Abstract
A substantial number of individuals have long-lasting adverse effects from a traumatic brain injury (TBI). Depression is one of these long-term complications that influences many aspects of life. Depression can limit the ability to return to work, and even worsen cognitive function and contribute to dementia. The mechanistic cause for the increased depression risk associated with a TBI remains to be defined. As TBI results in chronic neuroinflammation, and priming of glia to a secondary challenge, the inflammatory theory of depression provides a promising framework for investigating the cause of depression following a TBI. Increases in cytokines similar to those seen in depression in the general population are also increased following a TBI. Biomarker levels of cytokines peak within hours-to-days after the injury, yet pro-inflammatory cytokines may still be elevated above physiological levels months-to-years following TBI, which is the time frame in which post-TBI depression can persist. As tumor necrosis factor α and interleukin 1 can signal directly at the neuronal synapse, pathophysiological levels of these cytokines can detrimentally alter neuronal synaptic physiology. The purpose of this review is to outline the current evidence for the inflammatory hypothesis of depression specifically as it relates to depression following a TBI. Moreover, we will illustrate the potential synaptic mechanisms by which tumor necrosis factor α and interleukin 1 could contribute to depression. The association of inflammation with the development of depression is compelling; however, in the context of post-TBI depression, the role of inflammation is understudied. This review attempts to highlight the need to understand and treat the psychological complications of a TBI, potentially by neuroimmune modulation, as the neuropsychiatric disabilities can have a great impact on the rehabilitation from the injury, and overall quality of life.
Keywords: concussion, major-depressive disorder, chronic traumatic encephalopathy, inflammation, tumor necrosis factor α, interleukin 1, microglia, astrocytes, synaptic physiology, N-methyl-D-aspartic acid
Introduction
Traumatic brain injury (TBI) is a public health crisis, which by conservative estimates, affects 1.7 million Americans each year (National Center for Injury Prevention and Control, 2003; Faul et al., 2010). Consequences of brain injury are known to increase in prevalence proportionally to the severity of the TBI and with increasing instances of TBI. Some of these consequences include physical disability, memory impairments, and mood disorders (Blennow et al., 2016). In addition, factors such as age at the time of injury, sex, and genetic predisposition are associated with increased long-term complications from the TBI (Blennow et al., 2016).
As many as 90% of TBIs are classified as concussions, or mild TBI (Blennow et al., 2016). The vast majority of the time (80-90%), symptoms of the mild TBI will resolve within the first two weeks after the injury (Blennow et al., 2016). Even with the resolution of symptoms in the vast majority of people following a mild TBI, there is compelling evidence that progressive brain atrophy, as well as cognitive dysfunction, continue after a mild TBI (Gardner and Yaffe, 2015; Rabinowitz et al., 2015; Theadom et al., 2018). For those people with a mild TBI that have symptoms unresolved for longer than three months, the individuals are diagnosed with post-concussive syndrome. The symptoms associated with the post-concussive syndrome are varied and subjective: including sleep disturbances, headache, and mood disorders, such as anxiety, irritability, and depression (Blennow et al., 2016). Repeated brain injuries can increase the prevalence and severity of post-concussive symptoms and may lead to a neurodegenerative condition called chronic traumatic encephalopathy (CTE), which is a pathologically diagnosed disease.
Memory impairments, sleep disturbances, headache, and mood disorders caused by TBI are highly interrelated. Treatment in one area following TBI may be beneficial in improving other symptoms. For example, treating depression can reduce memory impairments, overall disability, and improve quality of life (Kumar et al., 2018). Among the various cognitive disturbances following mild TBI, depression severity is strongly associated with overall disability following a mild TBI (Mac Donald et al., 2017) and is more common in people that suffered a TBI compared to the general population (Blennow et al., 2016).
In general, responsiveness to depression treatment is typically inconsistent (Belmaker and Agam, 2008), and may be even more so for depression associated with a TBI (Blennow et al., 2016). For example, while prior anecdotal evidence has suggested that depression in individuals with a history of TBI are responsive to anti-depression medication, recent results show this may not always be the case. Specifically, a recent randomized controlled trial found that a selective serotonin reuptake inhibitor (SSRI), Sertraline (trade name: Zoloft), was no more effective than placebo in people with depression following a TBI (Fann et al., 2017). This single trial does not prove, or even suggest, that individuals with depression and a history of TBI will not benefit from an SSRI or other anti-depression medication. It is possible that depression associated with TBI is more likely to be treatment-resistant, but to the best of our knowledge, this association has not been tested.
Depression does not arise from a single known pathogenic mechanism (Belmaker and Agam, 2008). A prior history of mood disorder and other comorbidities, such as substance abuse, psychosocial factors, and cardiovascular disease may contribute to the risk of developing depression following a TBI (Blennow et al., 2016). However, without a complete understanding of the pathophysiological mechanism(s) that lead to depression following TBI, targeted disease-modifying therapies cannot be developed.
While a specific mechanism has not been established, accumulating evidence indicates that inflammatory pathways may contribute to the development of depression, at least in a subset of patients (Miller and Raison, 2016). Given that inflammation can persist for years after a TBI (Ramlackhansingh et al., 2011; Coughlin et al., 2017), it is logical to speculate that the chronic low-grade inflammation seen after a TBI could be mechanistically linked to the high rates of depression following a TBI. In recent years, a limited number of studies have provided intriguing preliminary evidence of a link between TBI-induced inflammatory biomarkers in the cerebrospinal fluid (CSF) and increased odds of developing depression (Juengst et al., 2015), as well as an increased association with suicidal endorsement (Juengst et al., 2014), and post-traumatic stress disorder (PTSD) (Speer et al., 2018).
The objective of this review is to discuss the current evidence for the inflammatory hypothesis of depression, as it relates to post-TBI depression. We will first describe the evidence for both acute and chronic inflammatory cytokine changes following a TBI. We will then summarize the prevalence of depression following TBI. Linking cytokines and depression, we will then provide a general overview of the inflammatory hypothesis of depression. Then focusing on the IL-1, and TNFα pathways, two of the cytokines which have been most consistently shown to be dysregulated by a TBI, we will summarize the potential cellular and molecular mechanisms of those cytokines that may contribute to post-TBI depression. As a whole, the concept of anti-inflammatory therapy for depression is compelling, but largely unexplored, particularly in the context of post-TBI depression. By summarizing the state of the field, we hope to stimulate inquiries into the inflammatory theory of post-TBI depression, as a promising area of treatment for the chronic complications of TBI.
Cytokine Changes Associated With a TBI
The inflammatory response to a TBI has been the subject of many in-depth reviews, and we recommend the following reviews for readers who would like a more general overview of neuroinflammation following TBI (Witcher et al., 2015; Burda et al., 2016; Faden et al., 2016; Hellewell et al., 2016; Loane and Kumar, 2016; Jassam et al., 2017; Simon et al., 2017; Ziebell et al., 2017; Kokiko-Cochran and Godbout, 2018). The goal of the current review is to highlight the function of inflammatory cytokines as a critical mediator of the inflammatory effects on neuronal dysfunction as it relates to post-TBI depression, and as such we will not provide a general overview of neuroinflammation.
Cytokines are a category of signaling proteins that act via binding to specific receptors. The dominant effects of cytokines occur by cell-to-cell interaction in the local tissue environment via paracrine signaling, or even acting on the releasing cell in an autocrine manner (Gulati, 2009). Chemokines, which are a subclass of cytokines, in contrast, have more global effects with important endocrine functions such as establishing gradients to chemoattract cells to the appropriate target.
Neurotransmitters were once thought to only act in the nervous system and cytokines were thought to be restricted to the immune system; however, there is now resounding evidence that both neurotransmitters and cytokines are used in both the nervous and immune systems (Liu and Quan, 2018). For example, in the brain, interleukin (IL)-1 is largely produced by microglia and astrocytes (Liu and Quan, 2018); interestingly, the receptor for IL-1 is found on endothelial cells and neurons (Liu and Quan, 2018). Recent evidence suggests that there is heterogeneity in the class of neurons that express the receptor for IL-1 (Liu and Quan, 2018). Thus, there is an exciting possibility that expression of different cytokine receptors may define unique subtypes of neurons and provide an additional level modulatory information at the level of synaptic transmission.
Cytokines historically have been linked to the innate and adaptive immune system where they are involved in defense against pathogens (Gulati, 2009). In the healthy organism, cytokines are produced at very low levels, but in response to activating stimulus (i.e., pathogen, or tissue damage) levels of cytokines can be rapidly induced to levels greater than a 100-fold of that found at the basal condition. This property of rapid and robust induction has caused cytokines to be a very active area for biomarker research, as we will describe in the following paragraph. The dramatic increase in cytokines after an activating stimulus is limited in time as high levels of circulating cytokines, such as in sepsis, can be fatal. The mechanisms for temporally and spatially limiting the cytokine response include, for example, unstable RNA (Stumpo et al., 2010; Vlasova-St Louis and Bohjanen, 2014), short half-life of the cytokine proteins, anti-inflammatory cytokines (i.e., IL-4 and IL-10), receptor internalization, degradation or shedding, decoy receptors (IL-1R2), and receptor antagonists (IL-1ra) (Garlanda et al., 2013). Thus, cytokines whisper to neighboring cells when conditions are good, but when things are not good, they can also yell.
To measure cytokine changes in people after a TBI four approaches have been used, including measuring cytokines in blood, cerebrospinal fluid (CSF), microdialysate, and biopsy or postmortem brain tissue. Blood biomarker measurements are the least invasive approach, with levels of cytokines determined in either serum, plasma, and in some instances by stimulating circulating immune cells with a mitogen. In the case of moderate-to-severe TBI, CSF that would otherwise be discarded during the management of intracranial pressure is collected for biomarker assays. Cerebral microdialysis probes are capable of sampling the local brain parenchymal concentrations of cytokines, and other molecules, but must be neurosurgically implanted (Helmy et al., 2011).
A few technical considerations must be taken into account when interpreting cytokine biomarker changes after an injury. The sensitivity of the assay used to measure the cytokines can affect the ability to detect low levels of cytokines. In our own experience, we have found that multiplex cytokine kits from different companies or even new versions of the kit from the same company can find different results concerning which cytokine is most abundantly expressed after an injury and if certain cytokines can be detected or not. Differences in processing the samples can result in degradation of the cytokines. How often the CSF is sampled, for example, can affect the ability to detect cytokine changes after TBI (Shore et al., 2004). Microdialysis can measure tissue levels of cytokines, but the probe itself can induce inflammation, and the location of the probe in relation to the areas of the brain that are injured can influence the results (Helmy et al., 2009). Regardless of which method is used to measure cytokine levels, there is clear evidence that cytokines are elevated following a TBI, as we describe below.
Only a few studies have reported cytokine changes in people following mild TBI (Mukherjee and Mobry, 1979; Ghezzi et al., 1996; Sharma et al., 2017), while the majority of studies measuring cytokine changes after injury have been done following moderate-to-severe TBI. An interesting recent study found in a military population that a mild TBI was associated with elevated IL-6 and tumor necrosis factor α (TNFα) in plasma. Intriguingly, the elevated IL-6 and TNFα were associated with PTSD and depression in this military population (Devoto et al., 2017). In multiple studies, a moderate-to-severe TBI was found to cause a significant elevation of IL-6 with the peak of the response occurring within the first day, and levels returning to baseline within the first week (Kossmann et al., 1995; Bell et al., 1997a, b; Pleines et al., 2001; Park et al., 2002; Hayakata et al., 2004; Shore et al., 2004; Chiaretti et al., 2005, 2008; Buttram et al., 2007; Frugier et al., 2010; Helmy et al., 2011; Mellergard et al., 2011; Roberts et al., 2013; Yan et al., 2014a; Aisiku et al., 2016; Kumar et al., 2016; Nwachuku et al., 2016). TNFα was found to be significantly elevated in some studies (Otto et al., 2000; Hayakata et al., 2004; Buttram et al., 2007; Frugier et al., 2010; Helmy et al., 2011; Roberts et al., 2013; Juengst et al., 2014; Yan et al., 2014a), and no significant difference was found in others (Helmy et al., 2011; Aisiku et al., 2016; Kumar et al., 2016; Nwachuku et al., 2016). Similarly, IL-1b was found to be elevated in some studies (Park et al., 2002; Chiaretti et al., 2005; Shiozaki et al., 2005; Buttram et al., 2007; Chiaretti et al., 2008; Helmy et al., 2011; Mellergard et al., 2011; Roberts et al., 2013; Nwachuku et al., 2016), while not in others (Frugier et al., 2010; Aisiku et al., 2016; Kumar et al., 2016)
The increases in cytokine levels occurring acutely after injury, as measured in the clinical biomarker studies, have been shown to correlate with injury severity, and can be predictive of functional recovery (Kumar et al., 2016; Nwachuku et al., 2016). These properties as a biomarker of injury severity do not provide direct evidence that these acute cytokine surges seen after the injury are themselves detrimental to long-term recovery. The increase in cytokines is likely proportional to the damage caused by the primary injury. While the clinical literature is limited on the use of anti-cytokine therapy as a neuroprotective following TBI, there is a fairly extensive preclinical literature showing inhibiting cytokines acutely after injury can be protective (Simon et al., 2017). To use an example from our own work, we have found that a small molecule inhibitor of cytokine overproduction was able to improve functional recovery in a learning and memory task following an experimental brain injury in mice (Bachstetter et al., 2015).
Prevalence of Depression Following TBI
The compelling relationship of TBI with cognitive impairment and dementia has led to a substantial focus investigating this association by the clinical and scientific communities. Significant psychological disabilities, including aggression, anxiety, substance abuse and depression, as well as, physical complications including pain, motor, and balance impairments are also complication of a TBI. Rarely do the cognitive, psychological, and physical complications of a TBI occur in isolation (Kumar et al., 2018). For more information on this topic, please refer to an excellent recent review and meta-analysis that illustrates the interrelationship of post-traumatic depression, and other common secondary complications of a moderate-to-severe TBI, such as cognitive function and pain (Kumar et al., 2018).
Depression is one of the common psychological sequelae of TBI, with as high as 56% of individuals having symptoms of depression at 10-week post-injury (Singh et al., 2018). Following a moderate-to-severe injury, the cumulative rate of major depressive disorder during the first year was found to be 53%, compared to the rate of major depressive disorder in the general population around 7% (Bombardier et al., 2010). Further, when studies are limited to concussions, still up to 22% of people develop depression during the first 6-months after the injury (Max et al., 2012). A history of concussion is associated with 3.3-fold greater risk for a diagnosis of depression in adolescents (Chrisman and Richardson, 2014), as well as a 2-fold increase in the elderly (Albrecht et al., 2015). On top of single injury risk, the risk for developing depression is greatly enhanced by the number of concussions that a person sustains. The relative risk of developing depression increases from 1.5 to 3 when individuals report less than 3 versus 3+ concussions (Guskiewicz et al., 2007). Injury severity, number of injuries, age at the time of injury, and other factors influence the relative likelihood of post-TBI depression, but few are spared from the risk that a TBI could contribute to depression.
Like depression in the general population, post-TBI depression has been found to affect many aspects of life. Global disability, which reflects both work and non-work related disability, was found at 6–12 months post-injury to be significantly correlated with depression severity in a recent study of combat-related concussive TBIs in US military personnel (Mac Donald et al., 2017). In civilians, those individuals that can return to work after the mild TBI, report that they are less productive and more error prone than before the injury (Silverberg et al., 2018). This reduction in job performance was associated with depression but not bodily pain (Silverberg et al., 2018), indicating that something specific about the mechanism of depression following injury is affecting other cognitive functions. A double-blind, placebo-controlled, randomized clinical trial provided promising evidence that an SSRI, Sertraline, could reduce the onset of depression following a TBI when Sertraline was given during the first 6 months after injury (Jorge et al., 2016). An independent double-blind, placebo-controlled study did not find a significant effect of Sertraline on major depression after a TBI; however, the study did find that cognitive function was improved (Fann et al., 2017). More research is clearly warranted to understand the mechanism of post-TBI depression as the evidence strongly suggest a critical role of depression in the squeal of TBI, including disability, work productivity, and cognitive function.
Inflammatory Theory of Depression
Could depression be an inflammatory-mediated and evolutionarily conserved mechanism to TBI? A recent review paper by Drs. Miller and Raison provided a compelling answer to this question (Miller and Raison, 2016). A primary tenet of their treatise is that inflammation signals to the organism to decrease activity, to allow time to fight infection, heal from wounds, and avoid further pathogen exposure. Arguably, everyone has experienced an inflammatory state of depression, typically during cold and flu season. This response is not limited to acute inflammatory responses but carries over to chronic inflammatory diseases. Indeed, sickness behavior shares many hallmarks of major depressive disorder, such as social withdrawal, and anhedonia (Dantzer et al., 2008; Miller and Raison, 2016). The difference between chronic inflammatory disease and acute inflammation associated with infection and their link with depression may involve the mechanisms of clearing the infection. The sickness behavior associated with a viral infection and its concomitant increase in inflammation, for example, is transient. Depressive-like symptoms resolve as the infection clears. In the case of aging, cardiovascular disease, stress, Alzheimer's disease, and TBI, however, the stimulus that is inducing the proinflammatory state and the linked depressive-like symptoms does not readily clear like a viral infection, instead a protracted or even chronic low-grade response remains. While the levels of inflammatory cytokines, for example, will be much lower in the chronic disease states, than following a serious infection, they are nevertheless elevated above physiological levels and thus should be considered as pathophysiological. Evidence in support of the involvement of inflammation in depression comes from three main observations, as described below.
The first line of evidence supporting the inflammatory theory of depression is correlative and relies on the presence of elevated cytokines in the blood of depressed individuals compared to healthy controls. While correlative, the results are nevertheless impressive, as summarized in a number of excellent reviews and meta-analyses (Kohler et al., 2017, 2018; O’Brien et al., 2007; Leighton et al., 2018). Specifically, a meta-analysis of 82 studies showed that peripheral levels of numerous cytokines and chemokines, including IL-6 and TNFα, were elevated in people with major depressive disorder compared to healthy controls (Kohler et al., 2017). A subsequent meta-analysis furthered the association between cytokines and depression as they found that the use of anti-depression medication was associated with a reduction in peripheral inflammatory cytokine levels (Kohler et al., 2018). It has also been found that increased proinflammatory molecules in systemic circulation are associated with reduced efficacy of anti-depressants (O’Brien et al., 2007; Leighton et al., 2018). While the evidence is persuasive, a number of questions are raised, the most critical being; how is the depression leading to elevated systemic inflammation, and how does systemic inflammatory response affect the neurocircuitry to induce depressive behaviors?
In the review by Drs. Miller and Raison, they propose three routes to transmit inflammatory signals between the periphery and the central nervous system (CNS): 1) a humoral route where cytokines, chemokines, and other inflammatory molecules move back and forth from the blood and brain, potentially through circumventricular organs; 2) a cellular route where activation of monocytes/macrophages by cytokines and chemokines leads to the recruitment of cells to the brain; 3) a neural route where peripheral nerves, such as the vagus, stimulate directly on the immune cells in the bone marrow and spleen, for example, to activate an immune response (Miller and Raison, 2016).
In the context of TBI, the elevation of cytokines is likely to first occur locally within the central nervous system by reactive microglia/macrophages and astrocytes (Figure 1). The CNS inflammation or the primary damaged caused by the TBI could also alter one of the bidirectional routes of communication between the periphery and the CNS, leading to a chronic dysregulation of the neuroinflammatory response. Regardless of the source of the inflammation, if elevated cytokines are seen in the blood chronically after TBI, this could be an indicator of individuals that should be evaluated for depression and may predict the success of treatment to reduce the depression.
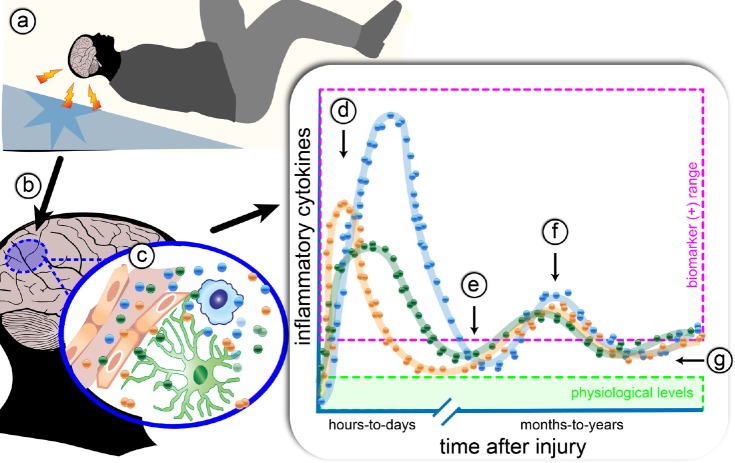
Figure 1.
The effect of traumatic brain injury (TBI) on acute cytokine response, and chronic cytokine dysregulation.
(a) A TBI causes local reactive microglia/macrophages and astrocytes (b). Humoral, cellular, and neural routes (c) lead to increase in local and systemic cytokines. The profile of the different cytokines (for example, interleukin (IL)-1, IL-6, and tumor necrosis factor α (TNFα)), indicated by the blue, orange, and green dots, have slightly different temporal patterns and max amplitude, but in general, follow a similar profile. (d) The acute inflammatory response to injury results in a surge of cytokines within the first hours-to-days after injury, which can be easily detected by biomarker assays (pink box). After the first week post-injury (e), cytokines fall below the level of sensitivity for most biomarker assays, but potentially remain above physiological levels (green box). Compounding factors, such as psychosocial stress and infection, may result in secondary spikes in inflammatory cytokines as measured in biomarker assays (f). Regardless, of the ability to measure cytokines in biomarker assays, therapeutically targeting the chronic dysregulated cytokines during the months-to-years after injury (g), could reduce cognitive, psychological, and physical complications of a TBI. Future studies are warranted to test this prediction. However, as cytokines have essential physiological functions, it will be necessary to have selective therapeutics that restore the physiological balance of cytokines, and not pan-immune suppression.
The second line of evidence comes from the therapeutic use of cytokines. Type I interferons used in the treatment of hepatitis and multiple sclerosis are strongly associated with the development of depression, with as much as 80% of patients on Type I interferon therapy developing depression (Raison et al., 2005). Not all individuals are equally susceptible to this interaction; for example, patients that have a prior history of mood disturbances are at a greater risk for developing depression with Type I interferon treatment (Raison et al., 2005). Similarly, in TBI having a prior history of pre-morbid psychiatric problems prior to the injury is also a risk factor for depression after the injury (Bowen et al., 1998; Rao et al., 2010; Rapoport, 2012). These findings suggest that some individuals, either due to genetics, age, injury, disease or psychosocial factors have a heightened or otherwise dysregulated response to inflammatory molecules. Thus, two hits – predisposition towards inflammation and an acute insult – could tip the balance towards the development of depression.
The third line of evidence comes from clinical trials where anti-cytokine treatment has been shown to reduce depression compared to control groups. Much of this evidence comes from studies where anti-cytokine therapy was used for the treatment of autoimmune disorders, such as psoriasis, rheumatoid arthritis, and Crohn's disease. As summarized in a systematic review and meta-analysis (Kappelmann et al., 2018), anti-cytokine treatment was found to have a significant reduction in depression symptoms compared to placebo, across the 16 studies included in the analysis, with an effect estimate of 0.4. To date a single randomized controlled trial has been completed using Infliximab, a TNFα-blocking antibody, in treatment-resistant depression. While blocking TNFα showed no significant effect on reducing depression, a secondary analysis showed that in a subgroup of participants that had elevated C-reactive protein levels (a protein associated with increases in inflammation), there was a significant effect of Infliximab treatment on depression symptoms (Raison et al., 2013). While not directly affecting cytokine signaling, other studies have found beneficial effects of adding nonsteroidal anti-inflammatory drugs (NSAIDs) to SSRI therapy (Kappelmann et al., 2018). NSAIDs, in addition to inhibition of prostaglandin synthesis, are able to cross the cell membrane to block various signaling cascades important for the inflammatory response (Diaz-Gonzalez and Sanchez-Madrid, 2015). Moreover, minocycline, an antibiotic with immunomodulatory properties, was shown in a recent meta-analysis to have significant antidepressant activity (Rosenblat and McIntyre, 2018).
As depression is a complex disorder, with many different potential comorbidities, but common symptomology, there is a need to consider multiple theories for the pathogenic mechanisms of depression. With the evidence presented here, the inflammatory theory of depression shows great promise. As such, there are currently a number of active clinical trials investigating anti-cytokine interventions for the treatment of depression (NCT03004443, NCT03006393, NCT02765100, NCT02456948). While there has been ample evidence of the involvement of cytokines following TBI and a link with post-traumatic depression, there is currently no information available about active or completed trials using anti-cytokine treatment for depression following a TBI.
Neuromodulatory Mechanism of TNFα and IL-1β in Regulating Synaptic Function
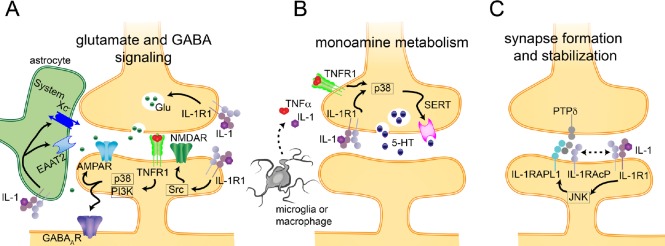
Beyond their role in immune function, cytokines have numerous roles in regulating neuronal function, which occur at the synapse (Figure 2). What is telling about the importance of cytokines as neuromodulators, is the highly specific mechanisms by which cytokines alter the function of different subsets of neurons. For instance, high levels of IL-1 and TNFα have been shown to impair long-term potentiation (LTP) induction and maintenance, but low levels of IL-1 and TNFα are necessary for LTP induction and maintenance (Murray and Lynch, 1998; Ross et al., 2003; Stellwagen and Malenka, 2006; Mori et al., 2014). Further, and specifically relevant for the role of cytokines in depression, IL-1 and TNFα have important roles in serotonergic signaling (Ramamoorthy et al., 1995; Mossner et al., 1998; Zhu et al., 2005, 2006, 2010; Steiner et al., 2008) (Figure 2). These cytokines have been shown to directly regulate the serotonin transporter (SERT) through a mechanism dependent on p38 mitogen activated protein kinase (MAPK) pathway, via increased production of SERT and increased localization of SERT to the membrane (Ramamoorthy et al., 1995; Mossner et al., 1998; Zhu et al., 2005, 2006, 2010; Steiner et al., 2008) (Figure 2B).
Figure 2.
Mechanisms by which interleukin 1 (IL-1) and tumor necrosis factor (TNF) can directly regulate neuronal synaptic function.
(a) (A) IL-1 and TNFα regulate the excitatory and inhibitory neurotransmitter balance. Hyperphysiological levels of cytokines, such as following a traumatic brain injury (TBI), increase excitatory (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMAPR) and N-methyl-D-aspartic acid receptor (NMDAR)) neurotransmission, while decreasing inhibitory (γ-aminobutyric acid (GABA) A receptor (GABAAR)) neurotransmission. Cytokines can also affect the release of glutamate (Glu) from neurons, and the uptake and release of glutamate from astrocytes. (B) IL-1 and TNFα also increase the activity of the serotonin transporter (SERT), leading to a decrease in serotonin (5-HT) at the synapse. (C) Members of the IL-1 family of proteins are also involved in synapse formation and stabilization, and IL-1 signaling can regulate this process. EAAT2: Excitatory amino acid transporter 2; IL-1R1: IL-1 receptor 1; IL-1RAPL1: IL-1-receptor accessory protein like 1; IL-1RAcP: IL-1 receptor accessory protein; TNFR1: TNF receptor 1; JNK: c-Jun terminal kinase; system XC -: cystine/glutamate antiporter system.
The effects of cytokines on neuronal modulation are concentration dependent, where physiological levels of cytokines are necessary to facilitate memory formation (Vezzani and Viviani, 2015). Acute increases in cytokines may increase awareness and sensory functions (Liu and Quan, 2018), while chronic increases cytokines impair synaptic function (Rogers et al., 2011; Bachstetter et al., 2012; Furman et al., 2012). What follows is a review of the mechanisms by which TNFα and IL-1 have been shown to modulate neuronal function at the synapse.
TNFα is produced as a transmembrane protein that can be cleaved from the membrane by TNFα converting enzyme (also known as ADAM17) to produce a soluble cytokine. TNFα signals via two membrane receptors, TNFR1 and TNFR2. The ubiquitously expressed tumor necrosis factor receptor (TNFR)1 can bind both the soluble and membrane-bound forms of TNFα. TNFR1 is implicated in apoptotic, proinflammatory, and pathological processes, as well as neuronal signaling, in response to TNFα activation (Konefal and Stellwagen, 2017). TNFR2 has a restricted expression, in comparison to TNFR1, and is found on endothelial cells, immune cells, and some neuronal populations (McCoy and Tansey, 2008). TNFR2 is implicated in prosurvival, and immune suppression. The membrane-bound form of TNFα is the most potent activator of TNFR2 (Grell et al., 1995). Following brain and spinal cord injury, TNFR2 has been shown to be neuroprotective and promote regeneration (Sullivan et al., 1999; Mironets et al., 2018). The effects of TNFα on synaptic function are thought to occur through TNFR1 signaling (Stellwagen et al., 2005; Pribiag and Stellwagen, 2013).
In the hippocampus, TNFα has significant effects on glutamate signaling, by altering the expression, composition, and phosphorylation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), which effect duration of AMPAR-mediated spontaneous excitatory postsynaptic currents (Bernardino et al., 2005; Stellwagen et al., 2005; Lai et al., 2006; Stellwagen and Malenka, 2006; Leonoudakis et al., 2008; Sama et al., 2012; Mandolesi et al., 2013). The effects of TNFα on AMPAR activity appear to be concentration and exposure length dependent. For example, following acute exogenous exposure of TNFα, a rapid increase in AMPAR surface expression and activity have been reported (Beattie et al., 2002; Stellwagen et al., 2005; Stellwagen and Malenka, 2006). In contrast, chronic exposure to low levels of TNFα, as in the case of normal aging, decreases AMPARs (Sama et al., 2012). Mechanistically, the regulatory effects of TNFα on AMPARs has been proposed to occur via phosphatidylinositol 3-kinase (PI3K), and p38 MAPK (Pribiag and Stellwagen, 2014) (Figure 2A). However, the full signal transduction pathway regulating the effects of TNFα on AMPAR remains to be defined. The effects of TNFα on glutamate signaling appear to be independent of the N-methyl-D-aspartic acid receptor (NMDAR), at least in the context of acute TNFα exposure (Beattie et al., 2002; He et al., 2012), and in the context of chronic TNFα seen with healthy aging (Sama et al., 2012).
In addition to the effects that TNFα has on the excitatory neurotransmission, via the AMPAR, TNFα also effects γ-aminobutyric acid (GABA) A receptor (GABAAR). GABAAR is the principal mediator of inhibitory neurotransmission in the brain. Specifically, TNFα, acting on neurons causes a rapid internalization of GABAAR, which decreases the inhibitory synaptic strength (Pribiag and Stellwagen, 2013). The signal transduction pathway regulating the effects of TNFα on GABAAR also requires p38 MAPK and PI3K (Pribiag and Stellwagen, 2013) (Figure 2A).
The IL-1 family includes two agonists, IL-1α and IL-1β, which bind to the same receptor (IL-1R1), to cause a similar biological response. IL-1α is released by all cells, when injured or dying, to rapidly activate an inflammatory response. IL-1β is mainly produced by immune cells and microglia. IL-1R2 is a decoy receptor not capable of signaling. In the brain, an alternative promoter in the IL-1R1 gene generates a functional brain-specific IL-1 receptor called, IL-1R3 (Qian et al., 2012). IL-1R1 is most highly expressed in the brain on endothelial cells, and neurons (Liu et al., 2015). The canonical IL-1R1 signaling pathway requires the IL-1R1 accessory protein subunit (AcP). In the brain, an alternative IL-1R1 co-receptor, called AcPb, was recently identified (Smith et al., 2009; Huang et al., 2011). IL-1R1 and the IL-1R1 accessory proteins (AcP, and AcPb) are highly expressed at the neuronal synapse (Prieto et al., 2015).
At the postsynaptic terminal, similar to TNFα, IL-1 has been shown to regulate AMPA receptor expression, phosphorylation, and activity (Stellwagen et al., 2005; Lai et al., 2006; Kawasaki et al., 2008; Mandolesi et al., 2013; Machado et al., 2018; Tong et al., 2018). In contrast to TNFα, IL-1 has also been found to have significant effects on NMDAR. IL-1-induces phosphorylation of the NR2A/B subunits of the NMDAR, as well as altering the cellular localization of AMPAR and NMDAR, via a Src kinase-dependent mechanism (Viviani et al., 2003; Davis et al., 2006a, b; Sanchez-Alavez et al., 2006; Ghosh et al., 2016; Bertani et al., 2017; Tong et al., 2018) (Figure 2A). IL-1R1 has also been shown to be physically associated with NR2B (Gardoni et al., 2011), further illustrating the interaction between IL-1 and modulating activity at the synapse. Also, at the postsynaptic terminal, chronic IL-1/IL-1R1 signaling pathway reduces GABAAR-mediated inhibitory postsynaptic currents (Kawasaki et al., 2008; Roseti et al., 2015), which reduces the counterbalance to the excitatory glutamatergic signaling, while acute stimulation with IL-1 increases membrane expression of GABAAR, via an AKT and p38 dependent pathway (Serantes et al., 2006; Wang et al., 2012).
At the presynaptic terminal, IL-1 can increase the release of glutamate (Casamenti et al., 1999). Astrocytes that surround the synapse are also involved in the IL-1 dependent regulation of glutamate signaling (Figure 2A). IL-1 decreases uptake of extracellular glutamate, via a reduction in the high-affinity glutamate transporters on the astrocyte, glial glutamate transporter 1/excitatory amino acid transporter 2 (GLT1/EAAT2). The reduction in GLT1/EAAT2 occurs through enhanced endocytosis, by a calcium/PKC dependent mechanism (Mandolesi et al., 2013; Yan et al., 2014b). Also at the astrocyte, IL-1 increases the activity of the cystine/glutamate antiporter System XC -, leading to increased release of glutamate from astrocytes (Fogal et al., 2007).
Physical interaction between pairs of presynaptic and postsynaptic adhesion molecules is known to regulate synapse formation and stabilization (Takahashi and Craig, 2013; Thalhammer and Cingolani, 2014). Currently, less than ten postsynaptic adhesion molecules have been identified. Interestingly, three of the postsynaptic adhesion molecules belong to the IL-1 family. Specifically, IL-1 receptor 1 accessory protein (IL-1R1AcP) and IL-1R1AcPb, as well as IL-1-receptor accessory protein like 1 (IL1RAPL1) are important postsynaptic adhesion molecules, which interact physically with protein tyrosine phosphatase (PTP) δ to regulate synaptogenesis and spine stabilization (Yoshida et al., 2012; Yamagata et al., 2015; Pozzi et al., 2018). IL-1 has been shown to alter dendritic morphology by signaling through the IL-1R1AcP(b)/IL-1R1APL1 complex, through a c-Jun terminal kinase (JNK) pathway (Pavlowsky et al., 2010; Montani et al., 2017) (Figure 2C).
When Can Cytokines Be Targeted Therapeutically after a TBI?
Following fundamental principles of pharmacology, it is often assumed that it is necessary to target cytokines after an injury during the peak of their response, or at least while they are elevated above basal levels. As described, the peak of cytokines occurs within hours to a day after an injury; thus, it can be difficult to target cytokines clinically during this short window. However, blocking cytokines during the first hours after injury, could prevent the onset of chronic neuroinflammation, and block excitatory damage to neurons occurring through dysregulation of the glutamate and GABA signaling (Figure 2).
As described, in blood and CSF, levels of cytokines stabilize at or near physiological levels within a week in people after a brain injury. Similarly, in animal models of TBI, cytokine levels in brain homogenates return to sham-injured levels typically within a week (Bachstetter et al., 2013, 2015; Webster et al., 2015). These data would suggest that the effect of cytokines in the long-term neurological sequelae of the TBI, is over after the first week of the injury, and treatments that target cytokines should be used during this period after the injury. However, we demonstrated in a mouse model of TBI, that we could promote functional recovery in a learning and memory task when we started the treatment with a small molecule cytokine inhibitor one week after the injury (Webster et al., 2015). These data suggest that there are likely local disruptions in the cytokine signaling network, possibly occurring at the synapse following a TBI, which last much longer than would be predicted by the biomarker data (Figure 1).
As cytokines act in an autocrine and paracrine manner in the local tissue milieu, it may not be possible using existing technology to see changes in the cytokines following TBI at the circuit level, specifically at the synapse. Neurons, for instance, are 1000 times more sensitive to IL-1, than other cell types (Huang et al., 2011), so that even changes in IL-1 levels that are too small to detect with current methods, could have a profound impact on neuronal physiology. An alternative hypothesis is that the primary injury or acute cytokine response to the injury increases the sensitivity of cells to the cytokines so that low amounts of cytokines are producing an exaggerated response in the injured brain compared to the uninjured brain (Figure 1). Much more work is warranted to test these potential mechanisms. Low levels of chronic cytokine dysregulation are associated with depression, and clinical evidence has demonstrated that reducing cytokine signaling has a significant impact on depression (Raison et al., 2013; Kappelmann et al., 2018).
Animal models of CNS injury have had mixed success in modeling psychiatric symptoms following a TBI, such as depressive-like symptoms using tasks such as the forced-swim test and sucrose preference test, as only a few studies have been able to detect depressive-like symptoms after the brain injury (Milman et al., 2005; Jones et al., 2008; Malkesman et al., 2013; Fenn et al., 2014, 2015; Petraglia et al., 2014; Wu et al., 2014; Rowe et al., 2016; Lim et al., 2017a, b; Tucker et al., 2017). However, depressive-like symptoms in animal models are likely associated with periods of remission and reestablishment, as seen in people. Moreover, as in people, animal models may require a secondary challenge for the TBI-induced depressive-like symptoms to be evident. For example, an eloquent study found that a subsequent immune challenge in mice, after the TBI-induced depressive-like behavior had resolved, caused reestablishment of the depressive-like symptoms (Fenn et al., 2014). As illustrated in Figure 1, during the chronic phase after the brain injury, a secondary challenge to the system, which has been primed by the TBI can lead to psychiatric and neurodegenerative conditions (for a review on inflammatory priming (Witcher et al., 2015)).
Conclusions
As a multifaceted disorder, depression has been associated with many medical conditions, including diabetes, cancer, and cardiovascular disease, as well as TBI, and Alzheimer's disease (Lang and Borgwardt, 2013). The biological underpinnings of depression are not fully understood and how depression interacts with these different diseases is a significant topic of study. For approximately one-third of people with depression, conventional antidepressants are not effective. Specifically, in major depression after a TBI, as described earlier, there are conflicting results on the effectiveness of SSRIs (Jorge et al., 2016; Fann et al., 2017). In these individuals, alternative methods of treatment have been proposed to individualize treatments to find the most effective pharmacotherapy to alleviate their symptoms. One such effort to predict response used computer modeling to improve the likelihood of treatment efficacy (Chekroud et al., 2016). Animal models have provided further evidence that increasing and decreasing inflammation after a TBI can have a reciprocal effect on depressive-like behavior (Fenn et al., 2014, 2015).
There are numerous theories for biological mechanisms of depression (Belmaker and Agam, 2008). Following at least thirty years of research since the macrophage theory of depression was initially postulated (Smith, 1991), there is now mounting evidence demonstrating that inflammatory pathways contribute to the pathophysiology of depressive disorders (Dantzer et al., 2008; Miller and Raison, 2016). As cytokine dysregulation is a hallmark pathology of TBI, the inflammatory theory of depression is particularly relevant to mood disorders that develop following a TBI. Determining how cytokines regulate mood is an exciting area of investigation and may have great potential for treating mood disorders in people that suffered a TBI.
Additional file: Open peer review report 1 (110.8KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: CNB was supported in part by a Kentucky Spinal and Head Injury Trust trainee fellowship. Research reported in this publication was supported by National Institutes of Health under award numbers R00 AG044445 (to ADB) and P30 GM110787 (to ADB).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Stephanie K Seidlits, University of California Los Angeles, USA.
Funding: CNB was supported in part by a Kentucky Spinal and Head Injury Trust trainee fellowship. Research reported in this publication was supported by National Institutes of Health under award numbers R00 AG044445 (to ADB) and P30 GM110787 (to ADB).
References
- Aisiku IP, Yamal JM, Doshi P, Benoit JS, Gopinath S, Goodman JC, Robertson CS. Plasma cytokines IL-6, IL-8, and IL-10 are associated with the development of acute respiratory distress syndrome in patients with severe traumatic brain injury. Crit Care. 2016;20:288. doi: 10.1186/s13054-016-1470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht JS, Kiptanui Z, Tsang Y, Khokhar B, Liu X, Simoni-Wastila L, Zuckerman IH. Depression among older adults after traumatic brain injury: a national analysis. Am J Geriatr Psychiatry. 2015;23:607–614. doi: 10.1016/j.jagp.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Rowe RK, Kaneko M, Goulding D, Lifshitz J, Van Eldik LJ. The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J Neurosci. 2013;33:6143–6153. doi: 10.1523/JNEUROSCI.5399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Webster SJ, Goulding DS, Morton JE, Watterson DM, Van Eldik LJ. Attenuation of traumatic brain injury-induced cognitive impairment in mice by targeting increased cytokine levels with a small molecule experimental therapeutic. J Neuroinflammation. 2015;12:69. doi: 10.1186/s12974-015-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Norris CM, Sompol P, Wilcock DM, Goulding D, Neltner JH, St Clair D, Watterson DM, Van Eldik LJ. Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of Alzheimer's disease-related pathology. J Neurosci. 2012;32:10201–10210. doi: 10.1523/JNEUROSCI.1496-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Whalen MJ, DeKosky ST. Comparison of the interleukin-6 and interleukin-10 response in children after severe traumatic brain injury or septic shock. Acta Neurochir Suppl. 1997a;70:96–97. doi: 10.1007/978-3-7091-6837-0_30. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Wisniewski SR, Whalen MJ, DeKosky ST. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma. 1997b;14:451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR, Vezzani A, Malva JO, Zimmer J. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005;25:6734–6744. doi: 10.1523/JNEUROSCI.1510-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani I, Iori V, Trusel M, Maroso M, Foray C, Mantovani S, Tonini R, Vezzani A, Chiesa R. Inhibition of IL-1β signaling normalizes NMDA-dependent neurotransmission and reduces seizure susceptibility in a mouse model of Creutzfeldt-Jakob disease. J Neurosci. 2017;37:10278–10289. doi: 10.1523/JNEUROSCI.1301-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic brain injuries. Nat Rev Dis Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen A, Neumann V, Conner M, Tennant A, Chamberlain MA. Mood disorders following traumatic brain injury: identifying the extent of the problem and the people at risk. Brain Inj. 1998;12:177–190. doi: 10.1080/026990598122656. [DOI] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol. 2016;275:305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RS, Kochanek PM. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer's disease. Neuroscience. 1999;91:831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- Chekroud AM, Zotti RJ, Shehzad Z, Gueorguieva R, Johnson MK, Trivedi MH, Cannon TD, Krystal JH, Corlett PR. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3:243–250. doi: 10.1016/S2215-0366(15)00471-X. [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Genovese O, Aloe L, Antonelli A, Piastra M, Polidori G, Di Rocco C. Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst. 2005;21:185–194. doi: 10.1007/s00381-004-1032-1. [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Antonelli A, Riccardi R, Genovese O, Pezzotti P, Di Rocco C, Tortorolo L, Piedimonte G. Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur J Paediatr Neurol. 2008;12:195–204. doi: 10.1016/j.ejpn.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisman SP, Richardson LP. Prevalence of diagnosed depression in adolescents with history of concussion. J Adolesc Health. 2014;54:582–586. doi: 10.1016/j.jadohealth.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, et al. Imaging of glial cell activation and white matter integrity in brains of active and recently retired National Football League players. JAMA Neurol. 2017;74:67–74. doi: 10.1001/jamaneurol.2016.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006a;98:1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- Davis CN, Mann E, Behrens MM, Gaidarova S, Rebek M, Rebek J, Jr, Bartfai T. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc Natl Acad Sci U S A. 2006b;103:2953–2958. doi: 10.1073/pnas.0510802103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto C, Arcurio L, Fetta J, Ley M, Rodney T, Kanefsky R, Gill J. Inflammation relates to chronic behavioral and neurological symptoms in military personnel with traumatic brain injuries. Cell Transplant. 2017;26:1169–1177. doi: 10.1177/0963689717714098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Gonzalez F, Sanchez-Madrid F. NSAIDs: learning new tricks from old drugs. Eur J Immunol. 2015;45:679–686. doi: 10.1002/eji.201445222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Wu JF, Stoica BA, Loane DJ. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 2016;173:681–691. doi: 10.1111/bph.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann JR, Bombardier CH, Temkin N, Esselman P, Warms C, Barber J, Dikmen S. Sertraline for major depression during the year following traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil. 2017;32:332–342. doi: 10.1097/HTR.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Atlanta, GA, USA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry. 2014;76:575–584. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, Eiferman DS, Godbout JP. Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J Neurotrauma. 2015;32:127–138. doi: 10.1089/neu.2014.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J Neurosci. 2007;27:10094–10105. doi: 10.1523/JNEUROSCI.2459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugier T, Morganti-Kossmann MC, O’Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010;27:497–507. doi: 10.1089/neu.2009.1120. [DOI] [PubMed] [Google Scholar]
- Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66:75–80. doi: 10.1016/j.mcn.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Boraso M, Zianni E, Corsini E, Galli CL, Cattabeni F, Marinovich M, Di Luca M, Viviani B. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1beta and NMDA stimulation. J Neuroinflammation. 2011;8:14. doi: 10.1186/1742-2094-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Torri V, Zaffaroni M. Isolated optic neuritis and its prognosis for multiple sclerosis: a clinical and paraclinical study with evoked potentials. CSF examination and brain MRI. Ital J Neurol Sci. 1996;17:325–332. doi: 10.1007/BF01999894. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Green MV, Krogh KA, Thayer SA. Interleukin-1beta activates an Src family kinase to stimulate the plasma membrane Ca2+ pump in hippocampal neurons. J Neurophysiol. 2016;115:1875–1885. doi: 10.1152/jn.00541.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Gulati P. Janeway's Immunobiology. In: Murphy K, Travers P, Walport M, editors. Biochemistry and Molecular Biology Education. 7th Edition. Wiley-Blackwell; 2009. p. 134. [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP Jr, Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Hayakata T, Shiozaki T, Tasaki O, Ikegawa H, Inoue Y, Toshiyuki F, Hosotubo H, Kieko F, Yamashita T, Tanaka H, Shimazu T, Sugimoto H. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22:102–107. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- He P, Liu Q, Wu J, Shen Y. Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons. FASEB J. 2012;26:334–345. doi: 10.1096/fj.11-192716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell S, Semple BD, Morganti-Kossmann MC. Therapies negating neuroinflammation after brain trauma. Brain Res. 2016;1640:36–56. doi: 10.1016/j.brainres.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Carpenter KL, Skepper JN, Kirkpatrick PJ, Pickard JD, Hutchinson PJ. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma. 2009;26:549–561. doi: 10.1089/neu.2008.0719. [DOI] [PubMed] [Google Scholar]
- Huang Y, Smith DE, Ibanez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31:18048–18059. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Cardamone L, Williams JP, Salzberg MR, Myers D, O’Brien TJ. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J Neurotrauma. 2008;25:1367–1374. doi: 10.1089/neu.2008.0641. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Acion L, Burin DI, Robinson RG. Sertraline for preventing mood disorders following traumatic brain injury: a randomized clinical trial. JAMA Psychiatry. 2016;73:1041–1047. doi: 10.1001/jamapsychiatry.2016.2189. [DOI] [PubMed] [Google Scholar]
- Juengst SB, Kumar RG, Arenth PM, Wagner AK. Exploratory associations with tumor necrosis factor-alpha, disinhibition and suicidal endorsement after traumatic brain injury. Brain Behav Immun. 2014;41:134–143. doi: 10.1016/j.bbi.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Juengst SB, Kumar RG, Failla MD, Goyal A, Wagner AK. Acute inflammatory biomarker profiles predict depression risk following moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2015;30:207–218. doi: 10.1097/HTR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335–343. doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, de Andrade NQ, Morris G, Fernandes BS, Brunoni AR, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55:4195–4206. doi: 10.1007/s12035-017-0632-1. [DOI] [PubMed] [Google Scholar]
- Kokiko-Cochran ON, Godbout JP. The inflammatory continuum of traumatic brain injury and Alzheimer's disease. Front Immunol. 2018;9:672. doi: 10.3389/fimmu.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konefal SC, Stellwagen D. Tumour necrosis factor-mediated homeostatic synaptic plasticity in behavioural models: testing a role in maternal immune activation. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160160. doi: 10.1098/rstb.2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann T, Hans VH, Imhof HG, Stocker R, Grob P, Trentz O, Morganti-Kossmann C. Intrathecal and serum interleukin-6 and the acute-phase response in patients with severe traumatic brain injuries. Shock. 1995;4:311–317. doi: 10.1097/00024382-199511000-00001. [DOI] [PubMed] [Google Scholar]
- Kumar RG, Rubin JE, Berger RP, Kochanek PM, Wagner AK. Principal components derived from CSF inflammatory profiles predict outcome in survivors after severe traumatic brain injury. Brain Behav Immun. 2016;53:183–193. doi: 10.1016/j.bbi.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RG, Gao S, Juengst SB, Wagner AK, Fabio A. The effects of post-traumatic depression on cognition, pain, fatigue, and headache after moderate-to-severe traumatic brain injury: a thematic review. Brain Inj. 2018;32:383–394. doi: 10.1080/02699052.2018.1427888. [DOI] [PubMed] [Google Scholar]
- Lai AY, Swayze RD, El-Husseini A, Song C. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J Neuroimmunol. 2006;175:97–106. doi: 10.1016/j.jneuroim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Lang UE, Borgwardt S. Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol Biochem. 2013;31:761–777. doi: 10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. 2018;23:48–58. doi: 10.1038/mp.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Zhao P, Beattie EC. Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity. J Neurosci. 2008;28:2119–2130. doi: 10.1523/JNEUROSCI.5159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SW, Sung KC, Shiue YL, Wang CC, Chio CC, Kuo JR. Hyperbaric oxygen effects on depression-like behavior and neuroinflammation in traumatic brain injury rats. World Neurosurg. 2017a;100:128–137. doi: 10.1016/j.wneu.2016.12.118. [DOI] [PubMed] [Google Scholar]
- Lim SW, Shiue YL, Liao JC, Wee HY, Wang CC, Chio CC, Chang CH, Hu CY, Kuo JR. Simvastatin therapy in the acute stage of traumatic brain injury attenuates brain trauma-induced depression-like behavior in rats by reducing neuroinflammation in the hippocampus. Neurocrit Care. 2017b;26:122–132. doi: 10.1007/s12028-016-0290-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Quan N. Microglia and CNS interleukin-1: beyond immunological concepts. Front Neurol. 2018;9:8. doi: 10.3389/fneur.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yamashita T, Chen Q, Belevych N, McKim DB, Tarr AJ, Coppola V, Nath N, Nemeth DP, Syed ZW, Sheridan JF, Godbout JP, Zuo J, Quan N. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. J Neurosci. 2015;35:2860–2870. doi: 10.1523/JNEUROSCI.3199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol. 2016;275:316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, Werner NJ, Adam OR, Rivet DJ, Flaherty SF, Oh JS, Zonies D, Fang R, Brody DL. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34:2206–2219. doi: 10.1089/neu.2016.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado I, Schiöth HB, Lasaga M, Scimonelli T. IL-1β reduces GluA1 phosphorylation and its surface expression during memory reconsolidation and α-melanocyte-stimulating hormone can modulate these effects. Neuropharmacology. 2018;128:314–323. doi: 10.1016/j.neuropharm.2017.09.041. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Tucker LB, Ozl J, McCabe JT. Traumatic brain injury - modeling neuropsychiatric symptoms in rodents. Front Neurol. 2013;4:157. doi: 10.3389/fneur.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi G, Musella A, Gentile A, Grasselli G, Haji N, Sepman H, Fresegna D, Bullitta S, De Vito F, Musumeci G, Di Sanza C, Strata P, Centonze D. Interleukin-1β alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci. 2013;33:12105–12121. doi: 10.1523/JNEUROSCI.5369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Schachar RJ, Saunders AE, Ewing-Cobbs L, Chapman SB, Dennis M, Yang TT, Levin HS. Depression in children and adolescents in the first 6 months after traumatic brain injury. Int J Dev Neurosci. 2012;30:239–245. doi: 10.1016/j.ijdevneu.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellergard P, Aneman O, Sjogren F, Saberg C, Hillman J. Differences in cerebral extracellular response of interleukin-1β, interleukin-6, and interleukin-10 after subarachnoid hemorrhage or severe head trauma in humans. Neurosurgery. 2011;68:12–19. doi: 10.1227/NEU.0b013e3181ef2a40. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Mironets E, Osei-Owusu P, Bracchi-Ricard V, Fischer R, Owens EA, Ricard J, Wu D, Saltos T, Collyer E, Hou S, Bethea JR, Tom VJ. Soluble TNFα signaling within the spinal cord contributes to the development of autonomic dysreflexia and ensuing vascular and immune dysfunction after spinal cord injury. J Neurosci. 2018;38:4146–4162. doi: 10.1523/JNEUROSCI.2376-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani C, Ramos-Brossier M, Ponzoni L, Gritti L, Cwetsch AW, Braida D, Saillour Y, Terragni B, Mantegazza M, Sala M, Verpelli C, Billuart P, Sala C. The X-linked intellectual disability protein IL1RAPL1 regulates dendrite complexity. J Neurosci. 2017;37:6606–6627. doi: 10.1523/JNEUROSCI.3775-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Nisticò R, Mandolesi G, Piccinin S, Mango D, Kusayanagi H, Berretta N, Bergami A, Gentile A, Musella A, Nicoletti CG, Nicoletti F, Buttari F, Mercuri NB, Martino G, Furlan R, Centonze D. Interleukin-1β promotes long-term potentiation in patients with multiple sclerosis. Neuromolecular Med. 2014;16:38–51. doi: 10.1007/s12017-013-8249-7. [DOI] [PubMed] [Google Scholar]
- Mossner R, Heils A, Stober G, Okladnova O, Daniel S, Lesch KP. Enhancement of serotonin transporter function by tumor necrosis factor alpha but not by interleukin-6. Neurochem Int. 1998;33:251–254. doi: 10.1016/s0197-0186(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Mukherjee BB, Mobry PM. Selective transcription of genomic sequences common to both N- and X-tropic endogenous retroviruses in BALB/c mouse tissues. J Gen Virol. 1979;43:723–728. doi: 10.1099/0022-1317-43-3-723. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlanta, GA, USA: Centers for Disease Control and Prevention; National Center for Injury Prevention and Control (2003) Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. [Google Scholar]
- Nwachuku EL, Puccio AM, Adeboye A, Chang YF, Kim J, Okonkwo DO. Time course of cerebrospinal fluid inflammatory biomarkers and relationship to 6-month neurologic outcome in adult severe traumatic brain injury. Clin Neurol Neurosurg. 2016;149:1–5. doi: 10.1016/j.clineuro.2016.06.009. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Otto VI, Heinzel-Pleines UE, Gloor SM, Trentz O, Kossmann T, Morganti-Kossmann MC. sICAM-1 and TNF-alpha induce MIP-2 with distinct kinetics in astrocytes and brain microvascular endothelial cells. J Neurosci Res. 2000;60:733–742. doi: 10.1002/1097-4547(20000615)60:6<733::AID-JNR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Park W, Li J, Song R, Messing J, Chen X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol. 2002;12:1484–1495. doi: 10.1016/s0960-9822(02)01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlowsky A, Zanchi A, Pallotto M, Giustetto M, Chelly J, Sala C, Billuart P. Neuronal JNK pathway activation by IL-1 is mediated through IL1RAPL1, a protein required for development of cognitive functions. Commun Integr Biol. 2010;3:245–247. doi: 10.4161/cib.3.3.11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, Huang JH. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma. 2014;31:1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleines UE, Morganti-Kossmann MC, Rancan M, Joller H, Trentz O, Kossmann T. S-100 beta reflects the extent of injury and outcome, whereas neuronal specific enolase is a better indicator of neuroinflammation in patients with severe traumatic brain injury. J Neurotrauma. 2001;18:491–498. doi: 10.1089/089771501300227297. [DOI] [PubMed] [Google Scholar]
- Pozzi D, Menna E, Canzi A, Desiato G, Mantovani C, Matteoli M. The Communication Between the Immune and Nervous Systems: The Role of IL-1beta in Synaptopathies. Front Mol Neurosci. 2018;11:111. doi: 10.3389/fnmol.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D. TNF-alpha downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J Neurosci. 2013;33:15879–15893. doi: 10.1523/JNEUROSCI.0530-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology. 2014;78:13–22. doi: 10.1016/j.neuropharm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Prieto GA, Snigdha S, Baglietto-Vargas D, Smith ED, Berchtold NC, Tong L, Ajami D, LaFerla FM, Rebek J, Jr, Cotman CW. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1β in the aged hippocampus. Proc Natl Acad Sci U S A. 2015;112:E5078–E5087. doi: 10.1073/pnas.1514486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Zhu L, Li Q, Belevych N, Chen Q, Zhao F, Herness S, Quan N. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proc Natl Acad Sci U S A. 2012;109:12189–12194. doi: 10.1073/pnas.1205207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz AR, Li X, McCauley SR, Wilde EA, Barnes A, Hanten G, Mendez D, McCarthy JJ, Levin HS. Prevalence and predictors of poor recovery from mild traumatic brain injury. J Neurotrauma. 2015;32:1488–1496. doi: 10.1089/neu.2014.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Ramamoorthy JD, Prasad PD, Bhat GK, Mahesh VB, Leibach FH, Ganapathy V. Regulation of the human serotonin transporter by interleukin-1 beta. Biochem Biophys Res Commun. 1995;216:560–567. doi: 10.1006/bbrc.1995.2659. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Rao V, Bertrand M, Rosenberg P, Makley M, Schretlen DJ, Brandt J, Mielke MM. Predictors of new-onset depression after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2010;22:100–104. doi: 10.1176/appi.neuropsych.22.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport MJ. Depression following traumatic brain injury: epidemiology, risk factors and management. CNS Drugs. 2012;26:111–121. doi: 10.2165/11599560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Jenne CN, Leger C, Kramer AH, Gallagher CN, Todd S, Parney IF, Doig CJ, Yong VW, Kubes P, Zygun DA. Association between the cerebral inflammatory and matrix metalloproteinase responses after severe traumatic brain injury in humans. J Neurotrauma. 2013;30:1727–1736. doi: 10.1089/neu.2012.2842. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, McIntyre RS. Efficacy and tolerability of minocycline for depression: A systematic review and meta-analysis of clinical trials. J Affect Disord. 2018;227:219–225. doi: 10.1016/j.jad.2017.10.042. [DOI] [PubMed] [Google Scholar]
- Roseti C, van Vliet EA, Cifelli P, Ruffolo G, Baayen JC, Di Castro MA, Bertollini C, Limatola C, Aronica E, Vezzani A, Palma E. GABAA currents are decreased by IL-1beta in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol Dis. 2015;82:311–320. doi: 10.1016/j.nbd.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Rowe RK, Ziebell JM, Harrison JL, Law LM, Adelson PD, Lifshitz J. Aging with traumatic brain injury: effects of age at injury on behavioral outcome following diffuse brain injury in rats. Dev Neurosci. 2016;38:195–205. doi: 10.1159/000446773. [DOI] [PubMed] [Google Scholar]
- Sama DM, Mohmmad Abdul H, Furman JL, Artiushin IA, Szymkowski DE, Scheff SW, Norris CM. Inhibition of soluble tumor necrosis factor ameliorates synaptic alterations and Ca2+ dysregulation in aged rats. PLoS One. 2012;7:e38170. doi: 10.1371/journal.pone.0038170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci U S A. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serantes R, Arnalich F, Figueroa M, Salinas M, Andres-Mateos E, Codoceo R, Renart J, Matute C, Cavada C, Cuadrado A, Montiel C. Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem. 2006;281:14632–14643. doi: 10.1074/jbc.M512489200. [DOI] [PubMed] [Google Scholar]
- Sharma R, Rosenberg A, Bennett ER, Laskowitz DT, Acheson SK. A blood-based biomarker panel to risk-stratify mild traumatic brain injury. PLoS One. 2017;12:e0173798. doi: 10.1371/journal.pone.0173798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki T, Hayakata T, Tasaki O, Hosotubo H, Fuijita K, Mouri T, Tajima G, Kajino K, Nakae H, Tanaka H, Shimazu T, Sugimoto H. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–410. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, Janesko KL, Bayir H, Jackson EK, Kochanek PM. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma. 2004;21:1113–1122. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- Silverberg ND, Panenka WJ, Iverson GL. Work productivity loss after mild traumatic brain injury. Arch Phys Med Rehabil. 2018;99:250–256. doi: 10.1016/j.apmr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Mason S, Lecky F, Dawson J. Prevalence of depression after TBI in a prospective cohort: The SHEFBIT study. Brain Inj. 2018;32:84–90. doi: 10.1080/02699052.2017.1376756. [DOI] [PubMed] [Google Scholar]
- Smith DE, Lipsky BP, Russell C, Ketchem RR, Kirchner J, Hensley K, Huang Y, Friedman WJ, Boissonneault V, Plante MM, Rivest S, Sims JE. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity. 2009;30:817–831. doi: 10.1016/j.immuni.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Speer K, Upton D, Semple S, McKune A. Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res. 2018;11:111–121. doi: 10.2147/JIR.S155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo DJ, Lai WS, Blackshear PJ. Inflammation: cytokines and RNA-based regulation. Wiley Interdiscip Rev RNA. 2010;1:60–80. doi: 10.1002/wrna.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Craig AM. Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 2013;36:522–534. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalhammer A, Cingolani LA. Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology. 2014;78:23–30. doi: 10.1016/j.neuropharm.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Theadom A, Starkey N, Barker-Collo S, Jones K, Ameratunga S, Feigin V, Group BIyR Population-based cohort study of the impacts of mild traumatic brain injury in adults four years post-injury. PLoS One. 2018;13:e0191655. doi: 10.1371/journal.pone.0191655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Prieto GA, Cotman CW. IL-1beta suppresses cLTP-induced surface expression of GluA1 and actin polymerization via ceramide-mediated Src activation. J Neuroinflammation. 2018;15:127. doi: 10.1186/s12974-018-1158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, Burke JF, Fu AH, McCabe JT. Neuropsychiatric symptom modeling in male and female C57BL/6J mice after experimental traumatic brain injury. J Neurotrauma. 2017;34:890–905. doi: 10.1089/neu.2016.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova-St Louis I, Bohjanen PR. Post-transcriptional regulation of cytokine signaling by AU-rich and GU-rich elements. J Interferon Cytokine Res. 2014;34:233–241. doi: 10.1089/jir.2013.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, Davies PA, Moss SJ, Lu WY, Orser BA. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell Rep. 2012;2:488–496. doi: 10.1016/j.celrep.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster SJ, Van Eldik LJ, Watterson DM, Bachstetter AD. Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J Neurosci. 2015;35:6554–6569. doi: 10.1523/JNEUROSCI.0291-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher KG, Eiferman DS, Godbout JP. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 2015;38:609–620. doi: 10.1016/j.tins.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci. 2014;34:10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata A, Yoshida T, Sato Y, Goto-Ito S, Uemura T, Maeda A, Shiroshima T, Iwasawa-Okamoto S, Mori H, Mishina M, Fukai S. Mechanisms of splicing-dependent trans-synaptic adhesion by PTPdelta-IL1RAPL1/IL-1RAcP for synaptic differentiation. Nat Commun. 2015;6:6926. doi: 10.1038/ncomms7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan EB, Satgunaseelan L, Paul E, Bye N, Nguyen P, Agyapomaa D, Kossmann T, Rosenfeld JV, Morganti-Kossmann MC. Post-traumatic hypoxia is associated with prolonged cerebral cytokine production, higher serum biomarker levels, and poor outcome in patients with severe traumatic brain injury. J Neurotrauma. 2014a;31:618–629. doi: 10.1089/neu.2013.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Yadav R, Gao M, Weng HR. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014b;62:1093–1109. doi: 10.1002/glia.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Shiroshima T, Lee SJ, Yasumura M, Uemura T, Chen X, Iwakura Y, Mishina M. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J Neurosci. 2012;32:2588–2600. doi: 10.1523/JNEUROSCI.4637-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell JM, Rowe RK, Muccigrosso MM, Reddaway JT, Adelson PD, Godbout JP, Lifshitz J. Aging with a traumatic brain injury: Could behavioral morbidities and endocrine symptoms be influenced by microglial priming? Brain Behav Immun. 2017;59:1–7. doi: 10.1016/j.bbi.2016.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.