Abstract
Background
The genetic architecture of birth size may differ geographically and over time. We examined differences in the genetic and environmental contributions to birthweight, length and ponderal index (PI) across geographical-cultural regions (Europe, North America and Australia, and East Asia) and across birth cohorts, and how gestational age modifies these effects.
Methods
Data from 26 twin cohorts in 16 countries including 57 613 monozygotic and dizygotic twin pairs were pooled. Genetic and environmental variations of birth size were estimated using genetic structural equation modelling.
Results
The variance of birthweight and length was predominantly explained by shared environmental factors, whereas the variance of PI was explained both by shared and unique environmental factors. Genetic variance contributing to birth size was small. Adjusting for gestational age decreased the proportions of shared environmental variance and increased the propositions of unique environmental variance. Genetic variance was similar in the geographical-cultural regions, but shared environmental variance was smaller in East Asia than in Europe and North America and Australia. The total variance and shared environmental variance of birth length and PI were greater from the birth cohort 1990–99 onwards compared with the birth cohorts from 1970–79 to 1980–89.
Conclusions
The contribution of genetic factors to birth size is smaller than that of shared environmental factors, which is partly explained by gestational age. Shared environmental variances of birth length and PI were greater in the latest birth cohorts and differed also across geographical-cultural regions. Shared environmental factors are important when explaining differences in the variation of birth size globally and over time.
Keywords: Birthweight, birth length, ponderal index, twins, genetics, pooled studies
Key Messages
Additive genetic factors contributing to birth size have a small but consistent effect across geographical-cultural regions (Europe, North America and Australia, and East Asia) and across birth cohorts.
Environmental factors shared by co-twins importantly contribute to the inter-individual variation in birthweight, length and ponderal index, which is partly explained by gestational age.
Shared environmental influences were smaller in East Asia than in Europe and North America and Australia.
Introduction
Birth size is an indicator of infant health and is associated with health-related traits in later life such as hypertension,1–3 obesity,4,5 and psychosocial distress.6 Moreover, low birthweight is associated with an increased risk of metabolic diseases including type 2 diabetes7 and cardiovascular diseases in adulthood.8,9 Both genetic and environmental factors influence birth size.10,11 Associations between fetal genotype and birthweight can in part reflect the indirect effects of the maternal genotype influencing birthweight via the intrauterine environment.12 Studying monozygotic (MZ) and dizygotic (DZ) twin pairs is a widely used method to decompose total variance into fractions explained by genetic and environmental differences between individuals. The environmental factors shared by co-twins include gestational age, total placental weight and maternal factors, such as maternal body size and smoking. Individual placental characteristics, such as placental function including nutrient capacity, anatomy and perinatal injuries, can lead to differences in birth size between co-twins and are thus part of the environment unique for each individual twin. A previous Dutch study found that the genetic factors explained almost an identical share of the total variation of birthweight and length when estimated by parent-offspring trios of singletons (26% and 26%, respectively) and MZ and DZ twins (29% and 27%, respectively), supporting the value of the twin design when studying birth size.13 Gestational age affects birthweight and, because it is shared by co-twins, may lead to the overestimation of shared environment, if not accounted for.14
Genetic and environmental variation of fetal growth may differ between populations because of differences in maternal dietary habits, other environmental exposures and the gene pool of population. A multinational twin study reported that genetic factors explained 17% of the variation of birthweight. This contribution was similar in Western and East Asian populations, but there were differences in the proportions of environmental factors both shared and unshared by co-twins.15
It is well known that maternal nutrition and other maternal factors affect birth size, and the determinants of birth size may have changed across birth cohorts over the 20th century.16,17 However, there are no previous studies which would have analysed how the roles of genetic and environmental factors on birth size have changed over time. Further, the only international comparison was based only on seven twin cohorts;15 larger studies would be warranted to get more precise estimates. Finally, it would be important to analyse also indicators of birth size other than birthweight, and gestational age should be adjusted for because otherwise the role of shared environment would be inflated. To address these questions, we used birthweight and length data available in the largest pooled database of twin cohorts in the world. We aimed to examine differences in genetic and environmental contributions to birthweight, length and ponderal index (PI) [PI = weight (kg)/height (m3)] across geographical-cultural regions (Europe, North America and Australia, and East Asia) and across birth cohorts from 1915 through 2013, and how gestational age modifies these effects.
Methods
Sample
The data were derived from the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) database.18 Information on birthweight was available in 26 cohorts from 16 countries, and birth length and gestational age were available in 14 and 17 of these cohorts, respectively. In the majority of cohorts, the birth-related measures were parentally reported (79% for birthweight, 87% for birth length and 83% for gestational age) or self-reported (14%, 2% and 8%, respectively); only in a few cohorts were they based on records from nurses or clinicians (7%, 11% and 9%, respectively). However, birthweights from maternal recall and medical records were found to be highly correlated.19 The participating twin cohorts are listed in Table 1 (footnote) and were previously described in detail.18 The prevalence of obesity and overweight is lowest in East Asia, thus representing a less obesogenic environment, and highest in North America and Australia, thus representing a more obesogenic environment.20 Obesogenic environment can affect maternal dietary habits and maternal size, which indirectly reflect birth size.21–23 Therefore, we divided these cohorts into three geographical-cultural regions: Europe, North America and Australia, and East Asia.20
Table 1.
Sample sizes, means and standard deviations of birthweight (kg) by sex, region, birth year, and zygosity
| Zygosity | Boys |
Girls |
|||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| All cohortsa | MZ | 20 596 | 2.52 | 0.55 | 22 806 | 2.41 | 0.53 |
| DZ | 36 212 | 2.60 | 0.57 | 35 612 | 2.50 | 0.55 | |
| Region | |||||||
| Europeb | MZ | 13 318 | 2.53 | 0.56 | 13 974 | 2.42 | 0.53 |
| Europeb | DZ | 24 616 | 2.63 | 0.56 | 23 598 | 2.52 | 0.54 |
| NA and Ausc | MZ | 5258 | 2.52 | 0.56 | 6592 | 2.40 | 0.54 |
| NA and Ausc | DZ | 9765 | 2.57 | 0.59 | 10 223 | 2.47 | 0.57 |
| East Asiad | MZ | 1910 | 2.48 | 0.51 | 2132 | 2.39 | 0.47 |
| East Asiad | DZ | 1421 | 2.49 | 0.51 | 1403 | 2.41 | 0.47 |
| Birth year | |||||||
| 1915 to 1939 | MZ | 174 | 2.49 | 0.68 | 374 | 2.44 | 0.65 |
| 1915 to 1939 | DZ | 133 | 2.85 | 0.84 | 353 | 2.64 | 0.66 |
| 1940 to 1949 | MZ | 758 | 2.60 | 0.56 | 1280 | 2.47 | 0.52 |
| 1940 to 1949 | DZ | 1092 | 2.77 | 0.57 | 1558 | 2.61 | 0.51 |
| 1950 to 1959 | MZ | 1166 | 2.62 | 0.56 | 1952 | 2.46 | 0.54 |
| 1950 to 1959 | DZ | 1384 | 2.79 | 0.58 | 1900 | 2.66 | 0.56 |
| 1960 to 1969 | MZ | 286 | 2.63 | 0.58 | 480 | 2.40 | 0.55 |
| 1960 to 1969 | DZ | 176 | 2.72 | 0.64 | 284 | 2.53 | 0.59 |
| 1970 to 1979 | MZ | 3068 | 2.62 | 0.52 | 1826 | 2.48 | 0.48 |
| 1970 to 1979 | DZ | 3274 | 2.74 | 0.53 | 2048 | 2.63 | 0.51 |
| 1980 to 1989 | MZ | 2734 | 2.56 | 0.52 | 3072 | 2.49 | 0.52 |
| 1980 to 1989 | DZ | 3698 | 2.71 | 0.53 | 3722 | 2.61 | 0.52 |
| 1990 to 1999 | MZ | 8338 | 2.48 | 0.57 | 9474 | 2.38 | 0.53 |
| 1990 to 1999 | DZ | 16 932 | 2.56 | 0.56 | 16 634 | 2.47 | 0.54 |
| 2000 to 2013 | MZ | 4072 | 2.46 | 0.55 | 4348 | 2.36 | 0.52 |
| 2000 to 2013 | DZ | 9523 | 2.53 | 0.58 | 9113 | 2.43 | 0.55 |
NA, North America; Aus, Australia.
Includes all cohorts in the footnotes b–d and Africa (one cohort, 108 twin pairs, Guinea-Bissau Twin Study) and Middle East (one cohort, 400 pairs, Longitudinal Israeli Study of Twins).
Europe (11 cohorts, 37 753 twin pairs): East Flanders Prospective Twin Survey, Finntwin12, Finntwin16, Gemini Study, Hungarian Twin Registry, Italian Twin Registry, Norwegian Twin Registry, Swedish Young Male Twins Study of Adults, Swedish Young Male Twins Study of Children, Twins Early Developmental Study and Young Netherlands Twin Registry.
North America and Australia (9 cohorts, 15 919 twin pairs): Australian Twin Registry, Boston University Twin Project, Carolina African American Twin Study of Aging, Colorado Twin Registry, Michigan Twins Study, Minnesota Twin Family Study, Minnesota Twin Registry, Peri/Postnatal Epigenetic Twins Study and Quebec Newborn Twin Study.
East Asia (4 cohorts, 3433 twin pairs): Japanese Twin Cohort, Mongolian Twin Registry, Qingdao Twin Registry of Children and West Japan Twins Registry.
There were 121 997 twin individuals with data on birthweight. We excluded individuals with birthweight <0.5 or >5 kg (n = 79) or without data on their co-twins (n= 6606) as well as those with intra-pair difference in birthweight >2 kg (22 pairs) or contrasting information on birth year between co-twins (21 pairs), leading to 57 613 twin pairs [38% MZ, 34% same-sex dizygotic (SSDZ) and 28% opposite-sex dizygotic (OSDZ) twins]. For the analyses on birth length and PI, individuals without data on birth length (n= 64 626), those with birth length <25 or >60 cm (n = 33), PI <12 or >38 kg/m3 (n = 675) or born before 1970 (n = 261), and co-twins with intra-pair difference in birth length >12 cm (three pairs) or PI >15 kg/ m3 (nine pairs) were removed, leading to 27 084 twin pairs (38% MZ, 33% SSDZ and 29% OSDZ twins).
We further standardized birthweight, length and PI for gestational age separately by sex and within the individuals included in each group of analyses. These three measures of birth size were expressed as standard deviation (SD) scores of the respective means/weeks of gestation (z-scores; i.e. mean = 0 and SD = 1) to estimate their relative value for a given gestational age. Individuals with gestational age <25 or >45 weeks were excluded. Outlying values for birthweight, length and PI values for a given gestational age were checked by visual inspection of histograms for each gestational week and removed (0.2% for birthweight and 0.4% for birth length and PI), resulting in 38 806 (birthweight) and 23 742 twin pairs (birth length and PI) for analyses.
All participants were volunteers and gave their informed consent when participating in their original studies. A limited set of observational variables and anonymized data was delivered to the data management centre at the University of Helsinki. The pooled analysis was approved by the ethical committee of the Department of Public Health, University of Helsinki.
Statistical analyses
The data were analysed using genetic structural equations modelling.24 MZ twins share virtually the same genomic sequence, whereas DZ twins share, on average, 50% of their genes identical-by-descent. On this basis, the total variance was decomposed into variance due to additive genetic factors (A: correlated 1.0 for MZ and 0.5 for DZ pairs), shared (common) environmental factors (C: by definition, correlated 1.0 for MZ and DZ pairs) and unique (non-shared) environmental factors (E: by definition, uncorrelated for MZ and DZ pairs). All genetic models were fitted by the OpenMx package (version 2.0.1) in the R statistical platform.25
A full model with A, C and E factors was fitted to the data. We allowed a shared environmental correlation to be less than 1 for OSDZ pairs, as compared with 1 expected for SSDZ and MZ pairs; this would suggest the presence of sex-specific shared environmental factors affecting size at birth. Since boys and DZ twins showed greater birth size than girls and MZ twins, different means for sex and zygosity groups were allowed. We then conducted the analyses in the three geographical-cultural regions and across the birth cohorts from 1915 through 2013, per decade. Moreover, the genetic and environmental variances of birthweight were analysed for each twin cohort. Birthweight, length and PI values (both unstandardized and standardized for gestational age) were first adjusted for twin cohort within each sex and geographical-cultural region/birth year groups using linear regressions, and the resulting residuals were used in the analyses.
Results
Birthweight was greater in European and North American and Australian than in East Asian newborns (Table 1). The variance of birthweight was greatest in North America and Australia and lowest in East Asia. Mean birthweight did not show any clear pattern across the birth cohorts until 1980–89, but started to decrease from 1990–99 onwards. Mean birth length in European and North American and Australian boys and girls was greater than in East Asians (Table 2). The variance showed a less clear pattern, but was greatest in European and lowest in East Asian boys and girls. In MZ and DZ twins, the means of PI in boys were similar to those in girls in all geographical-cultural regions, except for East Asia where MZ girls had the greatest PI. The mean PI of boys was similar between geographical-cultural regions, whereas the mean PI of girls was greater in East Asia than in Europe and North America and Australia. The variances of PI were greatest in Europe and lowest in East Asia in both sexes.
Table 2.
Sample sizes, means and standard deviations of birth length (cm) and ponderal index (kg/m3) by sex, region, birth year, and zygosity
| Birth length |
Ponderal index |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zygosity | Boys |
Girls |
Boys |
Girls |
|||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| All cohorts | MZ | 10 394 | 47.0 | 3.2 | 10 054 | 46.4 | 3.3 | 10 394 | 24.4 | 3.0 | 10 054 | 24.3 | 3.3 |
| DZ | 17 758 | 47.5 | 3.3 | 15 962 | 46.9 | 3.2 | 17 758 | 24.4 | 3.1 | 15 962 | 24.4 | 3.2 | |
| Region | |||||||||||||
| Europea | MZ | 8614 | 47.1 | 3.3 | 8062 | 46.5 | 3.3 | 8614 | 24.4 | 3.1 | 8062 | 24.3 | 3.4 |
| Europea | DZ | 16 040 | 47.6 | 3.3 | 14 276 | 47.0 | 3.3 | 16 040 | 24.4 | 3.2 | 14 276 | 24.4 | 3.3 |
| NA and Ausb | MZ | 350 | 47.0 | 3.3 | 348 | 46.6 | 2.8 | 350 | 24.3 | 2.8 | 348 | 23.9 | 2.8 |
| NA and Ausb | DZ | 540 | 47.9 | 3.1 | 506 | 46.9 | 3.1 | 540 | 24.0 | 2.9 | 506 | 24.1 | 3.1 |
| East- Asiac | MZ | 1418 | 46.4 | 2.8 | 1624 | 45.7 | 2.8 | 1418 | 24.2 | 2.5 | 1624 | 24.6 | 2.7 |
| East Asiac | DZ | 1096 | 46.2 | 2.9 | 1090 | 45.7 | 2.7 | 1096 | 24.5 | 2.6 | 1090 | 24.6 | 2.6 |
| Birth year | |||||||||||||
| 1970 to 1979 | MZ | 2650 | 47.2 | 2.7 | 1300 | 46.5 | 2.5 | 2650 | 24.8 | 2.5 | 1300 | 25.0 | 2.7 |
| 1970 to 1979 | DZ | 2997 | 47.7 | 2.7 | 1785 | 47.1 | 2.5 | 2997 | 25.1 | 2.6 | 1785 | 25.2 | 2.7 |
| 1980 to 1989 | MZ | 1802 | 47.1 | 2.7 | 1936 | 46.5 | 2.9 | 1802 | 24.5 | 2.8 | 1936 | 24.8 | 2.9 |
| 1980 to 1989 | DZ | 2916 | 47.7 | 2.7 | 2862 | 47.0 | 2.7 | 2916 | 25.0 | 2.6 | 2862 | 25.1 | 2.8 |
| 1990 to 1999 | MZ | 4486 | 46.9 | 3.6 | 5160 | 46.3 | 3.5 | 4486 | 24.0 | 3.3 | 5160 | 24.0 | 3.4 |
| 1990 to 1999 | DZ | 8790 | 47.5 | 3.5 | 8422 | 46.9 | 3.4 | 8790 | 24.0 | 3.3 | 8422 | 24.0 | 3.4 |
| 2000 to 2013 | MZ | 1456 | 46.8 | 3.5 | 1658 | 46.1 | 3.5 | 1456 | 24.3 | 3.3 | 1658 | 24.1 | 3.4 |
| 2000 to 2013 | DZ | 3055 | 47.2 | 3.6 | 2893 | 46.5 | 3.4 | 3055 | 24.3 | 3.1 | 2893 | 24.3 | 3.3 |
NA, North America; Aus, Australia.
Europe (eight cohorts, 23 496 twin pairs).
North America and Australia (three cohorts, 872 twin pairs).
East Asia (two cohorts, 2614 twin pairs).
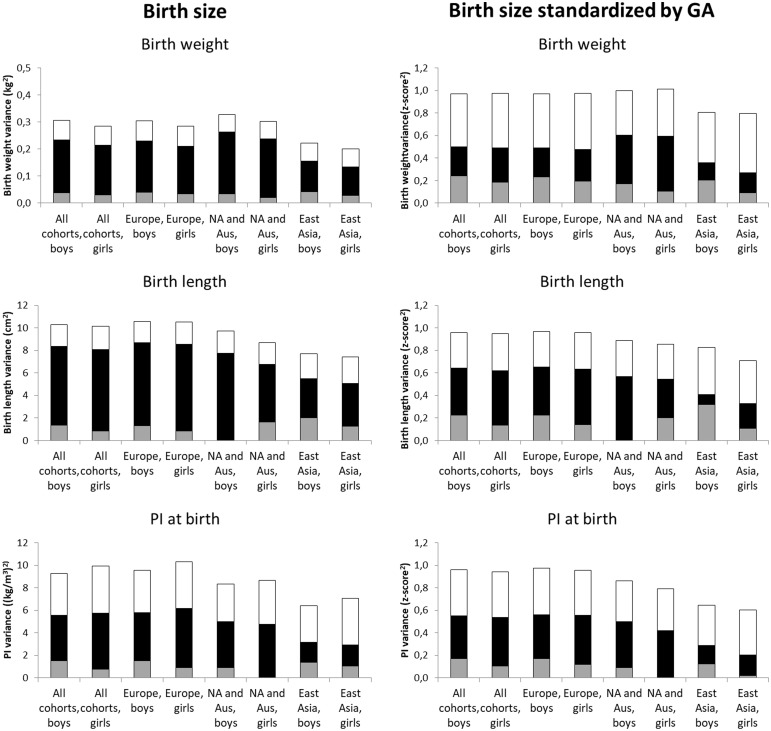
Figure 1 presents the additive genetic, shared environmental and unique environmental variances of birthweight, birth length and PI by cultural-geographical region; the exact point estimates and their 95% confidence intervals (CI) are available in Supplementary Tables 1 and 2, as Supplementary data at IJE online. Shared environmental factors explained the major part of the variation of birthweight and length, whereas shared and unique environmental factors explained roughly equal shares of the variation of PI. When comparing the cultural-geographical regions, the differences in the variances were mainly explained by shared environmental variances. For birthweight, the shared environmental variance was lower in East Asian boys (c2 = 0.11, 95% CI 0.09–0.14) and girls (c2 = 0.11, 95% CI 0.09–0.13) than found in Europe (c2 = 0.19, 95% CI 0.18–0.20 and 0.18, 95% CI 0.17–0.18, respectively) or North America and Australia (c2 = 0.23, 95% CI 0.22–0.24 and 0.22, 95% CI 0.21–0.23, respectively). Similar differences in the shared environmental variances were also found for birth length and PI. When the results were adjusted for gestational age, in particular the relative contribution of shared environmental variation to birthweight decreased. However, also in these analyses, the shared environmental variation was lower in East Asia than in the other regions. For birth length and PI, the relative decrease in shared environmental variance after the adjustment of gestational age was smaller than for birthweight.
Figure 1.
Additive genetic (grey), shared environmental (black) and unique environmental (white) variances of birth size measures before and after standardization for gestational age (GA) by geographic-cultural region.
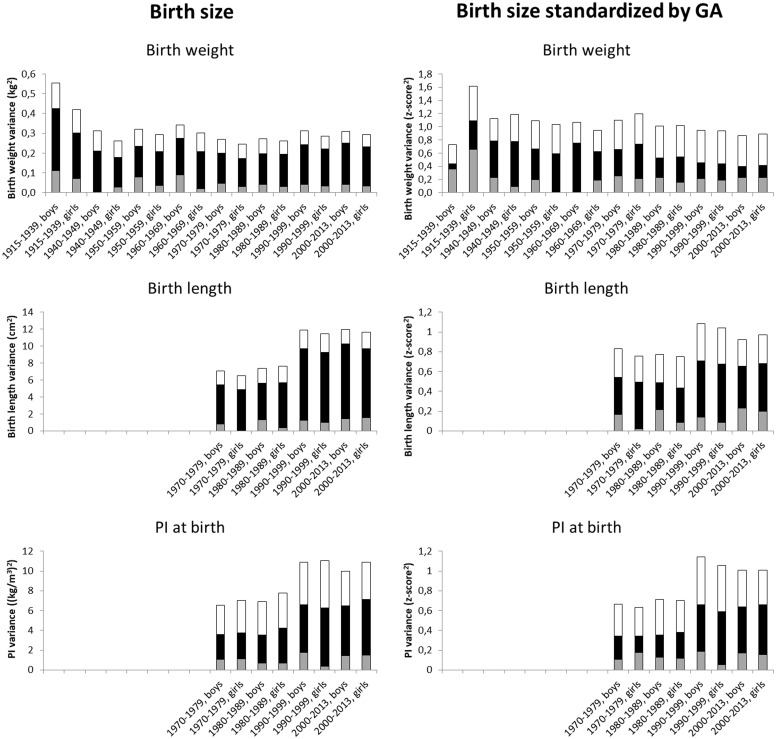
Figure 2 presents the corresponding results by birth cohorts (the exact point estimates and their 95% CIs are available in Supplementary Tables 1 and 2, available as Supplementary data at IJE online). For birth length and PI, the total variances were greater in the birth cohorts 1990–99 onwards as compared with the birth cohorts from 1970–79 to 1980–89. Adjusting the results for gestational age decreased in particular the proportions of shared environmental variance. After the adjustment for gestational age, systematic decrease in the shared environmental variance was found in the cohorts born in 1940–49 (c2 = 0.55, 95% CI 0.32–0.78 in boys and c2 = 0.68, 95% CI 0.46–0.87 in girls) up to 2000–13 (c2 = 0.17, 95% CI 0.10–0.26 and c2 = 0.18, 95% CI 0.11–0.27, respectively).
Figure 2.
Additive genetic (grey), shared environmental (black) and unique environmental (white) variances of birth size measures before and after standardization for gestational age (GA) by birth cohort.
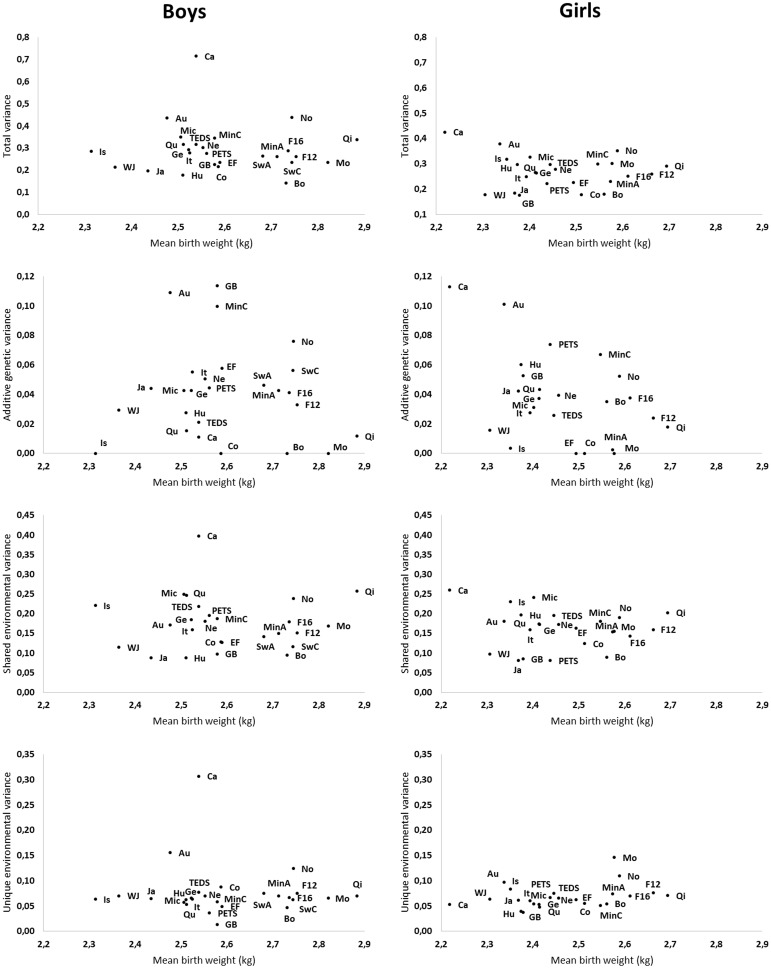
Figure 3 presents the variances of birthweight in each twin cohort according to the cohort mean birthweight (the exact point estimates with their 95% CIs are available in Supplementary Table 3, available as Supplementary data at IJE online). Some heterogeneity between the cohorts, especially in additive genetic variation, was found. However, this did not show any clear pattern according to the mean birthweight of cohort.
Figure 3.
Total, additive genetic, shared environmental and unique environmental variances of birthweight by twin cohort. Au, Australian Twin Registry; Bo: Boston University Twin Project; Ca, Carolina African American Twin Study of Aging; Co, Colorado Twin Registry; EF, East Flanders Prospective Twin Survey; F12, Finntwin12; F16, Finntwin16; Ge, Gemini Study; GB, Guinea-Bissau Twin Study; Hu, Hungarian Twin Registry; It, Italian Twin Registry; Ja, Japanese Twin Cohort; Is, Longitudinal Israeli Study of Twins; Mi, Michigan Twins Study; MinC, Minnesota Twin Family Study; MinA, Minnesota Twin Registry; Mo, Mongolian Twin Registry; No, Norwegian Twin Registry; PETS, Peri/Postnatal Epigenetic Twins Study; Qi, Qingdao Twin Registry of Children; Qu, Quebec Newborn Twin Study; SwA, Swedish Young Male Twins Study of Adults; SwC, Swedish Young Male Twins Study of Children; TEDS, Twins Early Developmental Study; WJ, West Japan Twins and Higher Order Multiple Births Registry; Ne, Young Netherlands Twin Registry.
Discussion
Using data from 57 613 complete twin pairs from 16 countries, the present study revealed that environmental factors shared by co-twins importantly contribute to the inter-individual variation in birthweight, birth length and PI. These factors also explained an important share of regional differences in the birthweight variation, as found also in previous studies.11,15,26 In the classical twin design, maternal effects shared by co-twins, including gestational age, would show up as a shared environmental variance. A previous international study of seven twin cohorts reported that from 50% to 70% of the total variance in birthweight was associated with maternal effects,15 which is close to the relative contribution of shared environmental variance found in our study before standardizing the results for gestational age. The standardization for gestational age decreased in particular the shared environmental variances for birthweight relative to the variances of birth length and PI, suggesting that birthweight is more influenced by the length of gestation than birth length and PI.27
The mean and total variance of birthweight and length were lower in East Asia than in the other regions, which corresponds with previous studies.28,29 The differences in the total variances were especially contributed by differences in shared environmental variance. It has been suggested that part of these maternal effects is due to maternal genes which regulate fetal growth, possibly through the intrauterine environment.30,31 Heritability estimates for the length of gestation were found to be over 30%,31,32 indicating that this is a heritable trait in European ancestry populations. Heritability of the length of gestation for East Asian populations is presently unknown, but if these differ from European ancestry estimates, this may partly explain these regional differences in shared environmental variances.
Various maternal genes have been shown to influence fetal growth, either directly or indirectly. A study examining genome-wide DNA methylation patterns in term human placentas showed that the patterns of DNA methylation were significantly associated with infant growth.33 Moreover, a multi-ancestry genome-wide association study indicated that two loci (INS–IGF2 and RB1), of the 60 genome-wide significant loci from maternal sources, fall within (or near) imprinted genes in fetal growth.12 If the frequencies of DNA methylation of gene and/or two loci among Asians differ from those among European ancestry,34 the genetic variability in maternal characteristics may explain some of the difference in shared environmental variance of birthweight between European ancestry and East Asians detected in the present study.
Mean PI was similar among boys across the geographical-cultural regions. However, mean PI was greater in East Asian than in European and North American and Australian girls. Gilson et al. (2015)27 indicated that PI varied between ethnicities. Moreover in the present study, shared environmental variance differed between these regions. The smaller shared environmental variance observed in East Asia than in the other regions may reflect differences in maternal nutrition, smoking and other environmental factors.
The means and variances of birthweight and length were lower in the cohorts born after than before 1990. In recent decades, the prevalence of preterm births among singletons and twins has increased in most industrialized countries, while at the same time perinatal mortality has decreased, mainly because of medically indicated preterm births.35–44 Gielen et al.44 (2010) reported that the frequency of infertility treatment and caesarean section, as well as advanced maternal age, have increased over the years, but none of these factors influenced the secular trends in birthweight. The decrease in birthweight and length found in the present study may reflect the decrease in mean length of gestation up to 32 weeks, as suggested by Gielen et al. (2010). Another factor with respect to time trends is the increasing survival of twin births. The survivors represent different proportions of twin pregnancies,45 and these proportions might be represented differentially in the distributions of birthweight and birth length. We found evidence for these explanations, since the results adjusted for gestational age did not show differences in the total variance of birthweight. This suggests that the increasing total variation over the birth cohorts is affected by increasing survival of babies with early gestational age. In the analyses adjusted for gestational age, shared environmental variance decreased over the birth cohorts. This may suggest that the variation in maternal factors has decreased at the same time as the general standard of living has increased.
When considering how well our results can be generalized, the assumptions made by the twin design need to be considered. MZ twins can either share one chorion and one amnion, or each fetus can have its own amnion, or they can each have their own chorion and amnion as for virtually all DZ twins. Previous Dutch and Belgian studies46,47 have reported somewhat lower correlations for mono-chorionic than di-chorionic MZ twins, which can lead to underestimation of additive genetic variance and overestimation of shared environmental variance. However, if there was extra variation because of more dissimilar intrauterine environments of MZ twins, it should have been seen as the higher trait variance in MZ twins, which was not the case in our study. One explanation is that very discordant pairs are not part of our study, because of higher neonatal mortality or other reasons. It would be important to estimate the contributions of genetic and environmental factors also by using other methods available for singleton pregnancies, to confirm how well our twin study results can be generalized to the whole population.
The main strength of our study is the very large sample size, allowing the investigation of differences on the genetic and environmental contributions to individual differences in birth size in much more detail than in previous studies. Pooling data from a large number of twin cohorts also permits analyses by geographical-cultural regions and birth cohorts born over 100 years. Further, we were able to analyse also birth length and PI and adjust the results for gestational age. Lack of information on gestational age, in particular, is a major limitation in previous studies, since it inflates shared environmental variation as demonstrated in our study. However, countries and/or geographical-cultural regions are not equally represented, and the database is heavily weighted towards populations following the Westernized lifestyle. There are few data available from the Middle East and Africa, and no data from South Asia or South America. It is also noteworthy that all countries have different historical developments, and thus the same birth cohorts can have been exposed to different environmental factores. This may well have diluted the differences between the birth cohorts in this study which reflects the average variances of different countries.
In conclusion, in contrast to the small contribution of genetic factors, environmental factors shared by co-twins importantly contribute to the inter-individual variation in birth size even after standardization for gestational age. The contributions of genetic effects on birth size were similar in the geographical-cultural regions, but unique environmental influences were slightly larger and shared environmental influences smaller in East Asia than in the other regions. This suggests that in the Westernized social context, there are features increasing variation in maternal nutrition and other maternal factors affecting birth size. Our results thus indicate that maternal factors importantly contribute to birth size and can then be a target for public health interventions to improve infant health.
Funding
This study was conducted within the CODATwins project (Academy of Finland #266592). The Australian Twin Registry is supported by a Centre of Research Excellence (grant ID 1079102) from the National Health and Medical Research Council, administered by the University of Melbourne. The Boston University Twin Project is funded by grants (#R01 HD068435 #R01 MH062375) from the National Institutes of Health to K.J.S. The Carolina African American Twin Study of Aging (CAATSA) was funded by a grant from the National Institute on Aging (grant 1RO1-AG13662–01A2) to KE.W. The Colorado Twin Registry is funded by NIDA-funded centre grant DA011015, & Longitudinal Twin Study HD10333; B.M.H. is supported by 5T32DA017637–11. Since its origin, the East Flanders Prospective Survey has been partly supported by grants from the Fund of Scientific Research, Flanders and Twins, a non-profit Association for Scientific Research in Multiple Births (Belgium). Data collection and analyses in Finnish twin cohorts have been supported by ENGAGE—European Network for Genetic and Genomic Epidemiology—FP7-HEALTH-F4–2007, grant agreement number 201413, the National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to R.J.R., the Academy of Finland Center of Excellence in Complex Disease Genetics (grant numbers: 213506, 129680), and the Academy of Finland (grants 100499, 205585, 118555, 141054, 265240, 263278 and 264146 to J.K.). Gemini was supported by a grant from Cancer Research UK (C1418/A7974). Anthropometric measurements of the Hungarian twins were supported by Medexpert Ltd, Budapest, Hungary. The Italian Twin Registry was partially supported by the Chiesi Foundation. The Longitudinal Israeli Study of Twins was funded by the Starting Grant no. 240994 from the European Research Council (ERC) to A.K. The Michigan State University Twin Registry has been supported by Michigan State University, as well as grants R01-MH081813, R01-MH0820–54, R01-MH092377–02, R21-MH070542–01 and R03-MH63851–01 from the National Institute of Mental Health (NIMH), R01-HD066040 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD), and 11-SPG-2518 from the MSU Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, the NICHD, or the National Institutes of Health. Data collection and research stemming from the Norwegian Twin Registry are supported, in part, by the European Union’s Seventh Framework Programmes ENGAGE Consortium (grant agreement HEALTH-F4–2007-201413, and BioSHaRE EU (grant agreement HEALTH-F4–2010-261433). The Netherlands Twin Register acknowledges the Netherlands Organization for Scientific Research (NWO) and MagW/ZonMW grants 904–61-090, 985–10-002, 912–10-020, 904–61-193, 480–04-004, 463–06-001, 451–04-034, 400–05-717, Addiction-31160008, Middelgroot-911–09-032, Spinozapremie 56–464-14192; VU University’s Institute for Health and Care Research (EMGO+ ); the European Research Council (ERC - 230374), the Avera Institute, Sioux Falls, South Dakota (USA). PETS was supported by grants from the Australian National Health and Medical Research Council (grant numbers 437015 and 607358 to J.C. and R.S.), the Bonnie Babes Foundation (grant number BBF20704 to J.M.C.), the Financial Markets Foundation for Children (grant no. 032–2007 to J.M.C.) and by the Victorian Government’s Operational Infrastructure Support Program. The Quebec Newborn Twin Study acknowledges financial support from the Fonds Québécois de la Recherche sur la Société et la Culture, the Fonds de la Recherche en Santé du Québec, the Social Science and Humanities Research Council of Canada, the National Health Research Development Program, the Canadian Institutes for Health Research, Sainte-Justine Hospital’s Research Center and the Canada Research Chair Program (Michel Boivin). The Twins Early Development Study (TEDS) is supported by a programme grant (G0901245) from the UK Medical Research Council, and the work on obesity in TEDS is supported in part by a grant from the UK Biotechnology and Biological Sciences Research Council (31/D19086). Currently TEDS is supported by MRC grant ‘MR/M021475/1’. The West Japan Twins and Higher Order Multiple Births Registry was supported by Grant-in-Aid for Scientific Research (B) (grant number 15H05105) from the Japan Society for the Promotion of Science.
Supplementary Material
Acknowledgement
We thank S Alexandra Burt and Kelly L Klump, Michigan State University, East Lansing, MI, USA, for contributing data to this project.
Conflict of interest: Reimbursement of travel expenses by Novonordic in 2015 and honorarium plus travel expenses from GSK in 2010 for H.B-N.
References
- 1. Gamborg M, Byberg L, Rasmussen F.. Birthweight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol 2007;166:634–45. [DOI] [PubMed] [Google Scholar]
- 2. IJzerman RG, Stehouwer CDA, Boomsma DI.. Evidence for genetic factors explaining the birthweight-blood pressure relation. Analysis in twins. Hypertension 2000;36:1008–12. [DOI] [PubMed] [Google Scholar]
- 3. Law CM, Shiell AW.. Is blood pressure inversely related to birthweight? The strength of evidence from a systematic review of the literature. J Hypertens 1996;14:935–41. [PubMed] [Google Scholar]
- 4. Sørensen HT, Sabroe S, Rothman KJ, Gillman M, Fischer P, Sorensen TI.. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ 1997;315:1137.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansson M, Rasmussen F.. Birthweight and body mass index in young adulthood: the Swedish young male twins study. Twin Res 2001;4:400–05. [DOI] [PubMed] [Google Scholar]
- 6. Cheung YB, Ma S, Machin D, Karlberg J.. Birthweight and psychological distress in adult twins: a longitudinal study. Acta Paediatrica 2004;93:965–68. [DOI] [PubMed] [Google Scholar]
- 7. Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D.. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 2000;133:176–82. [DOI] [PubMed] [Google Scholar]
- 8. Eriksson M, Wallander MA, Krakau I, Wedel H, Svardsudd K.. Birthweight and cardiovascular risk factors in a cohort followed until 80 years of age: the study of men born in 1913. J Intern Med 2004;255:236–46. [DOI] [PubMed] [Google Scholar]
- 9. Wang S-F, Shu L, Sheng J. et al. Birthweight and risk of coronary heart disease in adults: a meta-analysis of prospective cohort studies. J Dev Orig Health Dis 2014;5:408–19. [DOI] [PubMed] [Google Scholar]
- 10. Pietiläinen KH, Kaprio J, Räsänen M, Winter T, Rissanen A, Rose RJ.. Tracking of body size from birth to late adolescence: contributions of birth length, birthweight, duration of gestation, parents’ body size, and twinship. Am J Epidemiol 2001;154:21–29. [DOI] [PubMed] [Google Scholar]
- 11. Whitfield JB, Treloar SA, Zhu G, Martin NG.. Genetic and non-genetic factors affecting birth-weight and adult Body Mass Index. Twin Res 2001;4:365–70. [DOI] [PubMed] [Google Scholar]
- 12. Horikoshi M, Beaumont RN, Day FR.. Genome-wide associations for birthweight and correlations with adult disease. Nature 2016;538:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mook-Kanamori DO, van Beijsterveldt CE, Steegers EA. et al. Heritability estimates of body size in fetal life and early childhood. PLoS One 2012;7:e39901.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salas AA, Carlo WA, Ambalavanan N. et al. Gestational age and birthweight for risk assessment of neurodevelopmental impairment or death in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2016 Feb 19. doi: 10.1136/archdischild-2015-309670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hur YM, Luciano M, Martin NG. et al. A comparison of twin birthweight data from Australia, the Netherlands, the United States, Japan, and South Korea: are genetic and environmental variations in birthweight similar in Caucasians and East Asians? Twin Res Hum Genet 2005;8:638–48. [DOI] [PubMed] [Google Scholar]
- 16. Rugholm S, Baker JL, Olsen LW, Schack-Nielsen L, Bua J, Sørensen TIA.. Stability of the association between birthweight and childhood overweight during the development of the obesity epidemic. Obes Res 2005;13:2187–94. [DOI] [PubMed] [Google Scholar]
- 17. Schack-Nielsen L, Molgaard C, Sorensen TI, Greisen G, Michaelsen KF.. Secular change in size at birth from 1973 to 2003: national data from Denmark. Obesity (Silver Spring) 2006;14:1257–63. [DOI] [PubMed] [Google Scholar]
- 18. Silventoinen K, Jelenkovic A, Sund R. et al. The CODATwins project: the cohort description of collaborative project of development of anthropometrical measures in twins to study macro-environmental variation in genetic and environmental effects on anthropometric traits. Twin Res Hum Genet 2015;18:348–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen CB, Gamborg M, Heitmann B, Sørensen TIA, Baker JL.. Comparison of birthweight between school health records and medical birth records in Denmark: determinants of discrepancies. BMJ Open 2015;5:e008628.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng M, Fleming T, Robinson M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hjertholm KG, Iversen PO, Holmboe-Ottesen G. et al. Maternal dietary intake during pregnancy and its association to birth size in rural Malawi: a cross-sectional study. Matern Child Nutr 2018. Jan;14(1). doi: 10.1111/mcn.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crume TL, Brinton JT, Shapiro A. et al. Maternal dietary intake during pregnancy and offspring body composition: the Healthy Start Study. Am J Obstet Gynecol 2016;215:609.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chia AR, de Seymour JV, Colega M. et al. A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am J Clin Nutr 2016;104:1416–23. [DOI] [PubMed] [Google Scholar]
- 24. Posthuma D, Beem AL, de Geus EJ. et al. Theory and practice in quantitative genetics. Twin Res 2003;6:361–76. [DOI] [PubMed] [Google Scholar]
- 25. Neale MC, Hunter MD, Pritikin JN. et al. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika 2016;81:535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boomsma DI, Orlebeke JF, van Baal GC.. The Dutch Twin Register: growth data on weight and height. Behav Genet 1992;22:247–51. [DOI] [PubMed] [Google Scholar]
- 27. Gibson KS, Waters TP, Gunzler DD, Catalano PM.. A retrospective cohort study of factors relating to the longitudinal change in birthweight. BMC Pregnancy Childbirth 2015;15:344.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Urquia ML, Qiao Y, Ray JG, Liu C, Hjern A.. Birth outcomes of foreign-born, native-born, and mixed couples in Sweden. Paediatr Perinat Epidemiol 2015;29:123–30. [DOI] [PubMed] [Google Scholar]
- 29. Lagiou P, Hsieh CC, Trichopoulos D. et al. Birthweight differences between USA and China and their relevance to breast cancer aetiology. Int J Epidemiol 2003;32:193–98. [DOI] [PubMed] [Google Scholar]
- 30. Nance WE, Kramer AA, Corey LA, Winter PM, Eaves LJ.. A causal analysis of birthweight in the offspring of monozygotic twins. Am J Hum Genet 1983;35:1211–23. [PMC free article] [PubMed] [Google Scholar]
- 31. Van Den Oord EJ, Rowe DC.. A step in another direction: looking for maternal genetic and environmental effects on racial differences in birthweight. Demography 2001;38:573–76. [DOI] [PubMed] [Google Scholar]
- 32. Clausson B, Lichtenstein P, Cnattingius S.. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG 2000;107:375–81. [DOI] [PubMed] [Google Scholar]
- 33. Banister CE, Koestler DC, Maccani MA, Padbury JF, Houseman EA, Marsit CJ.. Infant growth restriction is associated with distinct patterns of DNA methylation in human placentas. Epigenetics 2011;6:920–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siffert W, Forster P, Jockel KH. et al. Worldwide ethnic distribution of the G protein beta3 subunit 825T allele and its association with obesity in Caucasian, Chinese, and Black African individuals. J Am Soc Nephrol 1999;10:1921–30. [DOI] [PubMed] [Google Scholar]
- 35. Joseph KS, Kramer MS, Marcoux S. et al. Determinants of preterm birth rates in Canada from 1981 through 1983 and from 1992 through 1994. N Engl J Med 1998;339:1434–49. [DOI] [PubMed] [Google Scholar]
- 36. Joseph KS, Allen AC, Dodds L, Vincer MJ, Armson BA.. Causes and consequences of recent increases in preterm birth among twins. Obstet Gynecol 2001;98:57–64. [DOI] [PubMed] [Google Scholar]
- 37. Kogan MD, Alexander GR, Kotelchuck M. et al. Trends in twin birth outcomes and prenatal care utilization in the United States, 1981-1997. JAMA 2000;284:335–41. [DOI] [PubMed] [Google Scholar]
- 38. Ananth CV, Joseph KK, Smulian JC.. Trends in twin neonatal mortality rates in the United States, 1989 through 1999: influence of birth registration and obstetric intervention. Am J Obstet Gynecol 2004;190:1313–21. [DOI] [PubMed] [Google Scholar]
- 39. Ananth CV, Joseph KS, Kinzler WL.. The influence of obstetric intervention on trends in twin stillbirths: United States, 1989-99. J Matern Fetal Neonatal Med 2004;15:380–87. [DOI] [PubMed] [Google Scholar]
- 40. Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM.. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol 2005;105:1084–91. [DOI] [PubMed] [Google Scholar]
- 41. Goldenberg RL, Culhane JF, Iams JD, Romero R.. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keirse MJ, Hanssens M, Devlieger H.. Trends in preterm births in Flanders, Belgium, from 1991 to 2002. Paediatr Perinat Epidemiol 2009;23:522–32. [DOI] [PubMed] [Google Scholar]
- 43. Hartley RS, Hitti J.. Increasing rates of preterm twin births coincide with improving twin pair survival. J Perinat Med 2010;38:297–303. [DOI] [PubMed] [Google Scholar]
- 44. Gielen M, van Beijsterveldt CE, Derom C. et al. Secular trends in gestational age and birthweight in twins. Hum Reprod 2010;25:2346–53. [DOI] [PubMed] [Google Scholar]
- 45. Vedel C, Oldenburg A, Worda K. et al. Short- and long-term perinatal outcome in twin pregnancies affected by weight discordance. Acta Obstet Gynecol Scand 2017;96:233–42. [DOI] [PubMed] [Google Scholar]
- 46. van Beijsterveldt CE, Overbeek LI, Rozendaal L. et al. Chorionicity and heritability estimates from twin studies: the prenatal environment of twins and their resemblance across a large number of traits. Behav Genet 2016;46:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vlietinck R, Derom R, Neale MC. et al. Genetic and environmental variation in the birthweight of twins. Behav Genet 1989;19:151–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.