Abstract
Fungal infections in CD4+ T-cell immunocompromised patients have risen sharply in recent years. Although vaccines offer a rational avenue to prevent infections, there are no licensed fungal vaccines available. Inactivated vaccines are safer but less efficacious, and require adjuvants that may undesirably bias toward poor protective immune responses. We hypothesized that reducing the TCR signaling threshold could potentiate anti-fungal CD8+ T cell responses and immunity to inactivated vaccine in the absence of CD4+ T cells. Here, we show that Cblb, a negative regulator of TCR signaling, suppresses CD8+ T cells in response to inactivated fungal vaccination in a mouse model of CD4+ T cell lymphopenia. Conversely, Cblb deficiency enhanced both the type 1 (e.g., IFNγ) and type 17 (IL-17A) CD8+ T cell responses to inactivated fungal vaccines, and augmented vaccine immunity to lethal fungal pneumonia. Furthermore, we show that immunization with live or inactivated vaccine yeast did not cause detectable pathology in Cblb−/− mice. Augmented CD8+ T cell responses in the absence of CBLB also did not lead to terminal differentiation or adversely affect the expression of transcription factors, T-bet, Eomes and RORγt. Additionally, our adoptive transfer experiments showed that CBLB impedes the effector CD8+ T cell responses in a cell-intrinsic manner. Finally, we showed that ablation of Cblb overcomes the requirement of HIF-1α for expansion of CD8+ T cells upon vaccination. Thus, adjuvants that target CBLB may augment inactivated vaccines and immunity against systemic fungal infections in vulnerable patients.
INTRODUCTION
Fungal infections in immunocompromised patients, such as those with AIDS and CD4+ T cell lymphopenia have sharply risen in recent years (1). Substantial evidence indicates that CD4+ T cells are necessary to control the fungal infections, but these cells are lacking in CD4+ lymphopenic individuals. We have previously shown that vaccine-induced antifungal cytokine-producing CD8+ T cells, both type 1 (e.g., IFNγ) and type 17 (IL-17A) cells, can compensate to provide sterilizing, vaccine-immunity in the absence of CD4+ T cells (2, 3). Although no licensed fungal vaccines are currently available, vaccines targeting CD8+ T cells in vulnerable immune-compromised patients would require an additional layer of safety. Subunits vaccines are highly desirable, but protective fungal epitopes have not been determined for CD8+ T cells. Inactivated vaccines are safe, but need adjuvants that may undesirably bias T-cell responses that support antibody production. Adjuvants for fungal vaccines tailored to CD4+ lymphopenic individuals should rather induce type 1 (IFNγ, TNFα, GM-CSF) and type 17 (IL-17A) CD8+ T-cell cytokine responses that are important for vaccine immunity against pathogenic fungi.
CD8+ T cells elicited by vaccination differentiate and clonally expand to become a large pool of effectors during the expansion phase, of which ~90% will die by apoptosis during the ensuing contraction phase. The remaining 5–10% of effectors, known as memory precursor effector cells, differentiate to become long-lasting memory cells. A threshold number of such memory cells is essential to rapidly clear infection upon re-exposure (4). Thus, the quantum of effector CD8+ T cells following expansion dictates the threshold number of memory cells, whereas the inflammatory milieu affects the type and quality of the response. The inflammatory cytokines dictate counter-regulatory types of CD8+ T cell responses; IL-12 promotes type1 responses and a combination of IL-6, TGFβ and IL-1 promotes type 17 responses (5). Moreover, antigen load and inflammatory milieu regulate the quality of memory CD8+ T cells. High antigen load causes the clonal deletion and functional exhaustion of effector CD8+ T cells, whereas an inappropriate inflammatory milieu guides their terminal differentiation and dysfunction (6, 7). Thus, both TCR signaling and inflammatory milieu are key targets for developing rational fungal vaccines.
Productive TCR signaling requires at least two signals: signal 1 from the peptide-MHC-TCR complex, and signal 2 from co-stimulatory signals such as CD28. Upon induction of TCR signaling, a cascade of signaling pathways associated with cell proliferation, differentiation and survival are activated involving tyrosine kinases, MAPKs and cytoskeleton remodeling that culminate in NFAT-NF-κB-mediated activation of CD8+ T cells (8). Casitas B-lymphoma-b (CBLB), an E3 ubiquitin ligase, is important for maintenance of peripheral T-cell tolerance, and its absence leads to augmented TCR signaling resulting in autoimmunity (9). However, CBLB negatively regulates T cell responses during infection, and its deletion enhances CD8+ T-cell mediated tumor immunity (10–12). CBLB controls TCR signaling pathways at various junctures by down-regulating the TCR complex, PI3K, PLCγ1 or Vav1, and is associated with T-cell anergy and generation of poor quality effector T cells (11, 13–16). Thus, Cblb deficient T cells can overcome the requirement of CD28 signaling, signal 2, for T cell activation (17). Thus, CBLB targeting has been shown to be beneficial in controlling chronic viral infections and tumors (11, 18).
Our past work has shown that live attenuated fungal vaccination, in the absence of CD4+ T cells, induces type1 and type17 CD8+ T cells that are required for vaccine immunity, and are distinct from anti-viral and –bacterial effector CD8+ T cells by portraying phenotypic attributes that portend stem-cell like behavior, and are maintained as long-lasting memory cells (19–21). In this study, we evaluated whether targeting CBLB would potentiate inactivated fungal vaccine immunity in a mouse model of CD4+ T cell lymphopenia. We assayed the quantity and quality of CD8+ T cell responses to inactivated Blastomyces and Histoplasma yeasts in comparison to Lymphocytic Choriomeningitis virus (LCMV). We also delineated the effect of CBLB on resistance following inactivated yeast vaccination. Finally, we established the T-cell intrinsic role of CBLB and present novel data that suggests Cblb deficiency can override the requirement of HIF-1α for vaccine-induced CD8+ T cell responses.
METHODS
Mice:
Cblb−/− mice were provided by P.S. Ohashi (University of Toronto, Ontario, Canada) with permission from Josef Penninger (IMBA, Austria). The C57BL/6, OT-I Tg (TCRα/TCRβ specific for OT-I epitope) and B6.PL-Thy1a/Cy/Thy1.1 (Thy1.1) were purchased from Jackson Laboratories. OT-I mice carrying Thy1.1 allele were generated by backcrossing OT-I Tg mice with Thy1.1 mice, which were then used to generate congenic Thy1.1+ OT-I Tg-Cblb−/− mice by backcrossing with Cblb−/− mice. All mice were maintained under specific-pathogen free conditions at the University of Wisconsin-Madison and at the University of Illinois at Urbana-Champaign and were used in accordance with the guidelines of the institutional animal care committees.
LCMV infection and DNA vaccination:
For acute viral infection, lymphocytic choriomeningitis virus (LCMV) Armstrong strain was given (105 pfu) by the intraperitoneal (i.p.) route. For chronic viral infection, LCMV Clone 13 strain was given (106 pfu) by the intravenous route (i.v.). To cause persistent infection (22), mice were infected with LCMV Clone 13 and were depleted of CD4+ cells with GK1.5 antibody (200μg i.p. twice on days 0 and 4 post-infection). For DNA vaccination, 200 μg of endotoxin-free pCMV-NP plasmid was given once via the intramuscular (anterior tibialis) route.
Adoptive transfer experiments:
Naïve Cblb+/+ and Cblb−/− OT-I cells (both Th1.1+) were purified from both draining lymph nodes (dLNs) and spleens using a CD8+ T cell enrichment kit (BD Biosciences). A total of 106 OT-I cells was adoptively transferred i.v. into naïve recipient mice.
Fungi, vaccinations and challenge infection:
Wild-type virulent strains of Blastomyces dermatitidis (#26199) and Histoplasma capsulatum (G217B) were obtained from ATCC. The isogenic attenuated strain of Blastomyces lacking the virulence factor BAD1 (#55) (23) was used for vaccination (105-106 CFU). Blastomyces was maintained as yeast on Middlebrook 7H10 agar supplemented with Oleic acid-albumin complex (Sigma) at 39° C. To measure OT-I T cell responses, recombinant #55 strain engineered to carry the OT-I epitope, SIINFEKL, was used for vaccination (106 CFUs) (19). Histoplasma capsulatum was maintained on Histoplasma Macrophage Medium (HMM) slants and used for vaccination (107 CFU). To study vaccine resistance, mice were challenged intratracheally (i.t.) with ~104 CFUs of virulent strain B. dermatitidis (#26199). Vaccinations were given subcutaneously (s.c.), divided at two sites, one dorsally and one at the base of tail (3, 24).
CD4+ T cell depletion:
Except for the LCMV infection studies (unless indicated), CD4+ T cells were depleted using 200 μg/mouse of GK1.5 mAb i.v., twice a week (BioXcell) for all experiments. The depletion efficiency was >99% as described previously (19).
Flow cytometry:
To assess vaccine-induced CD8+ T cell responses, dLNs and spleens were harvested on indicated days. To measure recall responses, lung tissues were harvested at day 4 after pulmonary challenge. Tissues were homogenized on BD cell strainers, and RBCs were lysed using 4% ammonium chloride containing buffer. Cells were re-stimulated with anti-CD3 (clone 145–2C11; 0.1ug/ml) and anti-CD28 (clone 37.51; 1ug/ml) antibodies in the presence of Golgi Stop (BD Biosciences) for 5 hr at 37° C. Following incubation, cells were washed and stained with flurochrome-conjugated antibodies for surface markers. Following fixation and permeabilization (BD CytoFix/CytoPerm buffer), cells were stained with antibodies against intracellular cytokine. All antibodies were purchased from BD Biosciences or eBioscience except anti-CD43 (Clone 1B11; Biolegend). Cells were analyzed by a BD LSRII or Cytek Aurora flow cytometer, and data was analyzed using FlowJo, LLC (Treestar). All flow cytometry data shown is after gating on lymphocyte gate followed by CD8α+ T cell gate.
CBLB and HIF-1α staining:
Single cell suspensions were antibody stained for surface markers followed by fixation and permeabilization (BD Cytofix & Cytoperm). Cells were stained with anti-mouse CBLB antibodies (#SC-1435 or #SC-8006; Santa Cruz Biotech) followed by staining with flurochrome conjugated rabbit anti-goat secondary antibody for SC-1435 clone (13) and rabbit anti-mouse HIF-1α (D1S7W, Cell Signaling). In some experiments, blocking peptide (SC-1435P, Santa Cruz) was used to block primary antibody binding sites before using for staining (13).
Cytokine Bead Array:
Blood was collected from mice when they were moribund and serum was separated. Serum cytokine concentrations were measured by flow cytometry using CBA kit (BD Biosciences) as per the manufacturer’s protocol.
Transcription factor staining:
Antibodies against T-bet, RORγt and Eomes were obtained from eBioscience, and cells were stained using a FoxP3 Staining Kit (eBioscience) along with cytokines staining.
HIF-1α inhibitor treatment:
Echinomycin was purchased from Cayman chemical and stocks were made in methanol (1mg/ml). Stocks were diluted in sterile PBS and administered to mice i.p. every other day (~3 μg/mouse) starting from day 4 post-vaccination.
Statistical analysis:
Statistical analysis of fungal load was measured by the non-parametric Mann-Whitney test, and all other analyses were performed using a two-tailed unpaired Student t-test. A two-tailed P value of ≤0.05 was considered statistically significant.
RESULTS
Antigenic load dictates CBLB dependent CD8+ T cell responses and immunopathology
We previously reported that Cblb deficiency augments TCR signaling in CD8+ T cells and enhances IFNγ production during an acute infection with the Armstrong strain of LCMV (resolved in 8–10 days) (22) (13). In contrast to an acute infection, in immunocompetent mice the Clone 13 strain of LCMV replicates to very high levels, induces varying levels of CD8+ T-cell dysfunction and results in a chronic viral infection lasting 3–6 months. Depletion of CD4+ T cells during a chronic LCMV infection leads to severe functional exhaustion of CD8+ T cells and life-long viral persistence (25). The expression levels of CBLB in CD8+ T cells in this setting were directly proportional to the viral antigen load (Fig. 1A). Thus, it was of interest to determine the role of CBLB in regulating CD8+ T cell responses under conditions of disparate antigenic loads that may be seen in immunocompromised hosts during systemic viral infection. To assess the effect of high viral load on immune regulation by CBLB, we evaluated CD8+ T cell responses in Cblb+/+ and Cblb−/− mice during chronic and persistent viral infections. Absence of CBLB significantly enhanced LCMV-specific (both NP396 & GP33 dominant epitopes) CD8+ T cell responses during both chronic and persistent viral infections (Fig. 1B). Cblb deficiency enhanced CD8+ T cell responses during chronic viral infection (18), but Cblb−/− mice succumbed within 8–9 days after LCMV infection (Supp. Fig. 1A). Remarkably, circulating levels of pro-inflammatory cytokines were sharply elevated following LCMV-Clone13 infection in Cblb−/− vs. Cblb+/+ mice (Supp. Fig. 1B), which suggested that cytokine storm might underlie the lethality of Cblb−/− mice. Thus, under conditions of high viral load during a chronic LCMV infection, CBLB protects against immunopathology by downregulating anti-viral T cell responses.
Figure 1. Role of CBLB during viral infections.
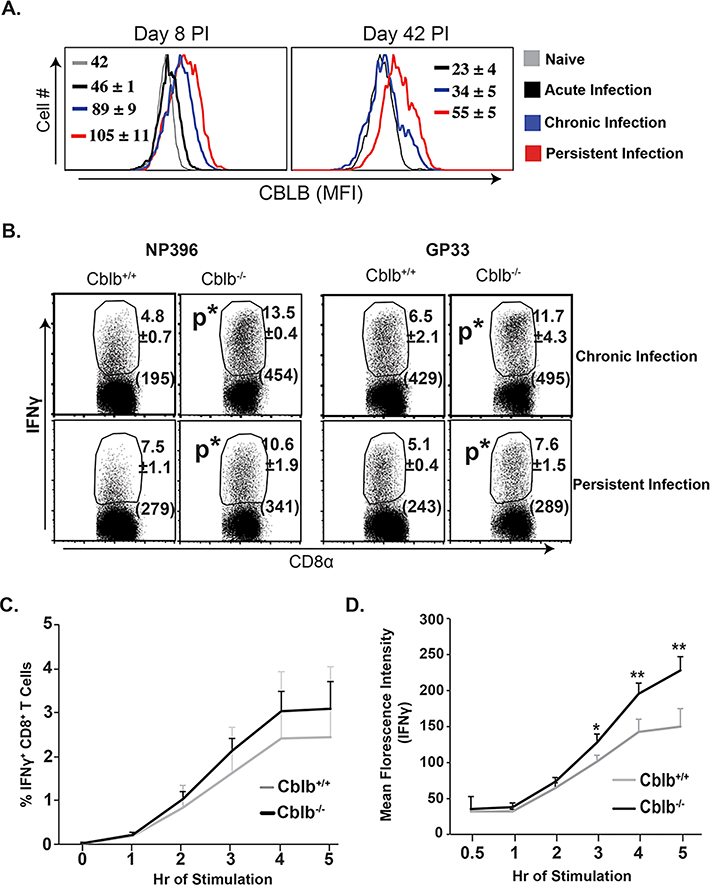
Naïve Cblb+/+ and Cblb−/− mice were inoculated with LCMV strains to produce acute (Arm), chronic (Clone 13) or persistent (Clone 13, CD4-depleted) infections. (A) Levels of CBLB expression. Values are Mean Florescence Intensity (MFI) ±SD. (B) LCMV-specific CD8+ T cell responses. Values are percent ± SD and MFI of IFNγ (parentheses). (C & D) Epitope-specific CD8+ T cell responses following recombinant DNA (LCMV-NP) vaccination. Percent (C) and IFNγ expression in MFI (D) of CD8+ T cell responses. N=4–5mice/group. *p≤0.05.
We next assessed the role of CBLB in regulating T cell responses under conditions of relatively low antigenic stimulation induced by a DNA vaccine. As shown in Fig 1C, following DNA immunization, the percentage and IFNγ-producing ability of antigen-specific CD8+ T cells were significantly augmented in Cblb−/− mice compared to Cblb+/+ mice. Together, these data suggest that CBLB augments the activation threshold of CD8+ T cells. By doing so, CBLB limits CD8+ T cell responses to weak antigenic stimuli, but protects against CD8+ T-cell-mediated immunopathology induced by a high antigen load.
Ablation of Cblb enhances anti-fungal CD8+ T cell responses following live yeast vaccination
As illustrated above, sustained high antigenic load during a chronic LCMV infection led to fatal immunopathology in Cblb−/− mice, and we have shown previously that fungal antigen persists for an extended period of time (~7 weeks) following vaccination with live attenuated Blastomyces yeast (19). First, we asked if CBLB is induced following fungal vaccination. As shown in Fig 2A, fungal vaccination induced high levels of CBLB in activated CD8+ T cells in both dLNs and spleens that waned in the ensuing weeks of rest (~8 wk post-vaccination) coinciding with the depletion of vaccine antigen (19). Next, we analyzed the response of Cblb−/− mice to vaccination with live attenuated yeast. We vaccinated Cblb+/+, Cblb+/− and Cblb−/− mice with live attenuated yeast and monitored their morbidity and survival. We did not observe overt signs of distress or mortality over 8-week post-vaccination in any of the groups. Lastly, we assessed the role of CBLB on anti-fungal CD8+ T cell responses. We have previously shown that both type 1 (IFNγ, GM-CSF, TNFα) and type 17 (IL-17A) responses were important for fungal vaccine-immunity mediated by CD8+ T-cells (2, 3). As shown in Fig. 2B, there was a significant increase in the frequency of both type 1 and type 17-cytokine producing CD8+ T cells in Cblb deficient mice compared to Cblb+/+ mice. Similarly, the number of type 1 and type 17 but not type 2 cytokine-producing CD8 T cells in dLNs and spleens were significantly higher in Cblb−/− mice compared to Cblb+/− and Cblb+/+ controls (Fig. 2C & Supp. Fig. 2A). Notably, even a single copy of Cblb was enough to suppress the CD8+ T cell responses. Collectively, these data suggest that Cblb deficiency enhances anti-fungal CD8+ T cell responses to live fungal vaccination without causing lethality or obvious adverse effects.
Figure 2. CBLB constrains anti-fungal CD8+ T cell responses.
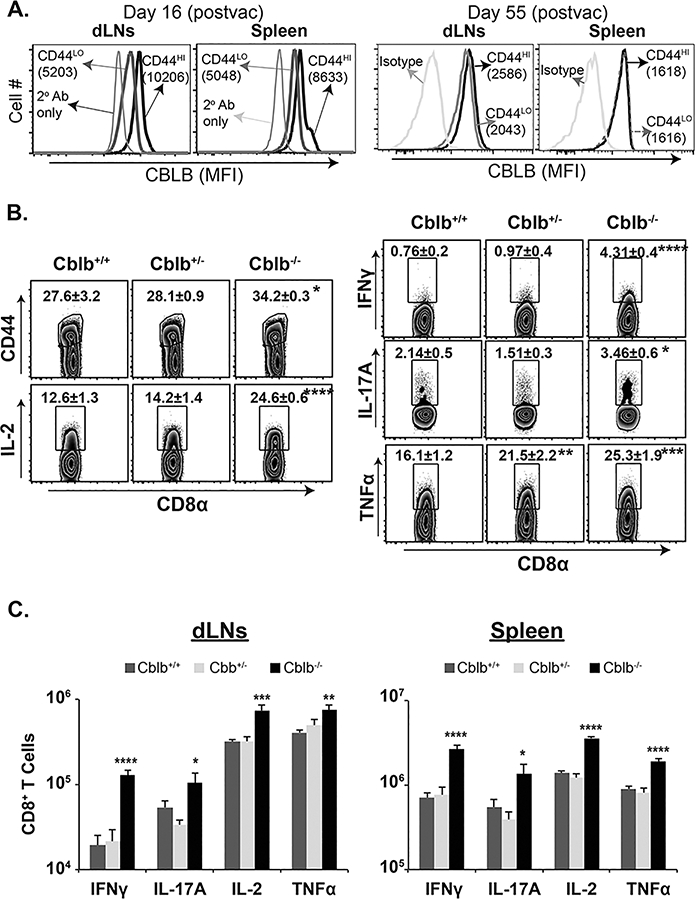
Naïve Cblb+/+, Cblb+/− and Cblb−/− mice were vaccinated s.c. with the live attenuated strain #55 of B. dermatitidis (105 cfu). On day 16, dLNs and spleens were harvested and cytokine-producing CD8+ T cells were analyzed by flow cytometry. (A) CBLB expression (MFI) in naïve (CD44lo) and activated (CD44hi) CD8+ T cells on indicated days post-vaccination. Frequency (B) and numbers (C) of cytokine-producing cells gated on activated (CD44hi) CD8+ T cells. Values are mean ± SD. N=4 mice/group. *p≤0.05. Data is representative of two independent experiments. CD4+ T cells were depleted throughout the experiment.
CBLB constrains anti-fungal CD8+ T cell responses to an inactivated vaccine
Inactivated vaccines are safer for immune-compromised individuals, but they often fail to elicit protective T cell responses. After finding above that persistent fungal vaccine antigen does not cause lethality or aberrant gross pathology in Cblb−/− mice, we investigated whether: (i) CBLB constrains CD8+ T cell responses to inactivated vaccine, and (ii) CBLB deficiency bolsters both type 1 and type 17 responses to inactivated vaccine. As shown in Figure 3A, vaccination with inactivated yeast, induced stronger Tc1 and Tc17 responses in Cblb−/− mice compared to Cblb+/+ and unvaccinated controls, and responses were augmented with a higher vaccine dose. Similarly, total numbers of both IFNγ+ (Tc1) and IL-17A+ (Tc17) CD8+ T cells in Cblb−/− mice vaccinated with inactivated yeast were significantly higher than in Cblb+/+ controls in both dLNs and spleens (Fig. 3B & Supp. Fig. 2B). CD43 expression (26), on cytokine producing cells in Cblb+/+ and Cblb−/− mice were similar (Supp. Fig. 2C), suggesting that CBLB doesn’t influence CD43 mediated effector cell functions (26). Collectively, these data suggest that CBLB down-regulates anti-fungal Tc1 and Tc17 cell responses to inactivated fungal vaccine.
Figure 3. CBLB limits CD8+ T cell responses to inactivated fungal vaccine.
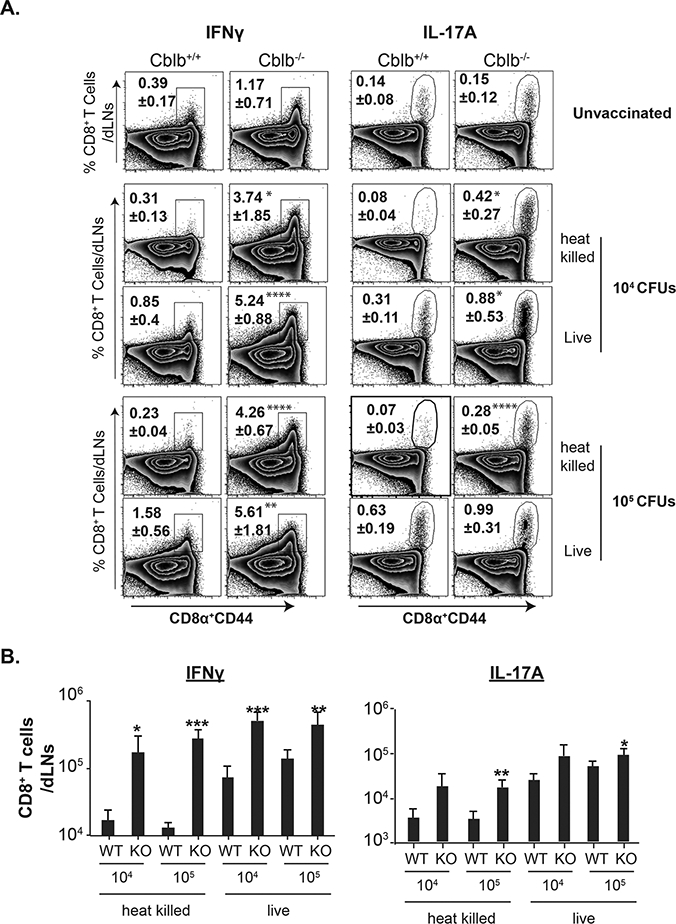
Naïve Cblb+/+ and Cblb−/− were vaccinated with either live or heat-killed strain #55 yeast. On day 18, dLNs were harvested to analyze CD8+ T cell responses by flow cytometry. Frequency (A) and numbers (B) of cytokine-producing CD8+ T cells. Values are mean ± SD. N=4–5 mice/group. *p≤0.05. CD4+ T cells were depleted throughout the experiment.
CBLB constrains inactivated vaccine-induced immunity against lethal fungal pneumonia
We next investigated whether enhanced CD8+ T cell responses to inactivated fungal vaccine in Cblb−/− mice translates to better resistance against lethal fungal pneumonia. Vaccination with heat-killed Blastomyces poorly protects Cblb+/+ mice from lethal pulmonary challenge (27). In line with this finding, Cblb+/+ mice vaccinated with inactivated yeast had only 5-fold les CFUs than unvaccinated controls after challenge. In sharp contrast, Cblb−/− mice that received inactivated vaccine had ~4 logs lower (1, 295-fold) CFUs compared to their unvaccinated controls (Fig. 4A). Interestingly, unvaccinated Cblb−/− mice had ~2 logs lower (169-fold) CFUs compared to unvaccinated Cblb+/+ mice, suggesting an innate, vaccine-independent role for CBLB. In concordance with CFU data, recall responses of cytokine-producing CD8+ T-cells in infected lungs were significantly higher in Cblb−/− mice compared Cblb+/+ mice (Fig. 4B). Thus, our data suggest that CBLB constrains antifungal immunity after vaccination with inactivated yeast and this finding correlates with poor induction and recall responses of CD8+ T cells.
Figure 4. CBLB regulates vaccine-immunity to inactivated fungal yeast.
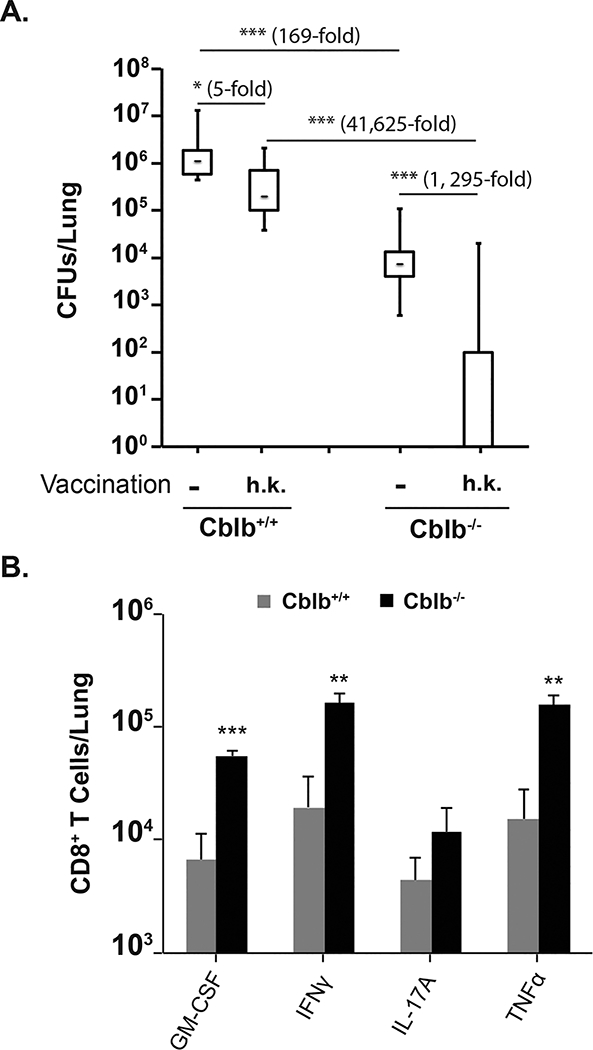
Naïve Cblb+/+ and Cblb−/− were vaccinated with 105 cfu of heat-killed (h.k) strain #55 yeast. (A) After a month rest, mice were challenged i.t. with a lethal dose (104 cfu) of virulent strain #26199. When unvaccinated mice were moribund (D16 post-infection; p.i.), lungs were harvested to quantify fungal burdens. CFUs are shown in whisker plots. N=4–14 mice/group. (B) Mice were vaccinated with killed yeast as above. Mice were challenged after 2 months rest and lungs were harvested (D4 p.i.) to enumerate cytokine-producing CD8+ T cells. N=2–4 mice/group. Data is representative of two independent experiments. *p≤0.05.
CBLB downregulates anti-fungal CD8+ T cell responses to inactivated Histoplasma yeast
To extend our observations to other pathogenic fungi, we evaluated the anti-fungal CD8+ T cell responses in Cblb+/+ and Cblb−/− mice following vaccination with inactivated Histoplasma yeast. Of note, Histoplasma induces strong type 1 (IFNγ) responses that are correlated with resistance (28–30). Figure 5A shows the frequency of cytokine-producing CD8+ T cells in Cblb+/+ and Cblb−/− mice vaccinated with either inactivated yeast or live yeast. As we observed with Blastomyces vaccination, the percentage of cytokine-producing CD8+ T cells (especially type I cells) was significantly higher in Cblb−/− mice compared to Cblb+/+ and unvaccinated mice (Fig. 5 & Supp. Fig. 3). Similar findings were present in live yeast vaccine groups except that Cblb−/− mice had significantly higher IL-17A+ CD8+ T cells compared to Cblb+/+ controls (Fig. 5B, Supp. Fig. 3B). In sum, these findings together suggest that CBLB negatively regulates CD8+ T cell responses to vaccination with multiple fungi.
Figure 5. CBLB limits CD8+ T cell responses following vaccination with live or inactivated Histoplasma yeast.
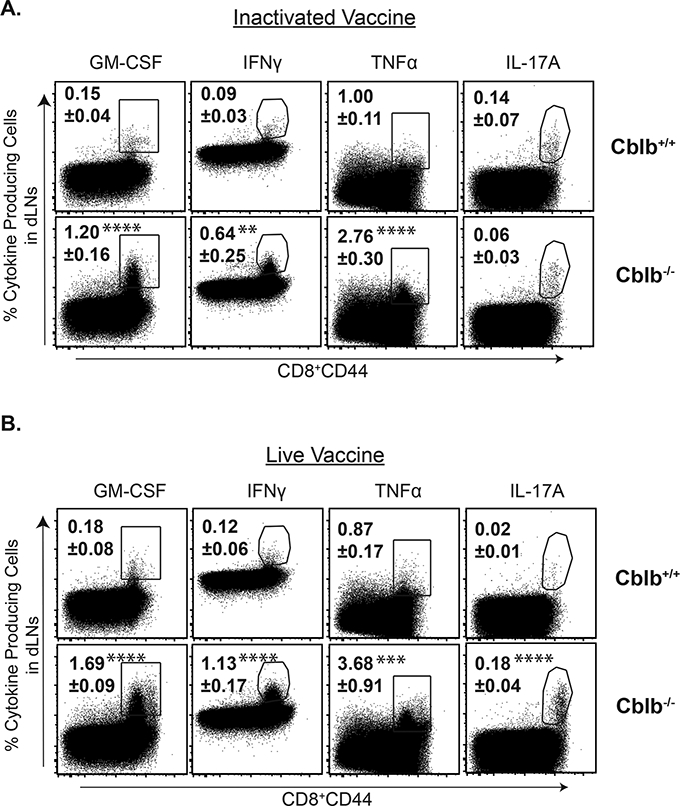
Naïve Cblb+/+ and Cblb−/− were vaccinated with either live or heat inactivated (h.k.) Histoplasma yeast (107 cfu). On day 26, dLNs were harvested to analyze CD8+ T cell responses by flow cytometry. Dot plots show the frequency of cytokine-producing CD8+ T cells. Values are mean ± SD. N=4–5 mice. *p≤0.05.
Ablation of Cblb bolsters cytokine-producing ability of CD8+ T cells without causing terminal differentiation
We next investigated whether robust activation of Cblb deficient CD8+ T cells causes their terminal differentiation or functional exhaustion, thereby potentially undermining long-term, vaccine-induced immunity. Figure 6A shows the expression of transcription factors in cytokine producing cells. Expression levels of prototypic transcription factors in Tc1 and Tc17 cells were similar in both Cblb+/+ and Cblb−/− mice (Fig. 6A). The ratio of T-bet/Eomes, a prognostic feature of terminal differentiation and memory development (31, 32), was also similar in both Cblb+/+ and Cblb−/− CD8+ T cells (Fig. 6B). Also, the expression levels of CD127 (pro-survival) and KLRG-1 (terminal differentiation) (33) were similar in Cblb+/+ and Cblb−/− mice (Supp. Fig. 4A). We also analyzed the quality of effector CD8+ T cells by comparing their cytokine-producing ability on a per-cell basis. The MFIs of IFNγ and IL-17A expression were significantly higher in Cblb−/− mice compared to Cblb+/+ mice following vaccination with inactivated yeast (Fig. 6C), suggesting a better qualitative signature cytokine imprinted on these cells in the absence of CBLB. These data suggest that CBLB deficiency promotes CD8+ T cell responses following vaccination with inactivated yeast without adversely causing their terminal differentiation.
Figure 6. Ablation of CBLB improves the quality of effector CD8+ T-cells.
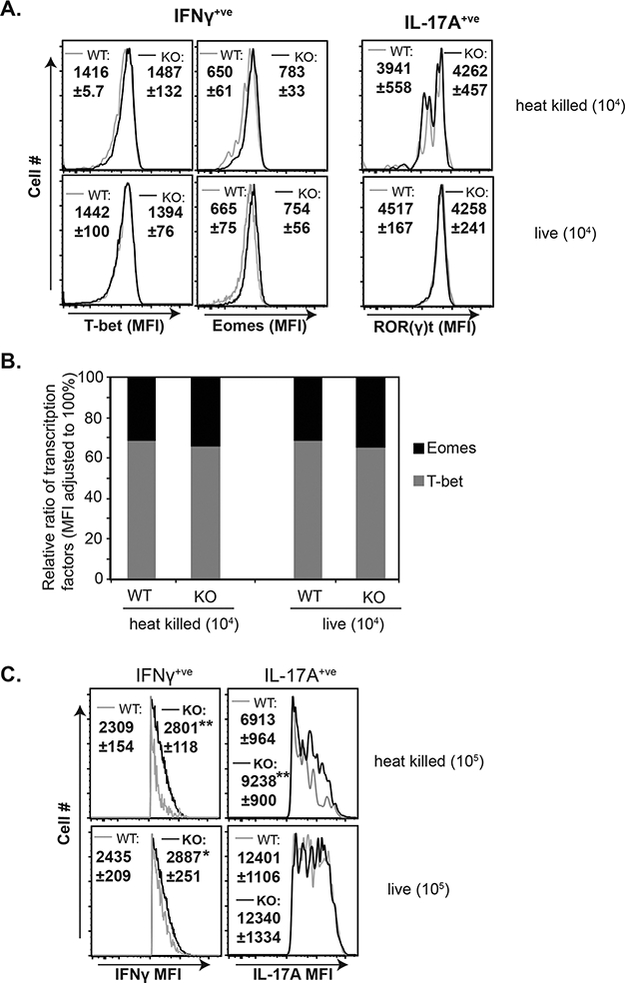
Naïve Cblb+/+ and Cblb−/− mice were vaccinated with either live or heat-killed strain #55 yeast. On day 18, dLNs were harvested to analyze CD8+ T cell responses by flow cytometry. (A) Histograms show transcription factor profile of cytokine-producing CD8+ T cells. (B) Relative proportions of expression of transcription factors in cytokine-producing CD8+ T cells; relative expression was calculated by dividing MFI of each transcription factor by the sum of both MFIs. (C.) Histograms show MFI of cytokines gated on cytokine-positive CD8+ T cells. Values are mean ± SD. N=4–5 mice. *p≤0.05.
CBLB downregulates anti-fungal CD8+ T cell responses in a T-cell intrinsic mechanism
CBLB is expressed not only in lymphoid cells but also in non-lymphoid cells, and affects their activation and functions (34). Although CBLB is a known negative regulator of TCR signaling, it is possible that CBLB expression in non-T cells affects the differentiation of anti-fungal CD8+ T cells in a cell-extrinsic manner in our models. To address this possibility, we did “crisscross” adoptive transfer experiments involving vaccination. As shown in Figure 7A, irrespective of the background of recipient mice, OT-I cells lacking CBLB showed a significantly higher frequency of cytokine responses (1.5–2 fold) than OT-I cells expressing CBLB, suggesting a stronger intrinsic role of CBLB. Likewise, the total numbers of cytokine-producing CD8+ T cells was significantly higher for Cblb−/−OT-I cells vs. Cblb+/+ OT-I cells in either Cblb+/+ and Cblb−/− recipients (Fig. 7B). Of note, although OT-I cells are known to resist becoming IL-17A+ cells, the total number of IL-17A+ Cblb−/− OT-I cells was significantly higher than Cblb+/+OT-I cells (Fig. 7B), even though the frequencies were similar between the groups. Thus, our findings indicate that CBLB has a prominent role in constraining anti-fungal CD8+ T cell responses in a cell-intrinsic manner.
Figure 7. Intrinsic role of CBLB for antifungal CD8+ T-cell responses.
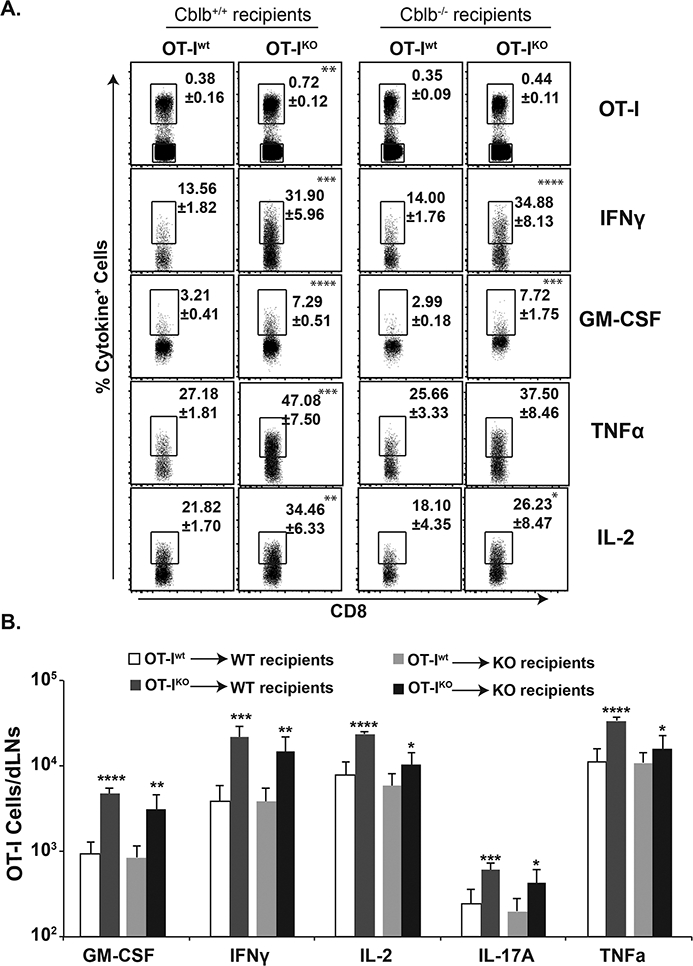
Enriched Thy1.1+ OT-I T cells (~106) from naïve Cblb+/+ or Cblb−/− mice were adoptively transferred into naïve Thy1.2+ Cblb+/+ or Cblb−/− mice. On the following day, recipients were vaccinated with strain #55 yeast engineered to express the OT-I epitope (106 CFUs). On day 22, dLNs were harvested to enumerate cytokine-producing CD8+ T cells. Frequency (A) and total numbers (B) of cytokine-producing OT-I cells. Values are mean ± SD. N=5–6 mice/group. Data is representative of two independent experiments. *p≤0.05, **p≤0.01, ***p≤0.001, and ****p≤0.0001, t-test comparing each cytokine-positive population between Cblb+/+ and Cblb−/− cells in the same recipient genotype.
Cblb deficiency overcomes the requirement of hypoxic factor, HIF-1α, for expansion of vaccine-induced CD8+ T cell responses
Hypoxia-inducible factor-1α (HIF-1α) is critical for survival of cells under the hypoxic conditions that can prevail during inflammation or tumor growth (35). We have recently shown that HIF-1α is necessary for the expansion of antifungal IFNγ+, but not IL-17A+, CD8+ T cell responses (21). Here, we investigated whether Cblb deficient CD8+ T cells, resistant to apoptosis (36), require HIF-1α for their response following fungal vaccination. We tested this hypothesis using the HIF-1α specific inhibitor, Echinomycin. In concert with prior findings (21), Echinomycin treatment did not affect the expansion of IL-17A+ (Tc17) CD8+ T cells in Cblb+/+ mice, but did significantly reduce IFNγ+ (Tc1) CD8+ T cell responses in these mice in both dLNs and spleens (Fig. 8 A & Supp. Fig. 4B). In contrast, Echinomycin treatment did not affect Tc1 (or Tc17) cells in Cblb−/− mice, suggesting a dispensable role for HIF-1α in the absence of CBLB. Next, we asked whether CBLB affects the protein levels of HIF-1α. Our direct ex vivo staining showed increased levels of HIF-1α in activated (CD44hi) CD8+ T cells over naïve (CD44lo) T cells in Cblb+/+ group (Fig. 8B & C), suggesting the induction of HIF-1α following vaccination. Direct ex vivo staining showed the similar levels of HIF-1α in both Cblb+/+- and Cblb−/−-activated (CD44hi) CD8+ T cells, suggesting a negligible role of CBLB on HIF-1α levels. Next, we evaluated the role of CBLB on the fate of HIF-1α following restimulation (upon engagement of TCR signaling mimicking infection) in the presence of Cyclohexamide (protein synthesis blocker; new HIF-1α synthesis) or MG132 (proteasome inhibitor; polyUb-mediated degradation of HIF-1α). We found higher levels of HIF-1α with MG132 and relatively lower levels with Cycloheximide in both Cbl-b+/+ and Cblb−/− CD8+ T cells proportionately (Fig. 8C), suggesting a minimal role of CBLB directly on HIF-1α protein levels. Thus, HIF-1α is redundant for expansion of CD8+ T cells in the absence of CBLB following fungal vaccination.
Figure 8. HIF-1α requirement for antifungal CD8+ T cell responses.
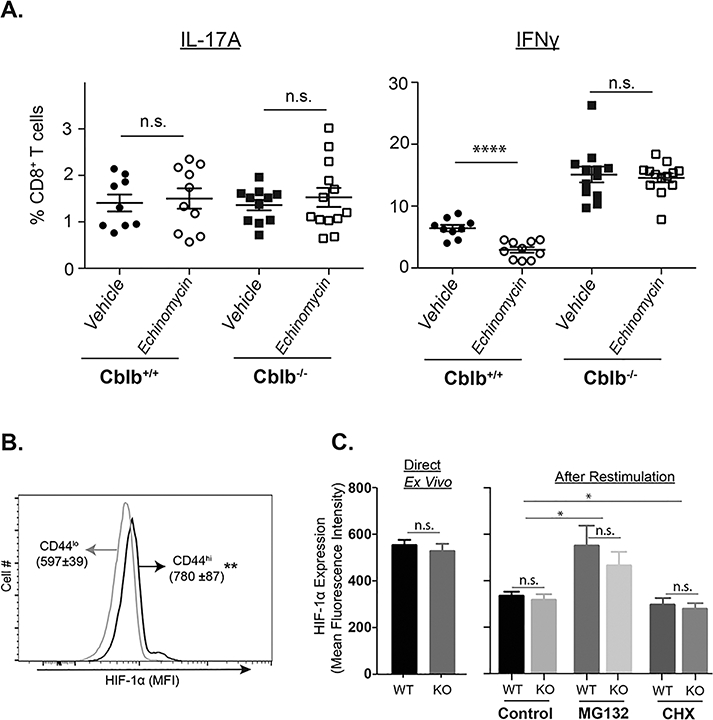
Naïve Cblb+/+ and Cblb−/− mice were vaccinated with attenuated strain #55 yeast. Cohorts of mice received either vehicle or Echinomycin (3 μg/mouse) every other day starting from day 4 post-vaccination (A). On day 14–16, dLNs were harvested to analyze cytokine-producing CD8+ T cells by flow cytometry. Scatter diagrams show frequency of cytokine-producing cells among CD8+ T cells. Each individual marker represents the value from a single mouse. Data is pooled from two independent experiments. (B & C) On day 16, dLNs were harvested to analyze HIF-1α levels in CD8+ T cells in the presence of Cyclohexamide (CHX; 10μM; EMD Millipore) or MG132 (50μM; EMD millipore). Data is representative of two independent experiments. Values are mean ± SD. N=4–5 mice/group. *p≤0.05, **p≤0.01 and ****p≤0.0001.
DISCUSSION
Despite the use of antifungals, individuals with deficiencies in CD4+ T cells, such as AIDS patients, remain susceptible to life-threatening fungal infections. Inactivated vaccines would be desirable for such patients, if effective, but such vaccines have limited effectiveness due to weaker T cell responses. In this study, we show that modulating signaling downstream of the TCR is a strategy for developing efficacious fungal vaccines tailored to vulnerable individuals. Knocking out a negative regulator of TCR signaling, e.g. Cblb, augments type1 and type 17 cytokine-producing CD8+ T cell responses required for effective antifungal vaccine immunity. Our data supports the premise that adjuvants targeting such a pathway can bolster vaccine-immunity elicited by inactivated fungi. CD8+ T-cell epitopes have not been identified for fungi; thus, the use of whole-cell based vaccines may be a rational method for inducing protective CD8+ T cell immunity against such eukaryotic pathogens (37, 38).
Systemic fungal infections can be deadly in AIDS or CD4+ lymphopenic individuals. Our study showed that chronic viral infections are lethal in Cblb−/− mice. However, inactivated fungal vaccination did not have this effect. Hence, perhaps one can vaccinate CD4+ T cell lymphopenic patients by targeting CBLB without undesirable effects. Our idea is to use such adjuvants in a spatial (subcutaneous) and temporal (limited timeframe) manner. Perhaps, this method may potentiate a pool of virus-specific T-cell responses and boost their ability to clear virus (39, 40).
Vaccine-induced CD8+ T cells compensate CD4+ T cells for mediating immunity against lethal fungal pneumonia. Antifungal CD8+ T cell immunity is correlated with the expression of both type 1 and type 17 cytokines (2, 3), and activation of neutrophils (3), an important leukocyte essential for killing yeast. Our study shows that CBLB deficiency fosters superior cytokine-producing CD8+ T cells (Fig. 3) that correlate with vaccine-immunity (Fig. 4). Interestingly, unvaccinated Cblb−/− mice showed increased resistance compared to unvaccinated Cblb+/+ mice, suggesting a role for innate (non-vaccine) immunity. Recent studies also showed that loss of CBLB promoted the activation of innate immune cells leading to superior Candida resistance (41–43). Nevertheless, our data indicate that vaccinated Cblb−/− mice are able to mediate much better resistance than vaccinated Cblb+/+ mice. Thus, adjuvants targeting CBLB may bolster the efficacy of inactivated fungal vaccines to enhance CD8+ T-cell immune correlates that govern vaccine-immunity. Our data also show that Cblb regulates vaccine-induced CD8+ T cell responses in a cell-intrinsic manner, with little effect from non-T cells.
Qualitatively superior memory CD8+ T cells are generated with optimal activation of TCR signaling (44). Overstimulation of TCR signaling, however, leads to CD8+ T cell clonal deletion or functional exhaustion, marked by increased T-bet, decreased Eomes, and poor cytokine production (45). In the absence of Cblb, TCR down-regulation is inhibited, leading to over-activation of TCR signaling and enhanced ERK phosphorylation (13). However, our data showed that vaccination with inactivated fungi did not cause terminal differentiation of Cblb deficient CD8+ T cells, but enhanced their cytokine producing ability on a per-cell basis.
HIF-1α is an important factor that facilitates survival, proliferation and tumor growth (35). Not surprisingly, tumor cells can thrive better in hypoxic niches by overexpressing HIF-1α, and it serves as a target for treatment against certain cancers (46). T-cell specific ablation of HIF-1α inhibits differentiation of Th17 cells by a RORγt dependent mechanism (47); and Vhl (negative regulator of HIF-1α) deficiency in CD8+ T cells enhances their effector functions, and causes immunopathology during persistent infection (48). We showed previously that antifungal Tc17 cells require HIF-1α during their differentiation following fungal vaccination, but not for their expansion, while the converse was true for Tc1 cell responses (21). Here, our data showed that Cblb deficiency overcame the requirement of HIF-1α for expansion of antifungal Tc1 cells. Perhaps, this may be one reason that Cblb−/− CD8+ T cells function better at tumor sites, a niche known to have hypoxia. Regardless, in sum, we show here that CBLB can be a potential target for enhancing antifungal CD8+ T cell responses following vaccination with inactivated yeast, thereby bolstering resistance against lethal fungal pneumonia.
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank Dr. Howard Steinberg, Dept. of Pathobiological Sciences at the University of Wisconsin-Madison for histopathological studies. We thank Kevin Galles, Dept. of Pediatrics at the University of Wisconsin-Madison and Mauricio Villamar, Dept. of Pathobiology at the University of Illinois at Urbana-Champaign for genotyping mouse strains. We also thank Animal Care Staff at UW-Madison and in the College of Veterinary Medicine at the University of Illinois at Urbana-Champaign for excellent animal care and management.
This work was supported by NIH grants AI119945 (SGN), AI124299, AI118326 (MS) and AI035681 & AI040996 (BSK). JSF received support from T32 AI055397 (NIH-NIAID).
AUTHORS CONTRIBUTIONS
SGN conceived the project, designed the experiments, executed and analyzed the data, and wrote the manuscript. JSF executed and analyzed the HIF-1α experiments and helped with editing the manuscript. MS and BSK provided conceptual and intellectual input for chronic viral infection and fungal vaccine experiments, respectively, and helped with editing the manuscript. SM executed experiments, analyzed the data, and helped with editing the manuscript.
DISCLOSURE
The authors have no financial conflicts of interest.
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, and White TC. 2012. Hidden killers: human fungal infections. Science translational medicine 4: 165–113. [DOI] [PubMed] [Google Scholar]
- 2.Wuthrich M, Filutowicz HI, Warner T, Deepe GS Jr., and Klein BS. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. The Journal of experimental medicine 197: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ, and Klein BS. 2012. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS pathogens 8: e1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed R, and Akondy RS. 2011. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunology and cell biology 89: 340–345. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Yamane H, and Paul WE. 2010. Differentiation of effector CD4 T cell populations (*). Annual review of immunology 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels MA, and Teixeiro E. 2015. TCR Signaling in T Cell Memory. Frontiers in immunology 6: 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JT, Wherry EJ, and Goldrath AW. 2014. Molecular regulation of effector and memory T cell differentiation. Nature immunology 15: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallner S, Gruber T, Baier G, and Wolf D. 2012. Releasing the brake: targeting Cbl-b to enhance lymphocyte effector functions. Clinical & developmental immunology 2012: 692639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin AE, and Mak TW. 2007. The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Current opinion in immunology 19: 665–673. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Zhou H, Langdon WY, and Zhang J. 2014. E3 ubiquitin ligase Cbl-b in innate and adaptive immunity. Cell Cycle 13: 1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz-Nicoladoni C, Wolf D, and Sopper S. 2015. Modulation of Immune Cell Functions by the E3 Ligase Cbl-b. Frontiers in oncology 5: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeser S, Loser K, Bijker MS, Rangachari M, van der Burg SH, Wada T, Beissert S, Melief CJ, and Penninger JM. 2007. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. The Journal of experimental medicine 204: 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamim M, Nanjappa SG, Singh A, Plisch EH, LeBlanc SE, Walent J, Svaren J, Seroogy C, and Suresh M. 2007. Cbl-b regulates antigen-induced TCR down-regulation and IFN-gamma production by effector CD8 T cells without affecting functional avidity. J Immunol 179: 7233–7243. [DOI] [PubMed] [Google Scholar]
- 14.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, and Penninger JM. 2004. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21: 167–177. [DOI] [PubMed] [Google Scholar]
- 15.Paolino M, and Penninger JM. 2010. Cbl-b in T-cell activation. Seminars in immunopathology 32: 137–148. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Qiao G, Ying H, Li Z, Zhao Y, Liang Y, Yang L, Lipkowitz S, Penninger JM, Langdon WY, and Zhang J. 2012. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell reports 1: 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, and Glant TT. 2002. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol 169: 2236–2240. [DOI] [PubMed] [Google Scholar]
- 18.Ou R, Zhang M, Huang L, and Moskophidis D. 2008. Control of virus-specific CD8+ T-cell exhaustion and immune-mediated pathology by E3 ubiquitin ligase Cbl-b during chronic viral infection. Journal of virology 82: 3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanjappa SG, Heninger E, Wuthrich M, Sullivan T, and Klein B. 2012. Protective antifungal memory CD8(+) T cells are maintained in the absence of CD4(+) T cell help and cognate antigen in mice. The Journal of clinical investigation 122: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanjappa SG, Hernandez-Santos N, Galles K, Wuthrich M, Suresh M, and Klein BS. 2015. Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia. PLoS pathogens 11: e1005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanjappa SG, McDermott AJ, Fites JS, Galles K, Wuthrich M, Deepe GS Jr., and Klein BS. 2017. Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNgamma cells. PLoS pathogens 13: e1006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matloubian M, Concepcion RJ, and Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. Journal of virology 68: 8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandhorst TT, Wuthrich M, Warner T, and Klein B. 1999. Targeted gene disruption reveals an adhesin indispensable for pathogenicity of Blastomyces dermatitidis. The Journal of experimental medicine 189: 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuthrich M, Filutowicz HI, and Klein BS. 2000. Mutation of the WI-1 gene yields an attenuated blastomyces dermatitidis strain that induces host resistance. The Journal of clinical investigation 106: 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahan SM, Wherry EJ, and Zajac AJ. 2015. T cell exhaustion during persistent viral infections. Virology 479-480: 180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, and Ahmed R. 2002. Dynamic regulation of T cell immunity by CD43. J Immunol 168: 6022–6031. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Li M, Hung CY, Sinha M, Lee LM, Wiesner DL, LeBert V, Lerksuthirat T, Galles K, Suresh M, DeFranco AL, Lowell CA, Klein BS, and Wuthrich M. 2016. MyD88 Shapes Vaccine Immunity by Extrinsically Regulating Survival of CD4+ T Cells during the Contraction Phase. PLoS pathogens 12: e1005787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanjappa SG, and Klein BS. 2014. Vaccine immunity against fungal infections. Current opinion in immunology 28: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemons KV, Darbonne WC, Curnutte JT, Sobel RA, and Stevens DA. 2000. Experimental histoplasmosis in mice treated with anti-murine interferon-gamma antibody and in interferon-gamma gene knockout mice. Microbes and infection 2: 997–1001. [DOI] [PubMed] [Google Scholar]
- 30.Deepe GS Jr. 1994. Role of CD8+ T cells in host resistance to systemic infection with Histoplasma capsulatum in mice. J Immunol 152: 3491–3500. [PubMed] [Google Scholar]
- 31.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, and Reiner SL. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology 6: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 32.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, and Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, and Ahmed R. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27: 670–684. [DOI] [PubMed] [Google Scholar]
- 34.Bachmaier K, Toya S, Gao X, Triantafillou T, Garrean S, Park GY, Frey RS, Vogel S, Minshall R, Christman JW, Tiruppathi C, and Malik AB. 2007. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nature medicine 13: 920–926. [DOI] [PubMed] [Google Scholar]
- 35.Rankin EB, and Giaccia AJ. 2016. Hypoxic control of metastasis. Science 352: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stromnes IM, Blattman JN, Tan X, Jeevanjee S, Gu H, and Greenberg PD. 2010. Abrogating Cbl-b in effector CD8(+) T cells improves the efficacy of adoptive therapy of leukemia in mice. The Journal of clinical investigation 120: 3722–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mordmuller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, Gmeiner M, Campo JJ, Esen M, Ruben AJ, Held J, Calle CL, Mengue JB, Gebru T, Ibanez J, Sulyok M, James ER, Billingsley PF, Natasha KC, Manoj A, Murshedkar T, Gunasekera A, Eappen AG, Li T, Stafford RE, Li M, Felgner PL, Seder RA, Richie TL, Sim BK, Hoffman SL, and Kremsner PG. 2017. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, and Hoffman SL. 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 39.Nanjappa SG, Kim EH, and Suresh M. 2011. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood 117: 5123–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, and Mak TW. 2011. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144: 601–613. [DOI] [PubMed] [Google Scholar]
- 41.Zhu LL, Luo TM, Xu X, Guo YH, Zhao XQ, Wang TT, Tang B, Jiang YY, Xu JF, Lin X, and Jia XM. 2016. E3 ubiquitin ligase Cbl-b negatively regulates C-type lectin receptor-mediated antifungal innate immunity. The Journal of experimental medicine 213: 1555–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirnsberger G, Zwolanek F, Asaoka T, Kozieradzki I, Tortola L, Wimmer RA, Kavirayani A, Fresser F, Baier G, Langdon WY, Ikeda F, Kuchler K, and Penninger JM. 2016. Inhibition of CBLB protects from lethal Candida albicans sepsis. Nature medicine 22: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Y, Tang J, Guo H, Zhao Y, Tang R, Ouyang S, Zeng Q, Rappleye CA, Rajaram MV, Schlesinger LS, Tao L, Brown GD, Langdon WY, Li BT, and Zhang J. 2016. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nature medicine 22: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, and Ahmed R. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28: 710–722. [DOI] [PubMed] [Google Scholar]
- 45.Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, and Wherry EJ. 2012. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37: 1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onnis B, Rapisarda A, and Melillo G. 2009. Development of HIF-1 inhibitors for cancer therapy. Journal of cellular and molecular medicine 13: 2780–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo WB, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, and Pan F. 2011. Control of T(H)17/T-reg Balance by Hypoxia-Inducible Factor 1. Cell 146: 772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, and Goldrath AW. 2013. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nature immunology 14: 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


