Abstract
Despite their popularity, little research has been performed on lightly cooked and raw diet formats for pets. Therefore, the objective of this study was to determine the apparent total-tract macronutrient digestibility (ATTD); fecal characteristics, metabolites, and microbiota; serum chemistry metabolites; urinalysis; and voluntary physical activity levels of adult dogs fed commercial diets differing in processing type. The diets included: 1) extruded dry kibble (EXT) diet; 2) high-moisture roasted refrigerated (RR) diet; 3) high-moisture grain-free roasted refrigerated (GFRR) diet; and 4) raw (RAW) diet. Eight dogs (mean age = 3.6; mean BW = 13.0 kg) were used in a replicated 4 × 4 Latin square design. Each period consisted of 28 d, with a 14-d adaptation phase followed by a 7-d phase for measuring voluntary physical activity, 1-d adaptation phase to metabolic cages, 5-d phase for fecal and urine collection, and 1 d for blood collection. Except for microbiota, all data were analyzed statistically by mixed models using SAS. Microbiota data were analyzed using Quantitative Insights Into Microbial Ecology (QIIME) and Statistical Analyses of Metagenomic Profiles (STAMP) software. Many differences in digestibility were observed, including greater (P < 0.05) ATTD of CP and fat in dogs fed GFRR and RR than dogs fed EXT. Dogs fed RAW had the lowest fecal pH and DM %, but fecal scores were not affected. Dogs fed RR had higher (P < 0.05) fecal indole and total phenol and indole concentrations than dogs fed the other diets. Dogs fed RAW had a higher (P < 0.05) fecal ammonia concentration than dogs fed the other diets. Fecal microbial diversity was altered by diet, with dogs fed GFRR and RAW having reduced species richness than dogs fed EXT. Dogs fed RR, GFRR, or RAW had lower (P < 0.05) Actinobacteria and higher (P < 0.05) Fusobacteria than dogs fed EXT. Dogs fed RAW or GFRR had higher (P < 0.05) Proteobacteria than dogs fed EXT or RR. Dogs fed RAW had higher (P < 0.05) Bacteroidetes and lower (P < 0.05) Firmicutes than dogs fed EXT. Serum triglycerides were within reference ranges, but greater (P < 0.05) in dogs fed EXT than dogs fed GFRR and RAW. All diets were well tolerated and dogs remained healthy throughout the study. In conclusion, the lightly cooked and raw diets tested were highly palatable, highly digestible, reduced blood triglycerides, maintained fecal quality and serum chemistry, and modified the fecal microbial community of healthy adult dogs.
Keywords: canine nutrition, diet processing, gastrointestinal microbiome, nutrient digestibility
INTRODUCTION
The relationship between humans and their pets has dramatically changed in recent years. What started out as a symbiotic relationship focused largely on hunting, protection, and various forms of work has evolved into companionship for most owners today, with a majority considering pets as important members of the family. Because of the emotional bond people have with their pets, the pet product industry has realized steady growth over the past decade, with continued increases in revenue from pet foods and snacks, supplies and medicine, veterinary care, and other pet services (e.g., grooming, boarding). In 2016, the U.S. pet product industry reached a revenue of $66.8 billion, with a large portion of it coming from pet food sales ($28.2 billion; APPA, 2017).
Early diets were primarily focused on providing all essential nutrients in a uniform format, most often in an extruded or retorted format (Aldrich, 2006). Extrusion, which uses heat, moisture, and pressure to cook food, has been used to process pet foods for several decades. Extrusion is a popular way to produce dry foods because it is a high-throughput, adaptable, and efficient technology that improves protein and starch digestibility and has positive effects on the stool quality of pets (Singh et al., 2007). Although extrusion and retort technologies still remain the most popular pet food processing methods, the sales of novel diet formats have increased.
Many new pet food formats, including fresh (refrigerated), raw, dehydrated, and freeze-dried have emerged and had significant growth in recent years (Beaton, 2014; Wall, 2018). Although there is evidence that raw diets have a higher digestibility than that of typical dry kibble diets (Beloshapka et al., 2012; Kerr et al., 2012; Bermingham et al., 2017) and wet foods may increase physical activity of pets (Deng et al., 2014), the popularity of these diet formats has probably more to do with anthropomorphism than scientific evidence. Regardless, if there is published evidence supporting their use, consumers are interested in diets that look and smell more similar to their own food, contain natural, organic, or functional ingredients, and/or that comes in convenient packaging.
Unfortunately, little research has been performed on the novel pet food formats mentioned above. Therefore, the objective of this study was to determine the apparent total-tract macronutrient digestibility (ATTD); fecal characteristics, metabolites, and microbiota; serum chemistry metabolites; urinalysis; and voluntary physical activity levels of adult dogs fed commercial diets differing in processing type. We hypothesized that a raw diet would have greater ATTD compared to mildly cooked diets and the mildly cooked diets would have greater ATTD than an extruded kibble diet. We also hypothesized that there would be no negative effects on fecal characteristics, metabolites, and microbiota; urinalysis; or serum chemistry profile. Lastly, we hypothesized that the raw and mildly cooked diets would produce greater voluntary physical activity levels than the low moisture kibble diet.
MATERIALS AND METHODS
All animal care procedures were approved by the University of Illinois Institutional Animal Care and Use Committee prior to animal experimentation (#15231).
Animals
Eight adult female beagles (mean age: 3.57 ± 0.29) were used in this study. Dogs were weighed (mean baseline BW: 13.0 ± 0.84) once a week prior to the 0800 h feeding during the adaptation phase and on the first and last days of the sample collection phase of each experimental period. During the first 21 d of each experimental period, dogs were housed individually in runs (1.0 × 2.1 × 1.8 m). From days 22 to 28, dogs were housed individually in stainless steel cages (0.9 × 0.9 × 0.8 m). Dogs were fed twice a day (0800 h; 1700 h) and had access to fresh water at all times.
Treatments
All dietary treatments were commercial diets formulated to meet all Association of American Feed Control Officials’ (AAFCO; 2016) nutrient recommendations for adult dogs. The treatments were as follows: 1) extruded dry kibble (EXT) diet (Purina Dog Chow; Nestle Purina PetCare Company, St. Louis, MO); 2) high-moisture roasted refrigerated (RR) diet (Freshpet Roasted Meals; Freshpet, Bethlehem, PA); 3) high-moisture grain-free roasted refrigerated (GFRR) diet (Freshpet Vital Balanced Complete Nutrition; Freshpet); and 4) raw (RAW) diet (Freshpet Vital Raw; Freshpet) (Table 1).
Table 1.
Analyzed chemical and energy composition of dog diets tested
| Item | Treatment | |||
|---|---|---|---|---|
| Extruded1 | Grain-free roasted refrigerated2 | Raw3 | Roasted refrigerated4 | |
| DM (%) | 93.33 | 38.63 | 47.28 | 42.23 |
| OM (%), DMB5 | 92.47 | 88.58 | 93.23 | 89.51 |
| CP (%), DMB | 24.07 | 45.69 | 25.13 | 31.08 |
| Acid-hydrolyzed fat (%), DMB | 13.30 | 30.30 | 33.90 | 27.82 |
| Total dietary fiber (%), DMB | 9.60 | 7.28 | 6.94 | 11.84 |
| GE5 (kcal/g), as-is | 4.58 | 2.32 | 3.07 | 2.39 |
| GE (kcal/g), DMB | 4.91 | 6.01 | 6.50 | 5.66 |
| ME5 (kcal/g), as-is | 3.02 | 1.63 | 2.15 | 1.77 |
| ME (kcal/g), DMB | 3.24 | 4.22 | 4.55 | 4.19 |
1Extruded: whole grain corn, meat and bone meal, corn gluten meal, animal fat preserved with mixed-tocopherols, soybean meal, poultry by-product meal, egg and chicken flavor, whole grain wheat, animal digest, salt, calcium carbonate, potassium chloride, mono and dicalcium phosphate, choline chloride, L-lysine monohydrochloride, zinc sulfate, yellow 6, vitamin E supplement, copper sulfate, calcium pantothenate, garlic oil, pyridoxine hydrochloride, vitamin B12 supplement, thiamine mononitrate, vitamin D3 supplement, riboflavin supplement, calcium iodate, menadione sodium bisulfite complex (source of vitamin K activity), folic acid, biotin, sodium selenite.
2Grain-free roasted refrigerated: chicken, chicken liver, beef, salmon, eggs, cranberries, spinach, pea protein, natural flavors, minerals (dicalcium phosphate, calcium carbonate, zinc proteinate, iron proteinate, manganese proteinate, copper proteinate, sodium selenite, calcium iodate), pea fiber, vinegar, salt, peas, carrageenan, potassium chloride, inulin, beta-carotene, vitamins (choline chloride, vitamin E supplement, niacin, calcium pantothenate, biotin, riboflavin, thiamine mononitrate, vitamin B12 supplement, vitamin D3 supplement, pyridoxine hydrochloride, folic acid), celery powder.
3Raw: chicken, sweet potatoes, kale, citrus fiber, water, sea salt, dicalcium phosphate, dextrose, celery powder, vitamin and minerals (choline chloride, zinc proteinate, iron proteinate, vitamin E supplement, copper proteinate, manganese proteinate, vitamin A supplement, niacin, calcium pantothenate, biotin, sodium selenite, thiamine mononitrate, riboflavin, vitamin B12 supplement, calcium iodate, vitamin D3 supplement, pyridoxine hydrochloride, folic acid), inulin, dried Pediococcus acidilactici fermentation product, cherry juice powder.
4Roasted refrigerated: chicken, chicken liver, ground oats, carrots, eggs, spinach, rice bran, natural flavors, minerals (dicalcium phosphate, calcium carbonate, potassium chloride, zinc proteinate, iron proteinate, manganese proteinate, copper proteinate, sodium selenite, calcium iodate), salt, vinegar, beta-carotene, vitamins (choline chloride, vitamin E supplement, niacin, calcium pantothenate, biotin, riboflavin, thiamine mononitrate, vitamin B12 supplement, vitamin D3 supplement, pyridoxine hydrochloride, folic acid), celery powder.
5DMB = DM basis; GE = measured by bomb calorimetry; ME = measured by GE − fecal energy − urinary energy.
In regards to processing, the mildly cooked diets, RR and GFRR, first had protein sources (meats) ground and emulsified and mixed into a homogenous blend with dry ingredients such as the pea protein, vitamin mix, and mineral mix. The blend then was formed into small meatball sized chunks that were pasteurized and chilled at approximately 4 °C. Once cooled, the chunks were mixed with whole vegetable pieces, packaged with gas flush and stored under refrigeration (1–4 °C) until used. The raw diet also had meat components ground and emulsified and mixed with the fiber source, vegetables, vitamin mix, mineral mix, and Pediococcus acidilactici fermentation product. After the log was incubated at room temperature (29–38 °C), it was chilled at approximately 4 °C. The diet was packaged with gas flush and refrigerated (1–4 °C) until used. Once the refrigerated packages are opened, shelf life is 7 d.
Because the dietary treatments tested were very different in terms of macronutrient composition and format, dogs were slowly adapted to new dietary treatments at the beginning of each experimental period to avoid gastrointestinal distress. The following feeding protocol was used in each experimental period: days 1–3: 75% kcal from prior dietary treatment + 25% kcal from new dietary treatment; days 4–6: 50% kcal from prior dietary treatment + 50% kcal from new dietary treatment; days 7–10: 25% kcal from prior dietary treatment + 75% kcal from new dietary treatment; and days 10–28: 100% kcal from new dietary treatment.
Experimental Design and Timeline
The study used a replicated 4 × 4 Latin square design. Using this design, each dog received all four treatments over the course of the experiment, serving as their own control and increasing statistical power (n = 8/treatment). The experiment was composed of four, 28-d periods, with each consisting of a 14-d adaptation phase, a 7-d phase for measuring voluntary physical activity using activity monitors, a 1-d adaptation to metabolic cages, a 5-d total fecal and urine collection phase, and 1 d for blood collection.
Fecal Sample Collection and Scoring
During the fecal collection phase, total fecal samples were collected, weighed, and scored using the following scale: 1 = hard, dry pellets, small hard mass; 2 = hard, formed, dry stool; remains firm and soft; 3 = soft, formed, and moist stool, retains shape; 4 = soft, unformed stool, assumes shape of container; and 5 = watery, liquid that can be poured. Samples were then frozen at −20 °C until further analysis. Fresh fecal samples were collected within 15 min of defecation. Fecal pH was measured immediately using an AP10 pH meter (Denver Instrument, Bohemia, NY) equipped with a Beckman Electrode (Beckman Instruments Inc., Fullerton, CA), and then aliquots were collected. Aliquots for phenol and indole measurement were frozen and stored at −20 °C until analysis. An aliquot for ammonia, short-chain fatty acid (SCFA), and branched-chain fatty acid (BCFA) measurement was placed in 2 N HCl and stored at −20 °C until analysis. An aliquot was collected for fecal DM determination. Finally, aliquots for microbiota measurement were transferred to sterile cryogenic vials (Nalgene, Rochester, NY), frozen on dry ice, and stored at −80 °C until analysis.
Urine Collection
During the urine collection phase, total urine output was measured. A fresh urine sample was collected for measurement of pH using an AP10 pH meter (Denver Instrument) equipped with a Beckman Electrode (Beckman Instruments Inc.) and specific gravity and total protein. Specific gravity was measured by the University of Illinois Veterinary Medicine Diagnostics Laboratory using a refractometer (Leica TS Meter Refractometer, Leica Microsystems Inc., Buffalo, NY). Fresh samples were collected into sterile cryogenic vials (Nalgene) and stored at 4 °C until analysis. Total urine samples were collected into vessels containing 2 N hydrochloric acid for immediate acidification upon urination to prevent loss of nitrogen. Acidified urine samples were subsampled (25% of each sample) and stored at −20 °C until analysis.
Blood Sample Collection
On day 28, 5 mL of blood was collected for serum metabolite concentrations and complete blood count via jugular and/or cephalic venipuncture. Samples were transferred immediately to appropriate vacutainer tubes [#367841 BD Vacutainer Plus plastic whole blood tube (lavender) with K2EDTA additive; #367974 BD Vacutainer Plus plastic serum tube (red/gray) with clot activator and gel for serum separation; BD, Franklin Lakes, NJ]. Red/gray tubes were centrifuged at 1,200 × g for 10 min at 4 °C for serum collection. Samples were then transported to the University of Illinois Veterinary Medicine Diagnostics Laboratory for serum chemistry and complete blood count analysis using Hitachi 911 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN).
Physical Activity
On days 15–21 (0800–0800 h), voluntary physical activity was evaluated using activity monitors (Actical monitors; Mini Mitter, Bend, OR), which were placed on collars and worn around dogs’ necks. Commercial software (Mini Mitter) analyzed the data compiled by the monitor and was expressed as activity counts per epoch (epoch length = 15 s). Values represent the mean epoch activity count over the 7-d measurement period during light hours (0700–2000 h), dark hours (2000–0700 h), and an average of daily activity. Dogs wore the same monitor throughout all four periods to minimize variability. Human interaction was limited as much as possible during the activity measurement week so that voluntary activity was not disrupted. Data were most variable during feeding times (0800 and 1700 h) and sanitary maintenance that occurred between 1200 h and 1400 h each day.
Chemical Analyses
To avoid nutrient degradation, high-moisture dietary treatments were lyophilized using a corrosion resistant Dura-Dry MP (FTS Systems; Stone Ridge, NY). Fecal samples were dried at 55 °C in a forced-air oven. All dried dietary treatments and feces were ground in a Wiley mill (model 4, Thomas Scientific, Swedesboro, NJ) through a 2-mm screen. Diet and fecal samples were analyzed for DM, OM, and ash according to Association of Official Analytical Chemists (AOAC, 2006; methods 934.01 and 942.05). Crude protein of diets, feces, and urine were determined by Leco Nitrogen/Protein Determinator (FP-2000, Leco Corp., St. Joseph, MI) total nitrogen values according to AOAC (2006; method 992.15). Total lipid content (acid-hydrolyzed fat; AHF) was determined according to the methods of the American Association of Cereal Chemists (AACC, 1983) and Budde (1952). Total dietary fiber was determined according to Prosky et al. (1992). Gross energy of dietary, fecal, and urine samples were measured using an oxygen bomb calorimeter (model 1261; Parr Instruments; Moline, IL).
Fecal SCFA and BCFA concentrations were determined by gas chromatography according to Erwin et al. (1961) using a gas chromatograph (Hewlett-Packard 5890A series II, Palo Alto, CA) and a glass column (180 cm × 4 mm i.d.) packed with 10% SP-1200/1% H3PO4 on 80/100+ mesh Chromosorb WAW (Supelco Inc., Bellefonte, PA). Nitrogen was the carrier with a flow rate of 75 mL/min. Oven, detector, and injector temperatures were 125, 175, and 180 °C, respectively. Fecal ammonia concentrations were determined according to the method of Chaney and Marbach (1962). Fecal phenol and indole concentrations were determined using gas chromatography according to the methods described by Flickinger et al. (2003).
Calculations
Apparent total-tract digestibility values were calculated using the equation as follows: [nutrient intake (g/d) − fecal output (g/d)/nutrient intake (g/d)] × 100. Dietary ME was calculated by the following equation: [GE intake (kcal/d) − fecal GE (kcal/d) − urinary GE (kcal/d)]/DMI (g/d). Data normality was checked using the univariate procedure and Shapiro–Wilk statistic, with log transformation being used when normal distributions were lacking.
Fecal DNA Extraction, Amplification, and Sequencing
Total DNA from fecal samples was extracted using Mo-Bio PowerSoil kits (MO BIO Laboratories, Inc., Carlsbad, CA). Concentration of extracted DNA was quantified using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY). 16S rRNA gene amplicons were generated using a Fluidigm Access Array (Fluidigm Corporation, South San Francisco, CA) in combination with Roche High Fidelity Fast Start Kit (Roche Diagnostics). The primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) that target a 252 bp-fragment of V4 region were used for amplification (primers synthesized by IDT Corp., Coralville, IA; Caporaso et al., 2012). CS1 forward tag and CS2 reverse tag were added according to the Fluidigm protocol. Quality of the amplicons was assessed using a Fragment Analyzer (Advanced Analytics, Ames, IA) to confirm amplicon regions and sizes. A DNA pool was generated by combining equimolar amounts of the amplicons from each sample. The pooled samples were then size selected on a 2% agarose E-gel (Life technologies) and extracted using a Qiagen gel purification kit (Qiagen, Valencia, CA). Cleaned size-selected pooled products were run on an Agilent Bioanalyzer to confirm appropriate profile and average size. Illumina sequencing was performed on a MiSeq using v3 reagents (Illumina Inc., San Diego, CA) at the W. M. Keck Center for Biotechnology at the University of Illinois.
Bioinformatics and Statistical Analyses for Assessing Fecal Microbial Communities
Forward reads were trimmed using the FASTX-Toolkit (version 0.0.13), and QIIME 1.9.1 (Caporaso et al., 2010) was used to process the resulting sequence data. Briefly, high-quality (quality value ≥ 20) sequence data derived from the sequencing process were demultiplexed. Sequences then were clustered into operational taxonomic units (OTU) using UCLUST (Edgar, 2010) through a closed-reference OTU picking strategy against the Greengenes 13_8 reference database (DeSantis et al., 2006) with a 97% similarity threshold. Singletons (OTU that were observed fewer than two times) and OTU that had less than 0.01% of the total observation were discarded. Taxonomic identity to each OTU was then assigned using UCLUST. A total of 795,339 16S rRNA-based amplicon sequences were obtained, with an average of 24,854 reads (range = 10,846–68,008) per sample. An even sampling depth (sequences per sample) of 10,846 sequences per sample was used for assessing alpha- and beta-diversity measures. Beta-diversity was calculated using weighted and unweighted UniFrac (Lozupone and Knight, 2005) distance measures. Statistical analysis was conducted via Statistical Analyses of Metagenomic Profiles (STAMP) software 2.1.3 (Parks et al., 2014) using ANOVA and Tukey–Kramer multiple comparison tests. All tests were corrected for multiple inferences using the Benjamini–Hochberg method to control for false discovery rate. Statistical significance was set at P < 0.05.
Statistical Analysis
Except for microbiota, all data were analyzed using SAS (version 9.4, SAS Institute, Cary, NC) using the Mixed Models procedure with dietary treatment being the fixed effect and animal being the random effect. Data normality was checked using the univariate procedure and Shapiro–Wilk statistic, with log transformation being used when normal distribution was lacking. When a main effect was significant, post hoc pairwise comparisons were performed using Tukey’s multiple comparison tests. Data were reported as means ± SEM with P < 0.05 considered significant.
RESULTS
The ingredient composition, chemical composition, and energy content of the experimental treatments are presented in Table 1. The experimental diets were dramatically different in terms of ingredient and chemical composition. EXT had the highest DM percentage and was much higher compared to RR, GFRR, and RAW that were all similar to one another. On a DM basis (DMB), the diets had similar OM percentages; however, GFRR had a higher CP concentration (DMB) compared to the other diets. EXT had a lower fat concentration (DMB) than the other three diets. Total dietary fiber (DMB) content was greater in RAW than in EXT, RR, and GFRR. Due to the low moisture content, as-is energy density was greater for EXT than RR, GFRR, and RAW. On a DMB, however, GFRR, RR, and RAW had a greater energy density than EXT.
Food intake, fecal characteristics, and ATTD of macronutrients and energy of dogs are presented in Table 2. As-is food intake (g/d) was greater (P < 0.05) in dogs fed RR than dogs fed GFRR or EXT, but not RAW. The DM and OM intake (g/d) of dogs fed RAW or RR was greater (P < 0.05) than those fed GFRR. The CP intake (g/d) of dogs fed GFRR was greater (P < 0.05) than those fed EXT or RAW, while CP intake (g/d) of dogs fed RR was greater (P < 0.05) than those fed EXT. The fat intake (g/d) of dogs fed RAW was greater (P < 0.05) than dogs fed the other diets. Dogs fed RR or GFRR had greater (P < 0.05) fat intake than dogs fed EXT. The caloric intake (kcal/d) of dogs fed RAW was greater (P < 0.05) than those fed EXT or GFRR.
Table 2.
Food intake, fecal characteristics, and total-tract apparent macronutrient and energy digestibility of dogs fed extruded, mildly cooked, and raw foods
| Item | Treatment | |||
|---|---|---|---|---|
| Extruded | Grain-free roasted refrigerated | Raw | Roasted refrigerated | |
| Food intake | ||||
| g food/d (as-is) | 176.3 ± 26.98c | 342.0 ± 26.98b | 391.3 ± 26.98ab | 426.0 ± 28.30a |
| g DM/d | 164.5 ± 14.20ab | 132.1 ± 14.20b | 185.0 ± 14.20a | 179.7 ± 14.90a |
| g OM/d | 152.1 ± 12.99ab | 117.0 ± 12.99b | 172.5 ± 12.99a | 160.8 ± 13.60a |
| g CP/d | 39.6 ± 4.09c | 60.4 ± 4.09a | 46.5 ± 4.09bc | 55.8 ± 4.28ab |
| g fat/d | 21.9 ± 3.55c | 40.0 ± 3.55b | 62.7 ± 3.55a | 49.9 ± 3.73b |
| kcal/d | 806.9 ± 79.63b | 794.5 ± 79.63b | 1202.7 ± 79.63a | 1015.7 ± 83.46ab |
| Fecal output | ||||
| Fecal output, as-is (g/d) | 84.3 ± 11.83ab | 52.3 ± 11.83b | 101.6 ± 11.83a | 77.6 ± 12.62ab |
| Fecal output, DM (g/d) | 29.4 ± 3.54 | 19.9 ± 3.54 | 29.6 ± 3.54 | 28.8 ± 3.76 |
| As-is fecal output (g/d)/DMI (g/d) | 0.48 ± 0.04 | 0.39 ± 0.04 | 0.55 ± 0.04 | 0.44 ± 0.05 |
| Nutrient and energy digestibility | ||||
| DM (%) | 82.6 ± 1.52 | 85.1 ± 1.52 | 83.6 ± 1.52 | 84.1 ± 1.63 |
| OM (%) | 87.8 ± 1.21 | 89.9 ± 1.21 | 86.2 ± 1.21 | 89.1 ± 1.29 |
| CP (%) | 85.1 ± 1.02c | 94.6 ± 1.02a | 88.3 ± 1.02bc | 92.0 ± 1.09ab |
| Fat (%) | 92.1 ± 0.38c | 97.2 ± 0.38ab | 97.5 ± 0.38a | 95.8 ± 0.41b |
| Energy (%) | 87.4 ± 0.93b | 92.7 ± 0.93a | 90.8 ± 0.93ab | 90.7 ± 1.00ab |
a–cMeans in the same row without common superscript letters differ (P < 0.05).
Fecal output (as-is basis) was greater (P < 0.05) in dogs fed RAW than those fed GFRR. The ATTD of CP was greater (P < 0.05) in dogs fed GFRR than those fed EXT or RAW and dogs fed RR had a greater (P < 0.05) CP ATTD than dogs fed EXT. The ATTD of fat was greater (P < 0.05) in dogs fed RAW than those fed EXT or RR. The ATTD of fat was also greater (P < 0.05) in dogs fed GFRR or RR was greater (P < 0.05) than those fed EXT. The ATTD of energy was greater (P < 0.05) in dogs fed GFRR than those fed EXT. Fecal output (DMB), the ratio of as-is fecal output and DMI, and ATTD of DM and OM were not different among treatments.
Fecal and urine characteristics of dogs are presented in Table 3. Fecal pH of dogs fed GFRR was greater (P < 0.05) than those fed RAW. Fecal DM percentage was greater (P < 0.05) in dogs fed EXT, GFRR, or RR than those fed RAW. Fecal acetate concentrations were greater (P < 0.05) in dogs fed RAW than dogs fed RR. Fecal indole and total phenol and indole concentrations were greater (P < 0.05) in dogs fed RR than those fed the EXT, GFRR, or RAW. Fecal ammonia concentrations were greater (P < 0.05) in dogs fed RAW than those fed all other diets, and greater (P < 0.05) in dogs fed RR than those fed EXT.
Table 3.
Fecal and urine characteristics of dogs fed extruded, mildly cooked, and raw foods
| Item | Treatment | |||
|---|---|---|---|---|
| Extruded | Grain-free roasted refrigerated | Raw | Roasted refrigerated | |
| Fecal characteristics | ||||
| pH | 6.22 ± 0.18ab | 6.78 ± 0.18a | 6.15 ± 0.18b | 6.59 ± 0.18ab |
| Fecal score1 | 2.40 ± 0.13 | 2.21 ± 0.13 | 2.08 ± 0.13 | 2.40 ± 0.13 |
| Fecal DM% | 37.16 ± 2.19a | 37.68 ± 2.19a | 28.82 ± 2.19b | 36.58 ± 2.34a |
| Fecal metabolites | ||||
| Acetate (µmol/g DMB) | 257.52 ± 24.18ab | 225.65 ± 24.18ab | 312.09 ± 24.18a | 214.52 ± 24.18b |
| Propionate (µmol/g DMB) | 116.00 ± 15.93 | 106.16 ± 15.93 | 127.33 ± 15.93 | 129.14 ± 15.93 |
| Butyrate (µmol/g DMB) | 43.59 ± 8.10 | 45.90 ± 8.10 | 44.64 ± 8.10 | 58.17 ± 8.10 |
| Total SCFA2 (µmol/g DMB) | 417.11 ± 38.44 | 377.71 ± 38.44 | 484.07 ± 38.44 | 401.83 ± 38.44 |
| Isobutyrate (µmol/g DMB) | 5.33 ± 0.70 | 5.93 ± 0.70 | 4.19 ± 0.70 | 6.68 ± 0.70 |
| Isovalerate (µmol/g DMB) | 9.66 ± 1.12 | 8.76 ± 1.12 | 6.19 ± 1.12 | 9.84 ± 1.12 |
| Valerate (µmol/g DMB) | 0.73 ± 0.11 | 0.51 ± 0.11 | 0.55 ± 0.11 | 0.83 ± 0.11 |
| Total BCFA2 (µmol/g DMB) | 15.72 ± 1.81 | 15.21 ± 1.81 | 10.94 ± 1.81 | 17.35 ± 1.81 |
| Phenol (µmol/g DMB) | 0.32 ± 0.23 | 0.51 ± 0.23 | 0.50 ± 0.23 | 1.09 ± 0.23 |
| Indole (µmol/g DMB) | 1.18 ± 0.21b | 1.10 ± 0.21b | 0.97 ± 0.21b | 2.26 ± 0.21a |
| Total P/I2 (µmol/g DMB) | 1.50 ± 0.39b | 1.60 ± 0.39b | 1.47 ± 0.39b | 3.35 ± 0.39a |
| Ammonia (µmol/g DMB) | 26.06 ± 4.23c | 36.26 ± 4.23bc | 61.43 ± 4.23a | 44.18 ± 4.23b |
| Urine characteristics | ||||
| Specific gravity | 1.042 ± 0.00 | 1.045 ± 0.00 | 1.042 ± 0.00 | 1.039 ± 0.00 |
| pH | 6.49 ± 0.16 | 6.56 ± 0.16 | 5.97 ± 0.16 | 6.44 ± 0.16 |
| Total protein | 1.19 ± 0.28 | 0.88 ± 0.28 | 0.81 ± 0.28 | 0.81 ± 0.28 |
BCFA = branched-chain fatty acid; DMB = DM basis.
1Fecal scores: 1 = hard, dry pellets; small hard mass; 2 = hard formed, dry stool; remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool; assumes shape of container; 5 = watery, liquid that can be poured.
2Total SCFA = acetate + propionate + butyrate; Total BCFA = valerate + isovalerate + isobutyrate. Total P/I = phenol + indole.
a–cMeans in the same row without common superscript letters differ (P < 0.05).
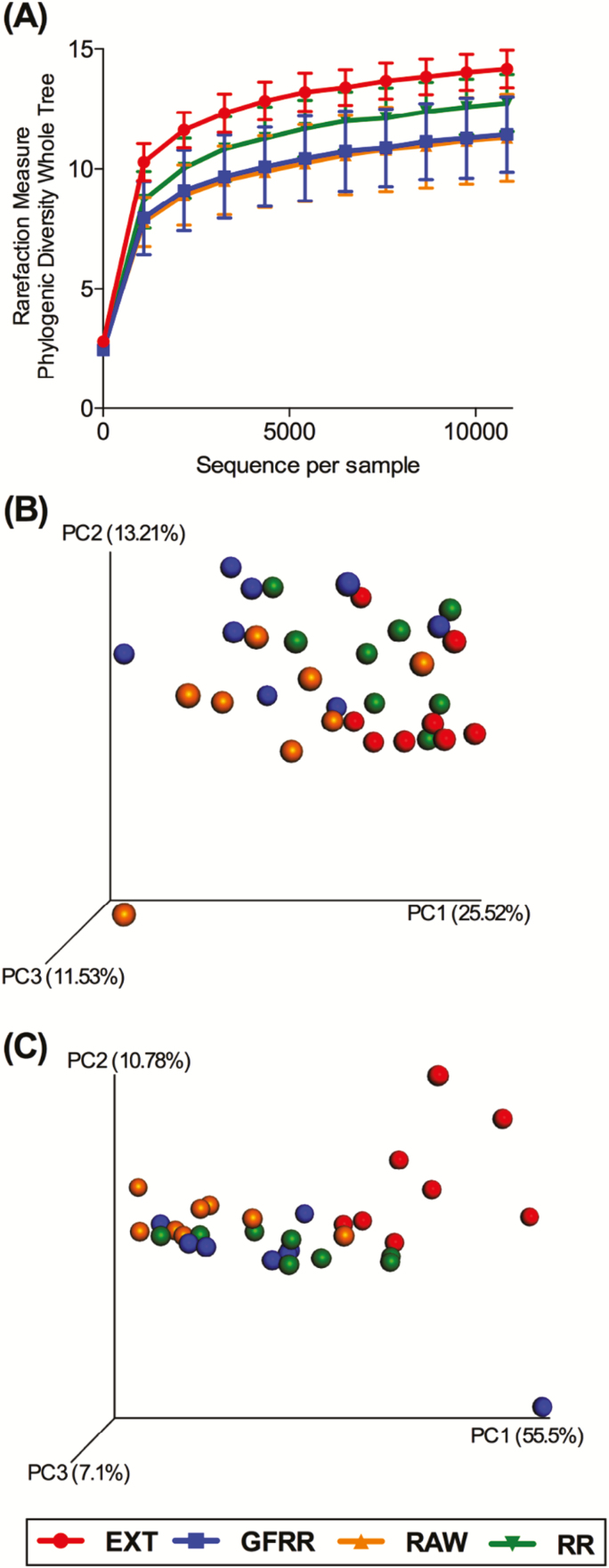
Fecal microbial communities were altered by dietary treatments (Figs 1–3). Alpha diversity measures suggested that species richness was lower (P < 0.05) in dogs fed GFRR and RAW than dogs fed EXT (Fig. 1). Beta-diversity measures, which are indicative of species richness among samples, are represented with principal coordinates analysis plots (Fig. 1). Unweighted UniFrac distances, which measure the presence or absence of microbial taxa, of fecal microbial communities revealed a tendency (P = 0.07) for dogs fed GFRR or RAW to differ from dogs fed EXT. Weighted UniFrac distances, which measure the presence and abundance of microbial taxa, of fecal communities revealed a tendency (P = 0.07) for dogs fed RAW or RR to differ from dogs fed EXT.
Figure 1.
Fecal microbial communities of dogs fed extruded, mildly cooked, and raw diets. (A) Alpha diversity measures suggested that species richness was lower (P < 0.05) in dogs fed the high-moisture grain-free roasted refrigerated (GFRR) diet or raw (RAW) diet than dogs fed the extruded dry kibble (EXT) diet. Principal coordinates analysis plots of unweighted (B) UniFrac distances of fecal microbial communities performed on the 97% OTU abundance matrix tended to reveal a separation (P = 0.07) between dogs fed GFRR or RAW from dogs fed EXT. Principal coordinates analysis plots of weighted (C) UniFrac distances of fecal microbial communities performed on the 97% OTU abundance matrix tended to reveal a separation (P = 0.07) between dogs fed the high-moisture roasted refrigerated (RR) diet or RAW from dogs fed EXT. Each dot represents a sample collected from each dog (n = 8/treatment). OTU = operational taxonomic units.
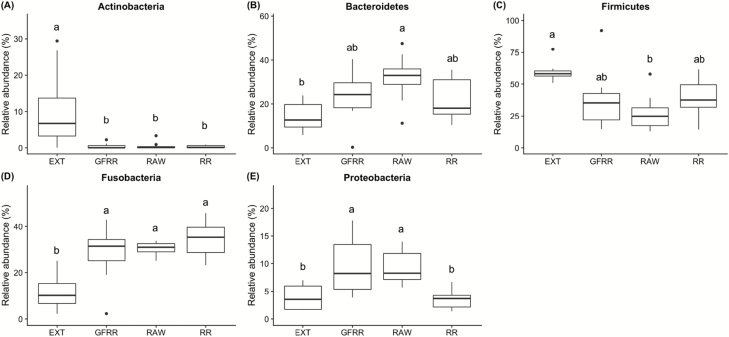
Figure 2.
Relative abundances of differentially abundant microbial phyla (P < 0.05) in feces of dogs fed an extruded dry kibble (EXT), high-moisture roasted refrigerated (RR), high-moisture grain-free roasted refrigerated (GFRR), or raw (RAW) diet (n = 8/treatment). False discovery rate corrected P values using the Benjamini–Hochberg method were calculated using Statistical Analyses of Metagenomic Profiles (STAMP) software, using ANOVA and a Tukey adjustment. Each box represents the 25% and 75% percentiles; error bars indicate 95% confidence interval of median.
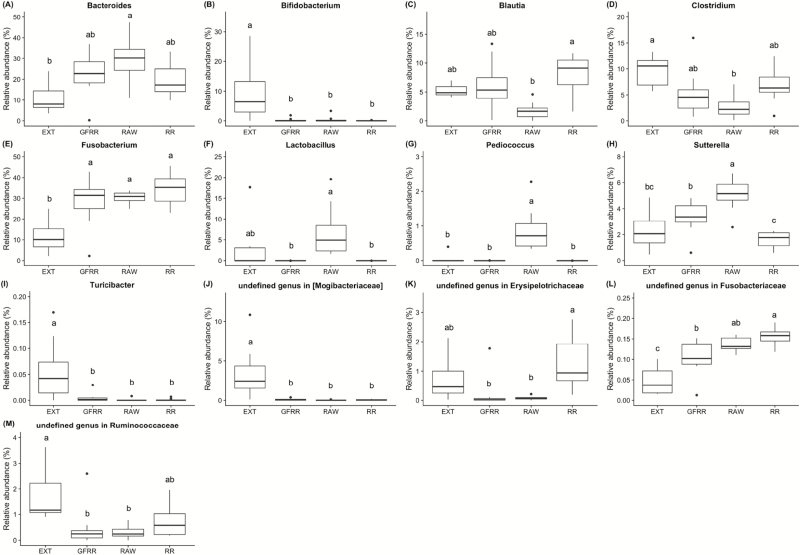
Figure 3.
Relative abundances of differentially abundant microbial genera (P < 0.05) in feces of dogs fed an extruded dry kibble (EXT), high-moisture roasted refrigerated (RR), high-moisture grain-free roasted refrigerated (GFRR), or raw (RAW) diet (n = 8/treatment). False discovery rate corrected P values using the Benjamini–Hochberg method were calculated using Statistical Analyses of Metagenomic Profiles (STAMP) software, using ANOVA and a Tukey adjustment. Each box represents the 25% and 75% percentiles; error bars indicate 95% confidence interval of median.
Five fecal bacterial phyla were altered by dietary treatment (Fig. 2). Specifically, dogs fed RR, GFRR, or RAW had lower (P < 0.05) fecal Actinobacteria relative abundance and higher (P < 0.05) fecal Fusobacteria relative abundance than dogs fed EXT. Dogs fed RAW or GFRR had higher (P < 0.05) fecal Proteobacteria relative abundance than dogs fed EXT or RR. Dogs fed RAW had higher (P < 0.05) fecal Bacteroidetes relative abundance and lower (P < 0.05) fecal Firmicutes relative abundance than dogs fed EXT.
Thirteen fecal bacterial genera were altered by dietary treatment (Fig. 3). Specifically, dogs fed RR, GFRR, or RAW had lower (P < 0.05) relative abundance of fecal Bifidobacterium, Turicibacter, and an undefined genus in [Mogibacteriaceae] and higher (P < 0.05) relative abundance of fecal Fusobacterium than dogs fed EXT. Dogs fed RAW had higher (P < 0.05) fecal Bacteroides relative abundance than dogs fed EXT, higher (P < 0.05) fecal Lactobacillus relative abundance than dogs fed GFRR or RR, higher (P < 0.05) fecal Pediococcus relative abundance than dogs fed EXT, GFRR, or RR, and lower (P < 0.05) fecal Clostridium relative abundance than dogs fed EXT. Dogs fed RAW also had higher (P < 0.05) fecal Sutterella relative abundance than dogs fed all other dietary treatments, and dogs fed GFRR had higher (P < 0.05) fecal Sutterella relative abundance than dogs fed RR. Dogs fed RR had higher (P < 0.05) fecal Blautia relative abundance than dogs fed RAW and higher (P < 0.05) relative abundance of an undefined genus in Erysipelotrichaceae in feces than dogs fed GFRR or RAW. Dogs fed RR also had higher (P < 0.05) relative abundance of an undefined genus in Fusobacteriaceae in feces than dogs fed GFRR or EXT, and dogs fed GFRR had higher undefined genus in Fusobacteriaceae in feces than dogs fed EXT. Dogs fed GFRR or RAW had lower (P < 0.05) relative abundance of an undefined genus in Ruminococcaceae in feces than dogs fed EXT.
Serum metabolites of dogs are presented in Table 4. Serum triglyceride concentrations were greater (P < 0.05) in dogs fed EXT than those fed GFRR or RAW. Serum chloride concentrations of dogs fed RAW were greater (P < 0.05) than those fed EXT. Serum alkaline phosphatase concentrations were greater (P < 0.05) in dogs fed EXT than those fed GFRR or RAW, and greater (P < 0.05) in dogs fed RR than those fed GFRR. All serum metabolites were within ranges except for creatine (0.48 mg/dL), which was just slightly out of range for GFRR (0.5–1.5 mg/dL). Blood cell counts (data not shown) were not different among treatments and were all within reference ranges.
Table 4.
Serum metabolites of dogs fed extruded, mildly cooked, and raw foods
| Item | Treatment | ||||
|---|---|---|---|---|---|
| Extruded | Grain-free roasted refrigerated | Raw | Roasted refrigerated | Reference range1 | |
| Creatinine (mg/dL) | 0.65 ± 0.15 | 0.48 ± 0.15 | 0.60 ± 0.15 | 0.78 ± 0.15 | 0.5–1.5 |
| BUN (mg/dL)2 | 14.88 ± 2.40 | 12.50 ± 2.40 | 11.13 ± 2.40 | 17.50 ± 2.40 | 6–30 |
| Total protein (g/dL) | 6.14 ± 0.17 | 6.34 ± 0.17 | 6.21 ± 0.17 | 6.19 ± 0.17 | 5.1–7.0 |
| Total bilirubin (mg/dL) | 0.18 ± 0.02 | 0.16 ± 0.02 | 0.16 ± 0.02 | 0.18 ± 0.02 | 0.1–0.3 |
| Albumin (g/dL) | 3.28 ± 0.08 | 3.53 ± 0.08 | 3.48 ± 0.08 | 3.39 ± 0.08 | 2.5–3.8 |
| Globulin (g/dL) | 2.86 ± 0.11 | 2.81 ± 0.11 | 2.74 ± 0.11 | 2.80 ± 0.11 | 2.7–4.4 |
| Albumin:globulin ratio | 1.15 ± 0.04 | 1.28 ± 0.04 | 1.28 ± 0.04 | 1.21 ± 0.04 | 0.6–1.1 |
| Ca (mg/dL) | 9.93 ± 0.18 | 9.89 ± 0.18 | 9.85 ± 0.18 | 9.98 ± 0.18 | 7.6–11.4 |
| P (mg/dL) | 4.51 ± 1.80 | 3.94 ± 1.80 | 3.46 ± 1.80 | 4.58 ± 1.80 | 2.7–5.2 |
| Na (mmol/L) | 144.12 ± 0.66 | 144.12 ± 0.66 | 144.50 ± 0.66 | 144.13 ± 0.66 | 141–152 |
| Cl (mmol/L) | 110.63 ± 0.67b | 112.25 ± 0.67ab | 113.50 ± 0.67a | 111.38 ± 0.67ab | 107–118 |
| K (mmol/L) | 4.73 ± 0.13 | 4.65 ± 0.13 | 4.75 ± 0.13 | 4.81 ± 0.13 | 3.9–5.5 |
| Na:K ratio | 30.63 ± 0.88 | 31.00 ± 0.88 | 30.50 ± 0.88 | 30.25 ± 0.88 | 28–36 |
| Bicarbonate (mmol/L) | 20.38 ± 0.79 | 19.38 ± 0.79 | 18.75 ± 0.79 | 18.50 ± 0.79 | 16–24 |
| Anion gap | 18.00 ± 0.96 | 17.38 ± 0.96 | 17.13 ± 0.96 | 19.13 ± 0.96 | 8–25 |
| ALP (U/L)2 | 38.13 ± 0.08a | 12.88 ± 0.08c | 16.00 ± 0.08bc | 29.38 ± 0.08ab | 7–92 |
| C-ALP (U/L)2 | 9.88 ± 2.89 | 2.50 ± 2.89 | 4.00 ± 2.89 | 8.00 ± 2.89 | 0–40 |
| ALT (U/L)2 | 30.50 ± 2.28 | 24.38 ± 2.28 | 25.25 ± 2.28 | 27.13 ± 2.28 | 8–65 |
| GGT (U/L)2 | 2.50 ± 0.38 | 2.63 ± 0.38 | 2.38 ± 0.38 | 1.88 ± 0.38 | 0–7 |
| Glucose (mg/dL) | 91.63 ± 6.29 | 91.38 ± 6.29 | 90.38 ± 6.29 | 99.75 ± 6.29 | 68–126 |
| Cholesterol (mg/dL) | 227.63 ± 19.30 | 224.38 ± 19.30 | 242.38 ± 19.30 | 251.75 ± 19.30 | 129–297 |
| Triglycerides (mg/dL) | 80.13 ± 5.97a | 53.50 ± 5.97b | 53.38 ± 5.97b | 60.25 ± 5.97ab | 32–154 |
1University of Illinois Veterinary Diagnostic Laboratory Reference Ranges.
2BUN = blood urea nitrogen; ALP = alkaline phosphatase; C-ALP = corticosteroid-induced alkaline phosphatase; ALT = alanine transaminase; GGT = gamma-glutamyltransferase.
a–cMeans in the same row without common superscript letters differ (P < 0.05).
Voluntary physical activity data (activity counts/epoch) are presented in Table 5. Activity during the dark period was greater (P < 0.05) in dogs fed EXT than those fed GFRR or RAW. The light:dark activity ratio was greater (P < 0.05) in dogs fed GFRR or RAW than those fed EXT.
Table 5.
Physical activity (activity counts/epoch)1 of dogs fed extruded, mildly cooked, and raw foods
| Item | Treatment | |||
|---|---|---|---|---|
| Extruded | Grain-free roasted refrigerated | Raw | Roasted refrigerated | |
| Total activity | 30.56 ± 3.79 | 26.70 ± 3.72 | 29.58 ± 3.72 | 29.27 ± 3.72 |
| Light period | 43.30 ± 6.27 | 38.68 ± 6.19 | 43.67 ± 6.19 | 43.09 ± 6.19 |
| Dark period | 13.39 ± 1.49a | 10.40 ± 1.46b | 10.21 ± 1.46b | 11.55 ± 1.46ab |
| Light:dark ratio | 3.55 ± 0.79b | 4.39 ± 0.79a | 4.49 ± 0.79a | 4.03 ± 0.79ab |
1Epoch: 15 s.
a,bMeans in the same row without common superscript letters differ (P < 0.05).
DISCUSSION
Pet owners are choosing to feed more premium and super-premium diets, including raw, fresh, and freeze-dried formats. To test the acceptance/palatability and effects on nutrient digestibility, serum metabolites, and fecal characteristics of these diet formats, the current study was conducted. The diets tested not only underwent different processing procedures, but included different ingredients and were formulated to contain different nutrient and energy concentrations. Therefore, differences due to the dietary treatments cannot be attributed to any specific ingredients or nutrient concentrations, but the diets as a whole.
Nutrient digestibility may be affected by many factors, including animal age, differences in ingredient source and form, and processing methods used to prepare the dietary treatments. Published data on raw diets designed for dogs or cats exist (Crissey et al., 1997; Vester et al., 2008, 2010; Beloshapka et al., 2012; Kerr et al., 2012; Bermingham et al., 2017; Kim et al., 2017; Sandri et al., 2017), but mildly cooked dog foods have been poorly studied. The ATTD of CP was lower in the raw diet tested in this study compared to Crissey et al. (1997; 90.26% in exotic cats), Vester et al. (2008; 92.94% in exotic cats), Vester et al. (2010; 91.7% in exotic cats), Beloshapka et al. (2012; raw beef: 91.35%; and raw chicken: 88.35% in dogs), Kerr et al. (2012; 93.3% in domestic cats), and Bermingham et al. (2017; 96.7%–99.2% in dogs), but was still very high for a pet food. The ATTD of fat in the raw diet tested in the current study and those tested in previous studies were similar and ranged from 93.0% to 97.8%.
Mild processing may increase nutrient digestibilities without damaging essential nutrients. Kerr et al. (2012) compared raw vs. cooked beef-based raw diets formulated for cats. In that study, the ATTD of macronutrients were similar in raw and cooked treatments (cooked diet: 92.9% CP and 95.3% fat; raw diet: 93.3 CP% and 95.5% fat) and similar to the mildly cooked diets in the present study. In all cases, the mildly cooked diets were highly digestible. Because ATTD of CP is not a true representation of what the host digests because of microbial metabolism in the hindgut, ileal-cannulated animals, or the cecectomized rooster assay may be used (Faber et al., 2010). A recent study evaluated the macronutrient digestibility and nitrogen-corrected true ME of chicken-based ingredients that had undergone different processing conditions using the precision-fed cecectomized rooster assay (Swanson et al., 2017). The processing conditions tested in that study were similar to those used to produce the mildly cooked diets (RR; GFRR) tested in this study. In that study, chicken meal had lower digestibility of DM (60.0%) and OM (65.9%), but higher AHF digestibility (90.3%) than the raw (DM: 75.9%; OM: 80.5%; AHF: 88.3%), steamed (DM: 76.5%; OM: 80.6%; AHF: 86.5%), and retorted (DM: 73.5%; OM: 77.8%; AHF: 83.5%) ingredients. For all essential and nonessential AA, steamed chicken had the highest digestibilities. For all essential AA and all but one nonessential AA (proline), raw and retorted chicken digestibilities were similar to one another and greater than that of chicken meal.
The diet composition, food intake, and nutrient digestibility may influence fecal output and characteristics, including consistency scores, fermentative end-product concentrations, and microbiota. Fecal scoring is a good measure of fecal quality and is used to evaluate consistency (Hernot, 2005; Nery et al., 2010). In the current study, dogs fed the raw diet had softer stools than dogs fed the other dietary treatments, but all were of acceptable quality. Fecal DM may also be a good measure of fecal quality. In other studies comparing extruded and raw diets (Vester et al., 2010; Kerr et al., 2012), cats had similar fecal DM content. In the present study, however, dogs fed the raw diet had lower fecal DM than those fed the other treatments. Our results are opposite of that of Bermingham et al. (2017), who observed a higher fecal DM in dogs fed a raw-meat diet (43%–57% DM) compared to those fed a kibble diet (31%–36% DM). Diet composition or species differences may be responsible for discordance among studies.
The authors are unaware of any studies conducted in dogs fed mildly cooked foods, but data in cats exist (Vester et al., 2010; Kerr et al., 2012). Kerr et al. (2012) evaluated extruded, cooked, and raw beef-based diets fed to domestic cats. Cats fed the extruded diet had greater fecal output (36.1 g/d as-is; 13.0 g/d DM) and fecal output (g as-is):food intake (g DM) ratio (0.6) compared to those fed the raw (17.6 g/d as-is; 6.7 g/d DM; 0.4 fecal output:food intake ratio) or cooked (17.4 g/d as-is; 7.2 g/d DM; 0.5 fecal output:food intake ratio) diets. This result has also been reported by Vester et al. (2010), who evaluated a high-protein extruded kibble diet with a commercial raw-meat–based diet fed to captive African wildcats. In that study, fecal output and fecal output (g as-is):food intake (g DM) ratio was greater in cats fed the kibble diet (32.0 g/d as-is; 12.9 g/d DM; 0.5 fecal output:food intake ratio) than those fed the raw diet (17.6 g/d as-is; 6.7 g/d DM 0.4 fecal output:food intake ratio). These results are likely due to the higher digestibility of raw diets compared to kibble diets. Although dogs fed the raw diet had a greater fecal output on an as-is basis than dogs fed the GFRR diet in the present study, it is important to note that the fecal output on a DMB or fecal output:food intake ratio was not different among treatments. Because dogs and cats may have different food intakes when eating diets of variable nutrient composition, caloric density, and moisture content, the fecal output:food intake ratio is probably the most appropriate comparison to make in such studies.
Fecal quality may also be evaluated according to the microbial communities present and the concentration of metabolites they produce. Fermentable substrates largely come from dietary carbohydrate and protein sources, but their impact on gut microbiota and metabolite production are quite different. Carbohydrates such as resistant starches, nonstarch polysaccharides, and nondigestible oligosaccharides are fermented by microbes and typically produce SCFA such as acetate, propionate, and butyrate. The SCFA have a number of functions within the gastrointestinal tract, serving as an important energy source to colonocytes, lowering pH to limit gut pathogen growth, and playing an important role in gut peptide synthesis and signaling. In contrast, phenols, indoles, and BCFA are an indication of protein fermentation occurring in the large intestine, contributing to fecal odor and have been associated with gastrointestinal diseases by some (Cummings and MacFarlane, 1991; Macfarlane and Macfarlane, 2012). Recent studies, however, suggest that indole may improve intestinal barrier function (Bansal et al., 2007, 2010; Valenzano et al., 2015). Therefore, greater indole and phenol in diseased states may be due to increased protein coming from blood or mucus entering the intestine in response to damage and not a cause of the condition. Fecal pH often coincides with SCFA and may be a good marker SCFA production. According to Wong et al. (2006), a decreased pH indicates an increase in SCFA production that indirectly influences the composition of colonic microbiota (e.g., acidic pH reduces pathogens).
In the current study, dogs fed the raw diet had a lower fecal pH and higher SCFA concentrations compared to the other dietary treatments. While this may have been due to microbial fermentation of carbohydrates, it may have been due to its original pH. The raw diet tested in this study underwent an acidification process using P. acidilactici, a bacterial taxa used in human and pet food products. Therefore, the lower fecal pH may have been due to the fact that it was an acidic product (pH < 5).
Branched-chain fatty acids, phenols, indoles, and ammonia are produced through protein fermentation. There are many types of protein in the large intestine and they occur partly from dietary residues, such as animal and plant proteins, but the host also produces a significant amount of protein sources in the form of oral, gastric, pancreatic, and small intestinal secretions (e.g., enzymes and glycoproteins; Macfarlane and Macfarlane, 2012). Phenols and indoles are deaminated aromatic AA, tyrosine, phenylalanine, and tryptophan. In the current study, there were no differences in phenol concentrations among the dietary treatments; however, one of the mildly cooked diets had greater indole and total phenol and indole concentrations when compared to the other dietary treatments.
Given how much the chemical composition, level of processing, and nutrient digestibility differed among diets, shifts in the fecal microbiota were expected in the current study. The fecal microbial shifts of dogs fed the mildly cooked and raw diets, all of which were rich in protein and fat, were similar to that observed in humans consuming high-fat, high-protein diets (David et al., 2014). Similar to the current study, David et al. (2014) identified shifts in numerous genera within the Bacteroidetes, Firmicutes, and Proteobacteria phyla in people consuming plant-based vs. animal-based diets that differed in protein and fat amount and source. They reported a similar increase in the relative abundance of fecal Bacteroides and decrease in the relative abundance of fecal Bifidobacterium in people eating an animal-based diet. They also reported an increase in the relative abundance of fecal Pediococcus and Lactobacillus in people consuming the animal-protein–based diet, and attributed this to their use as starter culture for fermented foods, including cheeses and cured meats. Bermingham et al. (2017) also reported greater fecal Lactobacillus in dogs fed a raw-meat diet compared to those fed an extruded diet. Because the raw-meat diet tested in that study did not contain starter cultures, other factors may be involved in the increased fecal Lactobacillus proportion observed in humans and animals fed such diets.
Beloshapka et al. (2011), Bermingham et al. (2017), Kim et al. (2017), and Sandri et al. (2017) evaluated the responses to consuming extruded kibble vs. raw-meat diets, with all reporting large shifts in the fecal microbial communities. Similar to the current study, all of those researchers reported a greater relative abundance of fecal Fusobacteria in dogs fed raw-meat vs. extruded diets. Beloshapka et al. (2011) and Sandri et al. (2017) also reported a greater relative abundance of fecal Proteobacteria in dogs fed raw-meat diets. Beloshapka et al. (2011) also reported a lower relative abundance of fecal Firmicutes in dogs fed raw-meat diets.
Not all differences were in agreement with the current study, however. For instance, Beloshapka et al. (2011) and Bermingham et al. (2017) both reported large reductions in relative abundances of fecal Prevotella and Faecalibacterium in dogs consuming raw-meat diets, but differences in these genera were not observed in the current study. Bermingham et al. (2017) also reported opposite effects of a raw-meat diet on fecal Bacteroides (lower in dogs fed raw-meat diet) and Clostridium (greater in dogs fed raw-meat diet) than that reported in the current study. Similarly, Sandri et al. (2017) reported an opposite effect of a raw-meat diet on fecal Actinobacteria (greater in dogs fed raw-meat diet) compared to that of the current study. Again, differences in dietary ingredient and nutrient composition, animals studied, or other experimental conditions may have contributed to the contradictory results among studies.
Hooda et al. (2013) and Deusch et al. (2014) both tested dry extruded diets that differed in protein:carbohydrate ratio. In those studies, consumption of a high-protein diet led to reductions in the relative abundances of Actinobacteria (i.e., Bifidobacterium) and increases in the relative abundances of Fusobacteria (i.e., Fusobacterium) and Proteobacteria, which were similar to dogs consuming the mildly cooked and raw diets in the current study. In contrast to the current study, a greater relative abundance of fecal Clostridium was reported in cats fed a high-protein diet (Hooda et al., 2013; Deusch et al., 2014).
Although all fecal microbial changes of the current and recent studies were not in line with one another, many of the fecal microbial shifts were in agreement. More research attention is required in the gastrointestinal microbiome field, including topics relevant to companion animal nutrition and health. One factor that is usually not controlled or measured, and in many cases not even considered as being important, is the substrate load reaching the large intestine. While the amount of protein reaching the large intestine may be similar among dietary treatments, it is often not the case, especially with raw or mildly cooked diets that are more digestible than highly processed diets. Therefore, a constant digestibility of nutrients cannot be assumed. Another item that deserves more attention is the identification of physiologically relevant changes in the fecal microbiota due to diet and how it may contribute to or protect against diseases of the host. Many of the recent studies testing raw-meat diets, for instance, have demonstrated large shifts in fecal microbiota, including reduced diversity and changes that are deemed negative in terms of health (e.g., greater Fusobacteria and Proteobacteria; lower Actinobacteria), yet all animals remained healthy throughout the testing period (Beloshapka et al., 2011; Hooda et al., 2013; Deusch et al., 2014; Bermingham et al., 2017). Long-term studies that control and measure accurate dietary intake, nutrient digestibility, and include longitudinal sampling and relevant physiological data may be useful in this regard.
Blood metabolite data suggest that animals were in good health throughout the study. Most metabolites remained within reference ranges throughout the study. The serum triglyceride results were curious, however. Interestingly, the dogs fed the kibble diet had much higher serum triglyceride concentrations than the dogs fed the raw and mildly cooked diets despite containing a much lower fat content (13% vs. 28%–34%). Beloshapka (2011) also evaluated blood triglycerides in dogs fed raw diets. The values reported for dogs fed raw chicken (37–38 mg/dL) and raw beef (45–46 mg/dL) diets in that study were more similar to the dogs fed the raw (53 mg/dL) and mildly cooked (53–60 mg/dL) diets than those fed the extruded diet (80 mg/dL) in the current study. More research needs to be done to understand the mechanism behind this.
In conclusion, all diets tested in this study were well tolerated and dogs remained healthy throughout the study. The mildly cooked and raw diets were highly palatable and maintained fecal quality and serum chemistry measures. Compared to the extruded diet, these diets had greater nutrient digestibility, resulted in reduced blood triglyceride concentrations, and shifted fecal microbiota populations and metabolite concentrations.
Footnotes
Funding provided by Freshpet, Bethlehem, PA 18017 USA. Presented as a poster presentation at the 2017 American Academy of Veterinary Nutrition Clinical Nutrition and Research Symposia, National Harbor, MD, June 2017.
LITERATURE CITED
- AAFCO 2016. Official Publication. Association of American Feed Control Officials, Inc, Oxford, IN. [Google Scholar]
- Aldrich G. 2006. Rendered products in pet food. Essential rendering. All about the animal by-product industry. National Renderers Association, Arlington, VA: p. 159–177. [Google Scholar]
- American Association of Cereal Chemists (AACC) 1983. Approved methods. 8th ed Amer. Assoc. Cereal Chem, St Paul, MN. [Google Scholar]
- American Pet Products Association (APPA) 2017. Pet industry market size and ownership statistics. APPA, Greenwich, CT. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 2006. Official methods of analysis. 17th ed Assoc. Off. Anal. Chem, Gaithersburg, MD. [Google Scholar]
- Bansal T., Alaniz R. C., Wood T. K., and Jayaraman A.. 2010. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. U. S. A. 107:228–233. doi: 10.1073/pnas.0906112107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T., Englert D., Lee J., Hegde M., Wood T. K., and Jayaraman A.. 2007. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 75:4597–4607. doi: 10.1128/IAI.00630-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton L. 2014. US petfood market update: specialty petfoods driving industry growth. Petfood Ind. 56:20–25. [Google Scholar]
- Beloshapka A. N. 2011. Thesis: Effects of inulin or yeast cell-wall extract on nutrient digestibility, fecal fermentative end-product concentrations, and blood metabolite concentrations in adult dogs fed raw meat–based diets. University of Illinois, Urbana, IL, p. 1–77. [DOI] [PubMed] [Google Scholar]
- Beloshapka A. N., Dowd S. E., Duclos L., and Swanson K. S.. 2011. Comparison of fecal microbial communities of healthy adult dogs fed raw meat-based or extruded diets using 454 pyrosequencing. J. Anim. Sci. 89(E-Suppl. 1):284. doi:10.2527/asasann.2017.228 [Google Scholar]
- Beloshapka A. N., Duclos L. M., Vester Boler B. M., and Swanson K. S.. 2012. Effects of inulin or yeast cell-wall extract on nutrient digestibility, fecal fermentative end-product concentrations, and blood metabolite concentrations in adult dogs fed raw meat-based diets. Am. J. Vet. Res. 73:1016–1023. doi: 10.2460/ajvr.73.7.1016 [DOI] [PubMed] [Google Scholar]
- Bermingham E. N., Maclean P., Thomas D. G., Cave N. J., and Young W.. 2017. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. Peerj. 5:e3019. doi: 10.7717/peerj.3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde E. F. 1952. The determination of fat in baked biscuit type of dog foods. J. Assoc. Off. Agric. Chem. 35:799–805. [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7:335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., Owens S. M., Betley J., Fraser L., Bauer M., et al. 2012. Ultra-high-throughput microbial community analysis on the illumina HiSeq and MiSeq platforms. Isme J. 6:1621–1624. doi: 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney A. L., and Marbach E. P.. 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. [PubMed] [Google Scholar]
- Crissey S. D., Swanson J. A., Lintzenich B. A., Brewer B. A., and Slifka K. A.. 1997. Use of a raw meat-based diet or a dry kibble diet for sand cats (Felis margarita). J. Anim. Sci. 75:2154–2160. doi:10.2527/1997.7582154x [DOI] [PubMed] [Google Scholar]
- Cummings J. H., and Macfarlane G. T.. 1991. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70:443–459. doi:10.1111/j.1365-2672.1991.tb02739.x [DOI] [PubMed] [Google Scholar]
- David L. A., Maurice C. F., Carmody R. N., Gootenberg D. B., Button J. E., Wolfe B. E., Ling A. V., Devlin A. S., Varma Y., Fischbach M. A., et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 505:559–563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P., Iwazaki E., Suchy S. A., Pallotto M. R., and Swanson K. S.. 2014. Effects of feeding frequency and dietary water content on voluntary physical activity in healthy adult cats. J. Anim. Sci. 92:1271–1277. doi: 10.2527/jas.2013-7235 [DOI] [PubMed] [Google Scholar]
- DeSantis T. Z., Hugenholtz P., Larsen N., Rojas M., Brodie E. L., Keller K., Huber T., Dalevi D., Hu P., and Andersen G. L.. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072. doi: 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deusch O., O’Flynn C., Colyer A., Morris P., Allaway D., Jones P. G., and Swanson K. S.. 2014. Deep illumina-based shotgun sequencing reveals dietary effects on the structure and function of the fecal microbiome of growing kittens. Plos One. 9:e101021. doi: 10.1371/journal.pone.0101021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 26:2460–2461. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Erwin E. S., Marco G. J., and Emery E. M.. 1961. Volatile fatty acid analysis of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. [Google Scholar]
- Faber T. A., Bechtel P. J., Hernot D. C., Parsons C. M., Swanson K. S., Smiley S., and Fahey G. C. Jr. 2010. Protein digestibility evaluations of meat and fish substrates using laboratory, avian, and ileally cannulated dog assays. J. Anim. Sci. 88:1421–1432. doi: 10.2527/jas.2009-2140 [DOI] [PubMed] [Google Scholar]
- Flickinger E. A., Schreijen E. M., Patil A. R., Hussein H. S., Grieshop C. M., Merchen N. R., and Fahey G. C. Jr. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x [DOI] [PubMed] [Google Scholar]
- Hernot D., Biourge V., Dumon H., Martin L., Sergheraert R., and Nguyen P.. 2005. Effect of dietary fermentable to non-fermentable fiber ratio on fecal putrefactive products in dogs varying in body size. Proceedings: Waltham Symposium, Washington, DC. Waltham Centre for Pet Nutrition, Melton Mowbray, UK. [Google Scholar]
- Hooda S., Vester Boler B. M., Kerr K. R., Dowd S. E., and Swanson K. S.. 2013. The gut microbiome of kittens is affected by dietary protein:carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br. J. Nutr. 109:1637–1646. doi: 10.1017/S0007114512003479 [DOI] [PubMed] [Google Scholar]
- Kerr K. R., Vester Boler B. M., Morris C. L., Liu K. J., and Swanson K. S.. 2012. Apparent total tract energy and macronutrient digestibility and fecal fermentative end-product concentrations of domestic cats fed extruded, raw beef-based, and cooked beef-based diets. J. Anim. Sci. 90:515–522. doi: 10.2527/jas.2010-3266 [DOI] [PubMed] [Google Scholar]
- Kim J., An J. U., Kim W., Lee S., and Cho S.. 2017. Differences in the gut microbiota of dogs (Canis lupus familiaris) fed a natural diet or a commercial feed revealed by the illumina MiSeq platform. Gut Pathog. 9:68. doi: 10.1186/s13099-017-0218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., and Knight R.. 2005. Unifrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G. T., and Macfarlane S.. 2012. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 95:50–60. doi:10.5740/jaoacint.SGE_Macfarlane [DOI] [PubMed] [Google Scholar]
- Nery J., Biourge V., Tournier C., Leray V., Martin L., Dumon H., and Nguyen P.. 2010. Influence of dietary protein content and source on fecal quality, electrolyte concentrations, and osmolarity, and digestibility in dogs differing in body size. J. Anim. Sci. 88:159–169. doi: 10.2527/jas.2008-1666 [DOI] [PubMed] [Google Scholar]
- Parks D. H., Tyson G. W., Hugenholtz P., and Beiko R. G.. 2014. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124. doi: 10.1093/bioinformatics/btu494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosky L., Asp N. G., Schweizer T. F., De Vires J. W., and Fruda I.. 1992. Determination of insoluble and soluble dietary fiber in foods and food products: collaborative study. J. AOAC. 75:360–367. [PubMed] [Google Scholar]
- Sandri M., Dal Monego S., Conte G., Sgorlon S., and Stefanon B.. 2017. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 13:65. doi: 10.1186/s12917-017-0981-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Gamlath S., and Wakeling L.. 2007. Nutritional aspects of food extrusion: a review. Inter. J. F. Sci. Tech. 42:916–929. doi:10.1111/j.1365-2621.2006.01309.x [Google Scholar]
- Swanson K. S., Utterback P. L., and Parsons C. M.. 2017. Chemical composition, nutrient digestibility, and true metabolizable energy of differentially processed chicken-based pet food ingredients using the precision-fed cecectomized rooster assay. J. Anim. Sci. 95(Suppl. 4):112–113. doi:10.2527/asasann.2017.228 [Google Scholar]
- Valenzano M. C., DiGuilio K., Mercado J., Teter M., To J., Ferraro B., Mixson B., Manley I., Baker V., Moore B. A., et al. 2015. Remodeling of tight junctions and enhancement of barrier integrity of the CACO-2 intestinal epithelial cell layer by micronutrients. Plos One. 10:e0133926. doi: 10.1371/journal.pone.0133926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester B. M., Burke S. L., Dikeman C. L., Simmons L. G., and Swanson K. S.. 2008. Nutrient digestibility and fecal characteristics are different among captive exotic felids fed a beef-based raw diet. Zoo Biol. 27:126–136. doi: 10.1002/zoo.20172 [DOI] [PubMed] [Google Scholar]
- Vester B. M., Burke S. L., Liu K. J., Dikeman C. L., Simmons L. G., and Swanson K. S.. 2010. Influence of feeding raw or extruded feline diets on nutrient digestibility and nitrogen metabolism of African wildcats (Felis lybica). Zoo Biol. 29:676–686. doi: 10.1002/zoo.20305 [DOI] [PubMed] [Google Scholar]
- Wall T. 2018. Raw pet food sales growing despite health warnings. Petfood Ind. 60:24–27. [Google Scholar]
- Wong J. M., de Souza R., Kendall C. W., Emam A., and Jenkins D. J.. 2006. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40:235–243. doi:10.1097/00004836-200603000-00015 [DOI] [PubMed] [Google Scholar]