lipin 1 involvement in human glucose metabolism. Hum Mol Genet 2006; 15: 377–386. [DOI] [PubMed] [Google Scholar]
- 38. Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin‐secreting cells by microRNAs. Biol Chem 2008; 389: 305–312. [DOI] [PubMed] [Google Scholar]
- 39. Seaman MN, Marcusson EG, Cereghino JL, et al Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol 1997; 137: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kooner JS, Saleheen D, Sim X, et al Genome‐wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet 2011; 43: 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukuda H, Imamura M, Tanaka Y, et al A single nucleotide polymorphism within DUSP9 is associated with susceptibility to type 2 diabetes in a Japanese population. PLoS ONE 2012; 7: e46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Sanger sequencing trace of six micro‐ribonucleic acid‐binding single‐nucleotide polymorphisms.
Table S1 | Reference of 28 selected genes.
Table S2 | Clinical characteristics of the participants.
Table S3 | Genotype distribution and gestational diabetes mellitus susceptibility in the recessive and dominant model.
Table S4 | Association between minor allele and 1‐h oral glucose tolerance test and 2‐h oral glucose tolerance test plasma glucose.


