Abstract
OBJECTIVES:
The Pancreatitis Activity Scoring System (PASS) has been derived by an international group of experts via a modified Delphi process. Our aim was to perform an external validation study to assess for concordance of the PASS score with high face validity clinical outcomes and determine specific meaningful thresholds to assist in application of this scoring system in a large prospectively ascertained cohort.
METHODS:
We analyzed data from a prospective cohort study of consecutive patients admitted to the Los Angeles County Hospital between March 2015 and March 2017. Patients were identified using an emergency department paging system and electronic alert system. Comprehensive characterization included substance use history, pancreatitis etiology, biochemical profile, and detailed clinical course. We calculated the PASS score at admission, discharge, and at 12 h increments during the hospitalization.
We performed several analyses to assess the relationship between the PASS score and outcomes at various points during hospitalization as well as following discharge. Using multivariable logistic regression analysis, we assessed the relationship between admission PASS score and risk of severe pancreatitis. PASS score performance was compared to established systems used to predict severe pancreatitis. Additional inpatient outcomes assessed included local complications, length of stay, development of systemic inflammatory response syndrome (SIRS), and intensive care unit (ICU) admission. We also assessed whether the PASS score at discharge was associated with early readmission (re-hospitalization for pancreatitis symptoms and complications within 30 days of discharge).
RESULTS:
A total of 439 patients were enrolled, their mean age was 42 (±15) years, and 53% were male. Admission PASS score >140 was associated with moderately severe and severe pancreatitis (OR 3.5 [95% CI 2.0, 6.3]), ICU admission (OR 4.9 [2.5, 9.4]), local complications (3.0 [1.6, 5.7]), and development of SIRS (OR 2.9 [1.8, 4.5]) as well as prolongation of hospitalization by a mean of 1.5 (1.3–1.7) days. For the prediction of moderately severe/severe pancreatitis, the PASS score (AUC = 0.71) was comparable to the more established Ranson’s (AUC = 0.63), Glasgow (AUC = 0.72), Panc3 (AUC = 0.57), and HAPS (AUC = 0.54) scoring systems. Discharge PASS score >60 was associated with early readmission (OR 5.0 [2.4, 10.7]).
CONCLUSIONS:
The PASS score is associated with important clinical outcomes in acute pancreatitis. The ability of the score to forecast important clinical events at different points in the disease course suggests that it is a valid measure of activity in patients with acute pancreatitis.
INTRODUCTION
Acute pancreatitis presents several challenges for clinicians and investigators alike, the foremost includes the variability in patient presentation and disease course. While patients with acute pancreatitis may initially appear to have mild disease, they may rapidly develop critical illness. Alternatively, even patients with what is considered mild disease may experience wide variation in their disease course ranging from full recovery within a few days to prolonged illness with protracted hospitalization secondary to pain and inability to tolerate resumption of oral intake. A major limitation to developing improved management strategies for patients with acute pancreatitis has been the lack of a widely accepted method to measure and monitor disease activity.
Interventional studies have targeted patients with predicted severe pancreatitis [1–4]. However, predicted severe pancreatitis has protean definitions ranging from various APACHE scores to C-reactive protein levels to clinical findings, such as abnormal chest roentgenography. Unfortunately, diversity of inclusion criterion makes it challenging to identify which groups of patients benefit from specific therapy. Additionally, while objective acute pancreatitis outcomes such as death are fortunately rare this necessitates the use of surrogate measures such as clinical improvement or changes in cytokine levels as study endpoints [1, 5]. The use of scoring systems that predict severity of disease have predominated in the acute pancreatitis field. However, there has been a limitation in quantitative scoring systems that encompass the overall physiologic status of the patient for studies.
The study of other disease states including inflammatory bowel disease has benefited from the development of quantitative scoring systems such as the Crohns Disease Activity Index that can be used to monitor the disease activity during its course [6, 7]. To address this need, a group of international experts recently developed the acute Pancreatitis Activity Scoring System (PASS), which was designed to provide an objective tool for measurement of disease activity in patients with acute pancreatitis [8].
A key step in validating any new disease assessment tool is to evaluate the relationship between the scoring system and clinical outcomes. Outcomes with high face validity in acute pancreatitis include the development of transient or persistent organ failure (moderately severe and severe pancreatitis) as well as local complications, such as pseudocysts and necrosis [9, 10]. In addition, early readmission (≤30 days) following discharge is an important benchmark for high quality and affordable care [11, 12].
Our aim was to assess the relationship between the PASS score and these important clinical outcomes in a large cohort of patients with acute pancreatitis. In addition, we sought to identify specific thresholds in the PASS score at admission and at discharge to provide a framework for applying the instrument in clinical practice as well as research settings.
METHODS
PASS instrument
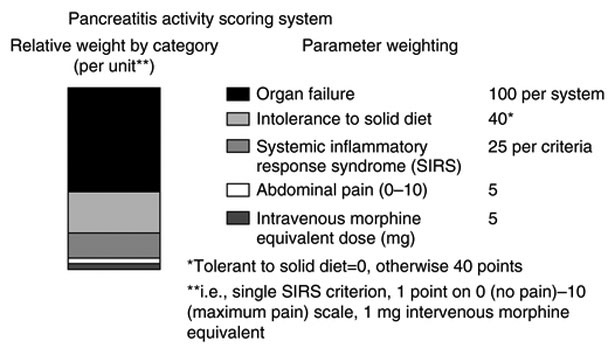
The PASS score was developed by systematic literature review to identify potential parameters followed by parameter selection by a group of international experts utilizing a modified Delphi process as previously reported [8]. This involved voting on a number of clinical domains (i.e., nutrition, inflammatory markers) by an expert panel; candidate markers were not derived by gauging their performance in a patient cohort. The PASS system applies a quantitative weight to five clinically important parameters (Fig. 1) and may be calculated sequentially during the pancreatitis admission.
Fig. 1.
Components of PASS score
Population
Institutional Review Board approval from the University of Southern California Health Sciences IRB was obtained for the prospective cohort. We evaluated all patients who presented to the Los Angeles County Hospital with acute pancreatitis between March 2015 and March 2017. The Los Angeles County Hospital is the largest acute care public hospital in Western United States. Its mission is not to function as a tertiary referral center but to provide nearly all inpatient care for a cohort of 1 million disadvantaged patients in central and eastern Los Angeles County. In comparison to the United States population, our population has lower socioeconomic status, has higher proportion of Hispanic, Asian, and African Americans, and is younger. In addition to a high number of admission for pancreatic disorders, there are a large number of admissions for alcohol overuse and gallbladder disease. Query of hospitalizations in 2014 revealed that there were 5100 admissions for complications of alcohol overuse and 980 cholecystectomies for symptomatic gallstone disease.
Our study team was alerted regarding potential patients with pancreatitis via a pager system activated in the emergency department (ED) and an electronic notification from the clinical laboratory reporting the medical record number of patients with elevated lipase. The diagnosis of acute pancreatitis was confirmed by two of the three criteria: lipase >3 times the upper limit of normal, characteristic epigastric pain, or cross-sectional imaging consistent with acute pancreatitis. Patients who were transferred from another hospital, left against medical advice, or had clinical or radiographic evidence of chronic pancreatitis were excluded. While the feasibility of determining PASS at the Los Angeles County Hospital/University of Southern California was described by Wu et al., this initial publication did not report the clinical outcomes from our center [8]. Thus this represents the initial assessment of the association of PASS with clinical outcomes in this cohort and the first attempt to validate PASS using a wide range of pancreatitis outcomes, including intensive care unit admission, time to tolerance of oral nutrition, and 30-day readmission in any cohort.
Disease parameters
We defined the patient’s first hospitalization between March 2015 and March 2017 as the index hospitalization and subsequent representations between March 2015 and July 2017 as either readmission or presentation to the ED. During the index hospitalization, we recorded 83 clinically relevant variables, including pancreatitis etiology; comorbidities; pancreatitis admission prior to March 2015; detailed alcohol and smoking history; outpatient medication use; Charlson score; body mass index; vital signs; visual analog pain score; and biochemical parameters, including blood urea nitrogen (BUN), creatinine, and hematocrit.
Over the course of the admission, we scored whether patients presented with or developed systemic inflammatory response syndrome (SIRS) following admission; SIRS was defined as two of the four criteria: heart rate >90 beats/min; respiration >20/min, or PaCO2 < 32 mmHg; temperature <36 or >38 °C; or white blood cell count <4000 or >12,000/mm3 [13]. The use of antibiotics, initiation of total parenteral nutrition, and development of local complications was recorded; the latter was defined as pseudocysts, necrotic collections, or walled off pancreatic necrosis [14]. We scored the episode as mild, moderately severe, and severe according to the revised Atlanta classification [14]. Development of organ failure was defined as modified Marshall organ failure score ≥2 [15]. We also captured admission to the intensive care unit (ICU), length of hospitalization, and time to initiation of oral diet. Given clinical benefit, cholecystectomy is performed on the same admission for those hospitalized with gallstone pancreatitis at our center and was also recorded [16].
This prospectively ascertained data set was used by two reviewers who calculated the PASS score at the time of admission, discharge, and every 12 h during the hospitalization. Information on laboratories, narcotic administration, and pain scores needed to calculate PASS score not available in the prospective data set was subsequently identified from the medical record.
Data analysis
We performed two sets of analyses to evaluate the relationship between the PASS score and inpatient as well as post-discharge outcomes in patients with acute pancreatitis.
PASS score and in-hospital outcomes.
We assessed for the relationship between admission PASS score and severity of acute pancreatitis. Disease severity was characterized as mild, moderately severe, and severe based on the revised Atlanta criterion, local complications, admission to the ICU, length of stay, and time to tolerance of oral nutrition. These outcomes were defined in the 'Disease parameters' section. We also studied the relationship between admission PASS and the development of SIRS. Development of SIRS was defined as the manifestation of ≥2 SIRS criterion (see 'Disease parameters' section) in those who did not have SIRS at the time of admission.
We also assessed for the relationship between the PASS score 24 h after admission and these clinical outcomes.
In order to compare tests characteristics among different scoring systems, we also assessed for the association between admission Ranson’s, Glasgow, Panc3, and Harmless Acute Pancreatitis Score (HAPS) scores for this cohort with subsequent development of moderately severe and severe pancreatitis according to the Revised Atlanta Criterion. We also determined the relationship between the admission Glasgow score for patients in our cohort and the requirement for ICU admission and the development of SIRS and local complications.
PASS score and readmission.
An additional aim of the study was to determine the association between discharge PASS score and readmission. Early readmission was defined as an admission to the inpatient unit ≤30 days following discharge from the index hospitalization for persistent symptoms related to the pancreatitis episode, complications of pancreatitis or therapy, or recurrent pancreatitis. Recurrent pancreatitis was defined as the development of characteristic pain and elevation of lipase ≥3 times the upper limit of normal in those whose symptoms resolved and lipase normalized following discharge from the index hospitalization.
We also studied the relationship between discharge PASS score and early evaluation in the ED and late readmission. The former was defined as ED evaluation in ≤30 days for pancreatitis symptoms, complications, treatment complications, and recurrent pancreatitis. Late readmission was defined as readmission to the inpatient unit >30 days for symptoms of pancreatitis, complications of the index episode and its treatment, or recurrent pancreatitis.
Statistical analysis
Categorical variables were reported as proportions and continuous parameters as mean (standard deviation/confidence interval (CI)) if normally distributed and medians (interquartile range (IQR)) if non-normal. Logistic regression was used to assess the relationship between PASS score and the clinical outcomes of moderately severe/severe pancreatitis, local complications, ICU admission, SIRS development as well as early and late readmission and ER presentation. Receiver operator characteristic (ROC) analysis was used to define the optimal cutoff point for admission PASS score to predict moderately severe and severe pancreatitis and discharge PASS score to predict early readmission. We assessed for the bivariate relationship between other clinical parameters and the outcomes (i.e., moderately severe or severe pancreatitis, early readmission) using χ2, Mann-Whitney U, and linear regression. We then introduced potential confounders as well as several a priori variables (sex, gender, age, pancreatitis origin) into logistic regression models to define the relationship between PASS score and the clinical outcomes. Locally weighted smoothing was used to verify linearity of continuous variables in these models.
To adjust for loss to follow-up, we performed a sensitivity analysis by introducing follow-up ≤30 days as a covariate in the multivariate analysis for early readmission. Because there were a large number of discharge PASS scores of zero, as an additional sensitivity analysis we re-ran the model assessing the relationship between PASS and early readmission after excluding a discharge PASS score of zero.
To study the association between length of hospitalization and admission PASS score, we used multivariate linear regression with logarithmic transformation of the output variable to adjust for the skewed outcome. The same approach was used to assess for the association between tolerance of oral nutrition and admission PASS score.
ROC/area under the ROC curve (AUC) analysis was used to assess for the relationship between admission Panc3, HAPS, Glasgow, and Ranson’s scores with moderately severe and severe pancreatitis when the algorithms were treated as continuous scales. Published cutoff values were used to compare their performance characteristics as discrete variables [9, 17, 18]. The same approach was used to study the association between admission Glasgow scores and organ failure, SIRS development, and local complications. All analyses were performed using SAS 9.4 (Cary, NC) and STATA. 14.2 (College Station, TX).
RESULTS
Patients and outcomes
Between March 2015 and March 2017, 439 unique patients were admitted to the Los Angeles County Hospital for acute pancreatitis. The most frequent etiology was gallstones, 81% were Hispanic, and 53% male (Table 1). The median follow-up was 4 (range 0–44) months and 3 patients (1%) died during the index hospitalization.
Table 1.
Characteristics of population
| Total population, N (%) |
Moderately severe or severe pancreatitis, N (%) |
Early (<30 days) readmitted, N (%) |
|
|---|---|---|---|
| Total | 439 | 76 | 37 |
| Female gender | 207 (47.1) | 29 (38.2) | 15 (40.5) |
| Hispanic ethnicity | 353 (80.8) | 58 (76.3) | 26 (70.3) |
| >20 alcoholic drinks/week | 74 (16.9) | 14 (18.4) | 6 (16.2) |
| >10 pack years tobacco | 31 (7.7) | 8 (10.5) | 4 (11.1) |
| Altered mental status | 18 (4.1) | 8 (10.5) | 1 (2.7) |
| Diabetes mellitus | 115 (26.2) | 27 (35.5) | 5 (13.5) |
| Prior acute pancreatitis | 69 (15.7) | 12 (15.8) | 10 (27.0) |
| SIRS on admission | 107 (24.4) | 38 (50) | 13 (35.1) |
| Alcohol | 109 (24.8) | 20 (26.3) | 11 (29.7) |
| Gallstone | 203 (46.2) | 30 (39.4) | 16 (43.2) |
| Other | 127 (28.9) | 26 (34.2) | 10 (27.3) |
| Comorbidities | 167 (38.0) | 37 (48.7) | 10 (27.0) |
| Obesity (BMI ≥ 30 kg m2) | 149 (33.9) | 30 (39.5) | 9 (24.3) |
| TPN | 8 (1.8) | 4 (5.3) | 3 (8.1) |
| Antibiotics | 149 (33.9) | 43 (56.4) | 12 (32.4) |
|
All patients, Mean (SD) |
Severe or moderately severe, Mean (SD) |
Readmitted within 30 days, Mean (SD) |
|
| Age | 41.9 (15.3) | 47.2 (2.0) | 41.9 (15.3) |
| Admission BUN | 15.5 (10.3) | 22.7 (17.8) | 15.4 (9.1) |
| Admission hematocrit | 40.8 (6.1) | 42.3 (7.2) | 39.7 (7.2) |
| Discharge BUN | 11.7 (9.1) | 14.7 (15.5) | 12.1 (8.2) |
| Discharge hematocrit | 37.8 (7.5) | 38.6 (8.2) | 37.4 (6.6) |
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Charlson score | 0(0–2) | 1 (0–3) | 0 (0–2) |
Moderately severe or severe pancreatitis developed in 76 (17%) of patients. Forty-nine (11%) patients developed local complications, including necrosis, pseudocysts, and walled off pancreatic necrosis. One hundred and seven (24%) patients presented with SIRS and an additional 116 (26%) developed SIRS following admission. The median length of hospitalization was 4 [2–7] days and ICU admission was necessary in 65 (15%) patients. The median time from admission to tolerance of oral nutrition was 3 [2–7] days.
Following their index hospitalization, 37 (9%) patients were readmitted within 30 days for pancreatitis. Of those, smoldering symptoms prompted readmission in 67%, local complications of pancreatitis or therapy in 25%, and recurrent pancreatitis in 8%. An additional 22 (5%) patients were evaluated in the ED within 30 days for smoldering pancreatitis symptoms (90%), complications of therapy (5%), or recurrent pancreatitis (5%). There were 25 (6%) patients who were readmitted for pancreatitis-related problems after 30 days. Patients with late readmission presented for recurrent pancreatitis in 56%, smoldering symptoms in 32%, and complications of pancreatitis or therapy in 12%.
PASS score and in-hospital patient outcomes
Moderately severe and severe pancreatitis.
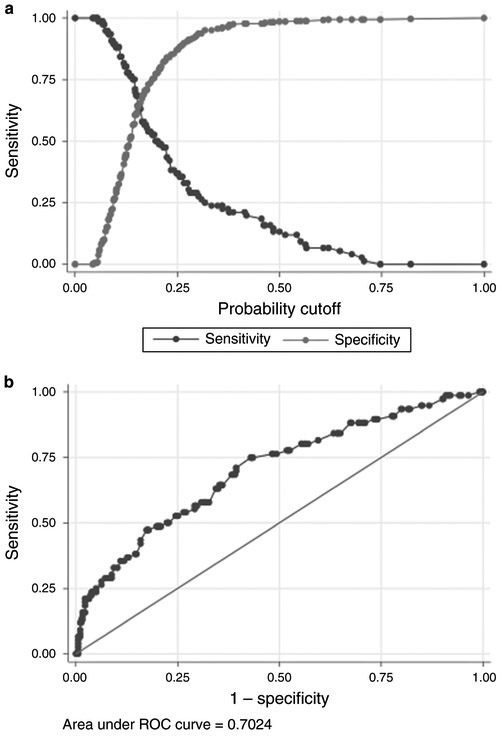
The overall median admission PASS score was 130 (IQR 95–174). We found that admission PASS score was strongly associated with moderate and severe pancreatitis (Fig. 2). The median admission PASS score among those with moderately severe and severe pancreatitis was 168 (133–222) compared to 125 (IQR 90–163) for those with mild disease. We observed a monotonic increase in the development of moderately severe and severe pancreatitis with higher admission PASS score (Table 2). ROC analysis demonstrated that an admission PASS cutoff point of 140 had optimal predictive value (Fig. 2) for moderately severe and severe pancreatitis with a sensitivity and specificity of 65% and an AUC of 0.7. In univariate analysis, BUN > 20, comorbidities, altered mental status, and SIRS all predicted moderately severe or severe pancreatitis (Table 3) though the latter was collinear with PASS score. Moderate or severe pancreatitis was not associated with other factors, including hematocrit >44, body mass index (BMI), tobacco, or heavy alcohol. The a priori variables age, gender, ethnicity, and origin of pancreatitis also were not associated with moderately severe and severe pancreatitis. After controlling for these factors, PASS >140 remained a significant predictor of moderately severe or severe pancreatitis, odds ratio (OR) 3.5 (95% CI 2.0–6.3). PASS scores at 12, 24, 36, and 48 h after admission predicted moderately severe and severe pancreatitis (p < 0.01) and the PASS scores were higher for patients with moderately severe and severe disease throughout the hospitalization (Fig. 3).
Fig. 2 a, b.
Receiver operator characteristic analysis for moderately severe and severe pancreatitis and admission PASS score
Table 2.
Admission PASS and inpatient outcome
| Admission PASS score |
N | Moderately severe or severe pancreatitis (%)a |
ICU admission (%) |
Local complications (%) |
Development of SIRS after admission (%) |
SIRS at presentation and after admission (%) |
|---|---|---|---|---|---|---|
| 0–50 | 30 | 3.3 | 0 | 0 | 3.3 | 3.3 |
| 50–100 | 96 | 8.3 | 5.2 | 5.2 | 12.5 | 21.9 |
| 100–150 | 162 | 14.8 | 9.9 | 9.9 | 26.5 | 46.3 |
| 150–200 | 89 | 20.2 | 27.0 | 7.9 | 46.1 | 80.9 |
| 200–250 | 38 | 23.7 | 26.3 | 23.7 | 26.3 | 84.2 |
| >250 | 24 | 66.8 | 41.7 | 50 | 37.5 | 91.7 |
Percentage of patients in each PASS score range who developed the clinical outcome
Table 3.
Predictors of moderately severe and severe pancreatitis
| Univariate OR (95% CI) |
Multivariate OR (95% CI) |
|
|---|---|---|
| Admission PASS > 140 | 3.2 (1.9, 5.4) | 3.5 (2.0, 6.3) |
| Altered mental status | 4.2 (1.6, 10.9) | 5.3 (1.7, 16.7) |
| Comorbidities | 1.7 (1.0, 2.8) | 1.2 (0.7, 2.2) |
| Admission BUN > 20 | 4.0 (2.3, 7.0) | 3.2 (1.7, 5.9) |
| Age | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) |
| Hispanic | 0.7 (0.4, 1.3) | 0.7 (0.4, 1.5) |
| Female | 0.6 (0.4, 1.1) | 0.8 (0.4, 1.5) |
| Etiology | ||
| Alcohol | Baseline 1.0 | Baseline 1.0 |
| Gallstone | 0.8 (0.4, 1.4) | 0.7 (0.5, 2.4) |
| Other | 1.1 (0.6, 2.2) | 0.5 (0.6, 2.9) |
Fig. 3.
PASS score by pancreatitis severity over time
ICU admission, local complications, SIRS development.
We found that there was a concordance of admission PASS score >140 with ICU admission (OR 4.9 [2.5, 9.4]) and local complications (OR 3.0 [1.6, 5.7]) (Table 2, Appendix 1). Admission PASS score >140 was also associated with SIRS development in those who did not have SIRS at admission (OR 2.9 [1.8, 4.5]) (Table 2, Appendix 1). This analysis was adjusted for a priori etiology and demographic factors as well as confounders that included hematocrit >44 for SIRS development (OR 1.7 [1.2, 3.3]) and hematocrit >44 (OR 2.4 [1.2, 4.6]), BUN > 20 (OR 2.4 [1.2, 4.8]), comorbidities (OR 2.3 [1.2, 4.4]), and altered mental status (8.9 [2.7, 29.6]) for ICU admission (Appendix 1). The likelihood of ICU admission, local complications, and development of SIRS increased with the level of the admission PASS score (Table 1).
Length of stay and time to oral nutrition.
The average length of hospitalization was also 1.5 (1.3–1.7) days longer in patients with PASS score >140 after controlling for age, etiology of pancreatitis, ethnicity, and gender. The tolerance of oral nutrition was also delayed by an average of 1.3 (1.2, 1.5) days in those with PASS score >140 after adjustment for these same covariates.
Comparison of PASS with established predictive scoring systems in cohort.
The performance of admission PASS (AUC 0.71; OR 3.2 [1.9–5.4]) was comparable to admission Glasgow (AUC 0.73; OR 4.1 [2.5–6.9]) and Ranson’s (AUC 0.63; OR 2.2 [1.2, 4.0]) and somewhat better than Panc3 and HAPS (Table 4). Additionally, admission PASS was comparable to Glasgow for prediction of ICU admission (both AUC 0.74) but performed somewhat better for prediction of SIRS development (AUC 0.66 vs. 0.56) and local complications (AUC 0.71 vs. 0.60) (Appendix 2).
Table 4.
Prediction of moderately severe/severe pancreatitis by established scoring systems and PASS
| AUC | Na | Cutoffb | OR | 95% CI | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Admission PASS | 0.71 | 439 | 140 | 3.2 | 1.9–5.4 | 0.44 | 0.84 | 0.37 | 0.87 |
| Glasgow | 0.73 | 432 | 2 | 4.1 | 2.5–6.9 | 0.32 | 0.92 | 0.47 | 0.86 |
| Admission Ranson’s | 0.63 | 436 | 2 | 2.2 | 1.2–4.0 | 0.05 | 0.99 | 0.50 | 0.83 |
| HAPS | 0.54 | 438 | 1 | 0.6 | 0.2–1.9 | 0.56 | 0.50 | 0.20 | 0.84 |
| Panc3 | 0.57 | 427 | 1 | 1.5 | 1.1–2.2 | 0.23 | 0.92 | 0.37 | 0.85 |
PASS score and outcomes following discharge
Readmission following index hospitalization.
Among the surviving patients, the discharge PASS score ranged from 0 to 373 with a median of 40 (IQR 0–73.3); 125 patients had a PASS score of 0. The median discharge PASS score for patients readmitted <30 days (early readmission) was 75 (IQR 50–105) vs. 39 (IQR 0–68) (p < 0.001) for patients not readmitted. The median discharge PASS score for patients presenting to the ED within 30 days for pancreatitis symptoms was 65 (IQR 50–104) vs. 40 (IQR 0–7)) (p < 0.001) for those who did not.
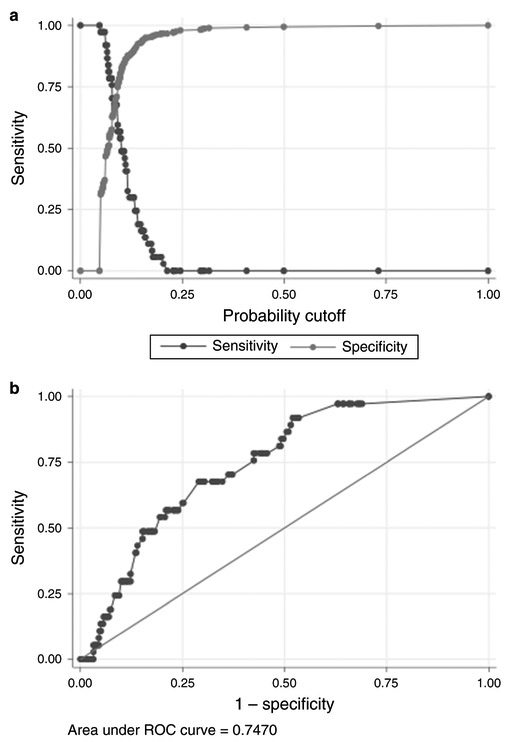
Discharge PASS score was significantly correlated with early readmission (Table 5) (p < 0.001). ROC analysis revealed that a discharge PASS score of >60 optimized performance characteristics (Fig. 4) with a sensitivity of 68%, specificity of 71%, and AUC of 0.75. When adding in potential covariates, we found that the use of total parenteral nutrition during hospitalization and prior pancreatitis also predicted readmission though the former was collinear with discharge PASS score. The severity of pancreatitis, development of local complications, ICU admission, Charlson score, obesity (BMI > 30), heavy alcohol use (>20 drinks/week), biochemical profile, and length of hospitalization did not predict readmission. Same admission cholecystectomy also did not predict early readmission, OR 0.6 (0.2, 1.7), but was included in the multivariate analysis given clinical importance. The a priori variables age, gender, ethnicity, origin of pancreatitis, and same admission cholecystectomy were not associated with pancreatitis readmission; after adjusting for these factors, discharge PASS score >60 remained a significant predictor of early readmission (OR 5.0 [2.4, 10.7]), showing strength of this cutoff (Table 6).
Table 5.
Discharge PASS score and readmission for pancreatitis
| Discharge PASS score |
N | Readmitted ≤30 days (%)a |
Readmitted or ED visit <30 days (%) |
Readmitted > 30 days (%) |
|---|---|---|---|---|
| 0 | 125 | 0.8 | 0.8 | 8.8 |
| 0–25 | 64 | 3.1 | 4.7 | 3.1 |
| 25–50 | 73 | 11.0 | 20.6 | 4.1 |
| 50–75 | 84 | 9.5 | 17.9 | 6.0 |
| 75–100 | 37 | 18.9 | 24.3 | 8.3 |
| 100–125 | 28 | 21.4 | 32.1 | 3.6 |
| 125–150 | 11 | 36.4 | 36.4 | 0 |
| >150 | 14 | 7.1 | 21.4 | 0 |
Percentage of patients in each PASS score range who developed the clinical outcome
Fig. 4 a, b.
Receiver operator characteristic analysis for early readmission and discharge PASS score
Table 6.
Predictors of early readmission
| Univariate OR (95% CI) |
Multivariate OR (95% CI) |
|
|---|---|---|
| Discharge PASS > 60 | 4.7 (2.3, 9.8) | 5.0 (2.4, 10.7) |
| Prior acute pancreatitis | 2.1 (1.0, 4.7) | 1.7 (0.7, 4.2) |
| Age | 1.0 (1.0, 1.0) | 1.0 (1.0,1.0) |
| Hispanic | 0.5 (0.3, 1.1) | 0.6 (0.3, 1.3) |
| Female | 0.7 (0.4, 1.5) | 0.7 (0.3, 1.6) |
| Same admission cholecystectomy | 0.6 (0.2, 1.7) | 0.8 (0.3, 2.5) |
| Etiology | ||
| Alcohol | Baseline 1.0 | Baseline 1.0 |
| Gallstone | 0.8 (0.3, 1.7) | 1.0 (0.4, 2.9) |
| Other | 0.8 (0.3, 1.9) | 0.8 (0.3, 2.4) |
Early (≤30 days) ED presentation and late readmission.
Discharge PASS score >60 was also correlated with ED presentation within <30 days of discharge for pancreatitis symptoms (OR 3.2 [1.3, 7.7]) (Appendix 3); severity of pancreatitis, local complications, and admission clinical parameters did not. There was no significant relationship between discharge PASS score and late readmission whether treated as a continuous (p = 0.8) or categorical (PASS > 60) variable (Appendix 3). Heavy alcohol use was associated with late readmission (OR 4.1 [1.8, 9.4] as was alcoholic pancreatitis relative to gallstone pancreatitis (Appendix 3).
Sensitivity analyses.
When follow-up of ≤30 days was included as a cofactor in the multivariate analysis, the relationship between discharge PASS score >60 and early readmission remained significant (OR 5.1 [95% CI 2.4–10.7]). Only 1 of the 125 patients with a discharge PASS score of zero was readmitted within 30 days. We repeated the analysis including ROC after excluding all patients with a score of zero and PASS score remained a significant predictor of early readmission (p < 0.01).
DISCUSSION
We have performed a prospective validation of a newly developed disease-activity instrument in acute pancreatitis. Specifically, in this well-characterized cohort of patients with acute pancreatitis the PASS score was strongly associated with sentinel clinical events that occur during hospitalization as well as following discharge. A PASS score >140 at admission was associated with the development of moderately severe and severe pancreatitis, SIRS, and local complications as well as prolonged length of stay and delayed resumption of oral nutrition. Meanwhile, a PASS score at discharge >60 was strongly correlated with readmission and ED presentation for smoldering symptoms and complications of pancreatitis. Promising performance at multiple points in the course of acute pancreatitis suggest its role as a true measurement of disease activity. This has important implications for its role in clinical care as well as prospective intervention trials for new treatments for patients with acute pancreatitis.
The development of pharmacologic and other therapy for acute pancreatitis requires the demonstration of quantifiable improvement in clinical outcomes [19]. Given its correlation with multiple aspects of the clinical course of pancreatitis, PASS represents a promising tool to gauge responses to therapy as well as to assess for the resolution of disease. Similarly, it may potentially be used as a system to determine eligibility; i.e., predicted severe could be defined as a PASS > 140 or another cutoff.
Admission and subsequent PASS score levels correlated with the development of established clinical outcomes in acute pancreatitis. Specifically, an admission score >140 was associated with substantially increased risk of transient and persistent organ failure (moderately severe and severe pancreatitis) as well as other clinical outcomes with high face validity including the development of local complications. Elevated admission PASS score was also linked to additional parameters such as increased length of hospitalization and need for intensive care unit admission, both of which have tangible financial and clinical implications.
As a measure of disease activity, the PASS score at discharge was linked to post-hospital outcome, in particular, risk of 30-day readmission. The 30-day readmission rate has received widespread attention as a correlate with adverse outcomes and death in congestive heart failure, coronary artery disease, and medical illnesses in general [20, 21]. It is a core quality metric of the Affordable Care Act and Centers for Medicare and Medicaid Services [22]. It is also the strongest predictor of death at 1 year following acute pancreatitis hospitalization [11].
Interestingly, discharge PASS score was associated with early readmission, whereas other parameters such as disease severity, length of stay, ICU admission, and local complications were not. This is consistent with previous literature that has linked specific components of the PASS including intolerance of oral nutrition, pain, abnormal vital signs, and high opiate requirements with increased risk of early readmission [23, 24]. Contrary to findings with respect to early readmission, the discharge PASS score was not associated with late readmission. The likely explanation is that late as opposed to early readmission was driven by recurrent episodes of acute pancreatitis in those who had recovered at the end of the index hospitalization. Our findings provide a quantitative correlate with prior reports that smoldering symptoms dominate early while recurrent pancreatitis episodes, particularly among those with alcoholic disease, account for late readmission [23, 25].
The PASS is distinct from prior prognostic scores that have focused on tools to predict outcomes at specific time points in the course of pancreatitis. A prior instrument to predict early readmission awarded points for ongoing symptoms, necrosis, antibiotic use, and pain at discharge [26]. In the validation cohort, which defined readmission as re-hospitalization and presentation to the ED, the score had a 71% sensitivity and 87% specificity for readmission [26]. Nevertheless, in a subsequent cohort at another academic medical center the specificity decreased to 56% [23]. At least nine scoring systems have been developed to predict severe pancreatitis and other adverse outcomes [9]. Laboratories, including BUN and creatinine, are also correlated with adverse outcomes [27, 28]. Nevertheless, work by Mounzer et al. comparing these individual algorithms found that they were equivalent, and while combinations could yield superior results, the requisite complexity made them impractical for clinical use [9]. Though not directly comparable to the study by Mounzer et al. given that our severity outcome was moderately severe and severe pancreatitis rather than persistent organ failure, our results demonstrated a similar trend, namely, that Glasgow (AUC of 0.73) performed slightly better than Ranson’s criterion (AUC 0.63). PASS was comparable to both scoring systems (AUC 0.71). Nevertheless, admission PASS performed somewhat better than Glasgow when compared across a wider range of outcomes. Furthermore, PASS has the advantage of being correlated with various pancreatitis outcomes at progressive stages of the disease and the ability to provide ongoing assessment of disease activity.
In addition, the development of the scoring system through a consensus-based process helps to ensure that routinely used clinical parameters predominated in the PASS score. The importance of qualitative metrics including pain and the ability to tolerate oral nutrition were recognized in its development and included in the scoring system. We hypothesize that inclusion of elements that reflect patient symptoms in addition to biochemical parameters underlies the score’s ability to predict length of hospitalization and early readmission. Future studies of PASS will be improved by correlating the score with additional quality-of-life measures.
The strength of our design is that cases were prospectively identified, carefully verified, and comprehensively characterized. This provided a much greater richness to the data set compared to those generated by interrogation of administrative data using International Classification of Disease or Current Procedural Terminology codes. Thus we were able to test and control for numerous potential confounders, including quantitative extent of alcohol use, BMI, and personal history of pancreatitis. Additionally, very few patients were transferred to the Los Angeles County Hospital whose primary mission is to provide inpatients' services for a million patient community health network. Thus our findings may provide greater generalizability than studies conducted exclusively at tertiary centers where most patients are referred for higher levels of care. Indeed, the somewhat lesser performance characteristics of HAPS and Panc3 relative to PASS in our cohort may reflect their development and validation at referral centers rather than a large community medical center.
There were several limitations to the present study. We did not contact patients to assess for readmission at other institutions, which may in part account for our lower rate of readmission (9%) than prior reports, 15–16% [11, 23, 24]. Nevertheless, our sensitivity analysis controlling for follow-up of <30 days did not materially alter our results. Additionally, our median follow-up was only 4 months. Thus, while we have detailed information regarding hospitalization and the immediate period following discharge, we were unable to assess correlation between PASS score and critical long-term outcomes, including chronic pancreatitis. Our population was primarily of Hispanic ethnicity, which may not reflect patients at other centers. Nevertheless, this group represents a rapidly increasing proportion of the United States population and has a diverse European, Amerindian, and African genetic admixture [29]. The discharge PASS cutoff of 60 derived to predict early readmission was used to assess other discharge outcomes and the admission cutoff of 140 derived to forecast moderately severe and severe pancreatitis was used for other inpatient outcomes, such as length of stay. Our rationale was that simplification tends to favor utilization of clinical tools. Finally, the AUCs for the various clinical outcomes was in the range of 0.7–0.8; nevertheless, this is similar to what has been observed for scores designed to predict specific outcomes at predefined time points [9, 10].
In summary, in this large prospective cohort of patients hospitalized for acute pancreatitis the PASS performed well at admission as well as at discharge in identifying patients at increased risk for multiple adverse in-hospital outcomes as well as early readmission, respectively. It appears to be a promising system to quantitatively gauge disease activity in patients with acute pancreatitis.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
The Pancreatitis Activity Scoring System derived by a modified Delphi process can be used in clinical practice and measures a variety of clinically relevant parameters.
WHAT IS NEW HERE
-
✓
Discharge PASS score is correlated with early (<30 days) hospital readmission and admission PASS score with severe pancreatitis, prolonged hospitalization, SIRS development, and local complications.
-
✓
The association of PASS with important clinical outcomes at different time points suggests that it is a valid measure of disease activity.
Acknowledgments
Financial support: This publication was supported by NIH/NCRR SC CTSI Grant Number UL1TR000130. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST
Potential competing interests: The authors declare that they have no conflict of interest.
Guarantor of the article: James Buxbaum.
SUPPLEMENTARY MATERIAL accompanies this paper athttps://doi.org/10.1038/s41395-018-0048-1
REFERENCES
- 1.Vege SS, Atwal T, Bi Y, et al. Pentoxifylline treatment in severe acute pancreatitis: a pilot, double-blind, placebo-controlled, randomized trial. Gastroenterology. 2015;149:318–20 e3. [DOI] [PubMed] [Google Scholar]
- 2.Isenmann R, Runzi M, Kron M, et al. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997–1004. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut 2001;48:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker OJ, van Brunschot S, van Santvoort HC, et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med 2014;371:1983–93. [DOI] [PubMed] [Google Scholar]
- 5.Buxbaum JL, Quezada M, Da B, et al. Early aggressive hydration hastens clinical improvement in mild acute pancreatitis. Am J Gastroenterol 2017;112:797–803. [DOI] [PubMed] [Google Scholar]
- 6.Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017;376:1551–60. [DOI] [PubMed] [Google Scholar]
- 7.Monteleone G, Neurath MF, Ardizzone S, et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med 2015;372:1104–13. [DOI] [PubMed] [Google Scholar]
- 8.Wu BU, Batech M, Quezada M, et al. Dynamic measurement of disease activity in acute pancreatitis: the pancreatitis activity scoring system. Am J Gastroenterol 2017;112:1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–82. quize15-6 [DOI] [PubMed] [Google Scholar]
- 10.Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010;105:435–41. quiz 442 [DOI] [PubMed] [Google Scholar]
- 11.Lee PJ, Bhatt A, Lopez R, et al. Thirty-day readmission predicts 1-year mortality in acute pancreatitis. Pancreas. 2016;45:561–4. [DOI] [PubMed] [Google Scholar]
- 12.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360: 1418–28. [DOI] [PubMed] [Google Scholar]
- 13.Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol 2009;7:1247–51. [DOI] [PubMed] [Google Scholar]
- 14.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995;23:1638–52. [DOI] [PubMed] [Google Scholar]
- 16.da Costa DW, Bouwense SA, Schepers NJ, et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet. 2015;386:1261–8. [DOI] [PubMed] [Google Scholar]
- 17.Lankisch PG, Weber-Dany B, Hebel K, et al. The harmless acute pancreatitis score: a clinical algorithm for rapid initial stratification of nonsevere disease. Clin Gastroenterol Hepatol 2009;7:702–5. quiz 607 [DOI] [PubMed] [Google Scholar]
- 18.Brown A, James-Stevenson T, Dyson T, et al. The panc 3 score: a rapid and accurate test for predicting severity on presentation in acute pancreatitis. J Clin Gastroenterol 2007;41:855–8. [DOI] [PubMed] [Google Scholar]
- 19.Afghani E, Pandol SJ, Shimosegawa T, et al. Acute pancreatitis-progress and challenges: a report on an International Symposium. Pancreas. 2015;44:1195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lum HD, Studenski SA, Degenholtz HB, et al. Early hospital readmission is a predictor of one-year mortality in community-dwelling older Medicare beneficiaries. J Gen Intern Med 2012;27:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuckerman RB, Sheingold SH, Orav EJ, et al. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med 2016;374:1543–51. [DOI] [PubMed] [Google Scholar]
- 23.Vipperla K, Papachristou GI, Easler J, et al. Risk of and factors associated with readmission after a sentinel attack of acute pancreatitis. Clin Gastroenterol Hepatol 2014;12:1911–9. [DOI] [PubMed] [Google Scholar]
- 24.Whitlock TL, Repas K, Tignor A, et al. Early readmission in acute pancreatitis: incidence and risk factors. Am J Gastroenterol 2010;105:2492–7. [DOI] [PubMed] [Google Scholar]
- 25.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol 2012;107:1096–103. [DOI] [PubMed] [Google Scholar]
- 26.Whitlock TL, Tignor A, Webster EM, et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin Gastroenterol Hepatol 2011;9:175–80. quize18 [DOI] [PubMed] [Google Scholar]
- 27.Wu BU, Bakker OJ, Papachristou GI, et al. Blood urea nitrogen in the early assessment of acute pancreatitis: an international validation study. Arch Intern Med 2011;171:669–76. [DOI] [PubMed] [Google Scholar]
- 28.Muddana V, Whitcomb DC, Khalid A, et al. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol 2009;104:164–70. [DOI] [PubMed] [Google Scholar]
- 29.Brown LA, Sofer T, Stilp AM, et al. Admixture mapping identifies an Amerindian ancestry locus associated with albuminuria in Hispanics in the United States. J Am Soc Nephrol 2017;28:2211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.