Abstract
The association between consumption of added or concentrated sugars and prostate cancer risk is unclear. We examined the association between concentrated sugars in beverages and desserts and prostate cancer risk among 22,720 men in the usual care arm of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, enrolled during 1993-2001. After a median follow-up of 9 years, 1996 men were diagnosed with prostate cancer. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) for prostate cancer risk and 95% confidence intervals (CIs), adjusting for potential confounding factors. Increased consumption of sugars from sugar-sweetened beverages was associated with increased risk of prostate cancer for men in the highest quartile of sugar consumption (HR: 1.21, 95% CI: 1.06, 1.39), and there was a linear trend (P<0.01). There were no linear associations between prostate cancer risk and consumption of sugars from fruit juices or dessert foods.
In conclusion, in this prospective substudy within the PLCO trial, consumption of sugars from sugar-sweetened beverages was associated with increased risk of prostate cancer among men receiving standard medical care. Our study suggests that limiting intake of sugars from beverages may be important in the prevention of prostate cancer.
Keywords: added sugars, proportional hazards regression, prostate cancer risk, prospective cohort
INTRODUCTION
Consumption of added sugars in America has increased considerably over time1. Dietary intake of caloric sweeteners including sucrose and high fructose corn syrup increased by nearly 40% between 1950-59 and 20002. Consumption of added sugars from beverages, particularly, has increased. Between 1977 and 2003 calories from added sugars in beverages increased by 90 kcal/day and those from added sugars in foods by 23 kcal/day in the U.S.3. In spite of a decline in consumption of absolute calories of added sugars, percentage of total energy intake from added sugars has remained high3. In light of the growing body of evidence highlighting unfavorable health effects of added sugars, the 2015 Dietary Guidelines Advisory Committee recommended that Americans limit sugar to no more than 10 percent of daily calories4. Intake of high fructose corn syrup or added sugars has been associated with metabolic syndrome, characterized by elevated blood pressure, triglycerides, LDL cholesterol, uric acid, and inflammation5–7. Not surprisingly, there is some evidence that dietary added sugars are associated with cancer, although the evidence is limited. Case-control and prospective studies have shown an association of consumption of sugary foods8, 9, and particularly beverages10, 11, with increased risk of pancreatic cancer, which may be mediated in part through induction of transketolase12. Additionally, sweet foods and beverages were shown to increase breast cancer risk by 27%13. Recently, we reported an association between sugary beverages (fruit juices and sugar- sweetened) and reduced survival among head and neck cancer patients14.
Little is known about the associations of dietary added and concentrated sugars with the development of prostate cancer, although it is understood that lifestyle plays an important role in prostate cancer prevention15. Because of the putative link between chronic inflammation and prostate cancer, dietary items that are potentially pro-inflammatory deserve particular attention. It has been shown, for example that heterocyclic amines promote the development of cancer and induce accumulation of inflammatory cells (lymphocytes and macrophages) in the prostate16, that processed meat or dietary fat from meat is associated with increased prostate cancer risk17, 18 and there is some evidence that dairy consumption may be associated with increased prostate cancer risk19. It is possible that the increased fructose and consequently triglycerides in items with concentrated sugars promotes an inflammatory response that supports DNA damage and genetic changes leading to neoplastic lesions of the prostate6, 20–22. We hypothesized that consumption of sugar-dense items, or items with concentrated sugars lacking the phytonutrients and fiber found in plant-based foods is particularly problematic, having a more detrimental impact on blood sugar, and ultimately promoting inflammation and prostate cancer growth.
The goal of the current study was to examine the association of concentrated sugars with prostate cancer risk. The term concentrated sugars was defined as sugars (in grams) from sugar- sweetened beverages and fruit juices as well sugars in refined and processed desserts, constituting at least 30% of total calories, with prostate cancer risk. Thus, this included added sugars in beverages and dessert foods, as well as natural sugars in fruit juices, which are naturally present in high amounts. These associations were evaluated in men receiving usual medical care in the prospective, population-based Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial.
METHODS
Study Population
This study utilized data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial, a large, prospective, randomized, multi-site study (Birmingham AL, Denver CO, Detroit MI, Honolulu HI, Marshfield WI, Minneapolis MN, Pittsburgh PA, Salt Lake City UT, St. Louis MO, and Washington DC) designed initially to examine the effects of cancer screening on cancer mortality23. Briefly, 10 screening centers across the U.S. enrolled men ages 55-74 to an intervention (screening) or control arm between 1993 and 2001. These men were recruited from the general population in the geographic area of the screening centers. A total of 38,343 men were randomized to the control arm, where they received usual medical care from their health care providers, unlike men in the screening arm who received digital rectal exams (DREs) and annual blood draws for prostate specific antigen (PSA). The follow up rate was 99.5% (Figure 1).
Figure 1.
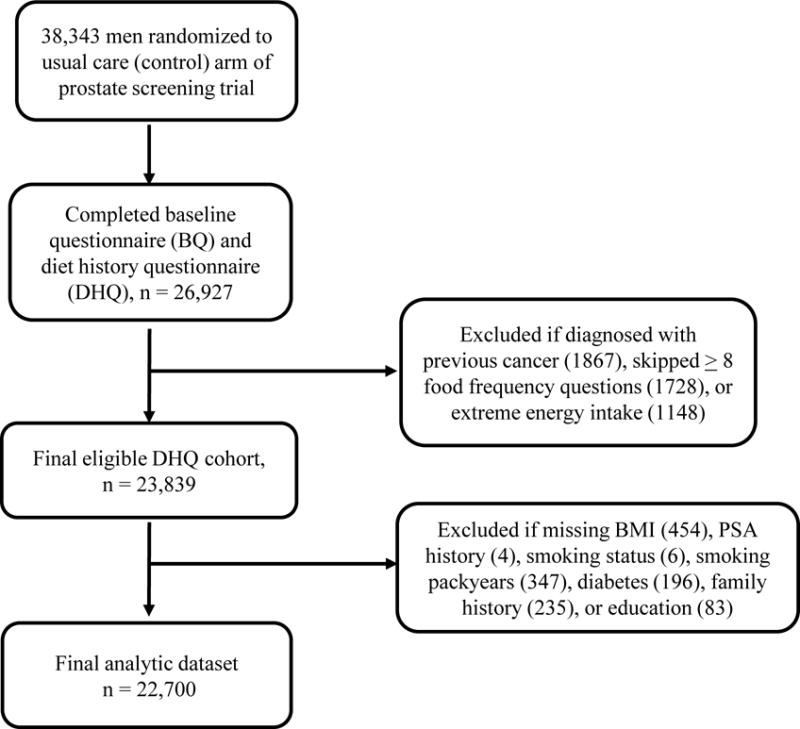
Study design and flowchart of participant selection.
Annual study update questionnaires were used to ascertain prostate cancer incidence and were sent to all study participants. For men reporting a prostate cancer diagnosis, or men with abnormal test results from screening, medical records were obtained and used to confirm the diagnosis, clinical stage and grade. In the current study, cancers of Gleason <7 were defined as low grade, and cancers of Gleason ≥ 7 were defined as high grade.
Of the 38,343 men in the usual care arm, 26,927 completed the baseline (BQ) and diet history (DHQ) questionnaires. After excluding participants who were diagnosed with any cancer before the questionnaires (1867), skipped 8 or more food frequency questions (1728), or were in the first or last percentile of caloric intake (< 553 kcal/d and >5619 kcal/d)(1,148), there were 23,839 men in the eligible DHQ cohort. Additional exclusion of men with missing data for smoking status, pack-years of smoking, education, family history, and diabetes history, which was found to be related to prostate cancer risk in this cohort24, resulted in an eligible cohort containing 22,720 men. Median follow-up for these men was 9 years (183,430 person-years) (Figure 1).
The study was reviewed and approved by the National Cancer Institute (NCI) institutional review board and screening centers (Clinicaltrials.gov identifier: NCT00002540). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data Collection
PLCO study participants completed a baseline questionnaire with information on demographics, medical history, and smoking history. Height and weight were measured at the randomization clinic visit and body mass index (BMI) was computed as weight (kg)/height (m)2. The DHQ, a food frequency questionnaire (FFQ) developed by the Risk Factor Monitoring and Methods Branch of the NCI, was introduced in December of 1998, five years into the trial. It included 156 questions on frequencies of consumption of various foods and beverages from which daily nutrient intake data were estimated. The food list and nutrient database used with the DHQ are based on national dietary data (USDA’s 1994-96 Continuing Survey of Food Intakes by Individuals [CSFII]. The DHQ has been validated and found to be as good as or superior to two widely used FFQs at the time the PLCO study was conducted25. Participants in the usual care arm randomized before 1998 were offered the DHQ in 1999 or 2000, around the anniversary of randomization, and individuals randomized after December 1998 were offered the DHQ at baseline. The food frequency questionnaires were self-administered and asked about frequency of consumption of desserts, sweetened beverages, fruit drinks, fruit juices, fruit, vegetables, and other items. For the purposes of this analysis, sugars from soft drinks and sodas, milkshakes, punch, and fruit drinks, and sugar or honey added to tea or coffee were summed to generate a composite variable for sugar-sweetened beverages. Sugars from fruit juices included sugars from orange, grapefruit, tomato, and “other” fruit or vegetable juices. Additionally, we considered common desserts determined to have concentrated sugars (comprising at least 30% of total calories per item), to generate a composite variable including sugars from cakes, cookies, pies, pastries, chocolate, candy, pudding, syrups, ice cream, and added sugar or sweet creams. Additionally, the sum of all added sugars was calculated, including sugar added to processed foods or used in baked goods or sodas and other beverages, in addition to sugar added “at the table”. The total amount of sugars in grams was calculated from the sum of fructose, galactose, glucose, lactose, maltose, and sucrose using DietCalc Software developed by the National Cancer Institute. The sugar variables were generated based on the Nutrition Data Systems for Research – Nutritional Analysis Software developed at the University of Minnesota.
Statistical Analysis
The main categories of added sugar considered for analyses were sugar-sweetened beverages, fruit juices, and desserts. Additionally, composite variables representing the sum of these three variables, as well as intake of all added sugars from the diet were generated. The association of daily consumption of concentrated sugars with prostate cancer risk was analyzed using the quartile distribution for sugar consumption for the final eligible DHQ cohort. Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Sugar consumption was additionally modeled linearly, as a continuous variable, representing a 10-unit increment in dietary intakes. Follow-up time was calculated as the interval between days from completion of the DHQ to prostate cancer diagnosis, death, or September 30, 2009, depending on which came first. Models were adjusted for study center, age, race (White vs. non-White), education (less than high school, high school graduate, post high school/some college, college graduate or more), cigarette status (never, current, former), pack- years of smoking, current BMI (at baseline), previous history of diabetes (yes/no), family history of prostate cancer (yes/no), number of prostate specific antigen (PSA) screens over the previous three years (none, once, more than once, unknown), and energy (kcal/day). These variables were included as they possibly confound the relationship between sugar consumption and prostate cancer risk, or were previously found to be associated with prostate cancer risk. Other variables considered were red and processed meat (g/day), fruit (servings/day), and vegetables (servings/day), but were not included in final models as they were found to be noninfluential on results. P-value for trend was calculated treating the exposure of interest as a continuous variable, based on the Wald statistic.
RESULTS
Baseline characteristics of the eligible study cohort according to intake of concentrated sugars from foods and beverages are shown in Table 1. Differences were noted for previous history of diabetes, with the most individuals with a previous history (47.8%) falling in the lowest quartile of sugar intake. Notable differences were also observed for race, with an increased percentage of Black participants in the highest quartile of intake of concentrated sugars (40.9%), and more Asians in the lowest quartile (40%) relative to the other quartiles. An increased proportion of men with high school or less education (~29%), as well as current smokers (~32%) were in the highest quartile of concentrated sugar consumption. A lower proportion of men in the highest quartile had multiple PSA screens (21.3%). Participants in the highest quartile of concentrated sugar consumption consumed the greatest amounts of sugar from sugar-sweetened beverages (which were the major source of concentrated sugars), fruit juices and desserts, as well as added sugars overall. Caloric intake was notably higher for men in the highest quartile (2550 kcal/day).
Table 1.
Baseline, demographic, and lifestyle characteristics of participants in the control arm of the PLCO study according to intake of concentrated sugars*,†.
| Total | Quartiles (g/day)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 23.47 (n=5680) | 23.47 - 40.20 (n=5680) | 40.21 - 65.93 (n=5678) | ≥65.94 (n=5682) | |||||||
|
| ||||||||||
| n | % | n | % | n | % | n | % | n | % | |
| Family history | 1625 | 7.2 | 359 | 22.1 | 386 | 23.8 | 443 | 27.3 | 437 | 26.9 |
| History of diabetes | 1842 | 8.1 | 880 | 47.8 | 473 | 25.7 | 282 | 15.3 | 207 | 11.2 |
| Ethnicity | ||||||||||
| White | 20633 | 90.8 | 5040 | 24.4 | 5190 | 25.2 | 5251 | 25.5 | 5152 | 25 |
| Black | 589 | 2.6 | 103 | 17.5 | 114 | 19.4 | 131 | 22.2 | 241 | 40.9 |
| Hispanic | 357 | 1.6 | 90 | 25.2 | 76 | 21.3 | 93 | 26.1 | 98 | 27.5 |
| Asian/Pacific Islander | 1089 | 4.8 | 436 | 40 | 286 | 26.3 | 192 | 17.6 | 175 | 16.1 |
| Other | 52 | 0.2 | 11 | 21.2 | 14 | 26.9 | 11 | 21.2 | 16 | 30.8 |
| Education | ||||||||||
| Less than 12 years | 1542 | 6.8 | 394 | 25.6 | 319 | 20.7 | 384 | 24.9 | 445 | 28.9 |
| High school | 4187 | 25.3 | 1005 | 24 | 988 | 23.6 | 990 | 23.6 | 1204 | 28.8 |
| Post-high school | 7392 | 57.9 | 1885 | 25.5 | 1786 | 24.2 | 1807 | 24.5 | 1914 | 25.9 |
| College graduate | 9557 | 42.1 | 2383 | 24.9 | 2577 | 277 | 2484 | 26 | 2113 | 22.1 |
| Smoking | ||||||||||
| Never | 8588 | 37.7 | 1881 | 22 | 2090 | 24.4 | 2232 | 26.1 | 2355 | 27.5 |
| Current | 2301 | 10.1 | 520 | 22.6 | 499 | 21.7 | 551 | 24 | 731 | 31.8 |
| Former | 11861 | 52.2 | 3279 | 27.7 | 3091 | 26.1 | 2895 | 24.4 | 2596 | 21.9 |
| Number of PSA screens‡ | ||||||||||
| None | 9956 | 43.4 | 2430 | 24.4 | 2401 | 24.1 | 2444 | 24.6 | 2681 | 26.9 |
| Once | 8518 | 37.5 | 2135 | 25.1 | 2167 | 25.4 | 2187 | 25.7 | 2029 | 23.8 |
| More than once | 2314 | 10.2 | 586 | 25.3 | 637 | 27.5 | 598 | 25.8 | 493 | 21.3 |
| Unknown | 1932 | 8.5 | 529 | 27.4 | 475 | 24.6 | 449 | 23.2 | 479 | 24.8 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 65.6 | 5.9 | 65.5 | 5.8 | 65.9 | 5.9 | 65.9 | 6 | 65 | 6 |
| Pack-years | 24.4 | 30.8 | 26.6 | 30.9 | 24.1 | 30.3 | 23 | 20.1 | 23.6 | 31.7 |
| Body mass index (kg/m2) | 27.5 | 4.1 | 27.9 | 4.3 | 27.5 | 3.9 | 27.3 | 4 | 27.5 | 4.1 |
| Sugar-sweetened | ||||||||||
| beverages (g/d) | 16 | 29.7 | 2 | 3.2 | 5.8 | 6.8 | 12.5 | 12.4 | 43.7 | 47.2 |
| Fruit juices (g/d) | 13.7 | 19.6 | 4.6 | 4.5 | 10.4 | 7.9 | 14.9 | 11.9 | 24.8 | 33 |
| Desserts (g/d) | 21.5 | 20.1 | 7.7 | 5.1 | 15.4 | 8.5 | 24.2 | 13.3 | 38.9 | 28.2 |
| All added sugars (tsp/d) | 14.3 | 10.3 | 6.4 | 3.1 | 10 | 3.6 | 14.4 | 4.5 | 26.4 | 12.6 |
| Calories (kcal/d) | 1989 | 816 | 1573 | 689 | 1784 | 658 | 2050 | 697 | 2550 | 857 |
Quartiles represent total sugars consumed from sugar-sweetened beverages, fruit juices, and desserts
P value family history = 0.01;
P value for all other variables < 0.0001
Screening over previous 3 years
Multivariable adjusted associations of consumption of concentrated sugars with prostate cancer risk are shown in Table 2. Consumption of sugars from sugar-sweetened beverages was associated with increased overall prostate cancer risk, with 21% increased risk for men in the top quartile of consumption (HR: 1.21, 95% CI: 1.06 – 1.39; P for trend < 0.01). Additionally, an association of sugar-sweetened beverages with increased risk of low grade prostate cancer was observed for individuals in the highest quartile (P=0.02). However, there was no statistical difference between sugar consumption and risk of low and high grade prostate cancer. Consumption of sugars from fruit juices was associated with increased overall prostate cancer risk in the upper second and third quartiles, but the association diminished thereafter. There were no significant associations between consumption of sugars from desserts and prostate cancer risk. There was additionally no association between consumption of concentrated sugars and prostate cancer risk when sugar consumption was analyzed as a continuous variable (not shown).
Table 2.
Multivariable associations of consumption of concentrated sugars (grams) from beverages with prostate cancer risk†,‡
| Sugar-sweetened beverages | Fruit juices | Desserts | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N Cases/Total Person-Years | HR | 95% CI | N Cases/Total Person-Years | HR | 95% CI | N Cases/Total Person-Years | HR | 95% CI | |
| Prostate Cancer (n=1996) | |||||||||
| Q1 | 398 /41955 | 1.00 | – | 433 /45505 | 1.00 | – | 481 /45662 | 1.00 | – |
| Q2 | 544 /48923 | 1.11 | 0.97, 1.26 | 524 /46618 | 1.14 | 1.01, 1.30 | 488 /45975 | 0.97 | 0.85, 1.10 |
| Q3 | 519 /45944 | 1.14 | 1.0, 1.31 | 514 /44009 | 1.15 | 1.01, 1.31 | 527 /45746 | 1.03 | 0.91, 1.18 |
| Q4 | 535 /46608 | 1.21** | 1.06, 1.39 | 525 /47298 | 1.07 | 0.94, 1.22 | 500 /46047 | 0.95 | 0.83, 1.10 |
| Low Grade (n=1014) | |||||||||
| Q1 | 202 /41032 | 1.00 | – | 218 /44466 | 1.00 | – | 262 /44596 | 1.00 | – |
| Q2 | 274 /47668 | 1.09 | 0.91, 1.31 | 271 /45336 | 1.17 | 0.97, 1.39 | 241 /44751 | 0.86 | 0.72, 1.03 |
| Q3 | 251 /44693 | 1.14 | 0.94, 1.37 | 262 /42822 | 1.2 | 0.95, 1.37 | 257 /44436 | 0.90 | 0.76, 1.08 |
| Q4 | 277 /45305 | 1.25* | 1.03, 1.51 | 263 /46074 | 1.05 | 0.87, 1.26 | 254 /44915 | 0.87 | 0.71, 1.05 |
| High Grade (n=960) | |||||||||
| Q1 | 193 /41020 | 1.00 | – | 212 /44534 | 1.00 | – | 217 /44493 | 1.00 | – |
| Q2 | 264 /47746 | 1.12 | 0.93, 1.36 | 248 /45378 | 1.13 | 0.94, 1.36 | 244 /44973 | 1.10 | 0.91, 1.33 |
| Q3 | 251 /44850 | 1.14 | 0.94, 1.39 | 243 /42915 | 1.13 | 0.94, 1.36 | 262 /44563 | 1.18 | 0.98, 1.43 |
| Q4 | 252 /45333 | 1.19 | 0.97, 1.45 | 257 /46122 | 1.09 | 0.90, 1.31 | 237 /44920 | 1.05 | 0.85, 1.29 |
Adjusted for age, race, study center, BMI, education, smoking, family history of prostate cancer, history of diabetes, PSA screening, and energy intake
Quartile cutpoints (g/d): SSB - 0.63, 4.83, 19.21; fruit juices - 2.55, 9.24, 20.01; desserts - 7.85, 16.18, 28.87
P for trend < 0.01,
P for trend < 0.05
There were no associations between servings of sugar-sweetened beverages and prostate cancer risk. There was also no discernable pattern between servings of fruit juices and prostate cancer risk (data not shown).
We sought to determine if the associations between sugars from sugar-sweetened beverages and prostate cancer risk were modified by race (White vs. non-White) or number of PSA screens (none vs. at least one) (Table 3). Number of PSA screens had no effect on the association between sugar consumption and prostate cancer risk, and there was no statistical interaction (Q4 vs. Q1, Pinteraction = 0.92). Similarly, race did not significantly modify the association between consumption of sugars from sugar-sweetened beverages and prostate cancer risk (Q4 vs. Q1, Pinteraction = 0.94), and positive associations with sugar consumption were noted for White men only, in the third and fourth quartiles. As diabetes history has previously been inversely associated with prostate cancer risk, and is likely associated with sugar consumption, we also examined the association between sugar-sweetened beverages and prostate cancer risk in individuals without previous history of diabetes. The trend was similar to that seen in the full analytic cohort (Q2, HR: 1.11, 95% CI: 0.97 – 1.26; Q3, HR 1.16, 95% CI: 1.02 – 1.33; Q4, HR: 1.20, 95% CI: 1.05 – 1.37) (not shown).
Table 3.
Multivariable associations of consumption of concentrated sugars (grams) from sugar-sweetened beverages with prostate cancer risk*,†
| No PSA Screens | ≥ 1 PSA Screen | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N Cases/Total Person-Years | HR | 95% CI | N Cases/Total Person-Years | HR | 95% CI | P‡ | |
|
|
|
|
|||||
| Q1 | 180/20059 | 1.00 | – | 216/24553 | 1.00 | – | |
| Q2 | 225/20123 | 1.16 | 0.95, 1.41 | 253/23447 | 1.04 | 0.86, 1.24 | 0.40 |
| Q3 | 219/20209 | 1.15 | 0.94, 1.40 | 271/21526 | 1.15 | 0.97, 1.38 | 0.95 |
| Q4 | 218/20511 | 1.18 | 0.97, 1.44 | 271/17630 | 1.17 | 0.97, 1.4 | 0.92 |
| White | Non-White | ||||||
| N Cases/Total Person-Years | HR | 95% CI | N Cases/Total Person-Years | HR | 95% CI | P‡ | |
|
|
|
|
|||||
| Q1 | 368/39120 | 1.00 | –– | 38/2835 | 1.00 | –– | |
| Q2 | 448/40082 | 1.22 | 0.98, 1.29 | 46/3365 | 1.07 | 0.70, 1.63 | 0.83 |
| Q3 | 488/41140 | 1.20 | 1.04, 1.37 | 34/4566 | 0.78 | 0.49, 1.22 | 0.07 |
| Q4 | 513/46084 | 1.19 | 1.03, 1.36 | 61/6238 | 1.21 | 0.80, 1.82 | 0.94 |
Adjusted for age, race, study center, BMI, education, smoking, family history of prostate cancer, history of diabetes, PSA screening, and energy intake
Quartile cutpoints (g/d): No PSA screens - 0.89, 5.95, 21.17; ≥ 1 PSA screen - 0.63, 4.13, 17.23; White - 0.63, 3.92, 16.42; Non-white 21.19, 7.74, 22.67
P value from heterogeneity test
We additionally examined multivariable-adjusted associations between consumption of all concentrated sugars (sugar-sweetened beverages, fruit juices, and desserts) or all dietary added sugars and prostate cancer risk (Table 4). No significant associations were found.
Table 4.
Multivariable associations of all concentrated or non-natural sugars with prostate cancer risk*
| Quartile | N Cases/Total Person-Years | HR | 95% CI |
|---|---|---|---|
| All concentrated† | |||
| Q1 | 451/45684 | 1.00 | – |
| Q2 | 525/45579 | 1.11 | 0.97, 1.26 |
| Q3 | 507/46021 | 1.04 | 0.91, 1.19 |
| Q4 | 513/46146 | 1.07 | 0.93, 1.23 |
| All added‡ | |||
| Q1 | 457/45316 | 1.00 | – |
| Q2 | 535/45766 | 1.12 | 0.99, 1.28 |
| Q3 | 488/45967 | 1.00 | 0.88, 1.15 |
| Q4 | 516/46381 | 1.08 | 0.93, 1.25 |
Adjusted for age, race, treatment center, BMI, education, smoking, family history of prostate cancer, history of diabetes, PSA screening, and energy intake
Includes desserts, sugar-sweetened beverages, and fruit juices; quartile cutpoints (g/d): 23.47, 40.20, 65.94
Quartile cutpoints (tsp/day): 7.43, 11.73, 18.15
DISCUSSION
In this prospective study of men within the usual care arm of the PLCO screening trial, we found that consumption of sugars from sugar-sweetened beverages was associated with increased prostate cancer risk for men in the upper third and fourth quartiles of sugar intake.
We did not find a similar trend with consumption of sugars from fruit juices. There was no linear association even after controlling for grams of tomato and vegetable juice consumed or exclusion of tomato and vegetable juices from the exposure variable. Thus the type of fruit juice consumed might be particularly relevant, but this is not discernible from the present study. Additional investigation revealed less than 30% overlap for consumption of sugars from fruit- juices and sugar-sweetened beverages comparing any one quartile, suggesting the association of consumption of fruit juices with prostate cancer risk was not confounded by sugar-sweetened beverages and vice versa.
Number of servings of sugar-sweetened beverages overall was low, and it was not possible to examine the quartile distribution of servings of these beverages without including servings of sugars added to tea and coffee, which included sugar and honey, which likely confounded results. Tea and coffee contain polyphenols and can potentially confound the association of sugar-rich beverages with prostate cancer risk. Moreover, it was not possible to separate sugars and honey added to tea or coffee. When analyzing sugars in grams of soft drinks only, excluding sugars added to tea and coffee, the association was still present but not as strong, and the linear trend persisted. This might be expected as grams of sugars added to tea and coffee constituted ~25% of sugars in the sugar-sweetened beverages category.
Other studies have examined the association between carbohydrate or added sugar intake and prostate cancer risk. In a prospective Swedish cohort study, it was found that some refined carbohydrates including cakes and biscuits, low-fiber cereals and rice and pasta were associated with low-grade or overall prostate cancer26. Increased consumption of sugar-sweetened beverages was associated with increased risk of symptomatic prostate cancer, characterized by malignancy-related symptoms (but not total or low-grade cancer). In a separate prospective study, an inverse association was reported between total fructose intake (fruit and non-fruit- derived) and prostate cancer risk27. Fructose, however, has been shown to promote growth of pancreatic cancer cells, and is believed to have an important role in inflammation6, 12. We did not observe an association between fructose and prostate cancer risk in our study (not shown). Neither was an association observed when considering a composite variable representing all added sugars from the diet. These findings emphasize the potential significance of concentrated added sugars on prostate cancer risk. In our study, sugar-sweetened beverages, and particularly sodas, contributed the greatest amount of sugar relative to the other categories.
Other dietary and behavioral factors may affect prostate cancer risk. Fruit and vegetable consumption has been shown to affect cancer risk, although evidence on the relative role of fruit and vegetables in prostate cancer susceptibility is inconclusive. It was previously reported that high vegetable consumption may be associated with reduced risk of aggressive prostate cancer in the PLCO study28. In our models, the effect of daily servings of vegetables and fruits on associations between sugar intake and prostate cancer risk was negligible.
It is plausible that body weight might interact with sugar to increase prostate cancer risk, but the relationship between obesity and prostate cancer is complex. There is evidence that obese men may have higher risk of high-grade cancers, although they may have lower PSA levels29–31. Additionally, increased intake of sugar sweetened beverages has been associated with increased weight gain32, 33, although an inverse association between BMI and sugar intake has also been reported for men, particularly34. For this reason BMI was included as a confounder when analyzing the association between dietary sugar and prostate cancer risk, although its effect on estimates was negligible. Comparisons at baseline revealed markedly increased energy intake for men in the highest quartile of sugar consumption, although mean BMI was not higher for these men. Regular or vigorous exercise might also be associated with reduced risk of prostate cancer, particularly advanced cancer, based on some evidence from large, prospective cohort studies35–37. It is unclear how physical activity affected the association between sugar consumption and prostate cancer risk in the usual care arm, as this information was not ascertainable from the DHQ.
Consumption of concentrated sugars may be related to prostate cancer risk through activation of inflammatory cytokines, such as interleukins, C-reactive protein, and tumor necrosis factor38, among others, as a result of elevated uric acid in the serum or another mechanism. Increases in uric acid, particularly, may lead to increased production of IL-1β, and chronic inflammation39. Alternatively, elevated triglycerides or cholesterol could be related to prostate cancer risk40 by inducing activation of signaling by NF-ĸB21 or protein kinase B, and ultimately other inflammatory factors41, 42. Furthermore, there is evidence that fructose, a common compound present at high concentrations in sugar-sweetened beverages and desserts, is converted to fat more rapidly than other sugars43. It is true that naturally occurring sugars and added sugars share the same chemical structure. However the difference to be noted is perhaps in the broader range of physiological effects that ultimately regulate inflammatory processes. It is important to highlight that processed sugar-dense goods may not have the same effect on the blood glucose. Absorption of sugars in plant foods occurs more slowly and is more regulated due to buffering by vitamins and fiber, and phytonutrients, such that there is not a spike in blood sugar and consequently, a heightened inflammatory response44. Therefore, studies examining consumption of total sugars may not report positive associations with chronic disease. In addition to the potentially greater relevance of concentrated sugars over total sugars, our study highlights a more detrimental role of concentrated sugars from beverages than desserts in the context of prostate cancer risk. Physiological events associated with digestion and metabolism of these sugars, particularly, could lead to increases or alterations in pro-inflammatory cytokines, and ultimately chronic inflammation.
Strengths of the study include the large number of study participants and high follow-up rate, detailed information on sugar consumption obtained from the food frequency questionnaire and subsequently thorough nutrient analysis, as well as complete information on demographic and clinical factors affecting prostate cancer risk. Limitations include possible measurement error in sugar intake due to misreporting or information bias, and limitations of the nutrient database in distinguishing natural from added sugars. Also, because dietary habits fluctuate, interpretation of findings of diet-related associations with cancer is more difficult in studies where the exposure assessed represents a single point in time. We were unable to accurately compare the role of sugar consumption with prostate cancer risk between the screening and usual care arms of the PLCO, as differences in the dietary assessment tools between study arms as well as the timing of administration of the DHQ precluded direct comparison. Furthermore, while risk of advanced stage prostate cancer or prostate cancer mortality are important clinically relevant endpoints, we were unable to examine the association of sugar consumption with these endpoints due to the very low number of men with advanced stage prostate cancer and limited number of prostate cancer-specific deaths. Therefore, conclusions of the current study are limited and consumption of concentrated sugars should be examined in other settings.
In conclusion, this study provides evidence for a positive association between sugars from sugar-sweetened beverages and increased risk of prostate cancer among men receiving usual medical care in the PLCO trial. Our findings highlight the potential significance of high consumption of added, concentrated sugars from beverages in prostate cancer etiology. Additional studies examining this association are warranted.
Acknowledgments
We would like to thank the National Cancer Institute for access to data collected in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. We declare that the statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by the National Cancer Institute.
FINANCIAL SUPPORT
This research was partially supported by the National Institutes of Health (Z.Z., and F.M., T32 CA09142), (F.M. and M.N., R25 CA092408); and the Alper Research Funds for Environmental (Z.Z., Genomics of the UCLA Jonsson Comprehensive Cancer Center).
Footnotes
CONFLICT OF INTEREST
None.
AUTHORSHIP
FM was responsible for writing and preparing the manuscript, developing the research questions, and analyzing data. MN and ZZ assisted in the statistical design, analytical approach and interpretation of data, and provided significant manuscript edits.
References
- 1.Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obes Res. 2003;11(11):1325–32. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- 2.USDA. Profiling Food Consumption in America. Agriculture Fact Book. :2001–2002. [Google Scholar]
- 3.Powell ES, Smith-Taillie LP, Popkin BM. Added Sugars Intake Across the Distribution of US Children and Adult Consumers: 1977-2012. J Acad Nutr Diet. 2016;116(10):1543–50.e1. doi: 10.1016/j.jand.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agriculture. USDoHaHSaUSDo. 2015-2020 Dietary Guidelines for Americans. 8th. Dec, 2015. [Google Scholar]
- 5.Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30(1):96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RJ, Sanchez-Lozada LG, Nakagawa T. The effect of fructose on renal biology and disease. J Am Soc Nephrol. 2010;21(12):2036–9. doi: 10.1681/ASN.2010050506. [DOI] [PubMed] [Google Scholar]
- 7.Jalal DI, Smits G, Johnson RJ, et al. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol. 2010;21(9):1543–9. doi: 10.1681/ASN.2009111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JM, Wang F, Holly EA. Sweets, sweetened beverages, and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control. 2009;20(6):835–46. doi: 10.1007/s10552-009-9323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson SC, Bergkvist L, Wolk A. Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. Am J Clin Nutr. 2006;84(5):1171–6. doi: 10.1093/ajcn/84.5.1171. [DOI] [PubMed] [Google Scholar]
- 10.Mueller NT, Odegaard A, Anderson K, et al. Soft drink and juice consumption and risk of pancreatic cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19(2):447–55. doi: 10.1158/1055-9965.EPI-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schernhammer ES, Hu FB, Giovannucci E, et al. Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2098–105. doi: 10.1158/1055-9965.EPI-05-0059. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Huang D, McArthur DL, et al. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res. 2010;70(15):6368–76. doi: 10.1158/0008-5472.CAN-09-4615. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw PT, Sagiv SK, Kabat GC, et al. Consumption of sweet foods and breast cancer risk: a case-control study of women on Long Island, New York. Cancer Causes Control. 2009;20(8):1509–15. doi: 10.1007/s10552-009-9343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles FL, Chang SC, Morgenstern H, et al. Association of sugary beverages with survival among patients with cancers of the upper aerodigestive tract. Cancer Causes Control. 2016;27(11):1293–300. doi: 10.1007/s10552-016-0792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heber D FS, Jones LW, Nelson WG. Nutrition, Exercise and Prostate Cancer. Santa Monica, CA: Prostate Cancer Foundation: 2009. [Google Scholar]
- 16.Nakai Y, Nelson WG, De Marzo AM. The dietary charred meat carcinogen 2-amino-1- methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007;67(3):1378–84. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]
- 17.Cross AJ, Peters U, Kirsh VA, et al. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005;65(24):11779–84. doi: 10.1158/0008-5472.CAN-05-2191. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Rimm EB, Colditz GA, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85(19):1571–9. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005;97(23):1768–77. doi: 10.1093/jnci/dji402. [DOI] [PubMed] [Google Scholar]
- 20.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welty FK. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr Cardiol Rep. 2013;15(9):400. doi: 10.1007/s11886-013-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60(1):199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 24.Leitzmann MF, Ahn J, Albanes D, et al. Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes Control. 2008;19(10):1267–76. doi: 10.1007/s10552-008-9198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 26.Drake I, Sonestedt E, Gullberg B, et al. Dietary intakes of carbohydrates in relation to prostate cancer risk: a prospective study in the Malmo Diet and Cancer cohort. Am J Clin Nutr. 2012;96(6):1409–18. doi: 10.3945/ajcn.112.039438. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Rimm EB, Wolk A, et al. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58(3):442–7. [PubMed] [Google Scholar]
- 28.Kirsh VA, Peters U, Mayne ST, et al. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. 2007;99(15):1200–9. doi: 10.1093/jnci/djm065. [DOI] [PubMed] [Google Scholar]
- 29.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103(5):1092–5. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 30.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 31.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17(8):989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 32.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 34.Macdiarmid JI, Vail A, Cade JE, et al. The sugar-fat relationship revisited: differences in consumption between men and women of varying BMI. Int J Obes Relat Metab Disord. 1998;22(11):1053–61. doi: 10.1038/sj.ijo.0800724. [DOI] [PubMed] [Google Scholar]
- 35.Giovannucci EL, Liu Y, Leitzmann MF, et al. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165(9):1005–10. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 36.Nilsen TI, Romundstad PR, Vatten LJ. Recreational physical activity and risk of prostate cancer: A prospective population-based study in Norway (the HUNT study) Int J Cancer. 2006;119(12):2943–7. doi: 10.1002/ijc.22184. [DOI] [PubMed] [Google Scholar]
- 37.Orsini N, Bellocco R, Bottai M, et al. A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer. 2009;101(11):1932–8. doi: 10.1038/sj.bjc.6605404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyngdoh T, Marques-Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6(5):e19901. doi: 10.1371/journal.pone.0019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y. Caught red-handed: uric acid is an agent of inflammation. J Clin Invest. 2010;120(6):1809–11. doi: 10.1172/JCI43132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allott EH, Howard LE, Cooperberg MR, et al. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2349–56. doi: 10.1158/1055-9965.EPI-14-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013;2013:697521. doi: 10.1155/2013/697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang L, Lin J, Lu ML, et al. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–31. [PubMed] [Google Scholar]
- 43.Parks EJ, Skokan LE, Timlin MT, et al. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138(6):1039–46. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebs-Smith SM, Cleveland LE, Ballard-Barbash R, et al. Characterizing food intake patterns of American adults. Am J Clin Nutr. 1997;65(4 Suppl):1264S–8S. doi: 10.1093/ajcn/65.4.1264S. [DOI] [PubMed] [Google Scholar]


