Abstract
Background and aims
Resilience and recovery are of increasing importance in the field of alcohol dependence (AD). This paper describes how imaging studies in man can be used to assess the neurobiological correlates of resilience and, if longitudinal, of disease trajectories, progression rates and markers for recovery to inform treatment and prevention options.
Methods
Original articles on recovery and resilience in alcohol addiction and its neurobiological correlates were identified from ‘PubMed’ and have been analyzed and condensed within a systematic literature review.
Results
Findings deriving from (f)MRI and PET studies have identified links between increased resilience and less task-elicited neural activation within the basal ganglia, and benefits of heightened neural prefrontal cortex (PFC) engagement regarding resilience in a broader sense, namely resilience against relapse in early abstinence of AD. Furthermore, findings consistently propose at least partial recovery of brain glucose metabolism and executive and general cognitive functioning, as well as structural plasticity effects throughout the brain of alcohol-dependent patients during the course of short, medium and long-term abstinence, even when patients only lowered their alcohol consumption to a moderate level. Additionally, specific factors were found that appear to influence these observed brain recovery processes in AD, e.g. genotype-dependent neuronal (re)growth, gender-specific neural recovery effects, critical interfering effects of psychiatric comorbidities, additional smoking or marijuana influences, or adolescent alcohol abuse.
Conclusions
Neuroimaging research has uncovered neurobiological markers that appear to be linked to resilience and improved recovery capacities that are furthermore influenced by various factors such as gender or genetics. Consequently, future system-oriented approaches may help to establish a broad neuroscience-based research framework for alcohol dependence.
Keywords: alcohol dependence, resilience, recovery, neuroimaging, functional, structural
Introduction
Imaging recovery and resilience
The toxic effects of alcohol are particularly seen in the brain as demonstrated by several post-mortem and in vivo neuroimaging studies in individuals with alcohol dependence (AD; e.g. (1–3)). Structural changes are clearly observed in the brain, including atrophy of gray and white matter with sulcal widening and ventricular enlargement. In addition, chronic alcohol consumption is accompanied by neural adaptations within different neurotransmitter systems, such as the dopamine system (cf. reviews (4–9)). These neural and molecular changes have been further shown to be associated with dysfunctional brain functions underlying psychological and behavioral processes in AD (10–19).
Once harmful alcohol use stops or is reduced, beneficial recovery processes can be observed regarding physical and mental health (see (20)), and in the brain using various neuroimaging techniques (21–24). One of the main questions for neuroimaging research in the field of addictive disorders is to characterize predictors of recovery and treatment outcome (25). It is notable however that a clear standard definition of the term “recovery” is not generally established yet. In this review, we will focus on structural and functional changes within the brain associated with reduction of alcohol intake or abstinence in AD investigated by studies using neuroimaging techniques as identified by our literature search.
Another consideration is to what extent abnormalities in brain structure and function are caused by the toxic effects of alcohol, or whether some of these differences might have been pre-existing and putatively predispose some individuals to develop alcohol dependence while others seem to have a protective effect i.e. confer resilience (26). Resilience is traditionally defined as the ability to adapt to adverse/ traumatic environments thus resulting in healthy long-term psychological functioning and better developmental outcomes (27–29). Resilience research also concentrates on high-risk groups, which do not develop the disorder of interest despite carrying risk genes and/or experiencing adverse environmental conditions. Studying those individuals already affected, however, adds a new perspective to the understanding of disease development, disease progression, and future potential treatment strategies by focusing on neurobiological factors that promote a good treatment outcome despite adversities. Thus, studies using neuroimaging techniques may help to identify such resilience mechanisms regarding the structural and functional markers of neural patterns associated with attenuating further disease progression and/or relapse in AD (10, 11, 30, 31). Such factors are not defined by the absence of vulnerability markers but rather by compensatory changes in biological markers that distinguish individuals with good treatment outcome from those who relapse and healthy controls.
We therefore reviewed the available literature to answer the following questions 1) why are some people less vulnerable in developing addictive disorders in comparison with others? 2) to what extent can recovery processes be observed? 3) why some individuals with alcohol dependence achieve and maintain abstinence better i.e. are more resilient than those who relapse?
Methods
Search strategy
We systematically reviewed the existing literature up to November 2017 using PUBMED electronic database for the identification of neuroimaging studies investigating recovery and/or resilience in alcohol dependence or alcohol dependence in humans, respectively. We therefore used the following search terms: imaging, neuroimaging, addiction, dependence, alcohol*, substance use*, substance use disorder, recovery, resilience. Bibliographies of relevant articles were additionally screened for further relevant information.
Study selection
We included peer-reviewed original studies irrespective of when the study was conducted and excluded single case studies, reviews and meta-analyses. For the sake of parsimony, we further excluded neuroimaging studies using imaging techniques other than functional magnetic resonance imaging (fMRI), structural MRI, diffusion tensor imaging (DTI), or positron emission tomography (PET). Additional exclusion criteria were: not in English, substances other than alcohol, neuropsychological studies without neuroimaging.
Extraction and quality assessment
One reviewer (KC) screened abstracts of articles identified for potential relevance. Then, two reviewers (AB and KC) independently extracted study data and further screened bibliographies of relevant articles. In the event of uncertainty or disagreement regarding criteria for eligibility between AB and KC, selected articles and manuscript draft were further discussed with the third and fourth reviewer (FWL and AH). Decisions on study selection were documented by AR.
Results
Search results
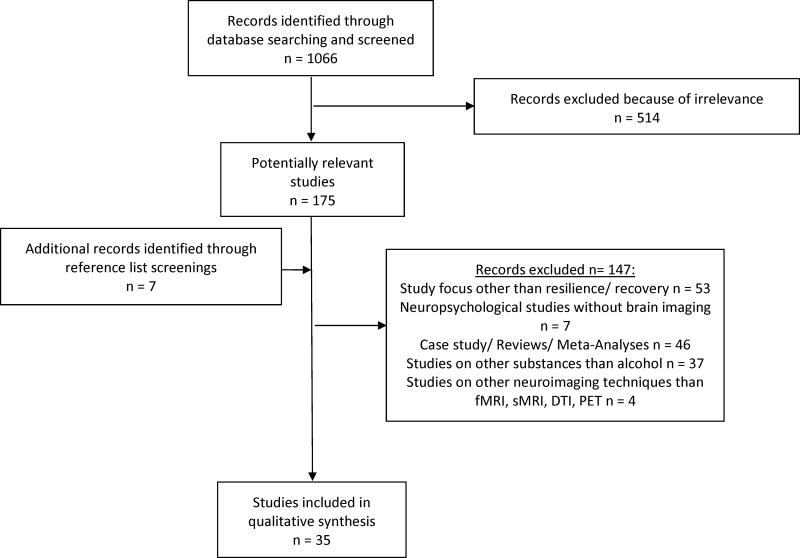
The initial term search identified a total of 1066 articles, of which 175 were considered potentially relevant. Additionally, 7 were identified through screening the reference lists of selected articles. Of those, 145 articles were further excluded as described in Figure 1 according to the PRISMA group (32). Finally, 35 studies were included in our review (in detail please see Table 1, Appendix).
Figure 1.
Flow diagram of the selection process of studies for the systematic review on imaging resilience and recovery in alcohol dependence, according to Moher et al. (2009)(32).
sMRI: structural magnetic resonance imaging, fMRI: functional magnetic resonance imaging, DTI: diffusion tensor imaging, PET: positron emission tomograph
Resilience and Recovery Markers detected by Functional Magnetic Resonance Imaging (fMRI)
We found 9 relevant fMRI studies (10–12, 33–38) investigating the role of cognitive functions commonly seen in AD such as executive, motivational aspects of behavior and emotion processing (for review see (4, 7, 39)).
Weiland et al. characterized resiliency as the ability for flexible adaptation of psychological control functions appropriate to the respective environmental context (36). Since low resiliency is known to be associated with later alcohol/drug problems and poor working memory performance (36), they investigated young healthy adolescents with and without a positive family history for alcohol dependence using a 2-Back working memory task and observers’ ratings based on the California Child Q-Sort as a measurement for resiliency. Resiliency negatively correlated with number of alcohol problems and illicit drugs used but did not differ regarding family history. This might point to the importance of environmental factors apart from genetic influences.
Another study reported that: in those with AD who became abstinent, higher functional engagement of brain areas within and outside of the “classical” working memory network (e.g. rostral/ventrolateral prefrontal cortex) was associated with executive behavioral control (11). This may constitute a resilience factor in terms of flexible recruitment of neural resources inside the classical working memory network and further compensatory processes associated with longer duration of abstinence. This is consistent with another fMRI study that also showed functional recruitment of neural working memory network in alcohol dependence (33) and suggests that such higher activity is productive rather than an impairment.
Drug-associated cue-reactivity has been associated with drug craving (e.g. (16, 40)) and risk of relapse after detoxification (e.g. (10, 14)). Two recent prospective studies reported altered cingulate cortex connectivity during individualized imaginary scripts provoking either alcohol-, stress-associated, or neutral states in AD (38). Those patients who showed greater posterior cingulate connectivity during alcohol imagery, or less anterior, mid-cingulate connectivity during neutral trials showed longer abstinence in the following 90-days and resembled healthy controls. These results emphasize the benefit of functional connectivity analyses in the investigation of neurobiological substrates and relapse risk in AD (38).
In their prospective study, Beck et al. (10) observed increased neural reactivity during presentation of alcohol-associated cues within midbrain/subthalamic nucleus as well as ventral striatum in those AD who achieved abstinence compared to relapsers (<3 months follow-up) (10). Further, patients who remained abstinent demonstrated increased functional connectivity between midbrain and amygdala as well as orbitofrontal cortex (OFC) during this alcohol-associated “cue reactivity” task compared to those patients who relapsed within 3 months. The authors argued that the increased connectivity between dopaminergic brain areas such as the midbrain and the amygdala/OFC might help to discriminate and signal aversive aspects of drinking alcohol and thus may support abstinence.
In the context of reward deficiency, Yau et al. observed reduced ventral striatal response during the anticipation of monetary reward and loss using a monetary incentive delay task (MID) in a group of healthy children of alcohol-dependent (COA) individuals (aged 18 to 22 years) compared with controls (37). In addition, in COAs only, activation of ventral striatum was positively correlated with externalizing behavior as well as current and lifetime alcohol consumption.
Another important, but rarely studied domain in addiction research regarding recovery or resilience is the neural basis of emotion processing. Heitzeg et al. (35) conducted a longitudinal cohort study to investigate externalizing behavioral problems and neural activation patterns during an fMRI task presenting emotional words in adolescents (16 to 20 years) with a family history of AD, who were considered vulnerable (risky drinking behavior) or resilient (no risky drinking behavior). These groups were compared to adolescents without any parental history of AD or risky drinking behavior (35). In response to emotional stimuli, increased activation in OFC, insula and putamen was observed in the resilient group. The vulnerable group showed more activation of dorsomedial prefrontal cortex (PFC) and less activation of ventral striatum and extended amygdala. Increased dorsomedial PFC activation and decreased subcortical activation were linked to greater externalizing behavior (35).
Another study by Charlet et al. (11) assessed brain responses during a face-matching task to investigate implicit emotion processing among detoxified AD and healthy controls. Greater activation of anterior cingulate cortex (ACC) during the processing of aversive faces correlated with longer subsequent abstinence and less subsequent binge drinking during the subsequent 6 months. This ACC response may indicate a possible resilience/recovery factor, presumably reflecting successful emotion regulation and error monitoring (12).
Taken together, findings derived from the fMRI studies indicate potentially important roles of basal ganglia and prefrontal brain. While 2 of 3 studies in COAs point to an increased resilience associated with less task-elicited neural activation within the basal ganglia (36, 37), those in AD patients showed that greater PFC engagement may underpin resilience against relapse in patients during early abstinence (cf. (41); (10–12, 33, 38)).
Resilience and Recovery Markers detected by Studies using Positron Emission Tomography
Despite the wealth of preclinical and clinical evidence about dopaminergic function in addiction (41, 43, 48), studies focusing on resilience and recovery in alcohol dependence are sparse (16, 44–47).
Two 11C-raclopride PET studies measured D2/D3 dopamine receptor availability in healthy young adults with either a positive (FHP) or a negative (FHN) family history of AD pre and post an amphetamine challenge. In both, unaffected FHP displayed higher level of striatal D2 (46) and D2/D3 (44) dopamine receptor availability in striatal regions compared with FHN. Interestingly, while amphetamine resulted in the expected increase in dopamine and positive subjective effects in FHN individuals, this was not found in FHP individuals (44). Such results support the hypothesis that high D2 receptor availability may serve as a protective biomarker compensating for the higher inherited vulnerability (p.1004; (46)). Further, striatal D2 receptor availability in FHP was also significantly linked to prefrontal glucose metabolism, which in turn was positively associated with emotional positivity (46). This suggests dopaminergic modulation of cognitive control over emotional responses protects against developing alcohol addiction.
In AD, PET studies have demonstrated lower levels of DA receptor availability and DA release compared with healthy controls (e.g. 16, 48).
Two early studies used PET to assess recovery of brain glucose metabolism during abstinence in AD. One reported a significant increase in brain glucose metabolism predominantly within 16–30 days, especially in frontal brain regions, whereas low metabolism persisted in the basal ganglia (47). Another study showed that the four patients who remained abstinent compared with two who relapsed showed partial recovery in brain metabolism within frontal cortex areas as well as significant improvement in general cognitive and executive functioning (45).
In sum, PET studies concentrating on recovery and resilience in alcohol dependence are sparse but do suggest that differences in dopaminergic function may result in vulnerability or resilience depending on the genetic background of an individual. Whilst high D2/D3 receptor availability may serve as protective non-alcoholic FHP, low D2 receptor availability may render individuals more vulnerable to alcohol abuse. Further, similarly to fMRI studies, normalization in metabolism is associated with abstinence.
Resilience and Recovery Markers detected by Structural Magnetic Resonance Imaging
We found 21 relevant studies investigating changes in brain structure during abstinence (21, 50–69) cf. Table 1)).
Table 1.
Summary of characteristics of 35 neuroimaging studies included for investigation of resilience and recovery in alcohol dependence
| Study | Study Design | Method | Follow- Up Period |
Age | Study Sample |
Main Findings | ||
|---|---|---|---|---|---|---|---|---|
| Neuroimaging | Paradigm | Other Tests | ||||||
| Alhassoon et al. 2012 | prospective cohort study | MRI/DTI | none | none | 1 year | AD: mean 51.4 SD (6) Controls: mean 51.8 SD (7.4) | n male= 30 (detoxified alcohol dependent ↔healthy controls) | Low FA and high RD in corpus callosum of AD group compared to controls. Significant improvement at follow up in AD group. |
| Alvanzo et al. 2015 | cross-sectional | MRI/PET | none | Family Tree Questionnaire | none | FHP: mean 23.1 SD (2.98) FHN: mean 22.7 SD (3.21) | n male = 51 n female = 33 (family history of AD ↔ no family history of AD) | Baseline [11C]raclopride BPND was generally higher in FHP compared with FHN subjects across striatal regions. Negative subjective drug effects were more pronounced in FHP than in FHN subjects. In FHN correlation between BPND and positive drug effects. This correlation was less pronounced in FHP. |
| Beck et al. 2012 | prospective cohort study | fMRI/Biological Parametric Mapping/PPI | alcohol cues | none | 3 months | AD: mean 39.37 SD (7.72) controls: mean 40.37 SD (6.68) | n= 92(detoxified AD ↔ healthy controls) | Abstinence related to increased activation in midbrain and VS. Stronger functional connectivity midbrain-left amygdala and midbrain - left orbitofrontal cortex. Relapse associated with atrophy in bilateral orbitofrontal cortex, right medial PFC and ACC as well as increased activity in left medial PFC. |
| Burt et al. 2016 | cross sectional | MRI/VBM | none | ESPAD, SDQ, DAWBA, LEQ | none | Mean 14.56 SD (0.44) | n male = 906 n female = 964 (competent (C/a), resilient (C/A), maladaptive (c/A), vulnerable (c/a)) | C/a and C/A showed larger left OFC volume. C/a and c/A larger fusiform gyrus volume than c/a and C/A groups. C/A group showed increased volume relative to other groups in right superior frontal and right middle frontal regions; in the right superior frontal region |
| Cardenas et al. 2007 | cross sectional/prospective cohort study | MRI/DBM | none | LDH | 8 months (AD) 12 months (LD) | AD : mean 49 SD (14) Controls: mean 45 SD (8) | n male = 60 n female = 5 (AD ↔ LD; AD longitudinal ↔ LD) | Atrophy in frontal and temporal lobe in AD group. Abstainers show faster recovery in parietal and frontal tissue than LD. Temporal lobes, thalamus, brainstem, cerebellum, corpus callosum, anterior cingulate, insula, and subcortical white matter was increased in abstainers compared to relapsers. Recovery predicted by baseline gray matter volumes. |
| Cardenas et al. 2011 | Prospective cohort study | MRI/DBM | None | LDH | 7.8 months | AD: mean 50 SD (10) Controls: Mean 47 SD (8) | n male = 104 n female = 11 (AD< healthy control; relapsers ↔ abstainers) | Abstainers vs. controls had smaller volume in left hippocampus, entorhinal cortex, amygdala and right thalamus but larger volume in left orbitofrontal region. Relapsers vs. abstainers smaller volume in lateral OFC, left posterior middle/temporal gyry and supramarginal gyrus. Relapsers had different pattern of volume loss than abstainers. |
| Chanraud et al. 2013 | cross sectional | fMRI/PPI/Resting state functional connectivity | working-memory task | none | none | AD: mean 40.1 SD (10.9) controls: mean 47.7 SD (12.29) | n male= 30 (detoxified AD ↔ healthy controls) | Recovery related to recruitment of dorsolateral prefrontal cortex (DLPFC)-cerebellar VIII system during rest and DLPFC-cerebellar VI system during working memory task. |
| Charlet et al. 2014 | prospective cohort study | fMRI/Biological Parametric Mapping | n-back task | Alcohol Timeline Follow-back | 7 months | AD: mean 44.9 SD (11.4) controls: mean 44.1 SD (12) | n male = 60 n female = 20 (detoxified AD ↔ Healthy controls) | High resilience (low relapse risk in alcohol dependence) associated with neural activation in lateral/medial premotor cortex, rostral/ventrolateral prefrontal cortex during n-back working memory task. |
| Charlet et al. 2014 | prospective cohort study | fMRI/Biological Parametric Mapping | Hariri faces task (modified) | LDH | none | AD: mean 44.8 SD (9.8) controls: mean 46.1 SD (9.8) | n male = 50 n female = 16 (detoxified AD ↔ healthy controls) | Increased ACC response to affective faces correlated to abstinence and less retrospective alcohol intake in alcohol dependent patients. |
| Chung et al. 2011 | cross sectional | event-related fMRI | antisaccade reward task | none | none | SUD: mean 17.0 SD (0.9) control: mean 16.9 SD (0.9) | n male/female = 12 (SUD [marijuana, alcohol, other] ↔ healthy controls) | During response preparation SUD showed increased activation in oculomotor control regions (FEF, SEF), DLPFC, regions in the parietal lobe, and areas in the frontal gyrus. |
| Deshmukh et al. 2005 | cross sectional | MRI/Volumetric data | none | none | none | AD: mean 49.4 SD (10.9) Controls: mean 45.2SD (13.9) Schizophrenia: mean 44.7 SD (8.6) Comorbid: mean 41.0 SD (7.5) | n male = 122 (AD detoxified ↔ schizophrenia ↔ AD/schizophrenia ↔ healthy controls) | Putamen and nucleus accumbens decrease greater in schizophrenia than AD, comorbid group fell between these groups. Schizophrenic patients treated with atypical medication showed greater volume decreases in putamen than those treated with typical medication. Recently sober (< 3 weeks) alcoholics had greater deficits in nucleus accumbens than AD with long-term sobriety. |
| Durazzo et al. 2015 | Prospective cohort study | MRI/Volumetric data | none | LDH, nerurocognitive battery | 7,5 months | AD smoking: mean 49 SD (9) AD non-smoking: mean 52 SD (11) Controls: Mean 47 SD (9) | n male = 103 n female = 11 | AD: volume increases in all GM and WM regions at FU; no significant predictors of regional volume change. Rates of GM gain greatest in first month. sAD showed less volume gain nsAD in frontal and total cortical GM. Improvement Processing Speed associated with increased volumes in nsAD, but not in sAD. After 7.5-months of abstinence, nsAD and sAD equal to controls on frontal GM volume |
| Gazdzinski et al. 2005 | cross sectional/prospective cohort study | MRI/Boundary Shift Integral | none | LDH | up to 12 months | AD: mean 50.6 SD (9.3) Controls: mean 45.0 SD (6.8) | n male = 37 n female = 3 (AD detoxified/longitudinal ↔ healthy controls) | Most tissue gain during the first abstinent month. Fastest volume recovery patients with greatest baseline brain shrinkage and drinking severity. Reversal of volume increases in non-abstinent individuals (modulated by duration of abstinence and non-abstinence periods, as well as recency of non-abstinence). |
| Gazdzinski et al. 2008 | cross sectional/prospective cohort study | MRI/short echo proton spectroscopy | none | BVMT | 1 month | smoking alcohol dependent: Mean 50.7 SD (9.0) Non-smoking alcohol dependent: Mean 50.2 SD (9.1) Non-Smoking controls: Mean 47.3 SD (8.2) | n male = 38 (smoking AD ↔ non-smoking AD ↔ non-smoking LD) | N-acetyl-aspartate normalized in the MTL of non-smoking AD group, remained low in the MTL of smoking AD group. Changes in both groups associated with improvements in visuospatial memory. Hippocampal volumes increased in both groups during abstinence, but increasing volumes correlated with visuospatial memory improvements only in non-smoking AD. |
| Gazdzinski et al. 2010 | prospective cohort study | MRI/DTI/spectroscopy | none | none | 1 year | smoking alcohol dependent: Mean 47.7 SD (9.5) Non-smoking alcohol dependent: Mean 51.5 SD (10.3) Non-smoking controls: Mean 48.3 SD (8.4) | n male = 53 n female = 5 (smoking AD ↔ non-smoking AD ↔ non-smoking LD) | Higher mean diffusivity in AD (smoking: frontal; non-smoking: parietal, frontal, temporal). Lower concentrations of N-acetyl-aspartate in AD (smoking: frontal; non-smoking: parietal). In non-smoking alcohol-dependent individuals increase in FA and decreases in mean diffusivity over 1 month of abstinence. White matter volume increase in frontal and temporal lobes in smoking AD group. |
| Heinz et al. 2004 | cross sectional | fMRI/PET | alcohol cues | Alcohol craving questionnaire | none | AD: mean 44.5 SD (6.5) controls: mean 43.2 SD (9.5) | n male = 24 (detoxified AD ↔ healthy controls) | In alcohol-dependent subjects higher activation of the medial pre-frontal cortex and striatum related to 1. less availability of D2-like receptors in VS, 2. Higher craving severity. |
| Heitzeg et al. 2008 | cross-sectional | fMRI | lexical emotional stimuli | YSR, Drinking and Drug History Form for Children | none | COAs resilient: mean 18.4 SD (1) COAs vulnerable : mean 17.5 SD (1.3) controls: mean 17.2 SD (1.6) | n male = 15 n female = 13 (COAs resilient ↔ COAS vulnerable↔ controls) | In response to emotional stimuli: Activation of orbital frontal gyrus and left insula/putamen greater in resilient than control and vulnerable groups. Vulnerable group had more activation of dorsomedial PFC and less activation of VS and extended amygdala. Increased dorsomedial prefrontal activation and decreased VS and amygdala activation correlated pos. with externalizing behaviors. |
| Hoefer et al. 2014 | cross sectional/pro spective cohort study | MRI/Volumetric data | none | LDH; Taqman genotyping assay; WAIS III; BVMT; AMNART | 7 months | smoking alcohol dependent: Mean 49.6 SD (9) Non-smoking alcohol dependent: Mean 53.6 (10.5) Non-smoking controls: Mean 45.6 SD (9.9) | n male = 144 n female = 12 (smoking alcohol dependent ↔ non-smoking alcohol dependent ↔ non-smoking controls) | AD had smaller hippocampi than healthy controls at all time points. Hippocampal volume at 1 month of abstinence correlated with lower visuospatial function. Smoking status did not influence volume or recovery. BDNF Val homozygotes had hippocampal volume increases over 7 months of abstinence, and Val homozygotes had significantly larger hippocampi than Met carriers at 7 months of abstinence. |
| Johnson-Greene et al. 1997 | pilot study | PET | none | neuropsych ological battery | up to 32 months | AD mean: 48.6 SD (10.2) | n male = 6 (AD longitudinal) | Abstinent group showed partial recovery of ICMRglc in two of three divisions of the frontal lobes and improvement on neuropsychological tests of general cognitive and executive functioning, whereas the patients who relapsed had further declines in these areas. |
| Kühn et al. 2014 | cross sectional/prospective cohort study | MRI/Volumetric data | none | LDH | 2 weeks | AD: mean 42.1 SD (11.6) Controls: mean 40.8 SD (3.4) | n male = 53 n female = 21 (AD detoxified ↔ healthy controls) | AD group had lower CA2+3 baseline volume and significant normalization of gray matter volume 2 weeks later. Neg. correlation between baseline CA2+3 volume and alcohol consumption and alcohol-withdrawal symptoms. AD patients with stronger withdrawal symptoms displayed the largest volume increase of CA2+3. |
| Mon et al. 2011 | prospective cohort study | MRI/Volumetric data/mathematical predictions | none | none | 222 days | AD: mean 50.7 SD (11.9) | n male = 13 n female = 3 | The data predicted from the formula were very similar to the experimentally measured data for all lobes and for both gray and white matter (intra-class correlation coefficients ↔ 0.95). |
| Mon et al. 2013 | cross sectional/pro spective cohort study | MRI/Volumetric data | none | LDH; Taqman genotyping assay; WAIS III | 5 weeks | AD: mean 50.8 SD (10.6) controls: mean 47.9 SD (7) | n male= 70 N female = 9 (AD detoxified/longitudinal ↔ LD) | VAL homozygote in AD group related to gray matter increase. VAL/MET heterozygote associated with white matter increases. Gray matter volume increases pos. correlated to neurocognitive measure increases. |
| Pfefferbaum et al. 1995 | prospective cohort study | MRI/Volumetric data | none | none | up to 12 months | AD: mean 45.0 SD (10.9) controls: mean 45.3 SD (14.2) | n male = 116 (AD detoxified/longitudinal ↔ controls) | From 1. to 2. scan, AD group showed declines in CSF volumes of lateral ventricles and posterior cortical sulci, and an increase in anterior cortical gray matter volume. From 2. to 3. scan third ventricular volumes decreased in the abstainers relative to the relapsers and controls; cortical white matter volume decreased in the relapsers. In the relapsers alcohol consumption predicted later vulnerability to white matter volume decline and third ventricular enlargement with relapse. |
| Pfefferbaum et al. 2001 | cross sectional | MRI/Volumetric data | none | LDH | none | AD male: mean 43.4 SD (8.4) AD female: mean 41.7 SD (9.5) Controls male: mean 44.6 SD (11.4) Controls female: mean42.9 SD (13.4) | n male = 92 n female = 79 (AD detoxified male/female ↔ healthy controls male/female) | Less brain shrinkage was found among alcoholic women than among alcoholic men. |
| Pfefferbaum et al. 2014 | Prospective cohort study | MRI/DTI/TBSS | None | Self-reported drinking histories | Up to 8 years | AD: mean 44.3 SD (9.2) Controls: mean 43.0 SD (10.1) | N male = 52 N female = 51 | FA of AD lower than that of healthy controls. Relapsing AD showed continued worsening, whereas abstaining AD showed improvement in fiber integrity. FA trajectories of relapsers exhibited faster aging relative to controls, whereas the trajectories of abstainers showed improvement toward normality |
| Ruiz et al. 2013 | cross sectional | MRI/Volumetric data | none | none | none | AD: mean 53.9 SD (11) Controls: mean 53.9 SD (12.4) | N = 44 N = 44 (AD detoxified male/female ↔ healthy controls male/female) | Female AD showed stronger positive associations between sobriety duration and white matter volume than men in first year of abstinence. Men showed this association more so than women after 1 year of abstinence. |
| Sameti et al. 2011 | cross sectional | MRI/Volumetric data | none | C-DIS | none | Long-term abstinent AD: mean 46.6 SD (6.7) Controls: mean 45.6 SD (6.5) | n male = 53 N female = 47 (long-term abstinent AD ↔ healthy controls) | Minimal differences in subcortical structure volumes between long-term abstinent AD and controls. In AD group differences in volume associated with current or lifetime psychiatric diagnosis. |
| Segobin et al. 2014 | cross sectional/prospective cohort study | MRI/Tensor-based Morphometry | none | none | 6 months | AD patients: mean 44.4 SD (6.07) Controls: mean 46.7 SD (4.25) | n male = 37 N female = 2 (AD ↔ healthy controls; AD/longitudinal) | Reduced thalamus volume associated with relapse. Recovery of cerebellum, striatum and cingulate gyrus even in AD patients with moderate alcohol intake but neg. correlated to amount of alcohol consumed over 6 months in AD group. |
| van Eijk et al. 2013 | cross sectional/prospective cohort study | MRI/VBM | none | none | 2 weeks | AD: mean 47.0 SD (10.1) Controls: mean 45.3 SD (11.9) | n male = 82 N female = 22 (AD detoxified/longitudinal ↔ healthy controls) | Gray matter volume (cingulate gyrus, middle and precentral prefrontal gyri, cerebellum, insula) smaller in AD compared with control group at baseline. Significant recovery after 2 weeks of abstinence. |
| Volkow et al. 1994 | prospective cohort study | PET | none | none | up to 2 months | AD: mean 41.0 SD (8) controls: mean 38.4 SD (3) | n male = 20 (AD detoxified/longitudinal ↔ healthy controls) | Metabolism increased predominantly in first 30 days of abstinence. Increases mainly in prefrontal regions. Metabolism negatively correlated to alcohol use. |
| Volkow et al. 2006 | Cross sectional | PET | None | Multidimensional Personality Questionnaire | none | FHP: mean 24 SD (3) FHN: mean 26 SD (4) | N male = 28 N female = 2 | FHP group had significantly higher measures of D2 receptor availability in caudate and VS. FHP subjects had lower metabolism in hippocampal gyrus, temporal pole and cerebellum. Metabolism in prefrontal cortex increased in FHP. Positive correlation between striatal D2 receptor availability and metabolism in OFC, ventral cingulate gyrus and PFC. D2 receptor and metabolism in left OFC was positively correlated to positive emotionality. |
| Wang et al. 2016 | Prospective cohort study | MRI/Volumetric data (CT, SA) | none | none | 14 days | AD: mean 47.02 SD (10) Controls: mean 46.65 SD (12.37) | N male = 47 N female = 12 (AD ↔ controls) | Lower subcortical volumes in AD in putamen, NA, amygdala and hippocampus. No subcortical volume regain at FU. Cortical volume recovery driven by an increase in CT. More CT reduction and recovery in sulci compared to gyri. |
| Weiland et al. 2012 | cross sectional | fMRI/PPI | n-back task | California Child Q-Sort | none | mean 20.2 SD (1.2) | N male = 43 N female = 24 (parental alcoholism ↔ no parental alcoholism) | High resilience: neg. correlated to STN, pallidum activation; pos. correlated to lower levels of substance use, fewer alcohol problems and better working memory performance. |
| Yau et al. 2012 | cross sectional | fMRI | MID task | Drinking and Drug History | none | COAs: mean 20.12 SD (1.2) control: mean 20.1 SD (1.3) | n male = 24 n female = 16 (COAs ↔ controls) | Resilience related to reduced ventral striatum activation in COAs. |
| Zakiniaeiz et al. 2016 | Prospective cohort study | fMRI/ICD | Individualized imagery paradigm | none | 90 days | Study 1 AD: mean 37.73 SD (1.16) Study 2 AD: mean 35.97 SE (0.08) Controls: mean 34.47 SE (1.55) | Study 1 N male = 35 N female = 10 Study 2 N male = 43 N female = 17 | AD showed decreased cingulate connectivity in responses to alcohol and stress cues compared to neutral cues. Weaker connectivity in ACC and MCC during neutral cue exposure related to longer abstinence. PCC connectivity during alcohol cues compared to stress cue conditions positively correlated to longer time to relapse. Cingulate connectivity significantly different between groups. AD showed reduced cingulate connectivity during alcohol and stress cues and increased cingulate connectivity during neutral cues. AD more similar to controls in cingulate connectivity had longer abstinence and better recovery. |
MRI = magnetic resonance imaging; DTI = diffusion tensor imaging; AD = alcohol dependent; SD = standard deviation; ↔ = versus; FA = fractional anisotropy; RD = radial diffusivity; PET = positron emission tomography; FHP = family history positive; FHN = family history negative; BPND = binding potential; FDG = 18F-fluorodeoxyglucose; fMRI = functional magnetic resonance imaging; PPI = psychophysiological interaction analysis; PFC = prefrontal cortex; ESPAD = european school survey project on alcohol and other drugs; SDQ = strengths and difficulties questionnaire; DAWBA = development and well-being assessment, LEQ = life events questionnaire; ACC = anterior cingulate cortex; DBM = deformation-based morphometry; LDH = lifetime drinking history; LD = light drinkers; OFC = orbitofrontal cortex; DLPFC = dorsolateral prefrontal cortex; SUD = substance use disorder; FEF = frontal eye field; SEF = supplementary eye field; GM = gray matter; WM = white matter; sAD = smoking alcohol dependent; nsAD = non-smoking alcohol dependent; BVTM = brief visuospatial memory test; MTL = medial temporal lobe; YSR = youth self-report; COA = children of alcoholics; VS = ventral striatum; pos. = positively; WAIS III = Wechsler adult intelligence scale; AMNART = American national adult reading test; BDNF = brain-derived neurotropic factor; ICMRGLC = regional cerebral glucose uptake; neg. = negative; c-DIS = computerized diagnostic interview schedule; FU = follow-up; CT = cortical thickness; STN = subthalamic nucleus; MID = monetary incentive delay; PCC = posterior cingulate gyrus; MCC = midcingulate cortex; ICD = intrinsic connectivity distribution
Smaller gray matter (GM) and white matter (WM) volumes have been found throughout the brain and were associated with relapse within 6 months after detoxification (67). Interestingly, increases in brain volumes were seen even in those patients with moderate alcohol consumption (<10g of pure alcohol per day) after detoxification. This indicates beneficial effects of reduced alcohol consumption in AD who are not ready or able to become abstinent (67). Some brain areas appeared to recover faster, such as the cingulate gyrus in comparison to the fusiform gyrus, which led the authors to propose that recovery in one area triggers recovery in other connected areas.
Along with ventricular volume recovery, significant volume increases in subcortical GM weremainly observed within the first month of abstinence in AD compared with the following 7.5 months of abstinence (55, 64). Indeed, frontal GM normalized to control level, though total cortical and regional GM volumes (e.g. parietal, temporal, thalamic) remained lower after 7.5 months of abstinence (55). Likewise, Gazdzinski, et al. (56) showed that recovery of brain tissue was six times faster in the first three weeks of abstinence than during the subsequent twelve months of abstinence (56). Brain volume gain was more prominent in heavier drinkers with less tissue at baseline (56). Partial recovery of cortical thickness was also found after only 2 weeks of sobriety with full normalization seen in medial OFC and rostral ACC. Regeneration of sulci was here more pronounced in all affected brain areas than in gyri (69). Another study showed significant normalization of hippocampal GM volume within the first two weeks of abstinence in AD, especially in those with greater withdrawal severity at baseline (21).
Other studies have also found smaller tissue volumes associated with greater previous alcohol intake (21, 53), e.g. in frontal and temporal cortices (53).
Mon et al. (60) mathematically modelled longitudinal brain structure changes in AD patients and found that in those with greater GM/WM atrophy at baseline (usually directly after detoxification), greater dynamic neuroplastic changes occurred within the first month of cessation of alcohol intake (60). Two studies by Cardenas et al., using deformation-based morphometry, reported that, one week after detoxification, patients had smaller frontal and temporal GM and WM volume but those that stayed abstinent regained WM and GM tissue in cortical and subcortical regions after 6–9 months (53). Apart from structural GM reductions in AD patients relative to controls, subsequent abstainers and relapsers showed different patterns of GM volume loss (52). In particular, future relapsers showed reduced GM in bilateral OFC in relation to abstainers, which might indicate conservation of GM in this region to benefit recovery in AD patients (52). In terms of subcortical regions, Deshmukh et al. (54) also discovered regional volume atrophy in caudate, putamen and nucleus accumbens in AD men abstinent for approximately 204 days compared to healthy controls, with greater volume deficits in the nucleus accumbens seen in the more recently abstinent patients (54).
Interestingly, some studies did not find significant WM differences between AD and controls (55, 69), although WM volume gain has been detected with abstinence. DTI is probably more sensitive to WM change than structural MRI as detailed architecture of white matter tissue can be analyzed by visualizing molecule diffusion patterns (50, 57). For example, a longitudinal study utilizing DTI reported improvement of white matter fibre tract coherence and myelin integrity in the corpus callosum of recently detoxified AD during one year of abstinence (50). Notably these WM indices in AD no longer differed from controls (50). However, there was no relationship between these WM changes with normalization of working memory function in the AD (50). Similarly, normalization of whole brain fibre tract integrity was observed in abstainers with multiple scans over the course of 8 years, while relapsers showed accelerated microstructural damage of the white matter, i.e. faster ageing (63).
Potential modulators
One potential mechanism underlying recovery could be related to genotype, such as has been shown for brain-derived neurotrophic factor (BDNF Val66Met (rs6265) polymorphism), a promyelination neurotrophin which serves as a neurobiological marker of neuronal growth and maintenance (61, 70). AD who are homozygous for Val demonstrated frontal, parietal and thalamic GM increase during the first 5 weeks of abstinence and greater hippocampal volume recovery over 7 months of sobriety (59). This was not seen in Val/Met heterozygotes though both, Val/Val and Val/Met carriers showed tissue gains in temporal GM (61). Interestingly, Mon et al. (61) observed significant increases in frontal WM volumes only in Val/Met heterozygotes but not in Val homozygotes, as well as subcortical volume decreases in caudate GM in Val but not Met carriers. Furthermore, Hoefer et al. found hippocampal volume changes to be associated with improvements in visuospatial memory performance only in BDNF Val homozygotes (but not in Met carriers) (59).
Structural atrophy and recovery may also vary between gender. Here, a recent study observed that the duration and quantity of heavy drinking was significantly related to WM reductions that regionally differed between male and female AD (65). Furthermore, stronger positive associations between duration of abstinence and WM volume were seen in women while men showed this association more so than women after 1 year of sobriety (65), confirming gender specific recovery processes (62, 71). Another gender-driven GM difference indicating heightened vulnerability to brain atrophy in women was observed by Sameti and coworkers (66): long-term abstinent alcohol-dependent women (mean 6.3 years) displayed smaller nucleus accumbens volumes compared to healthy women and male controls. However, no significant gender effects have also be detected such as in GM increases and cerebrospinal fluid (CSF) decreases in some brain areas observed within the first two weeks of alcohol abstinence (68).
Comorbid nicotine dependence is also important to consider because up to 80% of AD smoke (72, 73) and is itself neurotoxic (57, 58). Evidence is however inconsistent. Whilst non-smoking AD revealed faster microstructural recovery (i.e. in frontal, temporal, parietal and occipital lobes) compared with smoking alcohol dependent patients, faster macrostructural increases in frontal and temporal WM volume were seen in smokers only, with no changes of metabolic concentrations in both groups (57). Contrary to those WM volume findings, smoking AD were found to show less recovery with increasing age especially in frontal (and total cortical) GM volume. Moreover, beneficial effects regarding processing speed were associated with the found morphological GM increases, but again in non-smoking AD only (55). Another study could not support any of these smoking-dependent recovery findings (59).
Studying neurobiological underpinning of resilience and its predication of problematic alcohol use, a recent European adolescent study by Burt et al. including 1870 teens (average age 14.56 years), identified elevated GM volumes in prefrontal areas (BA 11, 10, 6) in resilient adolescents (high competence in academic, social and emotional domains despite experiencing adverse lifetime events in the past) compared with other peers, which also correlated negatively with problematic drinking. Thus, potentially preventing those teens from future AD development by the PFC regulating behavior with protective executive control (51).
In summary, structural neuroimaging studies demonstrate beneficial plasticity effects throughout the brain of AD during short-, medium- and long-term abstinence, even when patients only lower their alcohol consumption to a moderate level. However, recovery of neuronal tissue (GM vs. WM, or sulci vs. gyri) appears to recover variably across regions (frontal areas first in early abstinence) and at different time rates.
Discussion and Future Avenues for Research
Neuroimaging research has been key in shedding light on possible dysfunctional domains and affected brain regions in AD and their potential of recovery after alcohol cessation (or reduction). In summary, lower dopamine receptor availability as shown in PET studies, related to craving in AD patients (16) which in turn has been associated with relapse (10, 15). Moreover, functional MRI studies have linked deficient reward and emotion processing to negative treatment outcomes while structural MRI studies have shown that conserved PFC morphology in particular is linked to resilience and abstinence in AD patients. Altogether, investigations of morphology identified specific factors that influenced these observed brain recovery processes and should be considered in future studies on brain recovery in AD, e.g. genotype-dependent neuronal (re)growth (59, 61), gender-specific neural recovery effects (54, 62, 65, 66, 68, 71), additional smoking influences (58, 59, 74) or adolescent alcohol abuse (51).
Overall, the reviewed research suggests that volumetric brain tissue recovery processes follow non-linear trajectories, suggesting that faster reconstitution of regionally specific brain areas during early abstinence might trigger consecutively recovery of associated regions. Consistent with these results, additional lifetime and current psychiatric diagnoses (such as anxiety disorders including posttraumatic stress disorder or externalizing disorder) have been identified as a critical factor that interfere with morphometric brain recovery in alcohol dependence (66).
However, in reviewing these studies, one must be aware of some methodological diversity when trying to compare or summarize the existing study findings. Here, in addition to replication studies, meta-analyses that weigh findings by their effects sizes could be employed to preserve false positive findings or small effect sized results from overestimation. Also, usage of different self-report instruments (without verification by collateral information) to assess measures of alcohol consumption (e.g. lifetime drinking amount, onset and pattern of drinking) has to be regarded in light of potential bias toward socially desirable answers, which might cause underestimation of reported drinking due to embarrassment (e.g. (10–12)).
Future studies that aim at systematic investigation of factors that mediate recovery and resilience are at the focus of some system-oriented approaches (cf. (75)). On a functional level, different domains play a crucial role for the development and maintenance of addictive disorders and thus are important factors for recovery on one hand and resilience on the other: Executive functions including inhibitory control and working memory, reward processing as well as processing of emotional stimuli are potential targets for diagnosis, prognosis and therapy (10–12).
However, up to now most of imaging studies in this field of research are cross- sectional, and there is clear necessity of longitudinal studies for the characterization of disease trajectories, progression rates and markers for recovery and resilience to inform treatment options. Indeed, cohort studies as carried out by the IMAGEN consortium (e.g. (76)) can shed light on potential future research directions; here researchers from multiple European countries aim to identify neuronal predictors for developing addictive disorders as well as potential targets for AD prevention approaches. Additional application of machine learning algorithms may further help to generate models of current and future alcohol misuse by incorporating the assessed brain processes and structures, personality as well as cognitive factors, environmental conditions and finally genetic markers (76). Regarding the identification of intermediate phenotypes of resilience, clearly more studies are needed since this field of neurobiological research is rather unexplored. Here, investigations of individuals with and without heightened genetic or environmental risk for AD are needed to help disentangling resilience markers from vulnerability risk factors. Recent studies also introduced epigenetic mechanisms in AD, adding valuable information about modulating processes to the genotype-phenotype interaction (77). Those investigations should use appropriate study designs, such as comparisons of i) adolescent/young adult COAs with vs. without AD on their own or ii) adult AD patients vs. adult individuals without AD but with a positive family history of AD (e.g. first degree relatives of AD patients) vs. healthy individuals without familiar or own AD (as in the recent ongoing prospective cohort study “e:Med SysMed Alcoholism”; (75)), respectively. Clearly, findings testing neurobiological traits of vulnerability to AD (cf. (78–80)) may give rise to new hypotheses and research questions, but caution is warranted that vulnerability markers are not simply the opposite of resilience. Rather, vulnerability demonstrates conditions and aberrations which exist before AD and may facilitate developing AD but are not only caused by e.g. neurotoxic alcohol effects. Resilience, on the other hand, refers to factors that promote good treatment outcome despite negative effects of long-term alcohol intake on neural structure and function.
Further, future research should not only continue to strengthen knowledge about recovery processes and resilience markers (in high-risk groups without alcohol dependence as well as in already affected AD) but should also address whether they can be translated to various drugs of abuse in terms of general markers or can be characterized specifically for different substance classes.
Supplementary Material
Acknowledgments
This work has been supported by the German Ministry of Education and Research (BMBF; 01ZX1311E and 01ZX1311D/e:Med-program alcohol addiction, Spanagel et al., 2013; and in part by 01EE1406A) and the German Research Foundation (DFG; CH 1936/1-1; FOR 16/17; HE2597 13-1/2, 14-1/2, 15-1/2, Excellence Cluster Exc 257). Further, this work is part of the project Z99 AA999999 (NIH/NIAAA).
Footnotes
Declaration of Interests: None.
References
- 1.Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- 2.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 3.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol and alcoholism. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 4.Charlet K, Beck A, Heinz A. The dopamine system in mediating alcohol effects in humans. Curr Top Behav Neurosci. 2013;13:461–488. doi: 10.1007/7854_2011_130. [DOI] [PubMed] [Google Scholar]
- 5.Charlet K, Beck A, Heinz A. Drug Addiction. In: Shenton M, Mulert C, editors. MRI in Psychiatry. Heidelberg, Germany: Springer-Verlag; 2014. pp. 357–370. [Google Scholar]
- 6.Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction biology. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 9.Heinz A, Beck A, Wrase J, Mohr J, Obermayer K, Gallinat J, et al. Neurotransmitter systems in alcohol dependence. Pharmacopsychiatry. 2009;42(Suppl 1):S95–S101. doi: 10.1055/s-0029-1214395. [DOI] [PubMed] [Google Scholar]
- 10.Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, et al. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Archives of general psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- 11.Charlet K, Beck A, Jorde A, Wimmer L, Vollstadt-Klein S, Gallinat J, et al. Increased neural activity during high working memory load predicts low relapse risk in alcohol dependence. Addiction biology. 2014;19:402–414. doi: 10.1111/adb.12103. [DOI] [PubMed] [Google Scholar]
- 12.Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner CE, et al. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addiction biology. 2014;19:439–451. doi: 10.1111/adb.12045. [DOI] [PubMed] [Google Scholar]
- 13.Garbusow M, Schad DJ, Sebold M, Friedel E, Bernhardt N, Koch SP, et al. Pavlovian-to-instrumental transfer effects in the nucleus accumbens relate to relapse in alcohol dependence. Addiction biology. 2016;21:719–731. doi: 10.1111/adb.12243. [DOI] [PubMed] [Google Scholar]
- 14.Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 15.Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, et al. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. The American journal of psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- 16.Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. The American journal of psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 17.Kienast T, Schlagenhauf F, Rapp MA, Wrase J, Daig I, Buchholz HG, et al. Dopamine-modulated aversive emotion processing fails in alcohol-dependent patients. Pharmacopsychiatry. 2013;46:130–136. doi: 10.1055/s-0032-1331747. [DOI] [PubMed] [Google Scholar]
- 18.Sebold M, Schad DJ, Nebe S, Garbusow M, Junger E, Kroemer NB, et al. Don't Think, Just Feel the Music: Individuals with Strong Pavlovian-to-Instrumental Transfer Effects Rely Less on Model-based Reinforcement Learning. J Cogn Neurosci. 2016;28:985–995. doi: 10.1162/jocn_a_00945. [DOI] [PubMed] [Google Scholar]
- 19.Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Charlet K, Heinz A. Harm Reduction – A systematic review on effects of alcohol reduction on somatic and mental symptoms. Addiction biology. 2016 doi: 10.1111/adb.12414. in press. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn S, Charlet K, Schubert F, Kiefer F, Zimmermann P, Heinz A, et al. Plasticity of hippocampal subfield volume cornu ammonis 2+3 over the course of withdrawal in patients with alcohol dependence. JAMA Psychiatry. 2014;71:806–811. doi: 10.1001/jamapsychiatry.2014.352. [DOI] [PubMed] [Google Scholar]
- 22.Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcoholism, clinical and experimental research. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- 23.Mann K, Batra A, Gunthner A, Schroth G. Do women develop alcoholic brain damage more readily than men? Alcoholism, clinical and experimental research. 1992;16:1052–1056. doi: 10.1111/j.1530-0277.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 24.Mann K, Mundle G, Langle G, Petersen D. The reversibility of alcoholic brain damage is not due to rehydration: a CT study. Addiction. 1993;88:649–653. doi: 10.1111/j.1360-0443.1993.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbloom M, Pfefferbaum A. Magnetic Resonance Imaging of the Living Brain - Evidence for Brain Degeneration Among Alcoholics and Recovery With Abstinence. Alcohol Res Health. 2008;31:362–376. [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2014;76 Pt B:235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noeker M, Petermann F. Resilience functional adaptation to environmental adversity. Zeitschrift für Psychiatrie, Psychologie und Psychotherapie. 2008;56:255–263. [Google Scholar]
- 28.Werner EE. Risk, resilience and recovery: Perspectives from the Kauai longitudinal study. Development and Psychopathology. 1993;5:503–515. [Google Scholar]
- 29.Werner EE. Children and war: risk, resilience, and recovery. Dev Psychopathol. 2012;24:553–558. doi: 10.1017/S0954579412000156. [DOI] [PubMed] [Google Scholar]
- 30.Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, et al. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcoholism, clinical and experimental research. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 31.Jorde A, Bach P, Witt SH, Becker K, Reinhard I, Vollstadt-Klein S, et al. Genetic variation in the atrial natriuretic peptide transcription factor GATA4 modulates amygdala responsiveness in alcohol dependence. Biol Psychiatry. 2014;75:790–797. doi: 10.1016/j.biopsych.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanraud S, Pitel AL, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Remapping the brain to compensate for impairment in recovering alcoholics. Cerebral cortex. 2013;23:97–104. doi: 10.1093/cercor/bhr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung T, Geier C, Luna B, Pajtek S, Terwilliger R, Thatcher D, et al. Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug and alcohol dependence. 2011;115:43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcoholism, clinical and experimental research. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiland BJ, Nigg JT, Welsh RC, Yau WY, Zubieta JK, Zucker RA, et al. Resiliency in adolescents at high risk for substance abuse: flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcoholism: Clinical and Experimental Research. 2012;36:1355–1364. doi: 10.1111/j.1530-0277.2012.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, Constable RT. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. NeuroImage Clinical. 2017;13:181–187. doi: 10.1016/j.nicl.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nature reviews Neuroscience. 2011;12:400–413. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- 40.Bell RP, Garavan H, Foxe JJ. Neural correlates of craving and impulsivity in abstinent former cocaine users: Towards biomarkers of relapse risk. Neuropharmacology. 2014;85:461–470. doi: 10.1016/j.neuropharm.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinz A, Dufeu P, Kuhn S, Dettling M, Graf K, Kurten I, et al. Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol-dependent patients. Archives of general psychiatry. 1996;53:1123–1128. doi: 10.1001/archpsyc.1996.01830120061011. [DOI] [PubMed] [Google Scholar]
- 42.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 44.Alvanzo AA, Wand GS, Kuwabara H, Wong DF, Xu X, Mccaul ME. Family history of alcoholism is related to increased D2 /D3 receptor binding potential: a marker of resilience or risk? Addiction biology. 2015 doi: 10.1111/adb.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson-Greene D, Adams KM, Gilman S, Koeppe RA, Junck L, Kluin KJ, et al. Effects of abstinence and relapse upon neuropsychological function and cerebral glucose metabolism in severe chronic alcoholism. J Clin Exp Neuropsychol. 1997;19:378–385. doi: 10.1080/01688639708403866. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Archives of general psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 47.Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, et al. Recovery of brain glucose metabolism in detoxified alcoholics. The American journal of psychiatry. 1994;151:178–183. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, clinical and experimental research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 50.Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, et al. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcoholism, clinical and experimental research. 2012;36:1922–1931. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burt KB, Whelan R, Conrod PJ, Banaschewski T, Barker GJ, Bokde AL, et al. Structural brain correlates of adolescent resilience. Journal of child psychology and psychiatry, and allied disciplines. 2016;57:1287–1296. doi: 10.1111/jcpp.12552. [DOI] [PubMed] [Google Scholar]
- 52.Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry. 2011;70:561–567. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. NeuroImage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deshmukh A, Rosenbloom MJ, De Rosa E, Sullivan EV, Pfefferbaum A. Regional striatal volume abnormalities in schizophrenia: effects of comorbidity for alcoholism, recency of alcoholic drinking, and antipsychotic medication type. Schizophr Res. 2005;79:189–200. doi: 10.1016/j.schres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 55.Durazzo TC, Mon A, Gazdzinski S, Yeh PH, Meyerhoff DJ. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addiction biology. 2015;20:956–967. doi: 10.1111/adb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug and alcohol dependence. 2005;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain. 2010;133:1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoefer ME, Pennington DL, Durazzo TC, Mon A, Abe C, Truran D, et al. Genetic and behavioral determinants of hippocampal volume recovery during abstinence from alcohol. Alcohol. 2014;48:631–638. doi: 10.1016/j.alcohol.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mon A, Delucchi K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. A mathematical formula for prediction of gray and white matter volume recovery in abstinent alcohol dependent individuals. Psychiatry Res. 2011;194:198–204. doi: 10.1016/j.pscychresns.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mon A, Durazzo TC, Gazdzinski S, Hutchison KE, Pennington D, Meyerhoff DJ. Brain-derived neurotrophic factor genotype is associated with brain gray and white matter tissue volumes recovery in abstinent alcohol-dependent individuals. Genes Brain Behav. 2013;12:98–107. doi: 10.1111/j.1601-183X.2012.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. The American journal of psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- 63.Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, et al. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 2014;1:202–212. doi: 10.1016/S2215-0366(14)70301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism, clinical and experimental research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 65.Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism, clinical and experimental research. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sameti M, Smith S, Patenaude B, Fein G. Subcortical volumes in long-term abstinent alcoholics: associations with psychiatric comorbidity. Alcoholism, clinical and experimental research. 2011;35:1067–1080. doi: 10.1111/j.1530-0277.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Segobin SH, Chetelat G, Le Berre AP, Lannuzel C, Boudehent C, Vabret F, et al. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcoholism, clinical and experimental research. 2014;38:739–748. doi: 10.1111/acer.12300. [DOI] [PubMed] [Google Scholar]
- 68.Van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcoholism, clinical and experimental research. 2013;37:67–74. doi: 10.1111/j.1530-0277.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- 69.Wang GY, Demirakca T, Van Eijk J, Frischknecht U, Ruf M, Ucar S, et al. Longitudinal Mapping of Gyral and Sulcal Patterns of Cortical Thickness and Brain Volume Regain during Early Alcohol Abstinence. Eur Addict Res. 2016;22:80–89. doi: 10.1159/000438456. [DOI] [PubMed] [Google Scholar]
- 70.Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- 71.Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- 72.Kalman D, Kim S, Digirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30:12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spanagel R, Durstewitz D, Hansson A, Heinz A, Kiefer F, Kohr G, et al. A systems medicine research approach for studying alcohol addiction. Addiction biology. 2013;18:883–896. doi: 10.1111/adb.12109. [DOI] [PubMed] [Google Scholar]
- 76.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tawa EA, Hall SD, Lohoff FW. Overview of the Genetics of Alcohol Use Disorder. Alcohol and alcoholism. 2016;51:507–514. doi: 10.1093/alcalc/agw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baskin-Sommers AR, Foti D. Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. Int J Psychophysiol. 2015 doi: 10.1016/j.ijpsycho.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 79.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, et al. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.