Abstract
Emerging data highlight the critical role for the innate immune system in the progression of nonalcoholic fatty liver disease (NAFLD). Connexin 32 (Cx32), the primary liver gap junction protein, is capable of modulating hepatic innate immune responses and has been studied in dietary animal models of steatohepatitis. In this work, we sought to determine the association of hepatic Cx32 with the stages of human NAFLD in a histologically characterized cohort of 362 patients with NAFLD. We also studied the hepatic expression of the genes and proteins known to interact with Cx32 (known as the connexome) in patients with NAFLD. Last, we used three independent dietary mouse models of nonalcoholic steatohepatitis to investigate the role of Cx32 in the development of steatohepatitis and fibrosis. In a univariate analysis, we found that Cx32 hepatic expression associates with each component of the NAFLD activity score and fibrosis severity. Multivariate analysis revealed that Cx32 expression most closely associated with the NAFLD activity score and fibrosis compared to known risk factors for the disease. Furthermore, by analyzing the connexome, we identified novel genes related to Cx32 that associate with NAFLD progression. Finally, we demonstrated that Cx32 deficiency protects against liver injury, inflammation, and fibrosis in three murine models of nonalcoholic steatohepatitis by limiting initial diet‐induced hepatoxicity and subsequent increases in intestinal permeability. Conclusion: Hepatic expression of Cx32 strongly associates with steatohepatitis and fibrosis in patients with NAFLD. We also identify novel genes associated with NAFLD and suggest that Cx32 plays a role in promoting NAFLD development. (Hepatology Communications 2018;2:786‐797)
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BSA
bovine serum albumin
- CDAHFD
choline‐deficient, L‐amino acid‐defined, high‐fat diet
- Cx32
connexin 32
- Cx43
connexin 43
- HFHCD
high‐fat high‐cholesterol diet
- MCDD
methionine‐and‐choline‐deficient diet
- MGH
Massachusetts General Hospital
- mRNA
messenger RNA
- NAFLD
nonalcoholic fatty liver disease
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- WT
wild‐type
Nonalcoholic fatty liver disease (NAFLD) affects 30% of the U.S. population and has emerged as one of the most common causes of chronic liver disease.1 The progressive form of NAFLD, nonalcoholic steatohepatitis (NASH), affects up to 5%‐10% of the U.S. population and can lead to clinically significant sequelae, such as cirrhosis and hepatocellular carcinoma. Cirrhosis and hepatocellular carcinoma are collectively responsible for major morbidity and mortality and exact a substantial health and economic toll on society.2 Additionally, NASH cirrhosis is predicted to be the leading indication for liver transplantation in the United States by the year 2020.3 Despite the rising prevalence of NAFLD, its pathogenesis remains incompletely understood and treatment options are limited. Thus, there is a pressing need to improve our understanding of NAFLD pathogenesis and factors that contribute to its progression.
Innate immune activation is a key factor in triggering hepatic inflammation in murine models of NAFLD and is positively correlated with the severity of disease in patients with NASH.4, 5, 6 Gap junctions, intercellular channels composed of connexin proteins that directly connect the cytosol of coupled cells and allow rapid communication of cellular signals,7 represent a unique pathway for amplifying innate immunity.8, 9 The predominant hepatic gap junction, connexin 32 (Cx32), is widely expressed throughout the liver and is most highly expressed in zone 3, the area most vulnerable to injury from steatohepatitis.10 Multiple prior investigations have demonstrated the critical role of Cx32 gap junctions in establishing injury in various models of liver disease.11, 12, 13 In particular, recent data have highlighted that perturbations in Cx32 are associated with the development of NAFLD in dietary animal models.14, 15, 16, 17
Despite these compelling results supporting an association between Cx32 and NAFLD, no studies have examined the role of Cx32 in human NAFLD. Accordingly, the goal of this work was to comprehensively study, for the first time, the association between disease severity and expression of Cx32 and related genes in patients with NAFLD.
Methods
NAFLD PATIENT COHORT
Individuals were prospectively recruited for participation from the Massachusetts General Hospital (MGH) Fatty Liver Clinic as well as the MGH Weight Center. For patients who consented to participate, we collected blood for analysis of metabolic parameters. Additionally, we obtained excess liver tissue from clinically indicated liver biopsies from patients seen at the MGH Fatty Liver Clinic and from wedge liver biopsies obtained at the time of Roux‐en‐Y gastric bypass surgery from patients seen at the MGH Weight Center. For all patients, demographic data were obtained through electronic chart review.
HISTOLOGIC ANALYSIS
Histologic analysis was performed on liver sections stained with hematoxylin and eosin and trichrome. A single hepatopathologist (R.M.) at our institution scored all samples, rereading samples in a blinded fashion to ensure consistency of histologic data. To ensure internal validity, a second hepatopathologist scored 10% of the previously read samples. These secondary scores were found to be similar to our primary liver pathologist's review.
The severity of liver injury was determined using the NAFLD activity score (NAS) as described.18 Briefly, liver histology was scored for ballooning (0‐2), steatosis (0‐3), and inflammation (0‐3), and the sum of these scores was used to create the NAS. Fibrosis was scored as described.18 Patients were defined as having no liver disease if there was no evidence for steatosis, inflammation, ballooning, and fibrosis. Patients with histologic evidence for steatosis but no inflammation, ballooning, and/or fibrosis were designated as those with bland steatosis. Patients with any inflammation or ballooning but no fibrosis, regardless of steatosis status, were classified as NASH with no fibrosis. Finally, patients with any fibrosis, regardless of steatosis, inflammation, or ballooning score, were classified as NASH with fibrosis.
NANOSTRING ANALYSIS
Human samples were provided as flash‐frozen wedge biopsy tissue. RNA was extracted, and fragment size and distribution (RNA integrity number) were quantified by the Agilent Bioanalyzer. Nanostring analyses were performed as described.19 The custom NanoString probe set targeted genes in the connexome. Data were normalized by using the geometric mean of five genes with good median expression in the nCounter human reference panel gene list: succinate dehydrogenase complex flavoprotein subunit A (SDHA), clathrin heavy chain (CLTC), tubulin beta class I (TUBB), phosphoglycerate kinase 1 (PGK1), and glucuronidase beta (GUSB). We did not perform a background subtraction and did include positive control normalization. We performed replicate analysis of 10 RNA extractions as well as replicate analysis of two separate RNA extractions of duplicate samples from the same patient. For all technical replicates of the nanostring assay, R 2 exceeded 0.999 (Pearson's R 2). Log10‐log10‐transformed correlation coefficients all exceeded 0.96. For extractions of the duplicate sample, R 2 was 0.937 (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1179/full).
QUANTITATIVE REAL‐TIME POLYMERASE CHAIN REACTION
Mouse liver tissues were mechanically homogenized using the PowerGen 125 Homogenizer (Fisher Scientific, Hampton, NH). Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). Total RNA (500 ng) was converted into complementary DNA using the RT2 First Strand Kit (SA Biosciences, Valencia, CA). Quantitative real‐time polymerase chain reaction was performed using the RT2 Master Mix Kit (SA Biosciences) and the iQ5 system (Bio‐Rad, Hercules, CA). Quantitative real‐time polymerase chain reaction was performed for mRNA expression using primers designed by SA Biosciences (Qiagen).
MICROARRAY ANALYSIS
Isolated liver mRNA from wild‐type (WT) and Cx32‐deficient mice (Cx32KO) was used for hepatic mRNA microarray analysis on the Affymetrix GeneChip Mouse Gene ST 2.0 platform (Affymetrix Inc., Santa Clara, CA). CEL files were normalized to produce gene‐level expression values using the implementation of the Robust Multiarray Average algorithm in the affy package (version 1.36.1) included in the Bioconductor software suite (version 2.12) and an Entrez Gene‐specific probeset mapping (version 17.0.0) from the Molecular and Behavioral Neuroscience Institute (Brainarray) at the University of Michigan.20, 21, 22, 23
IN VIVO INDUCTION OF STEATOHEPATITIS
Male C57/BL6 mice aged 6‐8 weeks that were Cx32KO were created by K. Willecke (University of Bonn) and obtained from D. Paul (Harvard University). These mice and their WT littermates were used for all animal experimentation. To induce NASH, 6‐8‐week‐old mice were fed a methionine‐and‐choline‐deficient diet (MCDD) for 28 days and then euthanized. Additionally, mice were fed a choline‐deficient, L‐amino acid‐defined, high‐fat diet (CDAHFD) for 9 weeks, at which time they were euthanized.24 Finally, we also used a high‐fat high‐cholesterol diet (HFHCD) for 9 weeks. The HFHCD consists of a Surwit diet, comprised of 58 kcal% medium‐chain saturated fatty acids, and drinking water enriched with 42 g/L of high‐fructose corn syrup (55% fructose and 45% sucrose) equivalent.25 All experimental and control diets were purchased from Research Diets, Inc. (New Brunswick, NJ).
BIOCHEMICAL ANALYSIS OF LIVER INJURY
Immediately following mouse euthanasia, systemic blood was collected from the inferior vena cava. Serum was obtained by centrifugation of whole blood at 10,000 g for 10 minutes. To determine the extent of hepatocyte injury, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were quantified using Infinity ALT and AST Liquid Stable reagents (Thermo Scientific, Middletown, VA).
HEPATOCYTE ISOLATION AND CULTURE METHODS
Hepatocytes from WT and Cx32KO mice were isolated for use in in vitro experimentation. Specifically, catheterization of the inferior vena cava was performed to allow for flow of collagenase to the liver. Following whole liver digestion, centrifugation was used to separate hepatocytes from nonparenchymal cells.
Hepatocytes were plated on collagen‐coated plates at a density of 1.0 × 105 for 24 hours. The hepatocyte culture medium consisted of the following: Williams E medium was supplemented with 20 μg/L epidermal growth factor, 14.28 μg/L glucagon, 7.5 mg/L hydrocortisone, 0.05 U/L insulin, and 2% penicillin‐streptomycin. Hepatocytes were then exposed to palmitic acid‐contained media 24 hours after initial plating. This media was prepared by the following method: Briefly, stock solution of sodium palmitate was dissolved in an ethanol/water mixture (1:1, volume/volume) at 65°C for 15 minutes at a final concentration of 150 mM (43.5 mg/mL). Then, 50 μL of sodium palmitate solution was added to 1 mL fatty acid‐free bovine serum albumin (BSA; 10% in phosphate‐buffered saline) at 37°C and incubated at 55°C for 10 minutes. BSA‐palmitate was allowed to complex by incubation for 1 hour at 37°C, yielding a solution of 7.5 mM palmitate and 1.5 mM BSA. We then added 62.5 μL of sodium palmitate‐BSA solution to 937.5 μL Williams E media, resulting in a 0.5 mM sodium palmitate media. Cell culture supernatant was collected after 24 hours of hepatocyte exposure to the palmitic acid‐containing media. This supernatant was then analyzed for hepatocyte injury as detailed below.
LACTATE DEHYDROGENASE ASSAY
Lactate dehydrogenase levels in the hepatocyte supernatant were evaluated using CytoTox 96 Non‐Radioactive Cytotoxicity Assay (Promega Cat. No. G1780). We mixed 50 μL of media with 100 μL lactate dehydrogenase reagent and incubated this mixture at room temperature in the dark for 15‐20 minutes. At the end of this incubation, 50 μL stop solution was added and absorbance was measured at 490 nm.
IN VIVO INTESTINAL PERMEABILITY ASSAY
To determine paracellular intestinal permeability, serum levels of orally administered fluorescein isothiocynate‐4 kD dextran were measured. Mice were denied access to food and water for 8 hours prior to euthanasia, and fluorescein isothiocynate‐4 kD dextran (Sigma, St. Louis, MO) at 60 mg/100 g body weight was orally gavaged 4 hours later. Immediately following euthanasia, serum was collected and fluorescence intensity was measured (excitation, 490 nm; emission, 525 nm) using the Synergy 2 plate reader (BioTek, Winooski, VT).
STATISTICAL ANALYSES
All data are expressed as means ± SD. Data were analyzed by either analysis of variance for comparison across multiple groups or the Student t test for comparison between two groups. Two‐tailed P values were calculated, and P < 0.05 was defined as statistically significant. Risk factors for increased NAS and fibrosis were examined with the use of univariate and multivariate logistic regression; all factors in the univariate analysis were included in the multivariate analysis. A beta coefficient value was calculated to measure the degree to which each variable influenced the NAS score and fibrosis, with P < 0.05 considered significant. All analyses were conducted using Stata software, version 10.1 (StataCorp).
STUDY APPROVAL
All animal experiments were reviewed and approved by the MGH Subcommittee on Research Animal Care. For collection and analysis of patient data, written informed consent was received from participants prior to inclusion in the study.
Results
PATIENT DEMOGRAPHICS
Our cohort included 362 patients undergoing bariatric surgery or being followed at the MGH Fatty Liver Clinic (Table 1). Based on histologic criteria, this group represented the wide‐spectrum of NAFLD, including 22.7% of patients with no liver disease, 25.1% with bland steatosis, 20.4% with NASH and no fibrosis, and 21.8% with NASH and any fibrosis. Patients were similar in age, sex, and race. Despite the wide distribution of NAFLD severity in our cohort, body mass index was similar among all groups (median 45). Furthermore, we did not observe a difference in the frequency of kidney disease, coronary artery disease, or serum levels of low‐density lipoprotein and high‐density lipoprotein between groups. Patients with NASH and any fibrosis did, however, have a higher prevalence of diabetes as well as higher serum aminotransferases and triglyceride levels.
Table 1.
Patient Demographics
|
Normal (n = 82) |
Steatosis (n = 91) |
NASH (n = 74) |
NASH Fibrosis (n = 115) |
|
|---|---|---|---|---|
| Age, years mean (SD) | 42 (12) | 45 (12) | 44 (12) | 46 (13) |
| Female, n (%) | 68 (83) | 66 (73) | 61 (82) | 69 (60) |
| Race | ||||
| Hispanic, n (%) | 6 (8) | 6 (7) | 3 (4) | 13 (11) |
| Caucasian, n (%) | 39 (48) | 56 (62) | 49 (66) | 89 (78) |
| African American, n (%) | 36 (44) | 28 (31) | 22 (30) | 13 (11) |
| Diabetes, n (%) | 7 (9) | 22 (24) | 23 (31) | 66 (57) |
| CAD, n (%) | 7 (9) | 4 (4) | 4 (5) | 8 (7) |
| OSA, n (%) | 20 (24) | 41 (45) | 20 (27) | 54 (47) |
| HLD, n (%) | 19 (23) | 29 (32) | 28 (38) | 70 (61) |
| BMI, kg/m2 mean (SD) | 45 (6) | 46 (7) | 47 (8) | 46 (8) |
| ALT, IU/L mean (SD) | 30 (26) | 31 (16) | 35 (17) | 56 (41) |
| AST, IU/L mean (SD) | 22 (23) | 22 (11) | 21 (13) | 39 (27) |
| Cr, mg/dL mean (SD) | 0.9 (0.5) | 0.7 (0.2) | 0.8 (0.2) | 0.8 (0.2) |
| Total Chol, mg/dL mean (SD) | 174 (36) | 181 (36) | 178 (37) | 169 (40) |
| TG, mg/dL mean (SD) | 112 (70) | 132 (58) | 153 (109) | 168 (88) |
| LDL, mg/dL mean (SD) | 104 (31) | 108 (30) | 104 (28) | 97 (35) |
| HDL, mg/dL mean (SD) | 49 (15) | 46 (13) | 45 (11) | 40 (10) |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; Chol, cholesterol; Cr, creatinine; HDL, high‐density lipoprotein; HLD, hyperlipidemia; LDL, low‐density lipoprotein; OSA, obstructive sleep apnea; TG, triglycerides.
HEPATIC Cx32 EXPRESSION AND NAFLD
To gain insight into the clinical relevance of Cx32 in human liver disease, we analyzed hepatic expression of gap junction protein beta 1 (GJB1), the gene that encodes for Cx32, in our NAFLD cohort. We found that patients with NASH had lower hepatic expression of Cx32 than healthy patients (log2 fold change, –0.150; adjusted P = 0.0003). Moreover, patients with NASH had lower hepatic expression of Cx32 than those with bland steatosis (log2 fold change, –0.105; adjusted P = 0.02). In contrast, there was no significant difference in hepatic Cx32 expression between healthy patients and those with bland steatosis. In order to investigate the association of hepatic Cx32 and fibrosis, we studied hepatic expression of Cx32 in patients with NASH with and without fibrosis. We found that patients with NASH with fibrosis expressed Cx32 at lower levels than those without fibrosis (log2 fold change, –0.123; adjusted P = 0.04) (Table 2).
Table 2.
Hepatic Connexin 32 Expression Varies With NAFLD Histologic Subtype
| Log2 Fold Change | Adjusted P Value | |
|---|---|---|
| Bland steatosis versus heathy | −0.05 | 0.65 |
| NASH versus healthy | −0.15 | 0.0003 |
| NASH versus bland steatosis | −0.105 | 0.02 |
| NASH F1‐4 versus NASH F0 | −0.123 | 0.01 |
In a univariate analysis, we found that hepatic Cx32 expression significantly and negatively associated with the NAS (including its individual components of steatosis, inflammation, and ballooning) as well as with fibrosis (Fig. 1). To determine which characteristics of our cohort most closely associated with increased NAS and with fibrosis, we performed a multivariate logistic regression analysis that included the following: Cx32 hepatic expression, age, sex, body mass index, diabetes, and hyperlipidemia. After adjusting for these variables, only Cx32 expression significantly associated with NAS and fibrosis (Table 3). Notably, factors known to associate with NAS and fibrosis progression, such as diabetes, no longer achieved significance after accounting for Cx32 status.
Figure 1.
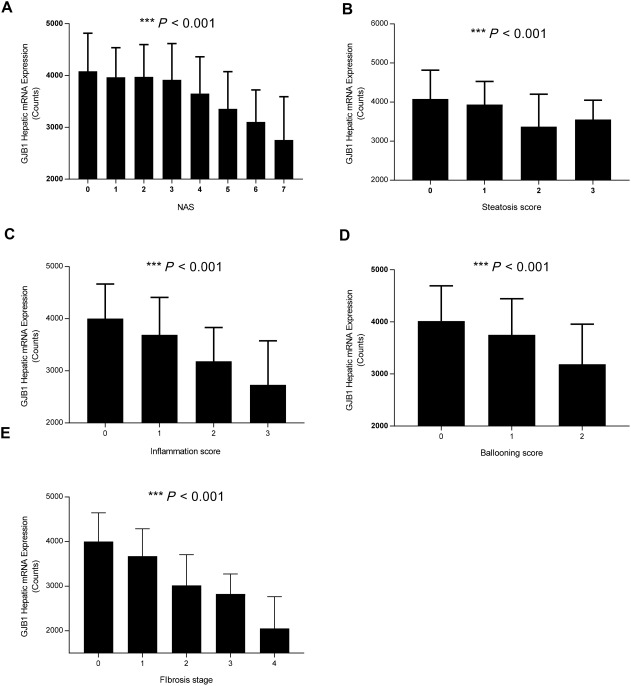
Hepatic GJB1 expression tracks with components of NAS activity score and fibrosis. Expression of GJB1, the gene that encodes for connexin 32, from liver biopsies taken from each patient in our NAFLD cohort was determined by real‐time quantitative polymerase chain reaction. Statistical testing for significance was performed using analysis of variance to determine the relationship between hepatic GJB1 and (A) NAS, (B) steatosis, (C) inflammation, (D) ballooning, and (E) fibrosis score. Data represented as mean ± standard deviation. Abbreviation: GJB1, gap junction protein beta 1.
Table 3.
Results of Multivariate Analysis for Factors Influencing NAS and Fibrosis
| Variable | Beta Coefficient | SE | P Value | |
|---|---|---|---|---|
| NAS | Hepatic Cx32 expression | –0.0004 | 0.0002 | 0.01 |
| Age | −0.0028 | 0.008 | 0.73 | |
| Sex | 0.1889 | 0.2229 | 0.41 | |
| BMI | 0.0098 | 0.0136 | 0.47 | |
| HLD | 0.3736 | 0.2261 | 0.099 | |
| Diabetes | 0.358 | 0.231 | 0.121 | |
| Fibrosis | Hepatic Cx32 expression | −0.0009 | 0.0002 | 0.0001 |
| Age | −0.0222 | 0.129 | 0.09 | |
| Sex | 0.0412 | 0.3244 | 0.89 | |
| BMI | −0.0119 | 0.0208 | 0.57 | |
| HLD | −0.9501 | 0.3486 | 0.79 | |
| Diabetes | 0.6377 | 0.3327 | 0.06 |
Abbreviations: BMI, body mass index; HLD, hyperlipidemia.
To assess whether the observed inverse association was specific to Cx32 and not seen with other hepatic gap junctions, we analyzed the relation between connexin 43 (Cx43) and NAS and fibrosis. We observed no significant difference in hepatic Cx43 expression between patients with varying degrees of NAFLD, nor did we find a significant association between Cx43 expression and NAS or fibrosis.
DEFINING THE CONNEXOME IN HUMAN NAFLD
In order to expand the list of novel drivers of NAFLD progression beyond those identified by previous genome‐wide association studies, we performed a targeted analysis of genes differentially expressed in hepatic microarray data comparing WT and Cx32KO mice at baseline (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1179/full). We hypothesized that many of these differentially expressed genes, in addition to those known to interact with Cx32 at the protein level (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1179/full), may have broader clinical relevance in NAFLD. We termed these genes related to Cx32 the “connexome.” A volcano plot for differentially expressed genes from our nanostring analysis is shown in http://onlinelibrary.wiley.com/doi/10.1002/hep4.1179/full.
Within the connexome, we identified a subset of genes that are significantly altered in patients with NASH versus steatosis (Table 4). Among those identified include genes involved in the host inflammatory response (interleukin‐7R [IL‐7R], interferon gamma inducible protein 16 [IFI16], and Tribbles pseudokinase 3 [TRIB3]), metabolism (aldehyde dehydrogenase 18 family member A1 [ALDH18A1], HD domain containing 3 [HDDC3], and aldo‐keto reductase family 1 member C3 [AKR1C3]), cellular regulation (MYC proto‐oncogene [MYC]), and those with no defined function (zinc finger 407 [ZNF407]).
Table 4.
Differentially Expressed Hepatic Genes Between Patients With NASH Versus Steatosis
|
Connexome Genes (FC, Adjusted P Value) |
Established Genes (FC; Adjusted P Value) |
|---|---|
| MYC (1.53; 0.001) | TM6SF2 (1.64; 0.01) |
| ABCC4 (1.45; 0.0005) | PNPLA3 (1.42; 0.02) |
| IL‐7R (1.35; 0.01) | PCSK9 (1.32; 0.01) |
| IFI16 (1.24; 0.0006) | |
| TRIB3 (1.23; 0.003) | |
| ZNF407 (1.17; 0.02) | |
| ALDH18A1 (1.11; 0.01) | |
| HDDC3 (0.91; 0.006) | |
| RPS13 (0.83; 0.0005) | |
| AKR1C3 (0.82; 0.01) |
Abbreviations: ABCC4, ATP binding cassette subfamily C member 4; FC, fold change; PCSK9, proprotein convertase subtilisin/kexin type 9; PNPLA3, patatin like phospholipase domain containing 3; RPS13, ribosomal protein S13; TM6SF2, transmembrane 6 superfamily member 2.
Cx32 DEFICIENCY PROTECTS AGAINST STEATOHEPATITIS IN MURINE MODELS
To further define the role of Cx32 in NAFLD, we examined the effect of Cx32 modulation in multiple dietary models of steatohepatitis. We studied the phenotype induced by two dietary models of steatohepatitis dependent upon deficiency of choline, the MCDD and the CDAHFD, as well as the HFHCD. We found that Cx32‐deficient (Cx32KO) mice exhibited significantly less hepatocellular injury compared to WT mice when exposed to these diets, based on serum levels of AST and ALT (Table 5). Although the degree of hepatic steatosis remained the same, histologic analysis of liver tissue showed significantly less inflammation in Cx32KO compared to WT mice (Table 5). Additionally, hepatic expression of tumor necrosis factor α (TNF‐α) and IL‐6, two proinflammatory cytokines, was attenuated in Cx32KO mice (Table 5). Taken together, these animal data demonstrate that Cx32 deficiency protects against liver injury in multiple models of steatohepatitis.
Table 5.
Connexin 32 Deficiency in Multiple Dietary Models Of Steatohepatitis
| MCDD | CDAHFD | HFHCD | ||||
|---|---|---|---|---|---|---|
| WT | Cx32 KO | WT | Cx32 KO | WT | Cx32 KO | |
| ALT, IU/L mean (SD) | 524 (98) | 247 (36)** | 812 (190) | 519 (92)** | 493 (88) | 192 (44)** |
| AST, IU/L mean (SD) | 510 (46) | 214 (99)** | 760 (332) | 336 (85)* | 395 (76) | 148 (83)* |
| Inflammation score, mean (SD) | 3.0 (0) | 1.7 (0.5)*** | 3.0 (0) | 1.5 (0.4)*** | 3.0 (0) | 1.7 (0.3)** |
| Steatosis score, mean (SD) | 2.8 (0.4) | 3.0 (0) | 3.0 (0) | 3.0 (0) | 3.0 (0) | 3.0 (0) |
| TNF‐α† (fold change) | 3.8 (0.9) | 2.3 (0.5)* | 4.5 (0.7) | 1.4 (0.3)* | 3.9 (1.0) | 1.8 (0.2)* |
| IL‐6† (fold change) | 4.4 (1.9) | 2.0 (0.6)* | 5.1 (0.8) | 1.9 (0.5)* | 4.1 (0.3) | 1.7 (0.7)* |
†Compared to WT mice fed a control diet. *P < 0.05, **P < 0.01, ***P < 0.001.
Given the association of hepatic Cx32 expression and fibrosis in our patient cohort, we sought to determine if Cx32 deficiency affected fibrosis development in the CDAHFD. We found that compared to WT mice, the livers from Cx32KO mice had significantly less trichrome staining of collagen, a reduced histologic fibrosis score, and lower expression of key fibrogenic genes collagen type I α1 (COL1A1) and transforming growth factor β (TGF‐β; Fig. 2). Taken together, these data demonstrate that hepatic gap junction deficiency blocks the progression of NASH and protects against fibrosis in multiple dietary models of steatohepatitis.
Figure 2.
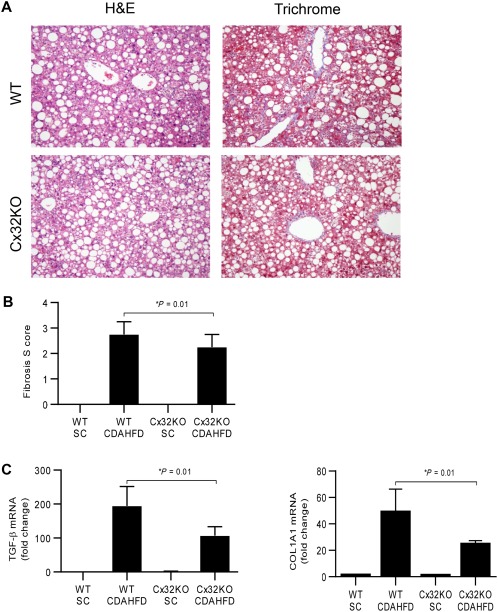
Connexin 32 deficiency protects against NASH fibrosis. WT and Cx32KO mice were given a CDAHFD for 9 weeks to induce NASH with fibrosis. (A,B) Histologic scoring of liver tissue for inflammation and fibrosis (H&E and trichrome, magnification ×20). (C) Hepatic expression of TGF‐β and COL1A1, key drivers of hepatic fibrosis, in WT and Cx32KO mice. Statistical testing for significance was performed using a Student t test, n = 15 mice per group. Data represented as mean ± standard deviation. Abbreviations: H&E, hematoxylin and eosin; SC, standard chow.
Last, we sought to define the temporal relationship between hepatic Cx32 expression and the onset of liver disease induced by these dietary models of steatohepatitis. We found that hepatic Cx32 expression decreased as WT mice were exposed to the diet for longer periods of time (Fig. 3A). To determine if this decrease was specific to Cx32 or the result of injury to hepatocytes, we assessed the hepatic expression of another hepatic gap junction, Cx43, and a protein produced exclusively in the liver, albumin. We found that neither Cx43 nor albumin mRNA expression decreased during the course of the MCDD (Fig. 3B), suggesting the observed decrease in hepatic Cx32 was not simply the result of cellular injury.
Figure 3.
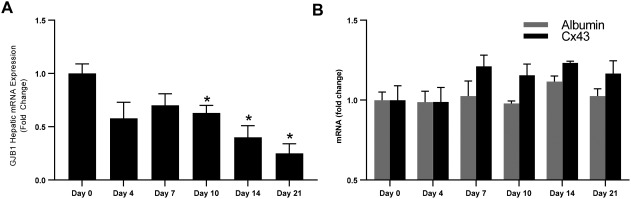
Hepatic GJB1 is down‐regulated during MCDD feeding. WT and Cx32KO mice were given a diet deficient in methionine and choline for 21 days, and hepatic (A) GJB1 and (B) albumin and Cx43 were measured at multiple time points during the diet. Statistical testing for significance was performed using a Student t test. Data represented as mean ± standard deviation. *P < 0.05.
Cx32 DEFICIENCY LIMITS INITIAL LIVER INJURY AND SUBSEQUENT INTESTINAL PERMEABILITY CHANGES INDUCED BY A DIETARY MODEL OF STEATOHEPATITIS
To gain further insight into the mechanism of protection observed in Cx32KO mice fed an MCDD, we first examined whether Cx32 deficiency in hepatocytes alone was sufficient for our observed in vivo protection. We isolated primary hepatocytes from WT and Cx32KO mice and then exposed them to increasing doses of palmitic acid, which induces steatosis and hepatic injury in cell culture. We found that Cx32KO hepatocytes exhibited less severe injury to these stimuli than WT hepatocytes (Fig. 4A), suggesting Cx32 deficiency in hepatocytes may account for the hepatoprotection seen in Cx32KO mice exposed to dietary models of steatohepatitis.
Figure 4.
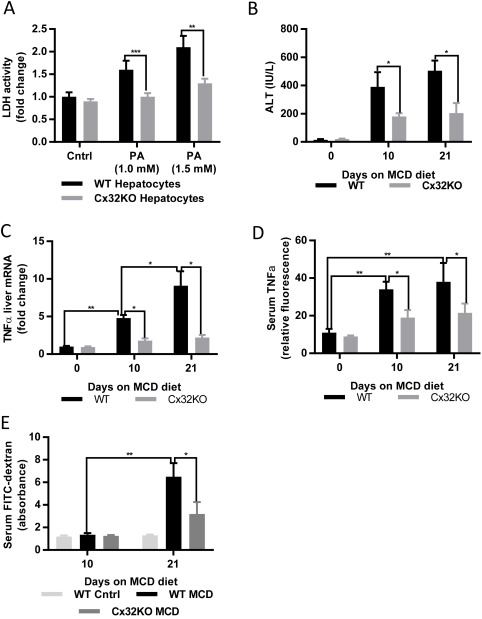
Connexin 32 deficiency limits initial liver injury and subsequent intestinal permeability changes induced by a dietary model of steatohepatitis. (A) Lactate dehydrogenase levels from the supernatant of hepatocytes isolated from WT and Cx32KO mice exposed to palmitic acid. WT and Cx32KO mice were exposed to an MCDD for 10 and 21 days. (B) Serum ALT, (C) serum FITC‐dextran levels, (D) hepatic TNF‐α, and (E) serum TNF‐α were measured at days 0, 10, and 21. Statistical testing for significance was performed using a Student t test. Data represented as mean ± standard deviation. Abbreviations: Cntrl, control; FITC, fluorescein isothiocyanate; LDH, lactate dehydrogenase; PA, palmitic acid.
Furthermore, our previous work has demonstrated the critical role for intestinal permeability in propagating the inflammatory response in the MCD model of steatohepatitis. Specifically, we previously showed that an initial liver injury leads to an increase in intestinal permeability and a subsequent microbial translocation that drives a secondary phase of liver injury.26 Here, we hypothesized that Cx32 deficiency will limit the initial liver injury, thereby blocking the secondary change in intestinal permeability and the resulting amplification of liver injury and inflammation. Indeed, we found that Cx32KO mice fed an MCDD were protected at an early point (day 10) compared to WT mice, based on serum ALT (Fig. 4B) and hepatic and systemic TNF‐α (Fig. 4C,D). While there was no difference in intestinal permeability at day 10 between WT mice and Cx32KO mice fed an MCDD, there was a marked reduction at day 21 (Fig. 4E). Taken together, these data suggest that Cx32 deficiency limits initial hepatocellular injury, leading to less hepatic and systemic inflammation, thereby preventing the subsequent increase in intestinal permeability, microbial translocation, and resulting secondary phase of inflammation and injury.
Discussion
In this work, we demonstrate for the first time that hepatic Cx32 expression decreases in patients with NAFLD as the histologic severity of inflammation and fibrosis increases. Furthermore, we show that Cx32 deficiency protects against inflammation and fibrosis in three independent murine dietary models of steatohepatitis. Our translational approach suggests Cx32 as a promising potential contributor to NAFLD pathogenesis.
Although we found that lower expression of hepatic Cx32 associated with the prevalence of more advanced disease in humans, in contrast Cx32KO mice were protected against diet‐induced NASH. To further understand these seemingly inconsistent observations, we studied the temporal expression pattern of connexins during the course of diet‐induced NASH. We found that expression of Cx32 but not Cx43 decreases over time with increasing liver injury in our MCDD‐fed NASH mouse models. œTaken together with the fact that complete elimination of Cx32 expression protects against diet‐induced steatohepatitis, we hypothesize that the liver might actively down regulate Cx32 expression through a negative feedback loop that is responding to parenchymal injury. Of course the mechanism by which this might be possible needs further evaluation. If this were the case, then patients with more advanced liver disease would be more likely to have lower Cx32 expression due to compensatory down‐regulation, which is consistent with our findings in the NAFLD patient cohort. Moreover, it should be emphasized that the activity of connexins, like other junctional proteins such as cadherins, is classically controlled through post‐translational modifications rather than transcriptional regulation. Thus, further studies are needed to shed light on the exact role of Cx32 and its variable expression in the pathogenesis of NAFLD.
Emerging data suggest that the various stages of NAFLD are associated with different rates of fibrosis progression. Accordingly, studies examining drivers of NAFLD pathogenesis in humans will benefit from histologic characterization of liver specimens in order to subclassify patients with NAFLD. A major strength of our study was the ability to fully characterize our NAFLD cohort and assess the association of hepatic Cx32 status with each histologic component of NAFLD. By doing so, we found that Cx32 was associated with inflammation and fibrosis but not with steatosis in patients. In parallel, our animal studies showed that Cx32 deficiency protected against inflammation and fibrosis but not steatosis in dietary models of steatohepatitis. Previous work has highlighted the ability of gap junctions to affect oxidative stress burden within the liver,11 and emerging data identify gap junctions as critical modulators of DNA sensing within the cell.8, 9 Accordingly, there is biological plausibility to support a contributory role of Cx32 in NAFLD progression.
Our work is consistent with multiple investigations demonstrating that Cx32 amplifies oxidative stress and inflammation.11, 12, 13 In line with our findings, Willebrords et al.16 found that specific inhibitors of hemichannels composed of Cx32 and Cx43 ameliorated steatohepatitis in mice fed a CDAHFD. There have been divergent observations made in two other studies, with possible confounders. Tiburcio et al.15 exposed WT and Cx32KO mice to the CDAHFD for 8 weeks, as we had done. In contrast, this group concluded that Cx32KO mice had increased liver injury and inflammation compared to WT mice. However, the WT mice fed the CDAHFD in that study developed only modest liver injury (ALT, ∼135 IU/L; AST, 49 IU/L; and histologic inflammation scores of ∼2), much lower than reported in multiple published studies showing that a CDAHFD induces severe hepatocellular injury and inflammation, as was the case in our study.24 Thus, it is unclear if the modest injury seen in the control group in their study was adequate to provide the dynamic range for appropriate comparisons between groups. Sagawa et al.14 used a murine model of NAFLD that combined the MCDD with the cancer‐promoting agent diethylnitrosamine. Using this model, they showed that Cx32 deficiency exacerbates liver injury. However, the addition of a cancer‐promoting agent makes this model system a less accurate reflection of human NAFLD and could certainly explain our seemingly conflicting results. Taken together, these dietary studies highlight the need for further research to better understand the mechanism by which Cx32 affects NAFLD pathogenesis.
Our understanding of the critical role of intestinal permeability and translocation of microbial products to the liver in the pathogenesis of NAFLD is evolving. Emerging data have highlighted the increased frequency of intestinal permeability changes seen in those with NASH compared to healthy controls,26 and mechanistic data have focused on the microbiome and specific microbial products that can engage the hepatic immune system to propagate hepatic inflammatory responses. While many studies have attempted to alter intestinal permeability by targeting the intestine itself, our data suggest that by limiting initial hepatic injury, secondary increases in intestinal permeability can be avoided. Further research is needed to decipher the complex relationship between the liver and intestinal system and the role of this liver–gut axis in NAFLD pathogenesis.
Intercellular communication through hepatic gap junctions represents a novel pathway that contributes to NAFLD pathogenesis. The role of gap junction‐dependent communication in liver diseases is evolving, and our data highlight this pathway as an attractive target for further study. Using a translational approach, we show that hepatic gap junctions may play a critical role in the progression of NAFLD.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1179/full.
Supporting Information 1
Potential conflict of interest: Dr. Warren is employed and owns stock in Glympse; Dr. Gala owns stock in New Amsterdam Genomics; Dr. Patel consults and has equity in Heprotech, Inc. The other authors have nothing to report.
Supported by an American Association for the Study of Liver Diseases Sheila Sherlock Clinical and Translation Research Award (to J.L.) and National Institutes of Health awards 5K23DK103119 (to M.K.G.), DK078772 (to R.T.C.), and 1R43AA023707‐01 (to S.J.P.).
REFERENCES
- 1. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462‐E468. [DOI] [PubMed] [Google Scholar]
- 2. Baumeister SE, Volzke H, Marschall P, John U, Schmidt CO, Flessa S, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5‐year observation. Gastroenterology 2008;134:85‐94. [DOI] [PubMed] [Google Scholar]
- 3. Charlton M. Cirrhosis and liver failure in nonalcoholic fatty liver disease: molehill or mountain? Hepatology 2008;47:1431‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuzaka T, Atsumi A, Matsumori R, Nie T, Shinozaki H, Suzuki‐Kemuriyama N, et al. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology 2012;56:2199‐2208. [DOI] [PubMed] [Google Scholar]
- 5. Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017;66:1037‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 2016;61:1294‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochem Biophys Acta 2004;1662:3‐21. [DOI] [PubMed] [Google Scholar]
- 8. Patel SJ, King KR, Casali M, Yarmush ML. DNA‐triggered innate immune responses are propagated by gap junction communication. Proc Natl Acad USA 2009;106:12867‐12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, et al. cGAS produces a 2′‐5′‐linked cyclic dinucleotide second messenger that activates STING. Nature 2013;498:380‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology 2012;55:622‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel SJ, Milwid JM, King KR, Bohr S, Iracheta‐Vellve A, Li M, et al. Gap junction inhibition prevents drug‐induced liver toxicity and fulminant hepatic failure. Nat Biotechnol 2012;30:179‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen ZY, Wang R, Huang F, Yuan DD, Li SR. Inhibition of gap junctions relieves the hepatotoxicity of TNF‐α. Genet Mol Res 2015;14:11896‐11904. [DOI] [PubMed] [Google Scholar]
- 13. Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, et al. The gap junction inhibitor 2‐aminoethoxy‐diphenyl‐borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c‐jun N‐terminal kinase activation. Toxicol Appl Pharmacol 2013;273:484‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sagawa H, Naiki‐Ito A, Kato H, Naiki T, Yamashita Y, Suzuki S, et al. Connexin 32 and luteolin play protective roles in non‐alcoholic steatohepatitis development and its related hepatocarcinogenesis in rats. Carcinogenesis 2015; 36: 1539‐1549. [DOI] [PubMed] [Google Scholar]
- 15. Tiburcio TC, Willebrords J, da Silva TC, Pereira IV, Nogueira MS, Crespo Yanguas S, et al. Connexin32 deficiency is associated with liver injury, inflammation and oxidative stress in experimental non‐alcoholic steatohepatitis. Clin Exp Pharmacol Physiol 2017;44:197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willebrords J, Cogliati B, Pereira IVA, da Silva TC, Crespo Yanguas S, Maes M, et al. Inhibition of connexin hemichannels alleviates non‐alcoholic steatohepatitis in mice. Sci Rep. 2017;7:8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hatoum IJ, Greenawalt DM, Cotsapas C, Daly MJ, Reitman ML, Kaplan LM. Weight loss after gastric bypass is associated with a variant at 15q26.1. Am J Hum Genet. 2013;92:827‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 19. Peloquin JM, Goel G, Kong L, Huang H, Haritunians T, Sartor RB, et al. Characterization of candidate genes in inflammatory bowel disease‐associated risk loci. JCI Insight 2016;1:e87899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Irizarry RA, Hobbs B, Collin F, Beazer‐Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4:249‐264. [DOI] [PubMed] [Google Scholar]
- 21. Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004;20:307‐315. [DOI] [PubMed] [Google Scholar]
- 22. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004;5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 2005;33:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsumoto M, Hada N, Sakamaki Y, Uno A, Shiga T, Tanaka C, et al. An improved mouse model that rapidly develops fibrosis in non‐alcoholic steatohepatitis. Int J Exp Pathol 2013;94:93‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, et al. High‐fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010:934‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell Mol Gastroenterol Hepatol 2015;1:222‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1179/full.
Supporting Information 1


