Abstract
The metabotropic glutamate receptors (mGlu receptors) are G protein-coupled receptors that bind to the excitatory neurotransmitter glutamate and are important in the modulation of neuronal excitability, synaptic transmission, and plasticity in the central nervous system. Trafficking of mGlu receptors in and out of the synaptic plasma membrane is a fundamental mechanism modulating excitatory synaptic function through regulation of receptor abundance, desensitization, and signaling profiles. In this review, we cover the regulatory mechanisms determining surface expression and endocytosis of mGlu receptors, with particular focus on post-translational modifications and receptor-protein interactions. The literature we review broadens our insight into the precise events defining the expression of functional mGlu receptors at synapses, and will likely contribute to the successful development of novel therapeutic targets for a variety of developmental, neurological, and psychiatric disorders.
Keywords: metabotropic glutamate receptors, trafficking, endocytosis, protein-protein interaction, post-translational modification, phosphorylation
1. Introduction
Metabotropic glutamate receptors (mGlu receptors) are class C G protein-coupled receptors (GPCRs) that modulate synaptic transmission and plasticity throughout the CNS. Like all GPCRs, mGlu receptors consist of seven transmembrane domains (TMs) joined by three extracellular and three intracellular loops. The N-terminal extracellular domain (ECD) contains a glutamate-binding Venus flytrap domain (VFD) and a cysteine rich domain. The cytoplasmic C-terminal domain (CTD) modulates receptor signaling, trafficking and G protein coupling. This region is a major target of protein phosphorylation and a hub for protein-protein interactions.
The nomenclature of mGlu receptors is numeric beginning with mGlu1, which was first isolated by expression cloning (Houamed et al., 1991; Masu et al., 1991). The cloning of subsequent mGlu receptors resulted in their sequential numbering. The mGlu receptors are divided into three sub-families based on sequence homology, second messenger coupling, and pharmacological properties. Group I (mGlu1 & mGlu5) receptors are coupled to Gq-like proteins and stimulate phospholipase C (PLC), leading to an elevation of intracellular Ca2+ and activation of protein kinase C (PKC). Group II (mGlu2 & mGlu3) and Group III (mGlu4, mGlu6, mGlu7 & mGlu8) receptors are coupled to Gi/Go proteins and inhibit adenylate cyclase (AC), which leads to a reduction of cAMP and inactivation of protein kinase A (PKA). The signaling cascades of mGlu receptors are far more complex than these canonical pathways (Niswender and Conn, 2010; Willard and Koochekpour, 2013). Group I mGlu receptors, for example, can modulate unconventional signaling pathways involving Gi/o and Gs (Aramori and Nakanishi, 1992; Francesconi and Duvoisin, 1998; Hermans et al., 2000; Thomsen, 1996). Furthermore, mGlu7 can stimulate PLC through the activation of a pertussis toxin (PTX)-sensitive or -resistant G protein (Martin et al., 2010; Perroy et al., 2000), and mGlu2 or mGlu4 can activate Gα15 and PLC (Gomeza et al., 1996).
The distribution and subcellular localization differs between the subtypes of mGlu receptors. Most mGlu receptors are broadly expressed in the brain, except for mGlu6, which is restricted to the retina. Some mGlu receptors are additionally found in glial cells, such as mGlu3 and mGlu5 (Aronica et al., 2003; Schools and Kimelberg, 1999). Group I mGlu receptors are mainly localized at postsynaptic sites, whereas Group II and Group III receptors are preferentially expressed in presynaptic axon terminals. Group II mGlu receptors are located away from the glutamate release sites, whereas Group III mGlu receptors are typically localized at the presynaptic active zones (Pinheiro and Mulle, 2008; Shigemoto et al., 1997).
This review focuses on the mechanisms that regulate mGlu receptor trafficking, including phosphorylation, protein interactions, and differential endocytic sorting and degradation. As with other GPCRs, second messenger-dependent protein kinases, such as PKA, PKC, and Ca2+/calmodulin-dependent protein kinase II (CaMKII), contribute to endocytosis and desensitization. Receptor phosphorylation additionally regulates binding to cytosolic proteins via their CTDs or intracellular loop (iL) domains (Dhami and Ferguson, 2006; Ritter and Hall, 2009). In this review, we discuss the current literature on mGlu receptor-specific binding partners that modulate surface expression and signaling.
2. Group I mGlu receptors
Group I mGlu receptors (mGlu1 and mGlu5) are Gαq-linked GPCRs, and binding of glutamate triggers a sequence of events: activation of Gαq/11 proteins leading to PLC activity that generates inositol 1,4,5–trisphosphate (IP3) and diacyl glycerol (DAG). IP3 binds to IP3 receptors leading to the release of intracellular Ca2+ from smooth endoplasmic reticulum (SER). DAG activates transient receptor potential canonical (TRPC)-mediated currents. Simultaneous binding of elevated Ca2+ and DAG activates PKC. mGlu1 and 5 are primarily expressed in the CNS and have distinct and sometimes overlapping distributions in different subtypes of neurons. Group I mGlu receptors are postsynaptic and are located in perisynaptic regions of the postsynaptic membrane of the excitatory neurons. mGlu1 and mGlu5 receptors share about 70% sequence homology, and also share many conserved signaling pathways and trafficking routes. There are many factors that have an impact on the trafficking of Group I mGlu receptors.
2.1. Alternative splice variants
Both mGlu1 and mGlu5 receptors undergo alternative splicing (Ferraguti et al., 2008; Pin and Duvoisin, 1995), with mGlu1 being the most extensively studied vis a vis alternative splicing and protein trafficking. mGlu1 has eight isoforms with CTDs of differing lengths (mGlu1a–e, mGlu1g, mGlu1g-393, mGlu1g-620, and mGlu1h) and a truncated ECD (taste-mGlu1, (San Gabriel et al., 2005)). A rare ATD (amino terminal domain) form of mGlu1E55 cloned from a mouse brain cDNA library has also been found in brain and heart tissue (Zhu et al., 1999). The CTDs of Group I mGlu receptors include many signaling elements, phosphorylation sites, and protein binding motifs. The longest and most prevalent form of mGlu1 receptor is mGlu1a, which has a long intracellular CTD and displays a relatively higher affinity for agonists compared to the other short forms of mGlu1 (Flor et al., 1996). Like mGlu1a, the CTD splice variants of mGlu1 are expressed postsynaptically; however, there is some variability with some located further away from the postsynaptic density than mGlu1a (Mateos et al., 1998; Mateos et al., 2000). The shorter forms of mGlu1 are missing many conserved regulatory motifs and are therefore, not unexpectedly, traffic differently.
One splice variant, mGlu1b, is characterized by a shorter CTD that derives from the insertion of an 61-bp exon (exon 9) with an in-frame stop codon, which produces 20 unique amino acids instead of a long CTD (Ferraguti et al., 2008). While mGlu1a is robustly expressed on the cell surface, surface expression of mGlu1b is weaker (Chan et al., 2001). Chan et al. were able to identify four positively charged amino acids, 877-RRKK-880, as an ER retention motif in the CTD of both mGlu1a and mGlu1b (Chan et al., 2001). C-terminal end truncation experiments of mGlu1a were also used to successfully map the region (amino acids 975 to 1098) of the CTD that can effectively mask ER-retention signal of mGlu1a. Interestingly, the RRKK motif can also regulate the dimerization of the receptors. As this RRKK motif is not conserved in mGlu5, mGlu5a and mGlu5b can exist as a heterodimer. Hetero-dimerization of mGlu1b with mGlu1a is inhibited resulting in the aberrant intracellular accumulation of mGlu1b (Remelli et al., 2008). However, Kumpost et al. has found that the long CTD of mGlu1a might neutralize the RRKK motif of an adjacent mGlu1b, reporting that mGlu1a co-expressed with mGlu1b readily forms heterodimers and promotes trafficking of mGlu1b to the cell surface (Kumpost et al., 2008). mGlu1b is found preferentially in complexes with mGlu1a in the brain and redistributed from soma to dendritic compartments upon co-expression with mGlu1a in cortical neurons (Techlovska et al., 2014). These results indicate that the ER-retention motif in mGlu1 regulates membrane trafficking of mGlu1 isoforms with differential effects based on splice variants and heterodimerization.
2.2. Domain structure and receptor trafficking
Membrane trafficking of Group I mGlu receptors has been focused almost entirely on the CTD of the receptors (see Table 1), particularly regarding phosphorylation sites, ER retention motifs, as well as a large number of protein binding sites as discussed in detail later in this review. Conversely, little attention has been focused on the ECD and the 7TM domains of mGlu receptors. It is worth noting that mGlu1 has many naturally occuring splice variants including a truncated form that consists of only the ECD. Like many other GPCRs, mGlu1 and 5 form stable, covalently and structurally linked homodimers with ligands bound to both dimer subunits (Kammermeier and Yun, 2005; Kniazeff et al., 2004). The closely related GABAB receptors 1 and 2 can form heterodimers; however, whether mGlu1 and mGlu5 can form heterodimers is still a topic for debate (Chang and Roche, 2017; Doumazane et al., 2011; Romano et al., 1996). We recently utilized a truncated form of mGlu5 to study dimerization, trafficking and ligand binding. Taking advantage of the strong homodimer formation between the two ECDs of mGlu5, we used distinctly tagged ECDs to study its trafficking and dimerization (Chang and Roche, 2017). We first found that the CTD is not essential for mGlu5 surface expression, whereas the 7th TM appears to be a critical factor influencing its surface expression. We also showed that the mGlu5 ECD could form a dimer with mGlu1 in reciprocal co-IP experiments (Chang and Roche, 2017). It is important to note that consistent differences in membrane trafficking of truncated and ligand binding site mutants of mGlu5 was observed when receptors were expressed in HEK293 cells as opposed to neurons. For instance, one of the ligand binding deficient mutants, mGlu5 T174A, traffics to the plasma membrane in HEK293 cells similar to the WT, whereas the mutation almost abolishes surface expression when the receptor is expressed in neurons. Furthermore, dominant negative effects of the ECD upon co-expression with full-length mGlu5 are observed in HEK293 cells, but not in neurons. These results indicate that in some cases neurons are more sensitive to truncations and mutations. Therefore, it is always important to include analyses of receptor trafficking in neurons and not rely solely on expression in heterologous systems.
Table 1.
A list of Group I mGlu receptor-interacting proteins that regulate cell surface expression of receptors.
2.3. Protein Phosphorylation
2.3.1. PKC phosphorylation and CaM/Siah-1A binding
Group I mGlu receptor signaling is regulated by membrane trafficking events such as endocytosis, as well as posttranslational modifications such phosphorylation. Receptor desensitization is governed by the activity of a variety of serine/threonine protein kinases including PKC, PKA, mitogen-activated protein kinases (MAPKs), CaMKII, and GPCR kinases (GRKs) upon agonist exposure (Kim et al., 2008). Among them, PKC has been extensively characterized as a key kinase acting on both the intracellular loops and the CTD of Group I mGlu receptors. Multiple PKC consensus phosphorylation sites are found on the intracellular loop 1 (iL1) and iL2 in addition to the CTD of Group I mGlu receptors. Phosphorylation by PKC regulates the constitutive and agonist-induced desensitization of mGlu1 (Mao et al., 2008). Specifically, PKC phosphorylates mGlu1 at T695 (T681 on mGlu5), which is responsible for the agonist-induced rapid desensitization of mGlu1. PKC phosphorylation at T695 selectively desensitizes Gq-coupled signaling pathways resulting in IP3 formation without affecting Gs-coupled cAMP signaling (Francesconi and Duvoisin, 2000). In the case of mGlu5, multiple potential PKC phosphorylation sites (T606, S613, T665, S881, and S890) play a role in the rapid desensitization of mGlu5. The T681A, non-phosphorylated, mutation of mGlu5 eliminates basal activity by a complete loss of PLC coupling, and T681 phosphorylation might therefore be responsible for the constitutive signaling or cell surface expression of mGlu5 (Gereau and Heinemann, 1998).
Both mGlu1a and mGlu5 activation leads to the release of Ca2+ from intracellular stores. However, mGlu1 induces a single transient peak of Ca2+, whereas mGlu5 activation elicits high frequency Ca2+ oscillations that are PKC-dependent (Kim et al., 2008). An early study demonstrated that a single mutation within the mGlu5 CTD that swaps the threonine residue to the analogous amino acid in mGlu1 (mGlu5 T840D, a phospho-mimetic mutant) dramatically changed the mGlu5 phenotype to mimic mGlu1 Ca2+ signaling (Kawabata et al., 1996). The assumption was that T840 was the critical PKC phosphorylation site; however, subsequent investigation revealed that T840 itself is not phosphorylated by PKC, but serves a permissive role allowing the phosphorylation of the adjacent S839 residue. Indeed, the phosphorylation of mGlu5 at S839 by PKC is critical for the generation of mGlu5-dependent high frequency Ca2+ oscillations (Kim et al., 2005).
PKC-induced phosphorylation of mGlu5 also affects receptor endocytosis and binding of various intracellular molecules such as calmodulin (CaM) and Siah-1A (seven in absentia homolog-1A). In particular, S901 on mGlu5 was identified as a major PKC phosphorylation site in close proximity to the critical Ca2+-CaM binding residue (Lee et al., 2008b). PKC-induced phosphorylation of S901 by agonist DHPG or PMA treatment increases the agonist-induced internalization of mGlu5 expressed in HeLa cells or endogenous mGlu5 in cultured hippocampal neurons. Agonist-induced internalization of mGlu5 is inversely regulated by CaM binding. Specifically, CaM inhibits the PKC-mediated S901 phosphorylation of mGlu5, whereas PKC-induced phosphorylation of S901 inhibits CaM binding to mGlu5. Thus, PKC phosphorylation and CaM reciprocally regulate mGlu5 trafficking in a Ca2+-dependent manner (Lee et al., 2008b; Minakami et al., 1997) (Fig. 1). CaM binding appears to be the critical factor regulating surface expression of mGlu5 rather than S901 phosphorylation itself, given that the mGlu5 S901A mutant, which is typically stably expressed on the cell surface, can be efficiently internalized by agonist upon knocking down CaM. In addition, PKC phosphorylation of mGlu5 S901 regulates mGlu5 signaling by prolonging intracellular Ca2+ oscillations most likely through increased density of surface mGlu5 (Lee et al., 2008b). CaM can additionally regulate DHPG-stimulated activation of extracellular signal-regulated kinases 1/2 (ERK1/2) and p70-S6 kinase 1 (S6K1) in hippocampal neurons (Sethna et al., 2016). PKC-induced phosphorylation can also regulate the endocytosis of mGlu1. The distal CTD of mGlu1a is important for the PKC- or CaMKII-mediated mGlu1a internalization, because PKC or CaMKII inhibitors suppress the agonist-induced internalization of mGlu1a, but not that of mGlu1b or mGlu1a truncated at V893 (Ciruela and McIlhinney, 1997; Mundell et al., 2002; Mundell et al., 2003).
Fig. 1.
Ca2+-activated CaM differentially regulates trafficking of mGlu5 versus mGlu7 receptors. In the postsynaptic compartments, Ca2+-CaM binds to mGlu5 at a single critical residue, L896, and stabilizes mGlu5 on the postsynaptic plasma membrane. Treatment with the agonist DHPG or PKC activation dissociates CaM binding, increases S901 phosphorylation of mGlu5, and induces mGlu5 endocytosis (Choi et al., 2011; Ko et al., 2012; Lee et al., 2008b). Siah-1A competes with CaM for mGlu5 binding, increases agonist-induced endosomal trafficking, and degrades mGlu5 in lysosomes (Ishikawa et al., 1999; Ko et al., 2012). CaMKIIα dissociates from mGlu5 in competition with CaM and increases agonist-induced receptor endocytosis upon mGlu5-stimulated Ca2+ release (Jin et al., 2013b; Raka et al., 2015). In the presynaptic compartment, CaM reduces PKC-induced phosphorylation of mGlu7 S862 and facilitates endocytosis of mGlu7. PICK1 augments the PKC-induced S862 phosphorylation of mGlu7, enhances stable surface expression of mGlu7, and competes with CaM for binding to mGlu7. PP1γ1 inhibits constitutive and agonist L-AP4-induced internalization of mGlu7 (Suh et al., 2013; Suh et al., 2008). At resting state, RIM1α and munc13-1 are sequestered by mGlu7. After prolonged mGlu7 activation, munc13-1, which may compete with CaM, is translocated from mGlu7 to associate with RIM1α and enhance synaptic vesicle exocytosis (Ferrero et al., 2016; Martin et al., 2010; Mochida, 2011; Pelkey et al., 2008). CaM also competes with Gβγ, CaBP1, MacMARCKS, MAP1B, and Norbin (Bertaso et al., 2006; Moritz et al., 2009; Nakajima, 2011; O’Connor et al., 1999; Wang et al., 2009).
Ca2+-CaM has long been known to bind to mGlu5 as characterized by in vitro binding assays (Minakami et al., 1997). For many years, it was assumed that mGlu1 would also bind to CaM. However, careful analyses demonstrated that CaM specifically binds to mGlu5, not mGlu1, and a single residue in mGlu5 (L896) is critical for CaM binding (Choi et al., 2011). The mGlu5 L896V mutant, which disrupts CaM binding, displays decreased surface expression. Conversely, the mGlu1 V909L mutation, which allows for CaM binding to mGlu1, results in increased surface expression of mGlu1 compared to wildtype (Choi et al., 2011). CaM binding to mGlu receptors stabilizes and increases their own cell surface density, which enhances mGlu-mediated intracellular signaling that favors AMPA receptor endocytosis. DHPG triggers rapid endocytosis of AMPA receptors, within 15 min, resulting in mGlu receptor-mediated long-term depression (LTD). On a slower time course, DHPG then causes the internalization of mGlu receptors after 30–90 min (Choi et al., 2011; Palmer et al., 1997; Snyder et al., 2001). Thus agonist-induced internalization of Group I mGlu receptors is regulated by CaM binding as is mGlu receptor-mediated AMPA receptor endocytosis, which is enhanced by CaM binding to Group I mGlu receptors in hippocampal neurons (Choi et al., 2011) (Fig. 1).
Siah-1A is a member of the E3 ubiquitin ligase family containing a RING-finger protein motif and the first identified ubiquitin ligase that plays a crucial role in the trafficking of mGlu receptors. Siah-1A directly interacts with Group 1 mGlu receptors, and induces receptor ubiquitination at multiple lysine residues resulting in receptor degradation via the ubiquitin-proteasome pathway. The Siah-1A binding site is present in mGlu1a (amino acids K905–P932), and mGlu1b and truncated mGlu1a missing the Siah-interacting domain are not regulated by Siah1A co-expression (Moriyoshi et al., 2004). Importantly, the binding regions of Siah-1A and CaM within the mGlu5 CTD overlap. Studies show that Siah-1A competes with CaM for mGlu5 binding. S901 phosphorylation of mGlu5 favors Siah-1A binding by displacing CaM in a Ca2+-dependent manner in hippocampal neurons (Ishikawa et al., 1999; Ko et al., 2012). Siah-1A binding decreases mGlu5 surface expression and increases agonist-induced endosomal trafficking, which leads to the lysosomal degradation of mGlu5 (Ko et al., 2012). Siah-1A-dependent ubiquitination is an important step regulating agonist-induced internalization of mGlu1a as well as mGlu5 in HEK293 cells and hippocampal neurons. In particular, K63-mediated polyubiquitination at K1112 in the mGlu1 CTD has been identified to be important in agonist-induced mGlu1a internalization (Gulia et al., 2017).
2.3.2. GRKs and β-arrestins
GPCR desensitization is the deactivation of GPCR-elicited signaling following prolonged or repeated agonist exposure. A major mechanism underlying desensitization is agonist-stimulated phosphorylation and subsequent endocytosis of the receptor. The second messenger-dependent protein kinases were originally regarded as the principal mediators of GPCR phosphorylation and desensitization. However, GRKs (originally called β-adrenoceptor kinases) have been shown to play a central role in the agonist-induced desensitization of many GPCRs since their discovery (Gurevich et al., 2012). The GRK family of serine/threonine kinases comprises seven members. Based on sequence homology, vertebrate GRKs are classified into three subfamilies: Visual or rhodopsin-kinases subfamily (GRK1 and GRK7), β-adrenergic receptor kinases subfamily (GRK2 and GRK3), and the GRK4 subfamily (GRK4, GRK5, and GRK6). The expression of GRK1 and 7 is restricted to the retina, whereas GRK4 is predominantly expressed in the testes, but has also been found in the brain. GRK2, 3, 5, and 6 are ubiquitously expressed. The GRK family members possess the N-terminal regulator of G protein signaling (RGS) homology (RH) domain implicated in GPCR binding, the catalytic domain containing S/T kinase activity, and the CTD responsible for plasma membrane targeting. GRK2 and GRK3 contain pleckstrin homology (PH) domains that interact with Gβγ subunits (Sato et al., 2015).
The canonical mechanism for GPCR desensitization involves phosphorylation of receptors by GRKs and subsequent recruitment of β-arrestins, which sterically hinders further heterotrimeric G protein activation (Dhami and Ferguson, 2006). β-arrestin-bound receptors initiate G protein-independent signaling and are subject to clathrin-mediated endocytosis (CME) and are sorted for receptor degradation or receptor recycling (Sato et al., 2015). β-arrestins were initially regarded as negative regulators of GPCRs by inducing receptor endocytosis and attenuation of receptor signaling. However, there is accumulating evidence that β-arrestins also contribute to the formation of fully engaged GPCR–G protein–β-arrestin supercomplexes that mediate sustained G protein activation from internalized receptors in endosomes (Ranjan et al., 2017). At the end of GPCR cycles, RGS acts as GTPase activating proteins (GAPs), leading to GTP hydrolysis and reversing the receptor to an initial resting state (Sato et al., 2015).
Different GRK subtypes combined with the action of β-arrestins can contribute to the endocytosis of Group I mGlu receptors in an agonist-dependent manner; however, the results are inconsistent (reviewed in Refs: (Iacovelli et al., 2013; Kim et al., 2008)). Both GRK2 and GRK4 facilitate the agonist-induced internalization of mGlu1a, which appears to require GRK2-mediated phosphorylation of the S869–V893 region of mGlu1a when expressed in HEK293 cells (Dale et al., 2001a; Iacovelli et al., 2003; Mundell et al., 2003; Sallese et al., 2000). These agonist-induced internalization processes are β-arrestin 1/2 and dynamin-dependent (Mundell et al., 2002; Mundell et al., 2001; Mundell et al., 2003). β-arrestin 1 appears to be important in mGlu1 endocytosis; however, the agonist-stimulated internalization of mGlu1a is observed only when β-arrestin 1 is co-expressed with either GRK2 or GRK5 in HEK293 cells. GRK2, GRK5, β-arrestin 1, or β-arrestin 2 individually has no significant effect on the internalization of mGlu1a (Dale et al., 2001a). The constitutive internalization of mGlu1a does not require GRK2 nor β-arrestin 1, but occurs by a clathrin-coated vesicle-mediated pathway (Dale et al., 2001a). In other studies, GRK4 has been shown to be critical for the rapid internalization of mGlu1a in HEK293 cells and in cerebellar Purkinje neurons (Iacovelli et al., 2003; Sallese et al., 2000). In this case, β-arrestin 1 does not seem to be essential for the agonist-induced internalization of mGlu1a (Iacovelli et al., 2003).
Much less is known about the role of GRKs in mGlu5 signaling and trafficking. GRK2 and GRK3, but not GRK4, GRK5 or GRK6, regulate mGlu5 signaling in a kinase-activity dependent manner, when mGlu5 signaling was monitored by measuring GIRK channel currents following DHPG application in transfected HEK293 cells (Sorensen and Conn, 2003). The membrane-proximal domain near G847, in particular S839, might contribute to mGlu5 signaling, since the T840A mutation alone did not reverse the effect of GRK2 (Sorensen and Conn, 2003). Recently it was reported that β-arrestin 2, not β-arrestin 1, is required for specific forms of mGlu receptor-dependent synaptic plasticity in the hippocampus. Specifically, endogenous mGlu1 and mGlu5 were shown to physically interact with β-arrestin 2 in hippocampus and cortex, but surface expression of mGlu5 was not altered in β-arrestin 2 knockout (KO) animals (Eng et al., 2016).
2.3.3. Other kinases and phosphatases
PKA
PKA potentiates constitutive (agonist-independent) signaling of mGlu1a by increasing IP3 formation in HEK293 cells, but does not alter the basal activity of mGlu1b (Francesconi and Duvoisin, 2000; Mundell et al., 2004). PKA increases the agonist-stimulated IP3 accumulation in mGlu1a- or mGlu1b-expressing HEK293 cells (Mundell et al., 2004). For receptor trafficking, PKA inhibits the agonist-induced internalization of both mGlu1a and mGlu1b by primarily acting on the domain spanning region S869–A886 through a mechanism that inhibits the agonist-induced association of receptors and GRK2/β-arrestin 1. In addition, PKA increases constitutive surface expression of mGlu1a, but not mGlu1b, in HEK293 cells (Mundell et al., 2004). An early study showed that PKA activation does not contribute to the agonist-induced desensitization of mGlu5 expressed in oocytes (Gereau and Heinemann, 1998). A recent study revealed that PKA directly phosphorylates S870 on mGlu5 in in vitro assays and in the neostriatum. PKA-mediated phosphorylation on mGlu5 S870 is necessary for agonist-triggered ERK1/2 activation and Ca2+ oscillations without altering receptor surface expression levels (Uematsu et al., 2015).
CaMKII
CaMKIIα increases the agonist-induced endocytosis of mGlu1 and mGlu5 (Jin et al., 2013a; Mundell et al., 2002; Raka et al., 2015). CaMKIIα directly binds to the membrane-proximal CTD region of mGlu1a (amino acids K841–N885) and promotes the phosphorylation of mGlu1a T871, which is part of a consensus CaMKII phosphorylation sequence (R/KXXS/T) (Jin et al., 2013a). In addition, CaMKIIα can bind to the iL2 region of Group I mGlu receptors in vitro (Raka et al., 2015). Agonist-induced activation of mGlu1a triggers a Ca2+-dependent activation of CaMKIIα and potentiates CaMKIIα binding to mGlu1a. CaMKIIα then desensitizes the mGlu1-mediated IP3 accumulation in striatal neurons (Jin et al., 2013a). In the case of mGlu5, there is evidence that inactive forms of CaMKIIα bind constitutively to the proximal CTD (amino acids L875–K917), which overlaps with the CaM binding region. Following mGlu5-stimulated Ca2+ release from intracellular calcium stores, CaMKIIα dissociates from mGlu5 in competition with CaM. The dissociated active CaMKIIα is subsequently recruited to the NMDA receptor where it can phosphorylate the GluN2B subunit (Jin et al., 2013b). CaMKIIα selectively attenuates the mGlu5a-stimulated ERK1/2 activation in a kinase activity-dependent manner in transfected HEK293 cells, but has no effect on mGlu1a-mediated ERK1/2 phosphorylation (Raka et al., 2015).
CaMKIIβ can regulate Group I mGlu receptor-mediated dendritic spine dynamics through its interaction with α-Actinin-4 (Actn4). Although Actn4 does not affect surface expression of Group I mGlu receptors, it is implicated in mGlu receptor-mediated dynamic remodeling of dendritic protrusions through the interaction with CaMKII (Kalinowska et al., 2015). Actn1, another α-actinin family protein, is associated with mGlu5b via a region that is conserved between mGlu5a and 5b (amino acids R931–A1002 of rat mGlu5b) in HEK293 cells and striatum. Actn1 increases the cell surface expression of mGlu5b and agonist-induced ERK1/2 phosphorylation in HEK293 cells (Cabello et al., 2007).
MAPK-ERK
MAPKs are serine/threonine kinases that include three subclasses: ERK1/2, c-Jun N-terminal kinases/stress-activated protein kinases (JNK/SAPK), and p38 MAPKs. Among these kinases, ERK has been demonstrated to phosphorylate Group I mGlu receptors directly and modulate synaptic transmission and plasticity (Mao and Wang, 2016). ERK1/2 are associated with the CTD of Group I mGlu receptors in vitro and in cerebellar neurons. The ERK2 binding site is a membrane-proximal region (amino acids K841–N885) in the mGlu1a CTD (Yang et al., 2016). As a proline-directed kinase, ERK phosphorylates mGlu1a (S1154) and mGlu5 (S1126) at a consensus phosphorylation motif (S/T-P) as detected in cortical and cerebellar neurons. The phosphorylation site is located within a conserved Homer binding motif (-PPSPF-), but is distant from the ERK binding region of mGlu1a (Mao and Wang, 2016; Yang et al., 2016). Because the Group I agonist DHPG increases ERK phosphorylation and activated ERK phosphorylates mGlu5 S1126 in an activity-dependent manner, mGlu receptor-ERK constitutes a positive feedback loop. The phosphorylation of mGlu5 at S1126 increases the affinity of Homer-1, and mGlu5 surface expression in striatal neurons (Park et al., 2013). ERK also positively stabilizes mGlu1a on the cell surface in cerebellar neurons, whereas inhibition of ERK by U0126 reduces mGlu1a surface expression (Yang et al., 2016).
Cdk5
Cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine/threonine kinase involved in neuronal development and synaptic plasticity. Cdk5 phosphorylates S/T residues within the Homer binding domain of mGlu5 in an in vitro kinase assay. CDK5-mediated phosphorylation of mGlu5 at T1123 and S1126 enhances its association with Homer, and appears to stabilize the receptor on cell surface (Orlando et al., 2009; Park et al., 2013).
Fyn
Fyn is a member of the Src family of nonreceptor tyrosine kinases and has been implicated in the direct phosphorylation, channel activity, and trafficking of GluN2B-containing NMDA receptors (Trepanier et al., 2012). Fyn also regulates mGlu1a by directly binding to the distal CTD (amino acids N925–T1000) of mGlu1a in GST pulldown assays and in cerebellar neurons. Fyn increases the surface expression of mGlu1a and agonist-stimulated IP3 production by phosphorylation of mGlu1a Y937 (Jin et al., 2017).
Protein phosphatases
Protein phosphatase 2A (PP2A) has been shown to regulate recycling of both mGlu1 and mGlu5. DHPG-induced internalization of mGlu1/5 occurs within 5 min, and increases over time in HEK293 and neuronal cell lines. Subsequent to ligand-dependent internalization, mGlu1 is localized in Rab11-positive recycling endosomes with low abundance in LAMP1-positive lysosomal compartments. Internalized mGlu1/5 is then recycled to the cell surface upon DHPG application, and recycling is abolished by PP2A inhibition (Mahato et al., 2015; Pandey et al., 2014). The constitutively internalized mGlu5 is also reported to recycle to the cell surface in HEK293 cells, without entering lysosomes (Trivedi and Bhattacharyya, 2012). Serine/threonine protein phosphatase 1γ1 (PP1γ1) binds to Group I mGlu receptors through KSVTW residues in their CTD (amino acids 891–895 in mGlu1a; 880–884 in mGlu5) as well as mGlu7 by GST pulldown and yeast two-hybrid assays. However, functional consequences of their binding remain unknown (Croci et al., 2003). The calcineurin inhibitor protein (CAIN) interacts with the iL2 and distal CTD of Group I mGlu receptors. CAIN inhibits the endocytosis of Group I mGlu receptors in transfected HEK293 cells, and attenuates mGlu1a signaling by disrupting Gαq/11-coupling to the receptor (Ferreira et al., 2009).
2.4. Binding proteins
2.4.1. Homer
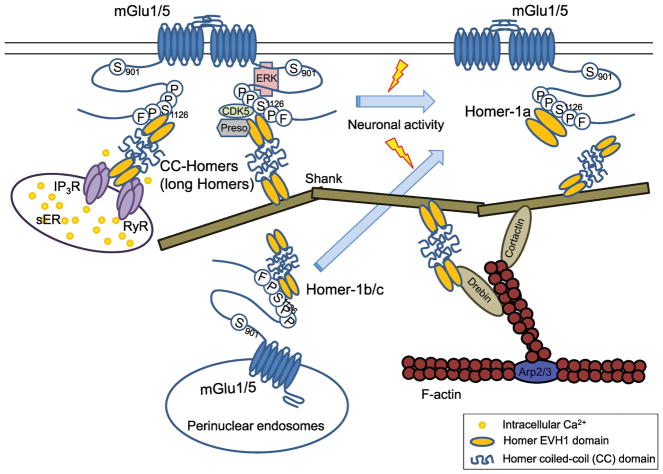
Homer, also known as Vesl (VASP/Ena-related protein induced during Seizure and LTP), is a postsynaptic scaffold protein bridging Group I mGlu receptors and intracellular signaling molecules or receptors at excitatory synapses. Homer-1a was first discovered as an immediate early gene, whose expression is rapidly induced by synaptic activation such as seizures or long-term potentiation (LTP) stimulation (Brakeman et al., 1997). Homer-1, 2, and 3 proteins are encoded by three independent mammalian genes, and alternative spliced variants from those genes give rise to Homer family members that share a similar structure. The N-terminal region of Homer isoforms contains an enabled/VASP homology 1 (EVH1) domain that interacts with a consensus binding motif of PPSPFR present on the CTD of mGlu1a and mGlu5 (Beneken et al., 2000; Brakeman et al., 1997; Tu et al., 1998). Unlike Homer-1a, long-form isoforms are expressed constitutively and contain a cytoplasmic domain of coiled-coil (CC) structure, which allows multimerization and links the CTD of Group I mGlu receptors to the IP3 receptors or ryanodine receptors (Feng et al., 2002; Kato et al., 1998; Rong et al., 2003; Tadokoro et al., 1999; Tu et al., 1998; Xiao et al., 1998; Xiao et al., 2000). The short-form Homer-1a lacks a CC domain and consequently cannot form multimers or interact with IP3 or ryanodine receptors, thereby functioning as a natural dominant negative. (Xiao et al., 1998). There is a direct competition between endogenous long Homers and short Homer-1a for mGlu1a binding (Ango et al., 2001). Disruption of long Homer-mGlu receptors interaction or expression of endogenous Homer-1a induces agonist-independent activation of Group I mGlu receptors (Ango et al., 2001) (Fig. 2). It was recently shown that Homer-1a and long Homers competitively interact with mGlu5 receptor to control NMDA-mGlu5 receptor cross talk and synaptic excitability in dendritic spines (Moutin et al., 2012).
Fig. 2.
Homer regulates trafficking of Group I mGlu receptors.
Long-form Homer proteins (CC-Homers) are postsynaptic scaffolds bridging Group I mGlu receptors and IP3/ryanodine receptors, Shank, or F-actin cytoskeleton, et cetera. They arguably act to decrease cell surface expression of Group I mGlu receptors (Ango et al., 2002; Hayashi et al., 2006; Roche et al., 1999). Following synaptic activation, Homer-1a, a short-form lacking a CC domain, is rapidly induced, competes with CC-Homers, and stabilizes cell surface targeting of mGlu1a (Ciruela et al., 1999; Minami et al., 2003). ERK and CDK5 phosphorylate S/T residues within a conserved Homer binding motif in Group I mGlu receptors, which leads to an increase in the affinity of Homer-1 and the stabilization of receptors on cell surface (Orlando et al., 2009; Park et al., 2013; Yang et al., 2016).
Homer organizes the trafficking and membrane clustering of Group I mGlu receptors. Homer-1b/c acts to decrease cell surface expression of Group I mGlu receptors. Homer-1b inhibits surface expression of mGlu5 or mGlu1a and retains the receptors within the ER via a direct protein-protein interaction in HeLa cells and cultured cerebellar granule cells (Ango et al., 2002; Hayashi et al., 2006; Roche et al., 1999). Homer-1c, but not Homer-1a diminishes surface expression of mGlu5 or mGlu1a and increases the formation of intracellular clusters of the receptors expressed in HEK293 cells (Abe et al., 2003; Coutinho et al., 2001). The reduction of Homer-3 increases surface expression of mGlu1a, and the degradation of mGlu1a can be facilitated by Homer-3 which binds to the S8 ATPase of the 26S proteasome in differentiated PC12 cells (Rezvani et al., 2012). Unlike long form Homers, Homer-1a has a relatively small effect on cell surface expression and clustering of Group I mGlu receptors (Ango et al., 2000; Ango et al., 2001; Ango et al., 2002; Ciruela et al., 1999; Roche et al., 1999). Homer-1a expression induced by neuronal activation reverses the intracellular retention of mGlu5 mediated by Homer-1b and promotes cell surface targeting of mGlu5 (Ango et al., 2002). Induction of Homer-1a expression by conditioning depolarization reduces the internalization and increases surface expression of mGlu1 in MAPK-dependent manner in HEK293 cells and cerebellar Purkinje neurons (Minami et al., 2003) (Fig. 2).
In contrast, Homer-1b/c has been shown, in some preparations, to increase cell surface expression and dendritic clustering of mGlu1a and mGlu5 in HEK293 cells and neurons, which are inhibited by overexpression of Homer-1a (Ciruela et al., 2000; Gama et al., 2001; Kammermeier, 2006; Serge et al., 2002; Tadokoro et al., 1999). Similarly, long Homer proteins, but not short Homer-1a, can induce surface clustering of Group I mGlu receptors and inhibit Group I mGlu receptor coupling to calcium channels in superior cervical ganglion (SCG) neurons (Beqollari and Kammermeier, 2013; Kammermeier, 2006; Kammermeier et al., 2000). Surface expression of mGlu5 increases in Homer-1a KO cortical neurons and a rescue expression of Homer-1a downregulates surface levels of mGlu5 (Hu et al., 2010).
Homer-dependent regulation of Group I mGlu receptor trafficking is also implicated in pathways involved in addiction. Homer-1 or Homer-2 KO mice, but not Homer-3 KO, display sensitized behavioral responses to cocaine (Szumlinski et al., 2004). Homer-2 deletion reduces total mGlu1a expression levels without altering mGlu5 levels in the Nucleus accumbens (NAc) (Szumlinski et al., 2004). Cocaine administration decreases surface expression of mGlu5 by elevating long Homers in the NAc (Fourgeaud et al., 2004). In contrast, a single dose of cocaine specifically induces Homer-1a, not long Homers, in the striatum and cocaine withdrawal leads to the reduction in the long Homer-1b/c and mGlu5 levels in the medial NAc (Swanson et al., 2001). Furthermore, upregulated Homer-1b/c in the NAc after extinction training reduces the surface expression of mGlu5 (Knackstedt et al., 2010). Overall, Homer has been demonstrated to play an important role in mGlu receptor trafficking with varying effects.
2.4.2. Other interactors
Tamalin
Tamalin (also termed GRP1-associated scaffold protein) has been isolated as a binding partner to the cytoplasmic domain of Group I mGlu receptors as well as mGlu2/3 by yeast two-hybrid screening (Kitano et al., 2002). Tamalin is a scaffolding protein composed of a PDZ domain, a leucine-zipper domain, four YXXL motifs involved in CME, a proline-rich region, and a carboxyl-terminal PDZ binding motif (Kitano et al., 2002; Kitano et al., 2003). The PDZ domain of tamalin interacts with Group I and II mGlu receptor CTDs and SAP90/PSD-95-associated protein (SAPAP) 1/3. A leucine-zipper domain of tamalin binds to guanine nucleotide exchange factor cytohesin-2, which promote surface trafficking of Group I mGlu receptors in heterologous cells and neurons (Kitano et al., 2002). The crystal structure of tamalin has revealed that tamalin undergoes the auto-inhibited self-assembly through its PDZ domain and C-terminus, which is converted to the released form by competitive binding of mGlu receptor CTD. This interaction liberates C-terminal PDZ binding domain of tamalin and facilitates the association with S-SCAM, and their association promotes mGlu receptor cell-surface trafficking (Sugi et al., 2007).
Band 4.1G
Band 4.1G cytoskeletal protein directly interacts with the mGlu1a CTD at the negatively charged glutamic acid-rich region (amino acids E1131–D1135), but not with mGlu1b in HEK293 cells and the cerebral cortex (Tateyama and Kubo, 2007). Band 4.1G co-localizes with mGlu1a and promotes mGlu1a surface expression in primary hippocampal neurons (Lu et al., 2004).
GluD2
The GluD2 receptor has roles in cerebellar LTD and synaptogenesis. It is a homologous protein to ionotropic glutamate receptors, but has long been considered as an orphan receptor without known endogenous ligands (Schmid and Hollmann, 2008). However, recent studies have demonstrated that GluD2 is indirectly gated by glutamate through mGlu1 receptor activation, and D-serine and Cbln1 (cerebellin 1 precursor) are endogenous ligands for GluD2 (Ady et al., 2014; Dadak et al., 2017; Yuzaki and Aricescu, 2017). The ECD of GluD2 interacts with neurexin through the secreted synapse organizer Cbln1 (Matsuda et al., 2010; Uemura et al., 2010). The mGlu1, PKCγ, and TRPC3 protein complex interact with GluD2 in the cerebellum. Loss of GluD2 increases surface expression of mGlu1b (Kato et al., 2012).
Norbin
Norbin (also known as Neurochondrin) has been identified as an mGlu5 binding molecule in a yeast two-hybrid screen, and increases cell surface expression and signaling of mGlu5 (Wang et al., 2009). Norbin physically interacts with the membrane proximal region of the CTD that overlaps with the calmodulin-binding site in mGlu5. CaM disrupts Norbin binding for mGlu5, but Norbin binding does not appear to affect CaM binding to mGlu5. Norbin positively regulates mGlu5-mediated IP3 formation and ERK1/2 phosphorylation, and prolongs mGlu5-elicited Ca2+ oscillations in HEK293 cells. The cell surface expression of mGlu5 increases upon co-expression of Norbin in N2a cells and is reduced in Norbin KO or knockdown cortical neurons. In addition, Norbin KO mice display the impaired mGlu5-dependent synaptic plasticity in the hippocampus and depressive-like behaviors (Wang et al., 2015; Wang et al., 2009).
Numb
Numb is a cell fate determinant maintaining neural stem cells during asymmetric cell division. Numb has been proposed as an endocytic protein because it locates at various endocytic organelles and co-traffics with the internalized receptors (Santolini et al., 2000). Numb appears to regulate mGlu1 trafficking in the cerebellar Purkinje neurons. Numb KO mice exhibit reduced synaptic expression of mGlu1 by altering agonist-induced internalization and recycling of mGlu1 in cerebellar Purkinje neurons, and display impaired motor coordination (Zhou et al., 2015).
Optineurin and Rab8
Optineurin (OPTN) is co-localized with huntingtin (Htt) in the Golgi apparatus, forms a complex with Rab8, and participates in the regulation of post-Golgi protein transport (del Toro et al., 2009; Esseltine and Ferguson, 2013). OPTN has been identified as a binding molecule to the mGlu1a CTD in a yeast two-hybrid screen (Anborgh et al., 2005). Rab8 functions in concert with OPTN to attenuate both mGlu1a internalization and G protein coupling. Rab8 is associated with mGlu1a, but not with mGlu1b. Agonist treatment increases their association and co-localization in HEK293 cells and hippocampal neurons. Rab8 attenuates the agonist-induced endocytosis of mGlu1a and increases cell surface receptor expression (Esseltine et al., 2012).
p11
p11 (also known as S100A10) is a Ca2+-insensitive multifunctional protein implicated in serotonin receptor signaling and depression (Svenningsson et al., 2013). p11 interacts directly with the membrane-proximal region of mGlu5 (amino acids F836–M844) through a consensus binding motif for p11 (S/T-S/T-V) (Lee et al., 2015). Co-expression of p11 increases the cell surface expression of mGlu5 and agonist-induced Ca2+ oscillations in HEK293 cells, whereas knockdown or KO of p11 decreases the surface availability of mGlu5 (Lee et al., 2015).
Preso1
Preso1 is a multi-domain scaffolding protein that contains WW, PDZ, FERM, Homer ligand, and PDZ ligand binding domains. Preso1 was first identified as a PSD-95-interacting protein (Lee et al., 2008a) and later a Homer-binding protein by yeast two-hybrid screening (Hu et al., 2012). In addition to binding to proline-directed kinases such as CDK5 and ERK, Preso1 also binds to the membrane-proximal region of Group I mGlu receptor CTD (K920–S1020) via its FERM domain. The Homer binding site on mGlu5 is additionally required for complete binding affinity for Preso1. Preso1 increases constitutive and agonist-induced mGlu5 S1126 phosphorylation and enhances Homer-mGlu5 binding by facilitating the association of proline-directed kinases with mGlu5. Preso1 inhibits Group I mGlu receptor coupling to L-type voltage-sensitive Ca2+ channels like the action of long Homer proteins (Hu et al., 2012). Although Preso1 stabilizes the interaction among Group I mGlu receptors, Homer, and proline-directed kinases, surface and total expression of mGlu5 was not altered in Preso1 KO mice or upon knockdown (Hu et al., 2012; Luo et al., 2013).
Spinophilin
Spinophilin is a multifunctional synaptic scaffolding protein that interacts with protein phosphatases and F-actin. Spinophilin reduces the agonist-induced internalization of Group I mGlu receptors and ERK1/2 phosphorylation via its association with the PDZ binding motif of the receptors in HEK293 cells and cortical neurons. Spinophilin KO mice exhibit enhanced agonist-induced endocytosis of mGlu5 in cortical neurons and a deficit of mGlu receptor-dependent LTD in the CA1 hippocampus (Di Sebastiano et al., 2016).
2.5. Trafficking pathways
2.5.1. Endocytic trafficking
Many GPCRs undergo both constitutive (agonist-independent) or regulated (agonist-induced) internalization. The subsequent endocytosis can be clathrin-dependent or clathrin-independent. CME is the most extensively characterized endocytic pathway, and it involves the formation of clathrin-coated pits composed of clathrin and adaptor protein complexes. In contrast, clathrin-independent endocytosis (CIE) is caveolin-dependent endocytosis that involves the formation of caveolae by caveolins and cavins. Caveolae are flask-shaped invaginated plasma membrane domains enriched in cholesterol and sphingolipids. Dynamin, a membrane scission GTPase, mediates fission at the neck of both clathrin- and caveolin-coated pits upon GTP hydrolysis. CLIC/GEEC (clathrin-independent carriers/GPI-anchored protein enriched early endosomal compartment) is an uncoated tubular shaped structure, which mediates clathrin- and caveolin-independent pathways. CLIC/GEEC endocytosis is regulated by Cdc42 and delivers glycosylphosphatidylinositol (GPI)-anchored proteins and CD44 as specific cargos. Another type of CIE is a MHCI/CD59/Arf6-positive pathway that might overlap with the CLIC/GEEC pathway (Johannes et al., 2015).
Group I mGlu receptors appear to use multiple endocytic pathways that act together for regulating the internalization and recycling of the receptors. Studies using a dominant negative (DN) mutant of Eps15 (EΔ95/295), which has been extensively regarded as a marker of CME, have revealed that the constitutive internalization of mGlu1a occurs via a clathrin-dependent pathway, whereas constitutive mGlu5 internalization occurs in a clathrin-independent pathway in heterologous cells and neurons (Dale et al., 2001a; Fourgeaud et al., 2003; Pula et al., 2004). However, constitutive endocytosis of both mGlu1a and mGlu5 were also shown to occur in a clathrin-dependent manner by Ral, a small GTP-binding protein in a complex with RalGDS (Ral guanine nucleotide dissociation stimulator), and PLD2 (phospholipase D2). The Ral/RalGDS/PLD2 complex are constitutively associated with mGlu1a and mGlu5, and are co-localized in clathrin-positive intracellular vesicles together with internalized mGlu1a or mGlu5 in HEK293 cells and neurons (Bhattacharya et al., 2004).
Agonist-independent endocytosis of Group I mGlu receptors might also be regulated by caveolin and a lipid raft-dependent process. Group I mGlu receptors co-fractionate in a detergent-resistant lipid raft-rich membrane (DRM) fraction and bind directly with caveolins via two caveolin-1 binding motifs present in the junction of iL1/3 and TM domains. The internalized Group I mGlu receptors co-localize with caveolin-1 and cholera toxin B, markers of the caveolar/raft pathway in neurons (Burgueno et al., 2004; Francesconi et al., 2009; Kumari et al., 2013). Caveolin-1 stabilizes mGlu1 at the cell surface, and disruption of caveolin-1 binding to mGlu1 drives rapid internalization of receptor in HEK293 cells and neurons. Depletion of membrane cholesterol also leads to the impairment of constitutive endocytosis of Group I mGlu receptors (Francesconi et al., 2009). However, a subsequent study reported that constitutive surface expression of Group I mGlu receptors is not altered in caveolin-1 KO hippocampus maybe due to the presence of compensatory trafficking mechanisms (Takayasu et al., 2010).
Agonist-induced endocytosis of Group I mGlu receptors involves both clathrin-dependent and caveolin-dependent pathways (Hong et al., 2009; Kumari et al., 2013; Luis Albasanz et al., 2002; Mundell et al., 2002; Mundell et al., 2001). As internalized mGlu1a was found in transferrin-positive endosomes in HEK293 cells, mGlu1a is probably endocytosed by clathrin-coated pits (Mundell et al., 2002; Mundell et al., 2001). Caveolar and lipid raft-dependent pathways also contribute to the agonist-induced internalization of mGlu1a; however, the role of caveolin itself is reported as inconsistent (see (Francesconi et al., 2009; Hong et al., 2009)). In particular, caveolin is phosphorylated and binds more to mGlu1a upon agonist stimulation, and then enhances internalization of mGlu1a rather than stabilizing the receptor on the cell surface (Hong et al., 2009). In a more recent study, agonist stimulation was shown to increase the association of Group I mGlu receptors with lipid rafts, which is dependent on membrane cholesterol content, not on caveolin-1 in HEK293 cells and cortical neurons (Kumari et al., 2013).
2.5.2. Nuclear trafficking
Nuclear entry of cell surface receptors has been reported for receptor tyrosine kinases (RTKs) such as EGFR and ErbB-2 and is thought to be important for some functions of those RTKs (Carpenter and Liao, 2013). It is well known that the intracellular CTD of these cell surface receptors is responsible for regulating receptor activity as well as subcellular localization. In fact, a nuclear localization signal (NLS) has been identified in the CTD of EGFR and ErbB-2 RTKs. Intriguingly, the CTD of one glutamate receptor, the NMDA receptor GluN1 subunit, has been reported to be present in the nucleus when expressed alone. And, another study has identified a bi-partite nuclear localization sequence in the C1 region of GluN1 (Holmes et al., 2002). There is also evidence that mGlu receptors can traffic to the nucleus. For example, mGlu2 has been found on the nuclear envelope of Golgi cells by immunogold electron microscopy (Lujan et al., 1997). Using immunocytochemical, ultrastructural, subcellular fractionation and intracellular Ca2+measuring techniques, O’Malley, K.L. et al. were among the first to demonstrate that mGlu5 not only exists on the nuclear membrane but can be activated with rapid oscillatory Ca2+ elevations in response to 1 mM glutamate (O’Malley et al., 2003). Activation of intracellular, but not cell surface, mGlu5 was found to be responsible for the increased phosphorylation of ERK1/2 in a downstream signaling study (Jong et al., 2009).
GPCR signaling at the nucleus is an emerging paradigm given several subsequent studies indicating that other GPCRs can also be expressed and function in the mitochondria, ER membrane, lysosomes, and on the nuclear membrane (Benard et al., 2012; Branco and Allen, 2015; Gobeil et al., 2006; Irannejad and von Zastrow, 2014; Oksche et al., 2000; Tadevosyan et al., 2012). In attempts to identify the NLS for mGlu5, Sergin, I. et al. have focused on the mGlu5 CTD and mapped a region of 25 amino acids (amino acids S852–S876) as important for inner nuclear membrane insertion, and mGlu5 appears to interact with chromatin directly (Sergin et al., 2017). As the NLS is thought to overlap with the Ca2+-CaM and Norbin binding sites, its regulation of mGlu5 entry to the nucleus may be far more complicated than originally thought since these sites have been proven to be involved with surface expression of the receptor (Chang and Roche, 2017; Choi et al., 2011; Kim et al., 2008; Ko et al., 2012; Lee et al., 2008b). Nevertheless, reports of nuclear trafficking of mGlu5 suggest mGlu5 is a membrane-crossing and multi-functional receptor, and its role in regulating and interacting with nuclear matrix proteins remains to be investigated.
3. Group II and III mGlu receptors
Activation of either Group II or III mGlu receptors inhibits AC activity via PTX-sensitive Gi/o, which leads to the inhibition of forskolin-stimulated cAMP formation, and activates the MAPK-ERK pathway. Although these receptors share a common signaling pathway, they differ in their expression patterns and subcellular distribution. For example, mGlu3 and mGlu7 are widely expressed (Ferraguti and Shigemoto, 2006), whereas mGlu6 is restricted to the retina. Group II and Group III mGlu receptors are known substrates for phosphorylation, both the second messenger-dependent protein kinases and GRKs, and phosphorylation affects receptor signaling and desensitization (reviewed in (Iacovelli et al., 2013)). However, very few studies demonstrate clear effects of phosphorylation on trafficking, with the exception of mGlu7. In this section we will discuss trafficking of Group II and III mGlu receptors with a major focus on the mechanisms underlying synaptic expression and endocytosis of mGlu7 (Table 2).
Table 2.
A list of Group III mGlu receptor-interacting proteins that regulate cell surface expression of receptors.
| Subtypes | Effect on surface expression | Interacting proteins | Interacting regionsa | References |
|---|---|---|---|---|
| mGlu4 | ||||
| Decrease | PKC | S859(?) | (Mathiesen and Ramirez, 2006) | |
| mGlu7 | ||||
| Stabilize | ||||
| PICK | L913–I915, S862 | (Suh et al., 2008) | ||
| PKC | S862 | (Suh et al., 2008) | ||
| SUMO1/3 | K889 | (Choi et al., 2016) | ||
| Decrease | ||||
| CaM | K858–M872 | (Suh et al., 2008) | ||
| PP1γ1 | K911–W915 (mGlu7b), S862 | (Suh et al., 2013) | ||
| mGlu8 | ||||
| Stabilize | ||||
| Band4.1B | K851–R854 | (Rose et al., 2008) | ||
| CRIP1a | N892–T898 (mGlu8a) | (Mascia et al., 2017) | ||
3.1. Group II mGlu receptors
Both mGlu2 and mGlu3 receptors are phosphorylated by PKA, and in vitro phosphorylation assays showed that PKA directly phosphorylates mGlu2 and mGlu3 at a single site (S843 and S845, respectively) within the CTD. There is another putative PKA consensus site (S675) on the iL2 of mGlu2, but assays reveal only weak phosphorylation by PKA, compared to the robust phosphorylation of S843 (Cai et al., 2001; Schaffhauser et al., 2000). S845 on mGlu3 is dephosphorylated by the serine/threonine protein phosphatase 2C (PP2C), which specifically binds to the membrane-proximal region of the mGlu3 CTD (amino acids T836–Q855), but does not bind to other mGlu receptors (Flajolet et al., 2003). Because S843 on mGlu2, S845 on mGlu3, S859 on mGlu4, S862 on mGlu7, and S855 on mGlu8 are conserved PKA phosphorylation sites (Cai et al., 2001), PKA may similarly contribute to the function of most Group II and Group III receptors. However, a direct role of PKA-induced phosphorylation in surface expression and trafficking of Group II/III mGlu receptors has not yet been addressed.
Group I and Group II mGlu receptors possess a common (S/T)x(V/L) motif that belongs to the class I PDZ binding motif, whereas Group III mGlu receptors contain PDZ binding motifs similar to the class II-IV (Hirbec et al., 2002; Songyang et al., 1997). PDZ domain-containing proteins act as scaffolds for the assembly of protein complexes via multiple protein-protein interacting PDZ domains. They play an important role in the regulation of trafficking, signaling, and clustering of GPCRs (Dunn and Ferguson, 2015; Sheng and Sala, 2001). Specifically, PDZ proteins such as PICK1 (Protein Interacting with C-Kinase), syntenin, and GRIP (glutamate receptor interacting protein) interact with Group II and III mGlu receptors in various affinities in in vitro binding assays (Hirbec et al., 2002). Tamalin and NHERF1/2 (Na+/H+ exchanger regulatory factors 1/2) were also identified as PDZ-dependent binding partners with mGlu2/3, as well as for Group I mGlu receptors (Kitano et al., 2002; Paquet et al., 2006; Ritter-Makinson et al., 2017). However, direct roles of these PDZ-containing proteins in regulating mGlu2/3 surface expression and trafficking remain unknown. Furthermore, phosphorylation has been shown to dramatically regulate protein interactions via PDZ binding domains, but this has also not been reported for these receptors.
3.2. Group III mGlu receptors
There has been a sustained effort by the pharmaceutical industry to make selective compounds to either activate or block distinct subtypes of mGlu receptors. In fact, the identification of agonists, antagonists and allosteric modulators has led to better insights into the varied functions of mGlu receptors. L-AP4 is a prototypical orthosteric agonist of Group III mGlu receptors. As mGlu7 is activated only by high concentrations of glutamate or L-AP4, mGlu7 becomes active only under conditions of sustained synaptic activity, thereby acting as an autoreceptor to inhibit further glutamate release (Niswender and Conn, 2010). However, recent studies suggest a different role for Group III mGlu receptors, namely the facilitation of presynaptic release following agonist-exposure. This switch from inhibition to facilitation might be mediated by agonist-induced receptor endocytosis, an altered signaling pathway, or the competitive interaction among the receptors, Ca2+-activated CaM, and the exocytic machinery (Ferrero et al., 2016; Martin et al., 2010; Mochida, 2011; Nakajima et al., 2009; Pelkey et al., 2008) (Fig. 1).
3.2.1. mGlu4 receptor
The internalization and desensitization of mGlu4 are independent of agonist treatment, but can be induced by PKC activation. Mathiesen and Ramirez found that L-AP4 fails to induce mGlu4 internalization even after a 60 min treatment in HEK293 cells, but rather slightly increases surface expression of mGlu4. However, mGlu4 is robustly internalized and desensitized when PKC pathways are activated in HEK293 cells (Mathiesen and Ramirez, 2006). It should be noted that another study successfully detected an agonist-induced, rapid and reversible internalization of mGlu4 in the same HEK293 cells by a GRK2-independent mechanism (Iacovelli et al., 2004).
Native mGlu4 is reported to interact with exocytic proteins such as Munc18-1, syntaxin, SNAP-25, and synapsin I and II in rat cerebellar lysates (Nakajima et al., 2009; Ramos et al., 2012). Munc18-1 binds to the membrane-proximal portion of mGlu4 CTD (amino acids E851–N871) that overlaps with the binding region of CaM. As Munc18-1 promotes soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated vesicle fusion, mGlu4 might facilitate synaptic vesicle release by regulating SNARE complex assembly through the competitive association between CaM and Munc18-1. Munc18-1 is sequestered by mGlu4 during basal transmission. After an action potential, Ca2+-activated CaM can liberate Munc18-1 from mGlu4, causing short-term synaptic facilitation (Mochida, 2011; Nakajima et al., 2009). Ca2+-activated CaM that can compete with exocytotic proteins for binding to Group III mGlu receptors has emerged as the likely molecule responsible for presynaptic facilitation and is reviewed elsewhere (Mochida, 2011).
3.2.2. mGlu7 receptor
Ca2+-CaM has been shown to bind to Group I and Group III mGlu receptors (except mGlu6), but not to Group II mGlu receptors (El Far et al., 2001; Lidwell et al., 2004). The minimal CaM binding consensus motif in mGlu7 (amino acids K858–M872) is conserved in Group III mGlu receptors other than mGlu6 (El Far et al., 2001; O’Connor et al., 1999). The heterotrimeric G protein βγ subunits are associated with the membrane-proximal region (amino acids Q857–F863) that partially overlaps with CaM binding domain in mGlu7 CTD. While the S862E, phospho-mimetic mutation in mGlu7 reduces binding of both Gβγ and CaM (El Far et al., 2001), the binding of Gβγ and CaM to mGlu7 is mutually exclusive such that CaM binding to the mGlu7 CTD displaces Gβγ from mGlu7 and liberates Gβγ subunits more available for a direct inhibition of P/Q-type Ca2+ channels or for further receptor signaling to regulate neurotransmitter release (Fagni et al., 2004; O’Connor et al., 1999). This competitive displacement of Gβγ subunits by CaM might be a general mechanism for regulating Gβγ signaling among presynaptic Group III mGlu receptors.
Ca2+-CaM binding to mGlu7 is regulated by the phosphorylation status of mGlu7 S862. This residue is located within the CaM-binding domain of mGlu7 and is phosphorylated by PKC, PKA, or PKG (Airas et al., 2001; Cai et al., 2001; Sorensen et al., 2002). S862 is the singular PKC phosphorylation site in mGlu7. When S862 is phosphorylated by PKC or mutated to a phospho-mimetic Glu(E) residue, CaM binding to mGlu7 is abolished. Conversely, overexpression of CaM reduces PKC-induced phosphorylation of mGlu7 S862 inan in vitro kinase assay or in transfected HeLa cells (Airas et al., 2001; Nakajima et al., 1999; Suh et al., 2008). In addition, CaM facilitates endocytosis of mGlu7 when bound to the CTD unphosphorylated at S862 (Suh et al., 2008). The phosphorylation of mGlu7 S862 critically regulates both constitutive and agonist-dependent mGlu7 trafficking along with the PDZ binding domain at the end of the mGlu7 CTD. The endocytosis of mGlu7 is markedly enhanced for mGlu7 S862A, whereas endocytosis is reduced for mGlu7 S862E (Suh et al., 2008). Phosphorylation of mGlu7 S862 and subsequent endocytosis of mGlu7 is regulated by the agonist L-AP4, the allosteric modulator AMN082, or neuronal activation (Pelkey et al., 2005; Pelkey et al., 2007; Suh et al., 2013; Suh et al., 2008). mGlu7 also undergoes constitutive endocytosis via a clathrin-independent pathway and is accumulated in ADP-ribosylation factor 6 (ARF6) positive endosomes, distinct from the transferrin receptor (Lavezzari and Roche, 2007).
The last three C-terminal amino acids of mGlu7 are essential for its interaction with the PDZ domain of PICK1 (Boudin and Craig, 2001; Dev et al., 2000; El Far et al., 2000; Enz and Croci, 2003; Hirbec et al., 2002; Perroy et al., 2002). This PDZ binding domain is important not only in the clustering of the receptor at the presynaptic active zone in hippocampal neurons (Boudin et al., 2000), but also in the control of mGlu7 endocytosis (Suh et al., 2008). As PICK1 recruits and interacts with PKCα, PICK1 augments the PKC-induced S862 phosphorylation of mGlu7, and enhances stable surface expression of mGlu7 (Suh et al., 2008). Interestingly, although S862 is ~50 amino acids away from the PDZ binding domain, PICK1 competes with CaM for binding to mGlu7 and regulates mGlu7 receptor trafficking (Fig. 1).
Agonist-induced dephosphorylation of S862 is primarily mediated by PP1γ1 in cortical and hippocampal neurons (Suh et al., 2013). The inhibition of PP1 increases constitutive receptor surface expression by promoting S862 phosphorylation, and the overexpression of PP1γ1 D95N, a catalytically inactive mutant, inhibits constitutive and L-AP4-induced internalization of mGlu7a. It has been shown that PP1γ1 binds only to the mGlu7b, but not to mGlu7a, through KSVTW residues (amino acids 911–915) in in vitro assays (Croci et al., 2003; Enz, 2002; Enz and Croci, 2003). However, it was reported that both mGlu7a and mGlu7b are co-immunoprecipitated with PP1γ1 and phosphorylated upon treatment with a PP1 inhibitor in HEK293 cells. Thus, PP1γ1 plays a role in S862 phosphorylation and receptor trafficking for both mGlu7a and 7b (Suh et al., 2013).
Agonist-induced mGlu7 endocytosis has been proposed as a key mechanism regulating a metaplastic switch at the mossy fiber-CA3 interneuron (MF-SLIN) synapses such that the presence or absence of mGlu7 on the presynaptic surface dictates LTD or LTP in response to high-frequency stimulation (HFS), respectively (Pelkey et al., 2005; Pelkey and McBain, 2007, 2008). At naive MF-SLIN synapses, HFS or L-AP4 treatment triggers presynaptic LTD by mGlu7 activation and subsequent inhibition of P/Q-type Ca2+ channels. This mGlu7-mediated LTD is lost in PICK1 KO mice (Pelkey et al., 2005; Suh et al., 2008). Following L-AP4-induced activation and internalization of mGlu7, the same HFS produces presynaptic LTP, which requires the activation of the AC-cAMP-PKA pathway and subsequent action of RIM1α released from mGlu7b (Pelkey et al., 2008). In addition, prolonged mGlu7 activation by L-AP4 pretreatment can switch mGlu7 signaling pathways from inhibition to potentiation of glutamate release. This pathway is dependent on a PTX-resistant G protein-PLC-PKC pathway and requires mGlu7-bound Munc13-1 translocation to promote interactions with RIM1α, thereby enhancing synaptic vesicle exocytosis and glutamate release (Ferrero et al., 2016; Martin et al., 2010).
Trafficking of mGlu7 is also implicated in synaptic dysfunction. The proper surface expression of mGlu7 appears to be required for blocking epilepsy, as disruption of the interaction between mGlu7a and PICK1 results in absence-like seizures and increased susceptibility to PTZ (pentylenetetrazole), a convulsant agent (Bertaso et al., 2008; Bockaert et al., 2010; Zhang et al., 2008). In addition, Elfn1, a cell adhesion protein that localizes to postsynaptic sites of somatostatin-positive interneurons, binds to mGlu7 in trans in the hippocampus. Elfn1 KO mice that have deficits in mGlu7 recruitment to synaptic sites display seizure-prone phenotypes (Tomioka et al., 2014).
Protein SUMOylation is a posttranslational modification that occurs most often at lysine residues within a consensus motif, ΦKX[D/E] (Φ, hydrophobic; X, any amino acid). All Group III mGlu receptors, except mGlu6, share a conserved SUMOylation motif. In addition, several PIAS (protein inhibitors of activated STATs) proteins that serve as SUMO E3 ligases, and Ubc9, an E2 enzyme have been found to interact with Group III mGlu receptor CTDs (Dutting et al., 2011; Tang et al., 2005). In addition, these SUMOylation machinery proteins are co-localized with mGlu8b in the retina (Dutting et al., 2011). All Group III mGlu receptors and mGlu2 have been found to be SUMOylated in a bacterial SUMOylation assay, whereas Group I mGlu receptors and mGlu3 were not SUMOylated (Dutting et al., 2011; Wilkinson and Henley, 2011; Wilkinson et al., 2008). Subsequent studies have identified K882/K903 on mGlu8b and K889 on mGlu7 as targets for SUMOylation in heterologous cells when Pias1 and/or Ubc9 are co-expressed (Choi et al., 2016; Dutting et al., 2011). SUMO-modifications participate in the stable surface expression of mGlu7. Endogenous mGlu7 is SUMOylated in hippocampus and cortical neurons, and SUMOylation of mGlu7 is reduced by the treatment of L-AP4. When mGlu7 is deSUMOylated, mGlu7 is internalized in hippocampal neurons. Furthermore, there is an interplay between SUMOylation and phosphorylation of receptor such that S862 phosphorylation of mGlu7 facilitates the SUMO conjugation of mGlu7 at K889 (Choi et al., 2016).
3.2.3. mGlu8 receptor
The membrane proximal region of the mGlu8 CTD is highly conserved with mGlu7, thus both receptors might be expected to share common trafficking mechanisms related to serine phosphorylation, Gβγ association, and Ca2+-CaM binding. As described above, S855 is a unique PKA phosphorylation site within mGlu8 (Cai et al., 2001) and mGlu8a signaling is inhibited by S855 phosphorylation in the hippocampus. However, the underlying molecular mechanism, for example receptor endocytosis, has not been analyzed (Cai et al., 2001). It is reported that PPG, an mGlu8 agonist, increases mGlu8 endocytosis in the enteric nervous system (Tong and Kirchgessner, 2003).
Band 4.1 proteins (4.1B, 4.1G, 4.1N, 4.1R) interact with the cytoplasmic domain of mGlu8a/b, but not with other Group III mGlu receptors in GST pulldown assays. The Band 4.1 protein family functions as adaptor proteins, linking membrane proteins to the actin cytoskeleton. Band 4.1B is co-localized with mGlu8, facilitates mGlu8 cell surface expression, and reduces mGlu8 receptor-mediated intracellular cAMP levels in HEK293 cells and hippocampal neurons (Rose et al., 2008). Band 4.1B binds to KRKR residues (amino acids K851–R854) in the membrane-proximal region of mGlu8, which is a possible consensus sequence for 4.1 binding partners (Rose et al., 2008). This region partially overlaps with the CaM and Gβγ binding region of mGlu7, but their competitive binding has not been analyzed.
Cannabinoid receptor interacting protein 1a (CRIP1a) is a binding partner of the CB1 receptor and negatively regulates endocytosis of CB1 receptors and mGlu8 coordinately. CRIP1a binding on the distal CTD (amino acids N892–T898) reduces the constitutive endocytosis of mGlu8a, but not of mGlu8b in HEK293 cells. The S894 residue on mGlu8a is responsible for the negative regulation of receptor endocytosis and protein-protein interactions (Mascia et al., 2017).
4. Conclusion
In conclusion, over the last two decades there has been a lot of progress in elucidating the mechanisms underlying mGlu receptor trafficking and functional regulation. The primary mechanisms identified are posttranslational modifications, such as phosphorylation, and C-terminal binding proteins that mediate synaptic expression of receptors. Although the majority of studies are focused on Group I mGlu receptors, many of the same themes apply to the Group II and Group III receptors.
Highlights.
Metabotropic glutamate receptors (mGlu receptors) undergo constitutive and agonist-induced endocytosis.
The surface expression and endocytosis of mGlu receptors are regulated by receptor phosphorylation and protein-protein interactions.
Many proteins involved in mGlu receptor trafficking bind to the intracellular C-terminus or cytoplasmic loop domains of mGlu receptors.
The intracellular trafficking pathways of mGlu receptors are remarkably diverse.
Acknowledgments
This work was support by the NINDS Intramural Research Program (K.C., K.W.R.), and the National Research Foundation of Korea (NRF) grants (NRF-2016R1D1A1B03930951, NRF-2017M3C7A1029611) (Y.H.S).
Abbreviations
- AC
adenylate cyclase
- Actn4
α-Actinin-4
- ARF6
ADP-ribosylation factor 6
- ATD
amino terminal domain
- CAIN
calcineurin inhibitor protein
- CaM
calmodulin
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- Cbln1
cerebellin 1 precursor
- CC
coiled-coil
- Cdk5
Cyclin-dependent kinase 5
- CIE
clathrin-independent endocytosis
- CLIC/GEEC
clathrin-independent carriers/GPI-anchored protein enriched early endosomal compartment
- CME
clathrin-mediated endocytosis
- CRIP1a
cannabinoid receptor interacting protein 1a
- CTD
C-terminal domain
- DAG
diacyl glycerol
- DN
dominant negative
- DRM
detergent-resistant lipid raft-rich membrane
- ECD
extracellular domain
- ERK1/2
extracellular signal-regulated kinases 1/2
- EVH1
enabled/VASP homology 1
- GAP
GTPase activating protein
- GPCR
G protein-coupled receptor
- GPI
glycosylphosphatidylinositol
- GRIP
glutamate receptor interacting protein
- GRK
GPCR kinase
- HFS
high-frequency stimulation
- Htt
huntingtin
- iL
intracellular loop
- IP3
inositol 1,4,5–trisphosphate
- JNK/SAPK
c-Jun N-terminal kinases/stress-activated protein kinase
- KO
knockout
- LTD
long-term depression
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- MF-SLIN
mossy fiber-CA3 interneuron
- mGlu receptor
metabotropic glutamate receptor
- NAc
Nucleus accumbens
- NECAB2
neuronal Ca2+ binding 2
- NHERF1/2
Na+/H+ exchanger regulatory factors 1/2
- NLS
nuclear localization signal
- OPTN
optineurin
- PH
pleckstrin homology
- PICK1
Protein Interacting with C-Kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PLD2
phospholipase D2
- PP1γ1
protein phosphatase 1γ1
- PP2A
protein phosphatase 2A
- PP2C
serine/threonine protein phosphatase 2C
- PTX
pertussis toxin
- PTZ
pentylenetetrazole
- RalGDS
Ral guanine nucleotide dissociation stimulator
- RGS
regulator of G protein signaling
- S6K1
p70-S6 kinase 1
- SAPAP
SAP90/PSD-95-associated protein
- SCG
superior cervical ganglion
- SER
smooth endoplasmic reticulum
- Siah-1A
seven in absentia homolog-1A
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TM
transmembrane domain
- TRPC
transient receptor potential canonical
- Vesl
VASP/Ena-related protein induced during Seizure and LTP
- VFD
Venus flytrap domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H, Misaka T, Tateyama M, Kubo Y. Effects of coexpression with Homer isoforms on the function of metabotropic glutamate receptor 1alpha. Molecular and cellular neurosciences. 2003;23:157–168. doi: 10.1016/s1044-7431(03)00052-6. [DOI] [PubMed] [Google Scholar]
- Ady V, Perroy J, Tricoire L, Piochon C, Dadak S, Chen X, Dusart I, Fagni L, Lambolez B, Levenes C. Type 1 metabotropic glutamate receptors (mGlu1) trigger the gating of GluD2 delta glutamate receptors. EMBO reports. 2014;15:103–109. doi: 10.1002/embr.201337371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airas JM, Betz H, El Far O. PKC phosphorylation of a conserved serine residue in the C-terminus of group III metabotropic glutamate receptors inhibits calmodulin binding. FEBS letters. 2001;494:60–63. doi: 10.1016/s0014-5793(01)02311-0. [DOI] [PubMed] [Google Scholar]
- Anborgh PH, Godin C, Pampillo M, Dhami GK, Dale LB, Cregan SP, Truant R, Ferguson SS. Inhibition of metabotropic glutamate receptor signaling by the huntingtin-binding protein optineurin. The Journal of biological chemistry. 2005;280:34840–34848. doi: 10.1074/jbc.M504508200. [DOI] [PubMed] [Google Scholar]
- Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Molecular and cellular neurosciences. 2002;20:323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. The European journal of neuroscience. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutierrez S, Martin-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, Lopez-Rodriguez ML, Grandes P, Rossignol R, Marsicano G. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nature neuroscience. 2012;15:558–564. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, Worley PF, Leahy DJ. Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron. 2000;26:143–154. doi: 10.1016/s0896-6273(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Beqollari D, Kammermeier PJ. The interaction between mGluR1 and the calcium channel Cav(2).(1) preserves coupling in the presence of long Homer proteins. Neuropharmacology. 2013;66:302–310. doi: 10.1016/j.neuropharm.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaso F, Lill Y, Airas JM, Espeut J, Blahos J, Bockaert J, Fagni L, Betz H, El-Far O. MacMARCKS interacts with the metabotropic glutamate receptor type 7 and modulates G protein-mediated constitutive inhibition of calcium channels. Journal of neurochemistry. 2006;99:288–298. doi: 10.1111/j.1471-4159.2006.04121.x. [DOI] [PubMed] [Google Scholar]
- Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, Lerner-Natoli M. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nature neuroscience. 2008;11:940–948. doi: 10.1038/nn.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Babwah AV, Godin C, Anborgh PH, Dale LB, Poulter MO, Ferguson SS. Ral and phospholipase D2-dependent pathway for constitutive metabotropic glutamate receptor endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8752–8761. doi: 10.1523/JNEUROSCI.3155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Perroy J, Becamel C, Marin P, Fagni L. GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annual review of pharmacology and toxicology. 2010;50:89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [DOI] [PubMed] [Google Scholar]
- Boudin H, Craig AM. Molecular determinants for PICK1 synaptic aggregation and mGluR7a receptor coclustering: role of the PDZ, coiled-coil, and acidic domains. The Journal of biological chemistry. 2001;276:30270–30276. doi: 10.1074/jbc.M102991200. [DOI] [PubMed] [Google Scholar]
- Boudin H, Doan A, Xia J, Shigemoto R, Huganir RL, Worley P, Craig AM. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–497. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Branco AF, Allen BG. G protein-coupled receptor signaling in cardiac nuclear membranes. Journal of cardiovascular pharmacology. 2015;65:101–109. doi: 10.1097/FJC.0000000000000196. [DOI] [PubMed] [Google Scholar]
- Burgueno J, Canela EI, Mallol J, Lluis C, Franco R, Ciruela F. Mutual regulation between metabotropic glutamate type 1alpha receptor and caveolin proteins: from traffick to constitutive activity. Experimental cell research. 2004;300:23–34. doi: 10.1016/j.yexcr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Cabello N, Remelli R, Canela L, Soriguera A, Mallol J, Canela EI, Robbins MJ, Lluis C, Franco R, McIlhinney RA, Ciruela F. Actin-binding protein alpha-actinin-1 interacts with the metabotropic glutamate receptor type 5b and modulates the cell surface expression and function of the receptor. The Journal of biological chemistry. 2007;282:12143–12153. doi: 10.1074/jbc.M608880200. [DOI] [PubMed] [Google Scholar]
- Cai Z, Saugstad JA, Sorensen SD, Ciombor KJ, Zhang C, Schaffhauser H, Hubalek F, Pohl J, Duvoisin RM, Conn PJ. Cyclic AMP-dependent protein kinase phosphorylates group III metabotropic glutamate receptors and inhibits their function as presynaptic receptors. Journal of neurochemistry. 2001;78:756–766. doi: 10.1046/j.1471-4159.2001.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G, Liao HJ. Receptor tyrosine kinases in the nucleus. Cold Spring Harbor perspectives in biology. 2013;5:a008979. doi: 10.1101/cshperspect.a008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Soloviev MM, Ciruela F, McIlhinney RA. Molecular determinants of metabotropic glutamate receptor 1B trafficking. Molecular and cellular neurosciences. 2001;17:577–588. doi: 10.1006/mcne.2001.0965. [DOI] [PubMed] [Google Scholar]
- Chang K, Roche KW. Structural and molecular determinants regulating mGluR5 surface expression. Neuropharmacology. 2017;115:10–19. doi: 10.1016/j.neuropharm.2016.04.037. [DOI] [PubMed] [Google Scholar]
- Choi JH, Park JY, Park SP, Lee H, Han S, Park KH, Suh YH. Regulation of mGluR7 trafficking by SUMOylation in neurons. Neuropharmacology. 2016;102:229–235. doi: 10.1016/j.neuropharm.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Choi KY, Chung S, Roche KW. Differential binding of calmodulin to group I metabotropic glutamate receptors regulates receptor trafficking and signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5921–5930. doi: 10.1523/JNEUROSCI.6253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, McIlhinney RA. Differential internalisation of mGluR1 splice variants in response to agonist and phorbol esters in permanently transfected BHK cells. FEBS letters. 1997;418:83–86. doi: 10.1016/s0014-5793(97)01353-7. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Soloviev MM, Chan WY, McIlhinney RA. Homer-1c/Vesl-1L modulates the cell surface targeting of metabotropic glutamate receptor type 1alpha: evidence for an anchoring function. Molecular and cellular neurosciences. 2000;15:36–50. doi: 10.1006/mcne.1999.0808. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Soloviev MM, McIlhinney RA. Co-expression of metabotropic glutamate receptor type 1alpha with homer-1a/Vesl-1S increases the cell surface expression of the receptor. The Biochemical journal. 1999;341( Pt 3):795–803. [PMC free article] [PubMed] [Google Scholar]
- Coutinho V, Kavanagh I, Sugiyama H, Tones MA, Henley JM. Characterization of a metabotropic glutamate receptor type 5-green fluorescent protein chimera (mGluR5-GFP): pharmacology, surface expression, and differential effects of Homer-1a and Homer-1c. Molecular and cellular neurosciences. 2001;18:296–306. doi: 10.1006/mcne.2001.1031. [DOI] [PubMed] [Google Scholar]
- Croci C, Sticht H, Brandstatter JH, Enz R. Group I metabotropic glutamate receptors bind to protein phosphatase 1C. Mapping and modeling of interacting sequences. The Journal of biological chemistry. 2003;278:50682–50690. doi: 10.1074/jbc.M305764200. [DOI] [PubMed] [Google Scholar]
- Dadak S, Bouquier N, Goyet E, Fagni L, Levenes C, Perroy J. mGlu1 receptor canonical signaling pathway contributes to the opening of the orphan GluD2 receptor. Neuropharmacology. 2017;115:92–99. doi: 10.1016/j.neuropharm.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Dale LB, Babwah AV, Bhattacharya M, Kelvin DJ, Ferguson SS. Spatial-temporal patterning of metabotropic glutamate receptor-mediated inositol 1,4,5-triphosphate, calcium, and protein kinase C oscillations: protein kinase C-dependent receptor phosphorylation is not required. The Journal of biological chemistry. 2001a;276:35900–35908. doi: 10.1074/jbc.M103847200. [DOI] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Seachrist JL, Anborgh PH, Ferguson SS. Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific. Molecular pharmacology. 2001b;60:1243–1253. doi: 10.1124/mol.60.6.1243. [DOI] [PubMed] [Google Scholar]
- del Toro D, Alberch J, Lazaro-Dieguez F, Martin-Ibanez R, Xifro X, Egea G, Canals JM. Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Molecular biology of the cell. 2009;20:1478–1492. doi: 10.1091/mbc.E08-07-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S. PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7252–7257. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacology & therapeutics. 2006;111:260–271. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano AR, Fahim S, Dunn HA, Walther C, Ribeiro FM, Cregan SP, Angers S, Schmid S, Ferguson SS. Role of Spinophilin in Group I Metabotropic Glutamate Receptor Endocytosis, Signaling, and Synaptic Plasticity. The Journal of biological chemistry. 2016;291:17602–17615. doi: 10.1074/jbc.M116.722355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- Dunn HA, Ferguson SS. PDZ Protein Regulation of G Protein-Coupled Receptor Trafficking and Signaling Pathways. Molecular pharmacology. 2015;88:624–639. doi: 10.1124/mol.115.098509. [DOI] [PubMed] [Google Scholar]
- Dutting E, Schroder-Kress N, Sticht H, Enz R. SUMO E3 ligases are expressed in the retina and regulate SUMOylation of the metabotropic glutamate receptor 8b. The Biochemical journal. 2011;435:365–371. doi: 10.1042/BJ20101854. [DOI] [PubMed] [Google Scholar]
- El Far O, Airas J, Wischmeyer E, Nehring RB, Karschin A, Betz H. Interaction of the C-terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1. The European journal of neuroscience. 2000;12:4215–4221. doi: 10.1046/j.1460-9568.2000.01309.x. [DOI] [PubMed] [Google Scholar]
- El Far O, Bofill-Cardona E, Airas JM, O’Connor V, Boehm S, Freissmuth M, Nanoff C, Betz H. Mapping of calmodulin and Gbetagamma binding domains within the C-terminal region of the metabotropic glutamate receptor 7A. The Journal of biological chemistry. 2001;276:30662–30669. doi: 10.1074/jbc.M102573200. [DOI] [PubMed] [Google Scholar]
- Eng AG, Kelver DA, Hedrick TP, Swanson GT. Transduction of group I mGluR-mediated synaptic plasticity by beta-arrestin2 signalling. Nature communications. 2016;7:13571. doi: 10.1038/ncomms13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R. The metabotropic glutamate receptor mGluR7b binds to the catalytic gamma-subunit of protein phosphatase 1. Journal of neurochemistry. 2002;81:1130–1140. doi: 10.1046/j.1471-4159.2002.00922.x. [DOI] [PubMed] [Google Scholar]
- Enz R, Croci C. Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. The Biochemical journal. 2003;372:183–191. doi: 10.1042/BJ20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseltine JL, Ferguson SS. Regulation of G protein-coupled receptor trafficking and signaling by Rab GTPases. Small GTPases. 2013;4:132–135. doi: 10.4161/sgtp.24304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseltine JL, Ribeiro FM, Ferguson SS. Rab8 modulates metabotropic glutamate receptor subtype 1 intracellular trafficking and signaling in a protein kinase C-dependent manner. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:16933–16942a. doi: 10.1523/JNEUROSCI.0625-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Ango F, Perroy J, Bockaert J. Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Seminars in cell & developmental biology. 2004;15:289–298. doi: 10.1016/j.semcdb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Feng W, Tu J, Yang T, Vernon PS, Allen PD, Worley PF, Pessah IN. Homer regulates gain of ryanodine receptor type 1 channel complex. The Journal of biological chemistry. 2002;277:44722–44730. doi: 10.1074/jbc.M207675200. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Crepaldi L, Nicoletti F. Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacological reviews. 2008;60:536–581. doi: 10.1124/pr.108.000166. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell and tissue research. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Ferreira LT, Dale LB, Ribeiro FM, Babwah AV, Pampillo M, Ferguson SS. Calcineurin inhibitor protein (CAIN) attenuates Group I metabotropic glutamate receptor endocytosis and signaling. The Journal of biological chemistry. 2009;284:28986–28994. doi: 10.1074/jbc.M109.050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero JJ, Ramirez-Franco J, Martin R, Bartolome-Martin D, Torres M, Sanchez-Prieto J. Cross-talk between metabotropic glutamate receptor 7 and beta adrenergic receptor signaling at cerebrocortical nerve terminals. Neuropharmacology. 2016;101:412–425. doi: 10.1016/j.neuropharm.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, Nairn AC, Greengard P. Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:16006–16011. doi: 10.1073/pnas.2136600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor PJ, Gomeza J, Tones MA, Kuhn R, Pin JP, Knopfel T. The C-terminal domain of the mGluR1 metabotropic glutamate receptor affects sensitivity to agonists. Journal of neurochemistry. 1996;67:58–63. doi: 10.1046/j.1471-4159.1996.67010058.x. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Bessis AS, Rossignol F, Pin JP, Olivo-Marin JC, Hemar A. The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. The Journal of biological chemistry. 2003;278:12222–12230. doi: 10.1074/jbc.M205663200. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. The Journal of biological chemistry. 1998;273:5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6185–6190. doi: 10.1073/pnas.97.11.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. The Journal of biological chemistry. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- Gereau RWt, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Canadian journal of physiology and pharmacology. 2006;84:287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Mary S, Brabet I, Parmentier ML, Restituito S, Bockaert J, Pin JP. Coupling of metabotropic glutamate receptors 2 and 4 to G alpha 15, G alpha 16, and chimeric G alpha q/i proteins: characterization of new antagonists. Molecular pharmacology. 1996;50:923–930. [PubMed] [Google Scholar]
- Gulia R, Sharma R, Bhattacharyya S. A Critical Role for Ubiquitination in the Endocytosis of Glutamate Receptors. The Journal of biological chemistry. 2017;292:1426–1437. doi: 10.1074/jbc.M116.752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacology & therapeutics. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Ames HM, Hayashi Y. Tetrameric hub structure of postsynaptic scaffolding protein homer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8492–8501. doi: 10.1523/JNEUROSCI.2731-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Saunders R, Selkirk JV, Mistry R, Nahorski SR, Challiss RA. Complex involvement of pertussis toxin-sensitive G proteins in the regulation of type 1alpha metabotropic glutamate receptor signaling in baby hamster kidney cells. Molecular pharmacology. 2000;58:352–360. doi: 10.1124/mol.58.2.352. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. The Journal of biological chemistry. 2002;277:15221–15224. doi: 10.1074/jbc.C200112200. [DOI] [PubMed] [Google Scholar]
- Holmes KD, Mattar P, Marsh DR, Jordan V, Weaver LC, Dekaban GA. The C-terminal C1 cassette of the N -methyl-D-aspartate receptor 1 subunit contains a bi-partite nuclear localization sequence. Journal of neurochemistry. 2002;81:1152–1165. doi: 10.1046/j.1471-4159.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- Hong YH, Kim JY, Lee JH, Chae HG, Jang SS, Jeon JH, Kim CH, Kim J, Kim SJ. Agonist-induced internalization of mGluR1alpha is mediated by caveolin. Journal of neurochemistry. 2009;111:61–71. doi: 10.1111/j.1471-4159.2009.06289.x. [DOI] [PubMed] [Google Scholar]
- Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O’Hara PJ, Mulvihill ER, Almers W, Hagen FS. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science (New York, NY) 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- Hu JH, Park JM, Park S, Xiao B, Dehoff MH, Kim S, Hayashi T, Schwarz MK, Huganir RL, Seeburg PH, Linden DJ, Worley PF. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010;68:1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Yang L, Kammermeier PJ, Moore CG, Brakeman PR, Tu J, Yu S, Petralia RS, Li Z, Zhang PW, Park JM, Dong X, Xiao B, Worley PF. Preso1 dynamically regulates group I metabotropic glutamate receptors. Nature neuroscience. 2012;15:836–844. doi: 10.1038/nn.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Capobianco L, Iula M, Di Giorgi Gerevini V, Picascia A, Blahos J, Melchiorri D, Nicoletti F, De Blasi A. Regulation of mGlu4 metabotropic glutamate receptor signaling by type-2 G-protein coupled receptor kinase (GRK2) Molecular pharmacology. 2004;65:1103–1110. doi: 10.1124/mol.65.5.1103. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Nicoletti F, De Blasi A. Molecular mechanisms that desensitize metabotropic glutamate receptor signaling: an overview. Neuropharmacology. 2013;66:24–30. doi: 10.1016/j.neuropharm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggio S, Sallese M, Porcellini A, Nicoletti F, De Blasi A. Role of G protein-coupled receptor kinase 4 and beta-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. The Journal of biological chemistry. 2003;278:12433–12442. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Current opinion in cell biology. 2014;27:109–116. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Nash SR, Nishimune A, Neki A, Kaneko S, Nakanishi S. Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes to cells : devoted to molecular & cellular mechanisms. 1999;4:381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013a;33:3402–3412. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Mao LM, Wang JQ. Differential regulation of CaMKIIalpha interactions with mGluR5 and NMDA receptors by Ca(2+) in neurons. Journal of neurochemistry. 2013b;127:620–631. doi: 10.1111/jnc.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Mao LM, Wang JQ. An Essential Role of Fyn in the Modulation of Metabotropic Glutamate Receptor 1 in Neurons. eNeuro. 2017:4. doi: 10.1523/ENEURO.0096-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L, Parton RG, Bassereau P, Mayor S. Building endocytic pits without clathrin. Nature reviews Molecular cell biology. 2015;16:311–321. doi: 10.1038/nrm3968. [DOI] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, O’Malley KL. Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. The Journal of biological chemistry. 2009;284:35827–35838. doi: 10.1074/jbc.M109.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowska M, Chavez AE, Lutzu S, Castillo PE, Bukauskas FF, Francesconi A. Actinin-4 Governs Dendritic Spine Dynamics and Promotes Their Remodeling by Metabotropic Glutamate Receptors. The Journal of biological chemistry. 2015;290:15909–15920. doi: 10.1074/jbc.M115.640136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ. Surface clustering of metabotropic glutamate receptor 1 induced by long Homer proteins. BMC neuroscience. 2006;7:1. doi: 10.1186/1471-2202-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Yun J. Activation of metabotropic glutamate receptor 1 dimers requires glutamate binding in both subunits. The Journal of pharmacology and experimental therapeutics. 2005;312:502–508. doi: 10.1124/jpet.104.073155. [DOI] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. The Journal of biological chemistry. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kato AS, Knierman MD, Siuda ER, Isaac JT, Nisenbaum ES, Bredt DS. Glutamate receptor delta2 associates with metabotropic glutamate receptor 1 (mGluR1), protein kinase Cgamma, and canonical transient receptor potential 3 and regulates mGluR1-mediated synaptic transmission in cerebellar Purkinje neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15296–15308. doi: 10.1523/JNEUROSCI.0705-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- Kim CH, Braud S, Isaac JT, Roche KW. Protein kinase C phosphorylation of the metabotropic glutamate receptor mGluR5 on Serine 839 regulates Ca2+ oscillations. The Journal of biological chemistry. 2005;280:25409–25415. doi: 10.1074/jbc.M502644200. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lee J, Lee JY, Roche KW. Metabotropic glutamate receptors: phosphorylation and receptor signaling. Journal of neuroscience research. 2008;86:1–10. doi: 10.1002/jnr.21437. [DOI] [PubMed] [Google Scholar]
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1280–1289. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano J, Yamazaki Y, Kimura K, Masukado T, Nakajima Y, Nakanishi S. Tamalin is a scaffold protein that interacts with multiple neuronal proteins in distinct modes of protein-protein association. The Journal of biological chemistry. 2003;278:14762–14768. doi: 10.1074/jbc.M300184200. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nature structural & molecular biology. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- Ko SJ, Isozaki K, Kim I, Lee JH, Cho HJ, Sohn SY, Oh SR, Park S, Kim DG, Kim CH, Roche KW. PKC phosphorylation regulates mGluR5 trafficking by enhancing binding of Siah-1A. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:16391–16401. doi: 10.1523/JNEUROSCI.1964-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Castillo C, Francesconi A. Agonist-dependent signaling by group I metabotropic glutamate receptors is regulated by association with lipid domains. The Journal of biological chemistry. 2013;288:32004–32019. doi: 10.1074/jbc.M113.475863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpost J, Syrova Z, Kulihova L, Frankova D, Bologna JC, Hlavackova V, Prezeau L, Kralikova M, Hruskova B, Pin JP, Blahos J. Surface expression of metabotropic glutamate receptor variants mGluR1a and mGluR1b in transfected HEK293 cells. Neuropharmacology. 2008;55:409–418. doi: 10.1016/j.neuropharm.2008.06.073. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, Roche KW. Constitutive endocytosis of the metabotropic glutamate receptor mGluR7 is clathrin-independent. Neuropharmacology. 2007;52:100–107. doi: 10.1016/j.neuropharm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Lee HW, Choi J, Shin H, Kim K, Yang J, Na M, Choi SY, Kang GB, Eom SH, Kim H, Kim E. Preso, a novel PSD-95-interacting FERM and PDZ domain protein that regulates dendritic spine morphogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008a;28:14546–14556. doi: 10.1523/JNEUROSCI.3112-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee J, Choi KY, Hepp R, Lee JY, Lim MK, Chatani-Hinze M, Roche PA, Kim DG, Ahn YS, Kim CH, Roche KW. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105:12575–12580. doi: 10.1073/pnas.0712033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Westin L, Kim J, Chang JC, Oh YS, Amreen B, Gresack J, Flajolet M, Kim D, Aperia A, Kim Y, Greengard P. Alteration by p11 of mGluR5 localization regulates depression-like behaviors. Molecular psychiatry. 2015;20:1546–1556. doi: 10.1038/mp.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidwell K, Dillon J, Sihota A, O’Connor V, Pilkington B. Determining calmodulin binding to metabotropic glutamate receptors with distinct protein-interaction methods. Biochemical Society transactions. 2004;32:868–870. doi: 10.1042/BST0320868. [DOI] [PubMed] [Google Scholar]
- Lu D, Yan H, Othman T, Rivkees SA. Cytoskeletal protein 4.1G is a binding partner of the metabotropic glutamate receptor subtype 1 alpha. Journal of neuroscience research. 2004;78:49–55. doi: 10.1002/jnr.20230. [DOI] [PubMed] [Google Scholar]
- Luis Albasanz J, Fernandez M, Martin M. Internalization of metabotropic glutamate receptor in C6 cells through clathrin-coated vesicles. Brain research Molecular brain research. 2002;99:54–66. doi: 10.1016/s0169-328x(02)00103-1. [DOI] [PubMed] [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. Journal of chemical neuroanatomy. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- Luo P, Yang Y, Liu W, Rao W, Bian H, Li X, Chen T, Liu M, Zhao Y, Dai S, Yan X, Fei Z. Downregulation of postsynaptic density-95-interacting regulator of spine morphogenesis reduces glutamate-induced excitotoxicity by differentially regulating glutamate receptors in rat cortical neurons. The FEBS journal. 2013;280:6114–6127. doi: 10.1111/febs.12531. [DOI] [PubMed] [Google Scholar]
- Mahato PK, Pandey S, Bhattacharyya S. Differential effects of protein phosphatases in the recycling of metabotropic glutamate receptor 5. Neuroscience. 2015;306:138–150. doi: 10.1016/j.neuroscience.2015.08.031. [DOI] [PubMed] [Google Scholar]
- Mao LM, Liu XY, Zhang GC, Chu XP, Fibuch EE, Wang LS, Liu Z, Wang JQ. Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology. 2008;55:403–408. doi: 10.1016/j.neuropharm.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang JQ. Regulation of Group I Metabotropic Glutamate Receptors by MAPK/ERK in Neurons. Journal of nature and science. 2016:2. [PMC free article] [PubMed] [Google Scholar]
- Martin R, Durroux T, Ciruela F, Torres M, Pin JP, Sanchez-Prieto J. The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13–1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. The Journal of biological chemistry. 2010;285:17907–17917. doi: 10.1074/jbc.M109.080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia F, Klotz L, Lerch J, Ahmed MH, Zhang Y, Enz R. CRIP1a inhibits endocytosis of G-protein coupled receptors activated by endocannabinoids and glutamate by a common molecular mechanism. Journal of neurochemistry. 2017 doi: 10.1111/jnc.14021. [DOI] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Mateos JM, Azkue J, Benitez R, Sarria R, Losada J, Conquet F, Ferraguti F, Kuhn R, Knopfel T, Grandes P. Immunocytochemical localization of the mGluR1b metabotropic glutamate receptor in the rat hypothalamus. The Journal of comparative neurology. 1998;390:225–233. doi: 10.1002/(sici)1096-9861(19980112)390:2<225::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mateos JM, Benitez R, Elezgarai I, Azkue JJ, Lazaro E, Osorio A, Bilbao A, Donate F, Sarria R, Conquet F, Ferraguti F, Kuhn R, Knopfel T, Grandes P. Immunolocalization of the mGluR1b splice variant of the metabotropic glutamate receptor 1 at parallel fiber-Purkinje cell synapses in the rat cerebellar cortex. Journal of neurochemistry. 2000;74:1301–1309. doi: 10.1046/j.1471-4159.2000.741301.x. [DOI] [PubMed] [Google Scholar]
- Mathiesen JM, Ramirez MT. The metabotropic glutamate receptor 4 is internalized and desensitized upon protein kinase C activation. British journal of pharmacology. 2006;148:279–290. doi: 10.1038/sj.bjp.0706733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, Watanabe M, Yuzaki M. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science (New York, NY) 2010;328:363–368. doi: 10.1126/science.1185152. [DOI] [PubMed] [Google Scholar]
- Minakami R, Jinnai N, Sugiyama H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. The Journal of biological chemistry. 1997;272:20291–20298. doi: 10.1074/jbc.272.32.20291. [DOI] [PubMed] [Google Scholar]
- Minami I, Kengaku M, Smitt PS, Shigemoto R, Hirano T. Long-term potentiation of mGluR1 activity by depolarization-induced Homer1a in mouse cerebellar Purkinje neurons. The European journal of neuroscience. 2003;17:1023–1032. doi: 10.1046/j.1460-9568.2003.02499.x. [DOI] [PubMed] [Google Scholar]
- Mochida S. Ca/Calmodulin and presynaptic short-term plasticity. ISRN neurology. 2011;2011:919043. doi: 10.5402/2011/919043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A, Scheschonka A, Beckhaus T, Karas M, Betz H. Metabotropic glutamate receptor 4 interacts with microtubule-associated protein 1B. Biochemical and biophysical research communications. 2009;390:82–86. doi: 10.1016/j.bbrc.2009.09.070. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Iijima K, Fujii H, Ito H, Cho Y, Nakanishi S. Seven in absentia homolog 1A mediates ubiquitination and degradation of group 1 metabotropic glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8614–8619. doi: 10.1073/pnas.0403042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutin E, Raynaud F, Roger J, Pellegrino E, Homburger V, Bertaso F, Ollendorff V, Bockaert J, Fagni L, Perroy J. Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. The Journal of cell biology. 2012;198:251–263. doi: 10.1083/jcb.201110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Matharu AL, Pula G, Holman D, Roberts PJ, Kelly E. Metabotropic glutamate receptor 1 internalization induced by muscarinic acetylcholine receptor activation: differential dependency of internalization of splice variants on nonvisual arrestins. Molecular pharmacology. 2002;61:1114–1123. doi: 10.1124/mol.61.5.1114. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. Journal of neurochemistry. 2001;78:546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Pula G, Carswell K, Roberts PJ, Kelly E. Agonist-induced internalization of metabotropic glutamate receptor 1A: structural determinants for protein kinase C- and G protein-coupled receptor kinase-mediated internalization. Journal of neurochemistry. 2003;84:294–304. doi: 10.1046/j.1471-4159.2003.01515.x. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Pula G, More JC, Jane DE, Roberts PJ, Kelly E. Activation of cyclic AMP-dependent protein kinase inhibits the desensitization and internalization of metabotropic glutamate receptors 1a and 1b. Molecular pharmacology. 2004;65:1507–1516. doi: 10.1124/mol.65.6.1507. [DOI] [PubMed] [Google Scholar]
- Nakajima Y. Ca2+-dependent binding of calcium-binding protein 1 to presynaptic group III metabotropic glutamate receptors and blockage by phosphorylation of the receptors. Biochemical and biophysical research communications. 2011;412:602–605. doi: 10.1016/j.bbrc.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Mochida S, Okawa K, Nakanishi S. Ca2+-dependent release of Munc18-1 from presynaptic mGluRs in short-term facilitation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18385–18389. doi: 10.1073/pnas.0910088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Yamamoto T, Nakayama T, Nakanishi S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. The Journal of biological chemistry. 1999;274:27573–27577. doi: 10.1074/jbc.274.39.27573. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual review of pharmacology and toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor V, El Far O, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A, Airas JM, Betz H, Boehm S. Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science (New York, NY) 1999;286:1180–1184. doi: 10.1126/science.286.5442.1180. [DOI] [PubMed] [Google Scholar]
- O’Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. The Journal of biological chemistry. 2003;278:28210–28219. doi: 10.1074/jbc.M300792200. [DOI] [PubMed] [Google Scholar]
- Oksche A, Boese G, Horstmeyer A, Furkert J, Beyermann M, Bienert M, Rosenthal W. Late endosomal/lysosomal targeting and lack of recycling of the ligand-occupied endothelin B receptor. Molecular pharmacology. 2000;57:1104–1113. [PubMed] [Google Scholar]
- Orlando LR, Ayala R, Kett LR, Curley AA, Duffner J, Bragg DC, Tsai LH, Dunah AW, Young AB. Phosphorylation of the homer-binding domain of group I metabotropic glutamate receptors by cyclin-dependent kinase 5. Journal of neurochemistry. 2009;110:557–569. doi: 10.1111/j.1471-4159.2009.06139.x. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Pandey S, Mahato PK, Bhattacharyya S. Metabotropic glutamate receptor 1 recycles to the cell surface in protein phosphatase 2A-dependent manner in non-neuronal and neuronal cell lines. Journal of neurochemistry. 2014;131:602–614. doi: 10.1111/jnc.12930. [DOI] [PubMed] [Google Scholar]
- Paquet M, Asay MJ, Fam SR, Inuzuka H, Castleberry AM, Oller H, Smith Y, Yun CC, Traynelis SF, Hall RA. The PDZ scaffold NHERF-2 interacts with mGluR5 and regulates receptor activity. The Journal of biological chemistry. 2006;281:29949–29961. doi: 10.1074/jbc.M602262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, Datko MC, Domingo RD, Reyes CM, Wang XJ, Etzkorn FA, Xiao B, Szumlinski KK, Kern D, Linden DJ, Worley PF. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell. 2013;154:637–650. doi: 10.1016/j.cell.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46:89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Differential regulation at functionally divergent release sites along a common axon. Current opinion in neurobiology. 2007;17:366–373. doi: 10.1016/j.conb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. The Journal of physiology. 2008;586:1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Yuan XQ, Lacaille JC, McBain CJ. State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron. 2008;60:980–987. doi: 10.1016/j.neuron.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Yuan X, Lavezzari G, Roche KW, McBain CJ. mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology. 2007;52:108–117. doi: 10.1016/j.neuropharm.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Perroy J, El Far O, Bertaso F, Pin JP, Betz H, Bockaert J, Fagni L. PICK1 is required for the control of synaptic transmission by the metabotropic glutamate receptor 7. The EMBO journal. 2002;21:2990–2999. doi: 10.1093/emboj/cdf313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy J, Prezeau L, De Waard M, Shigemoto R, Bockaert J, Fagni L. Selective blockade of P/Q-type calcium channels by the metabotropic glutamate receptor type 7 involves a phospholipase C pathway in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7896–7904. doi: 10.1523/JNEUROSCI.20-21-07896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nature reviews Neuroscience. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Pula G, Mundell SJ, Roberts PJ, Kelly E. Agonist-independent internalization of metabotropic glutamate receptor 1a is arrestin- and clathrin-dependent and is suppressed by receptor inverse agonists. Journal of neurochemistry. 2004;89:1009–1020. doi: 10.1111/j.1471-4159.2004.02387.x. [DOI] [PubMed] [Google Scholar]
- Raka F, Di Sebastiano AR, Kulhawy SC, Ribeiro FM, Godin CM, Caetano FA, Angers S, Ferguson SS. Ca(2+)/calmodulin-dependent protein kinase II interacts with group I metabotropic glutamate and facilitates receptor endocytosis and ERK1/2 signaling: role of beta-amyloid. Molecular brain. 2015;8:21. doi: 10.1186/s13041-015-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos C, Chardonnet S, Marchand CH, Decottignies P, Ango F, Daniel H, Le Marechal P. Native presynaptic metabotropic glutamate receptor 4 (mGluR4) interacts with exocytosis proteins in rat cerebellum. The Journal of biological chemistry. 2012;287:20176–20186. doi: 10.1074/jbc.M112.347468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Dwivedi H, Baidya M, Kumar M, Shukla AK. Novel Structural Insights into GPCR-beta-Arrestin Interaction and Signaling. Trends in cell biology. 2017 doi: 10.1016/j.tcb.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Remelli R, Robbins MJ, McIlhinney RA. The C-terminus of the metabotropic glutamate receptor 1b regulates dimerization of the receptor. Journal of neurochemistry. 2008;104:1020–1031. doi: 10.1111/j.1471-4159.2007.05034.x. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Baalman K, Teng Y, Mee MP, Dawson SP, Wang H, De Biasi M, Mayer RJ. Proteasomal degradation of the metabotropic glutamate receptor 1alpha is mediated by Homer-3 via the proteasomal S8 ATPase: Signal transduction and synaptic transmission. Journal of neurochemistry. 2012;122:24–37. doi: 10.1111/j.1471-4159.2012.07752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter-Makinson SL, Paquet M, Bogenpohl JW, Rodin RE, Chris Yun C, Weinman EJ, Smith Y, Hall RA. Group II metabotropic glutamate receptor interactions with NHERF scaffold proteins: Implications for receptor localization in brain. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nature reviews Molecular cell biology. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. The Journal of biological chemistry. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- Romano C, Yang WL, O’Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. The Journal of biological chemistry. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nature neuroscience. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Rose M, Dutting E, Enz R. Band 4.1 proteins are expressed in the retina and interact with both isoforms of the metabotropic glutamate receptor type 8. Journal of neurochemistry. 2008;105:2375–2387. doi: 10.1111/j.1471-4159.2008.05331.x. [DOI] [PubMed] [Google Scholar]
- Sallese M, Salvatore L, D’Urbano E, Sala G, Storto M, Launey T, Nicoletti F, Knopfel T, De Blasi A. The G-protein-coupled receptor kinase GRK4 mediates homologous desensitization of metabotropic glutamate receptor 1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:2569–2580. doi: 10.1096/fj.00-0072com. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Uneyama H, Yoshie S, Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate stimuli. Chemical senses. 2005;30(Suppl 1):i25–26. doi: 10.1093/chemse/bjh095. [DOI] [PubMed] [Google Scholar]
- Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP. Numb is an endocytic protein. The Journal of cell biology. 2000;151:1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiological reviews. 2015;95:377–404. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhauser H, Cai Z, Hubalek F, Macek TA, Pohl J, Murphy TJ, Conn PJ. cAMP-dependent protein kinase inhibits mGluR2 coupling to G-proteins by direct receptor phosphorylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:5663–5670. doi: 10.1523/JNEUROSCI.20-15-05663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SM, Hollmann M. To gate or not to gate: are the delta subunits in the glutamate receptor family functional ion channels? Molecular neurobiology. 2008;37:126–141. doi: 10.1007/s12035-008-8025-0. [DOI] [PubMed] [Google Scholar]
- Schools GP, Kimelberg HK. mGluR3 and mGluR5 are the predominant metabotropic glutamate receptor mRNAs expressed in hippocampal astrocytes acutely isolated from young rats. Journal of neuroscience research. 1999;58:533–543. doi: 10.1002/(sici)1097-4547(19991115)58:4<533::aid-jnr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Serge A, Fourgeaud L, Hemar A, Choquet D. Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:3910–3920. doi: 10.1523/JNEUROSCI.22-10-03910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergin I, Jong YI, Harmon SK, Kumar V, O’Malley KL. Sequences within the C Terminus of the Metabotropic Glutamate Receptor 5 (mGluR5) Are Responsible for Inner Nuclear Membrane Localization. The Journal of biological chemistry. 2017;292:3637–3655. doi: 10.1074/jbc.M116.757724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna F, Zhang M, Kaphzan H, Klann E, Autio D, Cox CL, Wang H. Calmodulin activity regulates group I metabotropic glutamate receptor-mediated signal transduction and synaptic depression. Journal of neuroscience research. 2016;94:401–408. doi: 10.1002/jnr.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annual review of neuroscience. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature neuroscience. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science (New York, NY) 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Conn PJ. G protein-coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology. 2003;44:699–706. doi: 10.1016/s0028-3908(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Macek TA, Cai Z, Saugstad JA, Conn PJ. Dissociation of protein kinase-mediated regulation of metabotropic glutamate receptor 7 (mGluR7) interactions with calmodulin and regulation of mGluR7 function. Molecular pharmacology. 2002;61:1303–1312. doi: 10.1124/mol.61.6.1303. [DOI] [PubMed] [Google Scholar]
- Sugi T, Oyama T, Muto T, Nakanishi S, Morikawa K, Jingami H. Crystal structures of autoinhibitory PDZ domain of Tamalin: implications for metabotropic glutamate receptor trafficking regulation. The EMBO journal. 2007;26:2192–2205. doi: 10.1038/sj.emboj.7601651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Park JY, Park S, Jou I, Roche PA, Roche KW. Regulation of metabotropic glutamate receptor 7 (mGluR7) internalization and surface expression by Ser/Thr protein phosphatase 1. The Journal of biological chemistry. 2013;288:17544–17551. doi: 10.1074/jbc.M112.439513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain CJ, Roche KW. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58:736–748. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nature reviews Neuroscience. 2013;14:673–680. doi: 10.1038/nrn3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Tadevosyan A, Vaniotis G, Allen BG, Hebert TE, Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. The Journal of physiology. 2012;590:1313–1330. doi: 10.1113/jphysiol.2011.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Tachibana T, Imanaka T, Nishida W, Sobue K. Involvement of unique leucine-zipper motif of PSD-Zip45 (Homer 1c/vesl-1L) in group 1 metabotropic glutamate receptor clustering. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13801–13806. doi: 10.1073/pnas.96.24.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu Y, Takeuchi K, Kumari R, Bennett MV, Zukin RS, Francesconi A. Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21778–21783. doi: 10.1073/pnas.1015553107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, El Far O, Betz H, Scheschonka A. Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. The Journal of biological chemistry. 2005;280:38153–38159. doi: 10.1074/jbc.M508168200. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. Coupling profile of the metabotropic glutamate receptor 1alpha is regulated by the C-terminal domain. Molecular and cellular neurosciences. 2007;34:445–452. doi: 10.1016/j.mcn.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Techlovska S, Chambers JN, Dvorakova M, Petralia RS, Wang YX, Hajkova A, Nova A, Frankova D, Prezeau L, Blahos J. Metabotropic glutamate receptor 1 splice variants mGluR1a and mGluR1b combine in mGluR1a/b dimers in vivo. Neuropharmacology. 2014;86:329–336. doi: 10.1016/j.neuropharm.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C. Metabotropic glutamate receptor subtype 1A activates adenylate cyclase when expressed in baby hamster kidney cells. Progress in neuro-psychopharmacology & biological psychiatry. 1996;20:709–726. doi: 10.1016/0278-5846(96)00042-5. [DOI] [PubMed] [Google Scholar]
- Tomioka NH, Yasuda H, Miyamoto H, Hatayama M, Morimura N, Matsumoto Y, Suzuki T, Odagawa M, Odaka YS, Iwayama Y, Won Um J, Ko J, Inoue Y, Kaneko S, Hirose S, Yamada K, Yoshikawa T, Yamakawa K, Aruga J. Elfn1 recruits presynaptic mGluR7 in trans and its loss results in seizures. Nature communications. 2014;5:4501. doi: 10.1038/ncomms5501. [DOI] [PubMed] [Google Scholar]
- Tong Q, Kirchgessner AL. Localization and function of metabotropic glutamate receptor 8 in the enteric nervous system. American journal of physiology Gastrointestinal and liver physiology. 2003;285:G992–g1003. doi: 10.1152/ajpgi.00118.2003. [DOI] [PubMed] [Google Scholar]
- Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. The FEBS journal. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- Trivedi RR, Bhattacharyya S. Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5) Biochemical and biophysical research communications. 2012;427:185–190. doi: 10.1016/j.bbrc.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Uematsu K, Heiman M, Zelenina M, Padovan J, Chait BT, Aperia A, Nishi A, Greengard P. Protein kinase A directly phosphorylates metabotropic glutamate receptor 5 to modulate its function. Journal of neurochemistry. 2015;132:677–686. doi: 10.1111/jnc.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Wang H, Warner-Schmidt J, Varela S, Enikolopov G, Greengard P, Flajolet M. Norbin ablation results in defective adult hippocampal neurogenesis and depressive-like behavior in mice. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9745–9750. doi: 10.1073/pnas.1510291112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Westin L, Nong Y, Birnbaum S, Bendor J, Brismar H, Nestler E, Aperia A, Flajolet M, Greengard P. Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science (New York, NY) 2009;326:1554–1557. doi: 10.1126/science.1178496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Henley JM. Analysis of metabotropic glutamate receptor 7 as a potential substrate for SUMOylation. Neuroscience letters. 2011;491:181–186. doi: 10.1016/j.neulet.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Nishimune A, Henley JM. Analysis of SUMO-1 modification of neuronal proteins containing consensus SUMOylation motifs. Neuroscience letters. 2008;436:239–244. doi: 10.1016/j.neulet.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SS, Koochekpour S. Glutamate, glutamate receptors, and downstream signaling pathways. International journal of biological sciences. 2013;9:948–959. doi: 10.7150/ijbs.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Current opinion in neurobiology. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yang JH, Mao LM, Choe ES, Wang JQ. Synaptic ERK2 Phosphorylates and Regulates Metabotropic Glutamate Receptor 1 In Vitro and in Neurons. Molecular neurobiology. 2016 doi: 10.1007/s12035-016-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzaki M, Aricescu AR. A GluD Coming-Of-Age Story. Trends in neurosciences. 2017;40:138–150. doi: 10.1016/j.tins.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CS, Bertaso F, Eulenburg V, Lerner-Natoli M, Herin GA, Bauer L, Bockaert J, Fagni L, Betz H, Scheschonka A. Knock-in mice lacking the PDZ-ligand motif of mGluR7a show impaired PKC-dependent autoinhibition of glutamate release, spatial working memory deficits, and increased susceptibility to pentylenetetrazol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8604–8614. doi: 10.1523/JNEUROSCI.0628-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Yang D, Wang DJ, Xie YJ, Zhou JH, Zhou L, Huang H, Han S, Shao CY, Li HS, Zhu JJ, Qiu MS, De Zeeuw CI, Shen Y. Numb deficiency in cerebellar Purkinje cells impairs synaptic expression of metabotropic glutamate receptor and motor coordination. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15474–15479. doi: 10.1073/pnas.1512915112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Ryan K, Chen S. Cloning of novel splice variants of mouse mGluR1. Brain research Molecular brain research. 1999;73:93–103. doi: 10.1016/s0169-328x(99)00239-9. [DOI] [PubMed] [Google Scholar]