Abstract
Spinal cord injury (SCI) from trauma or disease severely impairs sensory and motor function. Neurorehabilitation after SCI is a complex medical process that focuses on improving neurologic function and repairing damaged connections in the central nervous system. An increasing number of preclinical studies suggest that melatonin may be useful for the treatment of SCI. Melatonin is an indolamine that is primarily secreted by the pineal gland and known to be regulated by photoperiodicity. However, it is also a versatile hormone with antioxidative, antiapoptotic, neuroprotective, and anti-inflammatory properties. Here, we review the neuroprotective properties of melatonin and the potential mechanisms by which it might be beneficial in the treatment of SCI. We also describe therapies that combine melatonin with exercise, oxytetracycline, and dexamethasone to attenuate the secondary injury after SCI and limit potential side effects. Finally, we discuss how injury at different spinal levels may differentially affect the secretion of melatonin.
Keywords: spinal cord injury, melatonin, secondary damage, neuroprotection, antioxidative, antiapoptotic, anti-inflammatory, synergistic effects, neural regeneration
Introduction
Spinal cord injury (SCI) is a devastating neurologic disorder that often results in the loss of sensory and motor function. In severe cases, it can lead to paralysis and death (Gómez et al., 2018). In patients with SCI, primary injury from the initial trauma is followed by a secondary injury cascade of cellular and molecular events. The secondary injury exacerbates neurologic damage and loss of function (Hewson et al., 2018), which may be induced by the production of highly reactive species such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and free radicals to damage protein structure, DNA, and cell membranes (Liu et al., 2017). These changes also lead to oxidative stress, a major underlying pathophysiology of injury of central nervous system (CNS) (Naseem and Parvez, 2014). The consequences of the secondary injury include mitochondrial dysfunction, neurotransmitter accumulation, disruption of the blood-brain barrier and blood-spinal cord barrier (BSCB), apoptosis, excitotoxicity, and inflammatory and immune processes (Bains and Hall, 2012). Partially owing to the disruption of micro-circulation, activated neurons and glial cells often exhibit pro-inflammatory phenotypes after SCI. These neurotoxic actions can be further exacerbated by infiltrated peripheral leukocytes, resident microglia, and astrocytes (Rodrigues et al., 2018). Because SCI often results in permanent disability and poor quality of life, it generates an enormous financial burden on society, including the cost of medical care and loss of productivity (Yang et al., 2014). Unfortunately, the treatment of SCI remains a significant challenge to clinicians and a large unmet medical need.
Melatonin, also known as N-acetyl-5-methoxy tryptamine, is a methoxyindole derivative that is produced predominantly by the pineal gland during the dark phase of the circadian cycle. At a much lower amount, melatonin can also be produced by extra-pineal tissues and organs such as retina, Harderian gland, gut, bone marrow, platelets, astrocytes, and skin in both human and animals (Zhang et al., 2014; Ren et al., 2017). Melatonin is best known for its role in circadian rhythm, but it is also an antioxidant (Borin et al., 2016) that was found to be neuroprotective in rat models of traumatic brain injury and ischemic stroke. A recent study suggested that melatonin also exerts neuroprotective effects in the secondary pathophysiology of SCI (Yang et al., 2016). Melatonin could regulate the levels of malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), and myeloperoxidase (MPO), which showed abnormal changes after SCI (Ghaisas et al., 2011). Melatonin also exerts other biological actions, such as anti-inflammation, inhibition of apoptosis, and attenuation of edema which may protect tissues from secondary injury of SCI. This review focuses on the therapeutic potential and underlying mechanisms of melatonin for the treatment of SCI. We also briefly review the pathophysiologic mechanisms underlying SCI, possible synergistic effects of combining melatonin with other treatments, and the impact of spinal injury level on the secretion of melatonin (Additional Table 1 (99.8KB, pdf) ).
Characteristics of the major studies.
Reduction of Oxidative Stress
Oxidative stress occurs when there is an imbalance between prooxidants and antioxidants in the living system. It can be induced by free radical generation and lipid peroxidation, and plays an important role in the pathophysiology of many neurological disorders. Neurons in the CNS have very low capacity to attenuate the effects of oxidative stress, and hence are highly sensitive to oxidative stress and oxidative damage (Bains and Hall, 2012). Oxidative stress is also the hallmark of secondary injury after SCI, and oxidative injury after SCI leads to an increased production of lipid peroxidation (McDonald and Sadowsky, 2002). The degree of lipid peroxidation can be inferred by measuring the levels of MDA, GSH and SOD.
Erşahin et al. (2012) used a standard weight-drop method to induce a moderate injury to the spinal cord at the 10th thoracic vertebra (T10) in rats, and treated SCI rats with vehicle or melatonin (10 mg/kg, intraperitoneal (i.p.)) at 15 minutes after injury. A week later, blood, spinal cord, and urinary bladder tissue samples were harvested from these rats for biochemical and histological analysis. MDA content in SCI rats treated with vehicle was significantly increased from 1 day after SCI and reached a peak level around 7 days post-injury (Erşahin et al., 2012). In contrast, GSH level was significantly decreased after SCI. Importantly, the elevated MDA content after SCI was reduced by melatonin treatment, and the decreased GSH level was also restored by melatonin. In another study, SCI rats were treated by intraperitoneal administration of melatonin (7.5 mg/kg) immediately after laminectomy, and then once daily for 10 days (Erol et al., 2008). This study also found that melatonin treatment restored the decreased GSH level in the spinal cord due to SCI (Erol et al., 2008). Accordingly, melatonin may exert neuroprotective effects by reducing oxidative stress and damage to neurons during the progress of SCI.
Regulation of Nitric Oxide Synthase (NOS)
Nitric oxide (NO) is a free radical that can react with superoxide to form peroxynitrite (ONOO−), which is a potent and devastating oxidant. NO can be produced by three isoforms of NOS: neuronal (nNOS), endothelial (eNOS), and inducible (iNOS) (Drechsel et al., 2012). Adult male Sprague-Dawley rats (250–260 g) received contusion SCI and were maintained under 12-hour light/dark cycle or 24-hour constant light. Systemic melatonin (10 mg/kg, subcutaneous injection, twice daily) was administrated in these rats for 4 weeks after SCI (Park et al., 2012). The results showed that SCI causes motor disturbance in hind limbs that is associated with increased NO production. Importantly, melatonin treatment facilitated hind limb motor function recovery in rats exposed to a 12-hour light/dark cycle, but not in those exposed to 24-hour constant light. Moreover, motor function recovery correlated with decreased iNOS expression. This finding implicates that endogenous melatonin may also be important to the neuroprotective effect after SCI (Park et al., 2012). A recent study in an experimental model of spinal cord trauma further demonstrated that melatonin (30 mg/kg) inhibited the expression of iNOS (Paterniti et al., 2017). The effects of melatonin on NOS expression and NO synthesis were also examined in the lumbar spinal cord of neonatal rats after receiving a sciatic nerve transection at postnatal day (P) 2. Subcutaneous injections of melatonin (1 mg/kg) were given at 1 hour before transection, 1–2 hours after transection, and repeated once daily till P6. Animals in the control group received vehicle treatment after injury. Expressions of different isoforms of NOS were determined by both RT-PCR and immunohistochemistry from P2 to P7. Unlike a reduction of NOS by melatonin in adult SCI rats, the expression levels of nNOS and eNOS in neonatal rats after sciatic transection were not altered by melatonin compared with vehicle treatment (Rogério et al., 2006). Thus, melatonin may play an important role in attenuating NOS expression in adult rats after SCI, but not in neonatal rats after peripheral nerve injury.
Anti-inflammation
Inflammatory molecules trigger gene expression in reactive astrocytes and impair long-term recovery (Labombarda et al., 2011; Pekny and Pekna, 2016). Proinflammatory cytokines such as interleukin (IL)-1α, tumor necrosis factor (TNF)-α, and IL-1β are activated within a few hours after SCI (Ritz and Hausmann, 2008). These proinflammatory cytokines may exacerbate the secondary injury after SCI. Melatonin was suggested to play an important role in reducing inflammation. Recently, the effects of intraperitoneal injection of melatonin (10 mg/kg) on SCI were further examined in a mouse model of severe crush injury (Krityakiarana et al., 2016). In this study, mice were randomly separated into four groups: SCI, SCI + melatonin 1 (single treatment), SCI + melatonin 14 (once daily treatment for 14 days), and control. Immunohistochemical analysis showed that long term melatonin treatment attenuated the inflammatory response and decreased IL-1β and neuron/glial antigen 2 (NG-2) levels (Krityakiarana et al., 2016). These findings suggest that administration of melatonin soon after SCI may reduce the expression and release of proinflammatory mediators, thereby preventing the secondary inflammatory response and tissue damage.
Prolonged melatonin treatment may provide additional protective effects by preserving cell and nerve structures. Peroxisome proliferator-activated receptor α (PPAR-α) is a nuclear receptor protein. It functions as a transcription factor that can be activated by fatty acids. PPAR-α also plays an important role in the secondary inflammatory process after SCI. Researchers investigated the efficacy of melatonin (30 mg/kg) in experimental SCI through applying vascular clips (24 g force) to the dura via a four-level T5–8 laminectomy in mice and comparing PPAR-α (PPAR-α KO)-knockout mice with wild-type (WT) mice. Results revealed that melatonin-mediated anti-inflammatory activity was weakened in PPAR-α KO mice compared with WT controls. Melatonin alleviates post-traumatic injury associated with SCI by binding the PPAR-α receptors (Paterniti et al., 2017). Melatonin has been shown to attenuate histological damage, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha (IκB-α) degradation, nuclear factor κB (NFκB) activation, polymorphonuclear leukocyte infiltration, and mitogen-activated protein kinase (MAPK) activation in the injured spinal cord (Genovese et al., 2005).
Increasing amount of evidence suggests that TNF-α may play a role in the acute phase of SCI. Accordingly, Haddadi et al. (2013) evaluated the effects of melatonin on TNF-α expression in rats after radiation-induced SCI. Four groups of rats were studied: in group 1, rats received vehicle treatment (control); in group 2, rats were treated with vehicle followed by radiation; in group 3, rats were orally administered melatonin (100 mg/kg) followed by radiation; and in group 4, rats were orally administered melatonin (100 mg/kg) only. Melatonin (5 mg/kg) was also administered daily in groups 3 and 4. Three weeks after irradiation injury, TNF-α expression was significantly up-regulated in the spinal cord of rats in the group 2 compared with control group. TNF-α expression was significantly down-regulated in rats in the group 3 compared with control group. These findings suggest that oral melatonin may inhibit the upregulation of TNF-α expression after radiation-induced SCI (Haddadi and Fardid, 2015).
Promoting BSCB repair
Vascular endothelial growth factor (VEGF) plays an important role in BSCB disruption, vascular alterations, and the development of necrotic lessions (Wong and Van der Kogel, 2004). Previous in vitro studies have shown that melatonin can modulate the expression of VEGF in human neuroblastoma cells (González et al., 2017), serous papillary ovarian carcinoma cells (Zonta et al., 2017), and human gastric cancer cells (Wang et al., 2016). VEGF has been also shown to play an important role in radiation-induced SCI, and melatonin may exert a neuroprotective action through inhibiting VEGF expression (Haddadi et al., 2013). Both real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assays showed that VEGF expression was upregulated from 8 to 22 weels after irradiation injury in rats, as compared to that in control group. However, the increased VEGF expression after irradiation was significantly reduced in rats treated with melatonin compared with vehicle-treated rats (Haddadi et al., 2013). Melatonin can also reduce blood loss, facilitate structural and functional recovery, and increases the survival rate of irradiated animals (Haddadi et al., 2013).
A recent study (Wu et al., 2014) investigated the effects of melatonin (5, 10, 25, 50, 100 mg/kg, i.p.) on BSCB in a mouse model of SCI. Results revealed that at 48 hours post-SCI, BSCB permeability was significantly increased in vehicle-treated mice compared with melatonin (50 mg/kg)-treated mice. An immunohistochemical experiment indicated that melatonin may enhance and restore BSCB integrity by regulating tight junction proteins (Wu et al., 2014). Jing et al. (2014) directly investigated the effect of melatonin on microvessels after SCI. In their study, mice were randomly divided into three groups: (1) in the sham operated group, mice underwent a T10 laminectomy without injury; (2) in the SCI group, mice were treated with vehicle; and (3) in the SCI group, mice were treated with melatonin (10 mg/kg, i.p., twice daily for 2 weeks). Their findings suggested that melatonin ameliorated blood loss possibly due to an upregulation of angiopoietin 1 in pericytes. Angiopoietin 1 may attenuate inflammation and apoptosis and protect microvessels by increasing pericytes coverage (Jing et al., 2014). Thus, the neuroprotective effects of melatonin may also stem from its ability to promote BSCB repair and neurologic recovery, and to reverse the decrease of neuroplasticity-associated proteins, including brain-derived neurotrophic factor, synapsin I, and growth-associated protein-43 in the spinal cord.
The molecular mechanisms underlying these beneficial effects of melatonin, as well as the correlation between vascular preservation and neurologic protection should be further investigated (Jing et al., 2017). The optimal dose for the therapeutic effects of melatonin remains to be determined. Systemic administration of melatonin at 10 mg/kg has been reportedly to protect BSCB in both rats (Jing et al., 2017) and mice after SCI (Jing et al., 2014). Yet, Wu et al. (2014) treated SCI mice with 5, 10, 25, 50, and 100 mg/kg melatonin and found that only the 50 mg/kg dose improved the damaged BSCB. Therefore, high dose may not be beneficial to SCI animals.
Inhibition of Apoptosis
Apoptosis is a normal physiological process that maintains the stability of the internal environment through programmed cell death. It requires the activation, expression, and regulation of a series of genes (Wnuk and Kajta, 2017). The major executioners in the apoptotic program are proteases known as caspases (cysteine-dependent aspartate-specific proteases). Caspases can directly and indirectly orchestrate the morphologic changes of the cells during apoptosis (Meyer and Groseth, 2018). Caspases also play a pivotal role in the progression of many neurological disorders. In particular, exacerbated cell apoptosis after SCI was suggested to be a pathological process that contributes to further tissue damage. Melatonin is known to stabilize membranes and thus might limit cell damage and death (Yang et al., 2016). Intriguingly, in ischemic SCI rats that received melatonin treatment (50 mg/kg, subcutaneous), the expression of activated caspase-3 in the spinal cord tissue was significantly reduced, compared with SCI rats after vehicle treatment (Aydemir et al., 2016). The number of cells showing apoptosis was significantly higher in SCI rats (151.0 ± 9.7) than that in sham surgery group (11.3 ± 2.0). Importantly, the number of cells showing apoptosis in SCI rats was significantly reduced after melatonin treatment (82.3 ± 6.9), compared with that after vehicle treatment (Wu et al., 2014). Accordingly, melatonin may reduce the activity of caspases, and hence limits cell apoptosis and protects neurons from secondary injury after SCI. This may represent an important mechanism by which melatonin exerts neuroprotective effects after SCI.
Attenuation of Edema
Edema is another critical factor in the pathogenesis of secondary injury after SCI. When rats were promptly treated with systemic administration of melatonin (100 mg/kg) after SCI for 2 days, the water content in spinal cord tissue of SCI rats at 24, 48 and 72 hours after injury was significantly lower than that in vehicle-treated rats (Li et al., 2014). Immunohistochemical staining and western blot assays further demonstrated that melatonin suppressed the expression of aquaporin (AQP)-4 and glial fibrillary acidic protein (GFAP) in the spinal cord after acute SCI (Li et al., 2014). Accordingly, melatonin might alleviate secondary injury after SCI in part by downregulating the expression of AQP4 and reducing astrocytic swelling through downregulation of GFAP (Liu et al., 2015). In line with these findings, Nesic et al. (2008) reported that systemic administration of melatonin (10 mg/kg, subcutaneous) once daily for 14 or 35 consecutive days after SCI reduced AQP-1 expression in the spinal cord, which correlated with attenuation of behavioral and mechanical pain hypersensitivity, suggesting a possible role of AQP-1 in SCI-related pain. Together, these findings suggest that melatonin can attenuate edema in neurons and reduce astrocytic swelling.
Neuroprotective Effects of Melatonin in Other Tissues
SCI induces oxidative stress and can affect multiple organs. Akakin et al. (2013) examined the possible protective effect of melatonin on SCI-induced oxidative stress in renal tissue. Melatonin was administrated intraperitoneally (10 mg/kg) for 7 days in SCI rats. One week after sham operation or SCI induction, kidney tissues were collected for measuring MDA and GSH levels, and for determining MPO and SOD activities. Compared with vehicle-treated SCI rats, MDA and MPO activities were decreased, and GSH level was increased in melatonin-treated SCI rats. These results suggested that melatonin reduced oxidative stress in renal tissue after SCI, and might be a good candidate for managing renal problems after spinal cord trauma.
Erşahin et al. (2012) also reported that SCI rats treated with systemic administration of melatonin (10 mg/kg, i.p.) for a week showed a decreased level of tissue injury and improved bladder functions, compared with SCI rats that received vehicle treatment. Furthermore, this beneficial effect of melatonin is possibly through inhibition of oxidative stress and induction of nerve growth factor. Combining melatonin with tadalafil, a well-known phosphodiesterase inhibitor used to treat erectile dysfunction, prevented SCI-induced oxidative damage to cavernosal tissues and restored erectile function. These effects may be due to the anti-oxidant actions of melatonin as mentioned previously. Furthermore, combined treatment with melatonin and tadalafil produced a more pronounced functional recovery after SCI than treatment with either drug alone (Tavukçu Hasan et al., 2014). These findings suggest that melatonin not only plays a key role in neuroprotection, but also helps to preserve other tissue and organ functions after SCI, which may be related to its antioxidative effect and facilitation of nerve regeneration.
Regulation of Matrix Metalloproteinases (MMPs)
MMPs regulate developmental processes, maintain normal physiology in adulthood, and exert a reparative role after insults to the nervous system (Esposito et al., 2008). MMPs, especially MMP-2 and MMP-9, play an important role in the secondary inflammatory reaction and blood-brain barrier disruption after SCI. In particular, MMP-9/gelatinase B was considered to be the mediator of early secondary neuronal damage after SCI (Noble et al., 2002). It is hence important to note that a recent study suggested that exogenous melatonin administration after photothrombotic SCI in rats effectively attenuated the increased expression and activation of MMP-9, and led to an improvement in functional outcomes (Piao et al., 2014). In that study, SCI rats were divided into two groups receiving melatonin (50 mg/kg, i.p., starting at 1 hour after injury with a 12 hour interval) or weight-adjusted dose of vehicle for 7 days. The expressions and activities of MMP-9 were examined in spinal cord tissue using western blot assay and gelatin zymography from 6 hours to 3 days after SCI. The expressions and activities of MMP-9 were increased at 6, 24, 48, and 72 hours after SCI compared with that in sham operated group. However, the level of MMP-9 at 24, 48, and 72 hours after SCI was significantly lower in the melatonin-treated group than that in the vehicle-treated group (Piao et al., 2014). In a study from Wu et al. (2014), melatonin (5, 10, 25, 50, 100 mg/kg, i.p.) or vehicle was administered to mice immediately after SCI. At 48 hours after SCI, mice treated with melatonin (50 mg/kg) exhibited significantly lower levels of MMP-3, as compared to those treated with vehicle. These findings further support the notion that melatonin may be a promising candidate for ameliorating secondary damage after SCI through inhibiting MMPs.
Synergistic Effects of Melatonin with Other Treatments
Park et al. (2010) investigated the effects of combination therapy of melatonin (10 mg/kg) and exercise on the functional recovery after SCI. Rats were randomly divided into four groups: naïve control, SCI alone, SCI with exercise, and SCI with exercise and melatonin. Spinal cord tissues were harvested on days 1, 3, 7, 14, 21 and 28 after injury. Results showed that melatonin combined with exercise further improved motor function recovery, suppressed secondary inflammatory response, and enhanced neuronal regeneration after contusion SCI, compared with melatonin alone. The combined treatment also increased the endogenous neural stem/progenitor cells (nestin-positive eNSPCs) and reconstituted neuronal differentiation. Melatonin treatment together with exercise also reduced iNOS mRNA level in the spinal cord and decreased the degeneration of motor neurons. These beneficial effects promoted neuronal reconstruction and functional recovery after SCI (Park et al., 2010).
Another study investigated the effects of melatonin and tadalafil, alone or in combination, on SCI-induced erectile dysfunction (Tavukçu Hasan et al., 2014). In that study, male Wistar albino rats were divided into five groups: one sham-operated control group and four SCI-injured groups. Rats in the SCI-injured groups received vehicle, melatonin (10 mg/kg, i.p.), tadalafil (10 mg/kg, peroral), or a combination of melatonin and tadalafil). On day 7 after SCI, intracavernosal pressure (ICP) was measured and all rats were decapitated. As mentioned earlier, melatonin combined with tadalafil led to a greater functional recovery after SCI than the effect induced by either drug alone (Tavukçu Hasan et al., 2014).
Genovese et al. (2007) investigated the effects of treatment that combines melatonin with dexamethasone on SCI caused by vascular clip application. Mice were randomly allocated into the following six groups: (1) SCI + vehicle (mice were subjected to SCI followed by i.p. administration of vehicle at 1 and 4 hours after SCI); (2) SCI + dexamethasone (0.025 mg/kg) + melatonin (10 mg/kg) (mice were subjected to SCI followed by intraperitoneal administration of dexamethasone and melatonin); (3) SCI + dexamethasone; (4) SCI + melatonin; (5) sham + vehicle; (6) sham + dexamethasone + melatonin. Findings from this experiment confirmed that low dose melatonin (10 mg/kg) combined with dexamethasone (0.025 mg/kg) significantly attenuated tissue damage, infiltration of polymorphonuclear leukocytes in the spinal cord, TNF-α expression, nitration of tyrosine residues, iNOS expression, and apoptosis. Motor function recovery was also improved. These effects were not observed in animals treated with a single drug. The combined therapy decreased the effective dose of dexamethasone by approximately 10-fold (Genovese et al., 2007). Melatonin in combination with methylprednisolone improved neurologic recovery and resulted in a cumulative inhibitory effect on lipid peroxidation level in the subacute phase of injury. Thus, combined drug therapy may provide better outcome by inhibiting the occurrence of secondary injury after SCI. A better understanding of the dose-response relationship and mechanisms of combined drug treatment may offer better therapeutic options (Cayli et al., 2004).
Influence of SCI Level on Melatonin Secretion
SCI patients, especially those with tetraplegia, experience poor sleep quality, perhaps because of impaired circadian rhythmicity. Verheggen et al. (2012) examined the evening onset of melatonin secretion in patients with a complete cervical (tetraplegia) or thoracic (paraplegia) SCI. Samples of salivary melatonin were obtained during the evening hours and analyzed by enzyme linked immunosorbent assay (ELISA). They found that poor sleep quality was more prevalent in individuals with tetraplegia (83%) and paraplegia (75%) than in able-bodied controls (20%; P = 0.02). Interestingly, they identified an evening increase in melatonin concentration in control and paraplegia groups, but the increase was completely absent in the tetraplegia group. Since melatonin is mainly secreted by the pineal gland that is governed by nerves of cervical spine and thoracic spine (C8–T2), tetraplegia patients who have a traumatic SCI at or above the level of C8 spinal cord cannot retain sufficient melatonin release. Paraplegias patients with SCI below the level of T4 spinal cord may retain the rhythmicity of melatonin release. This finding provides biological insight into the poor sleep quality of patients with tetraplegia. Individuals with SCI show altered circadian variation in thermoregulatory control. To examine whether these findings are related to circadian thermoregulation, Jones et al. (2014) examined the correlations between evening changes in melatonin and core and skin temperatures in individuals with thoracic SCI, cervical SCI, and no injury. They found an inverse correlation between evening changes in melatonin and thermoregulation in both paraplegic and able-bodied controls. In contrast, changes in skin temperature below the level of the lesion were not correlated with changes in melatonin in patients with tetraplegia. Clinical studies also have suggested that patients with tetraplegia are more likely to lose their melatonin production at night than those with only mild paralysis (Zeitzer et al., 2005). These results indicate that neurologically complete cervical spinal injury results in a complete loss of pineal melatonin production. However, neither the loss of melatonin nor the loss of spinal afferent information disrupts the rhythmicity of cortisol or thyroid-stimulating hormone (TSH) secretion (Zeitzer et al., 2005). In a study involving rats with SCI, Gezici et al. (2010) reported that melatonin secretion began immediately after SCI but slowed a substantial level between 2 and 6 hours afterward. Tetraplegia rats that had complete injuries at the lower cervical spinal cord produced insufficient melatonin. In contrast, paraplegia rats that had complete injury at the upper thoracic spinal cord showed normal melatonin secretion (Gezici et al., 2010). The absence of nighttime melatonin secretion after cervical SCI may partially explain sleep disturbances. These findings raise the possibility that melatonin replacement therapy may improve sleep in patients with tetraplegia (Scheer et al., 2006).
Other Pathways Affected by Melatonin
Melatonin also induces activation of antioxidant enzymes, which reduce damage caused by free radicals and inhibit activation of a large number of peroxidases (Erten et al., 2003). Prophylactic zinc and melatonin administration not only suppressed lipid peroxidation by activating antioxidant systems but also exerted neuroprotective effects by specifically improving the neurologic and histopathologic conditions in a rabbit spinal cord ischemia model (Kalkan et al., 2007). Melatonin attenuated ischemia-induced necrosis and reduced injury after temporary aortic occlusion in this model (Korkmaz et al., 2008). Repeated treatment with melatonin also decreased phospho-p38 expression, phospho-c-Jun N-terminal kinase (JNK) expression, phospho-extracellular signal-regulated kinases (ERK)1/2 and phospho-ERK2 expression, and high mobility group box 1 (HMGB1) expression, and reduced histologic damage, motor deficit, and pro-inflammatory cytokine production after SCI (Esposito et al., 2009).
Recent findings suggested that melatonin might also modulate important transcription factors (Naseem and Parvez, 2014). Such activity would provide important insights into new mechanisms by which melatonin might regulate neural function. Shen et al. (2017) recently reported that melatonin reduced neuronal apoptosis and improved functional recovery in a rat model of SCI by activating the Wnt/β-catenin signaling pathway. Activation of Wnt/β-catenin signaling would suggest that melatonin might also be therapeutic for other neurodegenerative diseases (Shen et al., 2017). Gao et al. (2016) showed that treating mice with melatonin in combination with Wnt-4 promoted neurogenesis and neural cell differentiation. In contrast, inhibition of melatonin receptor 1 or Wnt-4 expression decreased the expression of neurogenesis-related genes in bovine amniotic epithelial cell-derived neural cells in vitro (Gao et al., 2016). These findings suggest that melatonin may play a protective role in SCI by promoting neurogenesis.
Oligodendrocytes are responsible for myelination in the CNS. In addition to neuronal damage, oligodendrocyte death and axon demyelination are observed after SCI. Oligodendrocyte survival and axon remyelination are closely related to the recovery of motor function. Therefore, it is important to reduce oligodendrocyte loss after SCI (Ward et al., 2014). Inhibition of cysteine protease can protect oligodendrocytes, so melatonin may protect neurologic function by inhibiting cysteine protease (Ray et al., 2011). However, whether melatonin receptor can exert neuroprotective effects after SCI through protecting oligodendrocytes with above mentioned mechanisms has not yet been demonstrated.
Conclusions
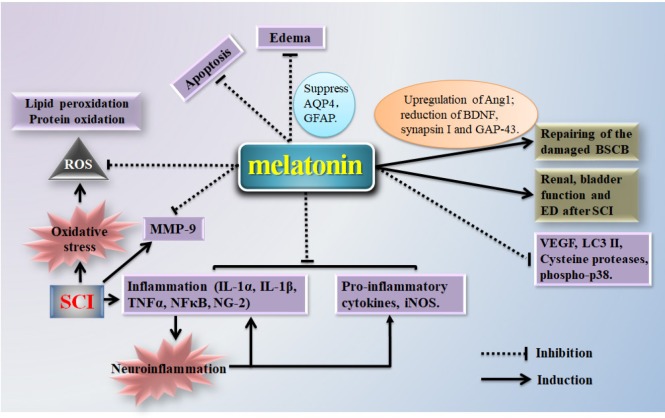
Preclinical studies suggest that melatonin may exert neuroprotective effects and promote the restoration of neurologic function after SCI (Figure 1). Multiple mechanisms are potentially involved, including antioxidant effects, regulation of iNOS, inhibition of proinflammatory cytokines, blood vessel repair, restoration of the blood-brain barrier, inhibition of cell apoptosis, edema resistance, inhibition of cell autophagy, and regulation of MMP, p38, JNK, ERK1/2, and MAPK pathways. The beneficial effects of melatonin have been shown in multiple animal models and in both sexes. Nevertheless, additional studies are needed to determine whether sex differences exist in the efficacy, therapeutic features, and mechanisms of action of melatonin. The clinical relevance of previous findings in animal SCI models remains obscure. Most clinical trials regarding melatonin in SCI patients aim to improve sleep disorder, for example, melatonin replacement therapy is used to improve sleep in patients with tetraplegia (Scheer et al., 2006). There is a pressing need for future clinical studies to examine and validate major findings in animal studies related to other major actions of melatonin, such as the antioxidant effect by regulating iNOS, inhibiting pro-inflammatory cytokines, repairing injured blood vessels, improving edema, and inhibiting cell autophagy. Future studies are also needed to clarify the cellular and molecular mechanisms that underlie the therapeutic effects of melatonin and to provide evidence-based rationales for the development of clinical therapies that can be used in SCI patients.
Figure 1.
Potential mechanisms underlying the beneficial effects of melatonin in spinal cord injury (SCI).
Oxidative stress can be induced by free radical generation and lipid peroxidation after SCI. Melatonin may act as a radical scavenger after SCI, as it moderates the increased reactive oxygen species (ROS). Melatonin can also reduce inducible nitric oxide synthase (iNOS) expression and neuroinflammation, and it plays an important role in promoting the repair of damaged blood-spinal cord barrier (BSCB). Furthermore, melatonin can inhibit apoptosis and reduce edema, which are both key elements in the pathogenesis of secondary injury after SCI. Melatonin also plays a protective role by reducing the expression of vascular endothelial growth factor (VEGF), microtubule-associated protein 1A/1B-light chain 3-phosphatidylethanolamine conjugate (LC3 II), cysteine proteases, phospho-p38, and matrix metalloproteinase-9 (MMP-9). Ang1: Angiopoietin 1; AQP4: aquaporin 4; BDNF: brain-derived neurotrophic factor; ED: erectile dysfunction; GAP-43: growth-associated protein-43; GFAP: glial fibrillary acidic protein; IL: interleukin; NFκB: nuclear factor kappaB; NG-2: neuron/glial antigen 2; TNFα: tumor necrosis factor alpha.
Additional files:
Additional Table 1 (99.8KB, pdf) : Characteristics of the major studies.
Additional file 1: Open peer reviewer report 1 (6KB, pdf) .
Acknowledgments
The authors thank Claire F. Levine, MS (scientific editor, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University) for editing the manuscript.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by the National Natural Science Foundation of China (No. 81671161; to ZJL). The funding body played no role in this work other than to provide funding.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Stephanie K Seidlits, University of California Los Angeles, USA.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81671161 (to ZJL).
(Copyedited by Yu J, Li CH, Song LP, Zhao M)
References
- Akakin D, Kiran D, Ozkan N, Erşahin M, Ozdemir-Kumral ZN, Yeğen B, Sener G. Protective effects of melatonin against spinal cord injury induced oxidative damage in rat kidney: A morphological and biochemical study. Acta Histochem. 2013;115:827–834. doi: 10.1016/j.acthis.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Aydemir S, Dogan D, Kocak A, Dilsiz N. The effect of melatonin on spinal cord after ischemia in rats. Spinal Cord. 2016;54:360–363. doi: 10.1038/sc.2015.204. [DOI] [PubMed] [Google Scholar]
- Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta. 2012;1822:675–684. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin TF, Arbab AS, Gelaleti GB, Ferreira LC, Moschetta MG, Jardim-Perassi BV, Iskander AS, Varma NR, Shankar A, Coimbra VB, Fabri VA, de Oliveira JG, Zuccari DA. Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J Pineal Res. 2016;60:3–15. doi: 10.1111/jpi.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayli SR, Kocak A, Yilmaz U, Tekiner A, Erbil M, Ozturk C, Batcioglu K, Yologlu S. Effect of combined treatment with melatonin and methylprednisolone on neurological recovery after experimental spinal cord injury. Eur Spine J. 2004;13:724–732. doi: 10.1007/s00586-003-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DA, Estévez AG, Barbeito L, Beckman JS. Nitric oxide-mediated oxidative damage and the progressive demise of motor neurons in ALS. Neurotox Res. 2012;22:251–264. doi: 10.1007/s12640-012-9322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol FS, Kaplan M, Tiftikci M, Yakar H, Ozercan I, Ilhan N, Topsakal C. Comparison of the effects of octreotide and melatonin in preventing nerve injury in rats with experimental spinal cord injury. J Clin Neurosci. 2008;15:784–790. doi: 10.1016/j.jocn.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Erşahin M, Özdemir Z, Özsavcı D, Akakın D, Yeğen Berrak Ç, Reiter Russel J, Şener G. Melatonin treatment protects against spinal cord injury induced functional and biochemical changes in rat urinary bladder. J Pineal Res. 2012;52:340–348. doi: 10.1111/j.1600-079X.2011.00948.x. [DOI] [PubMed] [Google Scholar]
- Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS. Protective effect of melatonin on experimental spinal cord ischemia. Spinal Cord. 2003;41:533–538. doi: 10.1038/sj.sc.3101508. [DOI] [PubMed] [Google Scholar]
- Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin regulates matrix metalloproteinases after traumatic experimental spinal cord injury. J Pineal Res. 2008;45:149–156. doi: 10.1111/j.1600-079X.2008.00569.x. [DOI] [PubMed] [Google Scholar]
- Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J Pineal Res. 2009;46:79–86. doi: 10.1111/j.1600-079X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- Gómez RM, Sanchez MY, Portela-Lomba M, Ghotme K, Barreto GE, Sierra J, Moreno-Flores MT. Cell therapy for spinal cord injury with olfactory ensheathing glia cells (OECs) Glia. 2018 doi: 10.1002/glia.23282. doi: 10.1002/glia.23282. [DOI] [PubMed] [Google Scholar]
- Gao Y, Bai C, Zheng D, Li C, Zhang W, Li M, Guan W, Ma Y. Combination of melatonin and Wnt-4 promotes neural cell differentiation in bovine amniotic epithelial cells and recovery from spinal cord injury. J Pineal Res. 2016;60:303–312. doi: 10.1111/jpi.12311. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Esposito E, Di Paola R, Muia C, Di Bella P, Bramanti P, Cuzzocrea S. Effects of combination of melatonin and dexamethasone on secondary injury in an experimental mice model of spinal cord trauma. J Pineal Res. 2007;43:140–153. doi: 10.1111/j.1600-079X.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Muià C, Bramanti P, De Sarro A, Cuzzocrea S. Attenuation in the evolution of experimental spinal cord trauma by treatment with melatonin. J Pineal Res. 2005;38:198–208. doi: 10.1111/j.1600-079X.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- Gezici AR, Karakaş A, Ergün R, Gündüz B. Rhythms of serum melatonin in rats with acute spinal cord injury at the cervical and thoracic regions. Spinal Cord. 2010;48:10–14. doi: 10.1038/sc.2009.73. [DOI] [PubMed] [Google Scholar]
- Ghaisas MM, Ahire YS, Dandawate PR, Gandhi SP, Mule M. Effects of combination of thiazolidinediones with melatonin in dexamethasone-induced insulin resistance in mice. Indian J Pharm Sci. 2011;73:601–607. doi: 10.4103/0250-474X.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A, González-González A, Alonso-González C, Menéndez-Menéndez J, Martínez-Campa C, Cos S. Melatonin inhibits angiogenesis in SH-SY5Y human neuroblastoma cells by downregulation of VEGF. Oncol Rep. 2017;37:2433–2440. doi: 10.3892/or.2017.5446. [DOI] [PubMed] [Google Scholar]
- Haddadi G, Shirazi A, Sepehrizadeh Z, Mahdavi SR, Haddadi M. Radioprotective effect of melatonin on the cervical spinal cord in irradiated rats. Cell J. 2013;14:246–253. [PMC free article] [PubMed] [Google Scholar]
- Haddadi GH, Fardid R. Oral administration of melatonin modulates the expression of tumor necrosis factor-alpha (TNF-alpha) gene in irradiated rat cervical spinal cord. Rep Pract Oncol Radiother. 2015;20:123–127. doi: 10.1016/j.rpor.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson DW, Bedforth NM, Hardman JG. Spinal cord injury arising in anaesthesia practice. Anaesthesia. 2018;73(Suppl 1):43–50. doi: 10.1111/anae.14139. [DOI] [PubMed] [Google Scholar]
- Jing Y, Bai F, Chen H, Dong H. Melatonin prevents blood vessel loss and neurological impairment induced by spinal cord injury in rats. J Spinal Cord Med. 2017;40:222–229. doi: 10.1080/10790268.2016.1227912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Wu Q, Yuan X, Li B, Liu M, Zhang X, Liu S, Li H, Xiu R. Microvascular protective role of pericytes in melatonin-treated spinal cord injury in the C57BL/6 mice. Chin Med J (Engl) 2014;127:2808–2813. [PubMed] [Google Scholar]
- Jones H, Eijsvogels TM, Nyakayiru J, Verheggen RJ, Thompson A, Groothuis JT, Atkinson G, Hopman MT, Thijssen DH. Within-subject correlations between evening-related changes in body temperature and melatonin in the spinal cord injured. Chronobiol Int. 2014;31:157–165. doi: 10.3109/07420528.2013.833516. [DOI] [PubMed] [Google Scholar]
- Kalkan E, Ciçek O, Unlü A, Abuşoglu S, Kalkan SS, Avunduk MC, Baysefer A. The effects of prophylactic zinc and melatonin application on experimental spinal cord ischemia-reperfusion injury in rabbits: experimental study. Spinal Cord. 2007;45:722–730. doi: 10.1038/sj.sc.3102035. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Oyar EO, Kardes O, Omeroglu S. Effects of melatonin on ischemic spinal cord injury caused by aortic cross clamping in rabbits. Curr Neurovasc Res. 2008;5:46–51. doi: 10.2174/156720208783565681. [DOI] [PubMed] [Google Scholar]
- Krityakiarana W, Sompup K, Jongkamonwiwat N, Mukda S, Pinilla FG, Govitrapong P, Phansuwan-Pujito P. Effects of melatonin on severe crush spinal cord injury-induced reactive astrocyte and scar formation. J Neurosci Res. 2016;94:1451–1459. doi: 10.1002/jnr.23930. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp Neurol. 2011;231:135–146. doi: 10.1016/j.expneurol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Li C, Chen X, Qiao SC, Liu XW, Liu C, Zhu DG, Su JC, Wang ZW. Melatonin lowers edema after spinal cord injury. Neural Regen Res. 2014;9:2205–2210. doi: 10.4103/1673-5374.147954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Schackel T, Weidner N, Puttagunta R. Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front Cell Neurosci. 2017;11:430. doi: 10.3389/fncel.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Y, Yang J, Liu Y, Zhou D, Hou M, Xiang L. Anti-edema effect of melatonin on spinal cord injury in rats. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:220–226. doi: 10.5507/bp.2015.012. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- Meyer B, Groseth A. Apoptosis during arenavirus infection: mechanisms and evasion strategies. Microbes Infect. 2018;20:65–80. doi: 10.1016/j.micinf.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal. 2014;2014:586270. doi: 10.1155/2014/586270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic O, Lee J, Unabia GC, Johnson K, Ye Z, Vergara L, Hulsebosch CE, Perez-Polo JR. Aquaporin 1 - a novel player in spinal cord injury. J Neurochem. 2008;105:628–640. doi: 10.1111/j.1471-4159.2007.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Lee Y, Park S, Lee S, Hong Y, Kil Lee S, Hong Y. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J Pineal Res. 2010;48:270–281. doi: 10.1111/j.1600-079X.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- Park S, Lee SK, Park K, Lee Y, Hong Y, Lee S, Jeon JC, Kim JH, Lee SR, Chang KT, Hong Y. Beneficial effects of endogenous and exogenous melatonin on neural reconstruction and functional recovery in an animal model of spinal cord injury. J Pineal Res. 2012;52:107–119. doi: 10.1111/j.1600-079X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Campolo M, Cordaro M, Impellizzeri D, Siracusa R, Crupi R, Esposito E, Cuzzocrea S. PPAR-alpha modulates the anti-inflammatory effect of melatonin in the secondary events of spinal cord injury. Mol Neurobiol. 2017;54:5973–5987. doi: 10.1007/s12035-016-0131-9. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta. 2016;1862:483–491. doi: 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Piao MS, Lee JK, Jang JW, Hur H, Lee SS, Xiao L, Kim HS. Melatonin improves functional outcome via inhibition of matrix metalloproteinases-9 after photothrombotic spinal cord injury in rats. Acta Neurochir (Wien) 2014;156:2173–2182. doi: 10.1007/s00701-014-2119-4. [DOI] [PubMed] [Google Scholar]
- Ray SK, Samantaray S, Smith JA, Matzelle DD, Das A, Banik NL. Inhibition of cysteine proteases in acute and chronic spinal cord injury. Neurotherapeutics. 2011;8:180–186. doi: 10.1007/s13311-011-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Liu G, Chen S, Yin J, Wang J, Tan B, Wu G, Bazer Fuller W, Peng Y, Li T, Reiter Russel J, Yin Y. Melatonin signaling in T cells: Functions and applications. J Pineal Res. 2017;62:e12394. doi: 10.1111/jpi.12394. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Rodrigues LF, Moura-Neto V, E Spohr TCLS. Biomarkers in spinal cord injury: from prognosis to treatment. Mol Neurobiol. 2018 doi: 10.1007/s12035-017-0858-y. doi: 10.1007/s12035-017-0858-y. [DOI] [PubMed] [Google Scholar]
- Rogério F, Teixeira SA, Júnior HJ, Maria CCJ, Vieira AS, de Rezende ACS, Pereira GAG, Muscará MN, Langone F. mRNA and protein expression and activities of nitric oxide synthases in the lumbar spinal cord of neonatal rats after sciatic nerve transection and melatonin administration. Neurosci Lett. 2006;407:182–187. doi: 10.1016/j.neulet.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Zeitzer JM, Ayas NT, Brown R, Czeisler CA, Shea SA. Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord. 2006;44:78–81. doi: 10.1038/sj.sc.3101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Zhou Z, Gao S, Guo Y, Gao K, Wang H, Dang X. Melatonin inhibits neural cell apoptosis and promotes locomotor recovery via activation of the Wnt/beta-catenin signaling pathway after spinal cord injury. Neurochem Res. 2017;42:2336–2343. doi: 10.1007/s11064-017-2251-7. [DOI] [PubMed] [Google Scholar]
- Tavukçu Hasan H, Şener Tarik E, Tinay İ, Akbal C, Erşahin M, Çevik Ö, Çadırcı S, Reiter Russel J, Şener G. Melatonin and tadalafil treatment improves erectile dysfunction after spinal cord injury in rats. Clin Exp Pharmacol Physiol. 2014;41:309–316. doi: 10.1111/1440-1681.12216. [DOI] [PubMed] [Google Scholar]
- Verheggen RJ, Jones H, Nyakayiru J, Thompson A, Groothuis JT, Atkinson G, Hopman MT, Thijssen DH. Complete absence of evening melatonin increase in tetraplegics. FASEB J. 2012;26:3059–3064. doi: 10.1096/fj.12-205401. [DOI] [PubMed] [Google Scholar]
- Wang RX, Liu H, Xu L, Zhang H, Zhou RX. Melatonin downregulates nuclear receptor RZR/RORγ expression causing growth-inhibitory and anti-angiogenesis activity in human gastric cancer cells in vitro and in vivo. Oncol Lett. 2016;12:897–903. doi: 10.3892/ol.2016.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RE, Huang W, Kostusiak M, Pallier PN, Michael-Titus AT, Priestley JV. A characterization of white matter pathology following spinal cord compression injury in the rat. Neuroscience. 2014;260:227–239. doi: 10.1016/j.neuroscience.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Wnuk A, Kajta M. Steroid and xenobiotic receptor signalling in apoptosis and autophagy of the nervous system. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004;4:273–284. doi: 10.1124/mi.4.5.7. [DOI] [PubMed] [Google Scholar]
- Wu Q, Jing Y, Yuan X, Zhang X, Li B, Liu M, Wang B, Li H, Liu S, Xiu R. Melatonin treatment protects against acute spinal cord injury-induced disruption of blood spinal cord barrier in mice. J Mol Neurosci. 2014;54:714–722. doi: 10.1007/s12031-014-0430-4. [DOI] [PubMed] [Google Scholar]
- Yang L, Yao M, Lan Y, Mo W, Sun YL, Wang J, Wang YJ, Cui XJ. Melatonin for spinal cord injury in animal models: a systematic review and network meta-analysis. J Neurotrauma. 2016;33:290–300. doi: 10.1089/neu.2015.4038. [DOI] [PubMed] [Google Scholar]
- Yang R, Guo L, Wang P, Huang L, Tang Y, Wang W, Chen K, Ye J, Lu C, Wu Y, Shen H. Epidemiology of spinal cord injuries and risk factors for complete injuries in Guangdong, China: a retrospective study. PLoS One. 2014;9:e84733. doi: 10.1371/journal.pone.0084733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Ayas NT, Wu AD, Czeisler CA, Brown R. Bilateral oculosympathetic paresis associated with loss of nocturnal melatonin secretion in patients with spinal cord injury. J Spinal Cord Med. 2005;28:55–59. doi: 10.1080/10790268.2005.11753798. [DOI] [PubMed] [Google Scholar]
- Zhang LM, Wang X, Gao WJ, Huang DS, Rong LM. Melatonin inhibits adipogenic differentiation of bone marrow mesenchymal stem cells through retinoid-related orphan receptor alpha. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:2968–2974. [Google Scholar]
- Zonta YR, Martinez M, Camargo IC, Domeniconi RF, Lupi Junior LA, Pinheiro PF, Reiter RJ, Martinez FE, Chuffa LG. Melatonin reduces angiogenesis in serous papillary ovarian carcinoma of ethanol-preferring rats. Int J Mol Sci. 2017;18:E763. doi: 10.3390/ijms18040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the major studies.