Abstract
Background
Currently while, topical minoxidil and oral finasteride are the only medications approved in androgenetic alopecia (AGA), the cause oriented treatment and immunsupressive treatment are being performed in telogen effluvium (TE) and alopecia areata (AA) respectively. Considering the inflammatory factors in the pathogenesis of these three nonscarring alopecia forms, we have formulated a mixture for topical usage composed of six different herbal extracts (HE) which have already known antiinflammatory and antioxidant features.
Materials and Methods
In addition to performing the phytochemical analysis of HE, we detected the gene expression level of IL-1α, the crucial hair loss mediator, for the putative efficacy in nonscarring alopecia. Cell proliferation assay was performed by XTT reagent. After determination of non-cytotoxic concentration, HaCaT cells were treated with HE. RNA isolations were carried out from both non-treated and treated cell groups by using TRI-reagent. Gene expressions of IL-1α and as control GAPDH were determined by RT-qPCR analysis.
Results
Results were represented as “IL-1α/GAPDH Fold Change”. HE solution caused statistically significant downregulation of IL-1α gene expressions (p<0.0001), compared to untreated control cells. HE treatment ended up with 0.1900 fold change for IL-1α.
Conclusion
IL-1α is a direct growth inhibitory agent in hair follicles and an important actor in the pathogenesis of AGA , TE, and AA. Considering together the vitamins, flavonoids, and trace elements identified in the phytochemical analyses and downregulation of IL-1α in HaCaT cells, our HE may be an auxiliary agent in the therapy of these three nonscarring alopecia forms.
Keywords: cosmeceuticals, herbal, topical, alopecia
1. INTRODUCTION
Although hair loss do not detorierate general health, it may constitute a ruined self confidence. Alopecic individuals generally have more frequent psychiatric disorders such as depression, anxiety or social phobia compared to whole society (1).
Typically men are affected by androgenetic alopecia (AGA), but it is a widespread dermatological problem affecting women also. Androgens have a significant role probably independent from genetic predisposition which is known as the main etiologic factor in AGA (2). 5α-reductase (5α-R) transforms testosterone to its more potent form dihydrotestosterone (DHT) in the AGA prone scalp which reveals augmented androgen receptor (AR) expression (3). In addition to the androgenic interaction, inflammatory processes are increasingly being emphasized as an inseparable part in the pathogenesis of AGA (3-7). In AGA, scalp biopsies taken from both men and women exposed follicular microinflammation and lymphocytic folliculitis, referring an immunological precipitating ground (6,7). This continuous inflammation of follicles and remodeling of the connective tissue lead to permanent hair loss in AGA (3).
Another frequent cause of diffuse hair loss is telogen effluvium (TE). Although it may be one of the manifestations of various chronic diseases; a connection between stress and hair loss is a general approval among clinicians (8,9). It was demonstrated the sensitivity of human hair follicles to key skin stress mediators: Organ cultured hair follicles responded to substance P by premature catagen development and degranulation of mast cells in the connective tissue sheath of hair follicles, indicating a neurogenic inflammation (10). Also, it was defined acute TE due to oxidative stress induced by ultraviolet radiation. Photoactivation of porphyrin compounds which were produced by the bacteria in the pilosebaceous duct, leaded to oxidative tissue injury and follicular microinflammation (11). It was demonstrated the immune status of murine skin changed substantially during hair follicle cycling, with increased immuno responses connected with telogen skin, while anagen skin was relatively immunoinhibited (12).
AA is admitted as an inflammatory disease already (13-17). The serum levels of interleukin-1 alpha (IL-1α) were found significantly elevated in AA patients with the localized form (15). It is a T cell dominated autoimmune disease of the hair follicle and histologically featured by focal inflammatory lesions with perifollicular T cell infiltrates (16,17).
Among the cytokines studied for inflammation in alopecia, IL-1α has been demonstrated as a direct growth inhibitory agent in hair follicles (4, 13,14,16). Currently while, topical minoxidil and oral finasteride are the only medications approved in AGA, the cause oriented treatment and immunsupressive treatment is being performed in TE and AA respectively. Considering the inflammatory factors in the pathogenesis explained above, we have formulated a mixture for topical usage composed of six different herbal extracts (HE) which have already known antiinflammatory and antioxidant features. Our hypothesis was if the mixture significantly decreases the activity of the major actor in the site of action in hair loss, it might also be effective in clinical manner. Therefore we detected the gene expression level of IL-1α, the crucial hair loss mediator, for the activity in the three nonscarring alopecia forms that are AGA, TE and AA. Here we studied with human keratinocyte cell line (HaCaT cells) both because the ectodermal keratinocytes are among the functional components of the hair follicle and they are also the major producers of IL-1α (4, 18, 19).
2. MATERIALS AND METHODS
Preparation of Herbal Extract
HE was prepared from six plants: Urtica Dioica Root Extract, Urtica Urens Leaf Extract, Equisetum Arvense Leaf Extract, Achillea Millefolium Aerial Part Extract, Matricaria Chamomilla Flower Extract and Ceratonia Siliqua Fruit Extract. These plants were bought from Martin Bauer. Dried plants were fine-cut. 40 g of plant mixture was extracted with 500 mL distilled water for 3 hours at 100°C using soxhlet extraction. The extract was filtered through a filter paper into a sterile bottle.
Phytochemical Analyses of Herbal Extract
Vitamin and flavonoid analyses were performed with ‘high pressure liquid chromatography’ (HPLC) in Phytolab, Vestenbergsgreuth, Germany. Trace elements analyses were performed with ‘inductively coupled plasma optical emission spectrometry’ (ICP-OES) in Saniter Lab., Istanbul, Turkey.
Cell Culture
HaCaT cells used in this study were in vitro spontaneously transformed keratinocytes from histologically normal skin. The cells were cultured in Dulbecco’s Modified Eagle’s medium with high glucose, supplemented with 10% heat-inactivated fetal bovine serum and 100 U/ml gentamicin. The cells were maintained at 37°C in a humidified atmosphere at 5% CO2 in Newbrunswick incubator. All supplements and media were purchased from Sigma Aldrich.
Cell Proliferation Assay
HaCaT cells were seeded into 96-well plates (1x104cells/well) and were subjected to different concentrations (100%, 10%, 5%, 3%, 1%, 0.2% and 0%) of HE solution to assess the cell proliferation. XTT and activator reagents (Roche) were added to the plates after 72 hour incubation period according to the manufacturer’s instructions. Then, cells were incubated at 37°C for 4 hours in order that XTT reagent was reduced to orange formazan compound. The optical density of soluble formazan compound was measured at 495 nm by microplate reader (Bio-Rad).
Ribonucleic Acid (RNA) Isolation and Reverse Transcription
Total RNA was extracted from cells treated with HE solution and from untreated cells by using TRI-reagent (an RNA, DNA and protein isolation reagent) according to manufacturer’s (Sigma Aldrich) instructions. The concentration and purity of isolated RNA samples were determined by measuring optical densities at 260 nm and 280 nm using BioSpec-nano. ‘’Transcriptor First Strand cDNA Synthesis Kit (Roche)’’ was used for reverse transcription. Complementary deoxyribonucleic acid (cDNA) synthesis was performed with 500 ng total RNA; 2 µM each final concentration of gene specific primers of IL-1α and GAPDH (Integrated DNA Technologies); 10 U of ‘’Transcriptor Reverse Transcriptase’’; 20 U of ‘’Protector RNase Inhibitor’’; 1mM each of ‘’dNTP mix’’ and ‘’Transcriptor Reverse Transcription Buffer (5X)’’ according to the manufacturer’s (Roche) instructions. Primer sequences (5’-3’) are given in Table 1.
Table 1. Primers (5’–3’) of the genes studied § Glyceraldehyde-3-phosphate dehydrogenase used as control.
| Primers | Forward primer | Reverse primer |
|---|---|---|
| IL-1α | ACCAGTGCTGCTGAAGGAGAT | GTGCCGTGAGTTTCCCAGAA |
| GAPDH § | ATGGGTGTGAACCATGAGAA | GTGCTAAGCAGTTGGTGGTG |
Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
RT-qPCR was carried out in ‘’Light Cycler 96 (Roche)’’. Amplification of products were detected via intercalation of the fluorescent dye SYBR green (Fast Start DNA Green Master Kit, Roche). Briefly, total volume of reaction mix was 20 µl; containing 10 µl ‘’SYBR Green Master Mix (2X)’’, 0.5 µM of reverse and forward primers, 2.5 ng cDNA and appropriate amount of nuclease free water. All samples were run as triplicates in each run including a non-template control and four standards (1:1, 1:10, 1:100, 1:1000). The PCR parameters were determined separately for each target according to melting and annealing temperatures of primers. Each parameter included a pre-incubation step for 10 minutes at 95°C and followed by 45 cycles of three amplification and melting step. Melting curve analysis was performed to verify specificity. For quantitation of RT-qPCR results, ΔΔCt method was used (2-ΔΔCt).
Statistical Analysis
All data are representative of three repeats (n = 3) and expressed as mean ± standard error of the means (SEM). Statistical evaluation was performed by unpaired t-test, using ‘’Graph Pad Prism 5 Software (USA)’’ and the results with p value less than 0.05 were accepted as significant.
2. RESULTS
Phytochemical Analyses (HPLC and ICP-OES)
The vitamins, flavonoids and trace elements found in phytochemical analyses of the herbal extract are depicted in Table 2. The quantities of all are expressed as mg/100g dry extract.
Table 2. The vitamins, flavonoids and trace elements found in phytochemical analyses of HE.
| mg/100g dry extract | |
|---|---|
| VITAMINS | |
| Thiamine (B1) | 7.4 |
| Riboflavin (B2) | 1.4 |
| Pyridoxine (B6) | 1.2 |
| Ascorbic acid (C) | <0.5 |
| FLAVONOIDS | |
| Myricetin | 1450 |
| Quercetin | 400 |
| Kaempherol | 600 |
| TRACE ELEMENTS | |
| Iron | 53.06 |
| Copper | 0.645 |
| Zinc | 2.048 |
Cytotoxicity Analysis (Cell Proliferation Assay)
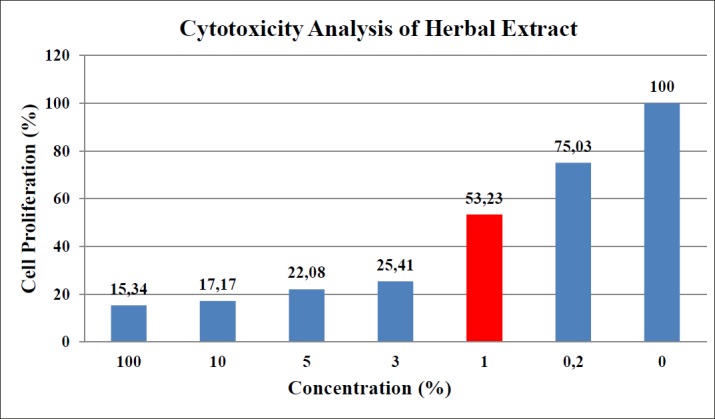
Based on cell proliferation ratios of treated cells with respect to the control cells, cytotoxicity levels of the HE solution were determined. Higher concentrations were found to be cytotoxic for HaCaT cells. For the subsequent analysis, the possible highest concentration was determined as 1% and HaCaT cells were incubated with 1% concentration of HE solution before total RNA isolation (Figure 1)
Figure 1. Cytotoxicity analysis result of HE solution.The values of bars represent the proliferation ratio of HaCaT cells in the relevant concentration of HE. Red bar is the concentration chosen for incubation.
Gene Expression Analysis (RT- qPCR)
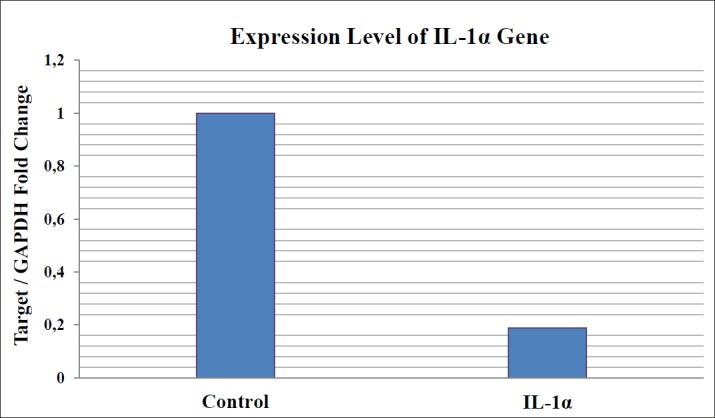
Results were represented as “Target/GAPDH Fold Change”. Results of gene expression analysis via RT-qPCR showed that HE solution caused statistically significant downregulation of IL-1α gene expressions (p<0.0001), compared to untreated control cells. HE treatment ended up with 0.1900 fold change for IL-1α (Figure 2).
Figure 2. Expression level of IL-1α gene compared to control.
3. DISCUSSION
The pilo-sebaceous unit is a ground of various cell and tissue interactions, involving epithelial cells, sebaceous gland, dermal papilla fibroblasts, melanocytes, endothelial cells and the immune system Langerhans’ cells (20). Although the mesenchyme derived papilla regulates the epithelial follicle in many aspects, intrinsic dermal-epidermal interactions are central to the development and growth of hair. Besides the inductive powers of dermal papilla cells, germinative epidermal cells of the lower follicle also can stimulate hair growth (21, 22). Together with the fact that the keratinocytes are the major producers of the inflammatory cytokine IL-1α (18, 19), HaCaT cells seem the best target to measure the effect of our HE in hair follicle.
Among the plants used in our formulation Urtica dioica (Ud) is the most widely studied one. Symptomatic benign prostate hyperplasia (BPH) is the best researched indication of this plant which is mainly due to its 5α-R inhibition activity (23, 24, 25). Inhibition of 5α-R precludes the conversion of testosterone to dihydrotestosterone (DHT) high levels of which are associated with BPH (26). The same pathogenesis is also valid for AGA (3,4). Although Ud leaves have traditionally been used for hair loss, confirmatory clinical trials are still lacking (26). In a study performed with a combination of herbal extracts including Ud, the combination was found to increase the proliferation of human dermal papilla cells significantly at concentrations ranging from 1.5% to 4.5% (27). Ud contains β sitosterol which stimulates angiogenesis by increasing vascular endothelial growth factor (VEGF) synthesis and supports new hair growth (28).
The leave extract of Urtica urens (Uu) contains a high amount of total phenolics, flavonoids, tannins, ortho-diphenols and flavonols (29,30). It decreased the paw oedema after carrageenin administration in rats, and enhanced the activities of catalase, superoxide dismutase, glutathione peroxidase, and malondialdehyde exhibiting a powerful antiinflammatory and antioxidant activity (30).
In a study performed with Equisetum arvense (Ea) alone and with combination of some other plants, Ea suppressed the superoxide anion levels in the xanthine/xanthine oxidase system and abolished the hydroxil radical. Ea also reduced reactive oxygen species (ROS) levels in phorbol myristate acetate stimulated neutrophils (31). Ea is one of the highest silicon accumulators among plant species. Silicon penetrates the hair follicles, enters in the hair matrix and makes the hair fibers thicker. Higher silicon content in the hair leads to a decrease in the rate of hair loss (32,33).
Leaves and flowers of Achillea millefolium (Am) have been used for centuries because of their antiinflammatory properties in some problems such as rheumatism, wound healing and skin inflammation (34). In an in vivo study performed with Am oil extract on artificially irritaded skin, the tested parameters (i.e pH, capacitance and erythema index) were restored to basal values after 3-7 days treatment (35). Am extract showed an improved expression profile of cytokeratin-10, transglutaminase-1 and filaggrin in cultured skin biopsies as well as an increase in epidermal thickness. Also in vivo a two month treatment with 2% Am extract significantly improved the appearance of wrinkles and pores compared to placebo (36).
Traditionally Matricaria chamomilla (Mc, German chamomile) oil has been used for the treatment of inflammatory skin disorders such as eczema (37). In a study performed with 3% Mc oil topically on the skin of mice showing its immunoregulatory potential, it alleviated the atopic dermatitis through influencing helper T cell 2 (Th2) activation (38). A pharmacologically active flavonoid apigenin which is contained by both Mc and Am, supports hair growth by suppressing transforming growth factor-β1 (TGF-β1) which stimulates the catagen phase in hair growth cycle (39,40).
Ceratonia siliqua (Cs) pod extract exhibits antioxidant properties due to the presence of catechin, epicatechin, epigallocatechin, epigallocatechin gallate, and epicatechin gallate, along with simpler phenolics, such as phloroglucinol, pyrogallol, catechol, and gallic acid. The high antioxidant level of Cs pod extract suggesting its potential development as a pharmaceutical product (41).
The phenolic compounds, vitamins and trace elements we identified in the phytochemical analysis of our formulation, may also give more specific support to the maintenance of healthy hair. Myricetin, quercetin, kaempherol and copper keep the hair follicle longer in anagen phase by inhibiting 5α-R and preventing DHT formation (42,43). Vitamin C (ascorbic acid), improves blood vessel formation and increases blood flow in the scalp by stimulating the synthesis of VEGF (44). Vitamin C might also improve the efficacy of therapeutic angiogenesis by cell transplantation (45). Also the derivatives of vitamin C are found hopeful in the treatment and prevention of DHT induced balding (46). It was demonstrated that high doses of environmental cigarette smoke induce alopecia in mice and this effect was prevented by administration of a mixture of l-cystine with vitamin B6 (pyridoxine) (47). Combinations of l-cystine and B vitamins are traditionally used in OTC products for the treatment of hair loss. Supplementation with l-cystine, pantothenic acid and vitamin B1 (thiamin) has been shown to increase the anagen rate in apparently healthy women with TE in a placebo-controlled study (48). Vitamin B2 (riboflavin) is known to enhance the metabolism of vitamin B6 (49). Zinc is found in the enzyme systems affecting hair formation and local zinc ions stimulate scalp cellular formation (44). Zinc metabolism disturbances play a key role in hair loss, especially in AGA and TE (50). Iron is a vital co-factor for proteins and enzymes involved in energy metabolism. Historically, it has long been known that iron is essential for healthy skin, mucous membranes, hair and nails. Iron deficiency causes dry, thin and fragile scalp hair clinically (51).
Apart from the phytochemical features explained above, the most significant result of this study is the inhibition of IL-1α which is a direct growth inhibitory agent in hair follicles and an important actor in the pathogenesis of AGA , TE, and AA (4,14-16,52). Besides the obvious role of androgens in AGA(2,3,16), the three clinical entities AGA, TE, and AA may gather in a common ground as they all involve hair follicles, and consist inflammatory mechanisms in their pathogenesis (3,6,7,10,11,16). Normal human epidermis is a rich source of biologically active IL-1α. Keratinocytes both synthesize this cytokine and respond to it via cell surface receptors, suggesting that the IL-1 system may play an important role in epidermal physiology and inflammation (18,19). Among various cytokines, IL-1 family and especially IL-1α is stated as the main hair growth inhibitor. It is reported that IL-1α exerts antiproliferative effect on hair follicles and causes inhibition of follicle growth as a secondary response (4, 13-16). Previously, due to the inhibitory role of IL-1α on human hair growth, it was suggested that identifying the ‘’inflammatory alopecic individual’’ might be of clinical importance to determine the individuals for whom anti-IL-1 strategies might be effective in AGA (4). Elements of the IL-1 signaling system show hair cycle dependent gene expression in murine skin. In murine skin IL-1α and IL-1β levels increased dramatically with the onset of spontaneous catagen and peaked during telogen (52). It is also reported the downregulation of IL-1α during anagen phase of hair cycle (53,54). Therefore we suggest this IL-1α suppression feature of our formulation may be beneficial for all three nonscarring alopecia forms because of the inflammatory components discussed in their pathogenesis.
4. CONCLUSION
Regarding together; the benefits cited in the literature; vitamins, flavonoids, and trace elements identified in the phytochemical analyses we performed, and downregulation of IL-1α in the gene expression analysis in HaCaT cells, our HE may be considered as a promising auxiliary agent in the therapy of AGA, TE and AA.
Conflict of interest
none declared.
REFERENCES
- 1.Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005;331:951–53. doi: 10.1136/bmj.331.7522.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010;11(8):1295–304. doi: 10.1517/14656561003752730. [DOI] [PubMed] [Google Scholar]
- 3.Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37(8-9):981–90. doi: 10.1016/s0531-5565(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 4.Mahé YF, Buan B, Billoni N, Loussouarn G, Michelet JF, Gautier B, Bernard BA. Pro-inflammatory cytokine cascade in human plucked hair. Skin Pharmacol. 1996;9(6):336–75. doi: 10.1159/000211447. [DOI] [PubMed] [Google Scholar]
- 5.Hirsso P, Rajala U, Hiltunen L, Jokelainen J, Keinanen-Kiukaanniemi S, Näyhä S. Obesity and low grade inflammation among young Finnish men with early onset alopecia. Dermatology. 2007;214(2):125–9. doi: 10.1159/000098570. [DOI] [PubMed] [Google Scholar]
- 6.El-Domyati M, Attia S, Saleh F, Abdel-Wahab H. Androgenetic alopecia in males: a histopathological and ultrastructural study. J Cosmet Dermatol. 2009;8(2):83–91. doi: 10.1111/j.1473-2165.2009.00439.x. [DOI] [PubMed] [Google Scholar]
- 7.Magro CM, Rossi A, Manhas-Bhutani S, Sadick N. The role of inflammation and immunity in the pathogenesis of androgenetic alopecia. J Drugs Dermatol. 2011;10(12):1404–11. [PubMed] [Google Scholar]
- 8.Arck PC, Handjiski B, Peters EM, Peter AS, Hagen E, Fischer A, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162(3):803–14. doi: 10.1016/S0002-9440(10)63877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malkud S. A hospital based study to determine causes of diffuse hair loss in women. J Clin Diagn Res. 2015;9(8):WC01–4. doi: 10.7860/JCDR/2015/14089.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters EM, Liotiri S, Bodo E, Hagen E, Biro T, Arck PC, et al. Probing the effects of stress mediators on the human hair follicle. Am J Pathol. 2007;171(6):1872–86. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trüeb RM. Is androgenetic alopecia a photoaggravated dermatosis? Dermatology. 2003;207(4):343–8. doi: 10.1159/000074111. [DOI] [PubMed] [Google Scholar]
- 12.Geyfman M, Plikus MV, Treffeisen E, Andersen B, Paus R. Resting no more: re-defining telogen, the maintenance stage of the hair growth cycle. Biol Rev Camb Philos Soc. 2015;90(4):1179–96. doi: 10.1111/brv.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon CS, Nevins TD. IL-1? inhibits human hair follicle growth and hair fiber production in whole organ cultures. Lymphokine Cytokine Res. 1993;12(4):197–203. [PubMed] [Google Scholar]
- 14.Philpott MP, Sanders DA, Bowen J, Kealey T. Effects of interleukins, colony stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135(6):942–8. doi: 10.1046/j.1365-2133.1996.d01-1099.x. [DOI] [PubMed] [Google Scholar]
- 15.Teraki Y, Imanishi K, Shiohara T. Cytokines in alopecia areata: Contrasting cytokine profiles in localised form and extensive form (alopecia universalis) Acta Derm Venereol. 1996;76(6):421–3. doi: 10.2340/0001555576421423. [DOI] [PubMed] [Google Scholar]
- 16.Tazi-Ahnini R, McDonagh AJ, Cox A, Messenger AG, Britton JE, Ward SJ, et al. Association analysis of IL-1? and IL-1? variants in alopecia areata. Heredity. 2001;87(2):215–9. doi: 10.1046/j.1365-2540.2001.00916.x. [DOI] [PubMed] [Google Scholar]
- 17.Garzorz N, Alsisi M, Todorova A, Atenhan A, Thomas J, Lauffer F, et al. Dissecting susceptibility from exogeneous triggers: the model of alopecia areata and associated inflammatory skin diseases. J Eur Acad Dematol Venereol. 2015;29(12):2429–35. doi: 10.1111/jdv.13325. [DOI] [PubMed] [Google Scholar]
- 18.Growes RW, Sherman L, Mizutani H, Dower SK, Kupper TS. Detection of interleukin-1 receptors in human epidermis. AJP. 1994;145(5):1048–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Growes RW, Mizutani H, Keiffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin-1? in basal epidermis. Proc Natl Acad Sci. 1995;92:11874–8. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard BA. [Molecular approach of hair biology] C R Seances Soc Biol Fil. 1994;188(3):223–33. [PubMed] [Google Scholar]
- 21.Cranwell W, Sinclair R. Male androgenetic alopecia. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc.; 2016. Feb 29, 2000. [Google Scholar]
- 22.Jahoda CA, Reynolds AJ. Dermal-epidermal interactions. Adult follicle derived cell populations and hair growth. Dermatol Clin. 1996;14(4):573–83. doi: 10.1016/s0733-8635(05)70385-5. [DOI] [PubMed] [Google Scholar]
- 23.Nahata A, Dixit VK. Ameliorative effects of stinging netle (Urtica dioica) on testosterone induced prostatic hyperplasia in rats. Andrologia. 2012;44(S1):396–409. doi: 10.1111/j.1439-0272.2011.01197.x. [DOI] [PubMed] [Google Scholar]
- 24.Lichius JJ, Lenz C, Lindemann P, Müller HH, Aumüller G, Konrad L. Antiproliferative effect of a polysaccharide fraction of a 20% methanolic extract of stinging nettle roots upon epithelial cells of the human prostate (LNCaP) Pharmazie. 1999;54(10):768–71. [PubMed] [Google Scholar]
- 25.Nahata A, Dixit VK. Evaluation of 5?-reductase inhibitory activity of certain herbs useful as androgens. Andrologia. 2014;46(6):592–601. doi: 10.1111/and.12115. [DOI] [PubMed] [Google Scholar]
- 26.Monograph. Altern Med Rev. 2007;12(3):280–4. Urtica dioica; Urtica urens (nettle) [PubMed] [Google Scholar]
- 27.Rastegar H, Ashtiani HA, Aghaei M, Barikbin B, Ehsani A. Herbal extracts induce dermal papilla cell proliferation of human hair follicles. Ann Dermatol. 2015;27(6):667–75. doi: 10.5021/ad.2015.27.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeidnia S, Manayi A, Gohari AR, Abdollahi M. The story of beta-sitosterol-a review. European J Med Plants. 2014;4(5):590–609. [Google Scholar]
- 29.Mzid M, Ben Khedir S, Ben Salem M, Regaieg W, Rebai T. Antioxidant and antimicrobial activities of ethanol and aqueous extracts from Urtica urens. Pharm Biol. 2017;55(1):775–81. doi: 10.1080/13880209.2016.1275025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mzid M, Ben Khedir S, Bardaa S, Sahnoun Z, Rebai T. Chemical composition, phytochemical constituents, antioxidant and anti-inflammatory activities of Urtica urens L leaves. Arch Physiol Biochem. 2017;123(2):93–104. doi: 10.1080/13813455.2016.1255899. [DOI] [PubMed] [Google Scholar]
- 31.Oka M, Tachibana M, Noda K, Inoue N, Tanaka M, Kuwabara K. Relevance of anti-reactive oxygen species activity to anti-inflammatory activity of components of eviprostat, a phytotherapeutic agent for benign prostatic hyperplasia. Pytomedicine. 2007;14(7-8):465–72. doi: 10.1016/j.phymed.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Vivancos J, Deshmukh R, Grégoire C, Rémus-Borel W, Belzile F, Bélanger RR. Identification and characterization of silicon efflux transporters in horsetail (Equisetum arvense) J Plant Physiol. 2016;200:82–9. doi: 10.1016/j.jplph.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Araújo LA, Addor F, Campos PM. Use of silicon for skin and hair care: an approach of chemical forms available and efficacy. An Bras Dermatol. 2016;91(3):331–5. doi: 10.1590/abd1806-4841.20163986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghobadian Z, Ahmadi MR, Rezazadeh L, Hosseini E, Kokhazadeh T, Ghavam S. In vitro evaluation of Achillea millefolium on the production and stimulation of human skin fibroblast cells (HFS-PI-16) Med Arch. 2015;69(4):212–7. doi: 10.5455/medarh.2015.69.212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tadić V, Arsić I, Zvezdanović J, Zugić A, Cvetković D, Pavkov S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflammatory potential in topical application. J Ethnopharmacol. 2017;199:138–48. doi: 10.1016/j.jep.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Pain S, Altobelli C, Boher A, Cittadini L, Favre-Mercuret M, Gaillard C, et al. Surface rejuvenating effect of Achillea millefolium extract. Int J Cosmet Sci. 2011;33(6):535–42. doi: 10.1111/j.1468-2494.2011.00667.x. [DOI] [PubMed] [Google Scholar]
- 37.Carle R, Gomaa K. The medicinal use of matricaria flos. Br J Phytother. 1992;2:147–53. [Google Scholar]
- 38.Lee SH, Heo Y, Kim YC. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J Vet Sci. 2010;11(1):35–41. doi: 10.4142/jvs.2010.11.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jesionek W, Móricz ÁM, Ott PG, Kocsis B, Horváth G, Choma IM. TLC-direct bioautography and LC/MS as complementary methods in identification of antibacterial agents in plant tinctures from the Asteraceae family. J AOAC Int. 2015;98(4):857–61. doi: 10.5740/jaoacint.SGE2-Choma. [DOI] [PubMed] [Google Scholar]
- 40.Huh S, Lee J, Jung E, Kim SC, Kang JI, Lee J, et al. A cell-based system for screening hair growth-promoting agents. Arch Dermatol Res. 2009;301(5):381–5. doi: 10.1007/s00403-009-0931-0. [DOI] [PubMed] [Google Scholar]
- 41.Karim AA, Azlan A. Fruit pod extracts as a source of nutraceuticals and pharmaceuticals. Molecules. 2012;17(10):11931–46. doi: 10.3390/molecules171011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiipakka RA, Zhang HZ, Dai W, Dai Q, Liao S. Structure-activity relationships for inhibition of human 5 alpha-reductases by poliphenols. Biochem Pharmacol. 2002;63(6):1165–76. doi: 10.1016/s0006-2952(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto Y, Lopez Solache I, Labrie F, Luu-The V. Cations inhibit specifically type I 5?-reductase found in human skin. J Invest Dermatol. 1995;104(5):775–8. doi: 10.1111/1523-1747.ep12606985. [DOI] [PubMed] [Google Scholar]
- 44.Semalty M, Semalty A, Joshi GP, Rawat MS. Hair growth and rejuvenation: an overview. J Dermatolog Treat. 2011;22(3):123–32. doi: 10.3109/09546630903578574. [DOI] [PubMed] [Google Scholar]
- 45.Takeshita Y, Katsuki Y, Katsuda Y, Kai H, Saito Y, Arima K, et al. Vitamin C reversed malfunction of peripheral blood-derived mononuclear cells in smokers through antioxidant properties. Circ J. 2008;72(4):654–9. doi: 10.1253/circj.72.654. [DOI] [PubMed] [Google Scholar]
- 46.Kwack MH, Kim MK, Kim JC, Sung YK. L-ascorbic acid 2-phosphate represses the dihydrotestosterone-induced dickkopf-1 expression in human balding dermal papilla cells. Exp Dermatol. 2010;19(12):1110–2. doi: 10.1111/j.1600-0625.2010.01143.x. [DOI] [PubMed] [Google Scholar]
- 47.D’Agostini F, Fiallo P, Pennisi TM, De Flora S. Chemoprevention of smoke-induced alopecia in mice by oral administration of L-cystine and vitamin B6. J Dermatol Sci. 2007;46(3):189–98. doi: 10.1016/j.jdermsci.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Lengg N, Heidecker B, Seifert B, Trüeb RM. Dietary supplement increases anagen hair rate in women with telogen effluvium: Results of a double-blind placebo-controlled trial. Therapy. 2007;4:59–65. [Google Scholar]
- 49.Kodentsova VM, Vrzhesinskaia OA, Sokol’nikov AA, Beketova NA, Spirichev VB. [The effect of riboflavin supply on metabolism of water-soluble vitamins] Vopr Med Khim. 1993;39(5):29–33. (in Russian) [PubMed] [Google Scholar]
- 50.Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol. 2013;25(4):405–9. doi: 10.5021/ad.2013.25.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright JA, Richards T, Srai SK. The role of iron in the skin and cutaneous wound healing. Front Pharmacol. 2014;5:156. doi: 10.3389/fphar.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann R, Happle R, Paus R. Elements of the interleukin-1 signalling system show hair cycle dependent gene expression in murine skin. Eur J Dermatol. 1998;8(7):475–7. [PubMed] [Google Scholar]
- 53.Boivin WA, Jiang H, Utting OB, Hunt DW. Influence of interleukin-1alpha on androgen receptor expression and cytokine secretion by cultured human dermal papilla cells. Exp Dermatol. 2006;15(10):784–93. doi: 10.1111/j.1600-0625.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 54.Hamada K, Hirotsu S, Uchiwa H, Yamazaki S, Suzuki K. Pro-inflammatory cytokine interleukin-1alpha is downregulated during anagen phase of hair cycle in vivo. J Dermatol Sci. 2003;33(3):195–8. doi: 10.1016/j.jdermsci.2003.08.001. [DOI] [PubMed] [Google Scholar]


