Abstract
Background:
This paper focuses on Periodontal Profile Class (PPC), a component of the Periodontal Profile Phenotype (P3) System that may be more representative of the periodontitis phenotype than current case definitions of periodontitis. Data illustrate the unique aspects of the PPC compared with other commonly used periodontal classification indices.
Methods:
Latent Class Analysis (LCA) identified discrete classes of individuals grouped by tooth-level clinical parameters. The analysis defined seven distinct periodontal profile classes (PPC A through G) and seven distinct tooth profile classes (TPC A through G). This LCA classification was an entirely data-derived agnostic process without any preconceived presumptions of what constituted disease.
Results:
Comparing the PPC with the Centers for Disease Control/American Academy of Periodontology (CDC/AAP) and European indices, the PPC is unique in that it contains four disease classes not traditionally used. Less than half of individuals classified as Healthy by both the CDC/AAP and European indices were Healthy using the PPC. About 25% of those classified as Severe by CDC/AAP and European indices were PPC-Severe. The remainder spread out over the High Gingival Index, Posterior Disease, Tooth Loss, and Severe Tooth Loss phenotypes.
Conclusions:
The PPC classification provides a significant departure from the traditional clinical case status indices that have been used, but has resulted in clinical phenotypes that are quite familiar to most clinicians who take notice of the distribution of missing teeth, areas of recession, diminished periodontal support, and other aspects of the dentition while conducting a periodontal examination. The mutually exclusive categories provided by the PPC system provide periodontal clinical summaries that can be an important component of precision dentistry.
Keywords: Diagnosis, epidemiology, gingivitis, periodontitis
1 |. INTRODUCTION
One major goal of the periodontology profession involves an evidence-based approach to assigning risk of future periodontal disease or disease progression to patients in order to provide individualized treatment (precision dentistry). The practice of precision dentistry requires optimal measures of clinical and biomarker assessments as well as a thoughtful analysis of the etiology and appropriate interventions to focus on the risks and treatment needs of the individual patient. The Periodontal Profile Phenotype (P3) System contributes to the practice of precision dentistry by providing a standardized method for classifying a patient, based upon history and clinical findings, that allows a practitioner to assess risk of future attachment and tooth loss. It does this without the benefit of genetic, biomarker, or other patient-specific parameters that will be needed to fully support the practice of precision dentistry.
The current authors have developed, validated, tested, and shown utility for risk profiling for tooth loss and attachment loss for a new classification of periodontal disease that may have greater utility for personalized dentistry than current case definitions of periodontitis.1 Importantly, this system uses clinical data to create three person-level measures and one tooth-level measure that we refer to as P3 (Fig. 1). The four key measures generated by the P3 algorithm include:
-
-
The Periodontal Profile Class (PPC), a person-level measure, provides a clinical, seven-class taxonomic system of the patient’s disease status.
-
-
The Tooth Profile Class (TPC) assigns each tooth present within a patient to one of seven possible statuses.
-
-
The Index of Periodontal Risk (IPR) incorporates the individual patient PPC and TPC composition and a longitudinal database of tooth loss and attachment loss to create a point estimate of future disease risk for the individual patient.
-
-
The Index of Periodontal Class (IPC) places the IPR risk score of the patient into a population context of low, moderate, or high risk.
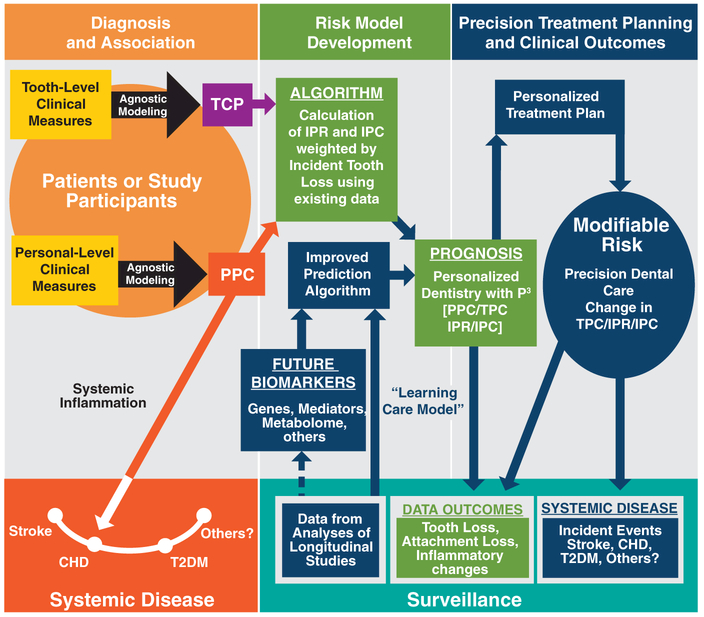
FIGURE 1.
Potential clinical utility of the University of North Carolina periodontal profile phenotype (P3) system. This figure provides a conceptual framework for the development and clinical utility of the P3 system containing the three major domains of Diagnosis, Risk. and Outcome. This is a work in progress, and the colors indicate areas supported by a publication. The dark blue color indicates the components of the P3 system that are under development.
P3 uses these four measures to potentially form the basis of an improved “healthcare learning system” for precision dentistry. There are four domains outlined in Fig. 1: 1) creating robust diagnostic “bins” of individuals with the PPC; 2) Risk Model Development that can lead to a more precise and probabilistic quantification of prognosis for an individual patient (using IPR for the subject risk and TPC for tooth-level risk; 3) using this information to lead to more precise Treatment Planning and provide more sensitive clinical outcome assessments; and 4) by including a Surveillance Platform, the risk modelling can be refined and will accommodate the addition of future biomarker data (e.g., genetics, inflammatory mediator, and other “omic” dimensions to optimize precision dentistry.
The current study aims to provide the reader with a more comprehensive understanding of the scope and current status of the P3 project, and to describe in detail the relationships between the PPC and TPC components of the P3 as shown within the orange circle under Diagnosis and Association in Fig. 1. The third aim is to illustrate the unique aspects of this system compared with other commonly used periodontal classification indices by providing a proof-of-principle demonstration for practitioners to eventually use the parts of this system that currently are under development. The overarching hypothesis is that the P3 System will have utility for establishing more precise dental prognoses and clinical outcome assessments, and potentially provide stronger associations with systemic diseases.
2 |. MATERIALS AND METHODS
2.1 |. Study samples
The Atherosclerosis Risk in Communities (ARIC) study2 enrolled 15,792 participants within the age group of 45 to 64 years in four different United States communities (Forsyth County [North Carolina], city of Jackson [Mississippi], suburbs of Minneapolis [Minnesota], Washington County [Maryland]). All participants provided written informed consent to a protocol reviewed and approved by the Institutional Review Board on research involving human subjects at the University of North Carolina and at each study performance site. In the current study, all participants who completed the fourth clinic visit (1996–1998) in ARIC (N = 11,656) were eligible for inclusion. Of the 11,656 ARIC participants seen at the fourth clinical visit, we excluded study participants who did not receive a periodontal examination. These exclusions resulted in 6,793 individuals who were included in this study as well as the latent class analysis (LCA) that resulted in the creation of PPC.3
The combined National Health and Nutrition Examination Surveys (NHANES 2009–2010, 2011–2012, and 2013–2014) were used as a validation study population. The technical details of the surveys, including sampling design, periodontal data collection protocols, and data availability, are described elsewhere.4–8 Briefly, periodontal measurements were collected for 3,750 individuals (NHANES 2009–2010), for 3,338 individuals (NHANES 2011–2012), and for 3,622 individuals (NHANES 2013–2014), yielding a total of 10,710. Periodontal measures were collected on six sites per tooth for all teeth present in the mouth except third molars.8–10
2.2 |. Measurement of exposures
2.2.1 |. Periodontal profile class (PPC)
The analytic approach implemented person-level LCA to identify discrete classes of individuals using seven tooth-level clinical parameters. These parameters were: ≥one site with interproximal clinical attachment level (iCAL) ≥3 mm, ≥one site with probing depth (PD) ≥4 mm, extent of bleeding on probing (BOP) (dichotomized at 50% or ≥three sites per tooth), gingival inflammation index11 (GI = 0 or GI ≥1), plaque index12 (PI = 0 or Pl ≥1), the presence/absence of full prosthetic crowns for each tooth, and tooth status (present or absent).3
Individuals were classified into mutually exclusive latent classes based upon their responses to a set of observed categoric variables. Criteria used to determine the optimal number of classes included the Akaike Information Criterion13 and the Bayesian Information Criterion,14 while ensuring that clinically relevant categories were maintained. Milligan and Cooper’s15 recommendation was used for the maximum number (n) of classes, which suggests stopping when the newly added class (n + 1) is not clinically distinct from the previous number (n) of identified classes. Additionally, it was verified that mean posterior probabilities of correct class assignment were > 0.7, which according to Nagan16 indicates adequate class separation. In the first step, the person-level LCA was used to classify individuals into seven latent classes based on 224 dichotomous variables (derived from seven tooth-level variables, using the clinical parameters referred to above for each of 32 teeth). The class membership probabilities represent the overall, unconditional proportions of individuals in each of seven latent classes. The model parameters from the first step were used to compute the posterior probabilities (the probability of event A occurring given that event B has occurred) of each individual’s membership into each class based upon the values of the 224 items, or as many of them as were observed for that individual.3
Because patients with periodontal disease have individual teeth with clinical signs ranging from health to severe disease, a tooth-level LCA analysis was carried out to capture the distribution of these tooth-specific classes within each PPC subgroup. This tooth-level analysis of each individual’s existing complement of teeth produced seven categories of teeth.
2.2.2 |. CDC/AAP and European indices
The Centers for Disease Control/American Academy of Periodontology (CDC/AAP) index17 along with the European Periodontal index18 may be the most frequently used indices and are a step forward in creating some consistency in periodontal disease case definitions. The current study used the CDC/AAP four-level index (healthy; mild; moderate; and severe disease)7 because it provided separation of the healthy and mild groups. The definitions of the levels of disease for both indices appear in Table 1. The European Index has three levels (healthy, incipient, and severe).18
TABLE 1.
Concordance (percent of subjects) of PPC status classification of periodontal disease with CDC/AAP and european classifications in dental ARIC and NHANES 2009–2014 Studies
| Study and Classification Used PPC Total N = 6,793 |
PPC-A Healthy N = 1,845 |
PPC-B Mild N = 1,047 |
PPC-C High GI N = 694 |
PPC-D Tooth Loss N = 800 |
PPC-E Posterior Disease N = 999 |
PPC-F Severe Tooth Loss N = 900 |
PPC-G Severe Disease N = 508 |
|---|---|---|---|---|---|---|---|
| Dental ARIC | Number (%) Study Participants | ||||||
| CDCa | |||||||
| Healthy N = 775 | 351 (45%) | 93 (12%) | 31 (4%) | 75 (10%) | 0 (0%) | 225 (29%) | 0 (0%) |
| Mild N = 2,035 | 867 (43%) | 402 (20%) | 286 (14%) | 204 (10%) | 50 (2%) | 207 (10%) | 19 (1%) |
| Moderate N = 2,799 | 582(21%) | 486 (17%) | 284 (10%) | 370 (13%) | 573 (20%) | 328 (12%) | 176 (6%) |
| Severe N = 1,194 | 45 (4%) | 66 (6%) | 93 (8%) | 151 (19%) | 376 (32%) | 140 (12%) | 313 (26%) |
| Total 6,793 | |||||||
| Europeanb | |||||||
| Healthy N = 749 | 379 (47%) | 98 (12%) | 28 (4%) | 75 (9%) | 0 (0%) | 219 (27%) | 0 (0%) |
| Incipient N = 5,021 | 1,465 (29%) | 939 (19%) | 587 (12%) | 575 (11%) | 818 (16%) | 388 (8%) | 249 (5%) |
| Severe N = 973 | 1 (0%) | 10 (1%) | 79 (8%) | 150 (15%) | 181 (19%) | 293 (30%) | 259 (27%) |
| Total = 6,793 | |||||||
| NHANES 2009–2014 | Number (%) Study Participants | ||||||
| PPC Total N = 10,710 | N = 5,878 | N = 674 | N = 962 | N = 1,197 | N = 506 | N = 693 | N = 800 |
| CDC | |||||||
| Healthy N = 2,521 | 2,277 (90%) | 0 (0%) | 5 (0%) | 101 (4%) | 0 (0%) | 138 (5%) | 0 (0%) |
| Mild N = 3,199 | 2,568 (80%) | 112(4%) | 145 (5%) | 237 (7%) | 17 (1%) | 115 (4%) | 5 (0%) |
| Moderate N = 3,791 | 1,015 (27%) | 505 (13%) | 709 (19%) | 605 (16%) | 317 (8%) | 370 (10%) | 270 (7%) |
| Severe N = 1,199 | 18 (2%) | 57 (5%) | 103 (9%) | 254 (21%) | 172 (14%) | 70 (6%) | 525 (44%) |
| Total N = 10,710 | |||||||
| European | |||||||
| Healthy N = 2,563 | 2 350 (92%) | 0 (0%) | 4 (0%) | 95 (4%) | 0 (0%) | 114 (4%) | 0 (0%) |
| Incipient N = 6,681 | 3 525 (53%) | 666 (10%) | 766 (11%) | 748 (11%) | 417 (6%) | 272 (4%) | 287 (4%) |
| Severe N = 1,466 | 3 (0%) | 8 (1%) | 192 (13%) | 354 (24%) | 89 (6%) | 307 (21%) | 513 (35%) |
| Total = 10,710 | |||||||
The definitions for levels of the CDC/AAP index are: No periodontitis, no evidence of mild, moderate, or severe periodontitis; Mild periodontitis, ≥two interproximal sites with AL ≥3 mm and ≥two interproximal sites with PD of ≥4 mm (not on same tooth) or one site with PD ≥5 mm; Moderate periodontitis, ≥two interproximal sites with AL ≥4 mm (not on same tooth) or ≥two interproximal sites with PD ≥5 mm (not on same tooth); Severe periodontitis, ≥two interproximal sites with AL ≥6 mm (not on same tooth) and ≥one interproximal site with PD ≥5 mm.
The definitions for levels of the European Index are: No periodontitis, no evidence of incipient or severe periodontitis; Incipient periodontitis, presence of proximal attachment loss of ≥3 mm in ≥two non-adjacent teeth; Severe periodontitis, presence of proximal attachment loss of ≥5 mm in ≥30% of teeth.
2.3 |. Statistical analysis
Table 1 and most of the figures presented in this paper are descriptive in nature and consist of means and proportions. Supplementary Figure 1 in the online Journal of Periodontology presents posterior probabilities that were produced as part of the LCA analysis13 using the Dental ARIC data set.
The average posterior probabilities were calculated for each of the seven person-level and tooth-level LCA classes. The average posterior probabilities for class assignment were calculated within each of the seven indices.
3 |. RESULTS
3.1 |. Concordance of PPC, CDC/AAP, and European classification systems
Table 1 displays similarities and differences in disease classifications between the PPC and both the CDC/AAP and the European Indexes. The PPC classification creates seven classes that are nominal categories arranged in order of increasing extent of interproximal attachment loss ≥3 mm. The PPC classes were given monikers (or names) based upon the dominant clinical feature of the teeth in that class. Although some of the monikers are familiar, the clinical status of the teeth in that class are different than expected. The mean clinical characteristics of each class are presented in Table 2 of an earlier article3 for readers interested in how traditional measures of periodontal disease are represented in each PPC.
For the PPC, 1,845 of 6,793 (27%) of the Dental ARIC participants were PPC healthy, 15% had mild disease, 10% had high gingival index, 12% had tooth loss, 15% had posterior disease, 13% had severe tooth loss, and 7% had severe disease. By contrast, the CDC/AAP index classified the dental ARIC participants as 11% healthy, 30% mild, 42% moderate, and 17% severe, and the European system classes were 11% healthy, 74% incipient, and 14% severe. Of participants classified as healthy by CDC/AAP and European indices, 45% and 47%, respectively, were classified healthy by the PPC. Of those not classified as healthy by the PPC, 29% CDC/AAP and 27% European were classified as having PPC-Severe Tooth Loss, indicating most subjects were near or completely edentate in the maxillae with only a few lower anterior/premolar teeth that were less diseased.3(Table 2) Of the remaining CDC/AAP and European “healthy” participants, about 12% were classified as “mild disease,” and 10% were classified as having “tooth loss” with the remaining 4% being High GI. Of those classified as “severe” by CDC/AAP, the PPC indicated 32% had “posterior disease,” and the majority of the remainder had “tooth loss” and “severe tooth loss.” Of those classified “severe” by the European index, most had “severe tooth loss, posterior disease, and tooth loss” (30%, 19%, and 15%, respectively). As one examines the extremes of Health versus Severe Disease as identified by the PPC in the dental ARIC dataset, 45% of the CDC/AAP Healthy are PPC-Healthy, while 26% of the CDC/AAP Severe are PPC-Severe. Very similar relationships are evident between the European and PPC indices. Thus, PPC identified four new categories of disease with distinct clinical traits in addition to the traditional healthy, mild, and severe categories. These new categories are composed of individuals who were in one of the CDC/AAP or European classification categories, but now represent previously “hidden” groupings of individuals with similar within-class clinical presentations.
The age range for the NHANES 2009–2014 sample was 30 to 85 years, which made it a younger group than the ARIC sample, where the youngest age was 52 years. However, the PPCs, when comparing the CDC/AAP to the European classifications, showed similar patterns even though this population had less overall disease. For example, in the NHANES studies, about 90% of study participants classified as “healthy” by both the CDC/AAP and European Indices were classified as “healthy” by PPC. Of those not classified as “healthy” by PPC, most were allocated to the two “tooth loss” categories. For those classified as “severe” by CDC/AAP and European indices, 44% and 35%, respectively, were classified as “severe” by PPC, while the remainder were spread among the other PPC categories.
3.2 |. PPC and TPC distributions
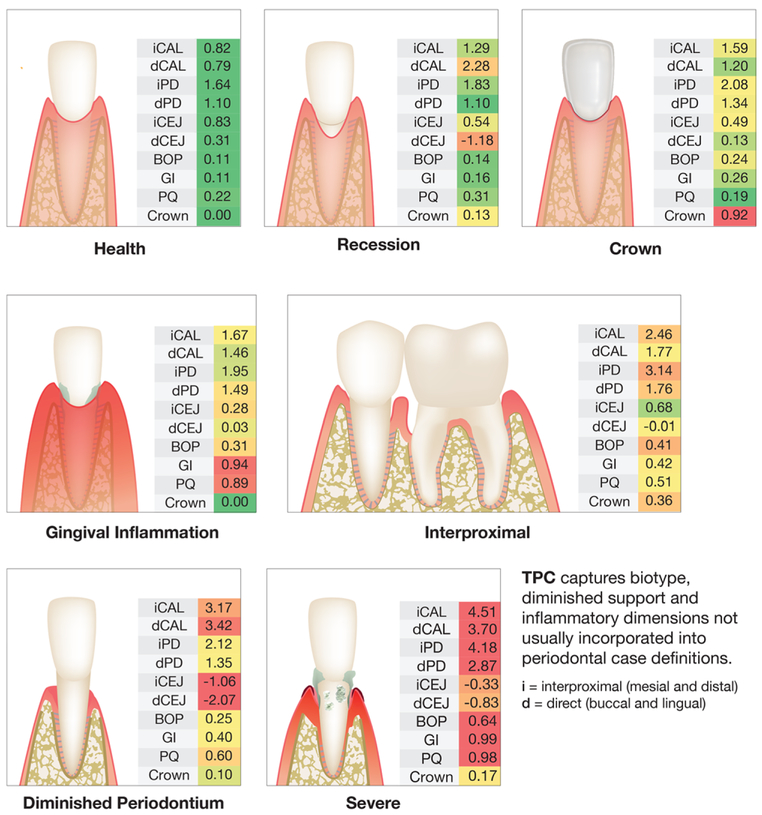
The tooth-based analysis in Fig. 2 presents the mean toothlevel clinical periodontal measures and proportion with crowns within each TPC in the form of multiple heat maps that allow the monikers of the seven TPC classes to become more apparent. For example, the clinical measures are green for Healthy teeth, indicating that there is little disease and no crowns. In contrast, teeth classified as Severe are mostly red, indicating high levels of interproximal and direct CAL and PD as well as high levels of BOP, GI, plaque, and recession. As an example, for TPC-Recession the mean clinical characteristics of the tooth is direct buccal and/or lingual recession (dCEJ), or circumferential loss of attachment with minimal PD to reflect a tooth with diminished periodontium, or high gingival inflammation and plaque that define a TPC-High GI. One can build a profile of a patient by considering the multiple possible combinations of TPC that might arise. The power of this approach is that any given individual can have a unique combination of these TPCs.
FIGURE 2.
Clinical measures for each tooth profile class. TPC, Tooth Profile Class; GI = Gingival Inflammation; Interprox, Interproximal Disease; DimPerio, Diminished Periodontium; iCAL, mean interproximal (i.e., adjoining tooth surfaces, four sites) attachment loss in mm; dAL = mean direct attachment loss in mm (buccal and lingual surfaces, two sites); iPD, mean interproximal probing depth (PD) in mm; dPD, mean direct PD in mm (buccal and lingual surfaces); iCEJ, mean distance from free gingival margin to cementoenamel junction (CEJ) in mm measured interproximally, negative indicates recession, i.e., CEJ exposed and above the free gingival margin; dCEJ, mean distance from free gingival margin to CEJ in mm measured directly at buccal and lingual surfaces (two sites), negative indicates recession, i.e., CEJ exposed and above the free gingival margin; BOP, mean bleeding on probing as percent of sites (six sites per tooth); GI, mean Gingival Index score as measured across buccal sites. GI is scored 0 to 3; PQ, mean Plaque index score measured across buccal sites. PQ is scored 0 to 3; Crown, proportion of teeth with crowns
For reference purposes, the heat map in supplementary Figure 1 in the online Journal of Periodontology displays another tooth-based analysis of the intraoral distribution of TPCs for all 145,497 teeth expressed as posterior probabilities of having that characteristic. The posterior probability is a conditional probability when all other variables are considered. This provides an intraoral map of probabilities of TPC status for the ARIC cohort.
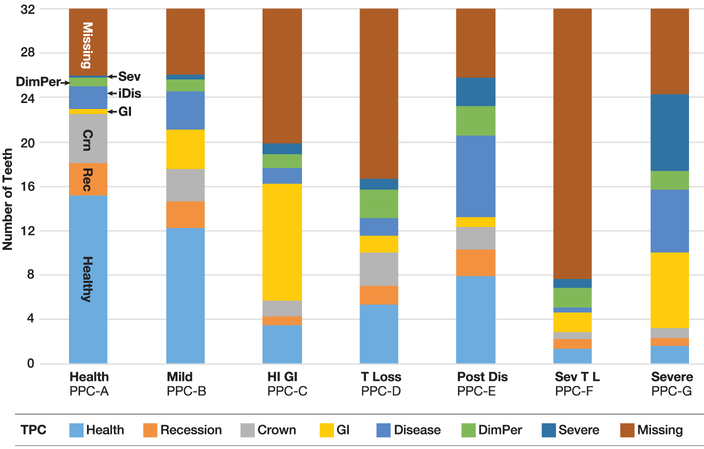
The identification of TPCs in addition to PPCs enables assignment to a tooth class for each existing tooth in a patient, as well as missing teeth. Fig. 3 presents the proportion of each of the seven TPCs and missing teeth within each PPC (0–32 teeth). This person-based analysis shows that PPC-Health individuals have few missing teeth (predominantly 3rd molars) and an average of 15 healthy teeth with very few teeth that are GI or iCAL. TPC-Severe is rare, but there are some recession TPCs (with local attachment loss in that area), and a few crowned teeth can be noted. As one examines the Mild, Posterior, and Severe disease PPCs, those phenotypes are associated with having a high number of diseased TPCs and fewer Healthy TPCs. The key difference here is that most teeth are present in these three PPCs and the PPC-Posterior Disease has fewer GI teeth and a greater number of teeth with iCAL, along with a few Severe teeth. PPC-Severe has more GI TPCs, more iCAL TPCs, more severe TPC teeth, and very few heathy TPC teeth. One should note that a few severe teeth in the PPC-Posterior Disease does not automatically shift the individual to the PPC-Severe class, as might be expected with other classifications. Furthermore, the PPC classification algorithm identifies three new PPCs: High GI that has more missing teeth and teeth with GI scores on the majority of the remaining teeth; Tooth Loss, and Severe Tooth Loss. There also is a refinement in what might be considered moderate periodontitis into Mild Disease and Posterior (interproximal) Disease.
FIGURE 3.
Distribution of tooth profile classes (TPC) by person profile class (PPC) in the Dental ARIC study. Sev, severe disease; iDis, interproximal disease; DimPer, diminished periodontium; GI, gingival inflammation; Crn, crown on tooth; Rec, recession; T, tooth; T L, tooth loss
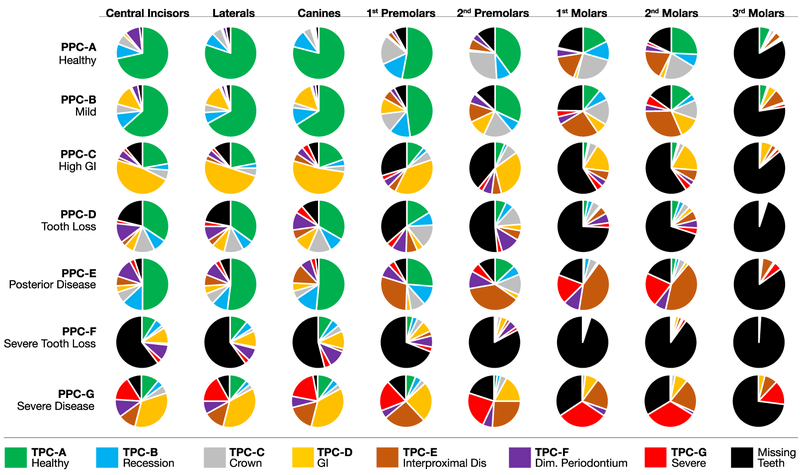
Fig. 4 shows the distribution of TPCs (including missing teeth) within each PPC, stratified by tooth type. This analysis combined all four quadrants of teeth (e.g., central incisors [#s 8+9+24+25]). The diagram shows the intraoral distribution of TPCs for the various PPC classifications. In other words, the distributions of tooth conditions are displayed considering the periodontal health of the individual (PPC). There are few surprises in that irrespective of PPC, there is a tendency for more disease and/or missing teeth moving from the anterior to posterior tooth positions. An exception is that the High GI individual presents with more GI TPCs in the anterior regions compared with posterior regions. The Interproximal Disease TPCs are mainly on molars in individuals classified as having Mild Disease, while the Interproximal Disease TPCs extend to the sextant to include the premolars in the individuals with Posterior Disease. Fig. 4 clearly demonstrates that missing canines are predominantly in the PPC-Severe Tooth Loss, consistent with the observation that canines are among the last teeth to be lost in a failing dentition.
FIGURE 4.
Intraoral distribution of tooth profile class (TPC) by periodontal profile class (PPC) by tooth type. GI, gingival index; Dis, disease; Dim, diminished
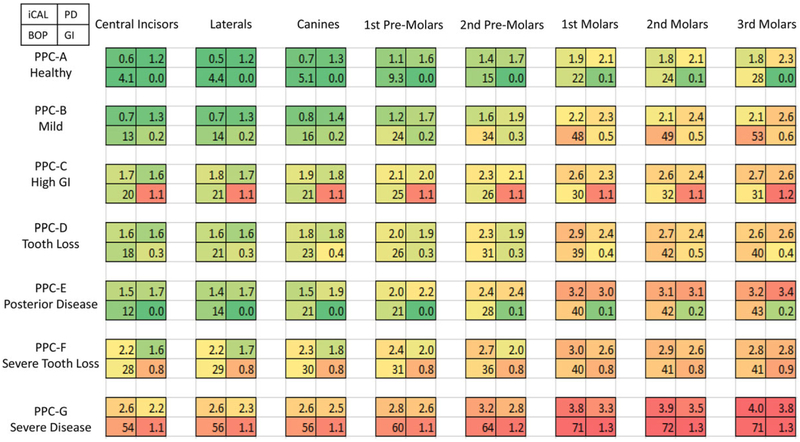
Fig. 5 displays the mean clinical values for iCAL, PD, extent (%) BOP, and mean GI, as a four-celled grid, for a 7 × 7 table of PPC by tooth type. Worsening mean values are seen transitioning from green to yellow to red which generally shows more severe disease from anterior to posterior. It is interesting to note that the PPC-High GI and the PPC-Severe have multiple GI TPCs throughout the mouth; however, the PPC-High GI has low/moderate BOP, whereas the PPC-Severe has high BOP. Meanwhile, the Severe TPC also has a high percent of GI and plaque as well as 64% with BOP. In contrast, Fig. 5 shows that among PPC-Mild Disease and PPC-Posterior Disease subjects, the higher extent of BOP scores are in the posterior teeth. However, the BOP scores increase dramatically from 54% to 71% anterior to posterior among PPC-Severe, with second molars displaying an average of 3.9 mm iCAL and 3.5 mm PD. When present, the third molars consistently displayed more disease across all PPCs.
FIGURE 5.
Mean interproximal attachment level, mean probing depth, extent bleeding on probing, and mean gingival index by periodontal profile class (PPC) and tooth type
4 |. DISCUSSION
Importantly, the reshuffling of subjects across domains of disease by differing classifications is, in itself, not meaningful unless the new classifications provide additional insight into risk or responses to treatment. Historically, a legion of indices, extent scores, severity scores, clinical measures, and study-specific distributions of attachment loss and PD have been used to describe the prevalence and incidence of periodontal diseases and their associations with individuallevel and group-level characteristics. These measures also have been useful for diagnosis and treatment planning. The assumption is that these tooth-based measures are useful for other objectives, such as assigning risk for future disease progression, establishing associations with systemic diseases and conditions, and practicing precision dentistry. This study questioned whether this is a valid assumption. For this reason, all a priori assumptions of what the periodontal phenotype should look like were abandoned in deriving the P3 system.
For many years, literature reviews, position papers, and reports have strongly stated that it is difficult to assess the state of our knowledge because of the variety of measures used to represent the periodontal phenotype.19–23 In addition, it is still not known how to value teeth that are lost due to periodontitis or other causes when assessing risk for disease progression and tooth loss. For these reasons, the various case status definitions used to describe the periodontal phenotype are narrowly focused and of limited utility when attempting to generalize across studies or apply them to other populations. Perhaps this problem is most profound when trying to establish a relevant case type for intervention studies. Inclusion criteria for case definitions are disparate, and responders and non-responders often are thought to be a result of the inclusion criteria. For example, a couple of severe teeth (TPC-Severe) might qualify a subject for study enrollment, but the TPC-Severe teeth do not intrinsically have similar risks for attachment loss when compared across PPCs.1
Table 1 provides a number of insights into how the PPC differs in the way it classified people compared with the CDC/AAP and the European indices. A larger number of PPC study participants are healthy and fewer are classified as Severe compared with the other indices. Of study participants classified as Healthy by the CDC/AAP, only 45% are Healthy by PPC and 12% are Mild. Another 10% are classified as Tooth Loss and another 29% are in the Severe Tooth Loss group. This pattern is similar for individuals classified by the European Index. These patterns show the influence of tooth loss, an event we have not been able to capture previously as part of a phenotype. Admittedly, a number of studies have adjusted for number of teeth in multivariable risk models; however, the PPC captures tooth loss, as well as high GI patterns, as separate subclasses of the phenotype. The TPC-Recession and TPC-Diminished Periodontium classes represent special types of attachment loss that likely capture the biotype of the subject. Our additional work has shown that High GI and the two tooth loss classes are at higher risk for future tooth loss and attachment loss.1 We note that, traditionally, when an individual’s periodontium is classified as having periodontitis, the GI status is ignored, i.e., there are no periodontitis subcategories, such as “periodontitis with extensive inflammation.” The LCA agnostically created this High GI classification, and it does perform well in predicting future attachment loss and tooth loss.1 It also is important to note that only slightly more than 25% of individuals classified as having Severe Periodontitis by the CDC/AAP and European indices were classified as Severe by the PPC because most of the individuals moved to other PPCs that had major tooth loss. Is this separation of the Severe Periodontitis case status meaningful? A companion paper indicates that future tooth loss and attachment loss rates differ by these three groups.1 This pattern could mean that the groups respond differently to treatment, which also has implications for selection of research volunteers in future clinical studies. Among the generally younger individuals in the NHANES studies, we see similar patterns when 44% CDC/AAP and 35% European participants classified as Severe Disease were classified as Severe Disease by the PPC. A good proportion (30% to 45%) of those not classified as Severe by PPC were allocated to the Tooth Loss and Severe Tooth Loss categories. It should be noted that although the PPC appears to work similarly in the younger NHANES database, these classifications are based on individuals who have chronic periodontitis. Whether the PPC categories also are appropriate for patients with aggressive periodontitis is not known at this time. However, among subjects aged 30 to 35 years in the NHANES data, there are proportionately higher numbers of PPC-Severe individuals among those with disease (i.e., 22% versus 8.7% for those aged > 60 years, data not shown). Thus, when disease is present among younger individuals, it tends to be more severe in clinical presentation and consistent with an aggressive periodontitis classification.
This difference between the PPC and other indices may have implications for clinical studies and trials, especially those trials involving systemic diseases and chronic conditions. If tooth loss itself conveys part of the risk for prevalent or incident systemic conditions, then current treatments for periodontal disease would not reduce the portion of the risk represented by tooth loss.
The current authors understand that the PPC is a more complex phenotype and the fact that a computer algorithm generated it may make some clinicians suspicious of its utility. However, the math has been calculated to harmonize group-level data to apply to an individual, such that a simple data entry of clinical signs by a practitioner will generate a total TPC profile and assign a PPC for the patient.3 Figs. 2 through 5 provide information on the more traditional clinical measures that underlie the different PPC and TPC monikers so that the reader can see the patterns and “get a feel” for the underlying clinical structure. For example, Fig. 3 shows that for an individual to be classified Healthy, there can be a few teeth with recession (and with concomitant attachment loss), a few missing teeth (mostly 3rd molars), a couple of teeth with interproximal disease, and, on average, less than one tooth with High GI, Diminished Periodontium, and Severe Disease; in sum, risk for disease progression is low. However, we do not suggest that individuals classified as “healthy” are not in need of periodontal care; the moniker refers to individuals with the lowest overall disease state and lowest risk for progression and tooth loss. Fig. 4 shows the influence of the individual’s PPC on dental condition for each tooth type. For example, the patterns of Healthy teeth in Healthy individuals differ dramatically for each tooth type compared with individuals classified as High GI. Looking down each column, the influence of the individual’s classification on the tooth health (TPC) of each tooth type can be seen. Fig. 5 presents the mean iCAL, PD, sites with BOP, and GI score for each tooth type for each PPC. Clinicians may find that all of these figures show patterns of oral health and disease that are familiar.
The P3 System, although still under development, was created so that it could be used by practitioners and researchers. We developed a web-based data entry system so that practitioners will be able to submit a patient’s clinical data and receive the patient’s PPC class along with a risk score for future tooth loss as part of a clinical record. Three example records are displayed as supplementary Figures 2A, 2B, and 2C in the online Journal of Periodontology. These figures show a typical record for patients classified as either PPC-Healthy, PPC-High GI, or PPC-Severe. After treatment, the practitioner can submit updated clinical information and receive a new score indicating changes in risk. The forms could be part of the patient’s electronic or paper dental record.
5 |. CONCLUSION
The PPC is the result of an agnostically, data-driven process creating definitions of disease that appear to have some face validity since it has resulted in phenotypes that are quite familiar to clinicians. In contrast with other indices that ignore tooth loss, the PPC creates two classes of tooth loss and a distinct High GI class that also has mild tooth loss. These classes result in a smaller “pure severe periodontitis group” that contains individuals with most of their teeth. As a result, the PPC creates more clinically homogeneous “bins” of individuals that may be very important when it comes to selecting individuals for clinical trials or refining therapeutic choices. The PPC system provides an opportunity for standardization of clinical definitions that currently appear to have broad utility in assessing risk for disease progression, designing therapy and monitoring clinical outcomes.
Supplementary Material
ACKNOWLEDGMENTS
The Atherosclerosis Risk in Communities Study (ARIC) is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN2682011 00005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268 201100010C, HHSN268201100011C, and HHSN26820 1100012C). The authors thank the staff and participants of the ARIC study for their important contributions. The ARIC Dental Study was funded by NIH/NIDCR R01-DE021418, and R01-DE021986, and NIH/NCRR UL1-TR001111. James Beck and Kevin Moss report no conflicts of interest. Thiago Morelli is funded by NIH/NIDCR K23-DE025093, and Steven Offenbacher is supported by R01-DE023836. They also report no conflicts of interest.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Morelli T, Moss KL, Preisser JS, et al. Periodontal profile classes predict periodontal disease progression and tooth loss. J Periodontal 2018;89:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 3.Morelli T, Moss K, Beck J, et al. Derivation and validation of the Periodontal and Tooth Profile Classification System for patient stratification. J Periodontal 2017;88:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res 2010;89:1208–1213. [DOI] [PubMed] [Google Scholar]
- 5.Dye BA, Li X, Lewis BG, Iafolla T, Beltran-Aguilar ED, Eke PI. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2009–2010. J Public Health Dent 2014;74:248–256. [DOI] [PubMed] [Google Scholar]
- 6.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. CDC periodontal disease surveillance workgroup. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012;91:914–920. [DOI] [PubMed] [Google Scholar]
- 7.Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontal 2015;86:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol 1990;61:521–528. [DOI] [PubMed] [Google Scholar]
- 9.Beck JD, Koch GG, Offenbacher S. Attachment loss trends over 3 years in community-dwelling older adults. J Periodontol 1994;65:737–743. [DOI] [PubMed] [Google Scholar]
- 10.Beck JD, Sharp T, Koch GG, Offenbacher S. A 5-year study of attachment loss and tooth loss in community-dwelling older adults. J Periodontal Res 1997;32:516–523. [DOI] [PubMed] [Google Scholar]
- 11.Loe H, Silness J. Periodontal Disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 1963;21:533–551. [DOI] [PubMed] [Google Scholar]
- 12.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 1964;22:121–135. [DOI] [PubMed] [Google Scholar]
- 13.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14:671–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz G Estimating the dimension of a model. Ann Statist 1978;6:461–464. [Google Scholar]
- 15.Milligan GW, Cooper M. An examination of procedures for determining the number of clusters in a data set. Psychometrika. 1985;50:159–179. [Google Scholar]
- 16.Nagan D Group-based modelling of development Harvard University Press; 2005. [Google Scholar]
- 17.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol 2007;78:1387–1399. [DOI] [PubMed] [Google Scholar]
- 18.Tonetti MS. Claffey N; European Workshop in Periodontology group C. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol 2005;32(Suppl. 6):210–213. [DOI] [PubMed] [Google Scholar]
- 19.Chapple ILC. Genco R; on behalf of working group 2. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol 2013;40:S106–S112. [DOI] [PubMed] [Google Scholar]
- 20.Tonetti MS. Van Dyke TE; working group 1 of the joint E.F.P.A.A.P. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013;84:S24–S29. [DOI] [PubMed] [Google Scholar]
- 21.Sanz M Kornman K: on behalf of working group 3. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol 2013;84:S164–S169. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Periodontol 2013;84:S70–S84. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Periodontology. Epidemiology of periodontal diseases: position paper. J Periodontol 2005;76:1406–1419. [DOI] [PubMed] [Google Scholar]
- 24.Beck JD, Moss KL, Morelli T, Offenbacher S. Periodontal profile class is associated with prevalent diabetes, coronary heart disease, stroke, and systemic markers of C-reactive protein and interleukin 6. J Periodontol 2018;89:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 2012;83:1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol 2007;78:1911–1925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.