Abstract
Escalating occurrences of emerging infectious diseases underscore the importance of understanding microbiome–pathogen interactions. The amphibian cutaneous microbiome is widely studied for its potential to mitigate disease-mediated amphibian declines. Other microbial interactions in this system, however, have been largely neglected in the context of disease outbreaks. European fire salamanders have suffered dramatic population crashes as a result of the newly emerged Batrachochytrium salamandrivorans (Bsal). In this paper, we investigate microbial interactions on multiple fronts within this system. We show that wild, healthy fire salamanders maintain complex skin microbiotas containing Bsal-inhibitory members, but these community are present at a remarkably low abundance. Through experimentation, we show that increasing bacterial densities of Bsal-inhibiting bacteria via daily addition slowed disease progression in fire salamanders. Additionally, we find that experimental-Bsal infection elicited subtle changes in the skin microbiome, with selected opportunistic bacteria increasing in relative abundance resulting in septicemic events that coincide with extensive destruction of the epidermis. These results suggest that fire salamander skin, in natural settings, maintains bacterial communities at numbers too low to confer sufficient protection against Bsal, and, in fact, the native skin microbiota can constitute a source of opportunistic bacterial pathogens that contribute to pathogenesis. By shedding light on the complex interaction between the microbiome and a lethal pathogen, these data put the interplay between skin microbiomes and a wildlife disease into a new perspective.
Keywords: host microbiome, disease, amphibians, chytridiomycosis, Batrachochytrium salamandrivorans, wildlife diseases
1. Introduction
Advances in the knowledge of symbiotic microbiomes are changing our understanding of vertebrate host biology and ecology [1,2]. Resident microbiotas of metazoans are intricately linked to host health, whether it be through participation in energy metabolism, immune system development, or contributing to defence against pathogens [1,3–6]. Symbiotic microbes can occupy a central role in host–pathogen interactions, eliciting protective effects against invading pathogens through space and nutrient competition, production of anti-pathogen compounds as well as immuno-modulatory stimulation [4,7]. Elucidating the role of the microbiota within host–pathogen systems is critical for understanding the ecology of the disease.
Pathogenic fungi are unprecedented players in emerging infectious diseases [8]. From plants to vertebrates, infectious diseases caused by fungi are threatening food resources and leading to biodiversity loss [8–10]. Wheat stem rust and rice blast disease threaten important crops upon which humans depend (Puccinia graminis, Magnaporthe oryzae [11]). Bees responsible for pollination are being devastated by colony collapse disorder (Nosema species [12]). Bat populations in North America are collapsing due to White-nose syndrome (Geomyces destructans [13]). Furthermore, amphibian chytridiomycosis, originally caused by the sole fungus, Batrachochytrium dendrobatidis [14,15], has been marked as a main culprit of amphibian declines within the so-called sixth mass extinction [16]. This cutaneous pathogen is considered the largest disease threat to the world's biodiversity as it has ravaged amphibian communities globally [16,17].
The recent emergence of a second amphibian-infecting chytrid, Batrachochytrium salamandrivorans (Bsal [18]) adds to the disease threat to these animals. Bsal poses a significant threat to western Palearctic salamanders, and, in particular, is responsible for severe declines of European fire salamanders, Salamandra salamandra [19,20]. Bsal invades keratinized amphibian skin, leading to superficial erosions and numerous deep ulcerations across the body of susceptible host species [18]. As infection escalates and induces chytridiomycosis it can result in death in less than one month [18–20]. Salamander hosts appear to have little ability to fight back against Bsal through host-based defences. Expression of immune genes remained unchanged during experimental infection of an Asian species, Tylototriton wenxianensis [21]. Furthermore, fire salamanders, S. salamandra, mounted no immune response after five cycles of exposure-clearance regimes [20].
To date, limited work has been conducted on the role of host microbiota in Bsal-infection dynamics [22], yet a thorough understanding of salamander-microbiome-Bsal interactions is clearly essential. It is plausible that resident skin microbiota contributes to the host's mucosal defences against Bsal, which would open new horizons for disease mitigation [23]. However, the massive destruction of the epidermis during Bsal infection may equally predispose opportunistic pathogens to cause fatal septicemia. These juxtaposing ideas raise the pivotal question: What is the role of skin bacteria in Bsal infection of fire salamanders? Are they a friend, foe or bystander?
Here, we combine evidence from field studies and laboratory experiments to understand the role of bacteria in Bsal infection dynamics of the highly susceptible fire salamander. We surveyed healthy populations of wild fire salamanders to determine their natural skin bacterial densities, and performed laboratory experiments to investigate the response of salamander skin microbiota to Bsal infection. We further evaluated the function of cultivable resident bacteria against Bsal and the ability of these bacteria to alter infection dynamics in vivo.
2. Methods
(a). Field sampling
Healthy, adult European fire salamanders (Salamandra salamandra) were sampled at multiple locations across Germany in 2015 (electronic supplementary material, tables S1 and S2). At the time of sampling, all amphibians screened for Bsal from these locations, including the fire salamanders in our study, were Bsal-negative [24]. Adults were captured with gloved hands and skin swabs were taken following standard methods [25,26] for either DNA-based analyses (qPCR estimation of bacterial abundance and 16S amplicon sequencing of bacterial community) or cultivation of skin microbes. Swabs for DNA-based analyses were stored dry, and swabs for cultivation were stored in 20% glycerol to maintain bacterial cell integrity. All samples were stored in ice and immediately frozen upon return to the laboratory.
(b). Culturing of skin bacteria
Skin bacteria were cultured from fire salamander populations across Germany (electronic supplementary material, table S2). Culturable skin swabs were processed as explained in [26].
(c). Bsal growth inhibition assays
A total of 708 bacterial isolates from fire salamander skin were tested in Bsal growth inhibition assays. Assay methodology followed a modified version of the 96-well assay method described in [27] (see electronic supplementary material, Methods). Inhibitory function against Bsal was determined by comparison of Bsal growth rate in the presence of bacterial CFS with that of the nutrient-depleted control (Bsal zoospores grown without additional nutrients) with FDR corrections. Enhancing function against Bsal was determined by comparison of Bsal growth rate in the presence of bacterial CFS with that of the positive control (Bsal zoospores growth in TGHL media) with FDR corrections. Selected bacteria (electronic supplementary material, table S3) were tested multiple times to explore functional consistency.
(d). Liver isolate cultivation
Bsal infection results in deep ulcerations of the skin surface. This breaching of the integrity of the skin barrier may result in bacterial invasion of internal organs and the blood. To investigate this, bacterial isolates from the livers of nine fire salamanders that died due to experimental Bsal infection and nine non-Bsal-infected salamanders were cultured on Columbia agar with sheep blood. Cultures were incubated at 15°C and isolated into pure culture. Morphologically distinct bacteria were identified using Sanger sequencing of the 16S rRNA gene.
(e). Bsal infection experiment for microbiome analysis
Twelve captive-bred fire salamanders (six control, six Bsal-exposed) were housed individually at 15 ± 1°C on moist tissue, with access to a hiding place (PVC pipe) and a water container. Salamanders were exposed to Bsal (AMFP13/01; 5000 zoospores ml−1) by dripping 1 ml of a zoospore suspension onto the salamander. Controls received 1 ml of artificial pond water. Animals were fed twice weekly with crickets. Individuals were swabbed as described in Bletz et al. [25] prior to exposure and 10 days post-exposure.
(f). Bacterial addition experiments
The bacterial addition experiment was conducted to evaluate the function of bacteria in an in vivo context and to understand if bacteria on the skin can alter Bsal-infection dynamics. Bacterial isolates for addition experiments were selected from in vitro Bsal growth assay results. Selection criteria are outlined in the electronic supplementary material, Methods.
Twenty-six captive-bred fire salamanders were housed individually and fed as explained above for a duration of 11 weeks. The following treatments were used: (i) daily addition of a Stenotrophomonas sp. (Bsal-enhancing, n = 7), (ii) daily addition of a Pseudomonas sp. (Bsal-enhancing, n = 7), (iii) daily addition of a sham treatment of 1 ml of sterile water with an agar swab (n = 7), and (iv) no treatment (n = 5). Once the bacteria had grown on the agar plate, a swab was used to collect bacterial cells from the plate. The collected cells were then suspended in 10 ml of sterile artificial pond water. The sham treatment with the agar swab was included as a control for this process. Bacterial cells were quantified by spectrophotometry that was verified by CFU counts. Bacterial treatments were administered daily by adding 1 × 108 bacterial cells suspended in 1 ml of sterile artificial pond water. Daily administration of treatments was completed to ensure the continual presence of these bacteria on the skin for the duration of the experiment (11 weeks/77 days). Bsal exposure was given as described above (except 1 × 104 zoospores ml−1). Individuals were swabbed on day 0 prior to any treatment, on day 3 prior to Bsal exposure, and at weekly intervals following Bsal exposure for 11 weeks or until animals were removed from the experiment. Swabs were used to quantify bacterial load, Bsal load, and to culture skin bacteria for Matrix-Assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometry (MALDi-TOF) identity confirmation. Animals were removed from the experiment when clinical signs indicated lethal disease (high Bsal load and morbidity). At this time, skin swabs were taken, and individuals were subsequently euthanized with MS-222 overdose.
(g). DNA extraction
Bacterial DNA was isolated following protocols in previous studies: for microbiome analyses of field swabs, and the analysis of microbiome response in the Bsal exposure experiment, we used the MoBio PowerSoil DNA Isolation kit [25]; for skin bacterial isolates we used a Chelex protocol [26]; and for the bacterial addition experiment, Prepman was used [18]. MoBio extraction methods were used for samples where exploration of the microbial community composition was the goal. Prepman extraction was used in the bacterial addition experiment because it is more cost-effective and microbiome analysis was not being performed. Importantly, no comparisons were made among samples from different extraction methods.
(h). Identification of bacterial isolates
A fragment of the 16S rRNA gene from cultured isolates was PCR-amplified with the primers 27F (AGAGTTTGATCCTGGCTCAG) and 907R (CCGTCAATTCMTTTGAGTTT). PCR products were sequenced at LGC Genomics in Berlin, Germany. Sequencing produced approximately 500–800 bp for each bacterial isolate. Sequences were cleaned in CodonCode Aligner, and aligned with PyNAST. Taxonomy was assigned with RDP in Quantitative Insights Into Microbial Ecology (QIIME) [28] and a phylogenetic tree was built with fasttree [29].
(i). Quantification of bacterial abundance and Bsal
Total bacterial abundance and Bsal infection intensities were estimated with qPCR. Total bacteria were quantified using a SYBR Green qPCR assay using the universal bacterial primers described in [30]. qPCR conditions were 10 min at 95°C, followed by 39 cycles of 60 s at 94°C, 60 s at 50°C, 60 s at 60°C, and a final elongation for 15 min at 60°C. Primer concentration was 0.5 µM. Within the experiments, bacterial densities were determined by calculating the surface area swabbed from measurements of the trunk length and width. Bsal infection intensities were determined using the qPCR assay described in [31]. qPCRs were performed using the CFX384 Bio-Rad detection system.
(j). 16S rRNA characterization of communities
16S rRNA gene amplicon sequencing was performed on field samples and on samples collected during the Bsal exposure experiment. The V4 region of the 16S rRNA gene was PCR-amplified with dual-indexed primers as described in [32]. Pooled amplicons were sequenced on an Illumina MiSeq with 2 × 250 paired-end technology at the Helmholtz Center for Infectious Research in Braunschweig, Germany. QIIME [33] was used to demultiplex and quality-filter the sequence data, and sequences were clustered into sub-operational taxonomic units (sOTUs) using Deblur [34]. Detailed methodologies for processing the amplicon sequencing data are provided in the electronic supplementary material, Methods.
(k). MALDi-TOF
During the bacterial addition experiment, MALDI-TOF MS direct cell profiling was used to confirm the presence of the administered bacteria [35]. More specifically, bacteria were re-isolated from the skin of treated fire salamanders and MALDI-TOF was used to compare the profile of each morphologically distinct, re-cultured isolate to that of the original inoculum. MALDI-TOF was performed using an Autoflex Biotyper MALDI-TOF mass spectrometer (Bruker Daltonik) using the direct transfer method and α-cyano-4-hydroxycinnamic acid (HCCA) as a matrix, according to the manufacturer's guidelines. Detailed methodologies are provided in the electronic supplementary materials.
(l). Scanning electron microscopy
Scanning electron microscopy (SEM) of the skin of Bsal-free fire salamanders (3) was carried out to provide a visual assessment of the density of skin microbes. SEM was performed after 2% paraformaldehyde and 2.5% glutaraldehyde fixation in 0.1 M phosphate buffer [18].
(m). Statistical analyses
All statistical analyses were performed in R (v. 3.4, [36]) unless otherwise stated. For the Bsal exposure experiment looking at microbiomes, bacterial density and alpha diversity were analysed with general linear mixed effect models (GLMMs, lmer4; [37]). Treatment, time, and the time by treatment interaction were included in GLMMs along with individual as a random factor to account for repeated sampling. Data were normalized with log transformations as needed. We used ADONIS2 [38] to perform a permutational multivariate analysis of variance to assess whether time and Bsal exposure explained significant portions of the observed variation in microbial community structure (i.e. beta diversity). Time, treatment, and the time by treatment interaction were included as explanatory variables. We used Linear discriminant analysis effect size method (LEfSe) to identify differentially abundant bacterial taxa between the microbial communities of Bsal-exposed and control salamanders at the post-exposure time point [39].
In the bacterial addition experiment, all analyses of bacterial density and Bsal infection loads were performed on samples collected from the first 28 days of the experiment when all salamanders were still in the experiment. Bacterial density was statistically analysed with GLMMs as described above. To explore main effects and significant interactions we performed pairwise post hoc comparisons among treatments and time points as necessary. Rate of change in Bsal infection intensity was calculated as the slope of the estimated raw Bsal loads for each individual between day 14 (first time point where Bsal was detected) and 28 (last time point when all individuals were still in the experiment). These calculated rates were then compared across treatments with a Kruskal–Wallis test. Host survival was evaluated with a Cox log-rank test using the survival package [40]. Boxplot and survival graphs were created with ggplot2 and the survminer package [41].
3. Results
(a). Bacterial diversity and density on Salamandra skin
Skin bacterial communities from 275 wild, healthy fire salamanders in Germany were characterized using 16S amplicon sequencing. The richness of sub-operational taxonomic units (sOTUs) on individuals across populations averaged 212.6 ± 9.56 s.e. The skin microbiota predominantly comprised Proteobacteria (48.8%), Bacteriodetes (24.8%), Actinobacteria (8.2%), Firmicutes (6.7%), Cyanobacteria (4.1%), Acidobacteria (2.8%) and Verrucomicrobia (2.7%) (electronic supplementary material, appendix, figure S1). Furthermore, we found that microbial community structure differed across locations (PERMANOVA, Pseudo-F = 4.18, p = 0.001; electronic supplementary material, figure S2).
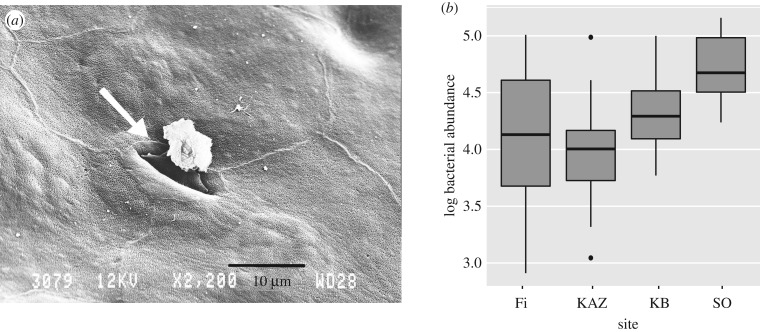
The average bacterial abundance on fire salamander skin (n = 94) across populations was 3.5 × 104 ± 6.7 × 103 s.e. rRNA copies/swab (figure 1b). The average bacterial abundance for the four populations was 2.34 × 104 ± 4.8 × 103 s.e., 1.43 × 104 ± 3.0 × 103, 2.63 × 104 ± 4.5 × 103 and 6.39 × 104 ± 1.3 × 103 for Fleischbach (Fi), Zweifallshammer (KAZ), Kallerbach (KB) and Solchbachtal (SO), respectively. A similarly low bacterial abundance on the skin surface was shown through SEM visualization, where often no or only a very limited number of bacterial cells can be seen (figure 1a).
Figure 1.
Natural densities of bacteria on the skin of fire salamanders. (a) Scanning electron microscopy image of salamander skin, showing a skin gland opening (arrow) and an overall lack of epidermal cell-associated bacteria. (b) Box plot showing qPCR estimates of bacterial abundance on fire salamander skin at four locations within the Eifel in western Germany. The upper and lower limits of the box represent the first and third quartiles, with the bold line representing the median. Whiskers extend to the minimum and maximum values, and points represent outliers.
(b). Bsal infection elicits subtle changes in Salamandra skin microbiome, which are associated with septicemic events
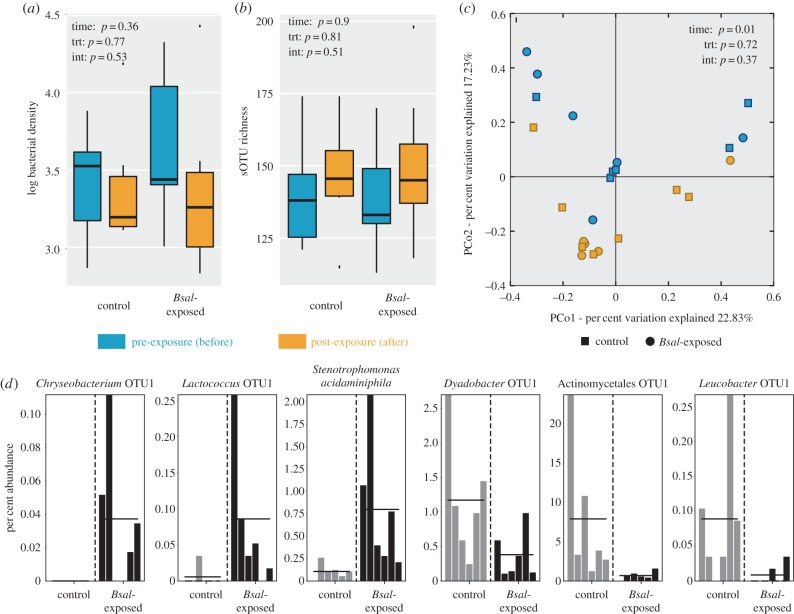
We characterized the cutaneous microbiome response to Bsal invasion using experimental infection of fire salamanders (Bsal-exposed: n = 6, control: n = 6) and 16S amplicon sequencing. Bsal infection had no effect on skin bacterial abundance, bacterial richness and diversity, or community structure. Average bacterial density on the salamander skin was 5.7 × 103 rRNA copies mm−2 (s.e. ± 1990.5) prior to exposure. There was no significant change as a result of Bsal infection or through time (GLMM: Treatment-F = 0.25, p = 0.63; Time-F = 0.63, p = 0.45; Interaction-F = 0.43, p = 0.53; figure 2a). Skin bacterial richness and diversity on fire salamanders before exposure averaged 142 ± 6.9 s.e., 81.7 ± 21.2, and 15.7 ± 1.1 for sOTU richness, effective number of sOTUs (exp(Shannon Index)), and Faith's phylogenetic diversity (PD), respectively. Bsal infection also had no significant effect on bacterial richness or diversity of skin communities; however, there was a significant increase through time in effective number of sOTUs (GLM: Effective number of sOTUs: Treatment-F = 0.02, p = 0.89; Time-F = 5.76, p = 0.04, Interaction-F = 1.35, p = 0.27; figure 2b; electronic supplementary material, figure S3). Bacterial community structure (i.e. beta diversity) also did not change as a result of Bsal infection, but significantly shifted through time (PERMANOVA: weighted Unifrac: Treatment–Pseudo-F = 0.67, p = 0.72; Time–Pseudo-F = 2.78, p = 0.01; Interaction–Pseudo-F = 1.016, p = 0.374; unweighted Unifrac: Treatment–Pseudo-F = 0.68, p = 0.86 Time–Pseudo-F = 1.73, p = 0.03; Interaction–Pseudo-F = 1.1119, p = 0.325; figure 2c).
Figure 2.
Response of fire salamander skin microbiota to Bsal infection. (a) qPCR estimates of bacterial density (rRNA copies/mm2), (b) species richness (sOTU richness) of skin bacterial communities and (c) bacterial community structure on control and Bsal-exposed salamanders. Bacterial community structure is visualized with Principal coordinate analysis of the weighted Unifrac distances. (d) Six representative sOTUs identified by LEfSe to be differentially abundant on Bsal-exposed individuals and control individuals.
While Bsal exposure elicited no significant change in alpha and beta diversity of fire salamander skin microbiota, particular bacterial taxa were found to be differentially abundant on infected versus control individuals using LEfSe. Seven bacterial sOTUs exhibited greater relative abundance on infected individuals, and three exhibited greater relative abundance on non-infected individuals at the post-exposure time point (figure 2d; electronic supplementary material, table S4). An Aeromonadaceae sp., a Chryseobacterium sp., a Fusobacteriacaeae sp., a Lactococcus sp. and Stenotrophomonas acidominiphila were differentially associated with Bsal-infected salamanders, whereas an Actinomycetales sp., a Dyadobacter sp., a Luecobacter sp. and a Pedobacter sp. were more common on control salamanders.
Moreover, Bsal-induced chytridiomycosis resulted in septicemic events, likely resulting from the breaching of the skin barrier by Bsal. Forty-five bacterial isolates were cultured and successfully identified from livers of nine infected fire salamanders. These liver-colonizing bacteria were from three phyla: Proteobacteria (21 isolates) Bacteriodetes (20 isolates) and Actinobacteria (four isolates) (electronic supplementary material, table S5). Most notably there were 15 isolates identified as Acinetobacter johnsonii and nine isolates identified as Chryseobacterium sp. (electronic supplementary material, table S5). No bacteria were isolated from the livers of fire salamanders that were not infected with Bsal.
(c). Increased density of Bsal-inhibitory bacteria dampens Bsal infection, but only marginally changes overall outcome
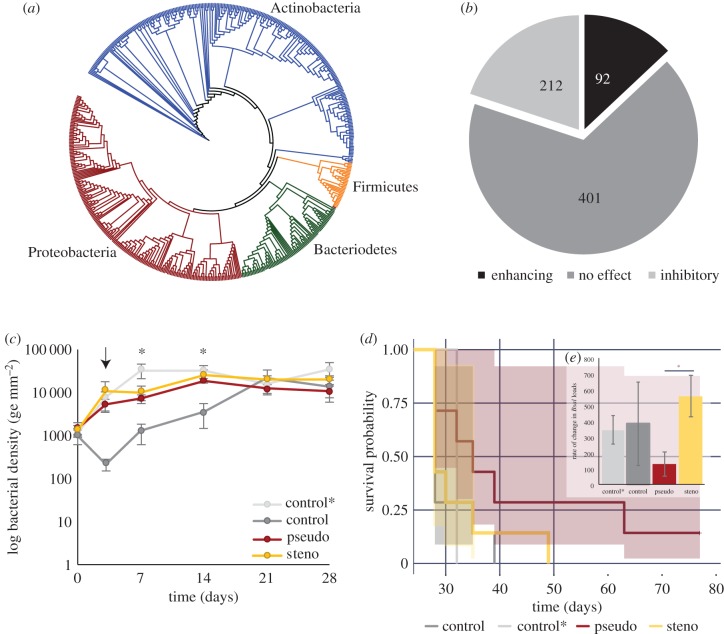
We evaluated the function of cutaneous bacteria on fire salamanders using in vitro culture-based approaches as well as in vivo experimentation. The cultured isolates (n = 708) were from the following phyla: Actinobacteria (44.6%), Proteobacteria (37.6%), Bacteriodetes (12.4%) and Firmicutes (5.4%) (figure 3a). We found that these resident skin bacteria exhibited a range of functional capacities against Bsal. Of the 708 isolates tested with in vitro growth assays, 30% inhibited Bsal growth, 13% enhanced Bsal growth and 57% had no effect (figure 3b). It is important to note, not all bacteria consistently exhibited the same function against Bsal. Re-testing of multiple skin bacterial isolates (n = 17) against Bsal resulted in variable functionality. This finding is potentially due to differences in the cell density of cultures when bacterial products were collected (electronic supplementary material, table S3).
Figure 3.
Phylogenetic distribution and function of cultured skin bacteria from all sampled salamanders and the effects of bacterial addition on Bsal-infection dynamics. (a) Phylogenetic distribution of skin bacterial isolates. The colours indicate bacterial phyla. (b) Distribution of functional capability of cultured skin bacterial isolates against Bsal. The numbers represent the total number of isolates with the respective function. (c) Bacterial density on salamander skin throughout the first 28 days of the experiment. The arrow indicates time of Bsal exposure. Treatments are labelled as follows: control* = daily addition of a sham treatment of 1 ml of sterile distilled water with an agar swab, control = no treatment, pseudo = daily addition of a Pseudomonas sp., and steno = daily addition of a Stenotrophomonas sp. Asterisks are used to denote time points where significant differences among treatments were detected. (d) Survival probability curves across experimental treatments. The pseudo treatment exhibited significantly greater survival compared to the control. (e) (Inset) Rate of change in Bsal infection intensity throughout the first 28 days (zoospores/day). The asterisk denotes significant differences among treatments. Error bars represent standard error of the mean (c and inset e), and shaded regions represent 95% confidence intervals (d).
Bacterial density at the start of the experiment (prior to experimental treatments) did not differ between experimental groups (n = 7 per treatment; KW-χ2 = 0.7851, p = 0.853). After bacterial addition began, bacterial density differed significantly among treatments (LMM, F = 12.67, p < 0.001) and through time (LMM, F = 49.57, p < 0.001) (figure 3c). There was also a significant interaction between time and treatment (LMM, F = 4.96, p = 0.003). To explore these main effects and the interaction we performed pairwise post hoc comparisons among treatments and time points. Daily addition of bacteria to fire salamander skin in both the inhibitory and enhancing treatment increased bacterial density in comparison to the no-treatment control (pseudo: t = −3.95, p < 0.001; steno: t = −5.23, p < 0.001), however, not in comparison to the agar-wash control (pseudo: t = 2.12, p < 0.152; steno: t = 0.69, p = 0.89). The mechanism for bacterial increase in agar-treated animals remains unclear. It is possible, for example, that the minimal agar media present in the inocula promoted bacterial growth; alternatively, the dilution of host skin factors could remove inhibition; to decipher this future work could consider including a water-only treatment control. Furthermore, this effect diminished over time; by day 21 there were no differences in bacterial density among treatments (KW χ2 = 1.95, p = 0.58). Re-isolation success of the added bacteria to confirm their presence on the skin proved highly inconsistent through time on a given individual. Nevertheless, MALDi-TOF identification of re-isolated bacteria confirmed the presence of the administered bacteria on the skin of 12 of 14 individuals on at least one time point throughout the experiment (electronic supplementary material, table S6).
The artificial addition of selected bacteria to salamander skin affected Bsal infection dynamics and survival. There was some evidence that the rate of increase in Bsal infection intensity throughout the first 28 days differed among treatments (KW χ2 = 7.24, p = 0.06; figure 3e). More specifically, when comparing the two bacterial treatments to address the hypothesis that bacteria can differentially affect infection dynamics, there was a significant difference among treatments, with the Bsal-inhibitory bacteria group exhibiting a lower rate of increase in infection intensity compared to the Bsal-enhancing bacteria group (KW χ2 = 3.92, p = 0.047). There was also evidence for a difference in survival probability among treatments (Cox log-rank test, χ2 = 7.76, p = 0.05; figure 3d); the Bsal-inhibitory bacteria group exhibited slower rates of mortality compared to the control treatment (pairwise log rank: p = 0.018, all other comparisons p > 0.1). One individual within this treatment group also survived the duration of the experiment, and was no longer positive for Bsal after 42 days.
4. Discussion
Advances in the understanding of symbiotic microbiomes are changing our perception of animal biology, including the ecology of disease in host–pathogen systems [3,5,42]. Our study investigated fire salamander skin microbial communities in the context of the emerging pathogen, Bsal, finding that microbial interactions can both elicit protection from disease and contribute to disease pathogenesis. While daily addition of Bsal-inhibiting bacteria was able to slow disease progression, the markedly low densities of cutaneous bacteria in unmanipulated settings likely limit their protective capacity. Moreover, selected bacteria that became more abundant following Bsal infection coincide with bacteria involved in septicemic events, suggesting a contributing role in disease pathogenesis.
Our study suggests that the abundance of bacteria on fire salamander skin is relatively low. There is limited knowledge in the literature on densities of skin microbiota on hosts, even for human skin; however, our estimates for salamander skin are some orders of magnitude lower than the available estimated density on human skin: 5.7 × 103 rRNA copies mm−2 versus < 1011 m−2 (approximately 108 mm−2) [43]. Interestingly, this estimate of bacterial density on human skin is derived solely from an old culture-based study (1987), which likely underestimates abundances. To date, few novel systematic studies have attempted to thoroughly address cutaneous microbial density. It is striking how scarce current data on cutaneous microbial density is, for human and amphibian systems alike, and clearly this topic warrants thorough study. It is also likely that these densities will differ among amphibian host species, further meriting comparative investigation.
Bacterial abundance on salamander skin may be low as a result of host investment into alternative defence strategies. A host's microbial community can be seen as a trait that is an extension of the host immune system [1,44]. Selective pressure on the host may lead to evolution of a mucosal environment that is particularly suitable for protective symbionts [45]. While microbial defences can be a significant component of a host's defence strategy, at times even replacing host-produced defences, investing in them can be costly for the host [7,46]. In this context, a host may only be able to invest resources in maintaining either defensive microbiota or host-based defences. The extent and nature of the epidermal mucosal layer undoubtedly differ among amphibian species, and likely shape density and potentially spatial distribution of skin microbes. Fire salamanders have a relatively minimal mucus layer on their skin in comparison to, for example, ranid frogs, which may not favour substantial microbial colonization and proliferation on the skin due to low resource availability. Alternatively, fire salamanders may maintain a strict, active control of microbial populations on their skin, investing resources in host-based defences rather than microbial-based defences, and thus limiting the development of a cutaneous environment that is conducive to microbial establishment and persistence.
Bacterial density and their spatial distribution can have strong implications on the function of these microbial residents [47,48]. In fact, microbial abundances rather than taxonomic shifts of particular taxa in the human gut microbiome have been found to be a fundamental driver of disease [49]. Amphibian skin microbiota are known to produce antifungal compounds [50] which can inhibit colonization and growth of fungal pathogens [51]. Many microbial taxa communicate via a form of cell-to-cell communication known as quorum sensing [52]. Such communication relies on signal build up from many bacterial cells and facilitates group behaviours that can lead to inhibition of colonization by other microbes [42,53]. Likewise, antibiotic activity against pathogens can be seen as a by-product of bacterial interference competition [54], and to participate in such competition bacteria most likely need to be in close proximity [48]. If microbial density is key, the low observed density on fire salamander skin could be inadequate to modulate quorum sensing or interference competition. Thus, the natural microbiota of these amphibians may be insufficient in eliciting large-scale effects on Bsal infection dynamics. Such a density-dependent effect is further supported by the artificial addition of Bsal-inhibitory bacteria to fire salamander skin instigating changes in infection dynamics. On the other hand, we do not fully know the spatial distribution of the bacterial residents; if spatially aggregated within specific skin regions, bacteria could engage in quorum sensing and/or interference competition. Even if the overall density is low, it may still be possible for bacteria to quickly proliferate under certain conditions, becoming locally abundant on particular skin regions and able to exert an important defensive effect.
Pathogen invasion is known to alter host microbial communities [55,56]. Cutaneous infection by the related amphibian fungus, Bd, has been documented to alter the diversity and composition of skin-associated microbiota on anuran hosts [57,58]. Experimental Bsal infection of fire salamanders counters this and did not alter total bacterial density, alpha diversity or community structure (i.e. beta diversity) of the skin bacterial communities as a whole. Despite the lack of overall effects on beta diversity, selected bacterial taxa were found to exhibit differential relative abundance between Bsal-exposed and control salamanders. In fact, a Chryseobacterium sp. found to be differentially abundant on the skin of Bsal-exposed individuals matched cultured liver isolates associated with observed septicemic events in Bsal-infected salamanders.
Septicemia may be a mechanism of mortality in Bsal-induced chytridiomycosis. Deep ulcerations induced by Bsal infection [18] result in breaching of the skin barrier, which likely allows bacteria to invade internal organs. The observed septicemic events may be a result of typically commensal skin bacteria becoming opportunistic pathogens and invading internal organs. Most of the bacteria isolated from the liver are not commonly known pathogens. Many were common residents of environmental substrates as well as in host skin and gut microbiomes; however, several of them have previously been documented as opportunistic pathogens, associated with cases of infection and bacteremia in fish, plants and humans (e.g. [59–62]; electronic supplementary material, table S2). Specifically, Acinetobacter, the most common bacterium isolated from the liver, has been associated with bloodstream infections (e.g. [42]), and also with skin lesions of another amphibian, the Hellbender [63].
Host-associated microbial communities are also known to affect disease dynamics [4,64–66]. In vitro, we found that cultured resident bacteria displayed a range of functional capacities against Bsal, including inhibition and enhancement. Such interactions between invading pathogens and host symbionts have been documented across multiple amphibian systems as well as other host systems [26,64,67–70]. In the conducted bacterial addition experiment we were able to, in part, replicate the in vitro function of these bacteria in vivo. We found that Bsal-enhancing bacteria had no effect on salamander survival. In general, Bsal-induced chytridiomycosis manifests itself very quickly on fire salamanders [19]. The average time until [death] in controls was 29.5 days, which is only 3–4 generations of Bsal; thus, in this hyper-susceptible host it is likely to be rather difficult to hasten the onset of disease, even if Bsal-enhancing bacteria are present in high abundance. This fact may explain the observed findings for the Bsal-enhancing treatment. On the other hand, the Bsal-inhibitory bacteria resulted in a slower build-up of Bsal infection intensity in comparison to the Bsal-enhancing treatment and a marginal increase in survival compared to the controls. These findings demonstrate that bacteria can differentially affect infection at least under certain conditions, which has also been observed in selected hosts infected with Bd (e.g. [47]). The inhibitory bacterium used was a Pseudomonas sp.; pseudomonads are known in multiple systems to be antifungal and have disease-suppressing properties [71–73]. Importantly, within the conducted experiment investigating in vivo function, skin bacterial densities on salamanders in bacterial addition groups were greater than those found to occur naturally on salamanders in this study; thus, density may be key for bacteria to elicit a protective function [47,49]. It is also worth highlighting that there was one individual within the Bsal-inhibitory bacteria group that survived the duration of the experiment (77 days), which has never been observed in untreated fire salamanders so far, neither in laboratory trials, nor in the wild.
The conducted experiment demonstrates that bacteria can shift infection dynamics within certain frameworks. The potential of bacteria to affect Bsal infection dynamics leaves the door to exploring probiotics open. In the sole survivor, Bsal infection was no longer present after 42 days, suggesting that clearance had occurred; such a prolonged course of infection, however, also involves prolonged pathogen shedding, which has been shown to be highly unfavourable to overall outcomes of disease outbreaks [74]. Any future work to develop probiotics, single species or probiotic mixtures, should focus on bacteria (or consortia) that prevent pathogen colonization or result in more rapid pathogen clearance. Such probiotics could cull disease progression and minimize transmission. The feasibility of achieving this on a large scale and thereby shifting disease dynamics at biologically meaningful levels, however, will certainly be a challenge.
Are skin bacteria on fire salamanders friends, foes or bystanders? Our data suggest that bacteria living on fire salamander skin may simply be bystanders, unable to provide sufficient protection against Bsal, perhaps due to low bacterial numbers combined with Bsal's ability to disseminate inside salamander skin, thus averting bacterial defences. Furthermore, disease-induced septicemia by potentially opportunistic pathogens within the skin microbiota presents selected bacteria as a clear foe to fire salamanders. However, dampening of Bsal infection by the addition of Bsal inhibitory bacteria suggests that skin bacteria can be a friend to the salamander under certain conditions. The multifaceted nature of host microbiota highlights the complex relationship of hosts, their microbiomes and disease, and underscores the importance for continued research in this field.
Supplementary Material
Acknowledgements
We thank Lutz Dalbeck, Andreas Nöllert and Dagmar Ohlhoff for help with field sampling of fire salamanders, and Stefan Lötters, Norman Wagner and Michael Veith for logistic support. We thank Meike Kondermann for assistance with laboratory work for 16S amplicon sequencing preparations, and the Genome Center at the Helmholtz Center for Infection Research for performing sequencing. We also thank Marjolein Couvreur for assistance with electron microscopy analyses.
Ethics
All work with animals has been approved by the ethical committee of the Faculty of Veterinary Medicine, Ghent University (EC2015/ 83 and EC 2016/73).
Data accessibility
All OTU tables, infection data andmetadata are available in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.m40t6g7 [74]. Illumina sequence data are available on SRA under Bioproject PRJNA477390 (SRA accession SRP151078). Bacterial isolate sequences have been archived on GenBank (MH512108-MH512801 & MH523105-MH523149).
Authors' contributions
M.C.B., M.V., S.S., F.P. and A.M. designed research; M.C.B., A.M., M.K., J.S.P., E.B., S.V.P., F.B. and W.B. performed research; M.C.B., A.M. and M.K. analysed data. All authors contributed to writing and revising the paper.
Competing interests
The authors declare having no competing financial interests.
Funding
We acknowledge funding received from Deutsche Bundesstiftung Umwelt, Deutsche Forschungsgemeinschaft (grant VE247/9-1) and Bundesamt für Naturschutz in Germany, FWO Vlaanderen (grant G0H2516N), and the European Commission Tender ENV.B.3/SER/2016/0028 that allowed completion of this work.
References
- 1.McFall-Ngai M, Hadefield M, Bosch TCG. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236. ( 10.1073/pnas.1218525110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapira M. 2016. Gut microbiotas and host evolution: scaling up symbiosis. Trends Ecol. Evol. 31, 539–549. ( 10.1016/j.tree.2016.03.006) [DOI] [PubMed] [Google Scholar]
- 3.Selber-Hnativ S, et al. 2017. Human gut microbiota: toward an ecology of disease. Front. Microbiol. 8, 1265 ( 10.3389/fmicb.2017.01265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stecher B, Hardt WD. 2008. The role of microbiota in infectious disease. Trends Microbiol. 16, 107–114. ( 10.1016/j.tim.2007.12.008) [DOI] [PubMed] [Google Scholar]
- 5.Sanford JA, Gallo RL. 2013. Functions of the skin microbiota in health and disease. Semin. Immunol. 25, 370–377. ( 10.1016/j.smim.2013.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belden LK, Harris RN. 2007. Infectious diseases in wildlife: the community ecology context. Front. Ecol. Environ. 5, 533–539. ( 10.1890/060122) [DOI] [Google Scholar]
- 7.King KC, Bonsall MB. 2017. The evolutionary and coevolutionary consequences of defensive microbes for host-parasite interactions. BMC Evol. Biol. 17, 190 ( 10.1186/s12862-017-1030-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. ( 10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KF, Sax DF, Lafferty KD. 2006. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 20, 1349–1357. ( 10.1111/j.1523-1739.2006.00524.x) [DOI] [PubMed] [Google Scholar]
- 10.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544. ( 10.1016/j.tree.2004.07.021) [DOI] [PubMed] [Google Scholar]
- 11.Pennisi E. 2010. Armed and dangerous. Science 327, 804–805. ( 10.1126/science.327.5967.804) [DOI] [PubMed] [Google Scholar]
- 12.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667. ( 10.1073/pnas.1014743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gargas A, Trest M, Christensen M, Volk TJ, Blehert DS. 2009. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon 108, 147–154. ( 10.5248/108.147) [DOI] [Google Scholar]
- 14.Longcore JE, Pessier AP, Nicholes DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nova chytrid pathogenic to amphibians. Mycologia 91, 219–227. ( 10.2307/3761366) [DOI] [Google Scholar]
- 15.Fisher MC, Garner TW, Walker SF. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 63, 291–310. ( 10.1146/annurev.micro.091208.073435) [DOI] [PubMed] [Google Scholar]
- 16.Wake DB, Vredenburg VT. 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA 105, 11 466–11 473. ( 10.1073/pnas.0801921105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330, 1503–1509. ( 10.1126/science.1194442) [DOI] [PubMed] [Google Scholar]
- 18.Martel A, et al. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl Acad. Sci. USA 110, 15 325–15 329. ( 10.1073/pnas.1307356110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martel A, et al. 2014. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 6209, 630–631. ( 10.1126/science.1258268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stegen G, et al. 2017. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544, 353–356. ( 10.1038/nature22059) [DOI] [PubMed] [Google Scholar]
- 21.Farrer RA, et al. 2017. Genomic innovations linked to infection strategies across emerging pathogenic chytrid fungi. Nat. Commun. 8, 14742 ( 10.1038/ncomms14742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muletz-Wolz CR, Almario JG, Barnett SE, DiRenzo GV, Martel A, Pasmans F, Zamudio KR, Toledo LF, Lips KR. 2017. Inhibition of fungal pathogens across genotypes and temperatures by amphibian skin bacteria. Front. Microbiol. 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KPC, Harris RN. 2013. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol. Lett. 16, 807–820. ( 10.1111/ele.12099) [DOI] [PubMed] [Google Scholar]
- 24.Spitzen-van der Sluijs A, et al. 2016. Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis. 22, 1286 ( 10.3201/eid2207.160109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bletz MC, Archer H, Harris RN, McKenzie VJ, Rabemananjara FCE, Rakotoarison A, Vences M. 2017. Host ecology rather than host phylogeny drives amphibian skin microbial community structure in the biodiversity hotspot of Madagascar. Front. Microbiol. 8, 1530 ( 10.3389/fmicb.2017.01530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bletz MC, Myers J, Woodhams DC, Rabemananjara FCE, Rakotonirina A, Weldon C, Edmonds D, Vences M, Harris RN. 2017. Estimating herd immunity to amphibian chytridiomycosis in Madagascar based on the defensive function of amphibian skin bacteria. Front. Microbiol. 8, 1751 ( 10.3389/fmicb.2017.01751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell SC, Alford RA, Garland S, Padilla G, Thomas AD. 2013. Screening bacterial metabolites for inhibitory effects against Batrachochytrium dendrobatidis using a spectrophotometric assay. Dis. Aquat. Organ. 103, 77–85. ( 10.3354/dao02560) [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. ( 10.1128/AEM.00062-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2 - Approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 ( 10.1371/journal.pone.0009490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DH, Zo YG, Kim SJ. 1996. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl. Environ. Microbiol. 62, 3112–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blooi M, Pasmans F, Longcore JE, Spitzen-Van Der Sluijs A, Vercammen F, Martel A. 2013. Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol. 51, 4173–4177. ( 10.1128/JCM.02313-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the Miseq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. ( 10.1128/AEM.01043-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. ( 10.1038/nmeth.f.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amir A, et al. 2017. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2, e00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singhal N, Kumar M, Kanaujia PK, Virdi JS. 2015. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 6, 1–16. ( 10.3389/fmicb.2015.00791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing. [Google Scholar]
- 37.Bates DM, Machler M, Bolker BM, Walker SC. 2014. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. (doi:10.18637/jss.v067.i01). [Google Scholar]
- 38.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB. 2017. vegan: Community Ecology Package. R package verion 2.4-2.
- 39.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 ( 10.1186/gb-2011-12-6-r60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Therneau TM, Grambsch PM. 2000. Modeling survival data: extending the cox model. New York: NY: Springer. [Google Scholar]
- 41.Wickham H. 2009. Ggplot2: elegant graphics for data analysis. New York: NY: Springer. [Google Scholar]
- 42.Cogen AL, Nizet V, Gallo RL. 2008. Skin microbiota: a source of disease or defence? Br. J. Dermatol. 158, 442–455. ( 10.1111/j.1365-2133.2008.08437.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sender R, Fuchs S, Milo R. 2016. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, 1–14. ( 10.1371/journal.pbio.1002533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abt M, David A. 2013. The dynamic influence of commensal bacteria on the immune response to pathogens. Curr. Opin. Microbiol. 16, 4–9. ( 10.1016/j.mib.2012.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke C, Steinberg P, Rusch DB, Kjelleberg S, Thomas T. 2011. Bacterial community assembly based on functional genes rather than species. Proc. Natl Acad. Sci. USA 108, 14 288–14 293. ( 10.1073/pnas.1101591108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kueneman JG, Woodhams DC, Van Treuren W, Archer HM, Knight R, McKenzie VJ. 2015. Inhibitory bacteria reduce fungi on early life stages of endangered Colorado boreal toads (Anaxyrus boreas). ISME J. 10, 934–944. ( 10.1038/ismej.2015.168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasumiba K, Bell S, Alford R. 2016. Cell density effects of frog skin bacteria on their capacity to inhibit growth of the chytrid fungus, Batrachochytrium dendrobatidis. Microb. Ecol. 71, 124–130. ( 10.1007/s00248-015-0701-9) [DOI] [PubMed] [Google Scholar]
- 48.Raynaud X, Nunan N. 2014. Spatial ecology of bacteria at the microscale in soil. PLoS ONE 9, e87217 ( 10.1371/journal.pone.0087217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandeputte D, et al. 2017. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551, 507. [DOI] [PubMed] [Google Scholar]
- 50.Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KPC. 2008. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J. Chem. Ecol. 34, 39–43. ( 10.1007/s10886-007-9352-8) [DOI] [PubMed] [Google Scholar]
- 51.Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KPC. 2009. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl. Environ. Microbiol. 75, 6635–6638. ( 10.1128/AEM.01294-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nunes-Alves C. 2015. Microbiome: taking advantage of quorum sensing. Nat. Rev. Micro 13, 252 ( 10.1038/nrmicro3477) [DOI] [PubMed] [Google Scholar]
- 53.Thompson JA, Oliveira RA, Xavier KB. 2016. Chemical conversations in the gut microbiota. Gut Microbes 7, 163–170. ( 10.1080/19490976.2016.1145374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheuring I, Yu DW. 2012. How to assemble a beneficial microbiome in three easy steps. Ecol. Lett. 15, 1300–1307. ( 10.1111/j.1461-0248.2012.01853.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hajishengallis G, et al. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506. ( 10.1016/j.chom.2011.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koskella B, Meaden S, Crowther WJ, Leimu R, Metcalf CJE. 2017. A signature of tree health? Shifts in the microbiome and the ecological drivers of horse chestnut bleeding canker disease. New Phytol. 215, 737–746. ( 10.1111/nph.14560) [DOI] [PubMed] [Google Scholar]
- 57.Jani AJ, Briggs CJ. 2014. The pathogen Batrachochytrium dendrobatidis disturbs the frog skin microbiome during a natural epidemic and experimental infection. Proc. Natl. Acad. Sci. USA 111, E5049–E5058. ( 10.1073/pnas.1412752111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker CG, Longo AV, Haddad CFB, Zamudio KR. 2017. Land cover and forest connectivity alter the interactions among host, pathogen and skin microbiome. Proc. R. Soc. B 284, 20170582 ( 10.1098/rspb.2017.0582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loch TP, Faisal M. 2015. Emerging flavobacterial infections in fish: a review. J. Adv. Res. 6, 283–300. ( 10.1016/j.jare.2014.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tronel H, Plesiat P, Ageron E, Grimont PAD. 2003. Bacteremia caused by a novel species of Sphingobacterium. Clin. Microbiol. Infect. 9, 1242–1244. ( 10.1111/j.1469-0691.2003.00801.x) [DOI] [PubMed] [Google Scholar]
- 61.Abro AH, Reza M, Shahmirzadi R, Jasim LM, Al Deesi Z. 2016. Sphingobacterium multivorum bacteremia and acute meningitis in an immunocompetent adult patient: a case report. Iran. Red Crescent Med. J. 18, 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seifert H, Strate A, Schulze A, Pulverer G. 1993. Vascular catheter-related bloodstream infection due to Acinetobacter johnsonii (Formerly Acinetobacter calcoaceticus var. lwoffii): Report of 13 Cases. Clin. Infect. Dis. 17, 632–636. [DOI] [PubMed] [Google Scholar]
- 63.Hernández-Gómez O, Kimble SJA, Briggler JT, Williams RN. 2016. Characterization of the cutaneous bacterial communities of two giant salamander subspecies. Microb. Ecol. 73, 445–454. ( 10.1007/s00248-016-0859-9) [DOI] [PubMed] [Google Scholar]
- 64.Fraune S, Anton-Erxleben F, Augustin R, Franzenburg S, Knop M, Schröder K, Willoweit-Ohl D, Bosch TC. 2014. Bacteria-bacteria interactions within the microbiota of the ancestral metazoan Hydra contribute to fungal resistance. ISME J. 9, 1543–1556. ( 10.1038/ismej.2014.239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker MH, et al. 2015. Composition of symbiotic bacteria predicts survival in Panamanian golden frogs infected with a lethal fungus. Proc. R. Soc. B 282, 20142881 ( 10.1098/rspb.2014.2881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. 2007. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 5, 355–362. ( 10.1038/nrmicro1635) [DOI] [PubMed] [Google Scholar]
- 67.Krediet CJ, Ritchie KB, Alagely A, Teplitski M. 2013. Members of native coral microbiota inhibit glycosidases and thwart colonization of coral mucus by an opportunistic pathogen. ISME J. 7, 980–990. ( 10.1038/ismej.2012.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau CSM, Chamberlain RS. 2016. Probiotics are effective at preventing Clostridium difficile -associated diarrhea: a systematic review and meta-analysis. Int. J. Gen. Med. 9, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Becker MH, et al. 2015. Phylogenetic distribution of symbiotic bacteria from Panamanian amphibians that inhibit growth of the lethal fungal pathogen Batrachochytrium dendrobatidis. Mol. Ecol. 24, 1628–1641. ( 10.1111/mec.13135) [DOI] [PubMed] [Google Scholar]
- 70.Woodhams DC, et al. 2015. Antifungual isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology 96, 595 ( 10.1890/14-1837.1) [DOI] [Google Scholar]
- 71.Haas D, Défago G. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3, 307–319. ( 10.1038/nrmicro1129) [DOI] [PubMed] [Google Scholar]
- 72.Kim JD, Kim B, Lee CG. 2007. Alga-lytic activity of Pseudomonas fluorescens against the red tide causing marine alga Heterosigma akashiwo (Raphidophyceae). Biol. Control 41, 296–303. ( 10.1016/j.biocontrol.2007.02.010) [DOI] [Google Scholar]
- 73.Hoyt JR, Cheng TL, Langwig KE, Hee MM, Frick WF. 2015. Bacteria isolated from bats inhibit the growth of Pseudogymnoascus destructans, the causative agent of white-nose syndrome. PLoS ONE 10, e0121329 ( 10.1371/journal.pone.0121329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canessa S, et al. 2018. Decision making for mitigating wildlife diseases: from theory to practice for an emerging fungal pathogen of amphibians. J. Appl. Ecol. 55, 1987–1996. ( 10.1111/1365-2664.13089) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All OTU tables, infection data andmetadata are available in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.m40t6g7 [74]. Illumina sequence data are available on SRA under Bioproject PRJNA477390 (SRA accession SRP151078). Bacterial isolate sequences have been archived on GenBank (MH512108-MH512801 & MH523105-MH523149).