Abstract
Study Design:
Narrative literature review.
Objectives:
Placental tissue, amniotic/chorionic membrane, and umbilical cord have seen a recent expansion in their clinical application in various fields of surgery. It is important for practicing surgeons to know the underlying science, especially as it relates to spine surgery, to understand the rationale and clinical indication, if any, for their usage.
Methods:
A literature search was performed using PubMed and MEDLINE databases to identify studies reporting the application of placental tissues as it relates to the practicing spine surgeon. Four areas of interest were identified and a comprehensive review was performed of available literature.
Results:
Clinical application of placental tissue holds promise with regard to treatment of intervertebral disc pathology, preventing epidural fibrosis, spinal dysraphism closure, and spinal cord injury; however, there is an overall paucity of high-quality evidence. As such, evidence-based guidelines for its clinical application are currently unavailable.
Conclusions:
There is no high-level clinical evidence to support the application of placental tissue for spinal surgery, although it does hold promise for several areas of interest for the practicing spine surgeon. High-quality research is needed to define the clinical effectiveness and indications of placental tissue as it relates to spine surgery.
Keywords: amniotic membrane, chorionic membrane, umbilical cord, spinal cord injury, intervertebral disc, epidural fibrosis, spinal dysraphism
Introduction
The use of mesenchymal stem cells (MSCs) in orthopedic and spinal surgery has become a popular topic in recent years with the introduction of numerous different products of various cellular origins, ranging from bone marrow, adipose tissue, synovial tissue, to placental tissue.1–5 Placental tissue has gained particular popularity as a tissue source identified as a rich source of MSCs as well as other growth factors beneficial to tissue repair and regeneration.6 Several commercially available products have been introduced recently as a clinical alternative for spinal injuries and pathologic conditions, ranging from intervertebral disc degeneration to traumatic spinal cord injuries. However, the evidence supporting the clinical application of these products often lags behind marketing strategies. The goal of this article is to review the science and evidence relating to the clinical application of placental tissue in treating various spinal pathology.
Placental Tissue
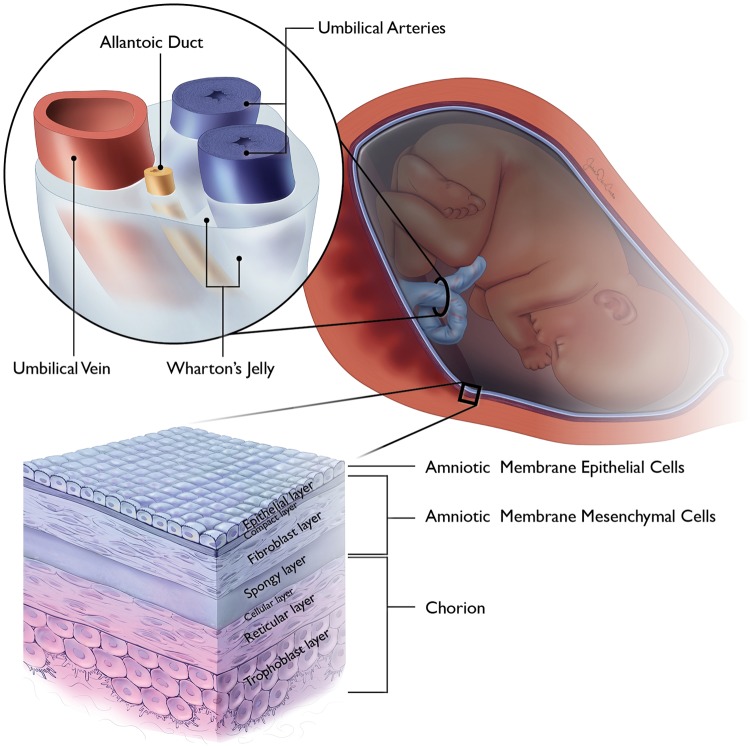
The membranes surrounding the developing fetus are a complex structure of various tissue subtypes that play a critical role in fetal development and sustenance. The membranes consist of 4 distinctly different structures: the amnion, chorion, umbilical cord, and placenta (Figure 1). Of these, the fetal-derived tissues, the amnion, chorion, and umbilical cord, have been investigated as potential source of MSCs for clinical application.
Figure 1.
Illustrative image depicting the placenta membranes and the cellular organization of the amnion, chorion, and umbilical cord.
Amnion
The amnion is a thin tissue, derived from the trophoblast that completely surrounds the developing fetus and facilitates fetal nutrition via diffusion.7,8 The amnion contains 3 distinct histologic layers: the epithelial layer, the basement membrane, and the mesenchymal layer (Figure 1).
The epithelial layer is the innermost layer of the amnion, consisting of a single layer of cuboidal amniotic epithelial cells (AECs). AECs are derived from the epiblast, developing on the eighth gestational day9 and have been shown to produce basic fibroblast growth factor, epidermal growth factor, keratinocyte growth factor, and hepatocyte growth factor.8,10 Through the production of these factors, AECs have been shown to promote epithelization,10 inhibit angiogenesis,11 inhibit fibroblast activation through the inhibition of transforming growth factor-β, promote neural differentiation,12–14 provide immunosuppression via inhibition of both innate and adaptive immune systems,15 promote tenocyte proliferation,16 as well as possess the capability for trilineage cell differentiation, or differentiation into all 3 germline lineages.17
The mesenchymal layer is a vascular layer that abuts the chorion and contains 3 distinct sublayers: the compact, fibroblast, and spongy layers (Figure 1).8,18 The fibroblast layer provides for the structural integrity via type I and III collagen, arranged in parallel bundles,18 and contains the embryonic mesoderm derived mesenchymal stromal cells(AMSCs).8 AMSCs have shown capabilities for trilineage cell differentiation.17 In addition to a higher proliferative rate than adult-derived MSC sources,19 AMSCs have been found to promote angiogenesis through the expression of proangiogenic factors8 and suppress the innate and adaptive immune systems.15 The spongy layer is abundant in proteoglycans and glycoprotenins, particularly hyaluronic acid, which is thought to serve as a primary inhibitor of transforming growth factor-β, resulting in cessation of scar formation.20 Additionally, the spongy layer possesses a loose, nonfibrillar collagen network connecting it to the adjacent chorion,18 which is exploited for tissue separation for various commercial graft products that are isolated amnion.
Combined, AECs and AMSCs have shown a particular capacity for osteogenic and chondrogenic cellular differentiation.21 Although numerous studies have investigated this osseous and chondral differentiation, the AEC and AMSC cells largely require induction using various mediums or growth factors.22–26
Chorion
The chorion is an extra-embryonic entity with a bilayered structure that represents the outermost layer of the placental tissue. Its villous trophoblast area serves as the primary location of nutrient exchange between the mother and developing fetus.6 As such, it has also been shown to have cross-contamination with maternal cells, present in approximately 30% of chorionic MSCs.27,28 Chorionic membrane cells have been identified as pluripotent with the capacity for trilineage germ line differentiation,29,30 but with a decreased capacity compared with other MSC cell sources.31 In addition, chorionic membrane cells demonstrate immunosuppressive effects as well as promote angiogenesis.27
Umbilical Cord
The umbilical cord is a unique structure of extra-embryonic origin that connects the fetal and maternal circulatory systems for nutrition and waste exchange.6 The umbilical cord is composed of the umbilical epithelial exterior, containing connective tissue, termed Wharton’s jelly, and blood vessels. MSCs have been isolated from both the epithelium and Wharton’s jelly32–35; however, Wharton’s jelly has been found to have the highest concentration of MSC, not just of the umbilical cord, but of all MSC sources.36 Umbilical cord–derived MSCs (UC-MSCs) have shown potential for multilineage cell differentiation, with a particular capacity for neural differentiation37–39 and immunosuppression.1,40,41
In addition to the umbilical cord tissue, the blood contained within the umbilical vessels has been identified as a source of MSCs, although present in significantly smaller quantity than other sources.36,42 The predominant stem cell population is CD34 positive hematopoietic stem cells, but this source has also been shown to express characteristics in common with bone marrow MSCs.43 In addition, umbilical cord blood has also shown potent production of motor-neuron-related markers, making it a prime target for motor nerve regeneration research.44
Methods
A literature search was conducted to retrieve previous publications regarding the use of placental tissue in various avenues relating to spine surgery. A PubMed search was performed using the terms “Amnion,” “Chorion,” “Amniotic Membrane,” “Umbilical,” and “Umbilical Cord” and “Spinal” or “Spine.” Using this broad search strategy, 4 main categories of clinical application were identified, consisting of intervertebral disc pathology, epidural fibrosis, spinal cord injury, and closure of spinal dysraphism. These 3 categories were individually inserted in a search strategy using PubMed and MEDLINE search engines between 1960 and 2017. Inclusionary criteria consisted of English language articles and investigating the application of human placental tissue for the 4 identified clinical applications. Review articles and non-English articles were excluded. Terminology for search parameters consisted of (1) “Amnion or Amniotic Membrane or Chorion” and (2) “Umbilical or Umbilical Cord,” with each of the 4 clinical applications. Search results are summarized in Table 1. The results and analysis of systematic review, broken down according to subgroup headings, are summarized in Appendix 1 (available in the online version of the article).
Table 1.
Summary of Basic Science Research as It Related to the Various Placenta Tissues and Their Cellular Capabilities.
| Amniotic Membrane Epithelial Cells | Amniotic Membrane Mesenchymal Cells | Chorion Cells | Umbilical Cord Tissue | Umbilical Cord Blood |
|---|---|---|---|---|
| Pro-epithelial, neural, and tenocyte | Trilineage cell differentiation | Trilineage cell differentiation | Trilineage cell differentiation | Hematopoietic proliferation |
| Anti-angiogenic | Angiogenic immunosuppressive | Angiogenic immunosuppressive | Immunosuppressive | Pro-neural proliferation |
| Anti-fibroblastic | Pro-neural proliferation | Immunosuppressive | Pro-neural proliferation | |
| Immunosuppressive | Immunosuppressive |
Epidural Fibrosis
Epidural fibrosis is considered as a cause of continued back pain following decompressive surgery, as well as a factor complicating revision spine surgery.45 Three articles were identified, investigating the application of amniotic membrane (AM) with and without the adjacent chorion on the development of epidural fibrosis (Table 2). Choi et al46 investigated the application of irradiated human AM grafts applied to a rat laminectomy model, compared to an untreated laminectomy cohort. Gross observation of surgical sites performed at 1, 3, and 8 weeks following surgery found a decreased overall amount of scarring in the AM cohort with less inflammatory cell infiltration and fibroblast proliferation on histologic examination. Similar findings were reported by Tao and Fan45 using an adult mongrel dog lumbar laminectomy model, comparing freeze-dried AM, cross-linked AM, autologous free fat, and untreated control. Gross observation and histology examination found decreased scar formation in the AM cohort cross-linked with glutaraldehyde. The freeze-dried AM membrane, interestingly, demonstrated no difference in comparison to the control samples on gross analysis with complete degradation of the graft demonstrated on histologic examination at 12 weeks following implantation. Quantitative analysis of fibroblasts at the surgical site found that the autologous free fat cohort has significantly less fibroblasts than the remaining cohorts, significantly lower than both AM groups.
Table 2.
Summary of Literature Review for Spine-Related Articles Investigating the Application of Placenta Tissue.
| Amnion/Chorion | Umbilical Cord | ||
|---|---|---|---|
| Intervertebral disc | Identified | 2 | 12 |
| Included | 2 | 7 | |
| Epidural fibrosis | Identified | 4 | 0 |
| Included | 3 | 0 | |
| Spinal cord injury | Identified | 60 | 549 |
| Included | 11 | 54 | |
| Spinal dysraphism | Identified | 0 | 3 |
| Included | 0 | 3 |
Subach and Copay47 were the only authors to report on the clinical application of a dehydrated combined amnion/chorion graft. They reviewed a case series of 5 patients undergoing revision lumbar surgery after implantation of a combined amnion/chorion graft performed during primary transforaminal lumbar interbody fusion. At the time of revision surgery, 80% (4/5) of the patients had easily detachable tissue adjacent to the dura, with the authors concluding that combined amnion/chorion grafts have favorable effects on epidural fibrosis.
Intervertebral Disc Pathology
Low back pain is a pervasive condition, affecting more than 70% of the adult population at least once during their lifetime.48 Disc degeneration is one progressive etiology that has gained considerable attention in therapeutic attempts to facilitate regeneration.49 Literature review identified one article investigating the in vitro application of placental tissue and 7 articles investigating umbilical cord blood and tissue for intervertebral (IV) disc repair (Table 2). Ni et al50 found that placental-derived MSCs could be facilitated toward nucleus pulposus-like cell differentiation with increased proliferation when cultured under hypoxic conditions, suggesting it as a possible agent for IV disc repair. Similar cell culture studies were identified for umbilical cord blood and tissue, indicated inducible proliferation of nucleus pulposus-like cells,51,52 with production of proteoglycan-rich extracellular matrix (ECM) in a chondrocyte-like phenotype.53 Beeravolu et al54 reported on the in vivo application of umbilical cord MSCs and chondroprogenitor cells (CPGs) derived from the umbilical cord MSCs to damaged IV discs in a rabbit model. The CPGs-treated animals demonstrated significant improvement in histologic appearance, as well as ECM protein and glycosaminoglycan (GAG) content production while having significantly higher expression of nucleus-pulposus specific markers.
Tam et al55 compared the intravenous and intradiscal delivery of multipotent stem cells derived from umbilical cord blood in a damaged IV disc mouse model. Analysis performed 14 weeks after treatment found limited homing ability of the implanted cells without engraftment or expansion of the cells. Direct injection was found to better preserve disc height with a slight decrease in histologic degeneration.
Leckie et al56 performed a blinded, randomized, placebo-controlled in vivo rabbit study using umbilical cord tissue–derived cells in a degenerative disc model. Animal were subdivided into 4 groups, un-punctured control, punctured without treatment, and punctured with umbilical cord tissue injection with or without a hydrogel carrier. Serial magnetic resonance imaging (MRI) was performed out to 12 weeks following treatment with histologic examination performed at 12 weeks. There was no difference in the MRI analyses between the treatment groups with qualitative analysis showing less degeneration overall when compared with the untreated punctured group. Histologically, treated IV discs showed some improvement in cellularity and disc architecture in comparison to the untreated punctured cohort but were distinctly different from the un-punctured control cohort.
Investigating the application of these grafts when the disc degeneration is beyond potential for repair, Goldschlager et al57 applied AECs to an ovine model of cervical discectomy and fusion with an interbody cage. They found that when AECs were combined with a hydroxyapatite-tricalcium phosphate graft, there was a significant negative effect on the fusion rate, with a 0% fusion rate at 3 months following surgery.
Two clinical studies were identified that investigated the application of placental tissue for discogenic pathology (Table 2). Pang et al49 reported the outcomes of 2 patients with chronic discogenic low back pain who underwent treatment with transplantation of UC-MSCs with 2-year follow-up. Visual analog pain scale measurements were found to significant decrease at 2-year follow-up with an improvement in function according to assessment with the Oswestry Disability Index (ODI). Anderson et al58 performed a prospective, randomized controlled trial in 80 subjects undergoing lumbar microdiscectomy with or without a cryopreserved amniotic tissue graft applied to the annular defect. Patients were monitored for 24 months using functional outcome measures and visual analog pain scales, as well as for the incidence of reherniation. They found that there was significant greater improvement in mean ODI scores for patients in the cryopreserved amniotic tissue cohort as well as improved Short Form-12 physical component scores. Overall, there were no reherniations in the amniotic tissue cohort, compared with 3 in the control group and 2 control patients requiring subsequent fusion for persistent symptoms.
A review of ongoing clinical trials (www.clinicaltrials.gov, accessed August 31, 2017) reviews that there is one current trial investigating the 5-year postoperative outcomes of patients undergoing discectomy with application of cryopreserved amniotic membrane and umbilical cord grafts. Additionally, there are 4 trials investigating the use of an amniotic membrane–derived allograft combined with various bone graft products for application in lumbar and cervical spine fusion procedures.
Spinal Dysraphism Closure
Spinal dysraphism closure, performed in utero, has proven to significantly decrease morbidity and mortality in comparison to postnatal repair.59 Grafts are occasionally required to facilitate dural closure with several graft subtypes described in the literature.60–62 Papanna et al60 reported on the application of cryopreserved umbilical cord patch during in utero spina bifida repair in a sheep model. In comparison to a biocellulose film adhesive, umbilical cord patch was found to be significantly superior for facilitating closure and subsequent healing, with a larger spinal cord area and greater number of preserved anterior horn cells. In a subsequent study, Papanna et al63 reported on the neurological outcomes in a separate cohort of umbilical cord patched sheep and compared with normal control and untreated spina bifida cohorts. The cryopreserved umbilical cord patch cohort was found to have improved neurologic outcomes with improved Texas Spinal Cord Injury Scale scores and improved bladder control.
A single clinical study was reported on the use of cryopreserved human umbilical cord for in utero myeloschisis repair in 2 fetuses in which primary closure was unattainable.64 Both pregnancies were uncomplicated following midgestation repair, with births occurring at 37 weeks by planned cesarean delivery. Both repair sites were intact without cerebrospinal fluid leakage, and normal function of the lower extremities.
Spinal Cord Injury
The role of regenerative interventions has expanded in recent years with regard to treatment of patients with spinal cord injuries (SCI). Placental tissues have been extensively investigated with regard to their role in facilitating neurologic recovery given their neurotrophic capabilities.12–14,37–39,44 AECs and umbilical cord tissue and cord blood have been particular targets in this line of research. In vivo animal models have shown that AECs can promote neural cell differentiation,65 reduce secondary neural damage associated with inflammation and apoptosis associated with SCI,66 modulate spinal cord microglia activity to suppress mechanical allodynia,67 promote remyelination of nerve fibers and promote sprouting of nerve fibers,68 and improve functional recovery.68–70
Umbilical cord tissue has similarly shown a capacity to facilitate axonal regeneration,71 increase the number of surviving neurons,72,73 minimize allodynia and hyperalgesia,74 alter the local SCI microenvironment to minimize IL-1 expression,75 and improve functional recovery.38,71,73–77 These physiologic effects can additionally be accentuated with the co-administration of various growth factors, to include brain-derived neutrophic factor,71 glial cell-line neutrophic factor,72 and neurotrophin-3.78
Umbilical cord blood is an additional source of mesenchymal and multipotent stem cells that has shown superiority in animal models for SCI. Ryu et al73 compared the effect of bone marrow–derived, adipose-derived, umbilical cord tissue, and umbilical cord blood MSCs on neural regeneration in a canine SCI model. All MSCs groups were found to have significant improvement in locomotion, with an increase in the number of surviving neurons and neurofilament-positive fibers. Although there was no difference in functional outcome, umbilical cord blood–derived MSCs induced significant more nerve regeneration, and anti-inflammatory activity with reduced IL-6 and COX-2 levels. In contrast to UC-MSCs, umbilical cord blood has shown improved release of neutrotrophic growth factors,79–86 downregulation of caspase-3 extrinsic pathway, Fas, and other apoptotic genes to produce an anti-apoptotic milieu at the injury site,87,88 increase residual white matter,89 upregulate matrix metalloprotease-2 to reduce glial scar formation,86,90 and facilitate oligodendricyte and neural cell differentiation.91
Clinical studies have attempted to extrapolate the preclinical data. Liu et al92 investigated the effect of a single or repeated intrathecal injection of UC-MSC in 22 patients with mixed cord–level lesions treated at an average of 56 months following injury (range 2-204 months). No patient with a complete cord injury demonstrated any treatment response, while 81% of patients with an incomplete injury demonstrated a treatment effect, defined as any improvement in American Spinal Injury Association (ASIA) sensory or motor score. No patient reported an adverse effect following treatment.
Cheng et al93 investigated the use of UC-MSCs in thoracolumbar SCI patients, using a cohort of 34 patients subdivided into a control group of rehabilitation only and a UC-MSC transplantation group, receiving 2 treatment cycles. Patients in the UC-MSC group showed improved urodynamic parameters with 70% of patients reporting a neurologic improvement. Zhao et al94 combined UC-MSC with a collagen scaffold that was surgically implanted in 8 patients with chronic SCI after undergoing surgical scar resection with clinical observation for 1 year. Patients demonstrated expansion of sensation and motor-evoked potential responsive area, improved finger control, enhanced truncal stability, and autonomic neural function recovery without developing any adverse effects.
Yao et al95 performed a controlled cohort analysis of either intravenous or intrathecal application of umbilical cord blood administration in 25 patients with chronic (>6 months) SCI, compared with 25 control patients treated with traditional rehabilitation alone. They did find that 24% of treated patients demonstrated improved urinary control and 36% had improved somatosensory evoked potentials, despite a lack of difference in ASIA score when compared to pretreatment data. Zhu et al96 reported the results from their Phase I-II clinical trial investigating the intrathecal application of umbilical cord blood of varying dosages in 28 patients with chronic SCI, C3-T11 level injuries. There was a lack of treatment uniformity (vast difference in MSC dosages) and a mixture in adjuvant treatment (methylprednisolone, lithium); 5 patients converted from complete to incomplete SCIs (2 sensory, 3 motor) with a significant improvement in ability to ambulate 10 meters (7% vs 75%) and improvement in urinary and bowel control (0% vs 60%).
A review of ongoing clinical trials (www.clinicaltrials.gov, accessed August 31, 2017) identified one study investigating the application of UC-MSCs in patients with spinal cord injury that is currently enrolling patients, with 3 recently completed clinical trials.
Conclusions
Human-derived placental tissues have been identified as a source of pluripotent cells, with promising potential for spinal surgical applications, most especially with traumatic SCIs. Specific tissue effects can be expected, understanding the underlying anatomical and biochemical differences of the various subtypes of placental tissues. However, there is currently no evidence to support the clinical application of placental tissues in spine surgery. High-quality research studies are needed to investigate the potential use of placental tissue in spine surgery.
Supplemental Material
Supplemental Material, Placenta_Appendix for The Science and Clinical Applications of Placental Tissues in Spine Surgery by K. Aaron Shaw, Stephen A. Parada, David M. Gloystein, and John G. Devine in Global Spine Journal
Footnotes
Authors’ Note: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the US government. The authors are employees of the US government. This work was prepared as part of their official duties and as such there is no copyright to be transferred.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: K. Aaron Shaw  http://orcid.org/0000-0002-3553-2889
http://orcid.org/0000-0002-3553-2889
Supplemental Material: The supplemental material is available in the online version of the article.
References
- 1. Capelli C, Gotti E, Morigi M, et al. Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy. 2011;13:786–801. [DOI] [PubMed] [Google Scholar]
- 2. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. [DOI] [PubMed] [Google Scholar]
- 3. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. [DOI] [PubMed] [Google Scholar]
- 4. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. [DOI] [PubMed] [Google Scholar]
- 5. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McIntyre JA, Jones IA, Danilkovich A, Vangsness CT., Jr The placenta: applications in orthopaedic sports medicine [published online April 1, 2017]. Am J Sports Med. doi:10.1177/0363546517697682. [DOI] [PubMed] [Google Scholar]
- 7. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349:447–458. [DOI] [PubMed] [Google Scholar]
- 8. Riboh JC, Saltzman BM, Yanke AB, Cole BJ. Human amniotic membrane-derived products in sports medicine: basic science, early results, and potential clinical applications. Am J Sports Med. 2016;44:2425–2434. [DOI] [PubMed] [Google Scholar]
- 9. Heckmann N, Auran R, Mirzayan R. Application of amniotic tissue in orthopedic surgery. Am J Orthop (Belle Mead NJ). 2016;45:E421–E425. [PubMed] [Google Scholar]
- 10. Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 11. Niknejad H, Paeini-Vayghan G, Tehrani FA, Khayat-Khoei M, Peirovi H. Side dependent effects of the human amnion on angiogenesis. Placenta. 2013;34:340–345. [DOI] [PubMed] [Google Scholar]
- 12. Zhou H, Mu Z, Chen X, et al. HAEC in the treatment of brain hemorrhage: a preliminary observation in rabbits. Int J Clin Exp Pathol. 2015;8:6772–6778. [PMC free article] [PubMed] [Google Scholar]
- 13. Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118:11–17. [DOI] [PubMed] [Google Scholar]
- 14. Wu Z, Hui G, Lu Y, Liu T, Huang Q, Guo L. Human amniotic epithelial cells express specific markers of nerve cells and migrate along the nerve fibers in the corpus callosum. Neural Regen Res. 2012;7:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21:2377–2395. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Li C, Jiang X, et al. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657–664. [DOI] [PubMed] [Google Scholar]
- 18. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. [DOI] [PubMed] [Google Scholar]
- 19. Roubelakis MG, Trohatou O, Anagnou NP. Amniotic fluid and amniotic membrane stem cells: marker discovery. Stem Cells Int. 2012;2012:107836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fairbairn NG, Randolph MA, Redmond RW. The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg. 2014;67:662–675. [DOI] [PubMed] [Google Scholar]
- 21. Topoluk N, Hawkins R, Tokish J, Mercuri J. Amniotic mesenchymal stromal cells exhibit preferential osteogenic and chondrogenic differentiation and enhanced matrix production compared with adipose mesenchymal stromal cells. Am J Sports Med. 2017;45:2637–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diaz-Prado S, Rendal-Vázquez ME, Muiños-López E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11:183–195. [DOI] [PubMed] [Google Scholar]
- 23. Krishnamurithy G, Shilpa PN, Ahmad RE, Sulaiman S, Ng CL, Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res A. 2011;99:500–506. [DOI] [PubMed] [Google Scholar]
- 24. Lindenmair A, Nurnberger S, Stadler G, et al. Intact human amniotic membrane differentiated towards the chondrogenic lineage. Cell Tissue Bank. 2014;15:213–225. [DOI] [PubMed] [Google Scholar]
- 25. Nogami M, Tsuno H, Koike C, et al. Isolation and characterization of human amniotic mesenchymal stem cells and their chondrogenic differentiation. Transplantation. 2012;93:1221–1228. [DOI] [PubMed] [Google Scholar]
- 26. Tan SL, Sulaiman S, Pingguan-Murphy B, Selvaratnam L, Tai CC, Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2011;12:59–70. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez PL, Carvajal C, Cuenca J, et al. Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Transl Med. 2015;4:1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heazlewood CF, Sherrell H, Ryan J, Atkinson K, Wells CA, Fisk NM. High incidence of contaminating maternal cell overgrowth in human placental mesenchymal stem/stromal cell cultures: a systematic review. Stem Cells Transl Med. 2014;3:1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Araujo AB, Salton GD, Furlan JM, et al. Comparison of human mesenchymal stromal cells from four neonatal tissues: amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy. 2017;19:577–585. [DOI] [PubMed] [Google Scholar]
- 30. Antoniadou E, David AL. Placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:13–29. [DOI] [PubMed] [Google Scholar]
- 31. Kwon A, Kim Y, Kim M, et al. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep. 2016;6:23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim IJ, Phan TT. Epithelial and mesenchymal stem cells from the umbilical cord lining membrane. Cell Transplant. 2014;23:497–503. [DOI] [PubMed] [Google Scholar]
- 33. Covas DT, Siufi JL, Silva AR, Orellana MD. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res. 2003;36:1179–1183. [DOI] [PubMed] [Google Scholar]
- 34. Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. [DOI] [PubMed] [Google Scholar]
- 35. Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vangsness CT, Jr, Sternberg H, Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: a literature review of different harvest sites. Arthroscopy. 2015;31:1836–1843. [DOI] [PubMed] [Google Scholar]
- 37. Ribeiro J, Gartner A, Pereira T, et al. Perspectives of employing mesenchymal stem cells from the Wharton’s jelly of the umbilical cord for peripheral nerve repair. Int Rev Neurobiol. 2013;108:79–120. [DOI] [PubMed] [Google Scholar]
- 38. Hu SL, Luo HS, Li JT, et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit Care Med. 2010;38:2181–2189. [DOI] [PubMed] [Google Scholar]
- 39. Zhuang H, Zhang R, Zhang S, Shu Q, Zhang D, Xu G. Altered expression of microRNAs in the neuronal differentiation of human Wharton’s Jelly mesenchymal stem cells. Neurosc Lett. 2015;600:69–74. [DOI] [PubMed] [Google Scholar]
- 40. Weiss ML, Medicetty S, Bledsoe AR, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. [DOI] [PubMed] [Google Scholar]
- 41. Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 43. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. [DOI] [PubMed] [Google Scholar]
- 44. Yousefi B, Sanooghi D, Faghihi F, Joghataei MT, Latifi N. Evaluation of motor neuron differentiation potential of human umbilical cord blood-derived mesenchymal stem cells, in vitro. J Chem Neuroanat. 2017;81:18–26. [DOI] [PubMed] [Google Scholar]
- 45. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18:1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Subach BR, Copay AG. The use of a dehydrated amnion/chorion membrane allograft in patients who subsequently undergo reexploration after posterior lumbar instrumentation. Adv Orthop. 2015;2015:501202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Melloh M, Röder C, Elfering A, et al. Differences across health care systems in outcome and cost-utility of surgical and conservative treatment of chronic low back pain: a study protocol. BMC Musculoskelet Disord. 2008;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pang X, Yang H, Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician. 2014;17:E525–E530. [PubMed] [Google Scholar]
- 50. Ni L, Liu X, Sochacki KR, et al. Effects of hypoxia on differentiation from human placenta-derived mesenchymal stem cells to nucleus pulposus-like cells. Spine J. 2014;14:2451–2458. [DOI] [PubMed] [Google Scholar]
- 51. Chon BH, Lee EJ, Jing L, Setton LA, Chen J. Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system. Stem Cell Res Ther. 2013;4:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruan D, Zhang Y, Wang D, et al. Differentiation of human Wharton’s jelly cells toward nucleus pulposus-like cells after coculture with nucleus pulposus cells in vitro. Tissue Eng Part A. 2012;18:167–175. [DOI] [PubMed] [Google Scholar]
- 53. Anderson DG, Markova D, An HS, et al. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013;92:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beeravolu N, Brougham J, Khan I, McKee C, Perez-Cruet M, Chaudhry GR. Human umbilical cord derivatives regenerate intervertebral disc [published online September 30, 2016]. J Tissue Eng Regen Med. doi:10.1002/term.2330. [DOI] [PubMed] [Google Scholar]
- 55. Tam V, Rogers I, Chan D, Leung VY, Cheung KM. A comparison of intravenous and intradiscal delivery of multipotential stem cells on the healing of injured intervertebral disk. J Orthop Res. 2014;32:819–825. [DOI] [PubMed] [Google Scholar]
- 56. Leckie SK, Sowa GA, Bechara BP, et al. Injection of human umbilical tissue-derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013;13:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goldschlager T, Ghosh P, Zannettino A, et al. A comparison of mesenchymal precursor cells and amnion epithelial cells for enhancing cervical interbody fusion in an ovine model. Neurosurgery. 2011;68:1025–1035. [DOI] [PubMed] [Google Scholar]
- 58. Anderson DG, Popov V, Raines AL, O’Connell J. Cryopreserved amniotic membrane improves clinical outcomes following microdiscectomy. Clin Spine Surg. 2017;30:413–418. [DOI] [PubMed] [Google Scholar]
- 59. Adzick NS, Thom EA, Spong CY, et al. ; MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Papanna R, Moise KJ, Jr, Mann LK, et al. Cryopreserved human umbilical cord patch for in-utero spina bifida repair. Ultrasound Obstet Gynecol. 2016;47:168–176. [DOI] [PubMed] [Google Scholar]
- 61. Brown EG, Saadai P, Pivetti CD, et al. In utero repair of myelomeningocele with autologous amniotic membrane in the fetal lamb model. J Pediatr Surg. 2014;49:133–138. [DOI] [PubMed] [Google Scholar]
- 62. Pedreira DA, Zanon N, de Sá RA, et al. Fetoscopic single-layer repair of open spina bifida using a cellulose patch: preliminary clinical experience. J Matern Fetal Neonatal Med. 2014;27:1613–1619. [DOI] [PubMed] [Google Scholar]
- 63. Papanna R, Mann LK, Snowise S, et al. Neurological outcomes after human umbilical cord patch for in utero spina bifida repair in a sheep model. AJP Rep. 2016;6:e309–e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Papanna R, Fletcher S, Moise KJ, Jr, Mann LK, Tseng SC. Cryopreserved human umbilical cord for in utero myeloschisis repair. Obstet Gynecol. 2016;128:325–330. [DOI] [PubMed] [Google Scholar]
- 65. Gao Y, Bai C, Zheng D, et al. Combination of melatonin and Wnt-4 promotes neural cell differentiation in bovine amniotic epithelial cells and recovery from spinal cord injury. J Pineal Res. 2016;60:303–312. [DOI] [PubMed] [Google Scholar]
- 66. Gao S, Ding J, Xiao HJ, et al. Anti-inflammatory and anti-apoptotic effect of combined treatment with methylprednisolone and amniotic membrane mesenchymal stem cells after spinal cord injury in rats. Neurochem Res. 2014;39:1544–1552. [DOI] [PubMed] [Google Scholar]
- 67. Roh DH, Seo MS, Choi HS, et al. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant. 2013;22:1577–1590. [DOI] [PubMed] [Google Scholar]
- 68. Xue H, Zhang XY, Liu JM, Song Y, Li YF, Chen D. Development of a chemically extracted acellular muscle scaffold seeded with amniotic epithelial cells to promote spinal cord repair. J Biomed Mater Res A. 2013;101:145–156. [DOI] [PubMed] [Google Scholar]
- 69. Meng XT, Li C, Dong ZY, et al. Co-transplantation of bFGF-expressing amniotic epithelial cells and neural stem cells promotes functional recovery in spinal cord-injured rats. Cell Biol Int. 2008;32:1546–1558. [DOI] [PubMed] [Google Scholar]
- 70. Wu ZY, Hui GZ, Lu Y, Wu X, Guo LH. Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chin Med J (Engl). 2006;119:2101–2107. [PubMed] [Google Scholar]
- 71. Zhang L, Zhang HT, Hong SQ, Ma X, Jiang XD, Xu RX. Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res. 2009;34:2030–2039. [DOI] [PubMed] [Google Scholar]
- 72. Jiao G, Lou G, Mo Y, et al. A combination of GDNF and hUCMSC transplantation loaded on SF/AGs composite scaffolds for spinal cord injury repair. Mater Sci Eng C Mater Biol Appl. 2017;74:230–237. [DOI] [PubMed] [Google Scholar]
- 73. Ryu HH, Kang BJ, Park SS, et al. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci. 2012;74:1617–1630. [DOI] [PubMed] [Google Scholar]
- 74. Yousefifard M, Nasirinezhad F, Manaheji SH, Janzadeh A, Hosseini M, Keshavarz M. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther. 2016;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li C, Chen X, Qiao S, et al. Effects of Wharton’s jelly cells of the human umbilical cord on acute spinal cord injury in rats, and expression of interleukin-1β and nerve growth factor in spinal cord tissues. Artif Cells Nanomed Biotechnol. 2016;44:1254–1258. [DOI] [PubMed] [Google Scholar]
- 76. Zhilai Z, Biling M, Sujun Q, et al. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426–435. [DOI] [PubMed] [Google Scholar]
- 77. Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yan-Wu G, Yi-Quan K, Ming L, et al. Human umbilical cord-derived Schwann-like cell transplantation combined with neurotrophin-3 administration in dyskinesia of rats with spinal cord injury. Neurochem Res. 2011;36:783–792. [DOI] [PubMed] [Google Scholar]
- 79. Zhao ZM, Li HJ, Liu HY, et al. Intraspinal transplantation of CD34+ human umbilical cord blood cells after spinal cord hemisection injury improves functional recovery in adult rats. Cell Transplant. 2004;13:113–122. [DOI] [PubMed] [Google Scholar]
- 80. Chen CT, Foo NH, Liu WS, Chen SH. Infusion of human umbilical cord blood cells ameliorates hind limb dysfunction in experimental spinal cord injury through anti-inflammatory, vasculogenic and neurotrophic mechanisms. Pediatr Neonatol. 2008;49:77–83. [DOI] [PubMed] [Google Scholar]
- 81. Chua SJ, Bielecki R, Yamanaka N, Fehlings MG, Rogers IM, Casper RF. The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine (Phila Pa 1976). 2010;35:1520–1526. [DOI] [PubMed] [Google Scholar]
- 82. Chung HJ, Chung WH, Lee JH, et al. Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats. J Vet Sci. 2016;17:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mukhamedshina YO, Garanina EE, Masgutova GA, et al. Assessment of glial scar, tissue sparing, behavioral recovery and axonal regeneration following acute transplantation of genetically modified human umbilical cord blood cells in a rat model of spinal cord contusion. PLoS One. 2016;11:e0151745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee JH, Chang HS, Kang EH, et al. Percutaneous transplantation of human umbilical cord blood-derived multipotent stem cells in a canine model of spinal cord injury. J Neurosurg Spine. 2009;11:749–757. [DOI] [PubMed] [Google Scholar]
- 85. Kao CH, Chen SH, Chio CC, Lin MT. Human umbilical cord blood-derived CD34+ cells may attenuate spinal cord injury by stimulating vascular endothelial and neurotrophic factors. Shock. 2008;29:49–55. [DOI] [PubMed] [Google Scholar]
- 86. Yeng CH, Chen PJ, Chang HK, et al. Attenuating spinal cord injury by conditioned medium from human umbilical cord blood-derived CD34+ cells in rats. Taiwan J Obstet Gynecol. 2016;55:85–93. [DOI] [PubMed] [Google Scholar]
- 87. Dasari VR, Spomar DG, Li L, Gujrati M, Rao JS, Dinh DH. Umbilical cord blood stem cell mediated downregulation of fas improves functional recovery of rats after spinal cord injury. Neurochem Res. 2008;33:134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dasari VR, Veeravalli KK, Tsung AJ, et al. Neuronal apoptosis is inhibited by cord blood stem cells after spinal cord injury. J Neurotrauma. 2009;26:2057–2069. [DOI] [PubMed] [Google Scholar]
- 89. Nishio Y, Koda M, Kamada T, et al. The use of hemopoietic stem cells derived from human umbilical cord blood to promote restoration of spinal cord tissue and recovery of hindlimb function in adult rats. J Neurosurg Spine. 2006;5:424–433. [DOI] [PubMed] [Google Scholar]
- 90. Veeravalli KK, Dasari VR, Tsung AJ, et al. Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury. Neurobiol Dis. 2009;36:200–212. [DOI] [PubMed] [Google Scholar]
- 91. Dasari VR, Spomar DG, Gondi CS, et al. Axonal remyelination by cord blood stem cells after spinal cord injury. J Neurotrauma. 2007;24:391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu J, Han D, Wang Z, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15:185–191. [DOI] [PubMed] [Google Scholar]
- 93. Cheng H, Liu X, Hua R, et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao Y, Tang F, Xiao Z, et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yao L, He C, Zhao Y, et al. Human umbilical cord blood stem cell transplantation for the treatment of chronic spinal cord injury: electrophysiological changes and long-term efficacy. Neural Regen Res. 2013;8:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhu H, Poon W, Liu Y, et al. Phase I-III clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transplant. 2016;25:1925–1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Placenta_Appendix for The Science and Clinical Applications of Placental Tissues in Spine Surgery by K. Aaron Shaw, Stephen A. Parada, David M. Gloystein, and John G. Devine in Global Spine Journal