Summary
A combination of several metabolic and hormonal adaptations has been proposed to control aging. Little is known regarding the effects of multiple deregulations of these metabolic and hormonal systems in modulating frailty and mortality in hospitalized elderly patients. We measured 17 biological serum parameters from different metabolic/hormonal pathways in 594 hospitalized elderly patients followed up to 1 year who were stratified into three groups according to their multidimensional impairment, evaluated by a Comprehensive Geriatric Assessment (CGA)-based Multidimensional Prognostic Index (MPI). The mortality incidence rates were 7% at 1 month and 21% at 1 year. Our data show that frailty and mortality rate were positively associated with chronic inflammation and with a down-regulation of multiple endocrine factors. Of the 17 biomarkers examined, blood levels of IGF-1, triiodothyronine, C-reactive protein, erythrocyte sedimentation rate, white blood cell and lymphocyte counts, iron, albumin, total cholesterol, and LDL-c were significantly associated with both MPI severity grade and mortality. In multivariate Cox proportional hazard model, the following biomarkers most strongly predicted the risk of mortality (adjusted hazard ratio (HR) per 1 quintile increment in predictor distribution): IGF-1 HR = 0.71 (95% CI: 0.63–0.80), CRP HR = 1.48 (95% CI: 1.32–1.65), hemoglobin HR = 0.82 (95% CI: 0.73–0.92), and glucose HR = 1.17 (95% CI: 1.04–1.30). Multidimensional impairment assessed by MPI is associated with a distinctive metabolic ‘signature’. The concomitant elevation of markers of inflammation, associated with a simultaneous reduction in multiple metabolic and hormonal factors, predicts mortality in hospitalized elderly patients.
Keywords: comprehensive geriatric assessment, IGF-1, inflammation, mortality, multidimensional prognostic index, testosterone
Introduction
Aging is associated with a multidimensional impairment that affects metabolic, physical, and cognitive function and increases vulnerability to disease and death (Rockwood et al., 2006). Despite the complexity of the biology of the aging process, recent studies clearly indicate that several metabolic and hormonal factors play crucial roles in modulating aging and survival in simple model organisms and mammals (Lamberts et al., 1997; Bartke, 2005; Antebi, 2012). In particular, a down-regulation of the insulin/IGF-1/mTOR pathway and of several other mitogenic and inflammatory pathways has been shown to prolong healthspan and lifespan in several dietary and genetic animal models of longevity (Bartke, 2005; Fontana & Klein, 2007; Fontana et al., 2010). However, to our knowledge, little is known regarding the effects of multiple deregulations of metabolic and hormonal systems in mediating frailty and mortality in hospitalized elderly patients.
There is substantial interest in the clinical use of biomarkers and multidimensional geriatric assessment to identify elderly patients who are at higher risk of frailty and death, to improve clinical decision-making (i.e., invasive screening and aggressive treatments, or comfort care, and compassionate end-of-life plans) and to better understand the biology of aging (McGinn et al., 2000; Lee et al., 2006; Willcox et al., 2006). Many individual biomarkers (i.e., C-reactive protein, IGF-1, insulin, thyroid hormones, DHEA-S, testosterone, etc.) have been related to frailty and increased risk of mortality in hospitalized elderly patients (Cohen et al., 1997; Harris et al., 1999; Maggio et al., 2005; Puts et al., 2005; Laughlin et al., 2008; Leng et al., 2009). However, to our knowledge, little is known regarding the effects of multiple and simultaneous deregulations of these metabolic and hormonal factors that are modified by aging and calorie restriction (CR) in modulating frailty and mortality in hospitalized elderly patients.
The main aim of the present study was to evaluate how the interaction among these aging-related factors affects frailty in a large group of patients aged 65 or older, who were admitted to our hospital. Serum concentrations of markers of inflammation (e.g., CRP, ESR, WBC), anabolic hormones (e.g., insulin, testosterone, DHEA-s), growth factors (e.g., IGF-1), and metabolic (e.g., glycemia, total, LDL and HDL cholesterol, triglycerides), and nutritional factors (e.g., albumin, iron, hemoglobin) were evaluated in 594 hospitalized elderly men and women (mean age = 78.7 7.1 years), who were stratified into three groups according to their multidimensional impairment, evaluated by a Multidimensional Prognostic Index (i.e., a well-accepted and validated nondisease-specific prognostic index) constructed from data derived from Comprehensive Geriatric Assessment (Pilotto et al., 2008; Siontis et al., 2011; Yourman et al., 2012). We also evaluated the relationship that exists between these biological parameters and 1-year mortality. Finally, we evaluated the prognostic usefulness of these biomarkers in predicting all-cause mortality in this study population.
Results
Functional and clinical characteristics of patients
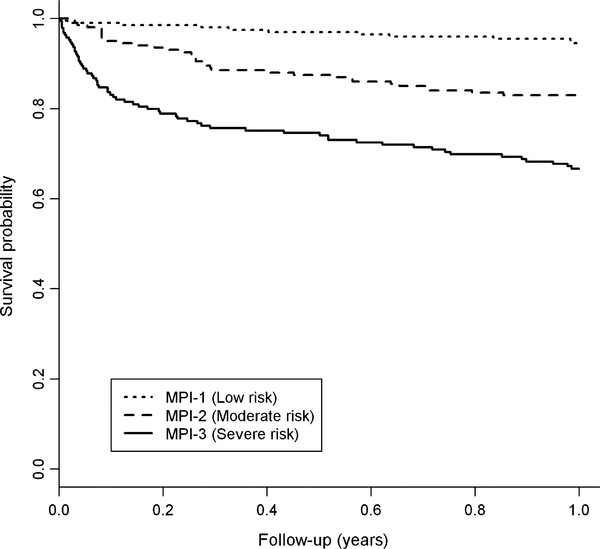
A total of 594 patients were included in this study, 231 (38.9%) men and 363 (61.1%) women, with a mean age of 78.7 ± 7.1, range from 65 to 99 years. Functional and clinical characteristics of the patients and mortality incidence rates are shown in Table 1. During the 1 year of follow-up, 109 of 594 patients (21%) died, of whom 63 were women and 46 were men. Female patients had significantly higher MPI mean values (P = 0.008), cognitive impairment (SPMSQ score, P < 0.001), prevalence of hypertension (P = 0.022) and a lower malnutrition score (MNA score, P < 0.001), risk of pressure sores (ESS score, P = 0.008), and cardiovascular diseases (P = 0.038) than males. Age, ADL, IADL, CIRS-CI mean values, number of drugs, prevalence of cerebrovascular diseases, neurodegenerative diseases, kidney diseases, respiratory failure, cirrhosis, and 1-month and 1-year mortality were not significantly different between male and female patients. Based on MPI values, 201 patients (33.8%) were included in the MPI-1 lowrisk group, 201 (33.8%) in the MPI-2 moderate risk group, and 192 (32.4%) in the MPI-3 severe risk group of mortality. As expected (Table 2 and Fig. 1), patients with higher MPI grade compared to patients in the lower MPI groups showed a progressively significant higher short- and long-term mortality incidence rates at 1 month (MPI-1 = 1.0% vs. MPI-2 = 4.6% vs. MPI-3 = 16.7%, P < 0.001) and 1 year (MPI-1 = 5.6% vs. MPI-2 = 19.7% vs. MPI-3 = 43.5%, P < 0.001).
Table 1.
Functional and clinical parameters, according to gender
| Total (N = 594) | Men (N = 231) | Women (N = 363) | P-value | |
|---|---|---|---|---|
| Age (years) | 78.71 7.12 | 78.18 7.04 | 79.04 7.14 | 0.137 |
| MPI | 0.46 0.24 | 0.43 0.24 | 0.48 0.24 | 0.008 |
| ADL | 3.57 2.49 | 3.73 2.52 | 3.47 2.47 | 0.105 |
| IADL | 3.31 3.13 | 3.27 3.06 | 3.34 3.18 | 0.952 |
| SPMSQ | 3.37 3.31 | 2.94 3.37 | 3.65 3.24 | < 0.001 |
| MNA | 20.57 5.76 | 21.36 5.86 | 20.08 5.66 | < 0.001 |
| CIRS | 3.25 1.82 | 3.29 1.82 | 3.22 1.83 | 0.553 |
| DRUGS | 4.60 2.87 | 4.70 3.06 | 4.53 2.75 | 0.481 |
| ESS | 15.08 3.81 | 15.55 3.84 | 14.77 3.77 | 0.008 |
| N (%) | N (%) | N (%) | ||
| Hypertension | 227 (38.22) | 75 (32.47) | 152 (41.87) | 0.022 |
| Cerebrovascular diseases | 197 (33.16) | 78 (33.77) | 119 (32.78) | 0.804 |
| Neurodegenerative diseases | 173 (29.12) | 66 (28.57) | 107 (29.48) | 0.213 |
| Heart diseases | 164 (27.61) | 75 (32.47) | 89 (24.52) | 0.038 |
| Respiratory failure | 41 (6.90) | 16 (6.93) | 25 (6.89) | 0.985 |
| Kidney diseases | 20 (3.37) | 8 (3.46) | 12 (3.31) | 0.917 |
| Liver cirrhosis | 11 (1.85) | 5 (2.16) | 6 (1.65) | 0.652 |
| Miscellaneous | 121 (20.37) | 44 (19.05) | 77 (21.21) | 0.601 |
| 1-month mortality (ev per pm, IR%) | 40/569 (7.0%) | 14/223 (6.3%) | 26/346 (7.5%) | 0.438 |
| 1-year mortality (ev per py, IR%) | 109/518 (21.0%) | 46/202 (22.8%) | 63/316 (20.0%) | 0.547 |
pm, person/months; py, person/years; IR%, incidence rate%.
MannWhitney U-test and chi-square test were assessed for continuous and categorical variables, respectively.
Table 2.
Biological serum parameters in older patients, according to MPI grades
| Multidimensional I Prognostic Index |
Correlations |
||||||
|---|---|---|---|---|---|---|---|
| MPI-1 (low risk) N = 201 | MPI-2 (moderate risk) N = 201 | MPI-3 (severe risk) N = 192 | Test for linear trend (P-value) | MPI-3 vs. MPI-1 (P-value) | Spearman's rho | P-value | |
| White blood cells (1000 μL−1) | 7.27 ± 2.18 | 7.84 ± 3.23 | 9.25 ± 4.44 | 0.004 | 0.005 | 0.205 | < 0.001 |
| Lymphocytes (1000 μL−1) | 2.05 ± 0.77 | 1.90 ± 0.95 | 1.68 ± 0.69 | 0.031 | 0.035 | −0.204 | < 0.001 |
| C-reactive protein (pg mL−1)* | 1.97 ± 4.00 | 2.85 ± 4.77 | 5.65 ± 7.49 | < 0.001 | < 0.001 | 0.358 | < 0.001 |
| Erythrocyte sedimentation rate (mm) | 30.19 ± 24.14 | 42.86 ± 28.73 | 52.28 ± 33.30 | < 0.001 | < 0.001 | 0.288 | < 0.001 |
| Iron (μg dL−1) | 71.64 ± 37.40 | 57.63 ± 33.44 | 49.26 ± 31.39 | < 0.001 | < 0.001 | −0.278 | < 0.001 |
| Hemoglobin (g dL−1) | 12.94 ± 2.01 | 12.17 ± 1.92 | 11.97 ± 2.09 | 0.191 | 0.176 | −0.233 | < 0.001 |
| Albumin (g dL−1) | 61.67 ± 4.52 | 60.48 ± 5.50 | 56.75 ± 7.19 | < 0.001 | < 0.001 | −0.292 | < 0.001 |
| Glycemia (mg dL−1)† | 104.33 ± 37.29 | 111.75 ± 50.30 | 118.58 ± 54.24 | 0.127 | 0.130 | 0.103 | 0.012 |
| Triglycerides (mg dL−1)‡ | 123.82 ± 86.84 | 122.13 ± 66.22 | 118.92 ± 62.60 | 0.903 | 0.887 | −0.012 | 0.769 |
| Total cholesterol (mg dL−1)‡ | 177.00 ± 47.02 | 160.24 ± 43.23 | 153.86 ± 44.57 | 0.006 | 0.006 | −0.225 | < 0.001 |
| HDL cholesterol (mg dL−1‡ | 43.47 ± 18.10 | 40.41 ± 13.35 | 37.56 ± 15.41 | 0.006 | 0.006 | −0.146 | < 0.001 |
| LDL cholesterol (mg dL−1)‡ | 105.89 ± 35.95 | 98.34 ± 37.80 | 90.19 ± 39.01 | 0.012 | 0.014 | −0.201 | < 0.001 |
| Insulin (μU mol−) | 9.22 ± 9.38 | 9.33 ± 7.03 | 11.99 ± 36.33 | 0.500 | 0.525 | −0.027 | 0.514 |
| FT3 (pg mL−1)$ | 2.89 ± 0.65 | 2.79 ± 0.61 | 2.49 ± 0.68 | < 0.001 | 0.001 | −0.270 | < 0.001 |
| DHEA-S (μg dL−1) | 63.78 ± 61.98 | 46.58 ± 37.90 | 47.39 ± 39.21 | 0.851 | 0.920 | −0.128 | 0.002 |
| Men | 85.01 ± 74.55 | 56.41 ± 39.93 | 57.42 ±51.27 | 0.039 | 0.041 | −0.201 | 0.002 |
| Women | 46.56 ± 42.66 | 40.48 ± 35.38 | 42.38 ± 30.53 | 0.125 | 0.154 | −0.035 | 0.504 |
| IGF-1 (ng mL−1) | 87.44 ± 39.88 | 82.25 ± 45.37 | 60.56 ± 29.95 | < 0.001 | < 0.001 | −0.305 | < 0.001 |
| Free testosterone (pg mL−1) | 35.85 ±38.14 | 23.18 ± 27.21 | 20.64 ± 30.47 | 0.050 | 0.036 | −0.143 | 0.001 |
| Men | 70.13 ± 30.40 | 48.65 ± 24.23 | 50.19 ± 38.69 | 0.021 | 0.022 | −0.366 | < 0.001 |
| Women | 8.86 ± 14.70 | 7.42 ± 13.64 | 6.52 ± 6.76 | 0.769 | 0.795 | 0.001 | 0.985 |
| N (%) | N (%) | N (%) | |||||
| 1-month mortality (ev per pm, IR%) | 2/198(1.0) | 9/197 (4.6) | 29/174(16.7) | < 0.001 | |||
| 1-year mortality (ev per py, IR%) | 11/196(5.6) | 35/177(19.7) | 63/145 (43.5) | < 0.001 | |||
pm, person/month; py, person/year; IR%, incidence rate%.
All analyses were adjusted for age, gender, and comorbidity. Subgroup analyses were adjusted for age and comorbidity only. Analyses were
also adjusted for statins and FANS
also adjusted for cortisone
also adjusted for statins
also adjusted for hormone therapy.
Fig. 1.
Kaplan–Meier curves of the survival probability, according to category of MPI
Biomarkers according to the MPI grade
As showed in Table 2, significant differences were observed among the three MPI groups in serum concentrations of several metabolic and hormonal biomarkers. In particular, patients with higher MPI grade had significantly lower blood levels of IGF-1 (P for trend < 0.001), FT3 (P for trend < 0.001), and higher levels of total CRP (P for trend < 0.001) and ESR (P for trend < 0.001) than patients in the lower MPI groups. In men only, patients with higher MPI grade showed significantly lower circulating concentrations of DHEA-S (P for trend = 0.039) and free testosterone (P for trend = 0.021) than men with lower MPI grade. In addition, patients with higher MPI grade had significantly higher blood levels of WBC (P for trend = 0.004) and lower numbers of circulating lymphocytes (P for trend = 0.031) than patients with lower MPI grade. A significant correlation between all the inflammatory parameters and the MPI considered as continuous variable was also observed (Table 2). Finally, patients with higher MPI grade had significantly lower serum levels of albumin (P for trend < 0.001), TC (P for trend = 0.006), HDL-c (P for trend = 0.006), and LDL-c (P for trend = 0.012) compared to patients with lower MPI grade. No significant differences among the three MPI groups were found in serum insulin, TG, and glucose concentration. As shown in Table 3, CRP and ESR were inversely correlated with the blood levels of IGF1, FT3, hemoglobin, iron, albumin, TC, LDL-c, HDL-c, and lymphocytes and positively correlated with blood levels of WBC in both men and women. All analyses were adjusted for age, sex, and comorbidity. Further adjustments for: (i) statins and nonsteroidal anti-inflammatory drugs; (ii) cortisone only; (iii) statins only; and (iv) hormone therapy only were included in the evaluation of CRP, glycemia, lipid levels (triglycerides, total cholesterol, HDL-c, LDL-c), and serum circulating concentrations of FT3 trends across MPI grades, respectively.
Table 3.
Spearman correlation coefficients for CRP and ESR
| CRP |
ESR |
|||
|---|---|---|---|---|
| Spearman coefficient | P-value | Spearman coefficient | P-value | |
| KjF-1 | ||||
| Male | −0.392 | <0.0001 | −0.178 | 0.008 |
| Female | −0.287 | <0.0001 | −0.170 | 0.0014 |
| FT3 | ||||
| Male | −0.307 | <0.0001 | −0.264 | <0.0001 |
| Female | −0.286 | <0.0001 | −0.145 | 0.0075 |
| Testosterone | ||||
| Male | −0.365 | <0.0001 | −0.286 | <0.0001 |
| Female | 0.158 | 0.0043 | 0.027 | 0.635 |
| DHEA-s | ||||
| Male | 0.058 | 0.376 | 0.035 | 0.601 |
| Female | 0.125 | 0.0175 | 0.082 | 0.1272 |
| WBC | ||||
| Male | 0.363 | <0.0001 | 0.288 | <0.0001 |
| Female | 0.307 | <0.0001 | 0.200 | 0.0002 |
| Lymphocytes | ||||
| Male | −0.251 | 0.0004 | −0.141 | 0.054 |
| Female | −0.282 | <0.0001 | −0.107 | 0.064 |
| Hemoglobin | ||||
| Male | −0.228 | 0.0005 | −0.520 | <0.0001 |
| Female | −0.228 | <0.0001 | −0.499 | <0.0001 |
| Iron | ||||
| Male | −0.569 | <0.0001 | −0.525 | <0.0001 |
| Female | −0.565 | <0.0001 | −0.442 | <0.0001 |
| Albumin | ||||
| Male | −0.551 | <0.0001 | −0.628 | <0.0001 |
| Female | −0.486 | <0.0001 | −0.558 | <0.0001 |
| Total cholesterol | ||||
| Male | −0.317 | <0.0001 | −0.280 | <0.0001 |
| Female | −0.272 | <0.0001 | −0.205 | 0.0002 |
| LDL cholesterol | ||||
| Male | −0.343 | <0.0001 | −0.238 | 0.0004 |
| Female | −0.259 | <0.0001 | −0.206 | <0.0001 |
| HDL diolesterol | ||||
| Male | −0.301 | <0.0001 | −0.273 | <0.0001 |
| Female | −0255 | <0.0001 | −0.261 | <0.0001 |
survival C-index, from 0.704 (95% CI: 0.660–0.748) to 0.748 (95% CI: 0.705–0.791, P = 0.002). Both models were calibrated, HL P-values being 0.948 and 0.416, respectively. The continuous net Reclassification Improvement (cNRI) was 0.668 (bootstrap 95%, CI: 0.345–0.949, P < 0.001) and 0.633 (bootstrap 95% CI: 0.431– 0.821, P < 0.001) after 1 month and 1 year of follow-up, respectively.
Biomarkers and mortality prediction
HRs for all the 17 biomarkers are reported in Table 4. At 1 year of follow-up, all biomarkers were significant predictors of death, except triglycerides, HDL-c, insulin, DHEA-S, and free testosterone. When performing the variable selection, according to the maximum increment of cNRI criteria (as described in Statistical Analysis section), four biomarkers were identified, providing additional improvements in mortality prediction when added to the MPI. The addition of CRP, hemoglobin, glycemia, and IGF-1 to the MPI produced a significant improvement in both 1-month survival C-index, from 0.757 (95% CI: MPI. 0.693–0.823) to 0.803 (95% CI: 0.738–0.868, P = 0.03), and 1-year
Table 4.
Mortality prediction of all biomarkers (HRs per one-quintile increment on1 year of follow-up)
| Biomarker | Ev/tot | HR (95% CI) | P-value |
|---|---|---|---|
| White blood cells (1000 lL1) | 104/571 | 1.322 (1.1461.524) | < 0.001 |
| Lymphocytes (1000 lL1) | 94/499 | 0.750 (0.6460.872) | < 0.001 |
| C-reactive protein (pg mL1) | 109/592 | 1.620 (1.4031.870) | < 0.001 |
| Erythrocyte sedimentation rate (mm) | 106/572 | 1.204 (1.0491.381) | 0.008 |
| Iron (lg dL1) | 104/572 | 0.652 (0.5610.758) | < 0.001 |
| Hemoglobin (g dL1) | 109/591 | 0.813 (0.7070.934) | 0.004 |
| Albumin (g dL1) | 107/563 | 0.693 (0.5990.801) | < 0.001 |
| Glycemia (mg dL1) | 109/591 | 1.179 (1.0311.347) | 0.016 |
| Triglycerides (mg dL1) | 107/586 | 0.919 (0.8041.050) | 0.214 |
| Total cholesterol (mg dL1) | 95/560 | 0.776 (0.6680.900) | < 0.001 |
| HDL cholesterol (mg dL1) | 109/592 | 0.910 (0.7941.041) | 0.170 |
| LDL cholesterol (mg dL1) | 109/592 | 0.832 (0.7250.954) | 0.009 |
| Insulin (lU mol1) | 105/582 | 0.929 (0.8101.066) | 0.294 |
| FT3 (pg mL) | 104/572 | 0.796 (0.6920.915) | 0.001 |
| DHEA-S (lg dL1) | 109/594 | 0.975 (0.8541.113) | 0.704 |
| IGF-1 (ng mL1) | 109/594 | 0.708 (0.6140.816) | < 0.001 |
| Free testosterone (pg mL1) | 103/526 | 1.011 (0.8841.157) | 0.870 |
Discussion
In this study, we evaluated the interactions among a number of metabolic/hormonal factors, that are thought to play a role in the biology of aging and in mediating the anti-aging effects of CR, in 594 hospitalized elderly patients followed up to 1 year. First, our data demonstrated that in older hospitalized patients multidimensional impairment assessed by MPI is associated with a distinctive metabolic ‘signature’, characterized by systemic inflammation and low levels of anabolic hormones/growth factors (i.e., low serum concentrations of IGF-1, testosterone and DHEAS) and FT3. Interestingly, no significant differences among the three MPI groups were found in serum insulin, TG and glucose concentration. Second, our findings showed a strong association between several biomarkers from diverse metabolic/hormonal pathways and mortality risk. We observed that the most informative biomarkers for predicting death were blood levels of IGF-1, CRP, hemoglobin, and glucose. In this population, the use of these four biomarkers alone provides a good discriminatory ability (i.e., C statistics at 1 year, 0.745). Adding the four biomarkers to the MPI further improves the 1-year mortality prediction accuracy.
It is well known that long-term CR, one of the most powerful interventions known to slow aging and prevent a wide range of chronic disease, is associated with a significant down-regulation of several inflammatory pathways in association with a reduction in multiple anabolic/growth factors (e.g., reduction in serum concentrations of IGF-1, insulin, testosterone, T3) (Fontana et al., 2004, 2006, 2008; Cangemi et al., 2010). In contrast, the findings of this study show that higher mortality risk and frailty were significantly associated with elevated inflammatory markers, multiple endocrine dysfunctions, and metabolic disorders. The preponderance of inflammatory/immune biomarkers (i.e., CRP, ESR, WBC, and lymphocyte counts) in modulating multidimensional impairment suggests that these primary mediators play a central role in predicting mortality in elderly hospitalized men and women. The simultaneous presence of several key neuroendocrine (i.e., IGF-1, FT3, and testosterone) and metabolic factors in modulating multidimensional impairment, and the negative relationship between inflammatory biomarkers and neuroendocrine/metabolic factors, points to the potential interacting influence of neuroendocrine/ metabolic and inflammatory factors in affecting health and mortality in elderly adults. Biomarkers of these pathways concurrently modulate the functions of a wide range of biological functions, including metabolism, growth, immune, and reproductive functions (Lamberts et al., 1997; Fontana et al., 2010; Cawthon et al., 2009; Black, 2003), and may therefore serve as an early warning indication of multidimensional impairment and later mortality.
Data from numerous studies have shown an association between chronic inflammation and individual domains of frailty such as functional and cognitive impairment (Reichlin, 1993; Cohen et al., 1997; Harris et al., 1999; Leng et al., 2009), increased morbidity (Reichlin, 1993) and mortality risk (Walston et al., 2002). To our knowledge, this is the first study that has demonstrated an association between chronic inflammation and global multidimensional impairment assessed with MPI. Indeed, a recent prospective multicenter study carried out on 2033 hospitalized older patients demonstrated that the MPI was a significantly higher predictive index for all-cause mortality compared with three other frailty instruments (Pilotto et al., 2012). There are a number of reasons that support the hypothesis that inflammation links with multidimensional impairment and increased risk of death: (i) inflammatory biomarkers increase with aging (Ershler et al., 1993), (ii) inflammation is linked with many age-associated chronic disease (e.g., atherosclerosis, cancer, dementia) (Ross, 1999; Coussens & Werb, 2002), (iii) chronic inflammation is associated with frailty (Maggio et al., 2005; Puts et al., 2005; Wyss-Coray, 2006), and (iv) healthy centenarians and long-term calorie-restricted individuals have low levels of inflammatory biomarkers (Franceschi & Bonafe, 2003; Fontana & Klein, 2007). The exact mechanisms linking inflammation with aging are not fully understood but could reflect the accumulation of DNA/cellular damage by stressed and malfunctioning tissues.
In addition, this study showed an association between higher mortality risk and several neuroendocrine dysfunctions. Patients with higher mortality risk (i.e., MPI-3) had lower blood levels of IGF-1, testosterone, and FT3 than patients with lower mortality risk (i.e., MPI-1 and MPI-2). It is known that circulating levels of IGF-1 and testosterone decrease with aging and may contribute to the development of multidimensional impairment through different mechanisms (Maggio et al., 2005; Gill et al., 2010; Friedrich et al., 2009; Laughlin et al., 2008). Our data suggest that the reductions in circulating IGF-1 and androgens may contribute to multidimensional impairment through their interactions with other systems (i.e., inflammation). Accumulating evidence supports the importance of a dynamic and reciprocal stimulation and suppression between the neuroendocrine and the inflammatory systems in modulating aging and several age-associated diseases (Black, 2003; Franceschi & Bonafe, 2003; Paganelli et al., 2006). Interestingly, in long-lived calorie-restricted animals, low blood levels of IGF-1, testosterone, and T3 are coupled with very low levels of inflammation (Weindruch & Walford, 1988; Fontana & Klein, 2007). In contrast, in this study, patients with high mortality risk have lower levels of IGF-1, testosterone, and FT3 and higher levels of inflammation, further suggesting the importance of the interaction (system biology) of multiple biomarkers from distinct metabolic/ hormonal pathways for the prediction of health status and mortality risk.
It is well known that MPI is a good predictor of short- and longterm mortality in older patients with a prognostic accuracy significantly higher than other prognostic tools (Pilotto et al., 2012; Yourman et al., 2012). Moreover, the MPI showed very good predictive power for all-cause mortality in older patients with several clinical conditions such as chronic heart failure (Pilotto et al., 2010), community-acquired pneumonia (Pilotto et al., 2009a), dementia (Pilotto et al., 2009b), and transient ischemic attack (Sancarlo et al., 2012). To further improve the overall prediction of mortality risk, we evaluated the combination of biomarkers and the MPI. This combination showed a significant improvement on short-term (one month) and long-term (1 year of follow-up) mortality prediction as evidenced by the significant increment in the survival C-index and the cNRI.
In conclusion, our data show that inflammation in association with a down-regulation of multiple hormonal factors predicts mortality and frailty in elderly hospitalized patients. Our findings may have implications for clinical practice and future clinical and translational research.
Materials and methods
Study population
All patients consecutively admitted to the Geriatrics Unit of the Istituto di Ricovero e Cura a Carattere Scientifico ‘Casa Sollievo della Sofferenza’, San Giovanni Rotondo (FG), Italy, from January 2007 to December 2008, were screened for inclusion in the study. Inclusion criteria were as follows: (i) age 65 years; (ii) ability to provide an informed consent or availability of a proxy for informed consent and willingness to participate in the study; (iii) a complete Comprehensive Geriatric Assessment (CGA) during hospitalization to calculate the Multidimensional Prognostic Index (MPI). Patients with a diagnosis of cancer (ICD-9 CM codes 140–199, 209, and 230– 239) or hematologic malignancies (ICD-9 CM codes 200–208), acute and/or chronic inflammatory and infectious diseases including sepsis (ICD-9 codes 001–0139, 245, 460–466, 487.0–487.8, 488, 714, 720, 995.1–2), and endocrine diseases (ICD-9 CM codes 250– 259) were excluded. At baseline, the following parameters were collected by a structured interview, clinical evaluation, and review of records from the patients’ general practitioners: date of birth, gender, clinical history, current pathologies, and medication history. All patients admitted to our unit received a standard CGA. Vital status up to December 31, 2009 was assessed by directly contacting the participants or consulting the Registry Offices of the cities where the patients were residents at the time of hospital admission. Dates of death were identified from death certificates.
The Comprehensive Geriatric Assessment (CGA)
Functional status was evaluated by Activities of Daily Living (ADL) Index (Katz et al., 1970) and by Instrumental Activities of Daily Living (IADL) Scale (Lawton & Brody, 1969). Cognitive status was assessed by the Short Portable Mental Status Questionnaire (SPMSQ) (Pfeiffer, 1975). Comorbidity was examined using the Cumulative Illness Rating Scale (CIRS) (Linn et al., 1968). Nutritional status was explored with the MNA (Guigoz & Vellas, 1999). The Exton Smith Scale (ESS) was used to evaluate the risk of developing pressure sores (Bliss et al., 1966). Medication use was defined according to the Anatomical Therapeutics Chemical Classification code system, and the number of drugs used by patients at admission was recorded. Cohabitation status, that is, living with family, institutionalized or living alone, was also recorded.
The Multidimensional Prognostic Index (MPI)
We used the MPI developed and validated in two independent cohorts of elderly hospitalized patients as previously reported (Pilotto et al., 2008). Briefly, a cluster analysis on CGA data was initially made for evaluating the independence of variables and identifying the most relevant domains of the CGA in predicting mortality outcome. Following a stepwise method, the domains of the CGA, one at a time, were progressively included in the model and Cox and logistic regression analyses performed. Thus, an ‘eight domain’ MPI was developed and yielded the best index for predicting 1-year mortality. For each domain, a 3-level score was used, that is, 0 = no problems, 0.5 = minor problems, and 1 = major problems as previously reported (Pilotto et al., 2008). The sum of the calculated scores from the eight domains was then divided by 8 to obtain a final score between 0 and 1. As previously reported (Pilotto et al., 2008), three grades of risk severity to stratify the examined population were considered: low risk (MPI-1 values 0.33), moderate risk (MPI-2 values between 0.34 and 0.66), and severe risk (MPI-3 values > 0.66) of mortality.
Biological markers
At baseline, in the morning after an overnight fast, blood was collected from an arm vein; serum was isolated using centrifugation. White blood cells (WBC), lymphocytes, erythrocyte sedimentation rate (ESR), total cholesterol (TC), triglycerides (TGD) and albumin were measured on fresh blood samples using routine clinical chemistry. HDL cholesterol (HDL-c), LDL cholesterol (LDL-c), dehydroepiandrosterone sulfate (DHEA-S), total testosterone (TT), insulin-like growth factor 1 (IGF-1), C-reactive protein (CRP) and FT-3 levels were assayed in frozen serum samples that were stored at −20°C for later batch analyses. HDL cholesterol (HDL-c) and LDL cholesterol (LDL-c) were assayed enzymatically using an in vitro test on a Roche automated clinical chemistry analyzer (Roche/Hitachi Diagnostics, Mannheim, Germany). Quantitative measurements of DHEA-S, TT, and IGF-1 in serum were performed using solid-phase, chemiluminescent immunoassays (IMMULITE 2000, Siemens Healthcare Diagnostics, Milan, Italy) on IMMULITE Analyzer. DHEA-S values were expressed in ng mL−1, the average intra-assay CV was 5.9%, and the analytical sensitivity was 3 μg dL−1. TT values were expressed in ng dL−1, the average intra-essay CV was 8.4%, and the analytical sensitivity was 15 ng dL−1. Free testosterone was calculated from total, sex hormone–binding globulin (SHBG), and albumin concentration using the Vermeulen equation. Results were expressed in ng mL−1. IGF-1 values were expressed in ng mL−1, the average intra-assay CV was 3.0%, and the analytical sensitivity was 20 ng mL−1. Serum FT3 concentration was measured using a commercial electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). Results were expressed in pg mL−1. CRP was assessed by immunonephelometry, and results were expressed in pg mL−1.
Statistical methods
Patients’ baseline characteristics were reported as mean ± standard deviation (SD) or frequencies and percentages for continuous and categorical variables, respectively. Baseline comparisons between men and women were made using the chi-square test and the Mann–Whitney U-test for categorical and continuous variables, respectively. Baseline differences according to MPI grades were assessed with ANCOVA models, adjusted for age, gender, and comorbidity and specific covariates with a P-value for linear trend. Post hoc comparisons for MPI grade 3 (severe risk) vs. MPI grade 1 (low risk) were also evaluated. Survival probability curves were constructed for subjects with low, intermediate, and high MPI with the use of the Kaplan–Meier method. In performing ANCOVA models, the following biological serum measures were log-transformed, due to their skewed distribution: IGF1, lymphocytes, triglycerides, DHEA-S, and CRP. Incidence rates for 100 person-months and for 100 person-years and according to MPI grades were reported, and a P-value for trend was assessed with a Poisson regression model. Starting from 1-year mortality risk, predictive clinical model with the only continuous MPI as predictor, a parsimonious set of biomarkers, which provided the best additional improvements in mortality risk prediction, was detected to achieve the highest continuous Net Reclassification Improvement (cNRI) values (Pencina et al., 2008, 2011). The final prediction model was assessed using an ‘ad hoc’ forward variable selection method: at each step, the procedure selected the predictor that yielded the maximum cNRI and stopped when no further statistically significant in cNRI was detected. All biomarkers were categorized in their quintiles before performing the model building. Multivariate proportional hazard regressions of clinical model and that with further inclusion of the selected biomarkers were assessed. Discriminatory power was assessed by estimating survival C-indices, along with their 95% confidence interval (95% CI), and comparison between C-indices was carried out following Pencina & D’Agostino approach (Fontana et al., 2004). The survival-based Hosmer–Lemeshow measure of calibration was also assessed. cNRI 95% CI were estimated following a bootstrap approach with 10 000 resampling with replacement. The selected parsimonious prediction model was also tested for 1-year mortality and 1-month mortality prediction. All proportional hazard regression models were assessed in a time horizon of 1 year, and results were reported as hazard ratio (HR) per one-quintile increment in each biomarker, along with their 95% CI. P-values < 0.05 were considered for statistical significance. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA).
Acknowledgments
There are no financial disclosures. The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Funding/Support
This work is fully supported by ‘Ministero della Salute’, IRCCS Research Program, Ricerca Corrente 2009–2011, Linea n. 2 ‘Malattie complesse’.
References
- Antebi A (2012) Regulation of longevity by the reproductive system. Exp. Gerontol doi: 10.1016/j.exger.2012.09.009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A (2005) Minireview: role of the growth hormone/insulin-like system in mammalian aging. Endocrinology 146, 3718–3723. [DOI] [PubMed] [Google Scholar]
- Black PH (2003) The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun 17, 350–364. [DOI] [PubMed] [Google Scholar]
- Bliss MR, McLaren R, Exton-Smith AN (1966) Mattresses for preventing pressure sores in geriatric patients. Mon. Bull. Minist. Health Public Health Lab. Serv 25, 238–268. [PubMed] [Google Scholar]
- Cangemi R, Friedmann AJ, Holloszy JO, Fontana L (2010) Effects of long-term calorie restriction on serum sex hormones concentration in men. Aging Cell 9, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, Stefanick ML, Cummings SR, Orwoll ES, Osteoporotic Fractures in Men (MrOS) Research Group (2009). Sex hormones and frailty in older men: the Osteoporotic Fractures in Men (MrOS) Study. J. Clin. Endocrinol. Metab 94, 3806–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS (1997) The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci 52, M201–M208. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, Klopp RG, Roecker EB, Daynes RA, Weindruch R (1993) Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 12, 225–230. [PubMed] [Google Scholar]
- Fontana L, Klein S (2007) Aging, adiposity and calorie restriction. JAMA 297, 986–994. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO (2004) Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl Acad. of Sci. USA 101, 6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN (2006) Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab 91, 3232–3235. [DOI] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal D, Klein S, Holloszy JO (2008) Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M (2003) Centenarians as a model for healthy aging. Biochem. Soc. Trans 31, 457–461. [DOI] [PubMed] [Google Scholar]
- Friedrich N, Haring R, Nauck M, Ludemann J, Rosskopf D, Spilcke-Liss E, Felix SB,€ Dorr M, Brabant G, V€ olzke H, Wallaschofski H (2009) Mortality and serum€ insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J. Clin. Endocrinol. Metab 94, 1732–1739. [DOI] [PubMed] [Google Scholar]
- Gill TM, Gahbauer EA, Han L, Allore HG (2010) Trajectories of disability in the last year of life. N. Engl. J. Med 362, 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigoz Y, Vellas B (1999) The Mini Nutritional Assessment (MNA) for grading the malnutrition states of elderly patients: presentation of the MNA, history and validation. Nestle. Nutr. Workshop Ser. Clin. Perform. Programme 1, 3–11. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr, Heimovitz H, Cohen HJ, Wallace R (1999) Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med 106, 506–512. [DOI] [PubMed] [Google Scholar]
- Katz S, Downs TD, Cash HR, Grotz RC (1970) Progress in the development of an index of ADL. Gerontologist 10, 20–30. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, van den Beld AW, van der Lely AJ (1997) The endocrinology of aging. Science 278, 419–424. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J (2008) Low serum testosterone and mortality in older men. J. Clin. Endocrinol. Metab 93, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186. [PubMed] [Google Scholar]
- Lee SJ, Lindquist K, Segal MR, Covinsky KE (2006) Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 295, 801–808. [DOI] [PubMed] [Google Scholar]
- Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP (2009) White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J. Gerontol. A Biol. Sci. Med. Sci 64, 499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn B, Linn M, Gurel L (1968) The cumulative illness rating scale. J. Am. Geriatr. Soc 16, 622–626. [DOI] [PubMed] [Google Scholar]
- Maggio M, Cappola AR, Ceda GP, Basaria S, Chia CW, Valenti G, Ferrucci L (2005) The hormonal pathway to frailty in older men. J. Endocrinol. Invest 28, 15–19. [PubMed] [Google Scholar]
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS (2000) Evidence-Based Medicine Working Group. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. JAMA 284, 79–84. [DOI] [PubMed] [Google Scholar]
- Paganelli R, Di Iorio A, Cherubini A, Lauretani F, Mussi C, Volpato S, Abate M, Abate G, Ferrucci L (2006) Frailty of older age: the role of the endocrine immune interaction. Curr. Pharm. Des 12, 3147–3159. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB (2004) Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat. Med 23, 2109–2123. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med 27, 157–172. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB, Steyerberg EW (2011) Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med 30, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E (1975) A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc 23, 433–441. [DOI] [PubMed] [Google Scholar]
- Pilotto A, Ferrucci L, Franceschi M, D’Ambrosio LP, Scarcelli C, Cascavilla L, Paris F, Placentino G, Seripa D, Dallapiccola B, Leandro G (2008) Development and validation of a multidimensional prognostic index for 1-year mortality from the comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 11, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Addante F, Ferrucci L, Leandro G, D’Onofrio G, Corritore M, Niro V, Scarcelli C, Dallapiccola B, Franceschi M (2009a) The Multidimensional Prognostic Index (MPI) predicts short and long-term mortality in older patients with community-acquired pneumonia. J. Gerontol. A Biol. Med. Sci 64, 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Sancarlo D, Panza F, Paris F, D’Onofrio G, Cascavilla L, Addante F, Seripa D, Solfrizzi V, Dallapiccola B, Franceschi M, Ferrucci L (2009b) The Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short- and long-term mortality in hospitalized older patients with dementia. J. Alzheimers. Dis 18, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Addante F, Franceschi M, Leandro G, Rengo G, D’Ambrosio P, Longo MG, Rengo F, Pellegrini F, Dallapiccola B, Ferrucci L (2010) A Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ. Heart Fail 3, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Rengo F, Marchionni N, Sancarlo D, Fontana A, Panza F, Ferrucci L, FIRI-SIGG Study Group (2012) Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS ONE 7, e29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P (2005) Endocrine and inflammatory markers as predictors of frailty. Clin. Endocrinol. 63, 403–411. [DOI] [PubMed] [Google Scholar]
- Reichlin S (1993) Neuroendocrine-immune interactions. N. Engl. J. Med 329, 1246–1253. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A, Song X, Steen B, Skoog I (2006) Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J. Am. Geriatr. Soc 54, 975–979. [DOI] [PubMed] [Google Scholar]
- Ross R (1999) Atherosclerosis–an inflammatory disease. N. Engl. J. Med 340, 115–126. [DOI] [PubMed] [Google Scholar]
- Sancarlo D, Pilotto A, Panza F, Copetti M, Longo MG, D’Ambrosio P, D’Onofrio G, Ferrucci L, Pilotto A (2012) A Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short- and long-term all-cause mortality in older hospitalized patients with transient ischemic attack. J. Neurol 259, 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siontis GC, Tzoulaki I, Ioannidis JP (2011) Predicting death: an empirical evaluation of predictive tools for mortality. Arch. Intern. Med 171, 1721–1726. [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, Cardiovascular Health Study (2002) Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch. Intern. Med 162, 2333–2341. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL (1988) The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C Thomas Publisher. [Google Scholar]
- Willcox BJ, He Q, Chen R, Yano K, Masaki KH, Grove JS, Donlon TA, Willcox DC, Curb JD (2006) Midlife risk factors and healthy survival in men. JAMA 296, 2343–2350. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med 12, 1005–1015. [DOI] [PubMed] [Google Scholar]
- Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK (2012) Prognostic indices for older adults: a systematic review. JAMA 307, 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]