Abstract
Objectives
To present the consensus recommendations and supporting literature for red blood cell (RBC) transfusions in general critically ill children from the Pediatric Critical Care Transfusion and Anemia eXpertise Initiative (TAXI).
Design
Consensus conference series of international, multidisciplinary experts in RBC transfusion management of critically ill children
Methods
The panel of 38 experts developed evidence-based and, when evidence was lacking, expert-based recommendations and research priorities regarding RBC transfusions in critically ill children. The subgroup on RBC transfusion in general critically ill children included six experts. Electronic searches were conducted using PubMed, EMBASE and Cochrane Library (CENTRAL) databases from 1980 to May 30, 2017 using a combination of keywords to define concepts of RBC transfusion and critically ill children. Recommendation consensus was obtained using the Research And Development/University of California Los Angeles (RAND/UCLA) appropriateness method. The results were summarized using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method.
Results
Three adjudicators reviewed 4,399 abstracts; 71 papers were read, and 17 were retained. Three papers were added manually. The general TAXI subgroup developed and all TAXI members voted on 2 good practice statements, 6 recommendations and 11 research questions; in all instances, agreement was reached (> 80%). The good practice statements suggest a framework for RBC transfusion in PICU patients. The good practice statements and recommendations focus on hemoglobin as a threshold and/or target. The research questions focus on hemoglobin and physiologic thresholds for RBC transfusion, alternatives and risk/benefit ratio of transfusion.
Conclusions
TAXI developed pediatric-specific good practice statements and recommendations regarding RBC transfusion management in the general PICU population, as well as recommendations to guide future research priorities. Clinical recommendations emphasized relevant hemoglobin thresholds and research recommendations emphasized a need for further understanding of physiological thresholds, alternatives to RBC transfusion, and hemoglobin thresholds in populations with limited pediatric literature.
Keywords: blood, child, critical care, erythrocyte, evidence-based, guidelines, hemoglobin, intensive care, pediatrics, practice, threshold, transfusion
INTRODUCTION
The Joint Commission on Accreditation of Healthcare Organizations identified five overused treatments that reduce patient safety; blood transfusion was ranked second (1). The overuse of red blood cell (RBC) transfusion is indeed a significant concern in pediatric intensive care units (PICU) (2–9). About 50% of children in North-American PICUs receive at least one RBC transfusion (2, 10).
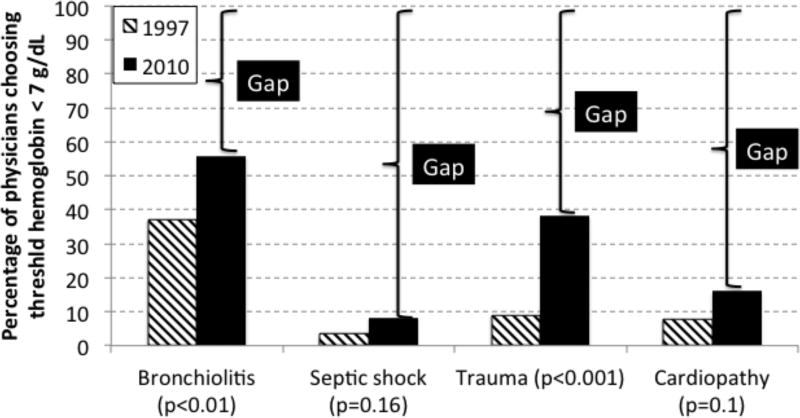
RBC transfusions are prescribed to treat anemia, which is observed in 74% of PICU patients (2, 11–13). Many papers have been published in the last decade with respect to the risk of anemia (14–24) and the risk/benefit ratio of RBC transfusion in PICU patients (25–42). A RBC transfusion is the only way to rapidly correct severe anemia; however, RBC transfusions have been associated with increased risk of mortality in critically ill patients in observational studies (5–8). Knowledge has improved recently with respect to the relative risk of uncorrected anemia (14–23) and of RBC transfusion in PICU patients (25–28, 32, 33, 37, 42). These data have led to conservative recommendations for transfusion decision-making. However, implementation of this knowledge is slow, large gaps in knowledge application persist and the prevalence of (likely) unnecessary transfusion remains high (43–46). For example, since 2007, it has been established that stable PICU patients do not require RBC transfusion if the Hb level is >7 g/dL (33). Yet, in a survey of North American and European intensivists in 2013, it was estimated that between 54% and 91% of stable PICU patients would receive RBC transfusions for Hb level above this aforementioned threshold (Figure 1) (46). Overuse of RBC transfusions is probably more common for children than for adults. A study involving 12 pediatric services at Johns Hopkins Medical Institutions reported that, depending on the service, 25% to 90% of transfused children received blood despite having an Hb >7 g/dL (47). Audits in the United Kingdom have consistently shown that approximately 20% of blood product usage is outside guideline recommendations (48). As for any treatment with potentially adverse effects, it is crucial to reduce unnecessary use of blood products. Clearly, there is room for improvement.
Figure 1.
Knowledge gap between stated practice pattern and evidence. In two surveys, practitioners were asked what hemoglobin concentration would prompt them to prescribe a red blood cell (RBC) transfusion to anemic critically ill children with bronchiolitis, septic shock, trauma or cardiopathy. We completed these surveys in 1999 and 2010 (46, 166), using the same scenarios. According to the TRIPICU study (33), which was published in 2007, no patients in the 4 scenarios need a RBC transfusion: thus the percentage of physicians choosing to prescribe a RBC transfusion only if the hemoglobin level is < 7 g/dL should be 100%. In this figure, each brace highlights the knowledge gap; the gaps decreased from year 1999 to 2010, but remain important.
In the ICU environment, knowledge translation interventions that include protocols, guidelines and/or decision trees are associated with improved clinical practice (49–56). The objective of the ‘Transfusion and Anemia eXpertise Initiative” (TAXI) was to scientifically develop guidelines that can help clinicians (e.g. pediatric intensivists, cardiologists, anesthesiologists, transfusion medicine specialists, etc.) in their decision-making process with respect to RBC transfusion in critically ill children. In this paper, we assess and summarize the current literature and ‘state of the science’ on the questions: what hemoglobin (Hb) concentration and what physiologic thresholds should guide the decision to prescribe a RBC transfusion in general critically ill children?
METHODS
A panel of 38 content experts met over the course of 2 years to develop evidence-based and (when evidence was lacking) expert-based recommendations regarding treatment and research priorities for transfusion in critically ill children. The subgroup on general PICU patients comprised six authors of this paper (AA, MB, JC, AD, JL, KR).
The details of the methodology are described elsewhere in this supplement of Pediatric Critical Care Medicine (7). Briefly, we searched PubMed, EMBASE, and Cochrane Library from 1980 to December 2015, with an update in May 2017, using a combination of medical subject heading terms and text words to define concepts of RBC transfusion and critically ill children. The search terms are provided in the supplemental digital data 1. We searched references from identified articles for additional publications. Three authors reviewed all citations independently. We used a standardized data extraction form to construct evidence tables and graded the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (57–58).
Recommendations developed and supporting literature were reviewed and scored by all panel members, using the Research And Development/University of California Appropriateness Method. All recommendations reached agreement (>80%). Final recommendations for RBC transfusion in general critically ill children based on hemoglobin and physiologic thresholds and were divided into three categories: good practice statements, clinical recommendations, and research recommendations.
RESULTS
Using the search strategy described above, we retrieved 4,399 abstracts. Seventy-one abstracts were retained for the final evaluation of screened papers. Three adjudicators (AA, JMC, KER) read and independently assessed the full text of these 71 papers. Fifty-four papers were excluded. The most frequent causes for exclusion were: the intervention was not RBC transfusion (17 papers), participants were not critically ill or at risk for critical illness (11), age >18 years of age (5), papers were not in English or unable to be found (5), data were not original (5), the outcome was not relevant (3), case report (3) or abstract only (3), age <36 weeks gestation (1) and duplicates (1). Seventeen papers were retained. Three papers were added manually and these twenty papers were used in the final process to generate good practice statements, recommendations and research questions (2, 19, 21, 22, 27, 28, 32, 33, 35, 37, 42, 59–67); a short summary of these 20 papers is provided in Supplemental Digital Data, Supplemental Table 1. Reviewer consensus was attained on the first round for 15 papers (75% agreement), and on the second round for the remaining five papers.
The TAXI members for general PICU patients generated two good practice statements, six recommendations and eight research questions. All recommendations reached a priori (>80%) agreement according to predefined definitions by the RAND UCLA appropriateness methodology. The voting data, including the number of voting experts and median score are provided for each recommendation. These recommendations apply to children ≥ 36 weeks gestational age and < 18 years of age (age-related determinant) who are critically ill (in PICU) or are at risk for critical illness (in hospital wards).
Good practice statements
Red blood cell transfusion: decision making
Good Practice Statement 1) When deciding to transfuse an individual critically ill child, we recommend considering not only the hemoglobin (Hb) concentration, but also the overall clinical context (e.g. symptoms, signs, physiological markers, laboratory results) and the risk, benefits, and alternatives to transfusion. Consensus panel expertise. Voting data (n=29): 97% agreement, median 9, IQR (9–9).
Good practice statement 1: supporting arguments – There is little empiric data to support this statement, which is based on practical experience, expert opinions and good common sense.
The presence and severity of anemia is diagnosed by measuring Hb concentration. Accordingly, while caring for PICU patients, practitioners use the Hb concentration as their primary determinant for RBC transfusion (2). However, the severity of anemia (low Hb level) is rarely the only relevant determinant for RBC transfusion. In 2003, during a brain-storming session, several pediatric intensivists elaborated a list of all potential justifications that come to mind when considering RBC transfusion; the generated list included more than 27 different items: young age (<1 year old), respiratory problems (respiratory insufficiency, low PaO2, low O2 delivery), cardiovascular problems (cardiovascular dysfunction, cyanotic heart disease, tachycardia), high serum lactate level, coagulopathy (increased prothrombin time or international normalized ratio, disseminated intravascular coagulation), bleeding (gastrointestinal bleeding, other acute blood loss), hematopoietic-related problems (bone marrow suppression defined as a white blood cell count < 500 cells/mm3, dedicated packed RBC units, autologous blood), surgery (emergency surgery, elective non trauma surgery, severe trauma), procedures (line insertion, exchange transfusion, plasmapheresis, extracorporeal membrane oxygenation (ECMO), hemodialysis, hemofiltration), and high severity of illness as measured by predictive scores like the Pediatric Risk of Mortality (PRISM) score (68). A prospective descriptive epidemiological study was subsequently conducted on the practice of RBC transfusion in 30 North-American PICUs (2). In this study, the attending physician and/or fellow who prescribed a RBC transfusion was asked within a few hours after a transfusion to choose up to three items enumerated above that justified their decision to transfuse; a low Hb level was the most important justification in 42% of cases, followed by acute blood loss (17%), cardiovascular insufficiency (9%), respiratory insufficiency (7%) and specific technologies (7%).
Good practice statement 1: summary of findings–Pediatric intensivists should not use the Hb level alone to drive the decision to administer RBC transfusions in PICU patients: they must also consider the patient’s general health (the patient’s ability to compensate for, or tolerate anemia). Moreover, they must remember that the relationship between Hb concentration and RBC number can be altered acutely by either hemoconcentration or hemodilution. Guidelines from many organizations emphasize that the decision to administer RBCs should not be based solely on Hb levels, but should involve sound clinical judgment (69, 70). There is a rationale behind such strategy. For example, the rate at which anemia develops influences anemia tolerance; as such, a Hb level as low as 3.5 g/dL may be well tolerated if the anemia is chronic, the patient is isovolemic and otherwise healthy and hematopoietic recovery is forthcoming (71). It is the presence of anemia and other risk factors that should prompt the practitioner to prescribe a RBC transfusion, such as severity of illness (severe enough to warrant hospitalization of patients, which is the case for all PICU patients), patient trajectory of illness (deteriorating or recovering), presence of severe bleeding, hemodynamic instability, congenital or acquired cardiac pathology, some congenital anemia, etc.
Hb measurement
Good Practice Statement 2) In critically ill children or those at risk for critical illness, we recommend measuring the hemoglobin (Hb) concentration before prescribing each RBC transfusion; knowledge of Hb concentration is not required before RBC transfusion if the patient has life threatening bleeding. Consensus panel expertise. Voting data (n=35): 100% agreement, median 9, IQR 8–9.
Good practice statement 2: supporting arguments–Unless a patient is in hemorrhagic shock, it is not rational to prescribe a RBC transfusion without knowing if the Hb level is low.
The gold standard method to estimate total Hb content in a patient’s blood is to measure the Hb level and the hematocrit as part of a complete blood count, which is typically done in the hematology laboratory, the blood bank, or with point of care instrumentation in the PICU. The Hb level measures the Hb concentration in a blood sample. The hematocrit measures the proportion of blood volume occupied by RBCs. Measurement of Hb level or hematocrit as part of a complete blood count is the most reliable means to estimate changes to the total amount of intra-cellular Hb circulating in blood. The Hb concentration can also be measured on blood gases and by pulse co-oximetry (SpHb Spot Check technology, Pronto Pulse CO-Oximeter®, Masimo, Corp., Irvine, CA). These methods are not as reliable as the Hb level measured with the standard technique, but the difference is small and not clinically significant; for example, the average difference between SpHb and Hb level measured in the laboratory is –0.15 ± 0.92 g/dL (72). However, the differences between peripheral Hb level (measured by SpHb) and central Hb (measured by blood tests, other than capillary samples) may vary widely during critical illness. The accuracy of Hb measurement on blood gases and by pulse co-oximetry is sufficient to be used in practice.
It is not mandatory to measure the Hb level or hematocrit after a RBC transfusion, but it can be useful to determine the degree to which the transfusion increased the Hb level, particularly in the setting of uncontrolled bleeding or ongoing hemolysis. Blood can be collected to measure the Hb concentration or hematocrit almost immediately after a transfusion: it takes not more than 15 to 30 minutes for the Hb level and the hematocrit to stabilize after a RBC transfusion (73). Good practice statement 2: summary of findings–Measurement of Hb concentration is essential to estimate the severity of anemia; this information may be useful after a transfusion. This measurement can be done as part of a complete blood count, on blood gas specimens and by pulse co-oximetry.
Recommendations: general critically ill child
Hb concentration < 5 g/dL
Recommendation 1.1. In critically ill children or those at risk for critical illness we recommend a RBC transfusion if the Hb concentration is < 5 g/dL. Strong recommendation. Low quality pediatric evidence (1C). Voting data (n=29): 97% agreement, median 9, IQR 8–9. (GRADE 1C based on upgrading for large treatment effect, and downgrading for imprecision and indirectness (observational studies).)
Recommendation 1.1: supporting arguments. – Many descriptive studies have reported that the risk of adverse outcomes of hospitalized children is significantly higher if their Hb level is below 5 g/dL (19, 21, 60, 64); a similar association has been reported in adults (14, 15).
An association does not ensure a cause-effect relationship. Demonstration that RBC transfusions improved the outcome of transfused patients when compared to similar non-transfused patients would be an important argument supporting a cause-effect relationship between anemia and adverse outcome, and would suggest that RBC transfusion might be useful in these patients; however, there is a remarkable dearth of data demonstrating the relationship between the degree of anemia and transfusion benefit. One study does suggest that RBC transfusion improves the outcome of severely ill anemic children. Lackritz (22) reported a prospective case-control study of 303 Kenyan children with Hb below 5 g/dL at hospitalization and of 303 control patients with Hb level ≥ 5 g/dL. Of the patients with Hb level < 5 g/dL, 116 (38%) did not receive a transfusion, mostly because of blood unavailability, while 187 (62%) were transfused given that blood was available; mortality was significantly higher in non-transfused children (41.4% vs 21.4%, p < 0.001). Of note, however, mortality was similar in children with Hb level < 5 g/dL who were transfused and in children with Hb level ≥ 5 g/dL who were not transfused (21.4% vs. 19.5%). Although this study was conducted in Africa, these results are, to an extent, generalizable to PICU patients elsewhere.
Recommendation 1.1: summary of findings. – The results of the study of Lackritz (22) suggest that RBC transfusion may improve the survival of anemic hospitalized patients with Hb level below 5 g/dL, but the specific threshold above 5 g/dL that should be used to prescribe a RBC transfusion is unknown.
RBC transfusion guided by physiologic markers
Recommendation 1.2. In critically ill children or those at risk for critical illness, we cannot recommend a specific RBC transfusion decision-making strategy that is based upon physiologic metrics and biomarkers. Consensus panel expertise. Voting data (n=35): 91% agreement, median 8, IQR 8–9.
Recommendation 1.2: supporting arguments. – RBC transfusion is primarily indicated to improve tissue O2 delivery in anemic patients with inadequate O2 consumption, or to relieve the physiologic stress of compensated anemia in patients with maintained O2 delivery homeostasis. Although RBC transfusion increases Hb and thereby blood O2 content (although, not instantaneously), it does not necessarily follow that tissue O2 delivery and O2 consumption is likewise increased — unless blood O2 content, itself, is rate limiting in transfer of O2 from lung to tissue. Constraint in O2 utilization may also arise from impairment in tissue respiration, itself; this pathology is not responsive to improving O2 content via transfusion. Such impairment of O2 utilization can be caused by mitochondrial dysfunction, which is typically observed, for example, during severe cases of sepsis and in other forms of severe critical illness (e.g., following systemic ischemia/reperfusion) (74, 75).
Historically, normal — or supranormal — Hb levels were presumed necessary during physiologic stress (76). Mounting evidence, however, has enabled better appreciation for anemia tolerance and donor RBC impairment. As such, conservative transfusion approaches have emerged, based upon non-inferiority trials of permissive anemia (e.g. restrictive Hb-based thresholds) (27, 28, 33, 61). This approach is evaluated, and recommendations are made elsewhere in this paper.
The Hb threshold strategy indicates that severely ill children should receive a RBC transfusion if their Hb concentration is < 5 g/dL (recommendation #1). However, in those with an Hb concentration above 5 g/dL, the Hb threshold strategy informs us only about those patients who do not require transfusion (e.g., evidence indicates permissive anemia is safe above specific Hb thresholds in some PICU population, such as a threshold Hb of 7.0 g/dL in hemodynamically stable critically ill children). Since it is not feasible to define specific (Hb) boundaries across the complex interaction of developmental-, condition-, and stress-specific situations encountered in the PICU, Hb alone cannot identify many patients for whom permissive anemia is unsafe, nor are these data sufficient to guide transfusion timing/volume.
Of note, in severely ill children with an Hb concentration > 5 g/dL, no single metric or biomarker in isolation should be expected to adequately predict whether the patient will or will not benefit from RBC transfusion (or, indicate how much blood should be given). Moreover, metric and/or biomarker trajectories (direction of change, rate of change or minimum change over time) are also likely to be as important in decision-making as set ‘threshold’ values. Tables 1 and 2 suggest metrics and biomarkers that may be useful in transfusion decision-making; however, to date, none have been validated as reliable and useful metrics to guide RBC transfusion therapy in PICU patients. Finally, the relative weighting (in decision-making) given to each metric and biomarker is likely to be context-sensitive (e.g. value of biomarkers may vary as a function of patient age, health-condition and illness trajectory).
TABLE 1.
Examples of Physiologic Metrics and Biomarkers Reporting Loss of Reserve in Compensating for O2 Delivery Insufficiency or Reporting Failure of O2 Delivery Homeostasis (e.g. Anaerobic Metabolism)
| Parameter | Normal values |
Suggested threshold* |
References | ||
|---|---|---|---|---|---|
| Pediatrics | General | ||||
| Physiologic Metric | Heart rate | Age-dependent | 120–130% of baseline? | (81, 90) | |
| Blood pressure | Age-dependent | 70–80% of baseline? | (90) | ||
| Respiratory rate/dyspnea | Age-dependent | 120–130% of baseline? | |||
| Capillary refill time (seconds) | ≤ 2 | > 3? | (113) | ||
| Core – peripheral temperature Δ | TBD | ||||
| StO2 (NIRS) | TBD | (97, 114–122) | |||
| Dynamic StO2 | TBD | TBD | (123) | ||
| O2 delivery (mL/O2/kg/min) | 11.0–14.0 | 7.0? | (124) | (125, 126) | |
| O2 consumption (mL/O2/kg/min) | 3.0–3.5 | < 3.0 or 80–90% of baseline? | (127–129) | (130) | |
| ScvO2 (%) | 65–75% | ~ 50–60%? | (131, 132) | (133–135) | |
| Systemic O2 extraction | 20–30% | 40–50%? | (124) | ||
| Pepripheral O2EXT | TBD | TBD | (136) | ||
| Heart rate variability (HRV) | TBD in children | TBD | (107, 137, 138) | ||
| Plethysmographic variability index | TBD | TBD | (139) | ||
| Functional capillary density | TBD | TBD | |||
| Biomarkers | Lactate (mmol/L) | < 1.5 | > 3.0 | (140–143) | (144) |
| Gastric tonometry (gastric intra-mucosal pH, pHi) | pHi > 7.35 or ΔPaCO2-PtoCO2 < 5 mmHg | pHi ≤ 7.35 or ΔPaCO2-PtoCO2 ≥ 5 mmHg? | (145) | (146) (147) | |
| Cytochrome oxidase redox | TBD | TBD | (148) | ||
Delta (Δ): change, difference or gradient; NIRS: near infrared spectroscopy; O2; oxygen; PaCO2: arterial PCO2; pHi: intramucosal gastric pH; PtoCO2: PCO2 measured by gastric tonometry; ScvO2: central vein oximetry; StO2: tissular O2 saturation, as measured by NIRS; TBD: to be determined.
The clinical usefulness of almost all thresholds listed in this table remains to be determined. Moreover, it must be underlined that change over time could even more informative than a given value: for example, a change (Δ) in lactate level from 7 to 4 mmol/L tell us that the patient is improving while a lactate level of 4 mmol/L suggests that the health status of the patient is alarming if the data is interpreted alone.
TABLE 2.
Examples of Physiologic Metrics and Biomarkers Reporting Vital Organ-Specific Evidence of Anemia Intolerance
| Organ | Parameter | Normal values |
Suggested threshold |
References | ||
|---|---|---|---|---|---|---|
| Pediatrics | General | |||||
| Physiologic metric or biomarkers | Heart | ST elevation | none | any | (82, 149, 150) | |
| Troponin | none | any | (151) | |||
| Brain | Cognitive impairment | normal | Δ from baseline | (90, 96, 152, 153) | ||
| Cerebral StO2, NIRS | ↓20% from baseline | (63, 91, 154) | (119, 155–159) | |||
| PbtO2 | TBD | TBD | (155, 160) | |||
| SjbO2 (%) | TBD | (161) | (162) | |||
| Small bowel | Stomach and/or gut StO2 | TBD | TBD | (92) | (147, 163, 164) | |
| Kidney | Renal perfusion | TBD | TBD | (165) | ||
| Muscle | StO2 | TBD | TBD | (114, 118, 120, 121, 123, 151) | ||
Delta (Δ): change, difference or gradient; NIRS: near infrared spectroscopy; O2; oxygen; PbtO2: PO2 in brain tissue; PO2: O2 partial pressure; ScvO2: central vein oximetry; SjbO2: jugular bulb SO2; StO2: tissular O2 saturation, as measured by NIRS; TBD: to be determined.
Recommendation 1.2: summary of findings. – We cannot recommend a specific RBC transfusion decision-making strategy that is based upon physiologic metrics and biomarkers because, to date, neither physiologic metrics nor biomarkers have been validated as reliable tools to support medical decision with respect to RBC transfusion.
Hb concentration ≥ 7 g/dL in hemodynamically stable PICU patients
Recommendation 1.3. In critically ill children or those at risk for critical illness, who are hemodynamically stable and who have an Hb concentration ≥ 7 g/dL, we recommend not administering a RBC transfusion. Strong recommendation, Moderate quality pediatric evidence (1B). (GRADE 1B based on downgrading for imprecision (single high quality RCT)). Voting data (n=29): 97% agreement, median 9, IQR 8–9.
Recommendation 1.3: supporting arguments. – The TRIPICU study showed that a restrictive RBC strategy (threshold Hb: 7 g/dL) is safe in hemodynamically stable or stabilized critically ill children. In TRIPICU, a patient was considered hemodynamically stable or stabilized if the mean arterial pressure was not less than two standard deviations below normal mean for age and if the cardiovascular support (vasopressors/inotropes and fluids) had not been increased in the last two hours (33).
One may expect a positive association between severity of illness and transfusion benefit in PICU patients. This was not observed, however, in the TRIPICU study: in stable critically ill children, a liberal RBC transfusion strategy was not associated with a lower risk of new or progressive multiple organ dysfunction syndrome (N/P MODS), regardless of the baseline severity of illness, as measured by the “pediatric risk of mortality” (PRISM) score (68): the absolute risk reduction (ARR) was +1.5% (95%CI: –6.3 to +9.4) when patients with PRISM score of zero (1st quartile) were compared to those with scores ≥ 8 (4th quartile).
Recommendation 1.3 is generalizable to all patients similar to those who were enrolled in the TRIPICU study. However, in the TRIPICU study, 5,399 patients were screened, and 4,751 (4,751/5,399 = 88%) were excluded for reasons such as expected to stay <24 hours in ICU (1,686 patients), no approval from physician (424), age <3 days or >14 years (414), refusal of consent by patient and/or parent (379), unstable hemodynamically (216) and acute blood loss (201). We do not know if recommendation 1.3 is applicable to patients similar to those who were excluded from TRIPICU.
Recommendation 1.3: summary of findings. – Recommendation 1.3 is applicable to all hemodynamically stable PICU patients, even those in septic shock (77, 78) and post-cardiac surgery (acyanotic patients and post biventricular repair), if older than 28 days (78, 79). However, the evidence does not support generalization of this recommendation to patients similar to those excluded from the TRIPICU study.
Hb concentration ≥ 7 g/dL in PICU patients with acute post-operative non-hemorrhagic anemia
Recommendation 1.4. In critically ill children with acute post-operative non-hemorrhagic anemia (excluding cardiac surgery), who are hemodynamically stable, we recommend not administering a RBC transfusion if the Hb concentration is ≥ 7 g/dL. Weak recommendation. Low quality pediatric evidence (2C). Voting data (n=29): 97% agreement, median 8, IQR 8–9. (GRADE 2C based on downgrading for imprecision (sub-analysis of RCT)).
Recommendation 1.4: supporting arguments. – An a priori subgroup analysis was planned for TRIPICU patients who underwent non-cardiac surgery: 124 such subjects were enrolled, 60 in the restrictive and 64 in the liberal group (37). Six and five new or progressive multiple organ dysfunction (N/P MODS) were observed in the two groups (ARR: +1.1%; 95%CI: –8.9%, +11%, p = 0.83). No clinically or statistically significant difference was observed with respect to the highest daily PELOD score (7.4 ± 9.6 vs 7.6 ± 8.8), PICU mortality (1 vs 0) and 28-day mortality (0 vs 1). More importantly, the trends observed in this subgroup were identical to the trend observed in other subgroups and in the whole TRIPICU study (32, 33, 42). Consistency of subgroups trends in an RCT supports the conclusion of the original trial (80). The subgroup analysis of non-cardiac surgical PICU patients enrolled in TRIPICU suggests that they are not different than other hemodynamically stable critically ill children, which supports recommendation 1.4. However, we do not know if recommendation 1.4 is applicable to surgical patients with severe hemorrhage because they were excluded from TRIPICU.
Recommendation 1.4: summary of findings. – A threshold Hb of 7 g/dL seems safe in stable critically ill children admitted to PICU after non-cardiac surgery, if they do not present with severe hemorrhage (37).
Hb concentration: between 5.0 and 7.0 g/dL
Recommendation 1.5. There is insufficient evidence to make a recommendation regarding transfusion thresholds for critically ill children who have an Hb concentration between 5 and 7 g/dL. However, it is reasonable to consider transfusion based on clinical judgment in these children. Consensus panel expertise. Voting data (n=29): 100% agreement, median 9, IQR 9–9.
Recommendation 1.5: supporting arguments. – The results of the TRIPICU study suggest that a threshold Hb of 7 g/dL is safe in hemodynamically stable PICU patients. The results of the study conducted by Lackritz (22) suggest that a RBC transfusion should be given in hospitalized children with an Hb level is < 5 g/dL.
Acute isovolemic reduction of blood Hb concentration to 5 g/dL in conscious healthy resting adults did not lead to evidence of inadequate systemic DO2 or VO2 or an increase in plasma lactate concentration (81). However, we do not know if it is safe to restrict RBC transfusion in adults with cardiac pathology (82) and in severely ill patients with Hb level between 5.0 and 7.0 g/dL (16). However, Weiskopf (81) reported that, although VO2 did not fall with progressive isovolemic anemia, VO2 rose along with a progressive increase in cardiac index, identifying the expected metabolic burden associated with physiologic compensation for anemia (likely from increased myocardial oxygen consumption linked to the observed compensatory increase in cardiac index). Clinical studies addressing this question should be performed, including RCTs.
Recommendation 1.5: summary of findings. – We do not know if patients with an Hb level between 5 and 7 g/dL require a RBC transfusion.
Hb concentration as a goal
Recommendation 1.6. In critically ill children or those at risk for critical illness who are hemodynamically stable, we recommend that the post-transfusion goal be to relieve the indication for transfusion and not necessarily to achieve normal hemoglobin for age. A reasonable hemoglobin goal post-transfusion is a range between 7.0 g/dL and 9.5 g/dL. Weak recommendation. Low quality pediatric evidence (2C). (GRADE 2C based on downgrading for imprecision (single RCT)). Voting data (n=29): 96% agreement, median 8, IQR 8–9.
Recommendation 1.6: supporting arguments. – Given that the goal (endpoint or target) of a RBC transfusion is to maintain or to improve O2 delivery (DO2) and O2 consumption (VO2), and since the metrics that can be used to estimate DO2 and VO2 are not easily measured directly, surrogate endpoints for transfusion must be selected (as for any intervention). The endpoint in current use, and for which outcome data exist, is Hb.
In the TRIPICU study, RBC transfusions in the restrictive arm were given to increase the Hb level from less than 7 g/dL to a target range of 8.5–9.5 g/dL, but not to increase Hb to the normal range (> 12 g/dL) (33). The TRIPICU study showed no difference in outcomes between transfused and non-transfused stable PICU patients with Hb level above 7 g/dL. A similar outcome has been observed in severely ill adults (83–86).
On the other hand, the results of one paper suggest that a certain minimum RBC volume may be required to achieve optimal transfusion outcomes in hospitalized children with very severe anemia. Olupot-Olupot (64) evaluated the safety and efficacy in hospitalized Ugandan children (median age: 36 months) of a higher whole blood volume (30 mL/kg; n = 78) against a lower volume (20 mL/kg; n = 82), the standard volume suggested by the World Health Organization. Median admission Hb was 4.2 g/dL (IQR 3.1 to 4.9). The Hb level rose above 6 g/dL in more children who received 30 mL/kg than those who received 20 mL/kg (90% and 74%, respectively). With respect to 28-day survival, there was one death in the 30 mL/kg group, and six in the 20 mL/kg group (1.3% vs. 7.3%; chi square with Yates correction: p < 0.05).
It is reasonable to target a given Hb concentration after a RBC transfusion to prevent under- as well as over-transfusion; we suggest targeting a post transfusion Hb level between 7.0 and 9.5 g/dL, given the data provided by the TRIPICU study. Of note, Hb level may not increase if there is ongoing severe bleeding or significant hemolysis; in such circumstances, serial transfusions may be indicated, until the original indication for transfusion is relieved.
Recommendation 1.6: summary of findings. – The target Hb level in critically ill children with severe anemia is not well determined, but the available data suggest that the targeted Hb should be over 7 g/dL, but lower than 9.5 g/dL.
Research questions
Physiologic thresholds
Research question R1.1. In critically ill children or those at risk for critical illness, we recommend creating clinical research programs specifically designed to determine the efficacy and safety of transfusion decision-making based upon physiologic metrics and biomarkers. Consensus panel expertise. Voting Data (n=35): 100% Agreement, Median 9, IQR 8–9.
Research question R1.1: supporting arguments. – A RBC transfusion is useful only if it improves recipient outcome. The relationship between (1) physiologic metrics and biomarkers that may be used as threshold and/or target, and (2) RBC transfusion versus clinically relevant outcomes is not yet determined with adequate precision to support clinical decision-making. For example, even if RBC transfusion normalizes a low ScvO2, it does not necessarily follow: (1) that this change results from improved O2 delivery, or instead from donor RBCs’ increased O2 affinity (which, in fact, may reduce O2 delivery), nor does it necessarily follow (2) that as a result, recipient outcomes will improve in proportion to ScvO2 ‘improvement’. There are many additional cases in which use of a non-validated physiologic metric or biomarker as surrogate outcome to guide a treatment might be misleading. For example, an observational prospective study indicated that hematocrit may not be a valid surrogate for survival among erythropoietin-treated renal failure population (87), and a large RCT reported that the administration of erythropoietin to raise the hematocrit to 42% was harmful in adults with clinically evident congestive heart failure or ischemic heart disease who are receiving hemodialysis (88). As such, putative endpoints should be formally evaluated against the ‘Prentice’ criteria for effective therapeutic endpoints, which specify that endpoints should demonstrate: (1) a direct relationship to the causal path between disease and outcome, and (2) a time-sensitive response to therapy in a fashion that (3) reliably predicts the effect of treatment upon outcome (89).
Research question R1.1: summary of findings. – A relevant cause-effect relationship between a given physiologic metric and/or biomarkers, RBC transfusion and clinically significant outcomes must be formally evaluated, prior to use of metrics/biomarkers in transfusion decision-making. Once such a relationship is proven, the usefulness of the physiologic metric and/or biomarkers to guide RBC transfusion therapy must be demonstrated before we can recommend using the parameter(s) at the bedside to guide RBC transfusion therapy.
Research Question R1.2. In children with critical illness or at risk for critical illness, we recommend investigation that identifies and evaluates biomarkers and/or physiologic measures that characterize anemia intolerance. Consensus panel expertise. Voting Data (n=35): 100% Agreement, Median 9, IQR 8–9.
Research Question R1.3 We recommend investigation to determine biomarkers or physiologic measures that identify anemia intolerance, defined as threat to O2 delivery and/or O2 consumption homeostasis, and manifested as an increase in global anaerobic metabolism. Consensus panel expertise 97% Agreement, (n=35), Median 8, IQR 8–9
Research questions R1.2 and R1.3: supporting arguments. – It appears reasonable to defer transfusion in anemic patients who are both (1) maintaining O2 delivery homeostasis and (2) tolerating the stress imposed by compensatory physiology. Inherent in this approach to transfusion decision-making is an understanding that any transfusion confers some risk to every patient and therefore, transfusion should only be administered when the anticipated improvement in outcome is ‘worth’ the anticipated risk. Moreover, it is also taken as a given that in many critically ill children (possibly most), compensatory physiologic reserve is adequate to dampen the effect of anemia upon the probability of recovery. Or stated otherwise, the effect of transfusion upon the probability of recovery is more dependent upon the relationship between the moment-specific severity (and trajectory) of illness and the remaining compensatory reserve than it is upon the degree of anemia (as defined by Hb level), alone. Therefore, this investigatory approach is designed to identify a suite of biomarkers that inform clinicians of the degree to which the relationship between illness severity, patient trajectory and compensatory reserve modify the effect of anemia correction (by transfusion) upon the probability of recovery.
Finding reliable markers of anemia intolerance could be useful in PICU patients; finding markers indicating that giving a RBC transfusion will improve the outcome of an anemic PICU patients will be even better. Table 1 provides a list of candidate biomarkers or physiologic measures that may help practitioners to identify PICU patients with systemic anemia intolerance, defined as threat to O2 delivery and/or O2 consumption homeostasis, and manifested as an increase in global anaerobic metabolism, and possibly, to predict a positive clinical response to a RBC transfusion. Moreover, as stated previously, it is unlikely that any single physiologic metric or biomarker will serve independently for the above stated purpose; it is anticipated that integrating multiple metrics/biomarkers along with their trajectories will improve performance, as predictors of anemia intolerance and/or transfusion benefit.
Research questions R1.2 and R1.3: summary of findings. – The reliability of markers to diagnose anemia intolerance and to predict that a RBC transfusion will improve recipient’s outcome should be studied.
Research question R1.4. We recommend investigation that identifies and evaluates biomarkers and/or physiologic metrics of anemia intolerance specific to individual vital organs, which may be present and indicate patient-specific likelihood of benefit from transfusion, even in the absence of measures indicating systemic impairment of O2 delivery and/or O2 consumption homeostasis. Consensus panel expertise. Voting Data (n=35): 97% Agreement, Median 9, IQR 8–9.
Research question R1.4: supporting arguments. – A single organ may suffer from anemia even in the absence of systemic impairment of O2 delivery and/or O2 consumption homeostasis. Such discrepancy between systemic and organ specific measurements of anemia intolerance may exist: this is the case for the brain (90, 91) and the digestive system (92) and one can easily envision the influence of organ specific pathology in this context (e.g. stroke, cardiomyopathies, etc.). Table 2 provides a list of candidate biomarkers or physiologic measures that may help practitioners to identify organ-specific anemia intolerance in PICU patients and, possibly, to predict a positive clinical response to a RBC transfusion.
Research question 1.4: summary of findings. – The reliability of markers to diagnose organ-specific anemia intolerance and to predict that a RBC transfusion will improve the outcomes of recipients should be studied.
Hemoglobin threshold
Research question R1.5. We recommend undertaking future studies aiming to identify the appropriate Hb concentration to guide administration of a RBC transfusion in hemodynamically unstable critically ill children. Consensus panel expertise, 91% Agreement, (n=35), Median 9, IQR 8–9.
Research question R1.6. We recommend undertaking future studies aiming to identify the appropriate Hb concentration to guide administration of a RBC transfusion in subpopulations of hemodynamically stable critically ill children or those at risk for critical illness. Consensus panel expertise, 91% Agreement, (n=35), Median 9, IQR 8–9.
Research questions 1.5 and 1.6: supporting arguments. – In the original TRIPICU study, 5,399 patients were screened, and 88% were excluded (see recommendation 3). We do not know for sure if the main TRIPICU recommendation — not giving RBC transfusion in hemodynamically stable PICU patients if their Hb concentration is > 7 g/dL — is generalizable to these subpopulations.
Research questions 1.5 and 1.6: summary of findings. – We recommend further research aiming to identify the appropriate Hb concentration in combination with physiologic markers (first research question) that should guide administration of RBC transfusion in combination in subpopulations of hemodynamically stable not covered in current recommendations, in hemodynamically unstable critically ill children (77), in patients with neurological insult (traumatic brain injury, stroke, intracranial hemorrhage) (93), in sickle cell with stroke or undergoing minor surgical procedures (94), in non-hemorrhagic shock undergoing acute resuscitation (77), in patients on ECMO or ventricular-assist device (VAD) (95).
Research Question R1.7 We recommend undertaking future studies aiming to identify the appropriate Hb concentration to guide administration of a RBC transfusion in hemodynamically stable critically ill children or those at risk for critical illness, when the Hb level is between 5 g/dL and 7 g/dL. Consensus panel expertise, 83% Agreement, (n=35), Median 8, IQR 7–8
Research question 1.7 supporting arguments: – There is insufficient evidence to guide decision making for RBC transfusions in critically ill children when the hemoglobin is between 5 g/dL and 7 g/dL, as was discussed in the clinical recommendation 1.5. We recommend undertaking clinical investigations and RCTs to help answer this question.
Alternatives to RBC transfusion
Research question R1.8. We recommend investigation that will inform priority (e.g. sequencing) of RBC transfusion relative to other interventions which may either: (a) improve anemia tolerance or (b) improve O2 delivery homeostasis by supporting physiologic compensation for anemia. Consensus panel expertise. Voting Data (n=35): 91% Agreement, Median 8, IQR 8–9.
Research question R1.8: supporting arguments. – Supportive interventions other than RBC transfusion can improve anemia tolerance or O2 delivery homeostasis. For example, O2 consumption can be blunted by (1) sedation of agitated patients, (2) optimization of mechanical ventilatory support in anemic patients with respiratory failure, or (3) fever control, while O2 delivery can be improved by (1) increasing Hb O2 saturation and PaO2 and (2) optimizing cardiac output and correction of cardiac dysrhythmias (90, 96–98). There is some evidence that such strategies are effective. For example, Weiskopf (90) demonstrated that increasing PaO2 in adults with acute anemia (Hb concentration: 5.7 g/dL) improved cardiovascular (heart rate, blood pressure) and cerebral function (memory) even if no RBC transfusion was given. Feiner (98) reported that high PaO2 decreased anemia-induced tachycardia equivalent to RBC transfusion in adults with a severe acute anemia (5 g/dL).
Research question R1.8: summary of findings. – Studies must be conducted to compare the efficacy of RBC transfusions and alternatives to RBC transfusion that are directed at improving O2 delivery/consumption relationships.
Transfusion-related adverse events
Research question R1.9. In addition to investigation of physiologic metrics and biomarkers likely to indicate patient-specific likelihood of benefit of transfusion in patients with anemia, we recommend investigation that seeks evidence of patient-specific likelihood of harm from transfusion (both acute and long term). Consensus panel expertise, Voting Data (n=35): 91% Agreement, Median 9, IQR 8–9.
Research question R1.9: supporting arguments. – Transfusion-transmitted infectious diseases and non-infectious serious hazards of transfusions (NISHOT) are now appreciated to be common in the general population (99, 100). Both transfusion transmitted infections and NISHOT can be severe; some can be life-threatening (100–102). Moreover, NISHOT are more frequent in children than in adults (100). Of note, the clinical impact of transfusion-related adverse events is not well characterized in PICU patients, but some data suggest that such events strongly influence the likelihood of favorable clinical outcome (59, 103–106). Moreover, the impact and the epidemiology of adverse events attributable to RBC transfusion amongst PICU sub-populations are unknown. Thus, it is not presently possible for clinicians to judiciously balance the risk/benefit of RBC transfusion for individual critically ill children.
Research question R1.9: summary of findings. – Studies on the impact and epidemiology of short- and long-term transfusion-related adverse events must be undertaken to guide practitioners in their transfusion decision-making to give or withhold a RBC transfusion in the general PICU population. Moreover, we recommend investigation that will seek evidence of patient-specific likelihood of harm from transfusion (both acute and long term).
Risk/benefit ratio of RBC transfusion
Research question R1.10. We recommend investigations that seek evidence on thresholds or triggers that would tell practitioners that the risk/benefit ratio tolerating anemia is higher than the risk/benefit ratio of giving a RBC transfusion in critically ill children. Consensus panel expertise. Voting Data (n=35): 94% Agreement, Median 9, IQR 8–9.
Research question R1.10: supporting arguments. – The risk/benefit ratio of anemia is quite easy to figure out. There is almost no benefit to being anemic, even though a low Hb level decreases blood viscosity; this benefit is usually not clinically useful because it is associated with increased cardiopulmonary work and increased myocardial oxygen demand (107). On the other hand, we know that a very low Hb level (about 5 g/dL) is associated with a higher risk of death in children who require hospitalization (22) as well as in adults who deny RBC transfusion following orthopedic surgery (14, 15, 84). Moreover, research is needed in less severe anemia — Hb level about 8 or 9 g/dL — in some specific populations, such as adults with cardiac disease (14, 15); this question is currently under study in the Myocardial Ischemia and Transfusion (MINT) trial (NCT02981407).
The risk/benefit ratio of RBC transfusion is not well known. We discussed above (research question 1.9) the lack of knowledge regarding the risk of RBC transfusion in PICU patients. There are almost no data on the clinical benefit of RBC transfusions.
Research question 1.10: summary of findings. – Studies are required that will tell us when the risk/benefit of anemia correction (RBC transfusion) is better than the risk/benefit of anemia tolerance in critically ill children (12).
Research question R1.11. We recommend investigation that seeks evidence that, once the decision to transfuse has been made, will inform a titrated approach to administering RBCs, to maintain the risk of transfusion as low as reasonably achievable, while monitoring for resolution of the original indication for transfusion. Consensus panel expertise. Voting Data (n=35): 97% Agreement, Median 9, IQR 8–9.
Research question R1.11: supporting arguments. – The research questions discussed above focus upon information that will enable practitioners to initiate a RBC transfusion; we also need to determine the information that will inform selection of the appropriate transfusion ‘dose’, e.g. what volume should be given once the decision is made to prescribe a RBC transfusion. Either administering too much or too little whole blood or packed RBC units can be ineffective, if not harmful (64, 108). Although there are data suggesting it is not necessary to target a post-transfusion Hb level in the normal range we do not know what Hb level must be targeted when a RBC transfusion is prescribed to anemic PICU patients. Again, as noted in other sections of this paper, for clinicians to appropriately titrate RBC transfusion therapy, it may be necessary to more broadly consider evidence (e.g. other than Hb level, alone) of both O2 delivery homeostasis and tolerance of compensatory physiology that has been activated in response to anemia.
Furthermore, giving only one RBC unit per transfusion limits the risk of exposure to multiple donors; this strategy is recommended in most guidelines unless more blood is required for specific clinical (e.g., severe uncontrolled bleeding) or technical reasons (e.g., ECMO initiation).
Research question R1.11: summary of findings. – Studies must be undertaken to identify appropriate approaches to guide transfusion amount and frequency, once the decision to transfuse has been made.
DISCUSSION
Ideally, the decision to transfuse RBCs should be based not only on the severity of anemia, as defined by the Hb concentration, but also on patient-related data like age, co-morbidity, severity of illness and physiologic metrics and/or biomarkers that may enable individual and context-specific assessment of the degree to which anemia contributes to tissue O2 consumption constraint. Moreover, the decision to transfuse must consider not only the benefits, but also the risks of RBC transfusion (12).
In many instances, however, anemia severity is the only information required. If the Hb level is < 5/dL in a PICU patient, a RBC transfusion should be given (22). On the other hand, if the Hb level is > 7 g/dL, a RBC transfusion is not indicated in most PICU patients who are hemodynamically stable (33). More data are needed before this threshold of 7 g/dL can be generalized to all PICU sub-populations (see other papers of this supplement), namely children with respiratory failure (109) acquired and congenital cardiac disease (79), shock (77), severe hemorrhage (110) neurological insult (93) congenital anemia (94), cancer (94) and children exposed to devices like ECMO, ventricular assist device (VAD) and renal replacement therapy (95). The approach to transfusion decision making is unclear not only for patients who belong to these subpopulations, such as patients with sickle cell disease, but also for PICU patients with Hb level between 5 and 7 g/dL and in hemodynamically unstable patients; as such, we need to undertake RCTs to address the question. Moreover, we must determine whether the use of physiologic metrics and biomarkers can inform and improve our decision making with respect to RBC transfusion in PICU. Ideally, such marker(s) would identify specific individuals for whom permissive anemia is unsafe. Many markers have been suggested for this role, but none have been validated. Further research is required to identify markers (and approaches to decision making) that optimize transfusion efficacy. Moreover, we need to know what threshold of each marker (or integrated/composite marker group) should be used to drive RBC transfusion in PICU patients, and what Hb concentration — possibly, in combination with marker response(s) — should be targeted after the RBC transfusion. Studies on transfusion-related complications in PICU patients are also required in order that practitioners can estimate the risk/benefit of giving or deferring a RBC transfusion in each patient; then, it will be possible to prescribe “the right dose of the right product, to the right patient for the right reason at the right time”, which would be the best possible “patient blood management” (111, 112).
CONCLUSIONS
The guidelines and decision tree developed by TAXI provide evidence-based, expert opinion regarding the state of the art with respect to RBC transfusion in PICU patients (78). Practice change and policy action should result, which will improve the outcomes of critically ill children and the effectiveness of the health system.
Supplementary Material
Acknowledgments
We thank all members of the TAXI initiative for their support and their comments. The study was supported by grants from the Washington University Children’s Discovery Institute (CDI-EI-2015-499), University of Massachusetts, CHU Sainte-Justine Foundation, National Institute of Child Health Development (1 R13 HD088086-01), National Heart, Lung and Blood Institute, and the Society for the Advancement of Blood Management. We also thank the World Federation of Pediatric Intensive and Critical Care Societies, Society for Critical Care Medicine and the AABB for their support of TAXI.
Sources of Funding
Copyright form disclosure: Drs. Doctor, Carson, Valentine, and Bateman received support for article research from the National Institutes of Health (NIH). Dr. Doctor’s institution received funding from the NIH, the Department of Defense, and KaloCyte. Dr. Argent received funding from the TAXI group that co-ordinated the preparation of these documents (provided funding for travel to and accommodation in Montreal for a meeting regarding the documents), N Kelly Attorneys, and he has received financial support for travel, accommodation and conference fees from a number of national and international conferences for attending as an invited speaker. Dr. Carson’s institution received funding from Terumo, and he received funding from the University of Massachussetts (supported travel with grant from NIH R13) and Children’s Discovery Institute from Washington University. Dr. Valentine’s institution received funding from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Heart, Lung, and Blood Institute (NHLBI) under award number 1 R13 HD088086-01; the Society for the Advancement of Blood Management SABM-Haemonetics Research Starter Grant; and Washington University Children’s Discovery Institute (DCI-E1-2015-499). Dr. Valentine received other support from the CHU-Sainte-Justine Foundation and the University of Massachusetts Medical School. She received other support for article research from the Society for the Advancement of Blood Management SABM-Haemonetics Research Starter Grant, CHU-Sainte-Justine Foundation, Washington University Children’s Discovery Institute (DCI-E1-2015-499) and the University of Massachusetts Medical School. Dr. Bateman’s institution received funding from NIH R13 from NICHD/NHLBI and the Society for the Advancement of Blood Management.
Appendix 1: Pediatric Critical Care Transfusion and Anemia eXpertise Initiative (TAXI) Members
(* for Executive Committee) Co-chairs: Stacey L. Valentine MD MPH* and Scot T. Bateman MD*, University of Massachusetts, USA, Content Experts: Section 1. General pediatric critical care patient based on physiologic and hemoglobin thresholds: Andrew Argent MD MBBBCh, University of Cape Town, South Africa, Jeffrey L. Carson MD, Rutgers Robert Wood Johnson Medical School, USA, Jill M. Cholette MD*, University of Rochester, USA, Allan Doctor MD*, Washington University of St. Louis, USA, Jacques Lacroix MD*, Universite de Montréal, Canada, Kenneth Remy MD, Washington University of St. Louis, USA, Section 2. Respiratory failure: Pierre Demaret MD MSc, CHC Liege, Belgium, Guillaume Emeriaud MD PhD, Université de Montréal, Canada, Nabil E. Hassan MD, University of Illinois, USA, Martin C.J. Kneyber MD PhD, University of Groningen, Netherlands, Marisa Tucci MD*, Université de Montréal, Canada, Section 3. Shock, excluding hemorrhagic shock: Nina Guzzetta MD, Emory University, USA, Mark W. Hall MD, Ohio State University, USA, Jennifer A. Muszynski MD MPH, Ohio State University, USA, Philip C. Spinella MD, Washington University of St. Louis, USA, Duncan Macrae MB ChB, Imperial College London, UK, Section 4. Hemorrhagic shock and non-life-threatening bleeding, Oliver Karam MD PhD, Virginia Commonwealth University, Robert T. Russell MD MPH, University of Alabama, USA, Philip C. Spinella MD*, Washington University of St. Louis, USA, Paul Stricker MD, University of Pennsylvania, USA, Adam M. Vogel MD, Texas Children’s Hospital, USA, Section 5. Acute brain injury: Philip C. Spinella MD*, Washington University of St. Louis, USA, Robert C. Tasker MA MD MBBS, Harvard University, USA, Alexis F. Turgeon MD MSc, Université Laval, Canada, Section 6. Acquired or congenital heart disease, Jill M. Cholette MD*, University of Rochester, USA, Steven M. Schwartz MD, University of Toronto, Canada, Ariane Willems MD, University of Brussels, Belgium, Section 7. Sickle cell/ oncologic disease, Cassandra D. Josephson MD, Emory University, USA, Naomi LC Luban MD, George Washington University, USA, Leslie E. Lehmann MD, Harvard University, USA, Robert I. Parker MD*, Stony Brook University, USA, Simon J. Stanworth MD, NHS Blood and Transplant, Oxford, UK, Marie E. Steiner MD MS*, University of Minnesota, USA, Nicole D. Zantek MD PhD, University of Minnesota, USA, Section 8. Receiving support from extracorporeal, ventricular assist and renal replacement therapy devices: Melania M. Bembea MD PhD*, Johns Hopkins University, USA, Timothy Bunchman MD, Virginia Commonwealth University, USA, Ira M. Cheifetz MD, Duke University, USA, James Fortenberry MD, Emory University, USA, Marie E. Steiner MD MS*, University of Minnesota, USA, Section 9. Selection and processing of red blood cell components: Meghan Delaney DO, MPH, Children’s National Health System USA, Cassandra D. Josephson MD, Emory University, USA, Robert I. Parker MD*, Stony Brook University, USA, Leo van de Watering MD, Leiden University, Netherlands, Nicole D. Zantek MD PhD, University of Minnesota, USA, Evidenced-Based Medicine: Karen A. Robinson PhD, Johns Hopkins University, USA, Melania M Bembea MD PhD*, Johns Hopkins University, USA, Implementation Science: Sara Small MS, Washington University of St. Louis, USA, Katherine Steffen MD, Stanford University, USA
Footnotes
Conflicts of Interest
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.The Joint Commission and the American Medical Association-Convened Physician Consortium. National Summit on Overuse. Physician Consortium for Performance Improvement® (PCPI®), editor. Proceedings from the National Summit on Overuse; 2013. http://www.jointcommission.org/overuse_summit/ [Google Scholar]
- 2.Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss and blood transfusion in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 3.Demaret P, Tucci T, Ducruet T, et al. Red blood cell transfusion in critically ill children. Transfusion. 2014;54:365–375. doi: 10.1111/trf.12261. [DOI] [PubMed] [Google Scholar]
- 4.Triulzi D. Are red cell transfusions harmful in critically ill patients? Crit Care Med. 2016;44:1014–1015. doi: 10.1097/CCM.0000000000001576. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix J, Luban NLC, Wong ECC. Blood products in the PICU. In: Nichols DG, editor. Rogers Textbook of Pediatric Intensive Care. 4. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. pp. 584–599. [Google Scholar]
- 6.Lacroix J, Tucci M, Gauvin F, et al. Transfusion medicine. In: Wheeler DS, Wong HR, Shanley TP, editors. Pediatric critical care medicine: Basic science and clinical evidence. London: Springer-Verlag; 2007. pp. 1263–1280. [Google Scholar]
- 7.Lacroix J, Tucci M, Tinmouth A, et al. Transfusion Medicine. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric Critical Care. 4. New York: Elsevier; 2011. pp. 1162–1176. [Google Scholar]
- 8.Orliaguet G, Gauvin F, Hume H, et al. Choc hémorragique. In: Lacroix J, Gauthier M, Hubert P, Leclerc F, Gaudreault P, editors. Urgences et soins intensifs pédiatriques. 2. Montréal et Paris: Éditions du CHU Sainte-Justine & Masson; 2007. pp. 167–186. [Google Scholar]
- 9.Roseff SD. In: Pediatric transfusion. 2. Gottschall JL, editor. Bethesda: AABB; 2006. p. 208. [Google Scholar]
- 10.Josephson CD, Mondoro TH, Ambruso DR, et al. One size will never fit all: The future of research in pediatric transfusion medicine. Pediatr Res. 2014;76:425–431. doi: 10.1038/pr.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee S, Wetterslev J, Sharma A, et al. Association of blood transfusion with increased mortality in myocardial infarction. Arch Intern Med. 2013;172:132–139. doi: 10.1001/2013.jamainternmed.1001. [DOI] [PubMed] [Google Scholar]
- 12.Du Pont-Thibodeau G, Harrington K, Lacroix J. Anemia and red blood cell transfusion in critically ill cardiac patients. Ann Intensive Care. 2014;4:16. doi: 10.1186/2110-5820-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw RE, Johnson CK, Ferrari G, et al. Blood transfusion in cardiac surgery does increase the risk of 5-year mortality: results from a contemporary series of 1714 propensity-matched patients. Transfusion. 2014;54:1106–1113. doi: 10.1111/trf.12364. [DOI] [PubMed] [Google Scholar]
- 14.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 15.Carson JL, Noveck H, Berlin JA, et al. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 16.Carson JL, Patel MS. Red blood cell transfusion thresholds: can we go even lower? Transfusion. 2014;54:2593–2594. doi: 10.1111/trf.12812. [DOI] [PubMed] [Google Scholar]
- 17.Carson JL, Spence RK, Poses RM, et al. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1:727–729. doi: 10.1016/s0140-6736(88)91536-x. [DOI] [PubMed] [Google Scholar]
- 18.Desjardins P, Turgeon AF, Tremblay MH, et al. Hemoglobin levels and transfusions in neurocritically ill patients: a systematic review of comparative studies. Crit Care. 2012;16:R54. doi: 10.1186/cc11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.English M, Ahmed M, Ngando C, et al. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet. 2002;359:494–495. doi: 10.1016/S0140-6736(02)07666-3. [DOI] [PubMed] [Google Scholar]
- 20.Hogervorst E, Rosseel P, van der Bom J, et al. Tolerance of intraoperative hemoglobin decrease during cardiac surgery. Transfusion. 2014;54:2696–2704. doi: 10.1111/trf.12654. [DOI] [PubMed] [Google Scholar]
- 21.Lackritz EM, Campbell CC, Ruebush TK, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524–528. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 22.Lackritz EM, Hightower AW, Zucker JR, et al. Longitudinal evaluation of severely anemic children in Kenya: The effect of transfusion on mortality and hematologic recovery. AIDS. 1997;11:1487–1494. doi: 10.1097/00002030-199712000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Shander A, Javidroozi M, Naqvi S, et al. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME) Transfusion. 2014;54:2688–2695. doi: 10.1111/trf.12565. [DOI] [PubMed] [Google Scholar]
- 24.Lelubre C, Bouzat P, Crippa IA, et al. Anemia management after acute brain injury. Crit Care. 2016;20:152. doi: 10.1186/s13054-016-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cholette JM, Henrichs KF, Alfieris GM, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med. 2012;13:290–299. doi: 10.1097/PCC.0b013e31822f173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cholette JM, Powers KS, Alfieris GM, et al. Transfusion of cell saver salvaged blood in neonates and infants undergoing open heart surgery significantly reduces RBC and coagulant product transfusions and donor exposures: results of a prospective, randomized, clinical trial. Pediatr Crit Care Med. 2013;14:137–147. doi: 10.1097/PCC.0b013e31826e741c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cholette JM, Rubenstein JS, Alfieris GM, et al. Children with single ventricle physiology do not benefit from higher hemoglobin levels following cavopulmonary connection: Results of a prospective, randomized controlled trial of a restrictive v. liberal red cell transfusion strategy. Pediatr Crit Care Med. 2011;12:39–45. doi: 10.1097/PCC.0b013e3181e329db. [DOI] [PubMed] [Google Scholar]
- 28.de Gast-Bakker DH, de Wilde RBP, et al. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients; a randomized controlled trial. Intensive Care Med. 2013;39:2011–2019. doi: 10.1007/s00134-013-3085-7. [DOI] [PubMed] [Google Scholar]
- 29.Du Pont-Thibodeau G, Tucci M, Robitaille R, et al., editors. Determinants of platelet transfusion in a paediatric critical care unit. 7th World Congress on Pediatric Intensive and Critical Care; 2014. Istanbul (Turqey) [Google Scholar]
- 30.Istaphanous GK, Wheeler DS, Lisco SJ, et al. Red blood cell transfusion in critically ill children; A narrative review. Pediatr Crit Care Med. 2011;12:174–183. doi: 10.1097/PCC.0b013e3181e30d09. [DOI] [PubMed] [Google Scholar]
- 31.Karam O, Tucci M, Bateman ST, et al. Association between length of storage of red blood cell units and outcome of critically ill children. Crit Care. 2010;14:R57. doi: 10.1186/cc8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karam O, Tucci M, Ducruet T, et al. Red blood cell transfusion thresholds in pediatric septic patients. Pediatr Crit Care Med. 2011;12:512–18. doi: 10.1097/PCC.0b013e3181fe344b. [DOI] [PubMed] [Google Scholar]
- 33.Lacroix J, Hébert PC, Hutchison JH, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 34.Lacroix J, Trottier H, Tucci M. Pour un meilleur usage des concentrés érythrocytaires dans les services de soins intensifs pédiatriques. Med Sci (Paris) 2009;25:963–966. doi: 10.1051/medsci/20092511963. [DOI] [PubMed] [Google Scholar]
- 35.Mazine A, Rached-D’Astous S, Ducruet T, et al. Blood transfusions adter pediatric cardiac operation: a North-American multicenter prospective study. Ann Thorac Surg. 2015;100:671–677. doi: 10.1016/j.athoracsur.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Ranucci M, Carlucci C, Isgro G, et al. Duration of red blood cell storage and outcomes in pediatric cardiac surgery: An association found for pump prime blood. Crit Care. 2009;13:R207. doi: 10.1186/cc8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouette J, Trottier H, Ducruet T, et al. Red blood cell transfusion threshold in post-surgical pediatric intensive care patients: a randomized clinical trial. Ann Surg. 2010;251:421–427. doi: 10.1097/SLA.0b013e3181c5dc2e. [DOI] [PubMed] [Google Scholar]
- 38.Strauss RG. 2008 Emily Cooley Memorial Lecture: lessons learnt from pediatric transfusion medicine clinical trials… a little child shall lead them. Transfusion. 2009;49:1996–2004. doi: 10.1111/j.1537-2995.2009.02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Székely A, Cserép Z, Sápi E, et al. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009;87:187–197. doi: 10.1016/j.athoracsur.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 40.Toledano B, Tucci M, Lacroix J. Red cell transfusion in pediatric cardiac patients: fact and fallacies. Pediatr Crit Care Med. 2011;12:107–108. doi: 10.1097/PCC.0b013e3181e289eb. [DOI] [PubMed] [Google Scholar]
- 41.White M, Barron J, Gornbein J, et al. Are red blood cell transfusions associated with nosocomial infections in pediatric intensive care units? Pediatr Crit Care Med. 2010;11:464–468. doi: 10.1097/PCC.0b013e3181ce708d. [DOI] [PubMed] [Google Scholar]
- 42.Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery. Crit Care Med. 2010;38:649–656. doi: 10.1097/CCM.0b013e3181bc816c. [DOI] [PubMed] [Google Scholar]
- 43.Netzer G, Liu X, Harris AD, et al. Transfusion practice in the intensive care unit: a 10-year analysis. Transfusion. 2010;50:2125–2134. doi: 10.1111/j.1537-2995.2010.02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dallman MD, Liu X, Harris AD, et al. Changes in transfusion practice over time in the PICU. Pediatr Crit Care Med. 2013;14:843–850. doi: 10.1097/PCC.0b013e31829b1bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy DJ, Pronovost PJ, Lehmann CU, et al. Red blood cell transfusion practices in two surgical intensive care units: a mixed methods assessment of barriers to evidence-based practice. Transfusion. 2014;54:2658–2667. doi: 10.1111/trf.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du Pont-Thibodeau G, Tucci M, Ducruet T, et al. Survey on stated transfusion practices in PICU. Pediatr Crit Care Med. 2014;15:409–416. doi: 10.1097/PCC.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 47.Klaus SA, Frank SM, Salazar JH, et al. Hemoglobin thresholds for transfusion in pediatric patients at a large academic health center. Transfusion. 2015;55:2890–7. doi: 10.1111/trf.13296. [DOI] [PubMed] [Google Scholar]
- 48.Hibbs SP, Nielsen ND, Brunskill S, et al. The impact of electronic decision support on transfusion practice: a systematic review. Transfus Med Rev. 2015;29:14–23. doi: 10.1016/j.tmrv.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Sinuff T, Muscedere J, Adhikari NK, et al. Knowledge translation interventions for critically ill patients: a systematic review. Crit Care Med. 2013;41:2627–2640. doi: 10.1097/CCM.0b013e3182982b03. [DOI] [PubMed] [Google Scholar]
- 50.Baer VL, Henry E, Lambert DK, et al. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion. 2011;51:264–269. doi: 10.1111/j.1537-2995.2010.02823.x. [DOI] [PubMed] [Google Scholar]
- 51.Stricker PA, Fiadjoe JE, Kilbaugh TJ, et al. Effect of transfusion guidelines on postoperative transfusion in children undergoing craniofacial reconstruction surgery. Pediatr Crit Care Med. 2012;13:e357–362. doi: 10.1097/PCC.0b013e31825b561b. [DOI] [PubMed] [Google Scholar]
- 52.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 53.Blood Observational Study Investigators of ANZICS-Clinical Trials Group. Westbrook A, Pettilä V, et al. Transfusion practice and guidelines in Australian and New Zealand intensive care units. Intensive Care Med. 2010;36:1138–1146. doi: 10.1007/s00134-010-1867-8. [DOI] [PubMed] [Google Scholar]
- 54.Rice TW, Morris S, Tortella BJ, et al. Deviations from evidence-based clinical management guidelines increase mortality in critically injured trauma patients. Crit Care Med. 2012;40:778–786. doi: 10.1097/CCM.0b013e318236f168. [DOI] [PubMed] [Google Scholar]
- 55.Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion. 2010;50:1227–1239. doi: 10.1111/j.1537-2995.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 56.Lacroix J, Demaret P, Tucci M. Red blood cell transfusion: decision making in pediatric intensive care units. Semin Perinat. 2012;36:225–231. doi: 10.1053/j.semperi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:327–330. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]
- 59.Acker SN, Partrick DA, Ross JT, et al. Blood component transfusion increases the risk of death in children with traumatic brain injury. J Trauma Acute Care Surg. 2014;76:1082–1087. doi: 10.1097/TA.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 60.Akech SO, Hassall O, Pamba A, et al. Survival and haematological recovery of children with severe malaria transfused in accordance to WHO guidelines in Kilifi, Kenya. Malar J. 2008;7:256. doi: 10.1186/1475-2875-7-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cholette JM, Swartz MF, Rubenstein J, et al. Outcomes using a conservative versus liberal red blood cell transfusion strategy in infants requiring cardiac operation. Ann Thorac Surg. 2017;103:206–214. doi: 10.1016/j.athoracsur.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 62.Gauvin F, Robillard P, Hume H, et al. Transfusion related acute lung injury in the Canadian paediatric population. Paediatrics & Child Health. 2012;17:235–240. [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo JA, Maher KO, Kirshbom PM, et al. Red blood cell transfusion for infants with single-ventricle physiology. Pediatr Cardiol. 2011;32:461–468. doi: 10.1007/s00246-011-9901-3. [DOI] [PubMed] [Google Scholar]
- 64.Olupot-Olupot P, Engoru C, Thompson J, et al. Phase II trial of standard versus increased transfusion volume in Ugandan children with acute severe anemia. BMC Med. 2014;12:67. doi: 10.1186/1741-7015-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajasekaran S, Sanfilippo D, Shoemaker A, et al. Respiratory impairment after early red cell transfusion in pediatric patients with ALI/ARDS. Crit Care Res Pract. 2012;2012 doi: 10.1155/2012/646473. Article IC number, 646473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Redlin M, Habazet H, Schoenfeld H, et al. Red blood cell storage duration is associated with various clinical outcomes in pediatric cardiac surgery. Transfus Med Hemother. 2014;41:146–151. doi: 10.1159/000357998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willems A, Van Lerberghe C, Gonsette K, et al. The indication for perioperative red blood cell transfusions is a predictive risk factor for severe postoperative morbidity and mortality in children undergoing cardiac surgery. Eur J Cardiothorac Surg. 2014;45:1050–1057. doi: 10.1093/ejcts/ezt548. [DOI] [PubMed] [Google Scholar]
- 68.Pollack MM, Ruttiman UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Gibson BE, Todd A, Roberts I, et al. Transfusion guidelines for neonates and older children. Br J Haematol. 2004;124:433–453. doi: 10.1111/j.1365-2141.2004.04815.x. [DOI] [PubMed] [Google Scholar]
- 70.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 71.Gould SA, Rice CL, Moss GS. The physiologic basis of the use of blood and blood products. Surg Ann. 1984;16:13–38. [PubMed] [Google Scholar]
- 72.Macknet MR, Allard M, Applegate RL, et al. The accuracy of noninvasive and continuous total hemoglobin measurement by pulse CO-Oximetry in human subjects undergoing hemodilution. Anesth Analg. 2010;111:1424–1426. doi: 10.1213/ANE.0b013e3181fc74b9. [DOI] [PubMed] [Google Scholar]
- 73.Elizalde JI, Clemente J, Marin JL, et al. Early changes in hemoglobin and hematocrit levels after packed red cell transfusion in patients with acute anemia. Transfusion. 1997;37:573–576. doi: 10.1046/j.1537-2995.1997.37697335150.x. [DOI] [PubMed] [Google Scholar]
- 74.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 75.Vanhorebeek I, De Vos R, Mesotten D, et al. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 76.Shoemaker WC, Appel PL, Kram HB, et al. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 77.Muszynski JA, Guzetta N, Hall MW, et al. Recommendations on red blood cell transfusion for critically ill children with non-hemorrhagic shock from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;19(Suppl) doi: 10.1097/PCC.0000000000001605. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valentine SL, Bateman ST, Bembea MM, et al. Consensus recommendations for red blood cell transfusion practice in critically ill children from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;1(Suppl) doi: 10.1097/PCC.0000000000001613. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cholette JM, Willems A, Schwartz S, et al. Recommendations on red blood cell transfusions in infants and children with acquired and congenital heart disease from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;19(Suppl) doi: 10.1097/PCC.0000000000001603. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yusuf S, Wittes J, Probstfield J, et al. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991;266:93–98. [PubMed] [Google Scholar]
- 81.Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 82.Leung JM, Weiskopf RB, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93:1004–1010. doi: 10.1097/00000542-200010000-00023. [DOI] [PubMed] [Google Scholar]
- 83.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 84.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 86.Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 87.Cotter DJ, Stefanik K, Zhang Y, et al. Hematocrit was not validated as a surrogate end point for survival among epoetin-treated hemodialysis patients. J Clin Epidemiol. 2004;57:1086–1095. doi: 10.1016/j.jclinepi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 89.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;(8):431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 90.Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology. 2002;96:871–817. doi: 10.1097/00000542-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 91.Figaji AA, Zwane E, Kogels M, et al. The effect of blood transfusion on brain oxygenation in children with severe traumatic brain injury. Pediatr Crit Care Med. 2010;11:325–331. doi: 10.1097/PCC.0b013e3181b80a8e. [DOI] [PubMed] [Google Scholar]
- 92.Bailey SM, Hendricks-Munoz KD, Wells JT, et al. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am J Perinatol. 2010;27:445–453. doi: 10.1055/s-0030-1247598. [DOI] [PubMed] [Google Scholar]
- 93.Tasker RC, Turgeon AF, Spinella PC, et al. Recommendations on red blood cell transfusion for critically ill children with acute brain injury from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;19(Suppl) doi: 10.1097/PCC.0000000000001589. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steiner ME, Zantek ND, Stanworth SJ, et al. Recommendations on red blood cell transfusion support in children with hematologic and oncologic diagnoses: recommendations from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;19(Suppl) doi: 10.1097/PCC.0000000000001610. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bembea MM, Cheifetz IM, Fortenberry JD, et al. Recommendations for red blood cell transfusions for the critically ill child receiving support from extracorporeal membrane oxygenation, ventricular assist and renal replacement therapy device from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;19(Suppl) doi: 10.1097/PCC.0000000000001600. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–920. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 97.van Beest PA, Vos JJ, Poterman M, et al. Tissue oxygenation as a target for goal-directed therapy in high-risk surgery: a pilot study. BMC Anesthesiol. 2014;14:122. doi: 10.1186/1471-2253-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feiner JR, Finlay-Morreale HE, Toy P, et al. High oxygen partial pressure decreases anemia-induced heart rate increase equivalent to transfusion. Anesthesiology. 2011;115:492–498. doi: 10.1097/ALN.0b013e31822a22be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 100.Bolton-Maggs PHB on behalf of the SHOT Steering Group. The 2015 Annual SHOT Report. London: Serious Hazards of Transfusion; 2016. [Google Scholar]
- 101.Looney MR, Roubinian N, Gajic O, et al. Prospective study on the clinical course and outcomes in transfusion-related acute lung injury. Crit Care Med. 2014;42:1676–1687. doi: 10.1097/CCM.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bellomo R, Mårtensson J, Kaukonen KM, et al. Epidemiology of RBC transfusions in patients with severe acute kidney injury: analysis from the randomized evaluation of normal versus augmented level study. Crit Care Med. 2016;44:892–900. doi: 10.1097/CCM.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 103.Gauvin F, Lacroix J, Robillard P, et al. Acute transfusion reaction in pediatric intensive care unit. Transfusion. 2006;46:1899–1908. doi: 10.1111/j.1537-2995.2006.00995.x. [DOI] [PubMed] [Google Scholar]
- 104.Church GD, Matthay MA, Liu K, et al. Blood product transfusions and clinical outcomes in pediatric patients with acute lung injury. Pediatr Crit Care Med. 2009;10:297–302. doi: 10.1097/PCC.0b013e3181988952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gauvin F, Lefebvre E, Jarlot C, et al. Transfusion-associated circulatory overload in a pediatric intensive care unit: different incidence rates with different interpretations of the same definition? Transf Med. 2013;23(suppl.1):19. [Google Scholar]
- 106.Kleiber N, Lefebvre É, Gauvin F, et al. Respiratory dysfunction associated with red blood cell transfusion in critically ill children: a prospective cohort study. Pediatr Crit Care Med. 2015;16:325–334. doi: 10.1097/PCC.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 107.Connes P, Hue O, Hardy-Dessources MD, et al. Hemorheology and heart rate variability: is there a relationship? Clin Hemorheol Microcirc. 2008;38:257–265. [PubMed] [Google Scholar]
- 108.Mpoya A, Kiguli S, Olupot-Olupot P, et al. Transfusion and treatment of severe anaemia in African children (TRACT): a study protocol for a randomised controlled trial. Trials. 2015;29:593. doi: 10.1186/s13063-015-1112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Demaret P, Emeriaud G, Hassan NE, et al. Recommendations for red blood cell transfusions in critically ill children with acute respiratory failure from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;19(Suppl) doi: 10.1097/PCC.0000000000001619. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karam O, Russell RT, Stricker P, et al. Recommendations on red blood cell transfusions in critically ill children with non-life threatening or life-threatening bleeding from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI) Pediatr Crit Care Med. 2018;19(Suppl) submitted. [Google Scholar]
- 111.Waters JH, Yazer MH. Patient blood management: where’s the bottom? Transfusion. 2015;55:700–702. doi: 10.1111/trf.13063. [DOI] [PubMed] [Google Scholar]
- 112.Frank SM, Johnson DJ, Resar LM. Ultramassive transfusion: give blood, save a life. Transfusion. 2016;56:546–548. doi: 10.1111/trf.13403. [DOI] [PubMed] [Google Scholar]
- 113.Raimer PL, Han YY, Weber MS, et al. A normal capillary refill time of ≤ 2 seconds is associated with superior vena cava oxygen saturations of ≥ 70% J Pediatr. 2011;158:68–72. doi: 10.1016/j.jpeds.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 114.Duret J, Pottecher J, Bouzat P, et al. Skeletal muscle oxygenation in severe trauma patients during haemorrhagic shock resuscitation. Crit Care. 2015;19:141. doi: 10.1186/s13054-015-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Creteur J, Neves AP, Vincent JL. Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care. 2009;13(Suppl 5):S11. doi: 10.1186/cc8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kiraly LN, Underwood S, Differding JA, et al. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67:29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 117.Orlov D, O’Farrell R, McCluskey SA, et al. The clinical utility of an index of global oxygenation for guiding red blood cell transfusion in cardiac surgery. Transfusion. 2009;49:682–628. doi: 10.1111/j.1537-2995.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- 118.McKinley BA, Marvin RG, Cocanour CS, et al. Tissue hemoglobin O2 saturation during resuscitation of traumatic shock monitored using near infrared spectrometry. J Trauma. 2000;48:637–642. doi: 10.1097/00005373-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 119.Torella F, Haynes SL, McCollum CN. Cerebral and peripheral oxygen saturation during red cell transfusion. J Surg Res. 2003;110:217–221. doi: 10.1016/s0022-4804(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 120.Smith J, Bricker S, Putnam B. Tissue oxygen saturation predicts the need for early blood transfusion in trauma patients. Am Surg. 2008;74:1006–1011. [PubMed] [Google Scholar]
- 121.Memtsoudis SG, Danninger T, Stundner O, et al. Blood transfusions may have limited effect on muscle oxygenation after total knee athroplasty. HSS J. 2015;11:136–142. doi: 10.1007/s11420-015-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sadaka F, Aggu-Sher R, Krause K, et al. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann Intensive Care. 2011;1:46. doi: 10.1186/2110-5820-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuruk K, Bartels SA, Milstein DM, et al. Red blood cell transfusions and tissue oxygenation in anemic hematology outpatients. Transfusion. 2012;52:641–646. doi: 10.1111/j.1537-2995.2011.03312.x. [DOI] [PubMed] [Google Scholar]
- 124.Lucking SE, Williams TM, Chaten FC, et al. Dependence of oxygen consumption on oxygen delivery in children with hyperdynamic septic shock and low oxygen extraction. Crit Care Med. 1990;18:1316–1319. doi: 10.1097/00003246-199012000-00002. [DOI] [PubMed] [Google Scholar]
- 125.Ronco JJ, Fenwick JC, Tweeddale MG, et al. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA. 1993;270:1724–1730. [PubMed] [Google Scholar]
- 126.Ronco JJ, Fenwick JC, Wiggs BR, et al. Oxygen consumption is independent of increases in oxygen delivery by dobutamine in septic patients who have normal or increased plasma lactate. Am Rev Respir Dis. 1993;147:25–31. doi: 10.1164/ajrccm/147.1.25. [DOI] [PubMed] [Google Scholar]
- 127.Fontana JL, Welborn L, Mongan PD, et al. Oxygen consumption and cardiovascular function in children during profound intraoperative normovolemic hemodilution. Anesth Analg. 1995;80:219–225. doi: 10.1097/00000539-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 128.Seear M, Wensley D, MacNab A. Oxygen consumption-oxygen delivery relationship in children. J Pediatr. 1993;123:208–214. doi: 10.1016/s0022-3476(05)81690-7. [DOI] [PubMed] [Google Scholar]
- 129.Shafer KM, Mann N, Hehn R, et al. Relationship between exercise parameters and noninvasive indices of right ventricular function in patients with biventricular circulation and systemic right ventricle. Congenit Heart Dis. 2015;10:457–465. doi: 10.1111/chd.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steffes CP, Bender JS, Levison MA. Blood transfusion and oxygen consumption in surgical sepsis. Crit Care Med. 1991;19:512–517. doi: 10.1097/00003246-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 131.McQuillen PS, Nishimoto MS, Bottrell CL, et al. Regional and central venous oxygen saturation monitoring following pediatric cardiac surgery: concordance and association with clinical variables. Pediatr Crit Care Med. 2007;8:154–160. doi: 10.1097/01.PCC.0000257101.37171.BE. [DOI] [PubMed] [Google Scholar]
- 132.Spenceley N, Krahn G, Skippen P, et al. Evaluation of a pediatric central venous oximetry catheter in critically ill children. Pediatr Crit Care Med. 2010;11:26–30. doi: 10.1097/PCC.0b013e3181a63e0c. [DOI] [PubMed] [Google Scholar]
- 133.Bronicki RA. Venous oximetry and the assessment of oxygen transport balance. Pediatr Crit Care Med. 2011;12(Suppl):S21–26. doi: 10.1097/PCC.0b013e3182211667. [DOI] [PubMed] [Google Scholar]
- 134.Fuller BM, Gajera M, Schorr C, et al. Transfusion of packed red blood cells is not associated with improved central venous oxygen saturation or organ function in patients with septic shock. J Emerg Med. 2012;43:593–598. doi: 10.1016/j.jemermed.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vallet B, Robin E, Lebuffe G. Venous oxygen saturation as a physiologic transfusion trigger. Crit Care. 2010;14:213. doi: 10.1186/cc8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baenziger O, Keel M, Bucher HU, et al. Oxygen extraction index measured by near infrared spectroscopy--a parameter for monitoring tissue oxygenation? Adv Exp Med Biol. 2009;645:161–166. doi: 10.1007/978-0-387-85998-9_25. [DOI] [PubMed] [Google Scholar]
- 137.Lauscher P, Kertscho H, Raab L, et al. Changes in heart rate variability across different degrees of acute dilutional anemia. Minerva Anestesiol. 2011;77:943–951. [PubMed] [Google Scholar]
- 138.Bravi A, Green G, Longtin A, et al. Monitoring and identification of sepsis development through a composite measure of heart rate variability. PLoS One. 2012;7:e45666. doi: 10.1371/journal.pone.0045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101:200–206. doi: 10.1093/bja/aen133. [DOI] [PubMed] [Google Scholar]
- 140.Shemie SD. Serum lactate predicts postoperative complications after pediatric cardiac surgery. Pediatrics. 1996;98:S550. [Google Scholar]
- 141.Shime N, Ashida H, Hiramatsu N, et al. Arterial ketone body ratio for the assessment of the severity of illness in pediatric patients following cardiac surgery. J Crit Care. 2001;16:102–107. doi: 10.1053/jcrc.2001.28786. [DOI] [PubMed] [Google Scholar]
- 142.Shime N, Kageyama K, Ashida H, et al. Application of modified sequential organ failure assessment score in children after cardiac surgery. J Cardiothorac Vasc Anesth. 2001;15:463–468. doi: 10.1053/jcan.2001.24983. [DOI] [PubMed] [Google Scholar]
- 143.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: The TOTAL randomized clinical trial. JAMA. 2015;314:2514–2523. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 144.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 145.Baines PBAPJT, Sills JA, et al. Gastric tonometry and cardiac function in children with meningococcal disease. Intensive Care Med. 1996;22:S163. [Google Scholar]
- 146.Silverman HJ, Tuma P. Gastric tonometry in patients with sepsis. Effects of dobutamine infusions and packed red blood cell transfusions. Chest. 1992;102:184–188. doi: 10.1378/chest.102.1.184. [DOI] [PubMed] [Google Scholar]
- 147.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 148.Typpo KV, Wong H, Finley S, et al. Monitoring severity of MODS: New technologies. Pediatr Crit Care Med. 2017;18(suppl):S24–31. doi: 10.1097/PCC.0000000000001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spahn DR, Smith LR, Schell RM, et al. Importance of severity of coronary artery disease for the tolerance to normovolemic hemodilution. Comparison of single-vessel versus multivessel stenoses in a canine model. J Thorac Cardiovasc Surg. 1994;108:231–239. [PubMed] [Google Scholar]
- 150.Spahn DR, Smith LR, Veronee CD, et al. Acute isovolemic hemodilution and blood transfusion. Effects on regional function and metabolism in myocardium with compromised coronary blood flow. J Thorac Cardiovasc Surg. 1993;105:694–704. [PubMed] [Google Scholar]
- 151.Heyer L, Mebazaa A, Gayat E, et al. Cardiac troponin and skeletal muscle oxygenation in severe post-partum haemorrhage. Crit Care. 2009;13(Suppl 5):S8. doi: 10.1186/cc8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Spahn DR, Madjdpour C. Physiologic transfusion triggers: do we have to use (our) brain? Anesthesiology. 2006;104:905–906. doi: 10.1097/00000542-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 153.Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92:1646–1652. doi: 10.1097/00000542-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 154.Liem KD, Hopman JC, Oeseburg B, et al. The effect of blood transfusion and haemodilution on cerebral oxygenation and haemodynamics in newborn infants investigated by near infrared spectrophotometry. Eur J Pediatr. 1997;156:305–310. doi: 10.1007/s004310050606. [DOI] [PubMed] [Google Scholar]
- 155.Yamal JM, Rubin ML, Benoit JS, et al. Effect of hemoglobin transfusion threshold on cerebral hemodynamics and oxygenation. J Neurotrauma. 2015;32:1239–1245. doi: 10.1089/neu.2014.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cem A, Serpil UO, Fevzi T, et al. Efficacy of near-infrared spectrometry for monitoring the cerebral effects of severe dilutional anemia. Heart Surg Forum. 2014;17:E154–159. doi: 10.1532/HSF98.2013293. [DOI] [PubMed] [Google Scholar]
- 157.Torella F, Haynes SL, McCollum CN. Cerebral and peripheral near-infrared spectroscopy: an alternative transfusion trigger? Vox Sang. 2002;83:254–257. doi: 10.1046/j.1423-0410.2002.00223.x. [DOI] [PubMed] [Google Scholar]
- 158.Aries MJ, Coumou AD, Elting JW, et al. Near infrared spectroscopy for the detection of desaturations in vulnerable ischemic brain tissue: a pilot study at the stroke unit bedside. Stroke. 2012;43:1134–1136. doi: 10.1161/STROKEAHA.111.636894. [DOI] [PubMed] [Google Scholar]
- 159.Moreau F, Yang R, Nambiar V, et al. Near-infrared measurements of brain oxygenation in stroke. Neurophotonics. 2016;3:031403. doi: 10.1117/1.NPh.3.3.031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zygun DA, Nortje J, Hutchison PJ, et al. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med. 2009;37:1074–1078. doi: 10.1097/CCM.0b013e318194ad22. [DOI] [PubMed] [Google Scholar]
- 161.Gayle MO, Frewen TC, Armstrong RF, et al. Jugular venous bulb catheterization in infants and children. Crit Care Med. 1989;17:385–388. doi: 10.1097/00003246-198905000-00001. [DOI] [PubMed] [Google Scholar]
- 162.Yoshitan iK, Kawaguchi M, Iwata M, et al. Comparison of changes in jugular venous bulb oxygen saturation and cerebral oxygen saturation during variations of haemoglobin concentration under propofol and sevoflurane anaesthesia. Br J Anaesth. 2005;94:341–346. doi: 10.1093/bja/aei046. [DOI] [PubMed] [Google Scholar]
- 163.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. [PubMed] [Google Scholar]
- 164.Kleen M, Habler O, Hutter J, et al. Effects of hemodilution on splanchnic perfusion and hepatorenal function. I. Splanchnic perfusion. Eur J Med Res. 1997;2:413–418. [PubMed] [Google Scholar]
- 165.Habler O, Kleen M, Hutter J, et al. Effects of hemodilution on splanchnic perfusion and hepatorenal function. II. Renal perfusion and hepatorenal function. Eur J Med Res. 1997;2:419–424. [PubMed] [Google Scholar]
- 166.Laverdière C, Gauvin F, Hébert PC, et al. Survey of transfusion practices in pediatric intensive care units. Pediatr Crit Care Med. 2002;3:335–340. doi: 10.1097/00130478-200210000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.