Abstract
Objective
Sham TMS coils isolate the ancillary effects of their active counterparts, but typically induce low-strength electric fields (E-fields) in the brain, which could be biologically active. We measured the E-fields induced by two pairs of commonly-used commercial active/sham coils.
Approach
E-field distributions of the active and sham configurations of the Magstim 70 mm AFC and MagVenture Cool-B65 A/P coils were measured over a 7-cm-radius, hemispherical grid approximating the cortical surface. Peak E-field strength was recorded over a range of pulse amplitudes.
Main results
The Magstim and MagVenture shams induce peak E-fields corresponding to 25.3% and 7.72% of their respective active values. The MagVenture sham has an E-field distribution shaped like its active counterpart. The Magstim sham induces nearly zero E-field under the coil’s center, and its peak E-field forms a diffuse oval 3–7 cm from the center. Electrical scalp stimulation paired with the MagVenture sham is estimated to increase the sham E-field in the brain up to 10%.
Significance
Different commercial shams induce different E-field strengths and distributions in the brain, which should be considered in interpreting outcomes of sham stimulation.
Keywords: transcranial magnetic stimulation, TMS, electric field, coil, active, sham
Introduction
In transcranial magnetic stimulation (TMS) studies, sham coils are used to isolate the impact of ancillary effects including scalp stimulation, auditory activation, and placebo response [1]. An ideal TMS sham would replicate these effects, without directly stimulating the brain. Reproducing scalp stimulation while reducing or eliminating the concomitant electric field (E-field) from the brain is the most challenging aspect of designing a sham [2–5]. Consequently, sham coils that induce some scalp stimulation typically also induce a residual E-field in the brain. Generally, this E-field is significantly weaker than in the active condition and may have a different spatial distribution. However, there is accumulating evidence that brain function may be affected by markedly subthreshold E-fields of less than 10% of the threshold to evoke action potentials [6–12]. Therefore, to inform investigations of the possibility that sham TMS contributes to subthreshold E-field effects, we measured the E-field distributions of two popular figure-8 coils and their complementary shams.
Methods
The coils characterized here include the Magstim 70 mm Double Air Film Coil (AFC; P/N: 3910-00; Magstim Co.), its complement sham (P/N: 3950-00), and the MagVenture Cool-B65 Active/Placebo (A/P) coil (P/N: 9016E0501; MagVenture A/S), which can be configured in active or sham mode by flipping the coil over. The Magstim coils were powered by a Magstim Rapid2, and the MagVenture coil was powered by a MagPro X100. In either case, the driving waveform was biphasic, and the inter-pulse frequency was 1 Hz.
E-field distributions were captured over a 1000-point, hemispherical grid using a robotic measurement tool with a probe comprised of two orthogonal wire loops forming isosceles triangles each with a 5.0 ± 0.1 mm base and 7 cm height [13, 14]. The resultant measurement surface approximates the superficial cortex. Each TMS coil was positioned and leveled 8.5 cm above the probe’s pivot point (its origin), a distance roughly equal to that between the center of the head and the scalp [15], with side loops bisected symmetrically by the probe’s y–z reference plane. This setup results in measurements that illustrate the shape and intensity of the E-field as if the coil were fixed at the vertex of the head. The distribution of E-field strength, quantified by the amplitude of the second phase of the cosine pulse waveform, was recorded at 50% of the maximum stimulator amplitude (MSA) for each coil. The probe was then positioned at the E-field spatial maximum, and E-field strength was recorded from 10% to 100% of MSA in 10% steps. In each measurement, the 6 degrees of freedom in relative position between the coil and probe assembly were constrained using laser-guided sight lines and levels.
Results
The measured E-field distributions are shown in figure 1A–D (the raw data are provided as supplementary material). The maximum E-field strength in active stimulation differs between the Magstim (133 V/m) and MagVenture (185 V/m) systems by 39.6%. This is expected, since the maximum energy-storage capacitor voltages and coil designs differ. Importantly, there are also significant differences in the E-field strength and distribution between the two shams. The Magstim sham induces a peak E-field of 34 V/m at 100% MSA, corresponding to 25.3% of the active maximum, whereas these respective values are only 14 V/m and 7.72% for the MagVenture system. The MagVenture sham has a figure-8 E-field distribution similar to its active counterpart, albeit with wider spacing between the zeros and less focality. In contrast, the Magstim sham induces an E-field that is nearly zero under the coil’s center, and its peak E-field forms a diffuse oval circulation pattern 3–7 cm from the coil’s center (supplementary figure S1 provides a clearer illustration of the E-field direction by normalizing the E-field magnitude for each coil configuration by its own maximum).
Figure 1.
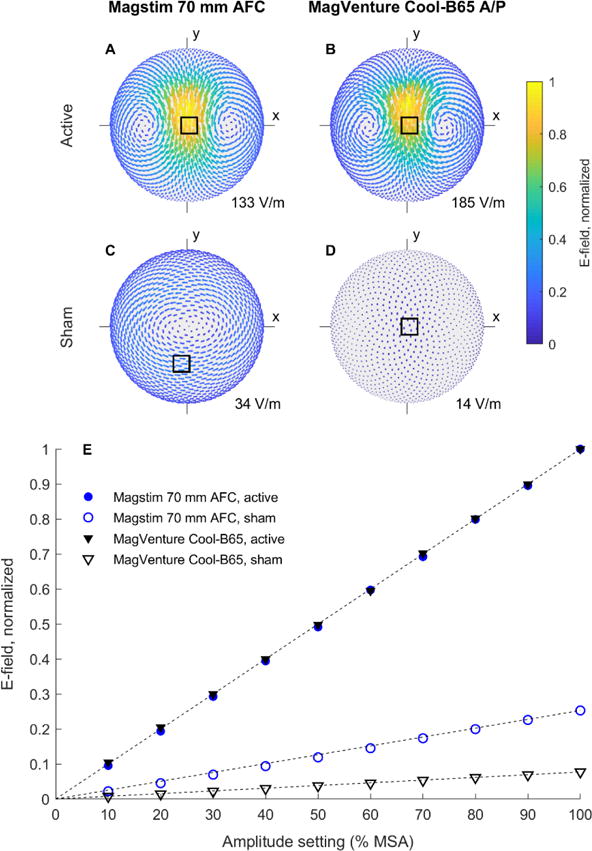
A–D: Measured E-field distributions for the active and sham coil configurations. For each coil type, the distributions are normalized by the maximum E-field strength of the active configuration (supplementary figure S1 shows all distributions normalized by their own maximum). The location of each maximum is denoted by a square, and the absolute E-field strength at this point for 100% maximum stimulator amplitude (MSA) is noted in the lower right corner of the plots. E: Measured peak E-field strength as a function of stimulator amplitude setting. Normalization is the same as in A–D. A dashed line is drawn from the origin to the normalized value at 100% MSA for each coil.
Figure 1E shows the E-field strength of each coil configuration over a range of stimulator output relative to 100% MSA of the corresponding active configuration. As expected, E-field strength is highly linear with respect to the device pulse amplitude setting (Pearson’s correlation coefficient > 0.95); therefore, the sham-to-active ratio is maintained across the output range.
The E-field pulse waveforms for each coil configuration are shown in figure 2. The active pulses for both the Magstim and MagVenture devices have an underdamped cosine shape, as expected. The sham waveforms have similar shape, albeit with some distortions. The Magstim sham pulse is 3.5% shorter in duration than its active counterpart, whereas the MagVenture waveforms have equal duration in the two modes. In all, the differences in waveform are small and unlikely to change substantially the E-field thresholds for neural activation between active and sham modes, supporting the validity of the relative E-field strength comparison in figure 1E.
Figure 2.
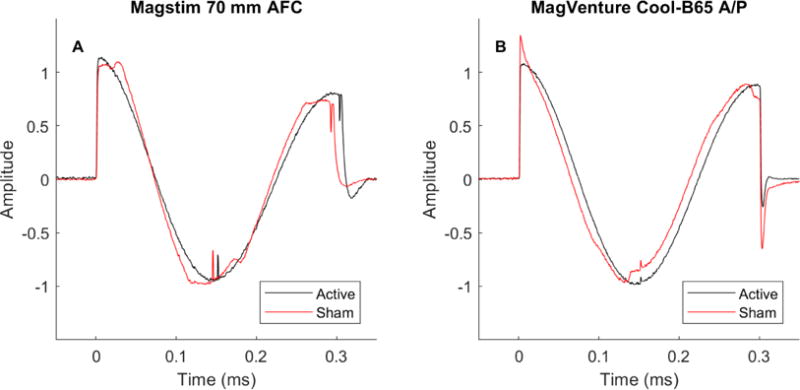
A–B: Measured E-field pulse waveforms for the two active and sham coil configurations. Each waveform is normalized by the peak value of the reverse phase.
Discussion
The Magstim sham coil effectively eliminates stimulation immediately under the coil’s center, where the active coil induces its peak E-field, a design that seems to maximize the contrast between the active and sham conditions. However, the E-field under the periphery of the Magstim coil is significantly stronger than the peak E-field of the MagVenture sham configuration, which is located under the coil’s center. Thus, the Magstim sham may produce more low-field effects in brain regions surrounding the target. Additionally, the direction of the E-field for the Magstim sham generally differs from the MagVenture sham, which may affect neuromodulation further [16]. While not directly measured, the E-field in the scalp would have spatial distribution similar to that in the underlying cortex, so these conclusions apply qualitatively to the magnetically-induced stimulation of the scalp as well.
The MagVenture Cool-B65 A/P coil can mimic scalp sensation by delivering electrical stimulation via a pair of surface electrodes, mounted under the center of the TMS coil [3, 4, 17]. Notably, this electrical stimulation also produces some E-field in the brain via current injection across the scalp, skull, and cerebrospinal fluid. In our lab, we use a pair of adhesive electrodes with 1.3-cm-diameter conductive area, separated by about 3.6 cm, and electrical stimulation settings that deliver 2–3 mA at typical TMS intensities of 40%–60% MSA [17]. Based on our prior simulations of the relationship between scalp electrode parameters and the resultant E-field [18], this electrical stimulation can produce 0.6–0.9 V/m (0.3 V/m/mA) in the brain, an increase of about 10% of the sham E-field or less than 1% of the active E-field at these intensities. It should be noted that the waveforms and spatial distributions of the magnetically and electrically induced pulses are different: The magnetically induced pulse has dampened cosine shape with a duration of 280 μs, whereas the electrical stimulus is a monophasic triangle with rise and fall times of 200 μs and 2000 μs, respectively [17]. Further, the spatial distribution of the electrical stimulation may add to or subtract from the magnetically induced E-field depending on the placement of the electrodes. Regardless, because of the relatively low amplitude of the electrically injected E-field, it is unlikely to substantially alter our conclusions about brain stimulation by sham TMS. At the same time, electrical stimulation substantially increases the scalp sensation to approximately match active TMS, which is the objective of this sham procedure.
Since the amplitudes of active and sham pulses are usually matched, TMS paradigms that use higher active intensities will also result in proportionally higher sham E-field strengths, making any unintended neural effects from sham stimulation more likely. Further, since sham conditions do produce E-field in the brain (as well as scalp and auditory stimulation), it may be more appropriate to refer to sham as a distinct stimulation condition when informing research subjects, institutional review boards, and scientific audiences.
Conclusions
The strength and distribution of the E-field induced by different commercial TMS shams in a subject’s head can vary. These differences may impact not only the reproduction of scalp sensation, but also potential neuromodulatory effects of the residual E-fields in the brain during sham. Completely blocking magnetic field delivery to the brain while reproducing the sensation of TMS with electrical scalp stimulation may be advantageous for minimizing the effects of E-field induced in the brain during sham.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants U01AG050618, RF1MH114253, and RF1MH114268. We thank Alexandra Brito, Hannah Palmer, and Rena Hamdan from Duke University for assistance in waveform measurements and information about sham TMS protocols and Yordan Todorov from MagVenture, Inc. for information about electrical stimulation in sham TMS.
J. Evan Smith has no relevant financial disclosures. A. V. Peterchev is inventor on patents and patent applications and has received research and travel support as well as patent royalties from Rogue Research; research and travel support, consulting fees, as well as equipment loan from Tal Medical/Neurex; patent application support and hardware donations from Magstim; as well as equipment loans from MagVenture, all related to TMS technology.
Footnotes
ORCiD iDs: JES: 0000-0003-4849-6220; AVP: 0000-0002-4385-065X
References
- 1.Duecker F, Sack AT. Rethinking the role of sham TMS. Front Psychol. 2015;6:210. doi: 10.3389/fpsyg.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davey KR, Riehl M. Suppressing the surface field during transcranial magnetic stimulation. IEEE Trans Biomed Eng. 2006;53(2):190–194. doi: 10.1109/TBME.2005.862545. [DOI] [PubMed] [Google Scholar]
- 3.Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, et al. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2007;118(3):709–716. doi: 10.1016/j.clinph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Arana AB, Borckardt JJ, Ricci R, Anderson B, Li X, Linder KJ, et al. Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: Does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimul. 2008;1(1):44–51. doi: 10.1016/j.brs.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng ZD, Peterchev AV. Transcranial magnetic stimulation coil with electronically switchable active and sham modes. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1993–1996. doi: 10.1109/IEMBS.2011.6090561. [DOI] [PubMed] [Google Scholar]
- 6.Carlezon WA, Jr, Rohan ML, Mague SD, Meloni EG, Parsegian A, Cayetano K, et al. Antidepressant-like effects of cranial stimulation within a low-energy magnetic field in rats. Biol Psychiatry. 2005;57(6):571–576. doi: 10.1016/j.biopsych.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Rohan M, Parow A, Stoll AL, Demopulos C, Friedman S, Dager S, et al. Low-field magnetic stimulation in bipolar depression using an MRI-based stimulator. Am J Psychiatry. 2004;161(1):93–98. doi: 10.1176/appi.ajp.161.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Rohan ML, Yamamoto RT, Ravichandran CT, Cayetano KR, Morales OG, Olson DP, et al. Rapid mood-elevating effects of low field magnetic stimulation in depression. Biol Psychiatry. 2014;76(3):186–193. doi: 10.1016/j.biopsych.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Rohan ML, Yamamoto RT, Ruzicka W, Copersino M, Fuller S, Murphy M, Wellons C, Cohen B. Sustained improvement in mood after 3 days of low field magnetic stimulation in subjects with bipolar depression. Biol Psychiatry. 2017;81:S260–S261. [Google Scholar]
- 10.Martiny K, Lunde M, Bech P. Transcranial Low Voltage Pulsed Electromagnetic Fields in Patients with Treatment-Resistant Depression. Biol Psychiatry. 2010;68(2):163–169. doi: 10.1016/j.biopsych.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Wang RL, et al. Effects of low-field magnetic stimulation on brain glucose metabolism. Neuroimage. 2010;51(2):623–628. doi: 10.1016/j.neuroimage.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opitz A, Legon W, Mueller J, Barbour A, Paulus W, Tyler WJ. Is sham cTBS real cTBS? The effect on EEG dynamics. Front Hum Neurosci. 2014;8:1043. doi: 10.3389/fnhum.2014.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieminen JO, Koponen L, Ilmoniemi RJ. Experimental characterization of the electric field distribution induced by TMS devices. Brain Stimul. 2015;8:582–589. doi: 10.1016/j.brs.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Shen MR, Deng Z-D, Smith JE, Tharayil JJ, Gurrey CJ, Gomez LJ, Peterchev AV. Redesigning existing transcranial magnetic stimulation coils to reduce energy: application to low field magnetic stimulation. J Neural Eng. 2018;15(3):036022. doi: 10.1088/1741-2552/aaa505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg TD, Salinas FS, Narayana S, Fox PT, Mogul DJ. Computational and experimental analysis of TMS-induced electric field vectors critical to neuronal activation. J Neural Eng. 2015;12(4):046014. doi: 10.1088/1741-2560/12/4/046014. [DOI] [PubMed] [Google Scholar]
- 17.MagVenture. Cool-Coil System: User Manual. 2017 Mar; P/N: 501-1282 (US), rev. 2.5. [Google Scholar]
- 18.Deng ZD, Lisanby SH, Peterchev AV. Controlling stimulation strength and focality in electroconvulsive therapy via current amplitude and electrode size and spacing: comparison with magnetic seizure therapy. J ECT. 2013;29(4):325–335. doi: 10.1097/YCT.10.1097/YCT.0b013e3182a4b4a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


