Klein et al. (1) report that global antibiotic consumption increased by 65% over the 2000–2015 period, with a 114% increase in low- and middle-income countries (LMICs). The authors conclude that global antibiotic consumption must be decreased to reduce the threat of antibiotic resistance, although reduction efforts must take into account the limitations in antibiotic access in LMICs and consider local and global resistance patterns.
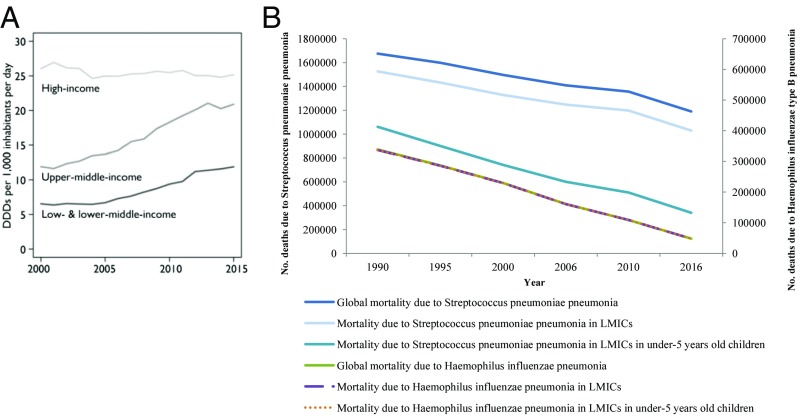
Considering the data from the 2016 Global Burden of Disease study (2), the worldwide mortality attributable to infectious agents dramatically decreased from ∼13 million deaths in 1990 to ∼10 million deaths in 2016. This decrease was mainly driven by the yearly decline of mortality caused by antibiotic-susceptible bacterial infections, including lower respiratory tract infections (LRTIs) (from ∼3.1 million to ∼2.4 million between 1990 and 2016, −23%), especially in LMICs (from ∼2.8 million to ∼2 million, −30%) (Fig. 1) (2, 3). More specifically, the mortality associated with pneumonia due to streptococcal and Haemophilus influenzae type B infections decreased, respectively, from ∼1.7 million to ∼1.2 million deaths (−29%) and from ∼339,000 to ∼48,000 deaths (−86%). These reductions can almost entirely be explained by the decreases in mortality observed in LMICs for the two species: from ∼1.5 million to ∼1 million deaths (−32%) for Streptococcus pneumoniae pneumonia and from ∼337,000 to ∼47,900 deaths (−86%) for H. influenzae type B pneumonia, (Fig. 1) (2, 3). In these countries, the yearly mortality of pneumococcal pneumonia declined by 68% (from ∼1.1 million to ∼340,000 deaths) in children younger than 5 y, while the decline of the mortality due to H. influenzae explained almost entirely the decrease in mortality in the aforementioned LMICs (from ∼337,000 to ∼47,900 deaths) (3).
Fig. 1.
(A and B) Yearly evolution of the mortality attributable to streptococcal and H. influenzae type B pneumonia, 1990–2016. Data were collected from the Global Burden of Disease Study 2016 (3). (A) Reprinted with permission from ref. 1. DDDPC, defined daily doses per capita.
Interestingly, first-line antibiotics used to treat community-acquired pneumonia are still in vitro and clinically efficient to treat pneumonia due to these important bacterial killers (4–6). Therefore, considering the fact that antibiotics are among the discoveries that have most contributed to the worldwide decrease in infectious diseases mortality (7), especially LTRIs (8), we think that the results of Klein et al. (1) are encouraging, as these increases mainly happened in LMICs, which are the countries the most affected by infectious diseases (2, 3). The burden of antibiotic consumption in LMICs paralleled that of life expectancy. However, to what extent can the decline in mortality due to infectious disease in LMICs be attributed to the increased use of antibiotics? A recent study may provide an answer. Among children aged 1–59 mo in sub-Saharan Africa, childhood mortality was reduced by 13.5% in communities randomly assigned to twice-yearly mass distribution of azithromycin (9). Therefore, a balanced ethical approach toward optimized antibiotic use in Africa should consider both the potential effect on the overall reduction of mortality and the possibility of antibiotic resistance. The future of antibiotic resistance and of new compounds remains unpredictable, and the worst is not inevitable. Additionally, we have seen in recent years the incredible efficiency of antibiotic dissemination. As quoted by Harlan Coben in a Yiddish proverb “Man plans, God laughs” (10).
Footnotes
The authors declare no conflict of interest.
References
- 1.Klein EY, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghavi M, et al. GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Study 2017 Global Burden of Disease Study 2016 (GBD 2016) Results (Institute for Health Metrics and Evaluation, Seattle). Available at ghdx.healthdata.org/gbd-results-tool. Accessed January 6, 2018.
- 4.Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20:368–389. doi: 10.1128/CMR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant GB, et al. World Health Organization Department of Child and Adolescent Health and Development Recommendations for treatment of childhood non-severe pneumonia. Lancet Infect Dis. 2009;9:185–196. doi: 10.1016/S1473-3099(09)70044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diene SM, Abat C, Rolain JM, Raoult D. How artificial is the antibiotic resistance definition? Lancet Infect Dis. 2017;17:690. doi: 10.1016/S1473-3099(17)30338-9. [DOI] [PubMed] [Google Scholar]
- 7.Rolain J-M, Abat C, Jimeno M-T, Fournier P-E, Raoult D. Do we need new antibiotics? Clin Microbiol Infect. 2016;22:408–415. doi: 10.1016/j.cmi.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Laxminarayan R, et al. Access to effective antimicrobials: A worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 9.Keenan JD, et al. MORDOR Study Group Azithromycin to reduce childhood mortality in sub-Saharan Africa. N Engl J Med. 2018;378:1583–1592. doi: 10.1056/NEJMoa1715474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coben H. Shelter. G. P. Putnam’s Sons; New York: 2011. [Google Scholar]